Abstract
The purpose of this review is to update and discuss key findings from in vitro and in vivo studies on apple and its biocompounds, with a special focus on its anticancer role. Several studies have proposed that apple and its extracts exhibit a variety of biological functions that may contribute to health benefits including beneficial effects against chronic heart and vascular disorders, respiratory and pulmonary dysfunction, diabetes, obesity, and cancer. In this review, we summarize the molecular mechanism(s) of various components in apple, as established in previous studies that indicated their growth-inhibitory effects in various cancer cell types. Moreover, an attempt is made to delineate the direction of future studies that could lead to the development of apple components as a potent chemo-preventive/chemotherapeutic agent against cancer.
Keywords: apple, cancer chemoprevention, phloretin, type 2 glucose transporter
1. Introduction
Apples (Malus sp., Rosaceae) are common fruits consumed worldwide. Apples are a rich source of dietary phytochemicals such as flavonoids. They also contain high levels of polyphenols and other phytochemicals [1]. Polyphenols in apples and their extracts (juices) have been studied in several human studies that have shown promising results related to their beneficial effects [2]. For example, consumption of at least one apple a day was reported to reduce the risk of colorectal cancer (odds ratio = 0.65, 95% confidence interval, 0.39–1.09) [3]. The study also predicted that the risk of colorectal cancer reduced by approximately 50% upon consumption of more than one apple a day (odds ratio = 0.53, 95% confidence interval, 0.35–0.79). In vitro and in vivo anticancer effects of apple extracts have been evaluated in many studies, including those of phytochemical compounds in these extracts [4,5] and apple juice fractions [6]. In our previous studies, we have demonstrated that phloretin isolated from apple peels exhibits significant antihepatic tumor proliferation capacity through in vivo inhibition of type 2 glucose transporter (GLUT2) [5]. We have further demonstrated that phloretin significantly potentiates paclitaxel-induced DNA laddering effects in a human liver cancer cell model [4]. This observation indicated that phytochemical components in apples exhibit a beneficial effect on human health. In this review, we include an overview of the positive association between apple juice extract and health benefits demonstrated in observational studies. We also discuss the extract’s basic antioxidant effects and mechanisms underlying cell growth cycle inhibition and cell death and its signaling.
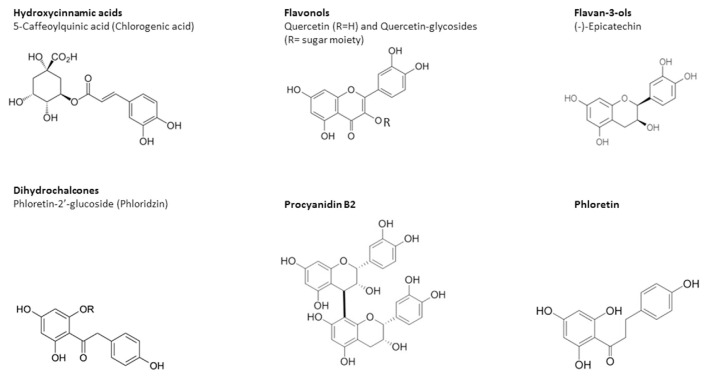
For cancer chemoprevention, dietary nutrients should be more readily available. Many studies have demonstrated the chemopreventive effects of dietary polyphenols, especially the most abundant subclasses, including flavonoids (60% of all polyphenols) and phenolic acids (30% of total polyphenols) [7]. Flavonoids are divided into various groups based on their molecular structure, several of which are present in significant quantities in apple, including flavanols, flavonols, and anthocyanidins as well as dihydrochalcones and hydroxycinnamic acids [8,9]. The chemical structures of several representative polyphenols present in apple are shown in Figure 1 [1].
Figure 1.
Chemical structures of some selected typical biocompounds in apple juice belonging to the structural classes of hydroxycinnamic acids, dihydrochalcones, flavan-3-ols (catechins and procyanidins), flavonols (quercetin-glycosides), and triterpenoids.
2. Antioxidant activity of apple polyphenols
Generation of oxygen radicals causes chronic diseases such as diabetes mellitus [10], retinal degeneration, neurodegenerative disorders, aging, and cancer. Several studies have demonstrated that apple polyphenols, including phloretin, exhibit promising antioxidants effects by playing a role in significant mechanisms responsible for the prevention of illnesses triggered by oxidative stress [11]. For example, in a previous study on Wistar rats, diabetes was induced by a single dose of streptozotocin. Rats in the diabetic group received either apple juice (15 mL/kg) or apple peel extract (1 g/kg) for 21 days. At the end of the study, lipid profile parameters were measured in serum samples and lipid peroxidation level, antioxidant enzyme activities, and level of inflammatory markers were evaluated in pancreatic tissue samples. The study concluded that supplementation with apple juice/extract may have protective effects against deleterious complications of diabetes mellitus due to its antioxidant effects [10]. In a different study on human participants, after 2 weeks of dietary intervention in 25 healthy individuals, the influence of apple and grape juices consumption on body antioxidant status was investigated. The results indicated that such a dietary consumption increased their plasma total antioxidant capacity and decreased their serum and plasma concentration of malondialdehyde [12].
3. Anticancer activity of apple polyphenols
3.1. Apple polyphenol and cell proliferation
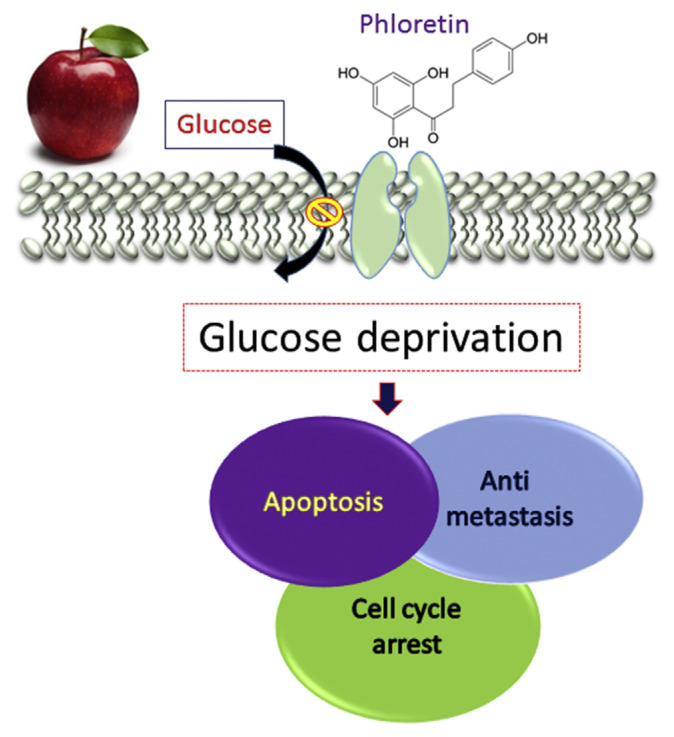
In addition to its antioxidant activity, we [4,5,13] and others [14–16] have demonstrated that apple polyphenols have significant effects in affecting signaling pathways that control cell survival, growth, and proliferation both in vitro and in vivo. The results have shown that phloretin inhibited proliferation and induced apoptosis in nonsmall cell lung cancer cells (A549, Calu-1, H838, and H520) in a dose-dependent manner; phloretin also suppressed the invasion and migration of these cells [14]. In our group, we found that phloretin (50–150μM) significantly potentiates paclitaxel (10nM)-induced DNA laddering formation in human hepatoma (Hep G2) cells. The antitumor therapeutic efficacy of phloretin (10 mg/kg body weight) was determined by combined treatment of cells with antitumor drug (paclitaxel, 1 mg/kg body weight) in an SCID mouse model [4]. Recently [5,13], we have also further demonstrated that apple phloretin inhibits human colorectal cancer and liver cancer cells through inhibition of GLUT2 (Figure 2). These results further provide evidence to the hypothesis that glucose deprivation therapy has some important beneficial effects on human cancer therapy [17]. To test this hypothesis, another group evaluated the antiproliferative activity of apple juices in vitro in MCF-7 and MDA-MB-231 human breast cancer cells. The study results showed that Pelingo apple juice has promising effects to inhibit breast cancer cell proliferation [18]. It was demonstrated that 3-beta-trans-cinnamoyloxy-2alpha-hydroxy-urs-12-en-28-oic acid, which is one of the main components of apple peels, showed potent in vitro and in vivo antitumor activity against mammary tumor in a nude mouse xenograft model at a dose of 50 mg/kg/d without body weight loss and mortality [19].
Figure 2.
Apple polyphenol phloretin inhibits growth of cancer cells through inhibition of type 2 glucose transporter. Our groups have demonstrated that phloretin can induce growth arrest of cells in the G0/G1 phase, induce apoptotic cell death, and inhibit tumor cells migration and metastasis. All these effects can be attributed to the phloretin-induced intracellular glucose deprivation.
3.2. Apple polyphenols inhibit cell migration and invasion
Antimetastasis effects of biocompounds in apple have been studied by our group. Our results have indicated that phloretin is an inhibitor of GLUT2 [4,5] and that targeting GLUT2 significantly inhibited COLO 205 colon cancer cell proliferation, migration, and invasion in vitro and in vivo [13]. In this study [13], p53-mediated signals were important. Inhibition of the wild-type p53 by dominant negative p53 will attenuate the phloretin-induced colon cancer migration and its related signals. In colorectal cancers, studies have demonstrated that the activation of nuclear factor-κB (NF-κB) occurs via lipopolysaccharide (LPS) binding to the Toll-like receptor 4 (TLR4). Modification of polysaccharide components in apple altered the LPS/TLR4/NF-κB pathway; consequently, supplementation of apple polysaccharide significantly inhibited the migratory ability in vitro on the LPS/TLR4/NF-κB pathway in colorectal cancer cells (HT-29 and SW620 cells) [20]. In a study on liver cancer cells, the effect of apple polyphenol extract on the proliferation and invasion of rat ascites hepatoma cell line (AH109A) was examined in vitro. The apple polyphenol extract suppressed both proliferation and invasion of the hepatoma cell line in a dose-dependent manner up to 200 μg/mL. In an in vivo study, apple polyphenol also reduced the growth and metastasis of solid hepatomas and significantly suppressed the serum lipid peroxide level in rats transplanted with AH109A [21].
3.3. Apple polyphenols induced apoptotic cancer cell death
Our previous results demonstrate that apple polyphenol phloretin (50–150μM) significantly potentiates paclitaxel (10nM)-induced DNA laddering formation in Hep G2 cells. We have also demonstrated that the caspases 3, 8, and 9 were involved in apoptosis, as evidenced by activity assays [4]. Previous studies in this area have also demonstrated that phloretin inhibited leukemia cell growth [22] and induced apoptosis of melanoma cells through deprivation of glucose uptake by inhibition of glucose transmembrane transport [23]. Using 18F-fluorodeoxyglucose micropositron emission tomography the effects of phloretin-induced suppression of liver tumor growth were demonstrated to involve regulation of glucose transportation [5]. The 18F-fluorodeoxyglucose uptake in the phloretin-treated Hep G2 tumor-bearing mice was significantly suppressed as compared with the control mice. Effects of phloretin on glioblastoma cancer cells have been investigated via induction of apoptosis and cells’ growth cycle arrest. The identified mechanisms demonstrated increased expression of p27 and decreased expression of cdk2, cdk4, cdk6, cyclin D, and cyclin E. Moreover, the phosphatidylinositol-3-kinase/Akt and the mammalian target of rapamycin (PI3K/Akt/mTOR) signaling cascades were suppressed by phloretin in a dose-dependent manner [24]. Phloretin-based combination treatment enhanced the anticancer effects of cisplatin on nonsmall cell lung cancer cell lines by suppressing the expression of Bcl-2, increasing the protein expression of cleaved caspases 3 and 9, and deregulating the expression of matrix metalloproteinase-2 and metalloproteinase-9 on gene and protein levels [14]. The results suggest that inhibition of intracellular glucose uptake was the most important mechanism responsible for the cancer cell killing effects. Because many cancer cells rely on aerobic glycolysis for energy production, Xintaropoulou et al [15] targeted this pathway as a potential strategy to inhibit cancer cell growth. In that study, inhibition of five glycolysis pathway molecules (GLUT1, HKII (hexokinase II), PFKFB3 (6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3), PDHK1 (pyruvate dehydrogenase kinase I), and LDH (lactate dehydrogenase)) using nine inhibitors (phloretin, quercetin, STF31 (Glut1 inhibitor), WZB117 (Glut1 inhibitor), 3PO (3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one, glycolytic inhibitor), 3-bromopyruvate, dichloroacetate, oxamic acid, NHI-1 (lactate dehydrogenase A inhibitor)) was investigated in panels of breast and ovarian cancer cell line models. Their results indicated that growth of breast and ovarian cancer cell lines was more sensitive to the glycolytic pathway, with increased sensitivity to the inhibitors under normoxic conditions [15].
4. Signaling molecules and disease protection effects of phloretin
As described earlier, apple polyphenols induced anticancer activity mainly through their antioxidant activity. Such results have been confirmed by basic in vitro studies. Moreover, phloretin-induced cell cycle arrest was associated with increased expression of p27 and decreased expression of cdk2, cdk4, cdk6, cyclin D, and cyclin E [24]. Inhibition of intracellular signaling pathways as well as the PI3K/Akt/mTOR and ERK/Nrf2 signaling cascades was suppressed by phloretin in a dose-dependent manner [25]. In addition, many previous studies have also proposed that phloretin triggered the mitochondrial apoptosis pathway [26–28] and generated reactive oxygen species (ROS) [29]. Most of these studies were accompanied by induction of cell growth arrest and apoptosis through upregulation of proapoptotic molecules such as Bax, Bak, and poly (ADP-ribose (adenosine diphosphate-ribose)) polymerase (cleaved) and downregulation of Bcl-2. The antioxidant agents N-acetyl-l-cysteine and glutathione weakened the effect of phloretin on glioblastoma cells. In conclusion, these results demonstrate that phloretin exerts a potent chemopreventive activity in human glioblastoma cells through the generation of ROS. Such effects may have some potential applications for clinical patients. For example, in acute hepatitis patients, liver damage is induced by several damaging factors, among which viral exposure, alcohol consumption, and drug and immune system issues are most popular [30]. In addition to antioxidant effects, phloretin is also able to modulate inflammatory responses. A previous study demonstrated that phloretin suppressed the activation and function of mouse dendritic cells [31]. The study results showed that phloretin disturbed the multiple intracellular signaling pathways in dendritic cells induced by the TLR4 agonist LPS, including ROS, mitogen-activated protein kinases (extracellular signal-regulated kinase, c-Jun N-terminal kinase, p38 mitogen-activated protein kinase), and NF-κB, thus reducing the production of inflammatory cytokines and chemokine [31].
5. Apple polyphenol-induced anticancer effects in animal model
The anticancer activity of biocompounds in apple has also been documented in animal models. We further examined the antitumor effect of phloretin in vivo by treating SCID mice bearing COLO 205 and Hep G2 tumor xenografts [5,13]. We have demonstrated that phloretin has significant effects on the inhibition of type 2 glucose 2 transporter as evidenced by micro-positron emission tomography imaging assay. The chemopreventive potential of apple extract following medium-term oral carcinogenesis assay induced by 4-nitroquinoline 1-oxide has been studied by histopathological analysis and gene expression of antioxidant enzymes and it was shown that the apple extract is able to modulate medium-term oral carcino-genesis assay due to its antioxidant ability [32]. Recently, a model of human microbiota-associated rats has been developed, where mice were fed with a human-type diet and injected with 1,2-dimethylhydrazine to induce colorectal tumor. The study results demonstrated that the number and size of 1,2-dimethylhydrazine-induced aberrant crypt foci were significantly higher in human microbiota-associated rats than in germ-free or conventional rats. Interestingly, the authors used this model to assess the protective effect of an apple proanthocyanidin-rich extract (APE) on colon carcinogenesis. In this model, aberrant crypt foci number and multiplicity were not reduced by APE at 0.001% and 0.01% concentration in drinking water. Such results provide evidence that the cross-talk between human microbiota and the colon epithelium should be considered in carcinogenesis models and that it is necessary to pay increased attention prior to using proanthocyanidin extracts as dietary supplements for humans [33]. Moreover, in vivo studies have shown the cancer prevention potential of apple extracts based on their ability to protect genotoxicity and inhibit the development of aberrant crypt foci experimentally induced in a rat model using dimethylhydrazine [34,35]. Using a pure compound isolated from apple peels, 3beta-trans-cinnamoyloxy-2alpha-hydroxy-urs-12-en-28-oic acid, another study showed potent in vitro antitumor activity against human tumor cells. In vivo experiments further demonstrated that 3beta-trans-cinnamoyloxy-2alpha-hy-droxy-urs-12-en-28-oic acid significantly inhibited the growth of mammary tumor in a nude mouse xenograft model at a dose of 50 mg/kg/d without body weight loss and mortality [19]. Apple polyphenol extracts were also evaluated for their in vivo effects on the growth and metastasis of rat ascites hepatoma cell line (AH109A) and the results were promising [21].
6. Summary and conclusion
This review provides a summary of recent studies suggesting an association between apple polyphenols and reduced risk of carcinogenesis and indicating multiple plausible mechanisms by which apple polyphenols might be protective in humans. Recent studies have demonstrated multiple beneficial effects of apple polyphenols, including antiproliferative, apoptotic, and antioxidative effects. These data suggest that apple polyphenol might have the potential to reduce the risk of several forms of cancer formation and metastasis. The identified antioxidant mechanisms have important implications to better understand the protective effect of apple polyphenols on cancer formation. Although these are preliminary results, the obtained results are intriguing, as they clearly demonstrate the potential of apple polyphenols to modulate some of these processes in animal models. The observations that apple intake might be associated with reduced risk of cancer have led to an expanded field of animal and in vitro research studies using cell models that mimic phases in the initiation, promotion, and progression of cancer.
Acknowledgments
Part of the study results reported in this review were supported by the Health and Welfare Surcharge on tobacco products (MOHW105-TDU-B-212-134001) to Dr Y.-S.H.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
REFERENCES
- 1. Hyson DA. A comprehensive review of apples and apple components and their relationship to human health. Adv Nutr. 2011;2:408–20. doi: 10.3945/an.111.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jedrychowski W, Maugeri U, Popiela T, Kulig J, Sochacka-Tatara E, Pac A, Sowa A, Musial A. Case-control study on beneficial effect of regular consumption of apples on colorectal cancer risk in a population with relatively low intake of fruits and vegetables. Eur J Cancer Prev. 2010;19:42–7. doi: 10.1097/CEJ.0b013e328333d0cc. [DOI] [PubMed] [Google Scholar]
- 3. Jaganathan SK, Vellayappan MV, Narasimhan G, Supriyanto E, Dewi DEO, Narayanan ALT, et al. Chemopreventive effect of apple and berry fruits against colon cancer. World J Gastroenterol. 2014;20(45):17029–36. doi: 10.3748/wjg.v20.i45.17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang KC, Tsai CY, Wang YJ, Wei PL, Lee CH, Chen JH, Wu CH, Ho YS. Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Mol Carcinog. 2009;48:420–31. doi: 10.1002/mc.20480. [DOI] [PubMed] [Google Scholar]
- 5. Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL, Lee CH, Liu RS, Lin SY. In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer. 2009;124:2210–9. doi: 10.1002/ijc.24189. [DOI] [PubMed] [Google Scholar]
- 6. Sudan S, Rupasinghe HP. Flavonoid-enriched apple fraction AF4 induces cell cycle arrest, DNA topoisomerase II inhibition, and apoptosis in human liver cancer HepG2 cells. Nutr Cancer. 2014;66:1237–46. doi: 10.1080/01635581.2014.951733. [DOI] [PubMed] [Google Scholar]
- 7. Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007;18:427–42. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 8. Bouayed J, Hoffmann L, Bohn T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: bioaccessibility and potential uptake. Food Chem. 2011;128:14–21. doi: 10.1016/j.foodchem.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 9. van der Sluis AA, Dekker M, Verkerk R, Jongen WM. An improved, rapid in vitro method to measure antioxidant activity. Application on selected flavonoids and apple juice. J Agric Food Chem. 2000;48:4116–22. doi: 10.1021/jf000156i. [DOI] [PubMed] [Google Scholar]
- 10. Fathy SM, Drees EA. Protective effects of Egyptian cloudy apple juice and apple peel extract on lipid peroxidation, antioxidant enzymes and inflammatory status in diabetic rat pancreas. BMC Complement Altern Med. 2016;16:8. doi: 10.1186/s12906-015-0957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim ES, Hong WK. An apple a day… does it really keep the doctor away? The current state of cancer chemoprevention. J Natl Cancer Inst. 2005;97:468–70. doi: 10.1093/jnci/dji103. [DOI] [PubMed] [Google Scholar]
- 12. Yuan L, Meng L, Ma W, Xiao Z, Zhu X, Feng JF, Yu H, Xiao R. Impact of apple and grape juice consumption on the antioxidant status in healthy subjects. Int J Food Sci Nutr. 2011;62:844–50. doi: 10.3109/09637486.2011.587399. [DOI] [PubMed] [Google Scholar]
- 13. Lin ST, Tu SH, Yang PS, Hsu SP, Lee WH, Ho CT, Wu CH, Lai YH, Chen MY, Chen LC. Apple polyphenol phloretin inhibits colorectal cancer cell growth via inhibition of the type 2 glucose transporter and activation of p53-mediated signaling. J Agric Food Chem. 2016;64:6826–37. doi: 10.1021/acs.jafc.6b02861. [DOI] [PubMed] [Google Scholar]
- 14. Ma L, Wang R, Nan Y, Li W, Wang Q, Jin F. Phloretin exhibits an anticancer effect and enhances the anticancer ability of cisplatin on non-small cell lung cancer cell lines by regulating expression of apoptotic pathways and matrix metalloproteinases. Int J Oncol. 2016;48:843–53. doi: 10.3892/ijo.2015.3304. [DOI] [PubMed] [Google Scholar]
- 15. Xintaropoulou C, Ward C, Wise A, Marston H, Turnbull A, Langdon SP. A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget. 2015;6:25677–95. doi: 10.18632/oncotarget.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park SY, Kim EJ, Shin HK, Kwon DY, Kim MS, Surh YJ, Park JH. Induction of apoptosis in HT-29 colon cancer cells by phloretin. J Med Food. 2007;10:581–6. doi: 10.1089/jmf.2007.116. [DOI] [PubMed] [Google Scholar]
- 17. Hong SM, Park CW, Kim SW, Nam YJ, Yu JH, Shin JH, Yun CH, Im SH, Kim KT, Sung YC, Choi KY. NAMPT suppresses glucose deprivation-induced oxidative stress by increasing NADPH levels in breast cancer. Oncogene. 2016;35:3544–54. doi: 10.1038/onc.2015.415. [DOI] [PubMed] [Google Scholar]
- 18. Schiavano GF, De Santi M, Brandi G, Fanelli M, Bucchini A, Giamperi L, Giomaro G. Inhibition of breast cancer cell proliferation and in vitro tumorigenesis by a new red apple cultivar. PLoS One. 2015;10:e0135840. doi: 10.1371/journal.pone.0135840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qiao A, Wang Y, Xiang L, Wang C, He X. A novel triterpenoid isolated from apple functions as an anti-mammary tumor agent via a mitochondrial and caspase-independent apoptosis pathway. J Agric Food Chem. 2015;63:185–91. doi: 10.1021/jf5053546. [DOI] [PubMed] [Google Scholar]
- 20. Zhang D, Li YH, Mi M, Jiang FL, Yue ZG, Sun Y, Fan L, Meng J, Zhang X, Liu L, Mei QB. Modified apple polysaccharides suppress the migration and invasion of colorectal cancer cells induced by lipopolysaccharide. Nutr Res. 2013;33:839–48. doi: 10.1016/j.nutres.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21. Miura D, Miura Y, Yagasaki K. Effect of apple polyphenol extract on hepatoma proliferation and invasion in culture and on tumor growth, metastasis, and abnormal lipoprotein profiles in hepatoma-bearing rats. Biosci Biotechnol Biochem. 2007;71:2743–50. doi: 10.1271/bbb.70359. [DOI] [PubMed] [Google Scholar]
- 22. Devi MA, Das NP. In vitro effects of natural plant polyphenols on the proliferation of normal and abnormal human lymphocytes and their secretions of interleukin-2. Cancer Lett. 1993;69:191–6. doi: 10.1016/0304-3835(93)90174-8. [DOI] [PubMed] [Google Scholar]
- 23. Kobori M, Shinmoto H, Tsushida T, Shinohara K. Phloretin-induced apoptosis in B16 melanoma 4A5 cells by inhibition of glucose transmembrane transport. Cancer Lett. 1997;119:207–12. doi: 10.1016/s0304-3835(97)00271-1. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Fan C, Pu L, Wei C, Jin H, Teng Y, Zhao M, Yu AC, Jiang F, Shu J, Li F, Peng Q, Kong J, Pan B, Zheng L, Huang Y. Phloretin induces cell cycle arrest and apoptosis of human glioblastoma cells through the generation of reactive oxygen species. J Neurooncol. 2016;128:217–23. doi: 10.1007/s11060-016-2107-z. [DOI] [PubMed] [Google Scholar]
- 25. Yang YC, Lii CK, Lin AH, Yeh YW, Yao HT, Li CC, Liu KL, Chen HW. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic Biol Med. 2011;51:2073–81. doi: 10.1016/j.freeradbiomed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 26. Vineetha VP, Soumya RS, Raghu KG. Phloretin ameliorates arsenic trioxide induced mitochondrial dysfunction in H9c2 cardiomyoblasts mediated via alterations in membrane permeability and ETC complexes. Eur J Pharmacol. 2015;754:162–72. doi: 10.1016/j.ejphar.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 27. Ullen A, Fauler G, Bernhart E, Nusshold C, Reicher H, Leis HJ, Malle E, Sattler W. Phloretin ameliorates 2-chlorohexadecanal-mediated brain microvascular endothelial cell dysfunction in vitro. Free Radic Biol Med. 2012;53:1770–81. doi: 10.1016/j.freeradbiomed.2012.08.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hume DA, Weidemann MJ, Ferber E. Preferential inhibition by quercetin of mitogen-stimulated thymocyte glucose transport. J Natl Cancer Inst. 1979;62:1243–6. [PubMed] [Google Scholar]
- 29. Andrisse S, Koehler RM, Chen JE, Patel GD, Vallurupalli VR, Ratliff BA, Warren DE, Fisher JS. Role of GLUT1 in regulation of reactive oxygen species. Redox Biol. 2014;2:764–71. doi: 10.1016/j.redox.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zuo AR, Yu YY, Shu QL, Zheng LX, Wang XM, Peng SH, Xie YF, Cao SW. Hepatoprotective effects and antioxidant, antityrosinase activities of phloretin and phloretin isonicotinyl hydrazone. J Chin Med Assoc. 2014;77:290–301. doi: 10.1016/j.jcma.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 31. Lin CC, Chu CL, Ng CS, Lin CY, Chen DY, Pan IH, Huang KJ. Immunomodulation of phloretin by impairing dendritic cell activation and function. Food Funct. 2014;5:997–1006. doi: 10.1039/c3fo60548e. [DOI] [PubMed] [Google Scholar]
- 32. Ribeiro FA, Peres RC, Oshima CT, Spolidorio LC, Maluf Lle S, Ribeiro DA. Antioxidant activity of apple extract protects against rat tongue carcinogenesis induced by 4-nitroquinoline 1-oxide. Toxicol Mech Methods. 2015;25:532–7. doi: 10.3109/15376516.2015.1053651. [DOI] [PubMed] [Google Scholar]
- 33. Lhoste EF, Bruneau A, Bensaada M, Cherbuy C, Philippe C, Bruel S, Sutren M, Rabot S, Guyot S, Duee PH, Latino-Martel P. Apple proanthocyanidins do not reduce the induction of preneoplastic lesions in the colon of rats associated with human microbiota. J Agric Food Chem. 2010;58:4120–5. doi: 10.1021/jf904010a. [DOI] [PubMed] [Google Scholar]
- 34. Barth SW, Faehndrich C, Bub A, Watzl B, Will F, Dietrich H, Rechkemmer G, Briviba K. Cloudy apple juice is more effective than apple polyphenols and an apple juice derived cloud fraction in a rat model of colon carcinogenesis. J Agric Food Chem. 2007;55:1181–7. doi: 10.1021/jf063078t. [DOI] [PubMed] [Google Scholar]
- 35. Barth SW, Fahndrich C, Bub A, Dietrich H, Watzl B, Will F, Briviba K, Rechkemmer G. Cloudy apple juice decreases DNA damage, hyperproliferation and aberrant crypt foci development in the distal colon of DMH-initiated rats. Carcinogenesis. 2005;26:1414–21. doi: 10.1093/carcin/bgi082. [DOI] [PubMed] [Google Scholar]