Abstract
Pregnancy requires rapid adaptations in the uterine microcirculation to support fetal development. Nanomaterial inhalation is associated with cardiovascular dysfunction, which may impair gestation. We have shown that maternal nano-titanium dioxide (nano-TiO2) inhalation impairs microvascular endothelial function in response to arachidonic acid and thromboxane (TXA2) mimetics. However, the mechanisms underpinning this process are unknown. Therefore, we hypothesize that maternal nano-TiO2 inhalation during gestation results in uterine microvascular prostacyclin (PGI2) and TXA2 dysfunction. Pregnant Sprague-Dawley rats were exposed from gestational day 10–19 to nano-TiO2 aerosols (12.17 ± 1.67 mg/m3) or filtered air (sham-control). Dams were euthanized on gestational day 20, and serum, uterine radial arterioles, implantation sites, and lungs were collected. Serum was assessed for PGI2 and TXA2 metabolites. TXB2, the stable TXA2 metabolite, was significantly decreased in nano-TiO2 exposed dams (597.3 ± 84.4 vs 667.6 ± 45.6 pg/ml), whereas no difference was observed for 6-keto-PGF1α, the stable PGI2 metabolite. Radial arteriole pressure myography revealed that nano-TiO2 exposure caused increased vasoconstriction to the TXA2 mimetic, U46619, compared with sham-controls (−41.3% ± 4.3% vs −16.8% ± 3.4%). Nano-TiO2 exposure diminished endothelium-dependent vasodilation to carbaprostacyclin, a PGI2 receptor agonist, compared with sham-controls (30.0% ± 9.0% vs 53.7% ± 6.0%). Maternal nano-TiO2 inhalation during gestation decreased nano-TiO2 female pup weight when compared with sham-control males (3.633 ± 0.064 vs 3.995 ± 0.124 g). Augmented TXA2 vasoconstriction and decreased PGI2 vasodilation may lead to decreased placental blood flow and compromise maternofetal exchange of waste and nutrients, which could ultimately impact fetal health outcomes.
Keywords: advanced materials, titanium dioxide, microcirculation, thromboxane, prostacyclin, uterine arterioles
Due to unique properties, such as increased strength, enhanced thermal/electric conductivity, and catalytic capacity, the incorporation of nanomaterials has increased in numerous products and processes (Matsoukas et al., 2015). Advanced materials exhibit novel or enhanced properties that are not dependent on nanoscale size (Kennedy et al., 2019). Nanomaterial exposures may occur throughout the life-cycle of advanced materials. This includes manufacturing, transport, use, and/or disposal (Giese et al., 2018). Products such as air/water filters, implantable devices, or contrast agents utilize nano-titanium dioxide (nano-TiO2; Abukabda et al., 2017; Matsoukas et al., 2015). We previously demonstrated that inhaled nano-TiO2 alters systemic arteriolar reactivity in the heart, spinotrapezius muscle, and mesentery (Abukabda et al., 2017; Knuckles et al., 2012; LeBlanc et al., 2010). The impact of nano-TiO2 inhalation in the adult microcirculation is established; however, its influence on biological events where rapid vascular growth and adaptation are requisite, such as gestation, is understudied.
The Developmental Origins of Health and Disease hypothesis proposes that a hostile gestational environment compromises fetal health and predisposes individuals to adult disease (Barker, 1990; Barker and Martyn, 1992). This suggests that negative impacts of maternal toxicant inhalation exposure during gestation may influence offspring health. Pregnancy requires plasticity, adaptability, and rapid responses within the growing uterus to support fetal growth. As implantation and placentation progress, the human maternal vasculature must respond to trophoblast invasion by initiating spiral artery remodeling. The spiral arteries shift from high-resistance, coiled vessels to dilated, low-resistance vessels (Bibeau et al., 2016; Boeldt and Bird, 2017; Osol and Mandala, 2009), allowing for increased blood flow to the forming placenta, and facilitating nutrient/waste exchange with the fetus. In conjunction with functional adjustments, anatomic adaptation, and enlargement of the uterine vascular tree, including uterine arterioles, occurs through angiogenesis and increasing vessel length (Bibeau et al., 2016; Boeldt and Bird, 2017; Osol and Mandala, 2009). In humans, disruption of vessel development, vascular growth, or spiral artery invasion are associated with adverse gestational outcomes such as preeclampsia or fetal growth restriction (Bibeau et al., 2016; Boeldt and Bird, 2017; Giordano et al., 2010).
A potential pathway by which toxicant exposure may impact vascular reactivity is via arachidonic acid (AA) metabolites, which are vasoactive and essential to pregnancy. Phospholipase A2 (PLA2) hydrolyzes membrane phospholipids to release AA, while the rate limiting enzymes, cyclooxygenase (COX)-1, and COX-2, metabolize AA into prostaglandin (PGH2) (Majed and Khalil, 2012). Specific PG synthases form prostacyclin (PGI2), PGD2, PGE2, PGF2α, or thromboxane A2 (TXA2; Majed and Khalil, 2012). Prostacyclin is a potent vasodilator, inhibitor of platelet aggregation (Moncada and Vane, 1978; Walsh, 2004), and is critical for trophoblast-mediated angiogenesis (Wang and Zhao, 2010). PGI2 effects are counterbalanced by TXA2, which functions as a potent vasoconstrictor and supports platelet aggregation (Boeldt and Bird, 2017; Moncada and Vane, 1978). Studies in humans and sheep have demonstrated that PGI2 and TXA2 are important for the regulation of vascular tone, blood pressure, and feto-placental blood flow (Darby et al., 2020; Mak et al., 1984; Sellers and Stallone, 2008). Rat studies on uteroplacental flow modifications in response to PGI2 and TXA2 have not been demonstrated; however, alterations to renal blood flow have been. Renal blood flow in pregnant rats was decreased with the addition of PGI2, whereas TXA2 had no effect on total peripheral resistance in pregnant rats, but increased mean arterial pressure (Jackson et al., 1982; Kriston et al., 1999). Disturbances in the balance between PGI2 and TXA2 have been associated with arterial thrombosis, vasotonic angina, pulmonary arterial hypertension, preeclampsia, and persistent pulmonary hypertension in the newborn (Majed and Khalil, 2012; Moncada and Vane, 1978; Walsh, 2004). In humans, preeclampsia has been associated with diminished PGI2 concentrations and increased TXA2 production (Walsh, 2004). Thus, an imbalance between PGI2 and TXA2 may contribute to the clinical symptoms observed in preeclampsia: hypertension, decreased uteroplacental blood flow, and platelet aggregation (Walsh, 2004).
We have reported (Knuckles et al., 2012) that in vivo spinotrapezius muscle arterioles had increased sensitivity to TX mimetic, U46619, and decreased sensitivity to the PGI2 analog, iloprost after nano-TiO2 inhalation exposure. Due to uterine microvascular reactivity changes after maternal nano-TiO2 inhalation exposure (Bowdridge et al., 2019), and the importance of PGI2 and TXA2 during pregnancy, we hypothesized that maternal nano-TiO2 inhalation during gestation alters uterine microvascular PGI2 and TXA2 reactivity. We tested this by examining the impacts of maternal nano-TiO2 inhalation during gestation on microvascular reactivity adaptations in uterine arterioles, serum metabolite levels, and COX and phospholipase A2 enzymatic activity.
MATERIALS AND METHODS
Animal model
Female Sprague-Dawley (SD) rats were purchased from Hilltop Laboratories (Scottdale, Pennsylvania) and single-housed in an American Association for Accreditation of Laboratory Animal Care (AAALAC) approved facility at West Virginia University (WVU). Rats were maintained under a regulated temperature of 20–26°C, relative humidity of 30–70%, and a 12:12-h light-dark cycle. Once acclimated for 48–72 h, rats were randomly assigned to either air (sham-control; N = 46) or nano-TiO2 (N = 46) exposure groups before mating. Rat cages were lined with standard bedding (0.25-inch corncob), and had ad libitum access to standard chow (2918X; Envigo, Indianapolis, Indiana) and water throughout the acclimation and mating periods. Mating males (N = 12) ranged in age from 3 to 9 months, and females (N = 92) were 2–4 months of age at mating. Females were vaginally swabbed to assess for stage of estrous. When found in estrus, females were placed with single-housed male for up to 24 h, and then swabbed to check for sperm within the reproductive tract.
To increase the likelihood of a viable pregnancy and pups to study, pregnant rats were exposed after implantation during mid to late gestation (gestational day [GD] 10–19) for a total of 6 days. On GD20, rats were weighed and then anesthetized with isoflurane gas (5% induction, <5 min to effect, 2–3.5% maintenance for subsequent procedures). Animals were placed on a warm heating pad and maintained at a rectal temperature of 37°C. Blood, uterine artery, and uterine radial arteriole samples were collected, and rats were euthanized. All procedures were approved by the WVU Institutional Animal Care and Use Committee.
Nanomaterial
Nano-TiO2 powder was obtained from Evonik (P25 Aeroxide TiO2, Parsippany, New Jersey) and is composed of a mixture of anatase (80%) and rutile (20%) TiO2. Particle characteristics have previously been determined, including primary particle size (21 nm), specific surface area (48.08 m2/g), and Zeta potential (−56.6 mV; Stapleton et al., 2018; Yi et al., 2013).
Inhalation exposure and aerosol characterization
Nano-TiO2 aerosols were created with a high-pressure acoustical generator (IEStechno, Morgantown, West Virginia). The generator output was fed into a Venturi pump (JS-60M, Vaccon, Medway, Massachusetts) which further de-agglomerates particles, after which the nano-TiO2 aerosol mix enters the whole-body exposure chamber. A personal DataRAM (pDR-1500; Thermo Environmental Instruments Inc, Franklin, Massachusetts) sampled air in the exposure chamber to determine aerosol mass concentration in real-time. Feedback loops within the software automatically adjust the acoustic energy needed to maintain a stable mass aerosol concentration throughout the exposure. Gravimetric aerosol sampling measurements were conducted with Teflon filters concurrently with the DataRAM measurements to obtain calibration factors. Gravimetric measurements were taken during each exposure to calculate the mass concentration measurement. Aerosol size distributions were measured in real-time in the exposure chamber at a target mass concentration of 12 mg/m3 via: (1) a high-resolution electrical low-pressure impactor (ELPI+; Dekati, Tampere, Finland); (2) a scanning particle mobility sizer (SMPS 3938; TSI Inc., St Paul, Minnesota); (3) an aerodynamic particle sizer (APS 3321; TSI Inc.); and (4) a micro-orifice uniform deposit impactor (MOUDI 115R, MSP Corp, Shoreview, Minnesota). Bedding material was soaked to maintain proper humidity (20–70%). Sham-control animals were exposed to high-efficiency particulate absorbance (HEPA)-filtered air only, with similar temperature and humidity conditions within an exposure chamber that was used only for sham-control animals.
Inhalation exposures were performed for 6 nonconsecutive days from GD 10 to 19 to maximize the chance of healthy gestations. Pregnant rats were exposed to a target concentration of 12 mg/m3, a concentration identical to that used previously for late gestation inhalation exposure studies (Stapleton and Nurkiewicz, 2014; Stapleton et al., 2013, 2018). To estimate lung deposition (dose) with nano-TiO2 aerosols, the equation D = F•V•C•T was used (Nurkiewicz et al., 2008) where F is the deposition fraction (10%), V is the minute ventilation (208.3 cc), C is mass concentration (mg/m3), and T equals the exposure duration (min) (Yi et al., 2013). The exposure paradigm (12 mg/m3, 6 h/exposure, 6 days) produced a calculated cumulative lung deposition of 525 ± 16 µg with the last exposure occurring 24-h prior to the study on GD19. The calculations represent total lung deposition and do not account for lung clearance (MPPD Software v 2.11, Arlington, Virginia).
Pressure myography with isolated uterine microvessels
Once placentas and pups were removed, uteri were placed into a dissecting dish with physiological salt solution (PSS, in mmol/l: 129.8 NaCl, 5.4 KCl, 0.5 NaH2PO4, 0.83 MgSO4, 19.0 NaHCO3, 1.8 CaCl2, and 5.5 glucose) and maintained at 4°C. A segment of the uterine radial arteriole was removed and transferred to a vessel chamber (Living Systems Instrumentation, Burlington, Vermont) containing oxygenated (21% O2/5% CO2) PSS, where it was cannulated with glass pipettes, and secured by nylon suture (11-0 ophthalmic, Alcon, UK). Arterioles were extended to their in-situ length, pressurized to 60 mm Hg, superfused with warmed (37°C) oxygenated PSS at a rate of 10 ml/min, and allowed to develop spontaneous tone. Internal and external arteriolar diameters were measured using video calipers (Colorado Video, Boulder, Colorado). Vascular tone ranges from 0% (maximum dilation) to 100% (maximal constriction), and only vessels with ≥20% spontaneous tone were included in this study. After equilibration, arteriolar vasoreactivity was analyzed.
Vasoreactivity assessments
Radial arterioles were treated abluminally with pharmacological agents to assess inner diameter changes via video calipers. Increasing concentrations of the endothelium-dependent vasodilator, acetylcholine (ACh: 10−9–10−4 M), endothelium-independent vasodilator, s-nitroso-N-acetyl-DL-penicillamine (SNAP: 10−9–10−4 M), and the COX metabolite vasodilator, carbaprostacyclin (10−20–10−4 M) were used. Vasoconstrictive agents included the α1-adrenergic vasoconstrictor, phenylephrine (PE: 10−9–10−4 M), and the COX metabolite vasoconstrictor, thromboxane (U46619, mimetic: 10−9–10−4 M). A selective, competitive antagonist for the prostacyclin receptor was also utilized (R01138452: 10−5 M) and the TXA2 receptor was inhibited by the selective, competitive antagonist SQ29 548 (10−8 M). Inhibitor doses were based on a preliminary dose-response study (R01138452: 10−9–10−3 M; SQ29 548: 10−14–10−8 M) to determine the single most effective dose to prevent uterine vascular response from occurring in the presence of carbaprostacyclin or U46619, respectively. Steady-state diameter was recorded for at least 2 min after each dose, or 10 min after the addition of receptor antagonists. Once the dose-response curve was completed, the vessel chamber was washed with PSS to remove any excess drug and PSS was replaced with Ca2+-free PSS until maximum passive diameter was established. As mentioned above, arterioles with <20% spontaneous tone were not analyzed.
Calculations
Data are expressed as mean ± standard error of the mean (SEM). Spontaneous tone was calculated by the following equation:
where Dm is the maximal diameter in response to calcium free and Di is the initial steady-state diameter recorded prior to the experiments.
Active responses to pressure were normalized to the maximal diameter using the following formula:
where Dss is the steady-state diameter recorded during each pressure change.
The experimental response to ACh, SNAP, and PGI2 are expressed as the following:
where Dcon is the control diameter recorded prior to the dose-response curve and Dss is the steady-state diameter at each dose of the curve.
The experimental response to PE and U46619 is expressed using the following equation:
Wall thickness (WT) was calculated from the measurement of both inner (ID) and outer (OD) steady-state arteriolar diameters at the end of the Ca2+ free PSS wash using the following equation:
Wall-to-lumen ratio (WLR) was calculated using the following equation:
Although assessing vascular responses to the COX metabolites, distinct subpopulations of vessels became apparent that differed in their responsiveness to both PGI2 and TXA2. To determine if a vessel qualified as hyperresponsive, the following equation was used:
where Dmax is the diameter at the maximum dose response and Dcon is the control diameter recorded prior to the dose-response curve. Once the responsiveness was calculated, vessels with percent maximal change >20% were considered hyperresponsive. Within the nano-TiO2 exposed groups, these hyperresponsive vessels made up nearly 50% of the vessels tested, whereas within sham-controls, they constituted around 5% of vessels (n = 2).
Enzyme-linked immunosorbent assays
Blood samples (3 ml) were obtained from carotid cannulas placed during anesthesia, and serum was collected. Enzyme-linked immunosorbent assays (ELISAs) were performed on maternal serum for 6-keto-PGF1α (stable PGI2 metabolite) and TXB2 (stable TXA2 metabolite). All assays were performed according to manufacturer recommendations (Cayman Chemical Company, Ann Arbor, Michigan).
Enzymatic activity assay
Uterine arteries, implantation sites, and lungs were collected and individually homogenized in PBS (Thermo Fisher Scientific, Waltham, Massachusetts) containing a protease and phosphatase cocktail (Thermo Fisher Scientific). Tissue was homogenized with a bead mill homogenizer containing 1.6 mm stainless steel beads (Next Advance, Troy, New York) and spun in a refrigerated tabletop centrifuge (Hettich Lab Instrument AB, Stockholm, Sweden) at 14 000 × g for 15 min. Supernatant was collected, kept on ice, and total protein concentrations measured according to the Bradford method (Bradford, 1976). Collected protein samples were stored at −80°C until assayed for PLA2 enzymatic activity (Thermo Fisher Scientific) and COX activity (Abcam, Cambridge, UK). To measure activity specific for cPLA2, a sPLA2 inhibitor (thioetheramide-PC) and an iPLA2 inhibitor (bromoenol lactone) were utilized (Yano et al., 2011). All assays were performed according to manufacturer recommendations.
mRNA analysis by real-time PCR
Uterine arteries, implantation sites, lungs, and ovarian tissue were collected, flash frozen in liquid nitrogen, and stored at −80°C until processed for mRNA extraction. Total RNA was extracted via the RNeasy Kit (Qiagen, Hilden, Germany) based on manufacturer’s recommendations. Total RNA was then transcribed to cDNA via the High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific).
Using real-time PCR, we assessed genes involved in the production of prostacyclin, thromboxane, and/or their receptors: COX-1 (Ptgs1), prostacyclin synthase (Ptgis), thromboxane synthase (TXAS; Tbxas1), prostacyclin receptor (Ptgir), and thromboxane receptor (Tbxa2r). Primers were purchased from Integrated DNA Technologies (Coralville, Iowa); GenBank accession numbers, primer sequences, and references are listed in Table 1. Samples were analyzed in triplicate using iTAQ Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, California). Relative fold changes in expression of candidate genes were obtained using the 2−ΔΔCt method (Livak and Schmittgen, 2001). The Ct values were used to calculate ΔCt values for genes of interest [Ct(test) – Ct(housekeeping)]. The housekeeping gene for normalization was β-actin (Actb). Statistical representation for each gene is based on fold change with respect to β-actin.
Table 1.
Real-Time PCR Primers, Their Sequence, Length, and References
| GenBank | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Length (bp) | Reference |
|---|---|---|---|---|
| Actb NM_031144 | GTAGCCATCCAGGCTGTGTTG | TGCCAGTGGTACGACCAGAG | 1296 | Zhang et al. (2017) |
| Ptgs1 NM_017043 | CTCACAGTGCGGTCCAAC | CCAGCACCTGGTACTTAAG | Bernard et al. (2000) | |
| Ptgis NM_031557 | TGGTGTGGGATCTGCGTACA | CCTCCACTCCATACAGGGTCA | 567 | Numaguchi et al. (1999) |
| Ptgir NM_001077644 | TGCTGGAACATCACCTACGT | GTTTCGAGCATAGGCCACAA | 422 | Numaguchi et al. (1999) |
| Tbxas1 D31798 | GGGGCTTCTCAAGTTCGAAGT | CCCAACTTCCTCAGTCTTGAG | 117 | Tang and Vanhoutte, (2008) |
| Tbxa2r NM_017054.1 | ATCTCCCATCTTGCCATAGTCC | CCGATGATCCTTGAGCCTAAAG | 1880 | Zhang et al. (2017) |
Primers were used in lung, ovarian, uterine artery, and implantation site tissue.
Statistics
Dose-response curves were analyzed using two-way analysis of variance (ANOVA) with repeated measures and a Šídák’s multiple comparisons analysis for point-to-point differences when main effect significance was found. Uterine radial arteriolar characteristics were assessed via one-way ANOVA utilizing a Tukey post hoc analysis when statistical significance was found. Litter characteristics were assessed via two-way ANOVA utilizing a Tukey post hoc analysis when statistical significance was found. Dam characteristics, ELISA concentrations, enzymatic activities, and mRNA analyses were assessed via unpaired t test with Welch’s correction. All statistical analyses were performed with Graph Pad Prism 9 (San Diego, California). Significance was set at p ≤ .05, with N representing the number of dams per group and n being the number of vessels. The data in this study are reported as the mean ± SEM, unless stated otherwise.
RESULTS
Nanoparticle Aerosol Characteristics
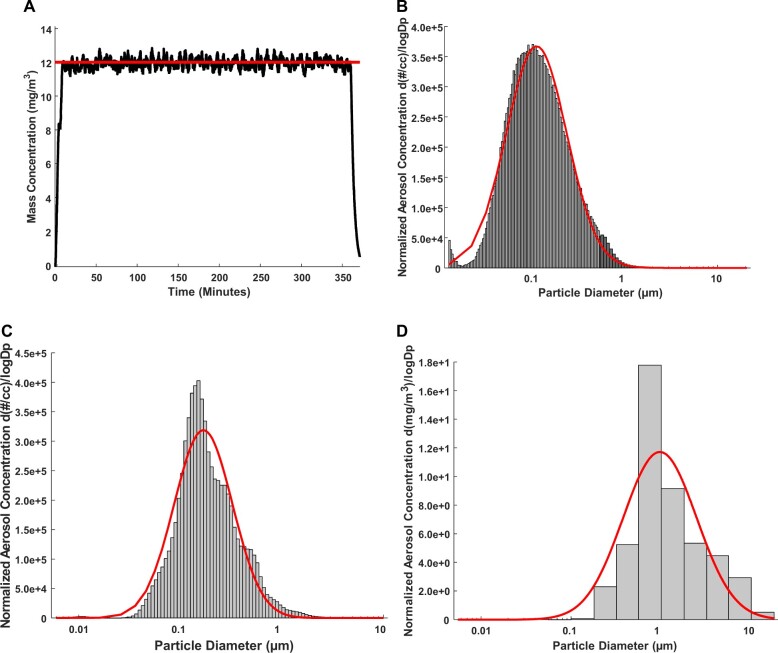
The average real-time aerosol mass concentration throughout exposures was 12.17 mg/m3 with a SD of 1.67 (Figure 1A). The aerosol mobility diameter measured via SMPS and APS, had a count median diameter (CMD) of 116 ± 2.11 nm (Figure 1B). The aerosol aerodynamic diameter, assessed via ELPI, had a CMD of 169 ± 1.94 nm (Figure 1C). Aerosol size distribution, assessed by the MOUDI, had a mass median aerodynamic diameter of 1.03 µm with a geometric SD of 2.57 (Figure 1D). We have extensively characterized the morphology of agglomerates via electron microscopy (Abukabda et al., 2019; Bowdridge et al., 2019).
Figure 1.
Nano-TiO2 aerosol real-time characterizations. Nano-TiO2 aerosol characteristics were monitored and verified throughout exposure. Red lines in the figures represent size distribution curves based on a log normal fit of the size data, unless otherwise specified. A, Aerosol mass concentration was controlled via software over the 6-h exposure paradigm. Target average concentration (red line) real-time nano-TiO2 aerosol mass concentration measurements (black line) were maintained at a target of 12 mg/m3. B, Aerosol agglomerate diameter was assessed by a SMPS (light gray) and an APS (dark gray). Based on results from the SMPS and APS the particle diameter, CMD = 116 nm, and had a GSD = 2.11. C, Electrical Low-Pressure Impactor (ELPI, light gray) assessed aerosol aerodynamic diameter at a CMD of 169 nm and a geometric SD of 1.94. D, Aerosol mass size distribution was evaluated by a Nano MOUDI; particles had an MMAD of 1.03 µm and a geometric SD of 2.57. Abbreviations: Nano-TiO2, nano-titanium dioxide; SMPS, scanning mobility particle sizer; APS, aerodynamic particle sizer; CMD, count median diameter; GSD, geometric standard deviation; MMAD, mass median aerodynamic diameter; MOUDI, Micro-Orifice Uniform Deposit Impactor. A color version of this figure appears in the online version of this article.
Pregnant Rat and Litter Characteristics
Dams showed no difference in age or weight on GD20 between groups (Table 2). There were no significant differences in litter size or number of pups per horn between treatments (Table 2). Resorption sites (right horn) were significantly decreased in the nano-TiO2 exposed group compared with sham-controls (Table 3). There was no difference between sham-control (N = 15) or nano-TiO2 exposed (N = 20) litters for wet pup weight, wet placenta weight, dry pup weight, dry placenta weight, and placental efficiency (Table 3). The ratio of male to female pups did not significantly differ between treatment groups. Litter characteristics were split based on fetal sex to determine if nano-TiO2 inhalation exposure during gestation negatively impacted fetal development in a sexually dimorphic manner. When the interaction of fetal sex and exposure were considered (Table 3), there was a significant decrease in nano-TiO2 fetal female wet pup weight (3.633 ± 0.064 g) compared with nano-TiO2 fetal males (3.998 ± 0.089 g) and sham-control fetal males (3.995 ± 0.124 g). The nano-TiO2 fetal female dry pup weight was also significantly decreased compared with the nano-TiO2 fetal male counterparts (0.4622 ± 0.014 vs 0.506 ± 0.013 g; Table 3). There was no significant difference for wet placenta, dry placenta, or placental efficiency when accounting for fetal sex. These data demonstrate that nano-TiO2 inhalation exposure during gestation negatively impacts fetal growth in a sexually dimorphic manner.
Table 2.
Dam Physical Characteristics on GD20
| Exposure | Dams (N) | Weight (g) | Age (Days) | Litter Size | Litter Distribution |
|
|---|---|---|---|---|---|---|
| Left Horn | Right Horn | |||||
| Sham-Control | 46 | 349.5 ± 5.0 | 86.8 ± 3.8 | 11.2 ± 0.5 | 5.5 ± 0.4 | 5.6 ± 0.4 |
| Nano-TiO2 exposed | 46 | 347.6 ± 4.7 | 83.2 ± 3.6 | 10.7 ± 0.6 | 5.3 ± 0.4 | 5.4 ± 0.4 |
There were no significant differences between sham-control and nano-TiO2 dams in weight, age, or litter size. Data are mean ± SEM.
Abbreviations: GD, gestational day; nano-TiO2, nano-titanium dioxide; SEM, standard error of the mean.
Table 3.
Litter Characteristics Include Wet Pup and Placenta Weight, Dry Pup and Placenta Weight, and the Litters Sex Ratio Between Groups
| Exposure | N | Wet Weight (g) |
Dry Weight (g) |
Placental Efficiency | Sex Ratio |
Resorption Sites |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pup | Placenta | Pup | Placenta | Male | Female | Left Horn | Right Horn | |||
| Sham-control | 15 | 3.760 ± 0.074 | 0.758 ± 0.033 | 0.483 ± 0.009 | 0.107 ± 0.004 | 5.056 ± 0.216 | 0.482 ± 0.035 | 0.504 ± 0.034 | 0.500 ± 0.154 | 0.750 ± 0.228 |
| Nano-TiO2 exposed | 20 | 3.720 ± 0.060 | 0.716 ± 0.027 | 0.493 ± 0.011 | 0.113 ± 0.008 | 5.152 ± 0.304 | 0.482 ± 0.035 | 0.504 ± 0.034 | 0.286 ± 0.122 | 0.143 ± 0.078 * |
| Sham-control fetal males | 15 | 3.995 ± 0.124 | 0.760 ± 0.032 | 0.499 ± 0.008 | 0.108 ± 0.005 | 5.168 ± 0.214 | — | — | — | — |
| Sham-control fetal females | 15 | 3.837 ± 0.107 | 0.757 ± 0.038 | 0.474 ± 0.009 | 0.109 ± 0.005 | 4.745 ± 0.184 | — | — | — | — |
| Nano-TiO2 exposed fetal males | 20 | 3.998 ± 0.089 | 0.709 ± 0.028 | 0.506 ± 0.013 | 0.104 ± 0.003 | 5.435 ± 0.288 | — | — | — | — |
| Nano-TiO2 exposed fetal females | 20 | 3.633 ± 0.064*,^ | 0.734 ± 0.29 | 0.462 ± 0.014^ | 0.106 ± 0.003 | 4.956 ± 0.297 | — | — | — | — |
The second portion is fetal characteristics based on the fetal sex for the litter average. N is the number of dams. Bold font is used to draw attention to the fact that the data points are significant. Data are mean ± SEM.
Abbreviations: GD, gestational day; nano-TiO2, nano-titanium dioxide; SEM, standard error of the mean.
p ≤ .05 versus sham-control fetal males;
p ≤ .05 versus nano-TiO2 fetal males.
When the uterine radial arteriole reactivity to PGI2 and TXA2 are considered, litter characteristics were split based on the corresponding uterine arteriole response to either PGI2 or TXA2. Based on the uterine arteriolar response, litter characteristics were split by augmented PGI2 nano-TiO2 exposed, nonaugmented PGI2 nano-TiO2 exposed, augmented TXA2 nano-TiO2 exposed, and nonaugmented PGI2 nano-TiO2 exposed. Litter characteristics based on corresponding uterine arteriolar response were not significantly different in any pup or placental measurements (data not included).
Arteriolar Characteristics
Resting characteristics of radial arterioles for sham-control and nano-TiO2 exposed groups are shown in Table 4. There were no differences between groups for inner diameter, outer diameter, WT, or WLR. Based on results obtained from uterine radial arteriole reactivity, it was determined there were subpopulations of vessels that displayed augmented or nonaugmented responses to TXA2 and PGI2. Due to these unique responses, arteriolar characteristics were split based on radial arteriole responses to TXA2 or PGI2. There was a significant increase in tone for PGI2 nonaugmented nano-TiO2 exposed vessels compared with sham-control and total nano-TiO2 exposed vessels. Passive inner and outer diameter were significantly decreased in the TXA2 augmented nano-TiO2 exposed vessels compared with both TXA2 nonaugmented nano-TiO2 exposed vessels and PGI2 augmented nano-TiO2 exposed vessels. These data provide evidence that maternal nano-TiO2 exposure during gestation leads to anatomical changes within the uterine arteriole vessels.
Table 4.
Uterine Radial Arteriolar Characteristics on GD 20
| Exposure | n | Inner Diameter (µm) | Outer Diameter (µm) | Tone % | Passive Diameter Inner (µm) | Passive Diameter Outer (µm) | Wall Thickness | Wall-to-Lumen Ratio |
|---|---|---|---|---|---|---|---|---|
| Sham-control | 28 | 97.00 ± 5.362 | 131.3 ± 7.570 | 22.64 ± 0.569 | 128.8 ± 6.837 | 156.3 ± 8.340 | 12.84 ± 0.949 | 0.082 ± 0.003 |
| Nano-TiO2 exposed | 33 | 88.52 ± 4.238 | 117.9 ± 4.721 | 23.45 ± 0.704 | 118.7 ± 4.952 | 144.2 ± 6.127 | 12.60 ± 0.813 | 0.090 ± 0.004 |
| Thromboxane nonaugmented nano-TiO2 exposed | 18 | 100.1 ± 6.835 | 137.9 ± 7.904 | 22.12 ± 0.837 | 144.3 ± 9.019 | 175.0 ± 11.09 | 14.47 ± 1.186 | 0.106 ± 0.008 |
| Thromboxane augmented nano-TiO2 exposed | 17 | 83.94 ± 6.185 | 112.8 ± 7.317 | 25.53 ± 1.186 | 100.5 ± 3.396Φ , † | 119.6 ± 4.071Φ , † | 9.938 ± 0.649 | 0.087 ± 0.005 |
| Prostacyclin nonaugmented nano-TiO2 exposed | 15 | 84.27 ± 5.027 | 121.3 ± 7.525 | 30.80 ± 2.909* , ^ | 123.7 ± 7.481 | 148.4 ± 9.294 | 12.37 ± 1.173 | 0.099 ± 0.007 |
| Prostacyclin augmented nano-TiO2 exposed | 19 | 106.2 ± 7.755 | 143.8 ± 9.594 | 23.66 ± 0.952 | 139.2 ± 10.38Φ | 167.0 ± 12.39Φ | 12.91 ± 1.133 | 0.120 ± 0.008 |
n represents the number of vessels assessed. Bold font is used to draw attention to the fact that the data points are significant. Data are mean ± SEM.
Abbreviations: GD, gestational day; nano-TiO2, nano-titanium dioxide; SEM, standard error of the mean; TXA2, thromboxane A2; PGI2, prostacyclin I2.
p ≤ .05 versus sham-control;
p ≤ .05 versus nano-TiO2 exposed;
p ≤ .05 versus TXA2 nonaugmented nano-TiO2 exposed;
p ≤ .05 versus PGI2 augmented nano-TiO2.
Uterine Radial Arteriole Reactivity
Arteriolar responses to the endothelium-dependent vasodilator ACh (Figure 2A), endothelium-independent vasodilator SNAP (Figure 2B), and the adrenergic vasoconstrictor PE (Figure 2C), did not differ significantly between groups.
Figure 2.
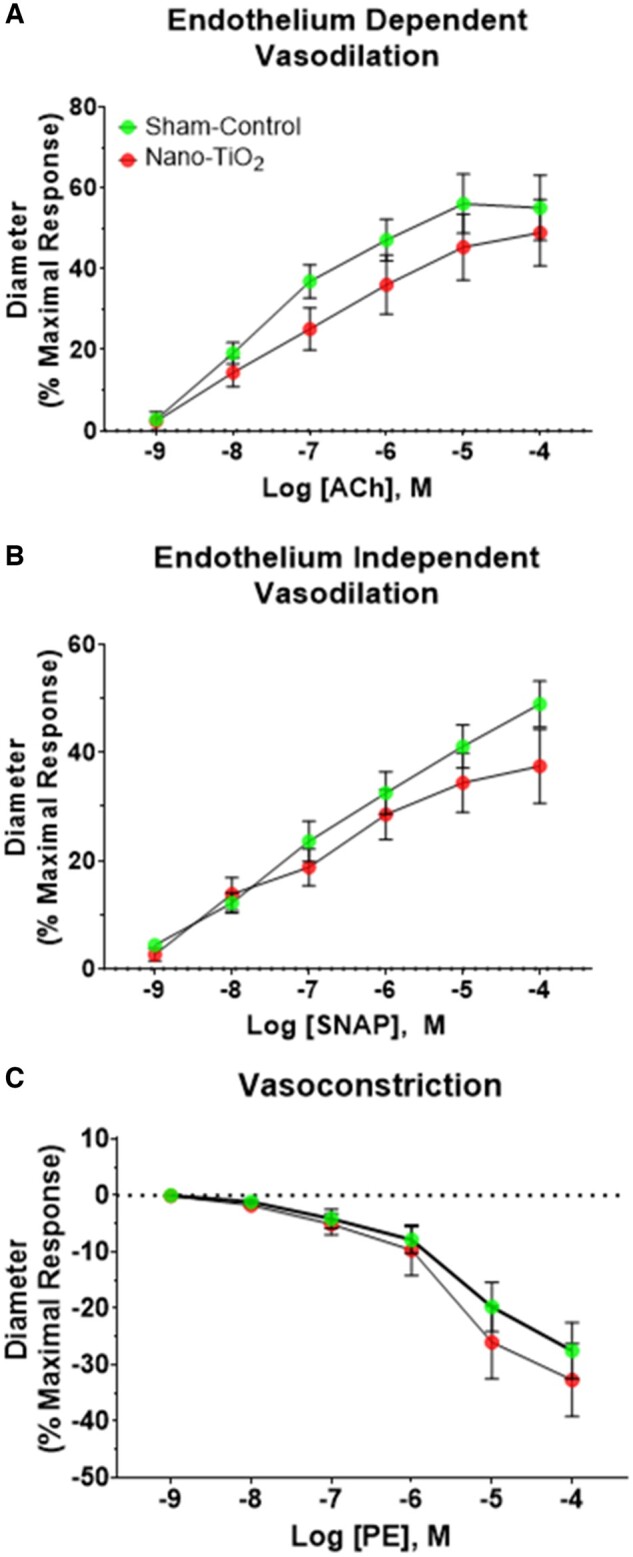
Vascular reactivity of uterine radial arterioles. Dose-response curves were performed to assess vascular reactivity of uterine radial arterioles (n = 29 for sham-control and n = 18 for nano-TiO2). A, Vascular reactivity following increasing doses of ACh, an endothelium-dependent vasodilator, (B) SNAP, an endothelium-independent vasodilator, and (C) PE, vasoconstrictor. *p ≤ .05 versus sham-control. Abbreviations: Nano-TiO2, nano-titanium dioxide; Ach, acetylcholine; SNAP, s-nitroso-N-acetyl-DL-penicillamine; PE, phenylephrine.
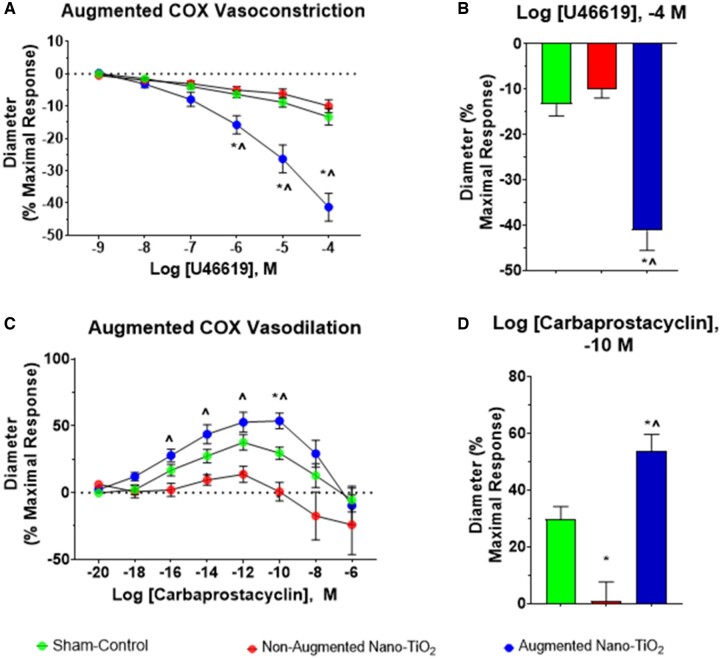
Within the exposed vessels, a subpopulation of vessels that displayed an augmented vasoconstrictive response to TXA2 was observed. We defined this as vessels that exceeded a 20% change in maximal response tone compared with their initial measurement before TXA2 treatment. The maximal vasoconstrictive response to TXA2 in this hyper-reactive subset of nano-TiO2 exposed vessels was significantly greater compared with sham-control vessels (−41.3% ± 4.3% vs −16.8% ± 3.4%). These augmented vessels also exhibited a significant increase in cumulative vasoconstriction compared with nonaugmented nano-TiO2 exposed vessels (−41.3% ± 4.3% vs −10.0% ± 2.0%). There was no difference between nonaugmented nano-TiO2 exposed vessels and sham-control vessels. (Figs. 3A and 3B).
Figure 3.
Cyclooxygenase metabolites vascular radial reactivity. Subpopulations of nano-TiO2 exposed uterine radial arterioles displayed hyperreactive responses to both COX metabolites. This subpopulation of vessels is shown herein as a separate group, and dose-response curves were assessed for vascular reactivity. A, Vascular responses following increasing doses of U46619, a TXA2 mimetic (n = 29 for sham-control, n = 8 for nonaugmented nano-TiO2, and n = 9 for augmented nano-TiO2). B, Maximum response to U46619 across the dose-response curve. C, Vascular reactivity following increasing doses of carbaprostacyclin, a stable PGI2 agonist (n = 29 for sham-control, n = 5 for nonaugmented nano-TiO2, and n = 11 for augmented nano-TiO2). D, Maximum response to carbaprostacyclin across the dose-response curve. *p ≤ .05 versus sham-control. ^p ≤ .05 versus nano-TiO2. Abbreviations: nano-TiO2, nano-titanium dioxide; COX, cyclooxygenase; TXA2, thromboxane A2; PGI2, prostacyclin I2.
A similar phenomenon occurred in nano-TiO2 exposed vessels in the presence of PGI2, with the augmented vessels presenting increased vasodilation (Figure 3C). At the maximal response dose, there was a significant difference between sham-controls and the augmented nano-TiO2 exposed group (30.0% ± 9.0% vs 53.7% ± 6.0%; Figure 3D). There was also a significant difference between sham-control and nonaugmented nano-TiO2 exposed vessels (30.0% ± 9.0% vs 1.0% ± 7.0%). Finally, the augmented nano-TiO2 exposed vessels were significantly different when compared with nonaugmented nano-TiO2 exposed vessels (53.7% ± 6.0% vs 1.0% ± 7.0%). It should be noted that 21% of all exposed vessels were hyperresponsive to PGI2 and TXA2, but the remainder only exhibited an augmented response to 1 agonist.
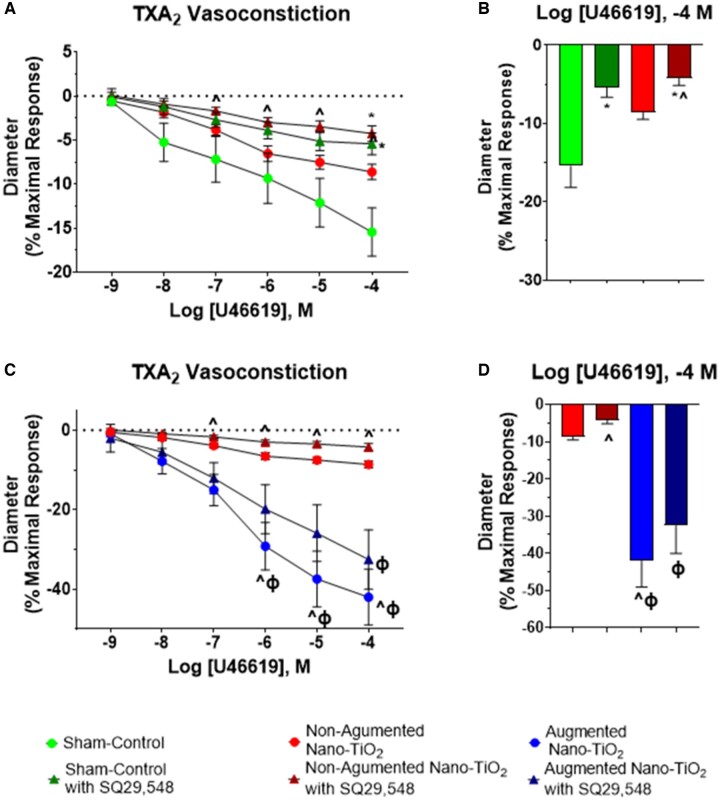
Arteriolar vasoconstriction to U46619 was tested in the presence of the thromboxane receptor antagonist, SQ29,548 for sham-control and nano-TiO2-exposed groups. This inhibitor was utilized to test for the nonreceptor mediated effects of TXA2. There was a significant difference between nonaugmented nano-TiO2 alone and nonaugmented nano-TiO2 with SQ29,548 vessels at the 10−6,−5,−4 doses (Figure 4A). At the maximal dose of U46619, there was a significant decrease in vasoconstriction of sham-control with SQ29,548 vessels compared with sham-control vessels alone (−5.4% ± 1.2% vs −15.4% ± 2.7%). At the maximal dose of U46619, there was also a significant decrease in vasoconstriction of nano-TiO2 with SQ29,548 vessels versus sham-control arterioles (−4.3% ± 0.9% vs −15.4% ± 2.7%; Figure 4B). Figure 4 also highlights the uterine radial arteriole vasoconstriction to U46619 in sham-control, nano-TiO2 nonaugmented, and augmented nano-TiO2 vessels with or without SQ29,548. When comparing nano-TiO2 exposed nonaugmented vessels to the augmented nano-TiO2 vessels, there was a significant increase in vasoconstrictive capabilities of the augmented vessels across the higher doses (Figure 4C). The augmented nano-TiO2 vessels also exhibited increased vasoconstriction compared with nano-TiO2 vessels with SQ29,548 amongst the higher doses in the dose-response curve (Figure 4C). At the maximal dose (Figure 4D), there was a significant increase in vasoconstriction for augmented exposed vessels compared with nano-TiO2 nonaugmented arterioles (−42.0% ± 7.0% vs −8.6% ± 0.9%). When compared with nano-TiO2 nonaugmented with SQ29,548, there was a significant increase in vasoconstriction for the augmented vessels (−4.2% ± 0.9% vs −42.0% ± 7.0%) and for the augmented vessels with SQ29,548 (−4.2% ± 0.9% vs −32.6% ± 7.5%).
Figure 4.
TXA2 vascular reactivity of uterine radial arterioles in the presence of antagonists. Uterine radial arterioles were exposed to a single dose of the thromboxane receptor antagonist, SQ29,548, and then a dose-response curve to U46619, a stable TXA2 mimetic, was performed. A, Sham-control (n = 12) versus nano-TiO2 (n = 11) vascular response following antagonist addition and increasing doses of U46619. B, Maximum response to U46619, in the presence of the receptor antagonist, across the dose-response curve. C, Nonaugmented nano-TiO2 (n = 11) versus augmented nano-TiO2 (n = 8) vascular response following antagonist addition and increasing doses of U46619. D, Maximum response to U46619 in the presence of the antagonist across the dose-response curve. *p ≤ .05 versus sham-control. ^p ≤ .05 versus nano-TiO2. Φp ≤ .05 versus nano-TiO2 with SQ29,548. Abbreviations: nano-TiO2, nano-titanium dioxide; TXA2, thromboxane A2.
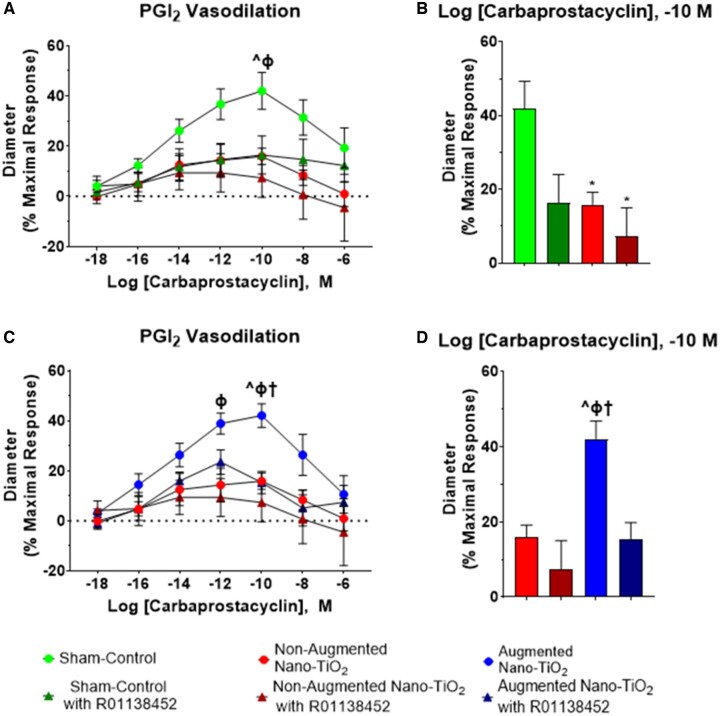
A significant decrease in uterine radial vasodilation was observed in sham-control versus all nano-TiO2 vessels (42.0% ± 7.3% vs 15.9% ± 3.5%), as well as in sham-control vessels compared with all nano-TiO2 with R01138452-treated arterioles (42.0% ± 7.3% vs. 7.3% ± 7.7%; Figure 5). Figure 5B highlights differences at the dose wherein sham-control vessels were most vasodilatory. When comparing augmented nano-TiO2 exposed vessels to sham-control arterioles, there was a significant increase in vascular vasodilation for the augmented nano-TiO2 vessels (Figure 5C). At the dose that elicited a maximal response in sham-control vessels, there was a significant increase in the vasodilation of augmented nano-TiO2 vessels compared with nano-TiO2 nonaugmented vessels (42.1% ± 4.7% vs 15.9% ± 3.3%). Figure 5D also highlights the significant increase in augmented radial arteriole vasodilation compared with nano-TiO2 nonaugmented vessels treated with R01138452 (42.1% ± 4.7% vs 7.3% ± 7.7%) and when compared with the nano-TiO2 augmented vessels treated with R01138452 (42.1% ± 4.7% vs 15.3% ± 4.4%). Overall, these data highlight how maternal nano-TiO2 inhalation exposure during gestation results in uterine radial arteriolar dysfunction in response to TXA2 and PGI2.
Figure 5.
PGI2 vascular reactivity of uterine radial arterioles in the presence of antagonists. Prostacyclin receptor antagonist, R01138452, was added to the bath as a single dose, and then a dose-response curve of carbaprostacyclin, a stable PGI2 agonist, followed. A, Sham-control (n = 12) versus nonaugmented nano-TiO2 (n = 8) vascular response following antagonist addition and then increasing doses of carbaprostacyclin. B, Maximum response to pretreated vessels with the prostacyclin receptor antagonist is represented in the bar graph. C, Nonaugmented nano-TiO2 (n = 8) versus augmented nano-TiO2 (n = 8) vascular response after R01138452 pretreatment to dose-response curve. D, Maximum response to carbaprostacyclin in the presence of the antagonist across the dose-response curve. *p ≤ .05 versus sham-control. ^p ≤ .05 versus nano-TiO2. Φp ≤ .05 vs nano-TiO2 with R01138,452. †p ≤ .05 versus augmented nano-TiO2 with R01138. Abbreviations: PGI2, prostacyclin I2; nano-TiO2, nano-titanium dioxide.
Serum 6-keto-PGF1α and TXB2
ELISA assays were utilized to measure 6-keto-PGF1α, and TXB2, the stable metabolites of PGI2 and TXA2, respectively. Circulating serum concentrations of 6-keto-PGF1α were not different between sham-control and nano-TiO2 exposed groups (597.3 ± 84.4 vs 667.6 ± 45.6 pg/ml; Figure 6A). Serum concentrations of TXB2 were significantly decreased in nano-TiO2 exposed dams (1483 ± 63.8 vs 1978 ± 313.2 pg/ml; Figure 6B). Therefore, maternal nano-TiO2 inhalation exposure appears to influences the gestational endocrine environment by decreasing circulating levels of TXB2.
Figure 6.
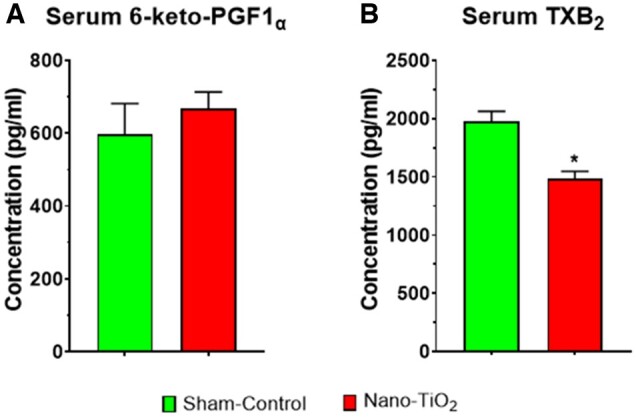
Circulating 6-keto-PGF1α and TXB2 serum concentration. Maternal sham-control (N = 9) versus nano-TiO2 (N = 9) circulating levels of 6-keto-PGF1α (A) and TXB2 (B) in pg/ml. *p ≤ .05 versus sham-control. Abbreviations: 6-keto-PGF1α, 6-keto-prostaglandin F1α; nano-TiO2, nano-titanium dioxide; TXB2, thromboxane B2.
Cytosolic Phospholipase A2 Enzymatic Activity
Cytosolic PLA2 (cPLA2) was measured as an indicator of AA production and COX activity. Uterine artery levels of cPLA2 were not different between sham-control and nano-TiO2 exposed groups (13.4 ± 8.7 vs 18.6 ± 12.6 U/mg; Figure 7A). Implantation sites were also assessed, with cPLA2 activity (Figure 7B) being significantly decreased in nano-TiO2 exposed dams (54.3 ± 2.7) when compared with sham-controls (59.6 ± 3.2). The cPLA2 activity changes seen in nano-TiO2 exposed dams demonstrate that maternal inhalation exposure during gestation exerts an effect on AA production specifically within the uterus at the implantation site of the embryo.
Figure 7.
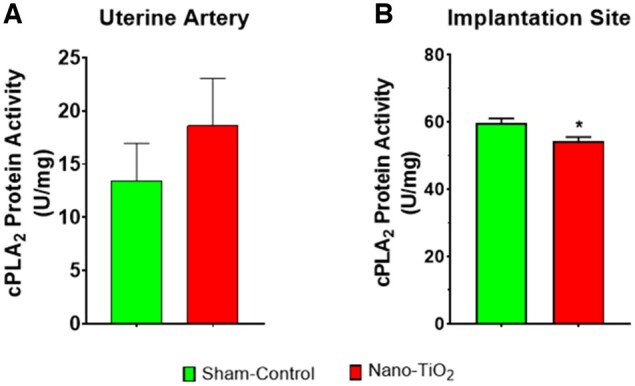
Cytosolic PLA2 enzymatic activity. Cytosolic PLA2 enzymatic activity (N = 5–8) in (A) uterine artery and (B) implantation sites in U/mg. *p ≤ .05 versus sham-control. Abbreviations: PLA2, phospholipase A2.
COX Enzymatic Activity
COX-2 activity within the lung was not significantly different between groups, but COX-2 activity tended (p = 0.067) to be lower in nano-TiO2 exposed lung tissue when compared with sham-control (2.8 ± 3.7 vs 12.3 ± 5.4 µU/mg; Figure 8A). Uterine artery COX-2 activity did not differ significantly between sham-control or nano-TiO2 exposed dams (49.1 ± 48.6 vs 19.8 ± 27.4 µU/mg; Figure 8B). COX-2 activity at implantation sites was not significantly different between sham-control and nano-TiO2 exposed groups (2.3 ± 1.7 vs 1.8 ± 0.3 µU/mg; Figure 8C). COX-2 activity was not significantly different between groups, which may be due to the lag time between the last exposure and tissue collection.
Figure 8.
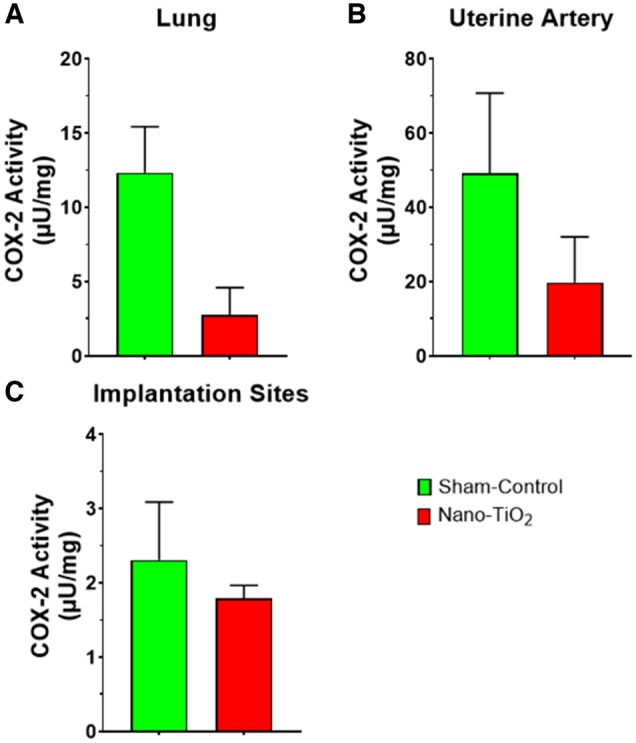
COX enzymatic activity. COX-2 enzymatic activity (N = 4–6) in (A) lung, (B) uterine artery, and (C) implantation sites in µU/mg. Abbreviations: COX, cyclooxygenase.
mRNA Expression of COX-1, PGIS, TXAS, PGR, and TXR
Expression of mRNA for COX-1, prostacyclin synthase, prostacyclin receptor, thromboxane synthase, and thromboxane receptor was determined in lung tissue from dams due to the direct exposure of that tissue to nano-TiO2 (Table 5). There was a significant decrease in mRNA fold-expression for prostacyclin receptor in nano-TiO2 exposed dams compared with sham-controls (1.0 ± 0.4 vs 0.3 ± 0.1). In contrast, prostacyclin synthase was significantly increased in lung tissue of nano-TiO2 exposed dams compared with sham-control dams (1.0 ± 0.9 vs 2.5 ± 0.3). Expression of mRNA for COX-1, thromboxane synthase, or thromboxane receptor was not significant between groups. Assessment of implantation sites failed to detect differences between groups for thromboxane synthase or thromboxane receptor. Although there was not a significant difference between groups for COX-1 (1.0 ± 0.5 vs 5.6 ± 0.9), prostacyclin synthase (1.0 ± 0.2 vs 2.2 ± 0.4), and prostacyclin receptor (1.0 ± 0.2 vs 1.8 ± 0.9) all 3 had elevated gene expression in the nano-TiO2 exposed tissue compared with sham-control. Assessment of placentas (sex not accounted for) was not significantly different between groups for COX-1, prostacyclin synthase, or thromboxane synthase. Although not significant, there was decreased prostacyclin receptor (0.46 ± 0.44 vs 1.00 ± 0.56) and increased thromboxane receptor (1.41 ± 0.38 vs 1.00 ± 0.37) gene expression in the nano-TiO2 exposed tissue compared with sham-control. Maternal ovaries were also examined due to the importance of the ovaries for production of prostaglandins and their critical role in the rat’s ability to maintain a pregnancy. There were no significant differences noted in the ovary for any of the genes examined (data not shown). Examination of uterine arteries gene markers likewise failed to produce significant differences between nano-TiO2 exposed and sham-control dams (data not shown). Maternal nano-TiO2 inhalation exposure affected gene expression in lung tissue, which is likely due to the direct insult delivered by nanoparticle deposition.
Table 5.
RT-PCR results for lung implantation site, and placental tissue
| N | Lung |
Implantation Sites |
Placenta |
||||
|---|---|---|---|---|---|---|---|
| Sham-Control | Nano-TiO2 | Sham-Control | Nano-TiO2 | Sham-Control | Nano-TiO2 | ||
| Cyclooxygenase-1 | 6 | 1.00 ± 0.61 | 0.73 ± 0.39 | 1.00 ± 0.52 | 5.57 ± 0.90 | 1.00 ± 0.42 | 1.09 ± 0.30 |
| Prostacyclin synthase | 6 | 1.00 ± 0.85 | 2.50 ± 0.28* | 1.00 ± 0.22 | 2.24 ± 0.37 | 1.00 ± 0.14 | 1.32 ± 0.27 |
| Prostacyclin receptor | 6 | 1.00 ± 0.39 | 0.31 ± 0.13* | 1.00 ± 0.23 | 1.78 ± 0.89 | 1.00 ± 0.56 | 0.46 ± 0.44 |
| Thromboxane synthase | 6 | 1.00 ± 0.28 | 0.48 ± 0.85 | 1.00 ± 0.51 | 0.56 ± 0.71 | 1.00 ± 0.14 | 0.78 ± 0.26 |
| Thromboxane receptor | 6 | 1.00 ± 0.49 | 0.98 ± 0.39 | 1.00 ± 0.08 | 0.84 ± 0.72 | 1.00 ± 0.37 | 1.41 ± 0.38 |
N represents the number dams used for tissue processing. Data are fold-change and represent mean ± SEM. Bold font is used to draw attention to the fact that the data points are significant.
Abbreviations: SEM, standard error of the mean; nano-TiO2, nano-titanium dioxide.
p ≤ .05 versus sham-control.
DISCUSSION
The primary aim of this study was to determine the effects of maternal nano-TiO2 inhalation during gestation on uterine microvascular reactivity in response to the COX metabolites, PGI2, and TXA2. The exposure paradigm used in this study with nano-TiO2 inhalation during gestation models and exposure pregnant women in an occupational setting would experience. This is mean to encompass the time periods in which these women may or may not be aware of their pregnancy and also continue to work until late gestation. We demonstrated that nano-TiO2 inhalation exposure during gestation impaired endothelium-dependent vasodilation, augmented TXA2-induced vasoconstriction in radial uterine arterioles, decreased fetal female pup mass, decreased resorption sites, increased lung prostacyclin synthase mRNA expression, decreased circulating TXB2 concentration, and decreased implantation site cPLA2. Decreased vasodilation as a result of inhalation exposure was an anticipated result, as it has been previously observed (Knuckles et al., 2012; LeBlanc et al., 2010). In a normal pregnancy, vascular tone decreases due to outward vascular remodeling, thus decreasing vascular resistance and increasing blood flow to the gravid uterus (Boeldt and Bird, 2017; Osol and Mandala, 2009). Vascular tone, which impacts vascular resistance and thus blood flow, is the product of a variety of factors including: myogenic activity, vasoconstrictor and vasodilatory influences, and flow or shear-stress (Hu and Zhang, 2021). It should be noted that arteriolar resistance is the result of two forms of tension regulatory elements: passive and active (Bohlen and Lash, 1994; Lash et al., 1991). Active tension is the dominant regulator of arteriolar diameter via physiologic signaling, such as vasoactive compounds, blood pressure, neurogenic activity, and metabolic state (Bohlen and Lash, 1994; Lash et al., 1991). Passive tension contributes broadly to arteriolar diameter and is associated with structural and anatomical characteristics of the arteriolar wall (Bohlen and Lash, 1994). Microcirculation heterogeneity is based on endothelial factors, smooth muscle cells, and the local microcirculation environment (Boegehold, 1998). Disruptions in vasoactive compound concentrations/activity (active tension), vascular remodeling (passive tension), and vascular resistance may ultimately disrupt blood flow and adversely impact fetal growth and development. Studies on rat uterine artery blood flow restriction found that decreased uterine blood flow via ligation, or due to ozone exposure, resulted in reduced fetal body weight (Miller et al., 2017; Wlodek et al., 2005). In this study, decreased vasodilation and increased vasoconstriction may have contributed to poor fetal health consequences as evidenced by decreased fetal female pup weights (Table 3).
The lung tissue collected from nano-TiO2 exposed dams exhibited increased gene expression of PGIS and decreased prostacyclin receptor (Table 5). Additionally, serum from dams contained elevated circulating levels of PGI2 (Figure 6). The increase in mRNA expression for PGIS in the lungs and elevated serum levels of PGI2 seen in our study is likely in response to inflammatory signals arising from the lungs, as it is the initial site of deposition. We have previously shown that pulmonary nano-TiO2 exposure is associated with increased interleukin (IL)-1β, 4, 5, 13, and tumor necrosis factor (TNF)-α in the plasma and bronchoalveolar lavage fluid, indicative of an innate inflammatory response (Abukabda et al., 2018). IL-1β has been reported to increase PGI2 production in hypoxic environments (Camacho et al., 2011). Due to the interdependency of the pulmonary and cardiovascular systems, inflammatory responses that begin in the lung may potentially enter the circulation and result in systemic effects. This is part of the hypothesis by which inhaled air pollution impart negative cardiovascular effects (Robertson and Miller, 2018). Considered together during pregnancy, inflammatory signals arising from the lungs could ultimately have detrimental effects on uteroplacental blood flow.
Traditionally, a lung insult and the subsequent inflammatory response results in a swift increase of TXA2 (Ishitsuka et al., 2004; Park and Christman, 2006; Wang et al., 2020). This is followed by increased PGI2 acting as an antiinflammatory agent to increase blood flow to the inflammatory site (Davies et al., 1984; Park and Christman, 2006). Given previous work, we anticipated that TXB2 would be increased after exposure, but this was not the case. However, thrombin and cytokines also stimulate production of PGI2, likely in an effort to balance any vasoconstrictive responses to TXA2 (Majed and Khalil, 2012). The increased PGI2, along with elevated nitric oxide (NO) activity, is known to oppose TXA2-induced vasoconstriction by desensitization of the thromboxane receptor (Goulopoulou, 2017). The increased TXA2 would result in more robust vasoconstriction at the uteroplacental level, thus decreasing blood flow to the placenta and hindering the nutrient-waste exchange.
PGI2 is mostly produced by endothelial cells but has an important role in the feedforward mechanism of parturition within the myometrium. TXA2 is produced by endothelial cells, activated platelets, and macrophages. Circulating blood levels of rat 6-keto-PGF1α is reported to be approximately 2.78 ± 0.05 ng/ml and rat TXB2 is reported to be approximately 9.34 ± 1.19 ng/ml (Verlohren et al., 2008). Maternal nano-TiO2 inhalation exposure decreased PGI2-induced vasodilation. This is consistent with observations in preeclamptic patients (Boeldt and Bird, 2017; Hu and Zhang, 2021). The decrease we observed in endothelium-dependent vasodilation, similar to what we have previously reported (Abukabda et al., 2018; LeBlanc et al., 2010), is potentially the result of decreased NO bioavailability (Nurkiewicz et al., 2009), ultimately impairing vasodilatory capacity. It should be noted that the AA pathway is complex and requires multiple cells and receptors to complete its steps, and as such, is highly dependent on the microenvironment around these cells (Boegehold, 1998). The heterogeneity of the microcirculation is important to this point, in that the vessels being studied can produce different responses based on their regional location (tissue), segmental location, and circulating factors. We speculate this heterogeneity contributes to disparities between this study and previous studies. In isolated coronary arterioles, we observed that nano-TiO2 inhalation had no significant effect on vasoconstriction to the TXA2 mimetic, U46619 (LeBlanc et al., 2010). This is different from our study herein in which uterine radial arterioles were studied and had increased vasoconstrictor responses to TXA2. Additionally, we found a subpopulation of vessels that were hyper-responsive to TXA2 and/or PGI2 (Figure 3). The increased TXA2-induced vasoconstriction of radial uterine arterioles (Figure 3C) is seen in other gestational disease states such as preeclampsia, or intrauterine growth restriction (IUGR). In preeclamptic women, the endothelium reduces vasodilator production and fails to become insensitive to vasoconstrictors (Boeldt and Bird, 2017). Thus, these women present with increased uterine vascular resistance, when there would normally be low resistance to encourage nutrient-waste exchange in the placenta. Preeclamptic women also exhibit increased circulating levels of TXB2, hypertension, proteinuria, and decreased fetal weight at term (Goulopoulou, 2017; Gunnarsdottir et al., 2018). Similarly, our model does show increased vasoconstriction (Figs. 3A and 4) and decreased fetal weight at term (Table 3). This may be evidence that uterine radial arterioles are compensating for decreased circulating TXA2 by being hyper-responsive to TXA2 stimulation. Increased vasoconstriction and decreased vasodilation, as seen in this study, have the potential to increase placental vascular resistance and ultimately lead to decreased blood flow to the placenta.
We observed a subpopulation of uterine radial arterioles that exhibited augmented vasodilation to PGI2 (Figs. 3A and 5). Vessels hyper-responsive to PGI2, unlike those that were hyper-responsive to TXA2, presented minimal dilatory responses to the prostacyclin receptor antagonist (Figure 5D). The hyper-responsiveness of the radial uterine arterioles was surprising, mainly because we have previously demonstrated decreased vasodilatory responses after nano-TiO2 inhalation exposures (Abukabda et al., 2018; LeBlanc et al., 2010). It should be noted that studies are lacking on these augmented responsive vessels to vasodilatory agents such as PGI2. A similar augmented vasodilatory phenomenon can be found in some forms of IUGR and may be compensatory mechanisms aimed at maintaining fetal blood flow to near normal levels (Cohen et al., 2015; Fleiss et al., 2019). In a rat model where IUGR is induced by a low-Na+ diet to reduce maternal blood volume expansion and uteroplacental perfusion, it was found that radial uterine artery relaxation to carbachol, a parasympathomimetic that stimulates muscarinic and nicotinic receptors, was increased in IUGR rats (Bigonnesse et al., 2018). This study also found that relaxation in IUGR radial arterioles was increased in response to NO donors (Bigonnesse et al., 2018). These studies indicate that when vasoconstriction is augmented, such as in our study, the body attempts to maintain a homeostatic balance by increasing vasodilatory responses, suggesting our results could reflect a compensatory mechanism. In the absence of this compensatory mechanism, fetal outcomes may be more pronounced due to perturbed blood flow and nutrient-waste exchange between, the uteroplacental unit.
Although sex ratio was not significantly different, there appears to be a relationship between wet pup weight and sex in exposure groups that should be further studied in the future. Neither fetal sex nor location within the uterine horn was accounted for during tissue collection in this study, which may explain at least in part, the large variability seen in gene expression within the placenta (Table 5), implantation site tissue (Table 5), and enzymatic activity (Figs. 7B and 8C). It has been shown that fetal and placental size is sexually dimorphic, with male fetuses and their placentas being larger throughout gestation (Gonzalez et al., 2018). Maternal vascular function and effects of toxic exposure during gestation are also influenced by fetal sex (Gabory et al., 2013). In normal pregnancies with a male fetus, microvascular vasodilation induced by corticotrophin is enhanced compared with pregnancies with a female fetus (Clifton et al., 2012). Based on this information, it is plausible that some of the variability seen in AA metabolite gene expression within implantation sites and placental tissue may be due to sexually dimorphic responses after maternal nano-TiO2 inhalation exposure during gestation. This would require further study to determine if toxicant exposure is causing sexually dimorphic changes to AA metabolites.
Maternal nano-TiO2 inhalation exposure during gestation results in increased TXA2-induced arteriolar vasoconstriction, decreased PGI2-induced vasodilation, and decreased female fetal weight. Our exposure paradigm also provides evidence that maternal nano-TiO2 inhalation exposure during gestation augments the microvascular response to thromboxane. Collectively, we speculate that alterations in vascular tone and reactivity compromise blood flow to the placenta thus decreasing placental nutrient and waste exchange. This environment is largely created by a balance of vasoactive elements, suggesting the modifications reported herein may be physiological adaptations in response to the insult of nano-TiO2 inhalation during gestation. Future studies should investigate uterine inflammatory changes and modifications of placental blood flow in response to thromboxane and prostacyclin after maternal inhalation exposure during gestation. This would provide extended evidence and insight into placental hemodynamic modifications to maternal inhalation exposures during pregnancy.
AUTHOR CONTRIBUTIONS
Study design: J.A.G./E.C.B./T.R.N. Data Collection: J.A.G./K.L.G./E.C.B./E.D./K.S. Data analysis and interpretation: J.A.G./E.C.B./S.H./T.R.N. Animal Exposures: J.A.G./K.L.G./E.C.B./K.J.E./T.P.B./W.T.G./K.W. Article draft: J.A.G. Critical revisions and final decision to submit: all authors.
ACKNOWLEDGMENTS
The authors are grateful to Dr Stan Hileman and Dr Eric Kelley for their review comments on the article.
FUNDING
National Institutes of Health (R01 ES015022 to T.R.N., R01 ES031253 to S.H., WV-CTSI U54 GM104942-05 to E.C.B., T32 AG 52375 to J.A.G., and WV-INBRE P20 GM103434).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Julie A Griffith, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA; Center for Inhalation Toxicology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
Krista L Garner, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA; Center for Inhalation Toxicology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
Elizabeth C Bowdridge, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA; Center for Inhalation Toxicology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
Evan DeVallance, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA; Center for Inhalation Toxicology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
Kallie J Schafner, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA; Center for Inhalation Toxicology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
Kevin J Engles, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
Thomas P Batchelor, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA; Center for Inhalation Toxicology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
William T Goldsmith, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA; Center for Inhalation Toxicology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
Kimberley Wix, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
Salik Hussain, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA; Center for Inhalation Toxicology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
Timothy R Nurkiewicz, Department of Physiology and Pharmacology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA; Center for Inhalation Toxicology, West Virginia University School of Medicine, Morgantown, West Virginia 26505-9229, USA.
REFERENCES
- Abukabda A. B., Bowdridge E. C., McBride C. R., Batchelor T. P., Goldsmith W. T., Garner K. L., Friend S., Nurkiewicz T. R. (2019). Maternal titanium dioxide nanomaterial inhalation exposure compromises placental hemodynamics. Toxicol. Appl. Pharmacol. 367, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abukabda A. B., McBride C. R., Batchelor T. P., Goldsmith W. T., Bowdridge E. C., Garner K. L., Friend S., Nurkiewicz T. R. (2018). Group II innate lymphoid cells and microvascular dysfunction from pulmonary titanium dioxide nanoparticle exposure. Part. Fibre Toxicol. 15, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abukabda A. B., Stapleton P. A., McBride C. R., Yi J., Nurkiewicz T. R. (2017). Heterogeneous vascular bed responses to pulmonary titanium dioxide nanoparticle exposure. Front. Cardiovasc. Med. 4, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. (1990). The fetal and infant origins of adult disease. BMJ 301, 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J., Martyn C. N. (1992). The maternal and fetal origins of cardiovascular disease. J. Epidemiol. Community Health 46, 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard N., Sacquet J., Benzoni D., Sassard J. (2000). Cyclooxygenases 1 and 2 and thromboxane synthase in kidneys of Lyon hypertensive rats. Am. J. Hypertens. 13, 404–409. [DOI] [PubMed] [Google Scholar]
- Bibeau K., Sicotte B., Béland M., Bhat M., Gaboury L., Couture R., St-Louis J., Brochu M. (2016). Placental underperfusion in a rat model of intrauterine growth restriction induced by a reduced plasma volume expansion. PLoS One 11, e0145982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigonnesse E., Sicotte B., Brochu M. (2018). Activated NO pathway in uterine arteries during pregnancy in an IUGR rat model. Am. J. Physiol. Heart Circ. Physiol. 315, H415–H422. [DOI] [PubMed] [Google Scholar]
- Boegehold M. A. (1998). Heterogeneity of endothelial function within the circulation. Curr. Opin. Nephrol. Hypertens. 7, 71–78. [DOI] [PubMed] [Google Scholar]
- Boeldt D. S., Bird I. M. (2017). Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 232, R27–R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen H. G., Lash J. M. (1994). Active and passive arteriolar regulation in spontaneously hypertensive rats. Hypertension 23, 757–764. [DOI] [PubMed] [Google Scholar]
- Bowdridge E. C., Abukabda A. B., Engles K. J., McBride C. R., Batchelor T. P., Goldsmith W. T., Garner K. L., Friend S., Nurkiewicz T. R. (2019). Maternal engineered nanomaterial inhalation during gestation disrupts vascular kisspeptin reactivity. Toxicol. Sci. 169, 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Camacho M., Rodríguez C., Guadall A., Alcolea S., Orriols M., Escudero J.-R., Martínez-González J., Vila L. (2011). Hypoxia upregulates PGI-synthase and increases PGI2 release in human vascular cells exposed to inflammatory stimuli. J. Lipid Res. 52, 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton V. L., Stark M. J., Osei-Kumah A., Hodyl N. A. (2012). Review: The feto-placental unit, pregnancy pathology and impact on long term maternal health. Placenta 33, S37–S41. [DOI] [PubMed] [Google Scholar]
- Cohen E., Baerts W., van Bel F. (2015). Brain-Sparing in intrauterine growth restriction: Considerations for the neonatologist. Neonatology 108, 269–276. [DOI] [PubMed] [Google Scholar]
- Darby J. R. T., Schrauben E. M., Saini B. S., Holman S. L., Perumal S. R., Seed M., Macgowan C. K., Morrison J. L. (2020). Umbilical vein infusion of prostaglandin I2 increases ductus venosus shunting of oxygen-rich blood but does not increase cerebral oxygen delivery in the fetal sheep. J. Physiol. 598, 4957–4967. [DOI] [PubMed] [Google Scholar]
- Davies P., Bailey P. J., Goldenberg M. M., Ford-Hutchinson A. W. (1984). The role of arachidonic acid oxygenation products in pain and inflammation. Annu. Rev. Immunol. 2, 335–357. [DOI] [PubMed] [Google Scholar]
- Fleiss B., Wong F., Brownfoot F., Shearer I. K., Baud O., Walker D. W., Gressens P., Tolcos M. (2019). Knowledge gaps and emerging research areas in intrauterine growth restriction-associated brain injury. Front. Endocrinol. 10, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabory A., Roseboom T. J., Moore T., Moore L. G., Junien C. (2013). Placental contribution to the origins of sexual dimorphism in health and diseases: Sex chromosomes and epigenetics. Biol. Sex Differ. 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese B., Klaessig F., Park B., Kaegi R., Steinfeldt M., Wigger H., von Gleich A., Gottschalk F. (2018). Risks, release and concentrations of engineered nanomaterial in the environment. Sci. Rep. 8, 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano R., Cacciatore A., Romano M., La Rosa B., Fonti I., Vigna R. (2010). Uterine artery Doppler flow studies in obstetric practice. J. Prenat. Med. 4, 59–62. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez T. L., Sun T., Koeppel A. F., Lee B., Wang E. T., Farber C. R., Rich S. S., Sundheimer L. W., Buttle R. A., Chen Y.-D. I., et al. (2018). Sex differences in the late first trimester human placenta transcriptome. Biol. Sex Differ. 9, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulopoulou S. (2017). Maternal vascular physiology in preeclampsia. Hypertension 70, 1066–1073. [DOI] [PubMed] [Google Scholar]
- Gunnarsdottir J., Cnattingius S., Lundgren M., Selling K., Högberg U., Wikström A.-K. (2018). Prenatal exposure to preeclampsia is associated with accelerated height gain in early childhood. PLos One 13, e0192514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Zhang L. (2021). Uteroplacental circulation in normal pregnancy and preeclampsia: Functional adaptation and maladaptation. Int. J. Mol. Sci. 22, 8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitsuka Y., Moriuchi H., Hatamoto K., Takase J., Irikura M., Irie T., Yang C., Golbidi S. (2004). Involvement of thromboxane A2 (TXA2) in the early stages of oleic acid-induced lung injury and the preventive effect of ozagrel, a TXA2 synthase inhibitor, in guinea-pigs. J. Pharm. Pharmacol. 56, 513–520. [DOI] [PubMed] [Google Scholar]
- Jackson E. K., Heidemann H. T., Branch R. A., Gerkens J. F. (1982). Low dose intrarenal infusions of PGE2, PGI2, and 6-keto-PGE1 vasodilate the in vivo rat kidney. Circ. Res. 51, 67–72. [DOI] [PubMed] [Google Scholar]
- Kennedy A., Brame J., Rycroft T., Wood M., Zemba V., Weiss C., Hull M., Hill C., Geraci C., Linkov I., et al. (2019). A definition and categorization system for advanced materials: The foundation for risk-informed environmental health and safety testing. Risk Anal. 39, 1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles T. L., Yi J., Frazer D. G., Leonard H. D., Chen B. T., Castranova V., Nurkiewicz T. R. (2012). Nanoparticle inhalation alters systemic arteriolar vasoreactivity through sympathetic and cyclooxygenase-mediated pathways. Nanotoxicology 6, 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriston T., Venuto R. C., Baylis C., Losonczy G. (1999). Hemodynamic and renal effects of U-46619, a TXA2/PGH2 analog, in late-pregnant rats. Am. J. Physiol.Regul. Integr. Comp. Physiol. 276, R831–R837. [DOI] [PubMed] [Google Scholar]
- Lash J. M., Bohlen H. G., Waite L. (1991). Mechanical characteristics and active tension generation in rat intestinal arterioles. Am. J. Physiol. Heart Circ. Physiol. 260, H1561–H1574. [DOI] [PubMed] [Google Scholar]
- LeBlanc A. J., Moseley A. M., Chen B. T., Frazer D., Castranova V., Nurkiewicz T. R. (2010). Nanoparticle inhalation impairs coronary microvascular reactivity via a local reactive oxygen species-dependent mechanism. Cardiovasc. Toxicol. 10, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Majed B. H., Khalil R. A. (2012). Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol. Rev. 64, 540–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak K. K., Gude N. M., Walters W. A., Boura A. L. (1984). Effects of vasoactive autacoids on the human umbilical-fetal placental vasculature. Br. J. Obstet. Gynaecol. 91, 99–106. [DOI] [PubMed] [Google Scholar]
- Matsoukas T., Desai T., Lee K. (2015). Engineered nanoparticles and their applications. J. Nanomater. 2015, e651273-2. [Google Scholar]
- Miller C. N., Dye J. A., Ledbetter A. D., Schladweiler M. C., Richards J. H., Snow S. J., Wood C. E., Henriquez A. R., Thompson L. C., Farraj A. K., et al. (2017). Uterine artery flow and offspring growth in long-evans rats following maternal exposure to ozone during implantation. Environ. Health Perspect. 125, 127005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. (1978). Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol. Rev. 30, 293–331. [PubMed] [Google Scholar]
- Numaguchi Y., Harada M., Osanai H., Hayashi K., Toki Y., Okumura K., Ito T., Hayakawa T. (1999). Altered gene expression of prostacyclin synthase and prostacyclin receptor in the thoracic aorta of spontaneously hypertensive rats. Cardiovasc. Res. 41, 682–688. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz T. R., Porter D. W., Hubbs A. F., Cumpston J. L., Chen B. T., Frazer D. G., Castranova V. (2008). Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part. Fibre Toxicol. 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz T. R., Porter D. W., Hubbs A. F., Stone S., Chen B. T., Frazer D. G., Boegehold M. A., Castranova V. (2009). Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol. Sci. 110, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osol G., Mandala M. (2009). Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24, 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G. Y., Christman J. W. (2006). Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L797–L805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S., Miller M. R. (2018). Ambient air pollution and thrombosis. Part. Fibre Toxicol. 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers M. M., Stallone J. N. (2008). Sympathy for the devil: The role of thromboxane in the regulation of vascular tone and blood pressure. Am. J. Physiol. Heart Circ. Physiol. 294, H1978–H1986. [DOI] [PubMed] [Google Scholar]
- Stapleton P. A., Nurkiewicz T. R. (2014). Maternal nanomaterial exposure: A double threat to maternal uterine health and fetal development? Nanomedicine (Lond) 9, 929–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton P. A., McBride C. R., Yi J., Abukabda A. B., Nurkiewicz T. R. (2018). Estrous cycle-dependent modulation of in vivo microvascular dysfunction after nanomaterial inhalation. Reprod. Toxicol. 78, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton P. A., Minarchick V. C., Yi J., Engels K., McBride C. R., Nurkiewicz T. R. (2013). Maternal engineered nanomaterial exposure and fetal microvascular function: Does the Barker hypothesis apply? Am. J. Obstet. Gynecol. 209, 227.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang E. H. C., Vanhoutte P. M. (2008). Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol. Genomics 32, 409–418. [DOI] [PubMed] [Google Scholar]
- Verlohren S., Niehoff M., Hering L., Geusens N., Herse F., Tintu A. N., Plagemann A., LeNoble F., Pijnenborg R., Muller D. N., et al. (2008). Uterine vascular function in a transgenic preeclampsia rat model. Hypertension 51, 547–553. [DOI] [PubMed] [Google Scholar]
- Walsh S. W. (2004). Eicosanoids in preeclampsia. Prostaglandins Leukot. Essent. Fatty Acids 70, 223–232. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao S. (2010). Vasoactivators and Placental Vasoactivity. In Vascular Biology of the Placenta, pp. 45–62. Morgan & Claypool Life Sciences, San Rafael, CA. [PubMed] [Google Scholar]
- Wang Y., Yu D., Yu Y., Liu X., Hu L., Gu Y. (2020). Association between inflammatory mediators and pulmonary blood flow in a rabbit model of acute pulmonary embolism combined with shock. Front. Physiol. 11, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodek M. E., Westcott K. T., O’Dowd R., Serruto A., Wassef L., Moritz K. M., Moseley J. M. (2005). Uteroplacental restriction in the rat impairs fetal growth in association with alterations in placental growth factors including PTHrP. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1620–R1627. [DOI] [PubMed] [Google Scholar]
- Yano T., Fujioka D., Saito Y., Kobayashi T., Nakamura T., Obata J-e., Kawabata K., Watanabe K., Watanabe Y., Mishina H., et al. (2011). Group V secretory phospholipase A2 plays a pathogenic role in myocardial ischaemia–reperfusion injury. Cardiovasc. Res. 90, 335–343. [DOI] [PubMed] [Google Scholar]
- Yi J., Chen B. T., Schwegler-Berry D., Frazer D., Castranova V., McBride C., Knuckles T. L., Stapleton P. A., Minarchick V. C., Nurkiewicz T. R., et al. (2013). Whole-body nanoparticle aerosol inhalation exposures. J. Vis. Exp. 75, e50263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Mi M., Xie Y.-H., Wang S.-W., Edvinsson L., Xu C.-B. (2017). Downregulation of thromboxane a2 receptor occurs mainly via nuclear factor-kappaB signaling pathway in rat renal artery. Adv. Pharmacol. Sci. 2017, e6507048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]