Abstract
Contaminants (or pollutants) that affect human health have become an important issue, spawning a myriad of studies on how to prevent harmful contaminant-induced effects. Recently, a variety of biological functions of natural dietary compounds derived from consumed foods and plants have been demonstrated in a number of studies. Natural dietary compounds exhibited several beneficial effects for the prevention of disease and the inhibition of chemically-induced carcinogenesis. Contaminant-induced toxicity and carcinogenesis are mostly attributed to the mutagenic activity of reactive metabolites and the disruption of normal biological functions. Therefore, the metabolic regulation of hazardous chemicals is key to reducing contaminant-induced adverse health effects. Moreover, promoting contaminant excretion from the body through Phase I and II metabolizing enzymes is also a useful strategy for reducing contaminant-induced toxicity. This review focuses on summarizing the natural dietary compounds derived from common dietary foods and plants and their possible mechanisms of action in the prevention/suppression of contaminant-induced toxicity.
Keywords: chemoprevention, environmental pollutants, metabolism, phytochemicals, xenobiotics
1. Introduction
Industrial pollution and food contamination (partially derived from environmental contaminants) have become increasingly serious matters as these factors elevate the risk of chemical contaminant-induced adverse health effects. Foreign chemicals, or xenobiotics, are difficult to avoid given their ubiquity in our global society. For example, several varieties of common xenobiotics are encountered in daily life, including dioxin, polycyclic aromatic hydrocarbons (PAHs), nicotine, and aflatoxins. Long-term exposure to these xenobiotics can gradually and negatively affect human health through the induction of disease development via various exposure routes. Indeed, epidemiological and investigational studies have shown that daily and/or occupational exposure to PAHs through skin contact, inhalation, or ingestion can induce inflammation, metabolic syndrome, cardiovascular disease, and cancers [1–3]. Additionally, aflatoxins, a type of food contaminant present in cereal and groundnuts produced by Aspergillus flavus and Aspergillus parasiticus, have been demonstrated to be strong inducers of hepatocellular carcinogenesis, thereby causing hepatic fibrosis, cirrhosis, and cancer [4,5].
Xenobiotics that enter the human body undergo four stages: absorption, distribution, metabolism, and elimination [6]. The metabolism stage plays a central role in the bioactivation/detoxification of xenobiotics. These processes are performed by xenobiotics/drug-metabolizing enzymes (XMEs), including Phase I (oxidation, reduction, or hydrolysis reactions) and Phase II (conjugation reaction) enzyme systems. The terms “Phase I” and “Phase II” enzymes were first established by Williams [7] in 1959. XMEs are important enzyme families that are present in the liver and extrahepatic organs, including the skin, lung, kidney, intestine, and colon/rectum, that interact with endogenous and exogenous chemicals and xenobiotics [8]. A typical xenobiotic metabolism involves the continuous biotransformation steps of oxidation, reduction or hydrolysis of parent substances to introduce reactive or polar groups, such as -NH2, -COOH, and -OH groups (Phase I), followed by conjugated with hydrophilic molecules (Phase II), such as glutathione, glucuronic acid, and sulfate, to increase the hydrophilicity of the substances, thereby rendering the substances suitable for renal or intestinal excretion [9]. However, in some instances, xenobiotics are metabolized by Phase I enzymes, which can form reactive or mutagenic metabolites that may induce DNA mutation and carcinogenesis. For example, aflatoxin B1 is metabolized to mutagenic aflatoxin-8,9-epoxide by CYP3A4 [10].
The majority of Phase I reactions are performed by cytochrome P450 (CYP) enzymes, particularly those in the CYP1, CYP2, and CYP3 families, which metabolize a vast range of xenobiotics [11]. It is well known that the CYP enzymes are regulated by farnesoid X receptor, liver X receptor, peroxisome proliferator activated receptor, constitutive androstane receptor, glucocorticoid receptor, pregnane X receptor, and aryl hydrocarbon receptor (AhR) [12,13].
Dietary chemoprevention is a potential strategy for preventing disease development and promoting health and is defined as the use of natural dietary compounds, also called phytochemicals [14–16]. Natural dietary compounds that are found in our diet are obtained from widespread and commonly consumed fruits, vegetables, medicinal plants, and derivatives, such as nobiletin from citrus peel, quercetin from onions, curcumin from turmeric, and resveratrol from red wine [17,18]. A number of studies have suggested that numerous natural dietary compounds are able to prevent/reduce xenobiotic-induced harmful effects on human health modulated through regulated XMEs and related signaling pathways [19–21].
In this review, we will discuss the regulative effects of natural dietary compounds on XMEs and provide a literature overview of natural dietary compounds as chemopreventive agents for preventing xenobiotic-induced toxicity.
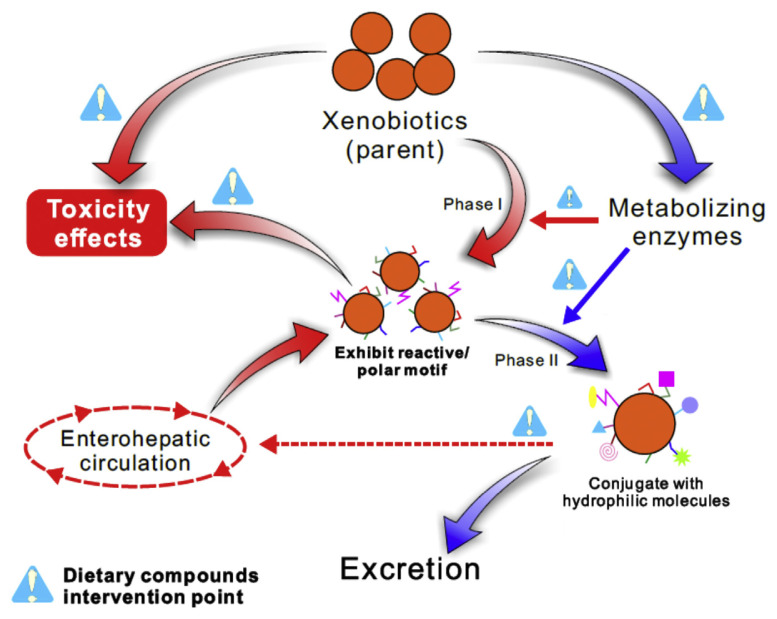
2. Potential strategies for reduced xenobiotics-induced toxicity effects by natural dietary compounds (Figure 1)
Figure 1.
Schematic representation of potential strategies for reduced xenobiotics-induced toxicity effects by natural dietary compounds.
Xenobiotics enter our body through metabolism and excretion. Nevertheless, the stage of xenobiotic metabolism contributes to the conversion of the parent substances for either metabolic bioactivation or detoxification [22]. The key enzymes for xenobiotic bioactivation are CYPs (Phase I), which catalyze the xenobiotics to generate reactive metabolites, such as a molecular ions, quinone, and epoxide. This is a necessary step and is followed by Phase II conjugation reaction [23]. Therefore, the principal strategy for the repression of xenobiotic bioactivation by natural dietary compounds is the modulation of the type and expression level of CYPs—although not complete inhibition—through the suppression of xenobiotic-induced receptor activation and related signaling transduction pathways, such as the AhR signaling pathway. Moreover, the use of natural dietary compounds as CYP inhibitors against CYP enzymatic activity is also a means of suppressing xenobiotic bioactivation. In contrast, Phase II enzymes, including glutathione S-transferases (GSTs), UDP-glucuronosyl-transferase (UGTs), sulfotransferases, nicotinamide adenine dinucleotide phosphate hydrogen (NADPH):quinone acceptor oxidoreductase 1, and quinone reductase, have been associated with xenobiotic detoxification and, ultimately, excretion [8]. Phase II enzymes induced by natural dietary compounds act as a detoxification strategy through the detoxification of reactive xenobiotic metabolites and the promotion of xenobiotic excretion.
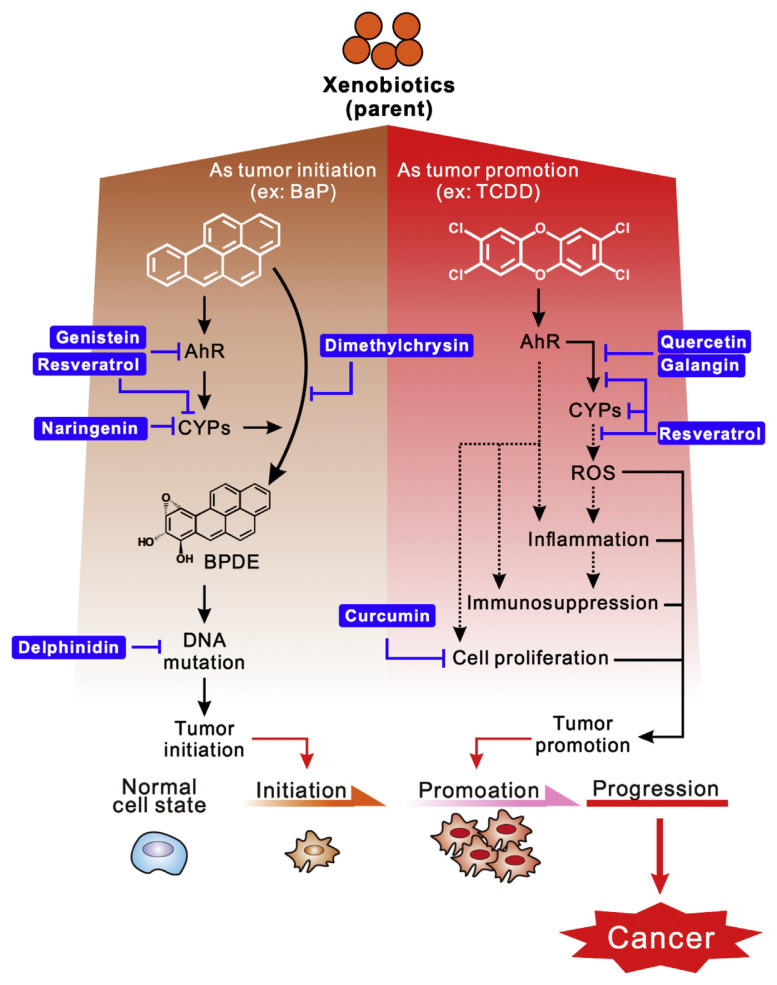
Furthermore, several studies have suggested the existence of a link between xenobiotics/reactive metabolites and the induction of inflammation and tumorigenesis mechanisms, such as inducing inflammatory cytokines expression, promoting cell proliferation and enhancing metastasis [24,25]. Hence, the ability of natural dietary compounds to suppress adverse mechanisms correlates with the effectiveness of these compounds at preventing/inhibiting xenobiotic-induced toxicity (Figure 2).
Figure 2.
Schematic mechanism of xenobiotics-induced carcinogenesis was suppressed by natural dietary compounds. AhR = aryl hydrocarbon receptor; BaP = benzo[a]pyrene; BPDE = benzo[a]pyrene diol epoxide; CYP = cytochrome P450; ROS = reactive oxygen species; TCDD = 2,3,7,8-tetrachlorodibenzo-p-dioxin.
3. Natural dietary compounds suppressed xenobiotics-induced toxicity
Most existing studies of natural dietary compounds that suppress xenobiotic-induced toxicity have been focused on two major contaminants, PAHs and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Indeed, TCDD is the most studied and toxic of all the dioxins. Several studies have also found that natural dietary compounds prevent less common xenobiotic-induced toxicity, such as 1,2-dimethylhydrazine (DMH), N-nitrosodiethylamine, 3-methylcholanthrene, and ferric nitrilotriacetate.
PAHs are one of the most common contaminants derived from environmental and food contamination, and recent epidemiological studies have established that the dietary intake of PAHs is the major route of daily human exposure [26,27]. Benzo[a]pyrene (BaP), a toxicological representative for carcinogenic PAHs, is formed by the incomplete combustion of organic materials, cigarette smoke, and process of roasting foods. The mutagenic activity of BaP has been correlated with CYP1s catalyzed by BaP that generate reactive diol epoxide and quinone metabolites, which were shown to initiate tumor development through the induction DNA adduct formation and the production of reactive oxygen species (ROS) generation. Furthermore, there is increasing recognition that CYP1B1 acts a PAH bioactivator, which may be attributed to the fact that the catalytic activity for the generation of BaP-diol epoxide was higher than CYP1A1. Furthermore, several studies have shown that the abolishment of CYP1B1 expression, but not CYP1A1, caused a reduction of the BaP-induced DNA adduct formation and mitochondrial dysfunction [28–30].
TCDD, a prototype of a group of highly toxic environmental pollutants, is formed as an unintended by-product of incomplete combustion and appear as persistent pollutants in the environment, foods, and milk [31]. Pitot et al. [32] documented that TCDD behaves as a nongenotoxic tumor promoter because TCDD was not able to interact with DNA and failed to reveal mutagenic activity in the Ames test [33]. TCDD is recognized a tumor promoter that is attributed to the blockage of normal cell regeneration, inhibition of damage cell leading to apoptotic cell death, suppression of cell contact inhibition, and induction of inflammation through direct activation of AhR, epidermal growth factor receptor, and nuclear factor-κB signaling pathways [34–36].
Many natural dietary compounds in fruits, vegetables, medicinal plants, and derivatives have been isolated and exhibit health-promoting properties. Natural dietary compounds acting as chemopreventive agents for disease have been characterized by a large number of investigational studies. Since CYPs were first discovered by Klingenberg [37] in 1958, the importance and functions of XMEs for biotransformation of xenobiotics and drugs have gradually become appreciated. Several studies also found that natural dietary compounds not only exhibited disease prevention activity but also displayed the ability to prevent and suppress xenobiotic-induced toxicity through regulated various XEMs and related signaling pathways [19–21,38]. The chemopreventive effects and molecular targets of selected natural dietary compounds in xenobiotic-induced toxicity are described below.
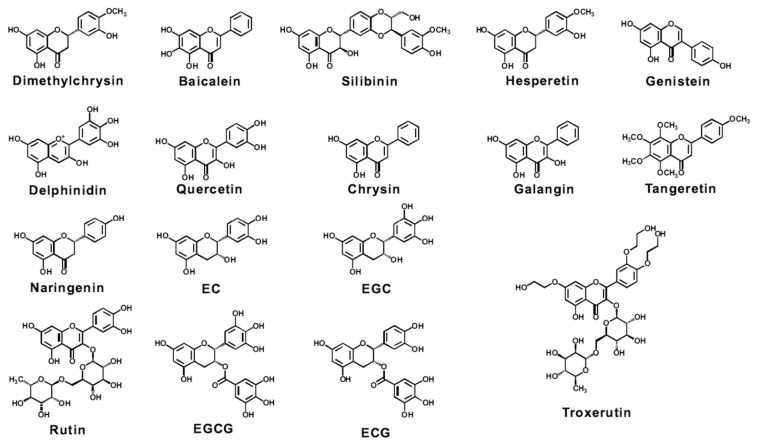
3.1. Flavonoids (Figure 3)
Figure 3.
Chemical structure of potential flavonoids for preventing/suppressing xenobiotic-induced toxicity. EC =(−)-epicatechin; ECG =(−)-epicatechin gallate; EGC =(−)-epigallocatechin; EGCG = epigallocatechin-3-gallate.
Flavonoids are a large family of natural polyphenolic compounds ubiquitous in fruits, vegetables, and medicinal plants. Predicated upon structural differences, flavonoids can be classified into seven groups: flavones, flavanones, flavonols, flavanonols, isoflavones, flavanols, and anthocyanidins [39]. Previous studies suggested that flavonoids possess many beneficial human health effects, such as antioxidation, anti-inflammation and anticarcinogenesis [39].
As proposed in previous chemopreventive strategies for the suppression of xenobiotic-induced toxicity, several flavonoids exhibited the ability to downregulate CYPs expression and activity towards the decreased formation of mutagenic metabolites. For instance, dimethylchrysin (5,7-dimethoxyflavone) reduced BaP-DNA binding activity through the inhibition of CYP1A1 activity and decreased the formation of reactive metabolites [40,41]. Baicalein, silymarin, and quercetin suppressed BaP-induced toxicity by inhibiting CYPs expression and activity and increasing Phase II enzyme expression, which promotes detoxification and elimination [42–44]. Furthermore, Liu et al. [44] found a synergistic effect of the combination quercetin and curcumin treatment, which suppressed BaP-induced lung carcinogenesis through the regulation of Phase I and Phase II enzymes. However, hesperetin and naringenin were demonstrated to suppress DMH-induced colorectal carcinogenesis and N-nitrosodiethylamine-induced hepatocarcinogenesis, respectively, attributed to the inhibition of CYPs activity and induction of GST and UGT expression [45,46]. In particular, metabolized xenobiotics are often conjugated with glucuronic acid in the liver by UGTs and returned to the intestine by biliary excretion. Glucuronide-conjugated xenobiotics excreted in the intestine can be cleaved by gut microbial β-glucuronidase and placed into enterohepatic recirculation, re-exposing organs to reactive metabolites and affecting xenobiotic detoxification and excretion [47–49]. Vinothkumar et al. [50] detected that oral administration of troxerutin significantly inhibited DMH-induced fecal microbiota β-glucuronidase and facilitated DMH excretion from feces. Moreover, several studies investigated the inhibition mechanism of CYPs enzymatic activity. For example, genistein exhibited as mixed-type CYP1A1 inhibition and competitive inhibition of CYP1B1 [51]. Muto et al. [52] suggested that epigallocatechin-3-gallate (EGCG) significantly inhibited CYP1A1 activity by mixed-type inhibition and as CYP3A4 activity by noncompetitive inhibition. Interestingly, only a few studies have discussed the ability of nobiletin and tangeretin, the most abundant flavonoids in citrus fruits, to regulate XMEs expression and activity [53–55]. Nobiletin and tangeretin have exhibited prevention/suppression of xenobiotic- or chemically-induced carcinogenesis through tumor promotion regulation; whereas those compounds that regulate XMEs are defective.
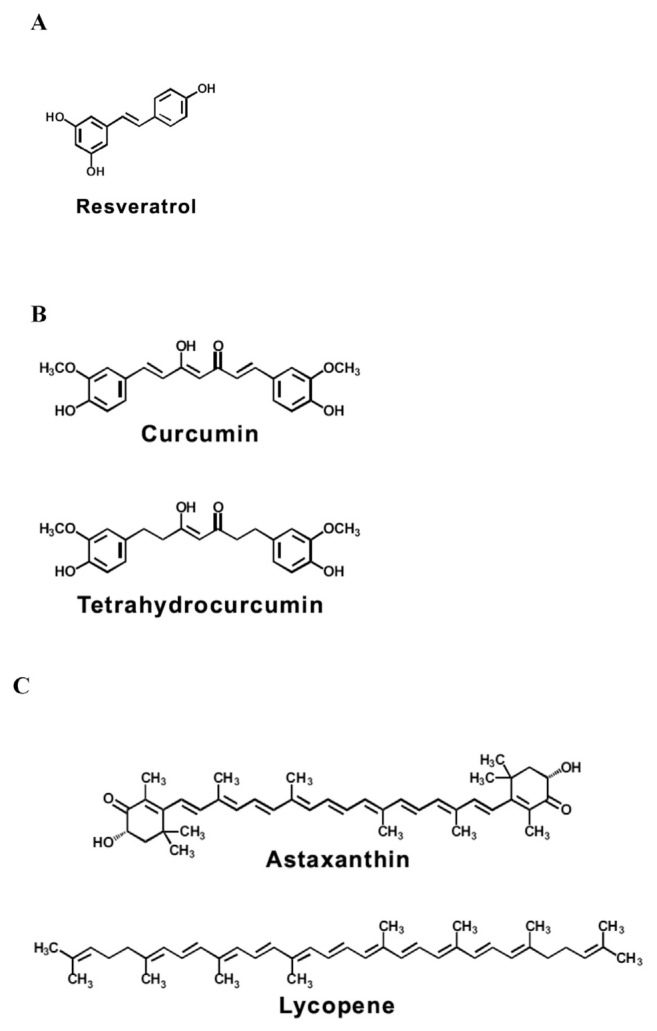
3.2. Stilbenes (Figure 4A)
Figure 4.
Chemical structure of potential (A) stilbene, (B) diarylheptanoids, and (C) carotenoids for preventing/suppressing xenobiotic-induced toxicity.
Stilbenes, such as resveratrol and pterostilbene, are natural nonflavonoid phenolic compounds present in grapevine organs, berries, and a number of plants [56,57]. It has been suggested that resveratrol displays several beneficial human health properties, including anti-high-fat diet-induced senescence, protective mycotoxin-induced cytotoxicity, reduced carbon monoxide-induced cardiotoxicity, and suppressed xenobiotic-induced toxicity through the regulation of xenobiotic-induced AhR activation [57–62]. Recently, studies have demonstrated that resveratrol acts a chemoprotective agent against a wide range of PAH-induced toxicities, including BaP, 7,12-dimethylbenz[a]anthracene, and dibenzo [a,l]pyrene, which is mediated through reduced production of reactive metabolites and DNA-adduct formation by specifically inhibiting CYP1A1 and 1B1 activation [40,63–65]. Additionally, resveratrol opposed PAH-induced oxidative stress through activation of the nuclear factor E2-related factor 2 (Nrf2) signaling pathway to produce antioxidation molecules, such as superoxide dismutase, glutathione, glutathione reductase, and catalase [63,65,66]. With respect to the chemoprevention of TCDD-induced toxicity, previous studies found that resveratrol exhibited as a chemopreventive agent for resisting TCDD triggered harmful effects by directly inhibiting CYP1As activity (half maximal inhibitory concentration = 1.46μM) and suppressed recruitment of active AhR and RNA polymerase II on promoter regions of the CYPs gene [59,61]. However, resveratrol also enabled the modulation of hepatotoxicity markers, XMEs, and oxidative stress induced by pyrogallol, which is used clinically as an antipsoriasis treatment [67]. Based on the observation that resveratrol exhibited the ability to inhibit CYPs activity, Aldawsari et al. [68] and Mikstacka et al. [69] showed that resveratrol is a potential backbone structure for the synthesis high efficacy CYPs inhibitors. This structural insight may provide an opportunity to discovery useful chemicals for the inhibition of xenobiotics that have been converted to carcinogenic metabolites by suppressing CYPs activity.
3.3. Diarylheptanoids (Figure 4B)
The chemical structure of diarylheptanoids, a small class of plant secondary metabolites, consist of two aromatic rings and one seven-carbon chain. These compounds are mainly present in zingiberaceous plants, including Curcuma longa Linn and Curcuma comosa Roxb [70]. Well-known diarylheptanoids are curcumin and its major metabolite, tetrahydrocurcumin. TCDD- and PAH-enhanced AhR activation and recruitment of xenobiotic response element are well-established mechanisms that contribute to increased CYPs expression. Choi et al. [71] demonstrated that curcumin not only affected TCDD-induced AhR activated and xenobiotic response element binding but also increased proteasomal degradation of AhR and Ah receptor nuclear translocator through an induction of the ROS-dependent pathway. Previous studies suggested that curcumin and tetrahydrocurcumin may have a beneficial potential for the remediation of xenobiotic-induced harmful effects through modulated CYPs expression [44,65,72,73].
3.4. Carotenoids (Figure 4C)
Carotenoids are a class of natural hydrophilic colorful plant pigments present in animals, fruits, vegetables, and algae, such as lycopene, β-carotene, astaxanthin, and lutein. Several studies reported that lycopene and astaxanthin induced protein antioxidation and Phase II enzyme expression via the activation of the Nrf2 signaling pathway, which contributes to the suppression of 7,12-dimethylbenz[a]anthracene-induced carcinoma development and homocysteine-induced oxidative stress, respectively [74,75].
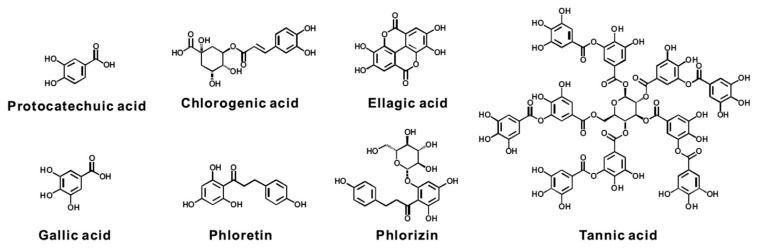
3.5. Phenolics (Figure 5)
Figure 5.
Chemical structure of potential phenolics for preventing/suppressing xenobiotic-induced toxicity.
Phenolics are a variety of chemicals that include mono-phenolic, diphenolic, and polyphenolic compounds. Indeed, most phenolics display antioxidant activity. The biological activity of these chemicals is not necessarily dependent on simple or complex structure. For instance, gallic acid (3,4,5-trihydroxybenzoic acid), a simple monophenolic phytochemical, is found in gallnuts, apple peel, grapes, and lemons. Giftson Senapathy et al. [76]. were reported that gallic acid significantly suppressed DMH-induced colorectal carcinogenesis through reduced CYPs expression and raised the expression level of GST, DT-diaphorase and γ-glutamyl transpeptidase in the liver and colonic mucosa. However, the detailed mechanisms of several natural phenolic dietary compounds that prevent/suppress xenobiotic-induced toxicity, and the harmful effects have not been clarified [74,77,78].
Despite this, these investigational research efforts may allow us to consider how to apply these commonly-found daily dietary phytochemicals for chemoprevention.
3.6. Crude extracts
Beneficial phytochemicals can be purified from fruits, vegetables, medicinal plants and crude extracts (complex composition) from the same sources. Moreover, a number of studies have shown that when two or more phytochemicals are combined as chemopreventive agents, such as curcumin combined with silymarin and EGCG combined with cranberry proanthocyanidins, a synergistic effect is observed that enhances the biological activity or preventive effect [79,80]. Sandur et al. [81] found that natural curcuminoid extracts from Curcuma longa exhibited more anti-inflammatory and antiproliferative activities than pure curcumin, curcumin derivatives and reconstituted curcuminoids. Recently, Suwannakul et al. [82] and Ilavarasi et al. [83] reported that extracts of purple rice bran and seaweed had the ability to attenuate the ABF1-induced initiation stage of hepatocarcinogenesis through alteration of XMEs and blockage of TCDD-induced toxicity, respectively.
4. Conclusion
Industrial pollution and food contamination have become increasingly serious due to adverse human health effects. This review article discusses the chemopreventive activity of various natural dietary compounds to prevent/suppress xenobiotics-induced toxicity (Table 1). These natural dietary compounds exhibited various biological functions for the regulation of xenobiotics metabolism, xenobiotic-induced harmful effects, and injury. Metabolism of xenobiotics and the regulation of xenobiotic-related signaling pathways are key factors for the activation of xenobiotic toxicity. Metabolism of xenobiotics and the first-pass effect are not only carried out in the liver but also in the intestines by gut mucosa and microbiota. The chemopreventive effects of natural dietary compounds discussed in this article are purported to occur by the regulation of XMEs expression and activity, reduction in the level of reactive metabolites and inhibition of xenobiotic-induced multiple signaling pathways.
Table 1.
Potential modulated targets of xenobiotics metabolized by natural dietary compounds. Natural dietary compounds regulated enzyme increased (▲) or decreased (▼) expression of mRNA/protein or enzyme activity.
| Category | Chemicals | Xenobiotics | Effect metabolizing targets | Reference no. |
|---|---|---|---|---|
| Flavonoids | Dimethylchrysin | BaP | CYP1A1▼ | [40,41] |
| Baicalein | BaP | CYPs, b5, CYPRs▼; GST, UGT, QR▲ | [42] | |
| Silibinin, hesperetin | DMH | CYPs, b5, 2E1, CYPRs, b5R▼; GST, UGT▲ | [46,84] | |
| Genistein | DMBA | CYP1A1, 1B1▼ | [51] | |
| Delphinidin | DBP | CYP1A1, 1A2, 1B1▼ | [63] | |
| Quercetin | BaP | CYPs, CYP b5▼; GST▲ | [44] | |
| Quercetin, rutin, chrysin | TCDD | CYP1A1▼ | [61,85] | |
| Galangin, tangeretin | TCDD | CYP1A1, 1A2 ▼ | [86] | |
| Troxerutin | DMH | CYPs, CYPRs, b5R▼; GST, UGT▲; β-glucuronidase, β-glucosidase (gut)▼ | [50] | |
| Naringenin | NDEA | CYPs▼; GST▲ | [45] | |
| Naringenin | DMBA | CYP1B1▼ | [87] | |
| EC, EGC, ECG, EGCG | BaP, PhIP, AFB1 | CYPs▼ | [52] | |
| EGCG | Paracetamol | CYP2E1, 3A4▼; GST▲ | [88] | |
| Stilbene | Resveratrol | BaP | CYPs, CYP b5▼; GST▲ | [40,65] |
| Resveratrol | BaP, DMBA | CYP1A1/1A2, 1B1, 2B▼; NQO1▲ | [78] | |
| Resveratrol | DMBA | CYP1A1, 1B1, UGT1A1▼ | [64] | |
| Resveratrol | DBP | CYP1A1, 1A2, 1B1▼ | [63] | |
| Resveratrol | TCDD | CYP1A1, 1A2, 1B1▼ | [59,61,89] | |
| Resveratrol | Pyrogallol | CYP1A2, 2E1▼; GST-ya, -yc▲ | [67] | |
| Diarylheptanoids | Curcumin | BaP | CYPs, CYP b5▼; GST▲ | [44,65] |
| Curcumin | TCDD | CYP1A1, 1B1▼ | [71] | |
| Curcumin, tetrahydrocurcumin | Fe-NTA | GST, NQO1▲ | [73] | |
| Carotenoids | Astaxanthin | DMBA | CYP1A1, 1B1▼; NQO1, GST▲ | [74] |
| Lycopene | Homocysteine | PON1, NQO1▲ | [75] | |
| Phenolics | Protocatechuic acid, chlorogenic acid, tannic acid | BaP, DMBA | CYP1A1/1A2, 1B1, 2B▼ | [78] |
| Protocatechuic acid | 3-MC | CYP2E1▼ | [77] | |
| Ellagic acid | DMBA | CYP1A1, 1B1▼; NQO1, GST ▲ | [74] | |
| Gallic acid | DMH | CYPs, b5▼; GST, DTD, GGT▲ | [76] | |
| Phloretin, phlorizin | TCDD | CYP1A1▼ | [85] | |
| Crude extract | Black tea extract | DMBA | CYP b5, 1A1, 1A2, 2B▼ | [90,91] |
| Red wine extract | Homocysteine | NQO1▲ | [92] | |
| Green/white tea extract | BaP | CYP1A1, 1B1▼; GST, QR▲ | [93] | |
| Green tea extract, Kava extract | TCDD | CYP1A1▼ | [61] | |
| Blueberry extract | DMBA | CYP1A1, 1B1▼; NQO1, GST ▲ | [74] | |
| Chokeberry extract | NDEA | CYP1A1/1A2, 2B1, 2E1▼ | [94] | |
| Apple juice extract | TCDD | CYP1A1▼ | [85] | |
| Purple rice bran extract | Aflatoxin B1 | CYP1A1, 1A2, 3A▼; GST, UGT▲ | [82] | |
| Black soybean seed coat Extract | BaP | CYP1A1▼; GSTs▲ | [95] | |
| Seaweed extract | TCDD | CYP1A1 activity▼ | [83] |
3-MC = 3-methylcholanthrene; AFB1 = aflatoxin B1; BaP = benzo[a]pyrene; CYP = cytochrome P450; DBP = dibenzo[a,l]pyrene; DMBA = 7,12-dimethylbenz[a]anthracene; DMH = 1,2-dimethylhydrazine; EC = (−)-epicatechin; ECG = (−)-epicatechin gallate; EGC = (−)-epigallocatechin; EGCG = epigallocatechin-3-gallate; Fe-NTA = ferric nitrilotriacetate; GSTs = glutathione S-transferases; LXR = liver X receptor; NDEA = N-nitrosodiethylamine; QR = quinone reductase; TCDD = 2,3,7,8-tetrachlorodibenzo-p-dioxin; UGTs = UDP-glucuronosyl-transferase.
Acknowledgments
This study was supported by the Ministry of Science and Technology 105-2628-B-002 -003 -MY3 and 105-2320-B-002 -031-MY3.
Funding Statement
This study was supported by the Ministry of Science and Technology 105-2628-B-002 -003 -MY3 and 105-2320-B-002 -031-MY3.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
REFERENCES
- 1. Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8:444–72. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 2. Ranjbar M, Rotondi MA, Ardern CI, Kuk JL. Urinary biomarkers of polycyclic aromatic hydrocarbons are associated with cardiometabolic health risk. PLoS One. 2015;10:e0137536. doi: 10.1371/journal.pone.0137536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu H, Kan H, Kearney GD, Xu X. Associations between exposure to polycyclic aromatic hydrocarbons and glucose homeostasis as well as metabolic syndrome in nondiabetic adults. Sci Total Environ. 2015;505:56–64. doi: 10.1016/j.scitotenv.2014.09.085. [DOI] [PubMed] [Google Scholar]
- 4. Shi J, He J, Lin J, Sun X, Sun F, Ou C, Jiang C. Distinct response of the hepatic transcriptome to Aflatoxin B1 induced hepatocellular carcinogenesis and resistance in rats. Sci Rep. 2016 doi: 10.1038/srep31898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118:818–24. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–68. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 7.Williams R. The metabolism and detoxication of drugs, toxic substances and other organic compounds. In: Tecwyn Williams R, editor. Detoxication mechanisms. New York: Wiley; 1959. [Google Scholar]
- 8. Gundert-Remy U, Bernauer U, Blomeke B, Doring B, Fabian E, Goebel C, Hessel S, Jackh C, Lampen A, Oesch F, Petzinger E, Volkel W, Roos PH. Extrahepatic metabolism at the body’s internal-external interfaces. Drug Metab Rev. 2014;46:291–324. doi: 10.3109/03602532.2014.900565. [DOI] [PubMed] [Google Scholar]
- 9. Mandlekar S, Hong JL, Kong AN. Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr Drug Metab. 2006;7:661–75. doi: 10.2174/138920006778017795. [DOI] [PubMed] [Google Scholar]
- 10. Kamdem LK, Meineke I, Godtel-Armbrust U, Brockmoller J, Wojnowski L. Dominant contribution of P450 3A4 to the hepatic carcinogenic activation of aflatoxin B1. Chem Res Toxicol. 2006;19:577–86. doi: 10.1021/tx050358e. [DOI] [PubMed] [Google Scholar]
- 11. Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol. 2003;43:149–73. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- 12. Zordoky BN, El-Kadi AO. Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr Drug Metab. 2009;10:164–78. doi: 10.2174/138920009787522151. [DOI] [PubMed] [Google Scholar]
- 13. Cederbaum AI. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015;4:60–73. doi: 10.1016/j.redox.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lai CS, Wu JC, Ho CT, Pan MH. Disease chemopreventive effects and molecular mechanisms of hydroxylated polymethoxyflavones. Biofactors. 2015;41:301–13. doi: 10.1002/biof.1236. [DOI] [PubMed] [Google Scholar]
- 15. Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol. 2010;80:1771–92. doi: 10.1016/j.bcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin S, Hou DX. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol Nutr Food Res. 2016;60:1731–55. doi: 10.1002/mnfr.201501017. [DOI] [PubMed] [Google Scholar]
- 17. Pan MH, Lai CS, Tsai ML, Wu JC, Ho CT. Molecular mechanisms for anti-aging by natural dietary compounds. Mol Nutr Food Res. 2012;56:88–115. doi: 10.1002/mnfr.201100509. [DOI] [PubMed] [Google Scholar]
- 18. Pan MH, Lai CS, Wu JC, Ho CT. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res. 2011;55:32–45. doi: 10.1002/mnfr.201000412. [DOI] [PubMed] [Google Scholar]
- 19. Ronis MJ. Effects of soy containing diet and isoflavones on cytochrome P450 enzyme expression and activity. Drug Metab Rev. 2016;48:1–11. doi: 10.1080/03602532.2016.1206562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Korobkova EA. Effect of natural polyphenols on CYP metabolism: implications for diseases. Chem Res Toxicol. 2015;28:1359–90. doi: 10.1021/acs.chemrestox.5b00121. [DOI] [PubMed] [Google Scholar]
- 21. Galal AM, Walker LA, Khan IA. Induction of GST and related events by dietary phytochemicals: sources, chemistry, and possible contribution to chemoprevention. Curr Top Med Chem. 2015;14:2802–21. doi: 10.2174/1568026615666141208110721. [DOI] [PubMed] [Google Scholar]
- 22. Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM. Xenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities. Toxicol Sci. 2011;120(Suppl 1):S49–75. doi: 10.1093/toxsci/kfq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Attia SM. Deleterious effects of reactive metabolites. Oxid Med Cell Longev. 2010;3:238–53. doi: 10.4161/oxim.3.4.13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henkler F, Brinkmann J, Luch A. The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancers (Basel) 2010;2:376–96. doi: 10.3390/cancers2020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang CS. Influences of dietary and other factors on xenobiotic metabolism and carcinogenesis—a review article in memory of Dr. Allan H. Conney (1930–2013) Nutr Cancer. 2015;67:1207–13. doi: 10.1080/01635581.2015.1081010. [DOI] [PubMed] [Google Scholar]
- 26. Bansal V, Kim KH. Review of PAH contamination in food products and their health hazards. Environ Int. 2015;84:26–38. doi: 10.1016/j.envint.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 27. Xia Z, Duan X, Qiu W, Liu D, Wang B, Tao S, Jiang Q, Lu B, Song Y, Hu X. Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Sci Total Environ. 2010;408:5331–7. doi: 10.1016/j.scitotenv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 28. Bansal S, Leu AN, Gonzalez FJ, Guengerich FP, Chowdhury AR, Anandatheerthavarada HK, Avadhani NG. Mitochondrial targeting of cytochrome P450 (CYP) 1B1 and its role in polycyclic aromatic hydrocarbon-induced mitochondrial dysfunction. J Biol Chem. 2014;289:9936–51. doi: 10.1074/jbc.M113.525659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nebert DW, Shi Z, Galvez-Peralta M, Uno S, Dragin N. Oral benzo[a]pyrene: understanding pharmacokinetics, detoxication, and consequences—Cyp1 knockout mouse lines as a paradigm. Mol Pharmacol. 2013;84:304–13. doi: 10.1124/mol.113.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shimada T, Gillam EM, Oda Y, Tsumura F, Sutter TR, Guengerich FP, Inoue K. Metabolism of benzo[a]pyrene to trans-7,8-dihydroxy-7, 8-dihydrobenzo[a]pyrene by recombinant human cytochrome P450 1B1 and purified liver epoxide hydrolase. Chem Res Toxicol. 1999;12:623–9. doi: 10.1021/tx990028s. [DOI] [PubMed] [Google Scholar]
- 31. Knerr S, Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol Nutr Food Res. 2006;50:897–907. doi: 10.1002/mnfr.200600006. [DOI] [PubMed] [Google Scholar]
- 32. Pitot HC, Goldsworthy TL, Moran S, Kennan W, Glauert HP, Maronpot RR, Campbell HA. A method to quantitate the relative initiating and promoting potencies of hepatocarcinogenic agents in their dose–response relationships to altered hepatic foci. Carcinogenesis. 1987;8:1491–9. doi: 10.1093/carcin/8.10.1491. [DOI] [PubMed] [Google Scholar]
- 33. Randerath K, Putman KL, Randerath E, Mason G, Kelley M, Safe S. Organ-specific effects of long term feeding of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 1,2,3,7,8-pentachlorodibenzo-p-dioxin on I-compounds in hepatic and renal DNA of female Sprague–Dawley rats. Carcinogenesis. 1988;9:2285–9. doi: 10.1093/carcin/9.12.2285. [DOI] [PubMed] [Google Scholar]
- 34. Chen RJ, Siao SH, Hsu CH, Chang CY, Chang LW, Wu CH, Lin P, Wang YJ. TCDD promotes lung tumors via attenuation of apoptosis through activation of the Akt and ERK1/2 signaling pathways. PLoS One. 2014;9:e99586. doi: 10.1371/journal.pone.0099586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vondracek J, Machala M. Environmental ligands of the aryl hydrocarbon receptor and their effects in models of adult liver progenitor cells. Stem Cells Int. 2016 doi: 10.1155/2016/4326194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Budinsky RA, Schrenk D, Simon T, Van den BM, Reichard JF, Silkworth JB, Aylward LL, Brix A, Gasiewicz T, Kaminski N, Perdew G, Starr TB, Walker NJ, Rowlands JC. Mode of action and dose-response framework analysis for receptor-mediated toxicity: the aryl hydrocarbon receptor as a case study. Crit Rev Toxicol. 2014;44:83–119. doi: 10.3109/10408444.2013.835787. [DOI] [PubMed] [Google Scholar]
- 37. Klingenberg M. Pigments of rat liver microsomes. Arch Biochem Biophys. 1958;75:376–86. doi: 10.1016/0003-9861(58)90436-3. [DOI] [PubMed] [Google Scholar]
- 38. Basheer L, Kerem Z. Interactions between CYP3A4 and dietary polyphenols. Oxid Med Cell Longev. 2015 doi: 10.1155/2015/854015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pan MH, Lai CS, Ho CT. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010;1:15–31. doi: 10.1039/c0fo00103a. [DOI] [PubMed] [Google Scholar]
- 40. Tsuji PA, Walle T. Benzo[a]pyrene-induced cytochrome P450 1A and DNA binding in cultured trout hepatocytes—inhibition by plant polyphenols. Chem Biol Interact. 2007;169:25–31. doi: 10.1016/j.cbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wen X, Walle UK, Walle T. 5,7-Dimethoxyflavone downregulates CYP1A1 expression and benzo[a]pyrene-induced DNA binding in Hep G2 cells. Carcinogenesis. 2005;26:803–9. doi: 10.1093/carcin/bgi015. [DOI] [PubMed] [Google Scholar]
- 42. Naveenkumar C, Raghunandakumar S, Asokkumar S, Binuclara J, Rajan B, Premkumar T, Devaki T. Mitigating role of baicalein on lysosomal enzymes and xenobiotic metabolizing enzyme status during lung carcinogenesis of Swiss albino mice induced by benzo(a)pyrene. Fundam Clin Pharmacol. 2014;28:310–22. doi: 10.1111/fcp.12036. [DOI] [PubMed] [Google Scholar]
- 43. Kiruthiga PV, Karthikeyan K, Archunan G, Pandian SK, Devi KP. Silymarin prevents benzo(a)pyrene-induced toxicity in Wistar rats by modulating xenobiotic-metabolizing enzymes. Toxicol Ind Health. 2015;31:523–41. doi: 10.1177/0748233713475524. [DOI] [PubMed] [Google Scholar]
- 44. Liu Y, Wu YM, Zhang PY. Protective effects of curcumin and quercetin during benzo(a)pyrene induced lung carcinogenesis in mice. Eur Rev Med Pharmacol Sci. 2015;19:1736–43. [PubMed] [Google Scholar]
- 45. Arul D, Subramanian P. Inhibitory effect of naringenin (citrus flavonone) on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Biochem Biophys Res Commun. 2013;434:203–9. doi: 10.1016/j.bbrc.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 46. Aranganathan S, Selvam JP, Sangeetha N, Nalini N. Modulatory efficacy of hesperetin (citrus flavanone) on xenobiotic-metabolizing enzymes during 1,2-dimethylhydrazine-induced colon carcinogenesis. Chem Biol Interact. 2009;180:254–61. doi: 10.1016/j.cbi.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 47. Long AS, Lemieux CL, Arlt VM, White PA. Tissue-specific in vivo genetic toxicity of nine polycyclic aromatic hydrocarbons assessed using the MutaMouse transgenic rodent assay. Toxicol Appl Pharmacol. 2016;290:31–42. doi: 10.1016/j.taap.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruby MV, Lowney YW, Bunge AL, Roberts SM, Gomez-Eyles JL, Ghosh U, Kissel JC, Tomlinson P, Menzie C. Oral bioavailability, bioaccessibility, and dermal absorption of pahs from soil-state of the science. Environ Sci Technol. 2016;50:2151–64. doi: 10.1021/acs.est.5b04110. [DOI] [PubMed] [Google Scholar]
- 49. Carmody RN, Turnbaugh PJ. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J Clin Invest. 2014;124:4173–81. doi: 10.1172/JCI72335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vinothkumar R, Vinoth KR, Sudha M, Viswanathan P, Balasubramanian T, Nalini N. Modulatory effect of troxerutin on biotransforming enzymes and preneoplasic lesions induced by 1,2-dimethylhydrazine in rat colon carcinogenesis. Exp Mol Pathol. 2014;96:15–26. doi: 10.1016/j.yexmp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 51. Chan HY, Leung LK. A potential protective mechanism of soya isoflavones against 7,12-dimethylbenz[a]anthracene tumour initiation. Br J Nutr. 2003;90:457–65. doi: 10.1079/bjn2003913. [DOI] [PubMed] [Google Scholar]
- 52. Muto S, Fujita K, Yamazaki Y, Kamataki T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat Res. 2001;479:197–206. doi: 10.1016/s0027-5107(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 53. Takanaga H, Ohnishi A, Yamada S, Matsuo H, Morimoto S, Shoyama Y, Ohtani H, Sawada Y. Polymethoxylated flavones in orange juice are inhibitors of P-glycoprotein but not cytochrome P450 3A4. J Pharmacol Exp Ther. 2000;293:230–6. [PubMed] [Google Scholar]
- 54. Quintieri L, Palatini P, Nassi A, Ruzza P, Floreani M. Flavonoids diosmetin and luteolin inhibit midazolam metabolism by human liver microsomes and recombinant CYP 3A4 and CYP3A5 enzymes. Biochem Pharmacol. 2008;75:1426–37. doi: 10.1016/j.bcp.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 55. Stuetz W, Prapamontol T, Hongsibsong S, Biesalski HK. Polymethoxylated flavones, flavanone glycosides, carotenoids, and antioxidants in different cultivation types of tangerines (Citrus reticulata Blanco cv. Sainampueng) from Northern Thailand. J Agric Food Chem. 2010;58:6069–74. doi: 10.1021/jf904608h. [DOI] [PubMed] [Google Scholar]
- 56. Pan MH, Lai CS, Tsai ML, Ho CT. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol Nutr Food Res. 2014;58:147–71. doi: 10.1002/mnfr.201300522. [DOI] [PubMed] [Google Scholar]
- 57. Donnez D, Jeandet P, Clement C, Courot E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009;27:706–13. doi: 10.1016/j.tibtech.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 58. Sang Y, Li W, Zhang G. The protective effect of resveratrol against cytotoxicity induced by mycotoxin, zearalenone. Food Funct. 2016;7:3703–15. doi: 10.1039/c6fo00191b. [DOI] [PubMed] [Google Scholar]
- 59. Beedanagari SR, Bebenek I, Bui P, Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci. 2009;110:61–7. doi: 10.1093/toxsci/kfp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hashemzaei M, Barani AK, Iranshahi M, Rezaee R, Tsarouhas K, Tsatsakis AM, Wilks MF, Tabrizian K. Effects of resveratrol on carbon monoxide-induced cardiotoxicity in rats. Environ Toxicol Pharmacol. 2016;46:110–5. doi: 10.1016/j.etap.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 61. Yueh MF, Kawahara M, Raucy J. Cell-based high-throughput bioassays to assess induction and inhibition of CYP1A enzymes. Toxicol. Vitro. 2005;19:275–87. doi: 10.1016/j.tiv.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 62. Zhang N, Li Z, Xu K, Wang Y, Wang Z. Resveratrol protects against high-fat diet induced renal pathological damage and cell senescence by activating SIRT1. Biol Pharm Bull. 2016;39:1448–54. doi: 10.1248/bpb.b16-00085. [DOI] [PubMed] [Google Scholar]
- 63. Russell GK, Gupta RC, Vadhanam MV. Effect of phytochemical intervention on dibenzo[a,l]pyrene-induced DNA adduct formation. Mutat Res. 2015;774:25–32. doi: 10.1016/j.mrfmmm.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leung HY, Yung LH, Shi G, Lu AL, Leung LK. The red wine polyphenol resveratrol reduces polycyclic aromatic hydrocarbon-induced DNA damage in MCF-10A cells. Br J Nutr. 2009;102:1462–8. doi: 10.1017/S0007114509990481. [DOI] [PubMed] [Google Scholar]
- 65. Liu Y, Wu YM, Yu Y, Cao CS, Zhang JH, Li K, Zhang PY. Curcumin and resveratrol in combination modulate drug-metabolizing enzymes as well as antioxidant indices during lung carcinogenesis in mice. Hum Exp Toxicol. 2015;34:620–7. doi: 10.1177/0960327114551396. [DOI] [PubMed] [Google Scholar]
- 66. Sahin K, Orhan C, Akdemir F, Tuzcu M, Iben C, Sahin N. Resveratrol protects quail hepatocytes against heat stress: modulation of the Nrf2 transcription factor and heat shock proteins. J Anim Physiol Anim Nutr (Berl) 2012;96:66–74. doi: 10.1111/j.1439-0396.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 67. Upadhyay G, Singh AK, Kumar A, Prakash O, Singh MP. Resveratrol modulates pyrogallol-induced changes in hepatic toxicity markers, xenobiotic metabolizing enzymes and oxidative stress. Eur J Pharmacol. 2008;596:146–52. doi: 10.1016/j.ejphar.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 68. Aldawsari FS, Elshenawy OH, El Gendy MA, Aguayo-Ortiz R, Baksh S, El-Kadi AO, Velázquez-Martinez CA. Design and synthesis of resveratrol-salicylate hybrid derivatives as CYP1A1 inhibitors. J Enzyme Inhib Med Chem. 2015;30:884–95. doi: 10.3109/14756366.2014.979347. [DOI] [PubMed] [Google Scholar]
- 69. Mikstacka R, Rimando AM, Dutkiewicz Z, Stefanski T, Sobiak S. Design, synthesis and evaluation of the inhibitory selectivity of novel trans-resveratrol analogues on human recombinant CYP1A1, CYP1A2 and CYP1B1. Bioorg Med Chem. 2012;20:5117–26. doi: 10.1016/j.bmc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 70. Suksamrarn A, Ponglikitmongkol M, Wongkrajang K, Chindaduang A, Kittidanairak S, Jankam A, Yingyongnarongkul BE, Kittipanumat N, Chokchaisiri R, Khetkam P, Piyachaturawat P. Diarylheptanoids, new phytoestrogens from the rhizomes of Curcuma comosa: isolation, chemical modification and estrogenic activity evaluation. Bioorg Med Chem. 2008;16:6891–902. doi: 10.1016/j.bmc.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 71. Choi H, Chun YS, Shin YJ, Ye SK, Kim MS, Park JW. Curcumin attenuates cytochrome P450 induction in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by ROS-dependently degrading AhR and ARNT. Cancer Sci. 2008;99:2518–24. doi: 10.1111/j.1349-7006.2008.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jearapong N, Chatuphonprasert W, Jarukamjorn K. Effect of tetrahydrocurcumin on the profiles of drug-metabolizing enzymes induced by a high fat and high fructose diet in mice. Chem Biol Interact. 2015;239:67–75. doi: 10.1016/j.cbi.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 73. Osawa T. Nephroprotective and hepatoprotective effects of curcuminoids. Adv Exp Med Biol. 2007;595:407–23. doi: 10.1007/978-0-387-46401-5_18. [DOI] [PubMed] [Google Scholar]
- 74. Kavitha K, Thiyagarajan P, Rathna NJ, Mishra R, Nagini S. Chemopreventive effects of diverse dietary phytochemicals against DMBA-induced hamster buccal pouch carcinogenesis via the induction of Nrf2-mediated cytoprotective antioxidant, detoxification, and DNA repair enzymes. Biochimie. 2013;95:1629–39. doi: 10.1016/j.biochi.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 75. Yefsah-Idres A, Benazzoug Y, Otman A, Latour A, Middendorp S, Janel N. Hepatoprotective effects of lycopene on liver enzymes involved in methionine and xenobiotic metabolism in hyperhomocysteinemic rats. Food Funct. 2016;7:2862–9. doi: 10.1039/c6fo00095a. [DOI] [PubMed] [Google Scholar]
- 76. Giftson Senapathy J, Jayanthi S, Viswanathan P, Umadevi P, Nalini N. Effect of gallic acid on xenobiotic metabolizing enzymes in 1,2-dimethyl hydrazine induced colon carcinogenesis in Wistar rats—a chemopreventive approach. Food Chem Toxicol. 2011;49:887–92. doi: 10.1016/j.fct.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 77. Krajka-Kuzniak V, Szaefer H, Baer-Dubowska W. Modulation of 3-methylcholanthrene-induced rat hepatic and renal cytochrome P450 and phase II enzymes by plant phenols: protocatechuic and tannic acids. Toxicol Lett. 2004;152:117–26. doi: 10.1016/j.toxlet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 78. Szaefer H, Krajka-Kuzniak V, Baer-Dubowska W. The effect of initiating doses of benzo[a]pyrene and 7,12-dimethylbenz [a]anthracene on the expression of PAH activating enzymes and its modulation by plant phenols. Toxicology. 2008;251:28–34. doi: 10.1016/j.tox.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 79. Montgomery A, Adeyeni T, San K, Heuertz RM, Ezekiel UR. Curcumin sensitizes silymarin to exert synergistic anticancer activity in colon cancer cells. J Cancer. 2016;7:1250–7. doi: 10.7150/jca.15690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lombardo Bedran TB, Palomari SD, Grenier D. Green tea polyphenol epigallocatechin-3-gallate and cranberry proanthocyanidins act in synergy with cathelicidin (LL-37) to reduce the LPS-induced inflammatory response in a three-dimensional co-culture model of gingival epithelial cells and fibroblasts. Arch Oral Biol. 2015;60:845–53. doi: 10.1016/j.archoralbio.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 81. Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–73. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 82. Suwannakul N, Punvittayagul C, Jarukamjorn K, Wongpoomchai R. Purple rice bran extract attenuates the aflatoxin B1-induced initiation stage of hepatocarcinogenesis by alteration of xenobiotic metabolizing enzymes. Asian Pac J Cancer Prev. 2015;16:3371–6. doi: 10.7314/apjcp.2015.16.8.3371. [DOI] [PubMed] [Google Scholar]
- 83. Ilavarasi K, Chermakani P, Arif NS, Sheeja MD, Pandima DK. Antioxidant compounds in the seaweed Gelidiella acerosa protects human peripheral blood mononuclear cells against TCDD induced toxicity. Drug Chem Toxicol. 2015;38:133–44. doi: 10.3109/01480545.2014.919582. [DOI] [PubMed] [Google Scholar]
- 84. Sangeetha N, Viswanathan P, Balasubramanian T, Nalini N. Colon cancer chemopreventive efficacy of silibinin through perturbation of xenobiotic metabolizing enzymes in experimental rats. Eur J Pharmacol. 2012;674:430–8. doi: 10.1016/j.ejphar.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 85. Pohl C, Will F, Dietrich H, Schrenk D. Cytochrome P450 1A1 expression and activity in Caco-2 cells: modulation by apple juice extract and certain apple polyphenols. J Agric Food Chem. 2006;54:10262–8. doi: 10.1021/jf061791c. [DOI] [PubMed] [Google Scholar]
- 86. Hamada M, Satsu H, Natsume Y, Nishiumi S, Fukuda I, Ashida H, Shimizu M. TCDD-induced CYP1A1 expression, an index of dioxin toxicity, is suppressed by flavonoids permeating the human intestinal Caco-2 cell monolayers. J Agric Food Chem. 2006;54:8891–8. doi: 10.1021/jf060944t. [DOI] [PubMed] [Google Scholar]
- 87. Poon CH, Wong TY, Wang Y, Tsuchiya Y, Nakajima M, Yokoi T, Leung LK. The citrus flavanone naringenin suppresses CYP1B1 transactivation through antagonising xenobiotic-responsive element binding. Br J Nutr. 2013;109:1598–605. doi: 10.1017/S0007114512003595. [DOI] [PubMed] [Google Scholar]
- 88. Yao HT, Yang YC, Chang CH, Yang HT, Yin MC. Protective effects of (−)-epigallocatechin-3-gallate against acetaminophen-induced liver injury in rats) Biomedicine (Taipei) 2015;5:15. doi: 10.7603/s40681-015-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ, Kim DH, Kang KS, Cho MH, Surh YJ. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25:2005–13. doi: 10.1093/carcin/bgh183. [DOI] [PubMed] [Google Scholar]
- 90. Kumaraguruparan R, Seshagiri PB, Hara Y, Nagini S. Chemoprevention of rat mammary carcinogenesis by black tea polyphenols: modulation of xenobiotic-metabolizing enzymes, oxidative stress, cell proliferation, apoptosis, and angiogenesis. Mol Carcinog. 2007;46:797–806. doi: 10.1002/mc.20309. [DOI] [PubMed] [Google Scholar]
- 91. Vidjaya LP, Chandra Mohan KV, Stegeman JJ, Gelboin HV, Hara Y, Nagini S. Pretreatment with black tea polyphenols modulates xenobiotic-metabolizing enzymes in an experimental oral carcinogenesis model. Oncol Res. 2008;17:75–85. doi: 10.3727/096504008784523649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Noll C, Dairou J, Ripoll C, Paul JL, Dupret JM, Delabar JM, Rodrigues-Lima F, Janel N. Effect of red wine polyphenol dietary supplementation on two phase II enzymes in liver of hyperhomocysteinemic mice. Food Chem Toxicol. 2011;49:1764–9. doi: 10.1016/j.fct.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 93. Kumar M, Jain M, Sehgal A, Sharma VL. Modulation of CYP1A1, CYP1B1 and DNA adducts level by green and white tea in Balb/c mice. Food Chem Toxicol. 2012;50:4375–81. doi: 10.1016/j.fct.2012.08.045. [DOI] [PubMed] [Google Scholar]
- 94. Krajka-Kuzniak V, Szaefer H, Ignatowicz E, Adamska T, Oszmianski J, Baer-Dubowska W. Effect of Chokeberry (Aronia melanocarpa) juice on the metabolic activation and detoxication of carcinogenic N-nitrosodiethylamine in rat liver. J Agric Food Chem. 2009;57:5071–7. doi: 10.1021/jf803973y. [DOI] [PubMed] [Google Scholar]
- 95. Zhang T, Jiang S, He C, Kimura Y, Yamashita Y, Ashida H. Black soybean seed coat polyphenols prevent B(a)P-induced DNA damage through modulating drug-metabolizing enzymes in HepG2 cells and ICR mice. Mutat Res. 2013;752:34–41. doi: 10.1016/j.mrgentox.2013.01.002. [DOI] [PubMed] [Google Scholar]