Abstract
Epidemiological studies have consistently shown that regular consumption of whole grain barley reduces the risk of developing chronic diseases. The presence of barley fiber, especially β-glucan in whole grain barley, has been largely credited for these health benefits. However, it is now widely believed that the actions of the fiber component alone do not explain the observed health benefits associated with the consumption of whole grain barley. Whole grain barley also contains phytochemicals including phenolic acids, flavonoids, lignans, tocols, phytosterols, and folate. These phytochemicals exhibit strong anti-oxidant, antiproliferative, and cholesterol lowering abilities, which are potentially useful in lowering the risk of certain diseases. Therefore, the high concentration of phytochemicals in barley may be largely responsible for its health benefits. This paper reviews available information regarding barley phytochemicals and their potential to combat common nutrition-related diseases including cancer, cardiovascular disease, diabetes, and obesity.
Keywords: barley, disease prevention, fiber, phytochemicals, whole grain
1. Introduction
Barley is among the most ancient cereal crops grown in the world today. Archeological evidence suggests the existence of barley in Egypt along the River Nile around 17,000 years ago [1]. It is one of the top most cultivated crops globally (12% of total cereal cultivated), ranking fourth among cereal grains after wheat, rice, and maize [2]. Barley outperforms other cereals under various environmental stresses due to its winter-hardy, drought-resistant, and early maturing nature and is thus generally more economical to cultivate [3]. Approximately 65% of cultivated barley is used for animal feed, 33% for malting, whereas only 2% is used directly for human consumption [4]. In 2014, over 48 million hectares of barley were cultivated globally, generating a harvest of 144 million metric tons, with the United States producing 3.8 million tons on 988,660 hectares of land during this period [5].
Epidemiological studies have associated the regular consumption of barley with its potential to reduce the risk of certain diseases, such as chronic heart disease [4,6,7], colonic cancer [8,9], high blood pressure [10], and gallstones [11,12]. Reports of barley’s role in maintaining a healthy colon [13], inducing immunostimulation [14], and generally boosting the immune system [15], among others, have been established. These therapeutic potentials are attributed to the presence of the bioactive components of vitamins, minerals, fiber, and other phytochemicals. Interestingly, among the myriads of bioactive substances present in barley, fiber component, especially β-glucan fiber, is mainly credited for barley’s health benefits [16–18].
Dietary fiber is a significant contributor to the health benefits of barley; however, sufficient evidence supports that phytochemicals also play important roles in preventing the development of chronic diseases [19]. Different classes of phytochemicals have been recognized, and their specific bioactivities have been reported. The major phytochemicals in barley that have shown health benefits include phenolic acids, flavonoids, lignans, vitamin E (tocols), sterols, and folates [20]. Phenolic compounds provide essential functions in reproduction and growth, act as defense mechanisms against pathogens, parasites, and predators, as well as contribute to the color of plants [21–23]. Sterols and tocols are mainly components of plant oils that provide benefits such as protection against toxins, neurological diseases like Alzheimer’s disease, and diabetes [24,25]. Barley competes well with other major cereal grains, such as wheat, oat, rye, and rice in terms of content and diversity of phytochemicals. In addition, barley has some unique phytochemical properties, such as the presence of all eight tocol vitamers, which are usually not complete in some cereals [26–28].
Considering the high beneficial potential of phytochemicals on human health and the absence of a critical review regarding the subject in barley, we summarized the recent findings about the phytochemical components of barley and their ability to modulate parameters related to human health, analyzed the limitations and ideas of recent studies, and proposed directions for future research.
2. Phytochemicals in barley
Barley contains an assortment of phytochemicals (non-nutrient components) in varying concentrations usually determined by genotypic or environmental factors, or the interaction of both factors [20]. Phytochemicals in barley may exist in free, conjugated, or bound forms and are categorized into several major classes, including phenolic acids, flavonoids, lignans, tocols, phytosterols, and folates [29].
2.1. Phenolic acids
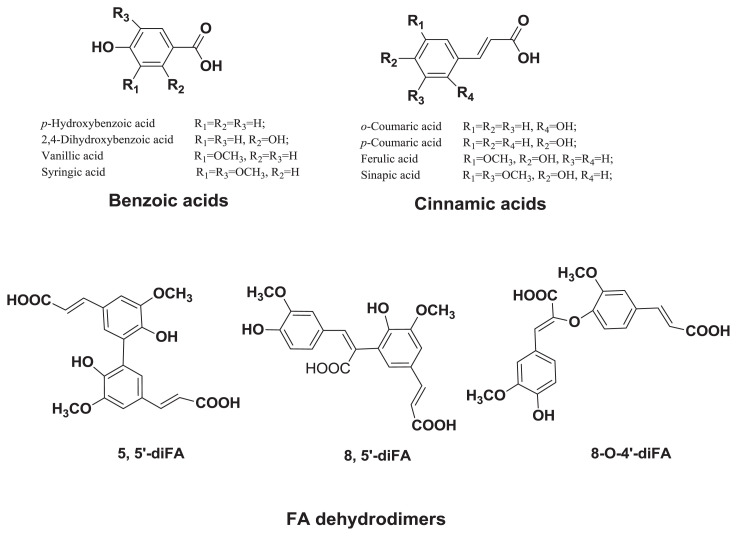
Phenolic acids (Figure 1), the dominant phenolic group of phytochemicals in barley are primarily located in the outer layers of the kernel [22]. They are subdivided into two groups: benzoic acid and cinnamic acid and their derivatives (Figure 1) [30–33]. Phenolic acids have been linked to chronic disease prevention partly due to the presence of unsaturated carboxylic group [34]. The abundant content of phenolic acids in barley, especially in the hulled variety, indicates that it may also serve as an excellent dietary source of natural antioxidants with antiradical and antiproliferative potentials [30,35].
Figure 1.
Structures of major phenolic acids in barley. FA = ferulic acid.
In barley, phenolic acids are found at highest concentrations in the bound form, followed by conjugated and free forms, respectively [31]. The free forms are often located in the outer part of the pericarp, whereas the bound forms are esterified to cell wall constituents, such as lignin, cellulose, arabinoxylans, polysaccharides, and hemicelluloses [36]. Free phenolic acids are usually a small portion of the total phenolic acid concentration. The phenolic acid concentration in barley approximately ranges between 4.6 μg/g and 23 μg/g for the free form, between 86 μg/g and 198 μg/g for the conjugated form, and between 133 μg/g and 523 μg/g for the bound form, whereas the total phenolic acid concentration ranges between 604 μg/g and 1346 μg/g [32,37]. The free forms of the major phenolic acids in barley are ferulic acid (FA; 27% of dry matter), vanillic acid (28%), syringic acid (17%), and p-coumaric acid (22%) [38].
FA is the most abundant and the main low molecular weight phenolic acid in barley [39]. It accounts for approximately 68% of total phenolic acids in barley, and its concentration in barley grains ranges between 149 μg/g and 413 μg/g [40]. Andersson et al [39] reported that the total concentration of FA in barley is about 270 μg/g of dry matter, whereas the average total concentrations of free, conjugated, and bound FA for different varieties of barley were approximately 2.7 μg/g, 33.21 μg/g, and 235 μg/g, respectively [39]. The major dehydrodimers of FA in barley are the (Z)-β-{4-[(E)-2-carboxyvinyl]-2-methoxyphenoxy}-4-hydroxy-3-methoxycinnamic acid (8-O-4′-DiFA), (E,E)-4,4′-dihydroxy-5,5′-dimethoxy-3′-bicinnamic acid (5,5′-DiFA) trans-5-[(E)-2-carboxyvinyl]-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran-3-carboxylic acid (8,5′-DiFA benzofuran form), and (E,E)-4,4-dihydroxy-3,5′-dimethoxy-β,3′-bicinnamic acid (8,5′-DiFA open form) (Figure 1). Among barleys FA dehydrodimers identified [41], 8-O-4′-DiFA (73–118 μg/g dry weight) was the major dehydrodimer, followed by 5,5′-DiFA (26–47 μg/g), 8,5′-DiFA benzofuran form (22–45 μg/g), and 8,5′-DiFA open form (10–23 μg/g) [41]. p-Coumaric acid is the second most abundant phenolic acid in barley ranging from 15 μg/g to 374 μg/g [32]. The concentrations of different forms of individual phenolic acids in barley expressed based on dry weight are presented in Table 1.
Table 1.
Content and composition of total, free, conjugated, and bound phenolic acids in barley.
| Phenolic acids | Free form (μg/g) | Conjugated form (μg/g) | Bound form (μg/g) |
|---|---|---|---|
| p-Hydrobenzoic acid | n.d. | 5.8–26.7 | 0.5–5.4 |
| Vanillic acid | 1.45–4.71 | 8.9–30.2 | 0.5–7.5 |
| Syringic acid | 0.45–3.74 | 2.2–10.0 | 0.0–3.0 |
| 2,4-Dihydroxybenzoic acid | 0.04–2.62 | 6.8–61.8 | 11.1–74.4 |
| Sinapic acid | n.d. | 12.4–24.4 | 8.9–17.8 |
| Ferulic acid | 1.32–5.87 | 21.7–42.5 | 104.3–365.4 |
| p-Coumaric acid | 0.57–7.01 | 1.7–13.1 | 2.7–109.7 |
| o-Coumaric acid | 0.27–1.31 | 1.2–3.2 | 2.7–4.7 |
Note. From “Phytochemical and dietary fiber components in barley varieties in the HEALTHGRAIN diversity screen,” by Andersson et al, J Agric Food Chem, 56, p. 9767–76. Copyright 2016, American Chemical Society. Adapted with permission.
n.d. = not determined.
2.2. Flavonoids
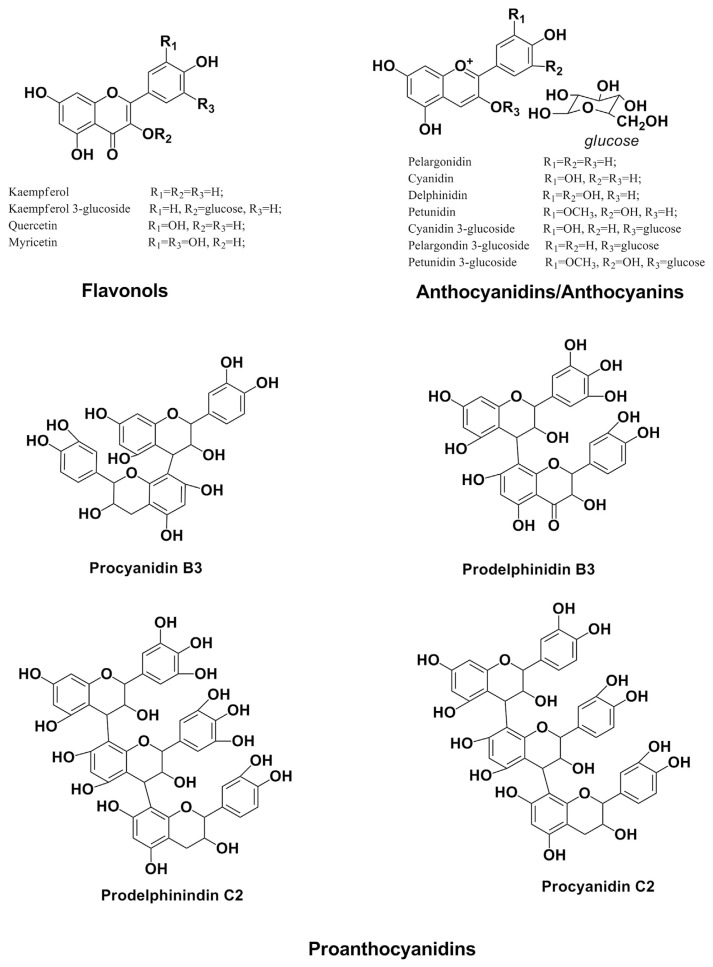
Flavonoids (Figure 2) are phytochemical compounds with a C6-C3-C6 skeleton (2 aromatic rings joined by a 3-carbon link). They are also believed to be UV-B absorbing compounds [42], which provide protection against UV radiation in response to “excess light” stress [43,44]. Clinical studies indicate that flavonoids may be the bioactive substances present in cereal grains responsible for the moderation of many diseases including cancer and coronary heart diseases [36,45].
Figure 2.
Structures of major flavonoids in barley.
Flavanols, anthocyanins, and proanthocyanidins (polymers of flavonoids) are the major types of flavonoids found in barley grains. Flavanols and anthocyanins are located in the pericarp of barley grains where they exist mostly as glycoside derivatives, including cyanidin-3-glucoside, penidin-3-glucoside, and delphinidin-3-glucoside [37,46]. Generally, the content of flavonoids in barley grains are proportional to the degree of color depth, and blue and purple barley grains have been discovered to possess the most flavonoid content among barley varieties [47].
Kim et al [48] studied the flavonoid content of 127 lines of hulled and unhulled colored barley wherein the total flavonoid content was found to range between 62.0 and 300.8 μg/g. Goupy et al [49] affirmed that monomeric, dimeric, and trimeric flavanol accounts for 58–68% of the total phenolic content of barley and that trimeric flavanols are the most abundant flavanols in barley. The proanthocyanidin content in barley studied by Kim et al [48] varied between 15.8 μg/g and 131.8 μg/g. The proanthocyanidin content of the unhulled barley group (75.9 μg/g) was higher than that of the hulled group (56.29 μg/g), whereas the proanthocyanidin content of the blue and purple group (83.0 μg/g) was significantly higher than that of the black group (55.3 μg/g). In a related study, Dvorakova et al [50] examined the proanthocyanidin content of 10 barley varieties (8 malt and 2 hulless barley varieties). From that study, the major proanthocyanidins reported included two proanthocyanidin dimers (prodelphinidin B3 and procyanidin B3) and four proanthocyanidin trimers (procyanidin C2, prodelphinidin C2, and 2 other prodelphinidin isomers). Prodelphinidin B3 (90–197 μg/g) accounted for majority of proanthocyanidin present in barley, whereas procyanidin C2 (5–19 μg/g) was reported to be present only in minor quantities [50].
Anthocyanins are water-soluble vacuolar pigments and the most studied flavonoids in barley. They are mainly present in the pericarp or the aleurone layers of barley grain causing purple or blue hues of kernel color. Anthocyanins in barley include cyanidin, cyanidin 3-glucoside, delphinidin, pelargonidin, pelargonidin glycosides, and petunidin 3-glucoside [46,51]. The most common anthocyanin in purple barley is cyanidin 3-glucosode (214.8 μg/g), followed by peonidin 3-glucoside and pelargonidin 3-glucoside. These three anthocyanins account for 50–70% of total anthocyanins reported in barley. Delphinidin 3-glucoside is usually the most abundant anthocyanin in blue (167.6 μg/g) and black (36.0 μg/g) barley varieties. In general, purple and blue barley (320.5 μg/g) contain higher average concentrations of anthocyanins than black barley (49.0 μg/g) [52,53]. Jende-Strid [54] reported that delphinidin and cyaniding were present in yellow, blue, and black barley varieties, whereas pelargonidin was present in purple barley.
Yang et al [55] analyzed the profiles of common flavonoids in unhulled purple barley, normal barley, and hulled purple barley. The bran-rich fraction of barley grain contained the most flavonoid content, whereas the hull fraction did not contain any significant flavonoid content. The total average content of flavonoids in hulled purple barley (124.8 μg/g) was significantly higher than that in unhulled purple barley (69.40 μg/g) and normal barley (48.50 μg/g). Total concentrations of catechin in the three types of barley studied varied from 0 μg/g to 21.85 μg/g. The total average catechin content in whole grain normal barley was significantly higher than that in hulled purple barley and unhulled purple barley. Whole grain myricetin content in hulled purple barley was significantly higher than that in unhulled purple barley and normal barley. The total average content of quercetin in hulled purple barley (60.98 μg/g) was significantly higher than that in the unhulled purple barley (24.35 μg/g) and normal barley (0.00 μg/g). The total average kaempferol content in unhulled purple barley (36.00 μg/g) was considerably higher than that in hulled purple barley (32.56 μg/g) and normal barley (26.65 μg/g).
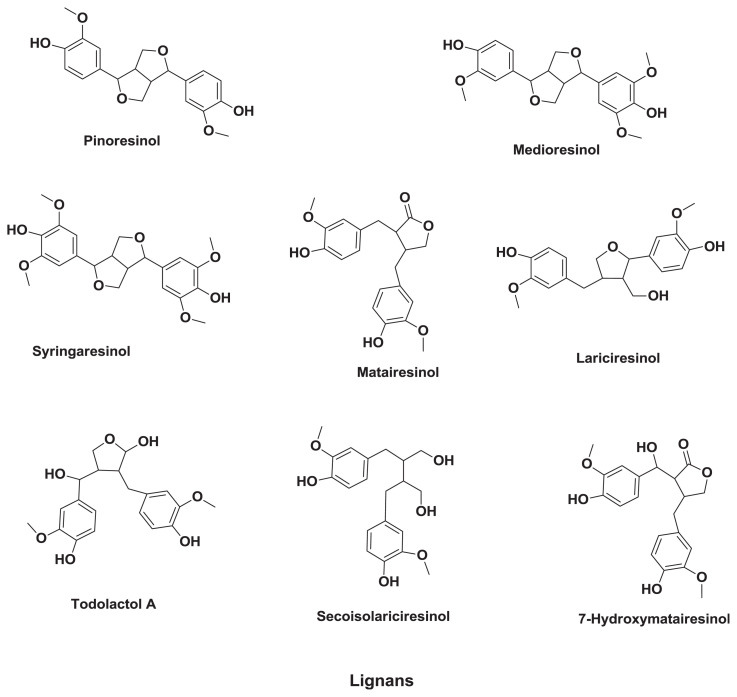
2.3. Lignans
Lignans (Figure 3) are natural polyphenols widely distributed in the plant kingdom as natural defense substances. They are bioactive as phytoestrogens because of their structural and functional similarity to 17β-estradiol. Lignans have been suggested to induce a wide range of biological effects, such as antioxidant, antitumor, antiviral, antibacterial, insecticidal, fungistatic, estrogenic, and antiestrogenic activities, and protect against coronary heart disease [56–58]. There is little information in the literature about lignans in barley. Smeds et al [59] studied the content of lignans in barley, which was reported to include pinoresinol (71 μg/100g), medioresinol (22 μg/100g), syringaresinol (140 μg/100g), lariciresinol (133 μg/100g), cyclolariciresinol (28 μg/100g), secoisolariciresinol (42 μg/100g), secoisolariciresinol-sesquilignan (24 μg/100g), matairesinol (42 μg/100g), oxomatairesinol (28 μg/100g), and 7-hydroxymatairesinol (541 μg/100g) as major lignans and todolactol (127 μg/100g), α-conidengrin acid (33 μg/100g), nor-trachelogenin (15 μg/100g), and lariciresinol-sesquillgnan (6.6 μg/100g) as minor lignans.
Figure 3.
Structures of major lignans in barley.
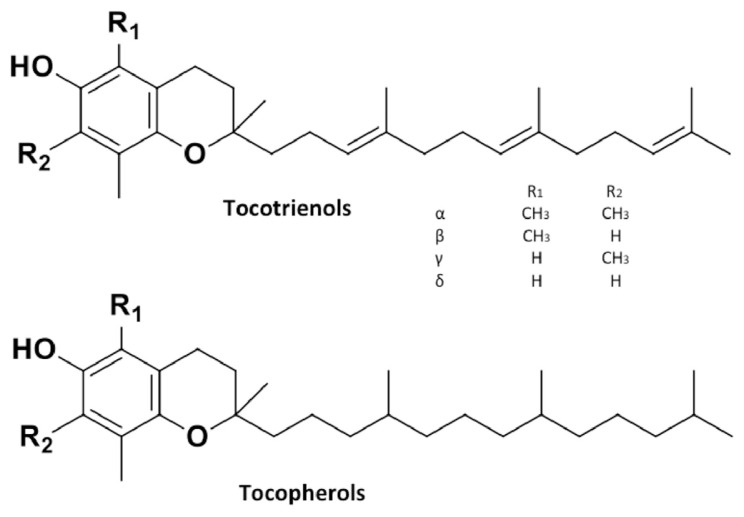
2.4. Tocols
Tocopherols and tocotrienols (collectively known as tocols; Figure 4) are a class of lipid-soluble phytochemicals found in barley. Tocols are recognized for their antioxidant properties, especially their ability to inhibit lipid peroxidation in biological membranes [60–63]. In addition to their antioxidant properties, tocols found in cereals proffer anticancer and cancer suppression effects [64,65], induce the immune system [66], moderate the risk factors of cardiovascular diseases (CVD) [67,68], and promote apoptosis induction [69]. One of the most striking discoveries about tocols is their ability to clear atherosclerotic blockages (stenosis) in the carotid artery, potentially reducing the risk of stroke [70].
Figure 4.
Structures of major tocols in barley.
Barley is one of the best sources of tocols among cereals due to a high concentration and favorable distribution of all eight biologically active vitamers [71]. Moreau et al [71] reported that levels of total phytosterols and total tocotrienols in the oils prepared from both whole kernels and scarified fines of barley ranged between 2911 μg/g and 6126 μg/g. This value is several-folds higher than that in palm oil (530 μg/g) and rice bran oil (770 μg/g), promoted as “high in tocotrienols”. Temelli et al [72] studied the total tocol content of whole grain barley varieties, which ranged between 40 μg/g and 151.1 μg/g. In whole grain barley, α-tocotrienol is the most individual tocol isomer, contributing about 47.7% of the total tocol content [39], followed by α-tocopherol (17.7–33.9%), γ-tocotrienol (10.4–20.2%), γ-tocopherol (1.9–9.2%), β-tocotrienol (2.9–7.8%), and δ-tocotrienol (2.7–6.7%) [72]. The average content of tocotrienol in barley is about 70.6–76.8% [40,71]. This signifies that barley could be one of the richest sources of tocotrienol among cereal grains.
The majority of barley’s tocopherols are located in its germ fraction, whereas the tocotrienols are mostly present in the endosperm and pericarp fraction of barley grains. Zielinski [28] reported that 95% of tocols occur in the endosperm, whereas 63% and 10% of tocols occur in the hull and germ components of barley, respectively. Hulled barley has been reported to possess more tocol content than hulless barley. Cavallero et al [73] reported higher tocol and α-tocotrienol contents in hulled barley (53–61 μg/g) than those in hulless barley (50.9–53.1 μg/g). This is attributed to the presence of tocols in the hull of barley. However, a study by Ehrenbergerova et al [74], showed that the hulless waxy variety, Washonubet, had a statistically higher total content of tocols (67.6 μg/g) and α-tocotrienol isomer (42.1 μg/g) than all other barley varieties studied, including hulled varieties. This gives credence to the variation between genotypes and the effect of growing location on tocol content.
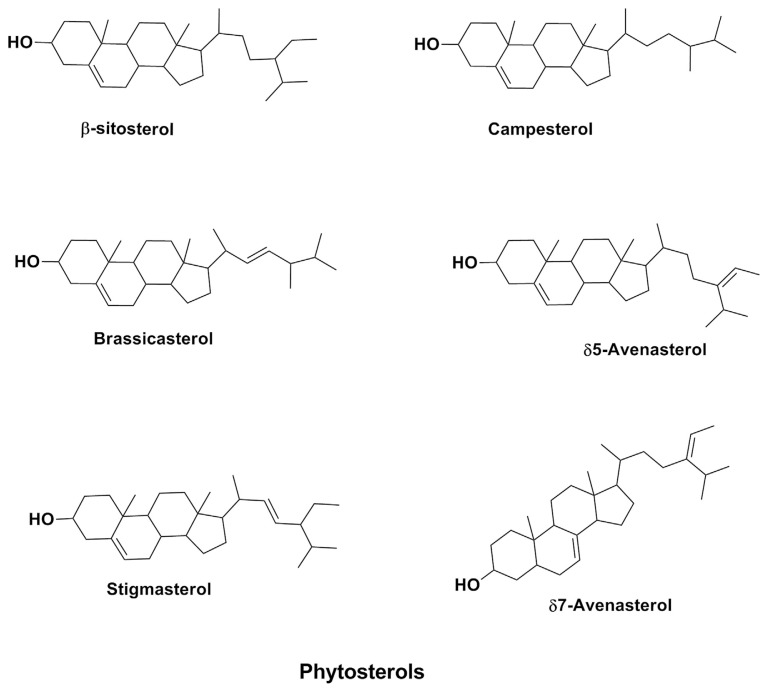
2.5. Phytosterols
Phytosterols (Figure 5) or plant sterol is an important structural component of plant membrane similar in structure to cholesterol, but different in configuration. Recent studies have shown that natural intake of dietary plant sterols can have a positive effect in decreasing serum cholesterol levels, protect against CVD, and prevent colon cancer [75–80]. Cereals are the main source of plant sterols, together with vegetable oils, contributing up to 40% of daily intake of plant sterols [81].
Figure 5.
Structures of major phytosterols in barley.
Barley is considered a good source of phytosterol although barley’s phytosterol level is moderate compared with other major grains [82]. Barley grains generally contain phytosterols in both free and bound forms, esterified to fatty acids, phenolic acids, steryl glucosides, or acylated steryl glycosides. The level of esterification varies among varieties and around different parts of barley grain [83]. Higher levels of phytosterols have been identified in the outer layers of the kernel. The content of sterols in 10 barley varieties studied by Andersson et al [39] ranged between 820 μg/g and 1153 μg/g. Piironen et al [84] analyzed and compared the phytosterol content of major cereal grains with the highest plant sterol content (mean, 955 μg/g) determined in rye, followed by barley (761 μg/g), wheat (690 μg/g), and oat (447 μg/g). As in most cereals, sitosterol is the most abundant sterol form in barley, contributing about 53–61% of total sterols, followed by campesterol (14–20%). Other reported forms of sterol in barley include brassicasterol, stigmasterol, δ5-avenasterol, stigmastenol, stigmastadienol, and δ7-avenasterol [85]. Lampi et al [85] studied the concentration of individual sterols in barley whole grain; β-sitosterol (476 ± 1 μg/g) was the most abundant, followed by campesterol (181 ± 2 μg/g), stigmasterol (39 μg/g), and other minor sterols (δ5- and δ7-avenasterols, δ7-stigmastenol, and stigmastadienol; 86 ± 1 μg/g).
The fully saturated subgroup form of phytosterol (phytostanols) has also been reported in barley; however, its concentration is generally lower than that of unsaturated sterols. The major phytostanols in barley are sistostanol and campestanol. According to Andersson et al [39], phytostanol makes up only about 1–3% of total phytosterol in barley. From that study, the concentration of phytostanol in 10 varieties of barley varied between 10–30 μg/g. Lampi et al [85] recorded the stanol concentrations of campestanol (11 μg/g) and sitostanol (5 μg/g) for the variety of barley studied.
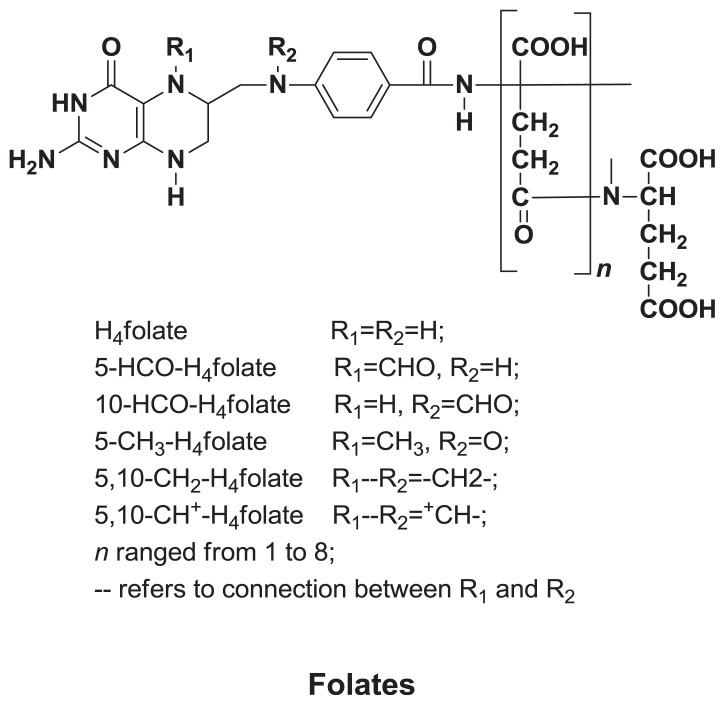
2.6. Folates
Folate (Figure 6) is a group of phytochemicals that represents an essential nutrition component (vitamin B). Folate is capable of performing the same biological activity as folic acid and is involved in many metabolic pathways [86]. Cereals are considered an important source of folate, though little is known about folate in barley. Folate is thought to be unevenly distributed in barley grain with more concentration around the outer layers than around the endosperm [87]. Edelmann et al [88] studied the distribution of folates in barley grain, which was found to accumulate in the bran and germ tissues of the grain. Andersson et al [39] studied the total folate content of 10 barley genotypes (518–789 ng/g of dry mass), which was found to be higher than that of wheat (323–774 ng/g, n = 130) [89], oat (495–604 ng/g, n = 5) [90], and close to that of rye (574–775 ng/g, n = 10). Edelmann et al [88] also reported a similar folate content of 598–664 ng/g for five hulled Finnish barley cultivars studied.
Figure 6.
Structures of major folates in barley.
The comparison between the occurrence and proportions of folate vitamers is difficult among cereal grain samples because of differences in the analytical methods and number of examined vitamers. Moreover, the harvesting time, growing conditions, and storage time may have an effect on the vitamer pattern. However, folate vitamers reported in barley include 5-methyltetrahydrofolate (5-HCO-H4folate), 5, 10-methylenetetrahydrofolate (5-CH3-H4folate), 5, 10-methenyltetrahydrofolate (5, 10-CH+-H4folate) and 10-formylfolic acid (10-HCO-PGA). Edelmann et al [88] found the major folate vitamer in barley to be 5-HCO-H4folate, which contributed an average of 27–42% of total folate content, followed by 5-CH3-H4folate and 10-CH+-H4folate. The proportion of 10-HCO-PGA was remarkable in the industrial milling fractions studied, with contributions of an average of 9–22% of total folate content.
3. Barley phytochemicals and human health
3.1. Cardiovascular disease (CVD)
CVD is a leading cause of death in the United States [91]. In 2011, the overall death rate attributed to CVD was 229.6 per 100,000 Americans, and in 2013, coronary heart disease alone caused approximately one of every seven deaths in the United States [92]. It is generally recognized that high blood cholesterol level is a significant risk factor in the occurrence of CVDs. In addition, there is evidence indicating that free radicals and other oxidants largely cause most neurodegenerative diseases linked with CVDs, such as Alzheimer’s disease and stroke [93–96]. Consumption of natural antioxidants, such as polyphenols, in daily diets can produce an effective protective action against oxidative processes generated by these compounds, thereby improving cardiovascular health [97–100].
Recent strategies for reducing the risk of CVDs utilize dietary restrictions to limit cholesterol intake and/or the use of a class of medication, statin, to lower serum cholesterol. Barley kernels are rich in phytosterols and are important for cardiovascular health. Consumption of barley grains rich in phytonutrients may help reduce the dependence on drug use or dietary restrictions for the moderation of CVD. Barley phytosterols can compete with cholesterol for micelle formation in the lumen of the intestine, thus inhibiting cholesterol absorption and increasing secretion and regulation of cholesterol levels. Studies show that the levels of total phytosterols in barley oils are sufficient (0.18–1.44 g/15 g oil) to significantly lower low-density lipoprotein (LDL) cholesterol at reasonable dosages of 15 mL/d (1 tablespoon/d) [71].
Polyphenols also play a major role in moderating CVDs due to their antiradical scavenging potential. Polyphenols extracted from black highland barley were found to have strong superoxide radical, hydroxyl radical, 2,2-diphenyl-1-picrylhydrazyl radical-scavenging activity, and antioxidant ability. Mice that were administered 600 μg black highland polyphenol extract per gram body weight showed significant reduction in total cholesterol (23.33%), LDL cholesterol (26.29%), and atherosclerosis index (38.70%), in addition to increasing high density lipoprotein cholesterol (HDL, 17.80%) [101]. Lee et al [102] also reported that barley sprout extract containing polyphenols regulated AMP-activated protein kinase, a cellular sensor of energy metabolism and a regulator for cholesterol metabolism. The barley sprout extract containing 19.65 mg/g of total polyphenol concentration reduced the intracellular total and free cholesterol concentrations in mice by 24% and 18%, respectively. Lignans, also present in barley, have been found to act as strong antioxidants comparable to FA and better than vitamin E [103]. They have also been reported to lower the risk of CVD [104].
Tocotrienols and tocopherols in barley are strong antioxidants and thought to be important for cardiovascular health. Among the most economically important cereals (wheat, barley, rice, rye, and oat), barley has been reported to have the highest amounts of tocols and phytosterols [39,87,105]. Tocols have been reported to lower serum cholesterol in humans and chicks [106]. Qureshi et al [107] identified α-tocotrienol as an inhibitor of cholesterol biosynthesis in the livers of experimental animals. The mechanism for this effect was α-tocotrienol’s ability to suppress 3-hydroxy-3-methyl-glutaryl-coenzyme reductase, a rate-limiting enzyme involved in cholesterol biosynthesis. Burger et al [108] reported that the administration of 0–20 ppm α-tocotrienol from barley for a 3-week period controlled the biosynthesis of cholesterol concentrations and reduced cholesterol levels by approximately 60%. Nonetheless, there have been conflicting reports on the ability of barley tocols to moderate cholesterol levels. Earlier data presented by Jadhav et al [109] showed no effect of barley oil on total serum cholesterol in rats fed a diet based on potato starch. Wang et al [106] also found no evidence in support of the cholesterol-lowering effect of barley oil and its nonsaponifiable constituents with the hamster model.
Folate has been associated with cardiovascular health. It is currently one of the most studied vitamins due to its essential role in amino acid metabolism and DNA methylation. It functions as a coenzyme, providing a carbon unit of nucleotide biosynthesis in humans. Its suboptimal presence in humans has been associated with the increased risk of cardiovascular ailment [110,111]. Barley grains enriched with folate are, therefore, a good and natural means of obtaining folate from diets and in turn preventing and reducing the risk of cardiovascular ailments.
3.2. Diabetes and obesity
Diabetes and obesity are public health problems with health and economic consequences that have raised global concern. Cost-effective mitigation strategies rather than containment are, therefore, of paramount importance for the prevention and treatment of these diseases. As a result, a plethora of lifestyle modification, diets, pills, and weight loss regimens have been recommended. Several epidemiological studies have linked the consumption of barley with the reduction of diabetes and obesity [112,113]. Nonetheless, the components of barley responsible for these benefits have not been clearly identified, but fiber and high nutrient components are considered leading contenders.
Notwithstanding scarce in vivo studies on effects of barley phytochemicals on diabetes and obesity, it is well known that one of the primary pathogenic factors leading to insulin resistance, β-cell dysfunction, impaired glucose tolerance, and ultimately diabetes occurrence is oxidative stress. Oxidative stress, which plays an important role in the pathology of diabetes and obesity, can be moderated by phytochemicals present in barley. An increase in the formation of reactive oxygen species and a decrease in antioxidant defense efficiency are characteristics of both diseases [114–116]. Therefore, phytochemicals may prevent the development and progression of obesity and diabetes by reducing oxidative stress [117]. Barley phytochemicals that may have a role in protecting against diabetes and obesity include various phenolic acids, flavonoids, phytosterols, and tocols. These compounds function as strong antioxidants. However, total phenolic acids are considered as the major components responsible for the antioxidative benefits of cereals including barley [38,118]. Phenolic acids in cereals have demonstrated similar or higher antioxidative activities than catechins and have been linked to prevention of chronic disease due to the presence of unsaturated carboxylic group [119]. Zielinski and Kozlowska [120] reported a positive correlation coefficient value of 0.96 between total phenolic compounds and total antioxidative activity of 80% methanolic extracts of barley whole grains. Goupy et al [49] studied the antioxidative properties of barley phytochemicals. The antioxidative properties of barley flavonoids, phenolic acids (especially FA), and tocolpherols (α, β, γ) determined by their capacity to react with 2,2-diphenyl-1-picrylhydrazyl (DPPH) were approximately 4.76, 0.34, and 0.89 antiradical power, respectively. In addition, the antioxidative activity determined by the inhibition of cooxidation of β-carotene in linoleate model system for different barley flavonoids studied ranged from 16.4% to 65.5%, whereas those for phenolic acids ranged from 12% to 31.2% and those of tocopherol ranged from 49.1 to 55.2%. Antioxidative activities of barley were also studied by their ability to inhibit lipoxygenase. The antioxidative activities recorded were 24.8–65.4% for flavonoids, 2.9–10.8% for phenolic acids, and 49.1–63.9% for tocopherols. Carotenoids in barley didn’t show as high antioxidant activities as other phytochemicals such as flavonoids and phenolic acids.
The antioxidative activity investigated for 80% methanolic extracts originating from different whole grains were in the hierarchy: barley > oat > wheat > rye [120]. This high anti-oxidative activity of phytochemicals present in barley makes it a useful natural means for the prevention of diabetes and obesity development and progression. Furthermore, systemic, low-grade inflammation, especially in adipose tissue, is a trademark of obesity and diabetes. In addition to barley phytochemicals’ antioxidant properties, barley phytochemical compounds have potent anti-inflammatory actions and could thereby moderate diabetes and obesity risk by this mechanism [121–123].
3.3. Cancer
Epidemiological data indicate that diet/lifestyle is responsible for approximately 20–80% human cancer mortality [124]. Dietary factors, especially those that reduce the impact of reactive oxygen species, can protect against DNA damage and stimulate the immune system, thus lowering cancer risks [125]. Barley and its products have bioactive compounds with antioxidative and immunomodulatory activities that are associated with cancer moderation. Most studies regarding the chemoprevention of carcinogenesis by barley have been in vitro and have mainly involved the effect of barley fiber, especially β-glucan, and the moderation of this disease. Kanauchi et al [126] investigated anti-carcinogenic benefits of germinated barley foodstuff (GBF), a prebiotic heterogeneous mixture of approximately 80% hemicellulose and insoluble glutamine-rich protein fiber. GBF also contains phytochemicals, especially phenolic acids, present in free or bound forms which contribute to its health benefits [127]. Kanauchi et al [126] reported that GBF affects the early stages of the pathogenesis of colon cancer and helps impede transforming hyperproliferative epithelia. GBF’s administration when compared with the control diet, induced the production of acetate (p < 0.05) and increased the luminal short chain fatty acid concentration, especially butyrate (p < 0.05), which is produced in the colon. The colonic mucosa of F344 rats in the GBF group showed a decrease in the production of succinate (p < 0.05) and a reduction in the expression of β-catenin (p < 0.05), aberrant crypt foci (p < 0.05), and β-catechin formations (p < 0.05). The activities of slc5a8, a tumor suppressor gene and a solute carrier, cecal B-glycosidase, and heat shock protein 25 (HSP25) positive cells content also increased in the GBF group compared with those in the control group (p < 0.05). Studies regarding the moderation of colonic carcinogenesis have shown similar changes in the contents and activities of acetate, butyrate, succinate, HSP25 positive cells, β-catenin, aberrant crypt foci, and other related biomarkers [128–133]. In another report by McIntosh et al [134] on the influence of different fiber-rich sources from wheat and barley in diets on tumor incidence, tumor burden, tumor mass index, and dimethyl induced tumor, these were reported to be more effectively prevented by dietary insoluble fiber from barley than from other soluble fiber-rich commercial bran from barley and oat [134]. Spent barley grain, a by-product of the brewing industry, produced the lowest tumor incidence (70%), tumor mass index (1.20), and the tumor burden per 10 rats (13).
Emerging research also suggests that polyphenols may offer more health benefits, such as enhancement of endothelial function, improvement of cellular signaling, and crucial intestinal protection from undigested polyphenols associated with fiber, than previously thought [135]. The abundant content of phenolic compounds in barley suggests that it may also serve as an excellent dietary source of natural antioxidants with antiradical and antiproliferative potentials [35]. Several studies have shown that FA has tumor inhibition properties [136,137]. There have also been reports of its ability to inhibit large bowel carcinogenesis [138].
Although a positive relationship exists between the intake of phenolic compounds and the reduced risk of certain chronic diseases, it remains unclear whether the protective effect of phenolic compounds arises from antioxidant activity or other mechanism.
High antioxidant activity due to the presence of phenolic acids in barley were reported by Madhujith and Shahidi [139] and Bellido and Beta [52]. Barley extracts with high phenolic acid content and corresponding high antioxidative activity led to the inhibition of cell proliferation of Caco-2 colon cancer by 29.3–51.2% after the administration of 0.5 mg/mL and 0.05 mg/mL of phenolic extracts from barley. Fermentation of barley (lactic acid) was also reported to produce a novel purple pigment called hordeumin, a type of anthocyanidin–tannin pigment whose scavenging activity increases with the length of fermentation. Deguchi et al [140] suggested that the free radical activity of hordeumin results from barley bran polyphenols. Phytochemicals present in barley may be beneficial in moderating the risk factors associated with cancer and therefore should be a subject of further studies.
4. Conclusion
Barley has an assortment of phytochemicals with the potential to impact human health. These benefits are due to inherent properties present in phytochemicals, such as high antioxidative activity against different free radicals, anti-inflammatory, and immunostimulation potentials, or the ability to inhibit LDL cholesterol, while increasing HDL cholesterol levels. However, there is a need for more systemic and detailed study on barley phytochemicals, thus establishing a chemical profile for phytochemicals in barley and possibly identifying unique compounds as have been done with avenanthramides in oat and alkylresorcinols in rye and wheat. In addition, since specific studies on health effects of phytochemicals in barley are limited, it is worthwhile to further study the efficacy and the underlying molecular mechanisms of barley phytochemicals, thereby promoting the use of barley as a functional food.
Acknowledgments
The authors would like to thank Aaron Yerke from North Carolina Agricultural and Technical State University for his suggestions on revisions and editing.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
REFERENCES
- 1. Badr A, Sch R, El Rabey H, Effgen S, Ibrahim H, Pozzi C, Rohde W, Salamini F. On the origin and domestication history of barley (Hordeum vulgare) Mol Biol Evol. 2000;17:499–510. doi: 10.1093/oxfordjournals.molbev.a026330. [DOI] [PubMed] [Google Scholar]
- 2. Schulte D, Close TJ, Graner A, Langridge P, Matsumoto T, Muehlbauer G, Sato K, Schulman AH, Waugh R, Wise RP, Stein N. The international barley sequencing consortium—at the threshold of efficient access to the barley genome. Plant Physiol. 2009;149:142–7. doi: 10.1104/pp.108.128967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook AH. Barley and malt: biology, biochemistry technology. New York: Academic Press; 2013. [Google Scholar]
- 4. Sullivan P, Arendt E, Gallagher E. The increasing use of barley and barley by-products in the production of healthier baked goods. Trends Food Sci Technol. 2013;29:124–34. [Google Scholar]
- 5.Shewry PR, Ullrich SE. Barley: chemistry and technology. American Association of Cereal Chemists, Inc (AACC)/CAB International; Wallingford: 2014. [Google Scholar]
- 6. Annapurna A. Health benefits of barley. Asian J Pharmaceut Res Health Care. 2011;3:22. [Google Scholar]
- 7. Bays H, Frestedt JL, Bell M, Williams C, Kolberg L, Schmelzer W, Anderson JW. Reduced viscosity Barley β-Glucan versus placebo: a randomized controlled trial of the effects on insulin sensitivity for individuals at risk for diabetes mellitus. Nutr Metab (Lond) 2011;8:1. doi: 10.1186/1743-7075-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dongowski G, Huth M, Gebhardt E, Flamme W. Dietary fiber-rich barley products beneficially affect the intestinal tract of rats. J Nutr. 2002;132:3704–14. doi: 10.1093/jn/132.12.3704. [DOI] [PubMed] [Google Scholar]
- 9. Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 10. Behall KM, Scholfield DJ, Hallfrisch J. Whole-grain diets reduce blood pressure in mildly hypercholesterolemic men and women. J Am Diet Assoc. 2006;106:1445–9. doi: 10.1016/j.jada.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 11. Hoang MH, Houng SJ, Jun HJ, Lee JH, Choi JW, Kim SH, Kim YR, Lee SJ. Barley intake induces bile acid excretion by reduced expression of intestinal ASBT and NPC1L1 in C57BL/6J mice. J Agric Food Chem. 2011;59:6798–805. doi: 10.1021/jf200681n. [DOI] [PubMed] [Google Scholar]
- 12. Zhang JX, Bergman F, Hallmans G, Johansson G, Lundin E, Stenling R, Theander OL, Westerlund ER. The influence of barley fibre on bile composition, gallstone formation, serum cholesterol and intestinal morphology in hamsters. APMIS. 1990;98:568–74. doi: 10.1111/j.1699-0463.1990.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 13. Kanauchi O, Fujiyama Y, Mitsuyama K, Araki Y, Ishii T, Nakamura T, Hitomi Y, Agata K, Saiki T, Andoh A, Toyonaga A, Bamba T. Increased growth of Bifidobacterium and Eubacterium by germinated barley foodstuff, accompanied by enhanced butyrate production in healthy volunteers. Int J Mol Med. 1999;3:175–84. doi: 10.3892/ijmm.3.2.175. [DOI] [PubMed] [Google Scholar]
- 14. Tada R, Ikeda F, Aoki K, Yoshikawa M, Kato Y, Adachi Y, Tanioka A, Ishibashi KI, Tsubaki K, Ohno N. Barley-derived β-D-glucan induces immunostimulation via a dectin-1-mediated pathway. Immunol Lett. 2009;123:144–8. doi: 10.1016/j.imlet.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 15. Kemp LP, Paik WC, Scheid M. Natural nutritional supplements. 20130280382A1. Google Patents, US. 2014
- 16. Baik BK, Ullrich SE. Barley for food: characteristics, improvement, and renewed interest. J Cereal Sci. 2008;48:233–42. [Google Scholar]
- 17. Thondre P, Ryan L, Henry C. Barley β-glucan extracts as rich sources of polyphenols and antioxidants. Food Chem. 2011;126:72–7. [Google Scholar]
- 18. Agostini S, Chiavacci E, Matteucci M, Torelli M, Pitto L, Lionetti V. Barley beta-glucan promotes MnSOD expression and enhances angiogenesis under oxidative microenvironment. J Cell Mol Med. 2015;19:227–38. doi: 10.1111/jcmm.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seo CR, Yi B, Oh S, Kwon SM, Kim S, Song NJ, Cho JY, Park KM, Ahn JY, Hong JW, Kim MJ. Aqueous extracts of hulled barley containing coumaric acid and ferulic acid inhibit adipogenesis in vitro and obesity in vivo. J Funct Foods. 2015;12:208–18. [Google Scholar]
- 20.Malik AH. Doctoral Thesis. Uppsala Swedish University of Agricultural Sciences; 2012. Governing grain protein concentration and composition in wheat and barley: use of genetic and environmental factors. [Google Scholar]
- 21.Lattanzio V, Lattanzio VM, Cardinali A. Phytochemistry: advances in research. Kerala: Research Signpost; 2006. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. [Google Scholar]
- 22. Dykes L, Rooney L. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World. 2007;52:105–11. [Google Scholar]
- 23. Mithöfer A, Boland W. Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol. 2012;63:431–50. doi: 10.1146/annurev-arplant-042110-103854. [DOI] [PubMed] [Google Scholar]
- 24. Bartłomiej S, Justyna RK, Ewa N. Bioactive compounds in cereal grains–occurrence, structure, technological significance and nutritional benefits–a review. Food Sci Technol Int. 2012;18:559–68. doi: 10.1177/1082013211433079. [DOI] [PubMed] [Google Scholar]
- 25. Shahidi F, Zhong Y. Lipid oxidation and improving the oxidative stability. Chem Soc Rev. 2010;39:4067–79. doi: 10.1039/b922183m. [DOI] [PubMed] [Google Scholar]
- 26. Panfili G, Fratianni A, Irano M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J Agric Food Chem. 2003;51:3940–4. doi: 10.1021/jf030009v. [DOI] [PubMed] [Google Scholar]
- 27.Lampi AM, Kamal-Eldin A, Piironen V. Functional foods: biochemical and processing aspects. Vol. 2. Boca Raton: CRC Press, LLC; 2002. Tocopherols and tocotrienols from oil and cereal grains; pp. 1–38. [Google Scholar]
- 28.Zielinski H. Tocotrienols: vitamin E beyond tocopherols. Vol. 1. Boca Raton: CRC Press; 2006. Tocotrienols: distribution and sources cereals–role in human health; pp. 23–42. [Google Scholar]
- 29. Fogarasi AL, Kun S, Tankó G, Stefanovits-Bányai É, Hegyesné-Vecseri B. A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 2015;167:1–6. doi: 10.1016/j.foodchem.2014.06.084. [DOI] [PubMed] [Google Scholar]
- 30. Quinde-Axtell Z, Baik BK. Phenolic compounds of barley grain and their implication in food product discoloration. J Agric Food Chem. 2006;54:9978–84. doi: 10.1021/jf060974w. [DOI] [PubMed] [Google Scholar]
- 31. Bonoli M, Verardo V, Marconi E, Caboni MF. Antioxidant phenols in barley (Hordeum vulgare L.) flour: comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J Agric Food Chem. 2004;52:5195–200. doi: 10.1021/jf040075c. [DOI] [PubMed] [Google Scholar]
- 32. Holtekjølen AK, Kinitz C, Knutsen SH. Flavanol and bound phenolic acid contents in different barley varieties. J Agric Food Chem. 2006;54:2253–60. doi: 10.1021/jf052394p. [DOI] [PubMed] [Google Scholar]
- 33. Tang Y, Li X, Zhang B, Chen PX, Liu R, Tsao R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015;166:380–8. doi: 10.1016/j.foodchem.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 34. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–8. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao Z, Moghadasian MH. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem. 2008;109:691–702. doi: 10.1016/j.foodchem.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 36. Tang Y, Zhang B, Li X, Chen PX, Zhang H, Liu R, Tsao R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J Agric Food Chem. 2016;64:1712–9. doi: 10.1021/acs.jafc.5b05761. [DOI] [PubMed] [Google Scholar]
- 37. Abdel-Aal ESM, Choo TM, Dhillon S, Rabalski I. Free and bound phenolic acids and total phenolics in black, blue, and yellow barley and their contribution to free radical scavenging capacity. Cereal Chem. 2012;89:198–204. [Google Scholar]
- 38. Gamel T, Abdel-Aal ESM. Phenolic acids and antioxidant properties of barley wholegrain and pearling fractions. Agric Food Sci. 2012;21:118–31. [Google Scholar]
- 39. Andersson AA, Lampi A-M, Nyström L, Piironen V, Li L, Ward JL, Gebruers K, Courtin CM, Delcour JA, Boros D, Fras A. Phytochemical and dietary fiber components in barley varieties in the HEALTHGRAIN diversity screen. J Agric Food Chem. 2008;56:9767–76. doi: 10.1021/jf802037f. [DOI] [PubMed] [Google Scholar]
- 40. Ward JL, Poutanen K, Gebruers K, Piironen V, Lampi A-M, Nyström L, Andersson AA, Boros D, Rakszegi M, Bedo Z, Shewry PR. The HEALTHGRAIN cereal diversity screen: concept, results, and prospects. J Agric Food Chem. 2008;56:9699–709. doi: 10.1021/jf8009574. [DOI] [PubMed] [Google Scholar]
- 41. Hernanz D, Nuñez V, Sancho AI, Faulds CB, Williamson G, Bartolomé B, Gómez-Cordovés C. Hydroxycinnamic acids and ferulic acid dehydrodimers in barley and processed barley. J Agric Food Chem. 2001;49:4884–8. doi: 10.1021/jf010530u. [DOI] [PubMed] [Google Scholar]
- 42. Tossi V, Lombardo C, Cassia R, Lamattina L. Nitric oxide and flavonoids are systemically induced by UV-B in maize leaves. Plant Sci. 2012;193:103–9. doi: 10.1016/j.plantsci.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 43. Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol. 2011;168:204–12. doi: 10.1016/j.jplph.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 44. Bashandy T, Taconnat L, Renou JP, Meyer Y, Reichheld JP. Accumulation of flavonoids in an ntra ntrb mutant leads to tolerance to UV-C. Molecular Plant. 2009;2:249–58. doi: 10.1093/mp/ssn065. [DOI] [PubMed] [Google Scholar]
- 45. Gani A, Wani S, Masoodi F, Hameed G. Whole-grain cereal bioactive compounds and their health benefits: a review. J Food Process Technol. 20122012 [Google Scholar]
- 46. Abdel-Aal ESM, Young JC, Rabalski I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J Agric Food Chem. 2006;54:4696–704. doi: 10.1021/jf0606609. [DOI] [PubMed] [Google Scholar]
- 47. Liu Z, Liu Y, Pu Z, Wang J, Zheng Y, Li Y, Wei Y. Regulation, evolution, and functionality of flavonoids in cereal crops. Biotechnol Lett. 2013;35:1765–80. doi: 10.1007/s10529-013-1277-4. [DOI] [PubMed] [Google Scholar]
- 48. Kim MJ, Hyun JN, Kim JA, Park JC, Kim MY, Kim JG, Lee SJ, Chun SC, Chung IM. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J Agric Food Chem. 2007;55:4802–9. doi: 10.1021/jf0701943. [DOI] [PubMed] [Google Scholar]
- 49. Goupy P, Hugues M, Boivin P, Amiot MJ. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J Sci Food Agric. 1999;79:1625–34. [Google Scholar]
- 50. Dvorakova M, Moreira MM, Dostalek P, Skulilova Z, Guido LF, Barros AA. Characterization of monomeric and oligomeric flavan-3-ols from barley and malt by liquid chromatography–ultraviolet detection–electrospray ionization mass spectrometry. J Chromatogr A. 2008;1189:398–405. doi: 10.1016/j.chroma.2007.10.080. [DOI] [PubMed] [Google Scholar]
- 51.Mazza G, Gao L. Blue and purple grains. In: Abdel-Aal E, Wood P, editors. Specialty grains for food and feed. St Paul: AACC International Inc; 2005. pp. 45–67. [Google Scholar]
- 52. Bellido GG, Beta T. Anthocyanin composition and oxygen radical scavenging capacity (ORAC) of milled and pearled purple, black, and common barley. J Agric Food Chem. 2009;57:1022–8. doi: 10.1021/jf802846x. [DOI] [PubMed] [Google Scholar]
- 53. Siebenhandl S, Grausgruber H, Pellegrini N, Del Rio D, Fogliano V, Pernice R, Berghofer E. Phytochemical profile of main antioxidants in different fractions of purple and blue wheat, and black barley. J Agric Food Chem. 2007;55:8541–7. doi: 10.1021/jf072021j. [DOI] [PubMed] [Google Scholar]
- 54. Jende-Strid B. Genetic control of flavonoid biosynthesis in barley. Hereditas. 1993;119:187–204. [Google Scholar]
- 55. Yang T, Duan CL, Zeng YW, Du J, Yang SM, Pu XY, Yang SC. HPLC analysis of flavonoids compounds of purple, normal barley grain. AMR. 2013;634:1486–90. [Google Scholar]
- 56.Saarinen N, Mäkelä S, Santti R, editors. From the Institute of Biomedicine, Department of Anatomy. Animal Cell Technology: Basic & Applied Aspects: Proceedings of the Fifteenth Annual Meeting of the Japanese Association for Animal Cell Technology (JAACT); Fuchu, Japan. November 11–15, 2002; Dordrecht: Springer Science & Business Media/Kluwer Academic Publishers; 2013. [Google Scholar]
- 57. Rhee Y. Flaxseed secoisolariciresinol diglucoside and enterolactone down-regulated epigenetic modification associated gene expression in murine adipocytes. J Funct Foods. 2016;23:523–31. [Google Scholar]
- 58. Prasad K, Jadhav A. Prevention and treatment of atherosclerosis with flaxseed-derived compound secoisolariciresinol diglucoside. Curr Pharm Des. 2016;22:214–20. doi: 10.2174/1381612822666151112151130. [DOI] [PubMed] [Google Scholar]
- 59. Smeds AI, Eklund PC, Sjöholm RE, Willför SM, Nishibe S, Deyama T, Holmbom BR. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J Agric Food Chem. 2007;55:1337–46. doi: 10.1021/jf0629134. [DOI] [PubMed] [Google Scholar]
- 60. Niki E, Kawakami A, Saito M, Yamamoto Y, Tsuchiya J, Kamiya Y. Effect of phytyl side chain of vitamin E on its antioxidant activity. J Biol Chem. 1985;260:2191–6. [PubMed] [Google Scholar]
- 61. Burton GW, Ingold KU. Vitamin E as an in vitro and in vivo antioxidanta. Ann N Y Acad Sci. 1989;570:7–22. doi: 10.1111/j.1749-6632.1989.tb14904.x. [DOI] [PubMed] [Google Scholar]
- 62. Tang Y, Li X, Chen PX, Zhang B, Hernandez M, Zhang H, Marcone MF, Liu R, Tsao R. Lipids, tocopherols, and carotenoids in leaves of amaranth and quinoa cultivars and a new approach to overall evaluation of nutritional quality traits. J Agric Food Chem. 2014;62:12610–9. doi: 10.1021/jf5046377. [DOI] [PubMed] [Google Scholar]
- 63. Tang Y, Li X, Chen PX, Zhang B, Hernandez M, Zhang H, Marcone MF, Liu R, Tsao R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015;174:502–8. doi: 10.1016/j.foodchem.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 64. Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med. 2007;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nesaretnam K, Yew WW, Wahid MB. Tocotrienols and cancer: beyond antioxidant activity. Eur J Lipid Sci Tech. 2007;109:445–52. [Google Scholar]
- 66.Becker F. Tocopherols in wheat and rye: a review of research within the subject. Institutionen för livsmedelsvetenskap; 2013. [Google Scholar]
- 67. Tiwari U, Cummins E. Nutritional importance and effect of processing on tocols in cereals. Trends Food Sci Technol. 2009;20:511–20. [Google Scholar]
- 68.Nawawi HM. Tocotrienols and atherosclerosis potential in cardioprotection. In: Tan B, Watson RR, Preedy VR, editors. Tocotrienols: Vitamin E Beyond Tocopherols. New York: CRC Press; 2012. p. 163. [Google Scholar]
- 69. Suman S, Datta K, Chakraborty K, Kulkarni SS, Doiron K, Fornace AJ, Kumar KS, Hauer-Jensen M, Ghosh SP. Gamma tocotrienol, a potent radioprotector, preferentially upregulates expression of anti-apoptotic genes to promote intestinal cell survival. Food Chem Toxicol. 2013;60:488–96. doi: 10.1016/j.fct.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 70. Upadhyay J, Misra K. Towards the interaction mechanism of tocopherols and tocotrienols (vitamin E) with selected metabolizing enzymes. Bioinformation. 2009;3:326–31. doi: 10.6026/97320630003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moreau RA, Flores RA, Hicks KB. Composition of functional lipids in hulled and hulless barley in fractions obtained by scarification and in barley oil. Cereal Chem. 2007;84:1–5. [Google Scholar]
- 72. Temelli F, Stobbe K, Rezaei K, Vasanthan T. Tocol composition and supercritical carbon dioxide extraction of lipids from barley pearling flour. J Food Sci. 2013;78:C1643–50. doi: 10.1111/1750-3841.12271. [DOI] [PubMed] [Google Scholar]
- 73. Cavallero A, Gianinetti A, Finocchiaro F, Delogu G, Stanca AM. Tocols in hull-less and hulled barley genotypes grown in contrasting environments. J Cereal Sci. 2004;39:175–80. [Google Scholar]
- 74. Ehrenbergerova J, Belcrediova N, Prýma J, Vaculova K, Newman C. Effect of cultivar, year grown, and cropping system on the content of tocopherols and tocotrienols in grains of hulled and hulless barley. Plant Foods Hum Nutr. 2006;61:145–50. doi: 10.1007/s11130-006-0024-6. [DOI] [PubMed] [Google Scholar]
- 75. Miettinen TA, Puska P, Gylling H, Vanhanen H, Vartiainen E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N Engl J Med. 1995;333:1308–12. doi: 10.1056/NEJM199511163332002. [DOI] [PubMed] [Google Scholar]
- 76. Hendriks H, Weststrate J, Van Vliet T, Meijer G. Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr. 1999;53:319–27. doi: 10.1038/sj.ejcn.1600728. [DOI] [PubMed] [Google Scholar]
- 77. Hallikainen MA, Sarkkinen ES, Uusitupa MI. Plant stanol esters affect serum cholesterol concentrations of hypercholesterolemic men and women in a dose-dependent manner. J Nutr. 2000;130:767–76. doi: 10.1093/jn/130.4.767. [DOI] [PubMed] [Google Scholar]
- 78. Jones PJ, Raeini-Sarjaz M, Ntanios FY, Vanstone CA, Feng JY, Parsons WE. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J Lipid Res. 2000;41:697–705. [PubMed] [Google Scholar]
- 79. Awad AB, Fink CS. Phytosterols as anticancer dietary components: evidence and mechanism of action. J Nutr. 2000;130:2127–30. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- 80. Rao AV, Janezic SA. The role of dietary phytosterols in colon carcinogenesis. Nutr Cancer. 1992;18:43–52. doi: 10.1080/01635589209514203. [DOI] [PubMed] [Google Scholar]
- 81. Valsta L, Lemström A, Ovaskainen ML, Lampi AM, Toivo J, Korhonen T, Piironen V. Estimation of plant sterol and cholesterol intake in Finland: quality of new values and their effect on intake. Br J Nutr. 2004;92:671–8. doi: 10.1079/bjn20041234. [DOI] [PubMed] [Google Scholar]
- 82. Frølich W, Åman P, Tetens I. Whole grain foods and health-a Scandinavian perspective. Food Nutr Res. 2013;57 doi: 10.3402/fnr.v57i0.18503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liu K, Moreau R. Concentrations of functional lipids in abraded fractions of hulless barley and effect of storage. J Food Sci. 2008;73:C569–76. doi: 10.1111/j.1750-3841.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- 84. Piironen V, Toivo J, Lampi AM. Plant sterols in cereals and cereal products. Cereal Chem. 2002;79:148–54. [Google Scholar]
- 85. Lampi AM, Moreau RA, Piironen V, Hicks KB. Pearling barley and rye to produce phytosterol-rich fractions. Lipids. 2004;39:783–7. doi: 10.1007/s11745-004-1296-1. [DOI] [PubMed] [Google Scholar]
- 86. Romano PS, Waitzman NJ, Scheffler RM, Pi RD. Folic acid fortification of grain: an economic analysis. Am J Public Health. 1995;85:667–76. doi: 10.2105/ajph.85.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Belobrajdic DP, Bird AR. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr J. 2013;12:62. doi: 10.1186/1475-2891-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Edelmann M, Kariluoto S, Nyström L, Piironen V. Folate in barley grain and fractions. J Cereal Sci. 2013;58:37–44. [Google Scholar]
- 89. Piironen V, Edelmann M, Kariluoto S, Bedo Z. Folate in wheat genotypes in the HEALTHGRAIN diversity screen. J Agric Food Chem. 2008;56:9726–31. doi: 10.1021/jf801066j. [DOI] [PubMed] [Google Scholar]
- 90. Shewry PR, Piironen V, Lampi AM, Nyström L, Li L, Rakszegi M, Fras A, Boros D, Gebruers K, Courtin CM, Delcour JA. Phytochemical and fiber components in oat varieties in the HEALTHGRAIN diversity screen. J Agric Food Chem. 2008;56:9777–84. doi: 10.1021/jf801880d. [DOI] [PubMed] [Google Scholar]
- 91. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD. Executive summary: heart disease and stroke statistics—2015 update a report from the American Heart Association. Circulation. 2015;131:434–41. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 92. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ. Executive summary: heart disease and stroke statistics—2016 update a report from the American Heart Association. Circulation. 2016;133:447–54. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 93. Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28:1685–96. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 94. Brennan ML, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003;14:353–9. doi: 10.1097/00041433-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 95. Daffu G, del Pozo CH, O’Shea KM, Ananthakrishnan R, Ramasamy R, Schmidt AM. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci. 2013;14:19891–910. doi: 10.3390/ijms141019891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stampfer M. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260:211–23. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 97. Ghafoor K. Optimized extraction of phenolic compounds from barley (Hordeum vulgare L.) seed and their radical scavenging properties. J Food Process Preserv. 2015;39:793–9. [Google Scholar]
- 98. Mahmoudi T, Oveisi MR, Jannat B, Behzad M, Hajimahmoodi M, Sadeghi N. Antioxidant activity of Iranian barley grain cultivars and their malts. Afr J Food Sci. 2015;9:534–9. [Google Scholar]
- 99. Lahouar L, El Arem A, Ghrairi F, Chahdoura H, Salem HB, El Felah M, Achour L. Phytochemical content and antioxidant properties of diverse varieties of whole barley (Hordeum vulgare L.) grown in Tunisia. Food Chem. 2014;145:578–83. doi: 10.1016/j.foodchem.2013.08.102. [DOI] [PubMed] [Google Scholar]
- 100.Frei B. Natural antioxidants in human health and disease. London: Academic Press; 2012. [Google Scholar]
- 101. Shen Y, Zhang H, Cheng L, Wang L, Qian H, Qi X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016;194:1003–12. doi: 10.1016/j.foodchem.2015.08.083. [DOI] [PubMed] [Google Scholar]
- 102. Lee JH, Lee SY, Kim B, Seo WD, Jia Y, Wu C, Jun HJ, Lee SJ. Barley sprout extract containing policosanols and polyphenols regulate AMPK, SREBP2 and ACAT2 activity and cholesterol and glucose metabolism in vitro and in vivo. Food Res Int. 2015;72:174–83. [Google Scholar]
- 103. Prasad K. Oxidative stress as a mechanism of diabetes in diabetic BB prone rats: effect of secoisolariciresinol diglucoside (SDG) Mol Cell Biochem. 2000;209:89–96. doi: 10.1023/a:1007079802459. [DOI] [PubMed] [Google Scholar]
- 104. Lucas EA, Lightfoot SA, Hammond LJ, Devareddy L, Khalil DA, Daggy BP, Smith BJ, Westcott N, Mocanu V, Arjmandi BH. Flaxseed reduces plasma cholesterol and atherosclerotic lesion formation in ovariectomized Golden Syrian hamsters. Atherosclerosis. 2004;173:223–9. doi: 10.1016/j.atherosclerosis.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 105. Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23:65–134. doi: 10.1017/S0954422410000041. [DOI] [PubMed] [Google Scholar]
- 106. Wang L, Behr SR, Newman RK, Newman CW. Comparative cholesterol-lowering effects of barley β-glucan and barley oil in golden Syrian hamsters. Nutr Res. 1997;17:77–88. [Google Scholar]
- 107. Qureshi AA, Burger W, Peterson D, Elson C. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J Biol Chem. 1986;261:10544–50. [PubMed] [Google Scholar]
- 108. Burger WC, Qureshi AA, Elson CE. Cholesterol lowering method of use. US4603142 A. Google Patents. 1986
- 109. Jadhav S, Lutz S, Ghorpade V, Salunkhe D. Barley: chemistry and value-added processing. Crit Rev Food Sci Nutr. 1998;38:123–71. doi: 10.1080/10408699891274183. [DOI] [PubMed] [Google Scholar]
- 110. Clarke R, Bennett DA, Parish S, Verhoef P, Dötsch-Klerk M, Lathrop M, Xu P, Nordestgaard BG, Holm H, Hopewell JC, Saleheen D. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9:e1001177. doi: 10.1371/journal.pmed.1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Santilli F, Davì G, Patrono C. Homocysteine, methylenetetrahydrofolate reductase, folate status and atherothrombosis: A mechanistic and clinical perspective. Vascul Pharmacol. 2016;78:1–9. doi: 10.1016/j.vph.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 112. Adolphe J, Fitzpatrick K. Barley and diabetes. Hemoglobin. 1:6.5. [Google Scholar]
- 113.Benkeblia N, Thondre PS. Barley β-Glucan: natural polysaccharide for managing diabetes and cardiovascular diseases. In: Benkeblia N, editor. Polysaccharides: natural fibers in food and nutrition. CRC Press; 2014. pp. 233–58. [Google Scholar]
- 114. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress–activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 115. Mertens A, Verhamme P, Bielicki JK, Phillips MC, Quarck R, Verreth W, Stengel D, Ninio E, Navab M, Mackness B, Mackness M. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice LCAT gene transfer decreases atherosclerosis. Circulation. 2003;107:1640–6. doi: 10.1161/01.CIR.0000056523.08033.9F. [DOI] [PubMed] [Google Scholar]
- 116. Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–41. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 117. Okarter N, Liu RH. Health benefits of whole grain phytochemicals. Crit Rev Food Sci Nutr. 2010;50:193–208. doi: 10.1080/10408390802248734. [DOI] [PubMed] [Google Scholar]
- 118. Adom KK, Liu RH. Antioxidant activity of grains. J Agric Food Chem. 2002;50:6182–7. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- 119. Sidhu JS, Kabir Y, Huffman FG. Functional foods from cereal grains. Int J Food Prop. 2007;10:231–44. [Google Scholar]
- 120. Zielinski H, Kozlowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48:2008–16. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]
- 121. Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, Manson JE, Liu S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism. 2008;57:437–43. doi: 10.1016/j.metabol.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11:1365–402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Salas-Salvadó J, Martinez-Gonzalez M, Bullo M, Ros E. The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21:B32–48. doi: 10.1016/j.numecd.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 124. Stewart B, Wild CP. World cancer report 2014 World. 2015 [Google Scholar]
- 125. Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–79. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 126. Kanauchi O, Oshima T, Andoh A, Shioya M, Mitsuyama K. Germinated barley foodstuff ameliorates inflammation in mice with colitis through modulation of mucosal immune system. Scand J Gastroenterol. 2008;43:1346–52. doi: 10.1080/00365520802245411. [DOI] [PubMed] [Google Scholar]
- 127. Floridi S, Montanari L, Marconi O, Fantozzi P. Determination of free phenolic acids in wort and beer by coulometric array detection. J Agric Food Chem. 2003;51:1548–54. doi: 10.1021/jf0260040. [DOI] [PubMed] [Google Scholar]
- 128. Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–84. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 129. Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J Biol Chem. 2004;279:13293–6. doi: 10.1074/jbc.C400059200. [DOI] [PubMed] [Google Scholar]
- 130. Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–75. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 131. Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–67. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cheng L, Lai MD. Aberrant crypt foci as microscopic precursors of colorectal cancer. World J Gastroenterol. 2003;9:2642–9. doi: 10.3748/wjg.v9.i12.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kim S, Guo J, O’Sullivan MG, Gallaher DD, Turesky RJ. Comparative DNA adduct formation and induction of colonic aberrant crypt foci in mice exposed to 2-amino-9H-pyrido [2, 3-b] indole, 2-amino-3, 4-dimethylimidazo [4, 5-f] quinoline, and azoxymethane. Environ Mol Mutagen. 2016;57:125–36. doi: 10.1002/em.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. McIntosh G, Jorgensen L, Royle P. The potential of an insoluble dietary fiber-rich source from barley to protect from DMH-induced intestinal tumors in rats. Nutr Cancer. 1993;19:213–21. doi: 10.1080/01635589309514252. [DOI] [PubMed] [Google Scholar]
- 135. Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med. 2010;31:435–45. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 136. Kumar N, Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnology Reports. 2014;4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Murakami A, Nakamura Y, Koshimizu K, Takahashi D, Matsumoto K, Hagihara K, Taniguchi H, Nomura E, Hosoda A, Tsuno T, Maruta Y. FA15, a hydrophobic derivative of ferulic acid, suppresses inflammatory responses and skin tumor promotion: comparison with ferulic acid. Cancer Lett. 2002;180:121–9. doi: 10.1016/s0304-3835(01)00858-8. [DOI] [PubMed] [Google Scholar]
- 138. Kawabata K, Yamamoto T, Hara A, Shimizu M, Yamada Y, Matsunaga K, Tanaka T, Mori H. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. doi: 10.1016/s0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 139. Madhujith T, Shahidi F. Antioxidative and antiproliferative properties of selected barley (Hordeum vulgarae L.) cultivars and their potential for inhibition of low-density lipoprotein (LDL) cholesterol oxidation. J Agric Food Chem. 2007;55:5018–24. doi: 10.1021/jf070072a. [DOI] [PubMed] [Google Scholar]
- 140. Deguchi T, Ohba R, Ueda S. Effects of reactive oxygen and temperature on the formation of a purple pigment, hordeumin, from barley bran-fermented broth. Biosci Biotechnol Biochem. 1999;63:1151–5. doi: 10.1271/bbb.63.1151. [DOI] [PubMed] [Google Scholar]