Abstract
Food for specified health use is a type of functional food approved by the Japanese government, with more than 1250 products in 10 health-claim categories being approved as of April 2016. Polyphenols are currently used as functional ingredients in seven of the 10 categories. Although they have not yet been used for the food-for-specified-health-use category of “gut health promotion,” polyphenols are expected to contribute to the future development of gut-modulating food. Intestinal functions include digestion/absorption, acting as a barrier, recognition of external factors, and signal transduction. Owing to incessant exposure to external stress factors including food substances, bacteria, and environmental chemicals, intestines are always inflammatory to some extent, which may cause damage to and dysfunction of intestinal tissues depending on the situation. We identified food factors that could suppress immoderate inflammation in the intestines. In addition to certain amino acids and peptides, polyphenols such as chlorogenic acid and isoflavones were found to suppress inflammation in intestinal cells. Intestinal inflammation is caused by various factors in diverse mechanisms. Recent studies revealed that activation of pattern recognition receptors, such as Toll-like receptors and nucleotide-binding oligomerization domain proteins, in epithelial cells triggers intestinal inflammation. Intracellular receptors or signaling molecules controlling the intestinal detoxification system are also involved in the regulation of inflammation. Differentiation of regulatory T cells by activating a transcription factor Foxp-3 is known to suppress intestinal inflammation. A variety of phytochemicals including polyphenols modulate these receptors and signaling molecules, and are thus anti-inflammatory. Polyphenols affect epigenetic changes occurring in intestinal tissues by interacting with the enzymes responsible for DNA methylation and histone acetylation. New types of anti-inflammatory food factors may be discovered by examining dietary substances that interact with the abovementioned target molecules.
Keywords: epithelial cell, functional food, intestinal inflammation, polyphenol
1. Food for specified health uses and polyphenols
Food for specified health use (FoSHU) is a functional food approved by the Consumer Affairs Agency of the Government of Japan [1]. The FoSHU system started in 1991, and over 1250 FoSHU products are currently approved. Major health-promoting functions of the current products include gut health promotion, regulation of intestinal absorption of nutrients, and metabolic regulation such as that of lipid and bone metabolism [2]. Major functional food substances used for these purposes include carbohydrates, proteins/peptides, lipids, minerals, vitamins, and polyphenols. Polyphenols have increasingly been used as a FoSHU ingredient in recent years. In particular, multifunctional properties of polyphenols are receiving increasing attention. As of the end of April 2016, polyphenols are used as functional ingredients in seven of 10 FoSHU categories (Table 1). This means that polyphenols are recognized as essential ingredients with enormous potential for the development of functional food products. It is well known that the intestines play crucial roles in health maintenance and disease prevention [3]. The promising future of dietary polyphenols as intestine-modulating substances is discussed in this review.
Table 1.
Food for specified health use categories using functional polyphenol ingredients.
| Health claim category (function) | Examples of functional polyphenol ingredients used |
|---|---|
| 1. Reduces blood glucose level | Guava tea polyphenol |
| 2. Reduces blood cholesterol level | Green tea polyphenol (catechin) |
| 3. Reduces blood neutral lipid level and body fat | Green tea polyphenol (catechin) Black tea polyphenol (theaflavin) Oolong tea polymerized polyphenol Apple polyphenol (procyanidin) Monoglucosylhesperidine Isoquercitrin Chlorogenic acid Tectorigenin |
| 4. Lowers blood pressure | Monoglucosylhesperidine Isoquercitrin Chlorogenic acid |
| 5. Promotes gut health | None |
| 6. Promotes tooth health | Green tea polyphenol (catechin) |
| 7. Promotes dental gum health | Macrocarpal Soybean isoflavone |
| 8. Promotes bone health | Soybean isoflavone |
| 9. Enhances mineral absorption | None |
| 10. Improves skin condition | None |
2. Functions of the intestines
Small and large intestines are important organs with a variety of functions (Figure 1), with food digestion and nutrient absorption being the most fundamental functions. Digestive enzymes secreted by pancreatic and gastrointestinal tissues as well as brush-border enzymes expressed at the surface of the intestinal epithelium are involved in gastrointestinal digestion of dietary substances. Food-derived low-molecular-weight digests, such as monosaccharides, amino acids, dipeptides, and fatty acids, are then absorbed by respective transporters or via other mechanisms present in the intestinal epithelium [4].
Figure 1.
Major functions of the intestines.
The intestinal epithelium also acts as a barrier against harmful substances such as pathogenic bacteria and food allergens. Epithelial cell monolayers stabilized by tight junctions provide a physical barrier, but the epithelium also has chemical and biological barrier systems [4]. The chemical barrier includes the detoxification system that metabolizes and detoxifies xenobiotic compounds such as environmental chemicals. Detoxified compounds will further be excreted from epithelial cells to the intestinal tract via efflux transporters. Below the epithelial cell monolayers, many immune cells, including T lymphocytes, monocytes, and dendritic cells, are present and form a unique immune system called the gut mucosal immune system [5]. This biological system recognizes pathogenic microorganisms invading from the apical side of the epithelium and prevents their invasion by secreting specific immunoglobulin A antibodies against them.
3. Inflammation in the intestines
Intestinal epithelial cells are always exposed to a variety of external stresses, including food-derived stimulants, environmental chemicals, and intestinal bacteria. Generating a proper response to these stimulants is one of the major roles of the epithelial cells. Immune cells present in the lamina propria, which is the space beneath the epithelial cell monolayer, also recognize external substances, including pathogens and allergens. To respond to these external stimulants, intestinal epithelial and immune cells are cooperatively activated, thereby producing cytokines and other bioactive compounds that reinforce and restore the intestinal barrier. These protective responses may, however, simultaneously induce inflammation. In other words, the intestinal epithelium is an inflammatory tissue by nature, always maintaining a moderate inflammatory state. This type of inflammation in normal intestines is mild and controllable, and is, therefore, called “controlled inflammation” [6]. However, if inflammatory reactions immoderately proceed because of excessive stress or the formation of a vicious reaction cycle, disruption of the epithelial tissues and dysfunction of the intestines will occur. A typical and severe example of such uncontrollable inflammation is inflammatory bowel disease, which includes Crohn’s disease and ulcerative colitis [7]. In Japan, The number of Crohn’s disease and ulcerative colitis patients were estimated to be approximately 40,000 and 180,000, respectively, in 2014. Treatment of inflammatory bowel disease with drugs such as aminosalicylate and anti-tumor necrosis factor-alpha antibody (infliximab) is now available; although these drugs effective, development of other treatments, including nutritional or food therapeutics, are also expected [8].
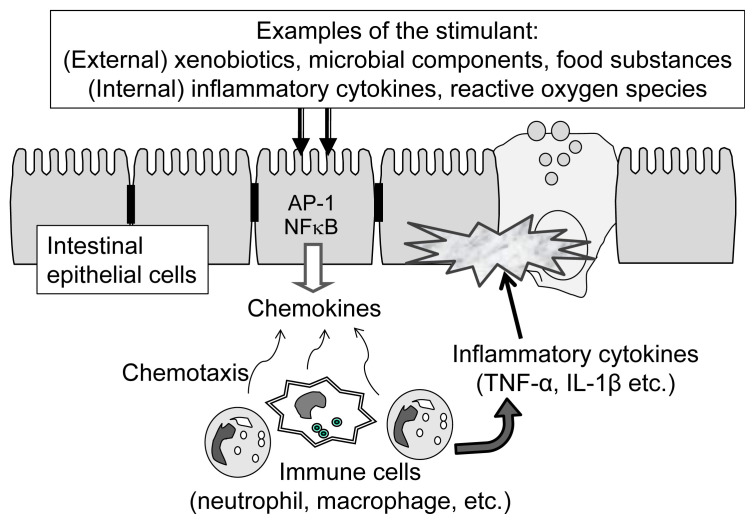
Although the molecular mechanism for inflammatory bowel disease is not fully understood, inflammatory reactions occurring in the intestinal epithelium can be outlined as shown in Figure 2. The reactions start with the stimulation of epithelial cells by external stimuli, including xenobiotic substances, microbial components, and active oxygen species. Stimulated epithelial cells produce chemokines, which attract immune cells such as macrophages and dendritic cells located in the lamina propria. Recruited immune cells are then activated near the epithelial cells, and immune cells secrete inflammatory cytokines, including interleukin (IL)-1 and tumor necrosis factor-alpha. Intestinal epithelial cells exposed to high concentrations of inflammatory cytokines are damaged, resulting in the destruction of the intestinal barrier [9].
Figure 2.
Major inflammation pathway in the intestinal epithelium upon stimulation by external and internal stress factors. AP-1 = activator protein-1; IL-1β =interleukin-1β; NFκB =nuclear factor κB; TNF-α =tumor necrosis factor-alpha.
4. Intestinal inflammation and food factors
One of the major roles of intestinal epithelial cells in the inflammation process is to secrete chemokines in response to stimulants. Suppression of chemokine secretion by the epithelial cells would, therefore, be an effective means to attenuate intestinal inflammation. In order to examine the effect of food factors on chemokine production, we constructed a simple in vitro experimental system using the human intestinal epithelial cell line Caco-2 [4]. As Caco-2 cells produce considerable amounts of IL-8, a typical chemokine in human intestines, we stimulated Caco-2 cells with hydrogen peroxide and tumor necrosis factor-alpha, and then examined the resultant production of IL-8 by the cells.
A variety of food substances were added to this experimental system to identify food factors that suppress IL-8 production, and several suppressive substances were found, including histidine [10], taurine [11], carnosine [12], lactoperoxidase [13], isoflavone [14], and chlorogenic acid (CHA) [15]. Anti-inflammatory activity of histidine was later confirmed by Andou et al [16] using an in vivo model. Suppression of intestinal inflammation by taurine was also confirmed in vivo using mice with dextran sulfate sodium-induced intestinal inflammation [11]. These studies indicated that our in vitro experimental system was, at least partly, useful in identifying anti-inflammatory food factors.
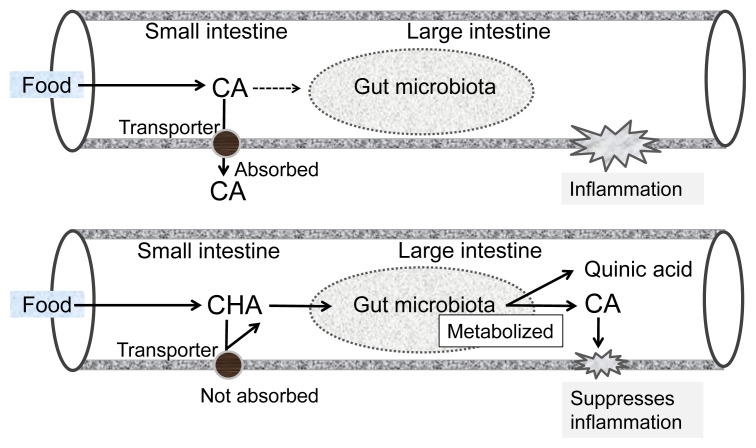
CHA, a major phenolic acid in coffee, also showed an anti-inflammatory effect on mice with dextran sulfate sodium-induced colitis [17]. We found that oral administration of CHA reduced mRNA expression of macrophage inflammatory protein-2 (MIP-2), a mouse homolog of human IL-8, in inflamed intestinal tissue. This suggests that CHA suppressed chemokine production by intestinal epithelial cells, which is in agreement with the results obtained using an in vitro Caco-2 cell system [15]. CHA consists of caffeic acid (CA) and quinic acid, with the former being responsible for the anti-inflammatory activity of CHA. Interestingly, the anti-inflammatory effect of CHA was more potent than that of CA when compared in an in vivo mouse system [17], whereas the activity of CA was more pronounced than that of CHA in our in vitro Caco-2 system [15]. This may be because CA is more easily absorbed in the small intestine [18] and, therefore, does not reach the large intestine, where the majority of inflammation occurs. In contrast, CHA is not easily absorbed by the small intestine, allowing the majority to reach the large intestine [18]. In the large intestine, CHA is converted to CA by intestinal bacteria and suppresses inflammatory reactions at the epithelium. A hypothetical mechanism for the anti-inflammatory function of CHA in the large intestine is illustrated in Figure 3. This may represent a typical example where the physiological functions of dietary polyphenols strongly depend on the rate of absorption and metabolic changes in the intestines.
Figure 3.
Hypothetical mechanisms for the higher anti-inflammatory activity of CHA than that of CA in the intestines. CA =caffeic acid; CHA =chlorogenic acid.
Regarding the molecular mechanism underlying the action of CHA, we found that the anti-inflammatory effect of CHA is dependent on the reactive oxygen species-scavenging activity of the catechol residue in the CA region (H.S. Shin et al unpublished result). As the nuclear factor κB pathway, which is responsible for chemokine production, is activated by reactive oxygen species [19], elimination of reactive oxygen species by CHA or CA in epithelial cells results in reduced chemokine expression in the cells.
5. Anti-inflammatory effect of polyphenols via receptors in the intestines
In addition to the antioxidant-related mechanism observed for CHA, regulating certain receptor-mediated signaling pathways is another mechanism for modulation of inflammatory reactions. One example is the modulation of immunity-related cells by polyphenols via pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain proteins (Figure 4A). PRRs recognize pathogen-related molecular structures and activate the innate immunity, an important part of the immune system that protects the host from pathogen invasion [20]. Over-activation of PRRs may, however, induce inflammation by increasing the expression of inflammatory mediators such as cytokines and cyclooxygenase-2. Recent studies revealed that certain phytochemicals can modulate PRR activation, which attenuates inflammatory reactions [21]. Phytochemicals such as curcumin [22] and isothiocyanate [23] are anti-inflammatory because they inhibit TLR4 dimerization, an essential step for TLR4 activation. Unlike curcumin and isothiocyanate, flavonoids such as resveratrol do not inhibit TLR activation, but instead suppress TLR4-mediated signal transduction by inhibiting TBK1, a kinase regulating the downstream reaction for cytokine expression [24]. Since PRRs are highly expressed in intestinal epithelial and immune cells, modulation of PRRs and PRR-mediated inflammatory reactions is a promising method of regulating intestinal inflammation, and polyphenols may play a role in modulating the inflammation process.
Figure 4.
Receptor-mediated inflammation and anti-inflammation pathways in the intestinal (A) epithelial cells and (B) T cells. Examples of dietary phytochemicals including flavonoids with anti-inflammatory activity are shown in italics. AhR =aryl hydrocarbon receptor; AP-1 = activator protein-1; COX-2 =cyclooxygenase-2; EGCG = epigallocatechin gallate; IL-10 =interleukin-10; NFκB =nuclear factor κB; PXR =pregnane X receptor; NOD = nucleotide-binding oligomerization domain; TLR =Toll-like receptor.
Nuclear receptors are involved in the detoxification system of the intestines. One important function of the intestinal epithelium is the detoxification/excretion of harmful environmental chemicals. Functional proteins involved in the detoxification/excretion system are classified as Phase 1 enzymes, Phase 2 enzymes, and Phase 3 transporters, expression of which is regulated by transcription factors such as the aryl hydrocarbon receptor (AhR) and pregnane X receptor (PXR) [25]. These receptors or xenosensors are activated by binding with their ligands such as environmental chemicals before being transferred to the nuclei, resulting in an increased expression of Phase 1/2 enzymes, such as cytochrome P-450s and uridine 5′-diphosphate (UDP)-glucuronic acid transferases, and Phase 3 transporters, such as multidrug resistance-associated proteins. This detoxification system acts as a chemical barrier in the intestines, and plays a crucial role in the detoxification and excretion of harmful xenobiotic chemicals from the body.
Interestingly, recent studies revealed that these detoxification systems interact with inflammatory reactions [26]. Activation of xenoreceptors such as PXR and CAR, for example, can affect inflammation by interfering with nuclear factor κB [27]. As certain dietary polyphenols and terpenoids activate PXR and AhR [28,29], these dietary compounds can also be expected to suppress intestinal inflammation. Therefore, identification of food factors that can modulate PXR and other xenobiotic receptors will be useful for the discovery of new anti-inflammatory food substances.
6. Induction of regulatory T cells by dietary polyphenols
The regulatory T cell (Treg) has been given increasing attention because it plays an important role in antiallergy and anti-inflammation [30]. Stimulation of naïve T cells by transforming growth factor-β enhances the expression of transcription factors such as Foxp3, which induce the differentiation of naïve T cells into Treg [31]. Since Treg induction in the intestine will reduce the expression of inflammatory cytokines and suppress inflammation, identifying food factors that can induce Treg is also beneficial for developing functional foods with anti-inflammatory effects.
Nguyen et al [32] demonstrated that AhR is involved in the differentiation of T cells because it activates Foxp3 expression and thus promotes Treg induction (Figure 4B). Considering that certain flavonoids can interact with AhR, flavonoids may be able to induce Treg. We identified flavonoids that could enhance AhR-dependent transcriptional activity and then measured their Treg induction activity [33]. Among eight flavonoids that facilitated AhR-dependent transcriptional activity, naringenin showed the most pronounced effect on Treg induction. We also showed that intracellular AhR was directly involved in Treg induction by naringenin.
7. Other potential roles of polyphenols in the modulation of intestinal inflammation
Physiological functions of dietary substances are broader and more diverse than was previously believed, with new mechanisms of action being reported in recent years. A notable example is the epigenetic function of food polyphenols. Inhibition of DNA methyltransferase by dietary phytochemicals was first reported with tea catechins [34]. Since then, epigenetic activities, including the inhibition of histone deacetylase and activation of histone acetyl transferase, have been reported with a variety of polyphenolic compounds [35,36]. Epigenetic regulation of cellular functions has been observed in a variety of tissues and organs, with dietary polyphenols believed to be involved in this regulation, particularly in the intestinal epithelium [37]. Interesting observations on the interaction between polyphenols and intestinal bacteria have also been reported [38]. The significance of polyphenols as functional ingredients for anti-inflammatory food is increasing.
REFERENCES
- 1.Shimizu M. History and current status of functional food regulation in Japan. In: Bagchi D, editor. Nutraceutical and functional food regulations in the United States and around the world. London, Waltham, San Diego: Elsevier Inc; 2014. pp. 257–63. [Google Scholar]
- 2. Shimizu M, Hachimura S. Gut as a target for functional food. Trends Food Sci Technol. 2011;22:646–50. [Google Scholar]
- 3. Schneeman BO. Gastrointestinal physiology and functions. Br J Nutr. 2002;88(Suppl 2):S159–63. doi: 10.1079/BJN2002681. [DOI] [PubMed] [Google Scholar]
- 4. Shimizu M. Interaction between food substances and the intestinal epithelium. Biosci Biotechnol Biochem. 2010;74:232–41. doi: 10.1271/bbb.90730. [DOI] [PubMed] [Google Scholar]
- 5. Jeon MK, Klaus C, Kaemmerer E, Gassler N. Intestinal barrier: molecular pathways and modifiers. World J Gastrointest Pathophysiol. 2013;4:94–9. doi: 10.4291/wjgp.v4.i4.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sartor RB. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn’s disease. Gastroenterol Clin North Am. 1995;24:475–507. [PubMed] [Google Scholar]
- 7. Singh D, Srivastava S, Pradhan M, Kanwar JR, Singh MR. Inflammatory bowel disease: pathogenesis, causative factors, issues, drug treatment strategies and delivery approaches. Crit Rev Ther Drug Carrier Syst. 2015;32:181–214. doi: 10.1615/critrevtherdrugcarriersyst.2015011095. [DOI] [PubMed] [Google Scholar]
- 8. Sarbagili-Shabat C, Singall-Boneh R, Levine A. Nutritional therapy in inflammatory bowel disease. Curr Opin Gastroenterol. 2015;31:303–8. doi: 10.1097/MOG.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 9. Blander JM. Death in the intestinal epithelium—basic biology and implications for inflammatory bowel disease. FEBS J. 2016;283:2720–30. doi: 10.1111/febs.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Son DO, Satsu H, Shimizu M. Histidine inhibits oxidative stress- and TNF-α-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005;579:4671–7. doi: 10.1016/j.febslet.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 11. Zhao Z, Satsu H, Fujisawa M, Hori M, Ishimoto Y, Totsuka M, Nambu A, Kakuta S, Ozaki H, Shimizu M. Attenuation by dietary taurine of dextran sulfate sodium-induced colitis in mice and of THP-1-induced damage to intestinal Caco-2 cell monolayers. Amino Acids. 2008;35:217–24. doi: 10.1007/s00726-007-0562-8. [DOI] [PubMed] [Google Scholar]
- 12. Son DO, Satsu H, Kiso Y, Totsuka M, Shimizu M. Inhibitory effect of carnosine on interleukin-8 production in intestinal epithelial cells through posttranscriptional regulation. Cytokine. 2008;42:265–76. doi: 10.1016/j.cyto.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 13. Matsushita A, Son DO, Satsu H, Takano Y, Kawakami H, Totsuka M, Shimizu M. Inhibitory effect of lactoperoxidase on the secretion of proinflammatory cytokine IL-8 in human intestinal epithelial Caco-2 cells. Int Dairy J. 2008;18:932–8. [Google Scholar]
- 14. Satsu H, Hyun JS, Shin HS, Shimizu M. Suppressive effect of an isoflavone fraction on tumor necrosis factor-α-induced interleukin-8 production in human intestinal epithelial Caco-2 cells. J Nutr Sci Vitaminol. 2009;55:442–6. doi: 10.3177/jnsv.55.442. [DOI] [PubMed] [Google Scholar]
- 15. Zhao Z, Shin HS, Satsu H, Totsuka M, Shimizu M. 5-Caffeoylquinic acid and caffeic acid down-regulate the oxidative stress- and TNF-α-induced secretion of interleukin-8 from Caco-2 cells. J Agric Food Chem. 2008;56:3863–8. doi: 10.1021/jf073168d. [DOI] [PubMed] [Google Scholar]
- 16. Andou A, Hisamatsu T, Okamoto S, Chinen H, Kamada N, Kobayashi T, Hashimoto M, Okutsu T, Shimbo K, Takeda T, Matsumoto H, Sato A, Ohtsu H, Suzuki M, Hibi T. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology. 2009;136:564–74. doi: 10.1053/j.gastro.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 17. Shin HS, Satsu H, Bae MJ, Zhao Z, Ogiwara H, Totsuka M, Shimizu M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015;168:167–75. doi: 10.1016/j.foodchem.2014.06.100. [DOI] [PubMed] [Google Scholar]
- 18. Konishi Y, Kobayashi S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal Caco-2 cell monolayers. J Agric Food Chem. 2004;52:2518–26. doi: 10.1021/jf035407c. [DOI] [PubMed] [Google Scholar]
- 19. Bhattacharyya S, Dudeja PK, Tobacman JK. ROS, Hsp27, and IKKbeta mediate dextran sodium sulfate (DSS) activation of IKappaBα, NFkappaB, and IL-8. Inflamm Bowel Dis. 2009;15:673–83. doi: 10.1002/ibd.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6:451–63. doi: 10.1038/mi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao L, Lee JY, Hwang DH. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr Rev. 2011;69:310–20. doi: 10.1111/j.1753-4887.2011.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang S, Zhao L, Kim K, Lee DS, Hwang DH. Inhibition of Nod2 signaling and target gene expression by curcumin. Mol Pharmacol. 2008;74:274–81. doi: 10.1124/mol.108.046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shibata T, Nakashima F, Honda K, Lu YJ, Kondo T, Ushida Y, Aizawa K, Suganuma H, Oe S, Tanaka H, Takahashi T, Uchida K. Toll-like receptors as a target of food-derived anti-inflammatory compounds. J Biol Chem. 2014;289:32757–72. doi: 10.1074/jbc.M114.585901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Youn HS, Lee JY, Fitzgerald KA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339–46. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- 25. Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–68. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 26. Xie W, Tian Y. Xenobiotic receptor meets NF-kappaB, a collision in the small bowel. Cell Metab. 2006;4:177–8. doi: 10.1016/j.cmet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27. Moreau A, Vilarem MJ, Maurel P, Pascussi JM. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm. 2007;5:35–41. doi: 10.1021/mp700103m. [DOI] [PubMed] [Google Scholar]
- 28. Satsu H, Hiura Y, Mochizuki K, Hamada M, Shimizu M. Activation of the pregnane X receptor and induction of MDR1 by dietary phytochemicals. J Agric Food Chem. 2008;56:5366–73. doi: 10.1021/jf073350e. [DOI] [PubMed] [Google Scholar]
- 29. Li L, Stanton JD, Tolson AH, Luo Y, Wang H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm Res. 2009;26:872–82. doi: 10.1007/s11095-008-9788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X, Zheng Y. Regulatory T cell identity: formation and maintenance. Trends Immunol. 2015;35:344–53. doi: 10.1016/j.it.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen NT, Hanieh H, Nakahama T, Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol. 2013;25:335–43. doi: 10.1093/intimm/dxt011. [DOI] [PubMed] [Google Scholar]
- 33. Wang HK, Yeh CH, Iwamoto T, Satsu H, Shimizu M, Totsuka M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J Agric Food Chem. 2012;60:2171–8. doi: 10.1021/jf204625y. [DOI] [PubMed] [Google Scholar]
- 34. Fang MZ, Eang Y, Ai N, Hou Z, Dun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70. [PubMed] [Google Scholar]
- 35. Busch C, Burkard M, Leischner C, Lauer UM, Frank J, Venturelli S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin Epigenetics. 2015;7:64–81. doi: 10.1186/s13148-015-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cuevas A, Saavedra N, Salazar LA, Abdalla DSP. Modulation of immune function by polyphenols: possible contribution of epigenetic factors. Nutrients. 2013;5:2314–32. doi: 10.3390/nu5072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karatzas PS, Gazouili M, Safioleas M, Mantzaris G. DNA methylation changes in inflammatory bowel disease. Ann Gastroenterol. 2014;27:125–32. [PMC free article] [PubMed] [Google Scholar]
- 38. Peluso I, Romanelli L, Palmery M. Interactions between prebiotics, probiotics, polysaturated fatty acids and polyphenols: diet or supplementation for metabolic syndrome prevention? Int J Food Sci Nutr. 2014;65:259–67. doi: 10.3109/09637486.2014.880670. [DOI] [PubMed] [Google Scholar]