Abstract
Cost-of-illness (COI) studies attempt to measure and describe the costs associated or attributed to a specific disease, but there are several considerations for measuring and interpreting drug costs estimates. The complexity of the pharmaceutical supply chain and contractual relationships between manufacturers, wholesalers, pharmacies and payers create challenges for researchers attempting to include drug costs in COI analyses. This article aims to provide contextual information for a general audience interested in conducting or evaluating COI studies that may include drug costs and to describe key factors to consider when reviewing drug costs in the peer-reviewed literature.
Keywords: burden-of-illness, cost-of-illness, drug costs, pharmacoeconomics
Introduction
Drug prices are the source of significant health policy debate around the globe, particularly with the concern that drug prices are too high or unaffordable for governments, payers and patients.1,2 When assessing the full value provided by a new pharmaceutical innovation, researchers typically rely on cost-effectiveness analyses that use a comparative evaluation approach for the new product compared to an older product or current standard of care.3,4 To obtain an accurate estimate of cost effectiveness, we need to have good information supporting costs and clinical benefits. For pharmaceuticals, the true price paid by a patient or a payer (government or private) will typically be much different than the original ‘list price’ stated by the manufacturer producing the product.5,6
Commentary
Background on cost-of-illness methods
Cost-of-illness (COI) studies are a subset of studies that measure disease burden, using an economic approach to measure and describe the costs associated or attributed to a specific disease.7 Since Rice’s seminal work in the 1960s, the methods used within COI studies have varied, making some experts question their reliability of cost-only estimates for healthcare decision-making.8–11 In the case of economic evaluations (e.g. COI, cost-effectiveness, cost–benefit) that incorporate drug costs, small variances in the drug price definition or methods used to estimate the cost attributed to medication utilization may yield very different results.5
First, COI methods are typically descriptive without a testable hypothesis.9,12 This is not to say that the analytics involved in cost estimates are simple or lack statistical rigor, but simply that the overall purpose of a COI study is to identify and measure all the costs of a particular disease. The body of COI evidence has been broadly categorized into two groups: (1) direct costs resulting from the disease and (2) other related costs, which may include non-health costs.13 Larg and Moss define a ‘traditional approach’ to COI that considers direct costs (health-related resource use), losses in productivity related to morbidity and mortality, and losses in quality and length of life (i.e. intangible costs).12 They further explain how tangible costs (e.g. direct costs, productivity losses) are frequently estimated and reported whilst intangible costs are less frequently monetized due to challenges in the objective valuation and validation of the estimates.12 Onukwugha (formerly Akobundu) et al. conducted multiple systematic reviews of published COI studies in 2006 and 2015 emphasizing how the design of a COI study often focuses on reporting total costs or incremental costs due to the disease in question.9,10 Onukwugha et al. also categorize these total and incremental costing approaches based on designs from the summation of all resources used, the resources used associated with a specific diagnosis, and use of control groups to compare these costs using various statistical techniques.9,10
Specific to pharmaceuticals, when COI studies aim to estimate a total or incremental cost associated with a disease, many of these design considerations may influence the results. This article aims to provide contextual information for a general audience interested in conducting or evaluating COI studies that may include drug costs and to describe key factors to consider when reviewing drug costs in recently published peer-reviewed COI studies.
Pharmaceutical supply chain and drug pricing terminology
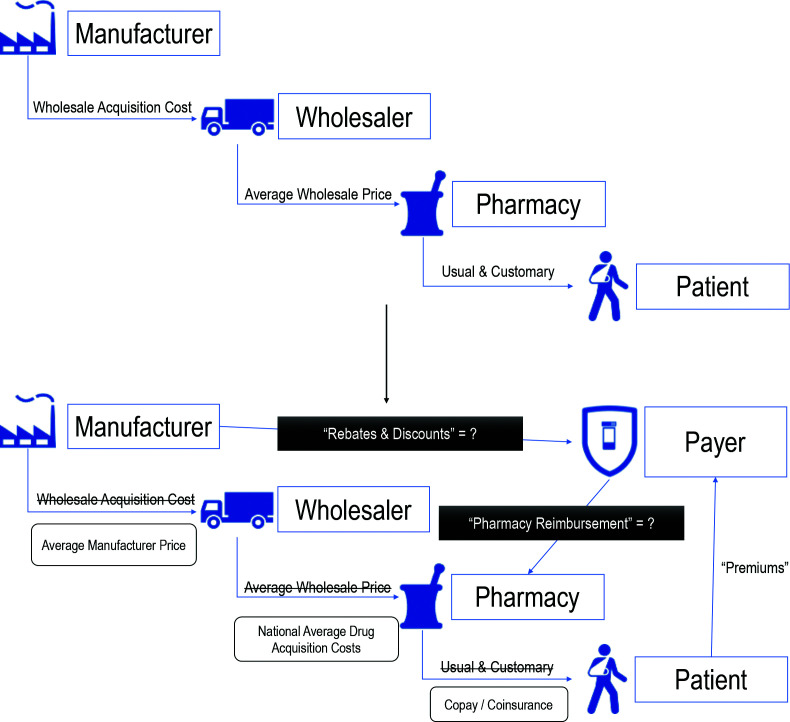
The full pharmaceutical supply chain from the synthesis of active pharmaceutical ingredients all the way to the final consumption of a prescribed medicine may include dozens of intermediaries and different stakeholders depending on the type of product. For the purposes of this article, we will simplify the supply chain and focus on selected transactions observed in the United States most relevant to measurements of drug pricing in COI studies (Figure 1).
Figure 1.
Drug pricing terms along pharmaceutical supply chain with added payer complexity.
Manufacturer to wholesaler
The transaction in the supply chain after the manufacture of pharmaceuticals occurs when manufacturer products are sold to a wholesale distributer or wholesaler. The wholesale acquisition cost (WAC) represents the manufacturer’s list price to the wholesaler before any discounts are applied.6 In research, WAC is a common drug price used because it is provided in drug price compendia such as the Redbook®.14 In the United States, the Centers for Medicare and Medicaid Services requires that pharmaceutical manufacturers submit quarterly reports of the average price paid by wholesalers to help capture these discounts, defined as the average manufacturer price.15
Wholesaler to pharmacy
Wholesalers provide national, regional and local distribution of pharmaceuticals to thousands of pharmacies and hospitals. A wholesaler will acquire the drug for a discounted price and sell to pharmacies at different rates based on volume or other contracted agreements. The average wholesale price reported in compendia, like WAC, can be thought of as a ‘suggested price’ wholesalers could charge pharmacies; however, true acquisition costs for pharmacies are much lower.5,16 To better estimate the pharmacy’s acquisition cost of a drug in the United States, the Centers for Medicare and Medicaid Services uses a survey of pharmacy invoices to estimate the National Average Drug Acquisition Cost.17
Pharmacy to patient
For the end consumer, the patient, the Usual and Customary price would be different across pharmacies, like other retail products. This would be the full price, also called the ‘cash price’, that the customer would pay without any subsidy or insurance benefit. When a patient has insurance, the out-of-pocket cost may vary based on plan design with some plans offering different tiered copayment or coinsurance expectations based on the type of product (e.g. brand, generic, specialty).
Third-party payer influence
This third-party payer design creates more complexity for health economists interested in assessing and comparing drug prices. Payers typically utilize Pharmacy Benefit Managers (PBMs) as intermediaries amongst pharmacies, insurance plans and prescription drug manufacturers.18,19 Over the past few decades, PBMs have expanded their role in the prescription drug supply chain. Currently, in the United States, the main functions of PBMs include designing drug formularies, negotiating rebates with drug manufacturers, building the pharmacy network, setting pharmacy reimbursement rates, and administering drug claims.20 In pharmacy claims data, a paid amount is often reported but it still does not capture any volume-based rebates or discounts built into the manufacturer–PBM contract. The additional layer of complexity creates scenarios where the net price ultimately paid by the payer (i.e. final price after all rebates and discounts are adjudicated) remains unknown to the public or research community as a proprietary trade secret.6
Considerations when including drug costs in COI studies
Study type and terminology
Observational cohort and cross-sectional research designs are commonly used in COI studies. For both cohort and cross-sectional COI analyses, researchers typically set the inclusion criteria to the diagnosis of the disease of interest. A cohort design typically implies that groups within the cohort will be defined based on an exposure and outcome of interest. Unfortunately, many COI studies may use the term cohort more generally as a sample or group defined by the disease. For example, Wittbrodt et al. identified two different cohorts of patients with type 2 diabetes in administrative claims based on the presence of specific ICD-10 diagnosis codes.21 Chamberlain et al. defined their cohort within administrative claims based on a diagnosis of cholangiocarcinoma plus previous (failed) treatment with either gemcitabine or fluorouracil to represent more advanced disease.22 Prospective cohort designs have also been used. Gharibpoor et al. identified a cohort of fibromyalgia patients in an outpatient clinic in Iran and followed them forward for 6 months.23 Cross-sectional COI designs typically refer to studies where the cost outcomes are assessed through a single survey tool. Yousif et al. used this approach to assess out-of-pocket payments by Sudanese patients with end-stage kidney disease on regular hemodialysis.24 Adane et al. employed a cross-sectional design with a semi-structured questionnaire given to 442 adult patients with hypertension from a clinic in Ethiopia.25
A challenge with describing a COI study using epidemiological terms in the methods may create confusion for reviewers expecting to see exposed versus unexposed groups within a cohort compared when many of these studies are analysed as a descriptive analysis of the included individuals with no comparator. This issue has been described well by Onukwugha et al., who further categorized COI study methods by descriptive approaches that ‘sum’ all costs or diagnosis-specific costs for a group of patients defined by a disease and approaches that use an ‘incremental’ design where patients with the disease are compared to patients without the disease.9 Unfortunately, many published COI studies still use a more general cohort or cross-section terminology in the manuscript abstract and first paragraph of methods. Onukwugha et al. further recommended the combination of design approaches to estimate a less biased cost estimate.9
Drug cost data sources
Once the study type and analysis design has been determined, deciding how data will be collected and how the data will be adjusted to estimate drug costs are the next major steps. Administrative pharmacy claims are frequently used in the United States and in countries where pharmacy claims are collected by the government or another third-party claims processor. For example, Colombo et al. identified 71,467 Italian patients with a diagnosis of osteoarthritis using claims data from IQVIA and estimated their drug costs by combining the drug utilization information from medical records and applied publicly available list prices from the Italian National Health Service.26 Jamil et al. used hospital records from the Cerner claims database representing over 90 United States health systems to study the COI for hepatorenal syndrome; however, their drug costs were based on hospital charges rather than on actual costs paid by insurers.27
Another common method of estimating drug costs focuses on medications reported via survey or questionnaires. When researchers survey patients or caregivers to assess drug costs, they typically capture utilization over a period (e.g. previous week or month) and then apply price assumptions from another source. For example, Schönfelder et al. conducted a survey of adult patients with amyotrophic lateral sclerosis for drug utilization over the previous week and then estimated total drug costs using available drug prices in Germany.28 Armour et al. built a 99-question survey tool to assess the COI of endometriosis and chronic pelvic pain in Australia asking patients to simply report what they spent on medications.29 Murota et al. used a survey approach to assess the COI of atopic dermatitis in Japan, but they surveyed both physicians (for direct medical costs) and patients (for over-the-counter or ‘self-medication’ costs) and then applied this to the full Japanese atopic dermatitis population for country-level estimates.30
In addition to surveys, researchers have also used semi-structured interviews to capture drug cost information. Radoičic et al. interviewed 97 patients with low back pain from a Serbian clinic where they could also review medical records to estimate drug costs.31 Gharibpoor et al. had patients fill out ‘cost diaries’ over a 1-month period prior to face-to-face interviews to assess the medication utilization for patients with fibromyalgia in Iran and multiplied the unit estimates by Iranian private service tariffs for the total costs.23
Health system structure, payer type and international considerations
COI studies frequently focus on one country or within a localized area. This single-country approach reduces the challenges of comparing across health systems with very different policies, payer structures, out-of-pocket expectations and clinical practice. In the United States, the fragmented nature of healthcare insurance and differences across the 50 state jurisdictions with varying Medicaid policies for pharmacy benefits creates additional problems for estimating a single cost estimate for the entire country. Countries with a high level of health services provisions through a universal health insurance scheme delivered by the national government allow a more uniform environment for a country-level drug cost estimate. Unfortunately, drug cost estimates outside the United States may be of little relevance for cost calculations inside the United States.
When attempting to compare drug costs across countries, we must first consider the delivery of pharmacy services at the patient level. Whilst universal healthcare may be reported for a given country, the inclusion of pharmacy services may be vastly different. Additionally, the availability to purchase certain drug products over the counter without a prescription may need to be considered. Many middle-income and low-income countries may have a government health insurance system but lack comprehensive coverage for pharmaceuticals, creating larger out-of-pocket burdens for patients in these countries with respect to medicines.32,33
Once differences in country-level insurance coverage and pharmacy services delivery are accounted for, any cost comparisons should be adjusted to a common currency for comparison. Turner et al. provide a methodological approach to consider three different methods to adjust cost estimates collected from different countries in different years: (1) exchanging the local currency to US dollars or international dollars and then inflating the costs using United States inflation rates; (2) inflating costs using the local currency and local inflation rates before exchanging to US dollars or international dollars; and (3) a mixed approach that separates different goods where tradable resources are treated with Method 1 and non-tradable resources are treated with Method 2.34
Assessing risk of bias and usefulness of drug cost estimates in COI studies
Charge versus paid amount
Assessing risk of bias in different methods of estimating drug costs requires the consideration of the data source and the required analytical steps previously discussed. When researchers utilize administrative claims, the cost variable reported in the data may represent a ‘charge’ or a ‘paid’ amount. This phenomenon has been widely discussed in the context of hospital charges versus the ‘allowed amount’ for the facility, which may be influenced by a variety of factors.35,36 Whilst the paid amount for a drug seems straightforward when that variable is included in a dataset, there may still be some need to adjust for potential manufacturer rebates to the payer that are received in lump sum payments rather than fully attributed to that single claim. As previously discussed, administrative claims for outpatient pharmacy costs that include a paid amount typically only reflect a PBM–pharmacy contract price prior to any inclusion of manufacturer rebates or additional price concessions from the pharmacy after the point of sale, referred to as pharmacy direct and indirect remuneration fees.6,37
Accounting for rebates and price concessions to payers
If manufacturer rebates and pharmacy direct and indirect remuneration fees were uniform, this systematic overestimation would have little impact on comparative analyses. However, manufacturer rebates are heterogeneous and may depend on a variety of factors.6 For example, a 2010 task force on drug costs suggested a base-case rebate of 15%, with a range of 5–25%, potentially accounting for the degree of in-class drug competition and formulary placement.6,38 If we take two hypothetical drug products (Drug A and Drug B) with substantially different post-point-of-sale rebates (10% and 50%), the reported paid amount within the pharmacy claim will overestimate the cost of Drug B to a greater extent than Drug A. Not accounting for this differential treatment of rebates at the drug level will make Drug A more favourable in a cost-effectiveness analysis. Levy et al. suggested using National Average Drug Acquisition Cost survey estimates as the upper-limit and drug prices reported in the US Veterans Affairs Federal Supply Schedule as the lower-bound estimates for economic analyses.5 This assumes the US Veterans Affairs Federal Supply Schedule achieves the best price compared to other large private payers. Feldman et al. reviewed several methods of estimating rebates and other discounts to Medicare Part D claims using approaches such as the summed-discount approach and net-to-gross method by utilizing Medicare Trustee Reports.39 Ippolito and Levy also provided guidance on estimating net drug prices from data collected by SSR Health, LLC, for branded drugs using publicly available tax filings from major pharmaceutical companies.40 Whilst these different approaches have different strengths and weaknesses articulated by the authors, they all provide reasonable alternatives to using drug cost inputs that do not account for these rebates and price concessions that occur after the point of sale.
Conclusion
Drug costs are typically estimated in COI studies using observational methods and are often reported descriptively rather than comparatively or incrementally against a control. Researchers either use a reported cost within administrative claims or apply a unit cost from a different source to the total number of units of a drug utilized by the patients in the study. When attempting to compare drug cost estimates across studies, there are a variety of methods to account for potential bias from the sources of drug cost data and cost approach. When comparing drug cost estimates across countries, health system differences must be accounted for in addition to standard inflation or currency conversions.
Acknowledgements
None.
Footnotes
Contributions: All authors contributed equally to the preparation of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Correct attribution: Copyright © 2022 Mattingly II TJ, Weathers S. https://doi.org/10.7573/dic.2022-5-4. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/drug-costs-in-context-assessing-drug-costs-in-cost-of-illness-analyses
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Disclosure and potential conflicts of interest: TJM reports consultant fees from the Arnold Foundation and PhRMA, all unrelated to this review. TJM also reports research grant support from PhRMA, the Patient-Centered Outcomes Research Institute, and the Food and Drug Administration unrelated to this review. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/06/dic.2022-5-4-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
References
- 1.Emanuel EJ. When is the price of a drug unjust? The average lifetime earnings standard. Health Aff. 2019;38(4):604–612. doi: 10.1377/hlthaff.2018.05052. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Guideline on Country Pharmaceutical Pricing Policies. WHO Document Production Services; 2015. [Accessed July 11, 2022]. https://apps.who.int/iris/handle/10665/335692 . [Google Scholar]
- 3.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses. J Am Med Assoc. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 4.Neumann PJ, Willke RJ, Garrison LP. A health economics approach to US value assessment frameworks – Introduction: An ISPOR Special Task Force Report [1] Value Health. 2018;21(2):119–123. doi: 10.1016/j.jval.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Levy J, Rosenberg M, Vanness D. A transparent and consistent approach to assess US outpatient drug costs for use in cost-effectiveness analyses. Value Health. 2018;21(6):677–684. doi: 10.1016/j.jval.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattingly TJ, II, Levy JF, Slejko JF, Onwudiwe NC, Perfetto EM. Estimating drug costs: How do manufacturer net prices compare with other common US price references? Pharmacoeconomics. 2018;36(9):1093–1099. doi: 10.1007/s40273-018-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom BS, Bruno DJ, Maman DY, Jayadevappa R. Usefulness of US cost-of-illness studies in healthcare decision making. Pharmacoeconomics. 2001;19(2):207–213. doi: 10.2165/00019053-200119020-00007. [DOI] [PubMed] [Google Scholar]
- 8.Rice DP. Estimating the cost of illness. Am J Public Health. 1967;57(3):424–440. doi: 10.2105/AJPH.57.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onukwugha E, McRae J, Kravetz A, Varga S, Khairnar R, Mullins CD. Cost-of-illness studies: An updated review of current methods. Pharmacoeconomics. 2015;34(1):43–58. doi: 10.1007/s40273-015-0325-4. [DOI] [PubMed] [Google Scholar]
- 10.Akobundu E, Ju J, Blatt L, Mullins CD. Cost-of-illness studies: a review of current methods. Pharmacoeconomics. 2006;24(9):869–890. doi: 10.2165/00019053-200624090-00005. [DOI] [PubMed] [Google Scholar]
- 11.Currie G, Kerfoot KD, Donaldson C, Macarthur C. Are cost of injury studies useful? Inj Prev. 2000;6(3):175–176. doi: 10.1136/ip.6.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. Pharmacoeconomics. 2011;29(8):653–671. doi: 10.2165/11588380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Rice DP. Cost of illness studies: what is good about them? Inj Prev. 2000;6(3):177–179. doi: 10.1136/ip.6.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redbook Online [online database] [Accessed January 8, 2021]. http://www.micromedexsolutions.com/micromedex2/librarian/
- 15.McRae J, Vogenberg FR, Beaty SW, Mearns E, Varga S, Pizzi L. A review of US drug costs relevant to medicare, medicaid, and commercial insurers post-affordable care act enactment, 2010–2016. Pharmacoeconomics. 2017;35(2):215–223. doi: 10.1007/s40273-016-0458-0. [DOI] [PubMed] [Google Scholar]
- 16.Mattingly TJ., II . Understanding drug pricing. US Pharmacist; [Accessed May 5, 2022]. https://www.uspharmacist.com/article/understanding-drug-pricing . [Google Scholar]
- 17.Centers for Medicare & Medicaid Services. [Accessed February 19, 2020];NADAC (National Average Drug Acquisition Cost) https://www.medicaid.gov/medicaid/prescription-drugs/pharmacy-pricing/index.html . [Google Scholar]
- 18.Bai G, Sen AP, Anderson GF. Pharmacy benefit managers, brand-name drug prices, and patient cost sharing. Ann Intern Med. 2018;168(6):436–437. doi: 10.7326/M17-2506. [DOI] [PubMed] [Google Scholar]
- 19.Kang SY, Bai G, Distefano MJ, Socal MP, Yehia F, Anderson GF. Comparative approaches to drug pricing. Annu Rev Public Health. 2019;41:499–512. doi: 10.1146/annurev-publhealth-040119-094305. [DOI] [PubMed] [Google Scholar]
- 20.Lyles A. Pharmacy benefit management companies: do they create value in the US healthcare system? Pharmacoeconomics. 2017;35(5):493–500. doi: 10.1007/s40273-017-0489-1. [DOI] [PubMed] [Google Scholar]
- 21.Wittbrodt E, Bhalla N, Andersson Sundell K, et al. Assessment of the high risk and unmet need in patients with CAD and type 2 diabetes (ATHENA): US healthcare resource utilization, cost and burden of illness in the Diabetes Collaborative Registry. Endocrinol Diabetes Metab. 2020;3(3):e00133. doi: 10.1002/edm2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamberlain CX, Faust E, Goldschmidt D, et al. Burden of illness for patients with cholangiocarcinoma in the United States: a retrospective claims analysis. J Gastrointest Oncol. 2021;12(2):658–668. doi: 10.21037/jgo-20-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gharibpoor F, Nasrollahzadeh E, Ghavidel-Parsa B, Ghaffari ME, Bidari A. High cost of illness in fibromyalgia patients in Iran, irrespective of disease severity: a prospective cost study. Int J Rheum Dis. 2021;24(5):671–680. doi: 10.1111/1756-185X.14094. [DOI] [PubMed] [Google Scholar]
- 24.Yousif AO, Idris AKM, Awad MM, El-Samani EFZ. Out-of-pocket payments by end-stage kidney disease patients on regular hemodialysis: cost of illness analysis, experience from Sudan. Hemodial Int. 2021;25(1):123–130. doi: 10.1111/hdi.12895. [DOI] [PubMed] [Google Scholar]
- 25.Adane E, Atnafu A, Aschalew AY. The cost of illness of hypertension and associated factors at the university of gondar comprehensive specialized hospital northwest Ethiopia, 2018. Clin Outcomes Res. 2020;12:133–140. doi: 10.2147/CEOR.S234674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colombo GL, Heiman F, Peduto I. Utilization of healthcare resources in osteoarthritis: A cost of illness analysis based on real-world data in Italy. Ther Clin Risk Manag. 2021;17:345–356. doi: 10.2147/TCRM.S301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamil K, Huang X, Lovelace B, Pham AT, Lodaya K, Wan G. The burden of illness of hepatorenal syndrome (HRS) in the United States: a retrospective analysis of electronic health records. J Med Econ. 2019;22(5):421–429. doi: 10.1080/13696998.2019.1580201. [DOI] [PubMed] [Google Scholar]
- 28.Schönfelder E, Osmanovic A, Müschen LH, Petri S, Schreiber-Katz O. Costs of illness in amyotrophic lateral sclerosis (ALS): A cross-sectional survey in Germany. Orphanet J Rare Dis. 2020;15:149. doi: 10.1186/s13023-020-01413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armour M, Lawson K, Wood A, Smith CA, Abbott J. The cost of illness and economic burden of endometriosis and chronic pelvic pain in Australia: a national online survey. PLoS One. 2019;14(10):e0223316. doi: 10.1371/journal.pone.0223316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murota H, Inoue S, Yoshida K, Ishimoto A. Cost of illness study for adult atopic dermatitis in Japan: a cross-sectional web-based survey. J Dermatol. 2020;47(7):689–698. doi: 10.1111/1346-8138.15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radoičic MJ, Božovic BV, Ilić KDP, Janković SM, AnĐelković JZ, Kostić MJ. Pharmacoeconomic aspects of low back pain treatment: cost of illness study in the republic of Serbia. Acta Med Port. 2019;32(4):272–278. doi: 10.20344/amp.10910. [DOI] [PubMed] [Google Scholar]
- 32.Danzon PM, Towse A, Mulcahy AW. Setting cost-effectiveness thresholds as a means to achieve appropriate drug prices in rich and poor countries. Health Aff. 2011;30(8):1529–1538. doi: 10.1377/hlthaff.2010.0902. [DOI] [PubMed] [Google Scholar]
- 33.Shahrawat R, Rao KD. Insured yet vulnerable: out-of-pocket payments and India’s poor. Health Policy Plan. 2012;27(3):213–221. doi: 10.1093/heapol/czr029. [DOI] [PubMed] [Google Scholar]
- 34.Turner HC, Lauer JA, Tran BX, Teerawattananon Y, Jit M. Adjusting for inflation and currency changes within health economic studies. Value Health. 2019;22(9):1026–1032. doi: 10.1016/j.jval.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Cooper Z, Craig S, Gaynor M, Harish NJ, Krumholz HM, Van Reenen J. Hospital prices grew substantially faster than physician prices for hospital-based care in 2007–14. Health Aff. 2019;38(2):184–189. doi: 10.1377/hlthaff.2018.05424. [DOI] [PubMed] [Google Scholar]
- 36.Cooper Z, Craig SV, Gaynor M, Van Reenen J. The price ain’t right? Hospital prices and health spending on the privately insured. Q J Econ. 2019;134(1):51–107. doi: 10.1093/qje/qjy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattingly TJ, II, Bai G. Reforming pharmacy direct and indirect remuneration in the medicare Part D program. Health Affairs Forefront. [Accessed May 5, 2022]. [DOI]
- 38.Mansley EC, Carroll NV, Chen KS, et al. Good research practices for measuring drug costs in cost-effectiveness analyses: a managed care perspective: the ISPOR drug cost task force report – Part III. Value Health. 2010;13(1):14–17. doi: 10.1111/j.1524-4733.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- 39.Feldman WB, Rome BN, Raimond VC, Gagne JJ, Kesselheim AS. Estimating rebates and other discounts received by medicare Part D. JAMA Health Forum. 2021;2(6):e210626. doi: 10.1001/jamahealthforum.2021.0626. [DOI] [PubMed] [Google Scholar]
- 40.Ippolito B, Levy JF. Best practices using SSR health net drug pricing data. [Accessed May 5, 2022];Health Affairs Forefront. doi: 10.1377/forefront.20220308.712815. [DOI] [Google Scholar]