Abstract
Diet polyphenols—primarily categorized into flavonoids (e.g., flavonols, flavones, flavan-3-ols, anthocyanidins, flavanones, and isoflavones) and nonflavonoids (with major subclasses of stilbenes and phenolic acids)—are reported to have health-promoting effects, such as antioxidant, antiinflammatory, anticarcinoma, antimicrobial, antiviral, and cardioprotective properties. However, their applications in functional foods or medicine are limited because of their inefficient systemic delivery and poor oral bioavailability. Epigallocatechin-3-gallate, curcumin, and resveratrol are the well-known representatives of the bioactive diet polyphenols but with poor bioavailability. Food macromolecule based nanoparticles have been fabricated using reassembled proteins, crosslinked polysaccharides, protein–polysaccharide conjugates (complexes), as well as emulsified lipid via safe procedures that could be applied in food. The human gastrointestinal digestion tract is the first place where the food grade macromolecule nanoparticles exert their effects on improving the bioavailability of diet polyphenols, via enhancing their solubility, preventing their degradation in the intestinal environment, elevating the permeation in small intestine, and even increasing their contents in the bloodstream. We contend that the stability and structure behaviors of nanocarriers in the gastrointestinal tract environment and the effects of nanoencapsulation on the metabolism of polyphenols warrant more focused attention in further studies.
Keywords: bioavailability, encapsulation, macromolecules, nanoparticles, polyphenols
1. Introduction
Hippocrates, the father of medicine, once said “Let food thy medicine and medicine thy food.” Certain foods possess medicinal functions that prevent chronic diseases. Since ancient times, numerous beneficial medical treatments have been attributed to plant-derived compounds, which are used as an important source of materials to treat various diseases. As shown by epidemiological studies and meta-analyses, diets rich in fruit and vegetables can reduce the incidence of several chronic diseases, including type 2 diabetes [1], cardiovascular disease [2], and even several cancers [3,4]. Furthermore, the consumption of polyphenols, either in plant based medicine or in diets rich in fruit and vegetables, has been observed to have health-promoting effects [5].
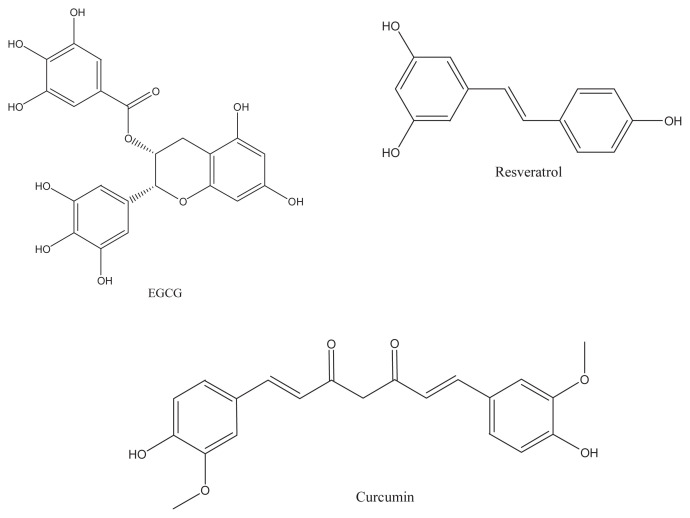
Epigallocatechin-3-gallate (EGCG) from green tea, curcumin isolated from turmeric, and resveratrol in wine (Figure 1) are the well-known representatives of the bioactive polyphenols that have been extensively studied for their preventive properties (including antioxidant, antiinflammatory, anticarcinoma, antimicrobial, antiviral and cardioprotective properties) against chronic diseases [6–9]. These food polyphenols can reduce the risk of chronic diseases, because of their inhibition effects on enzyme activities and signal transduction pathways during the course of disease development.
Figure 1.
The chemical structure of epigallocatechin-3-gallate, resveratrol, and curcumin.
However, the inefficient systemic delivery and poor oral bioavailability of bioactive polyphenols have largely limited their applications to humans [10]. Low solubility, instability under conditions encountered in the gastrointestinal (GI) tract (pH, enzymes, presence of other nutrients), insufficient gastric residence time, and the difficulty for many polyphenols to diffuse across the cells through the lipid-bilayer cell membranes in the intestine account for the low bioavailability of diet polyphenols [11,12].
Nanoparticles had been formally referred to as sphere-like substrates with dimensions ranging between 1 nm and 100 nm, which have been extended to range from 1 nm to 1000 nm especially in the biomedical fields. Polymer-based delivery nanoparticle systems that encapsulate bio-functional ingredients within networks have been developed extensively for the biomedical and functional food sectors to protect and transport them to target functions [12–14]. The biomacromolecular based nanoparticles enhance the absorption and bioavailability of bioactive molecule mainly through the following pathways: (1) protection of the bioactive molecule from the harsh environment of the GI tract, (2) prolongation of the residence time in the gut by mucoadhesion, (3) endocytosis of the particles, and/or (4) permeabilizing effect of the polymer [15]. For the purpose of oral consumption and minimizing carrier-induced undesirable cytotoxicity in the delivery of food polyphenols, we believe that there is no better option than food-grade macromolecules that are generally recognized as safe, which are suitable for developing such delivery systems. Macromolecules of food origin are natural sources of biopolymeric soft materials—they are not only biodegradable and biocompatible, but are also biofunctional [16,17]. Lipid based nanoencapsulator, nanoemulsions, biopolymeric nanoparticles, nanocomplexes formed with food-grade ingredients including food biopolymers (proteins, carbohydrates), fats, and copolymers (protein–carbohydrate conjugates) have been used to deliver a range of functional ingredients in pharmacy and foods [18,19].
In this review, the classification of food polyphenols and the factors influencing their bioavailability during oral consumption are described. Then, recent studies on enhancing the bioavailability of polyphenols, mainly EGCG, curcumin, and resveratrol, through encapsulation with food grade macromolecule nanoparticles are summarized.
2. Classification of polyphenols
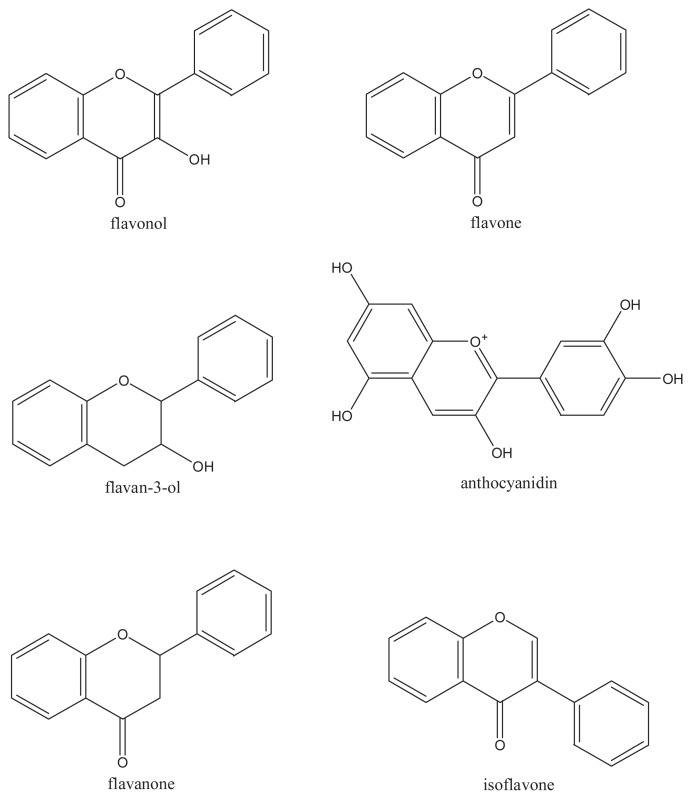
Polyphenols can be categorized primarily into flavonoids and nonflavonoids, which constitute a diverse class of secondary plant compounds, or phytochemicals. The main subclasses of dietary flavonoids are flavonols, flavones, flavan-3-ols, anthocyanidins, flavanones, and isoflavones (Figure 2). The nonflavonoids include diverse classes of polyphenols such as stilbenes and phenolic acids. The basic flavonoid skeleton (C6–C3–C6) can have numerous substituents. Numerous flavonoids commonly exist naturally as glycosides with sugars as the substituent [20]. The flavonoids become increasingly water-soluble with the substituent of sugars and hydroxyl groups; other substituents, such as methyl groups and isopentyl units, make flavonoids lipophilic.
Figure 2.
Basic chemical structures of the dietary flavonoids.
2.1. Dietary flavonoids
Flavonols, which are the most widespread flavonoids, are dispersed throughout the plant kingdom. The distribution and structural variations of flavonols are extensive. The main dietary flavonols are most commonly found as O-glycosides in nature, including myricetin, quercetin, kaempferol, and isorhamnetin.
Flavones are a class of flavonoids based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one) shown in Figure 2, which include apigenin and luteolin. Otherwise, a wide range of substitutions may occur in the molecules of flavones, which include hydroxylation, methylation, O- and C-alkylation, and glycosylation [21–23]. Polymethoxylated flavones, such as tangeretin and nobiletin, have been reported in citrus species [24]. Compared with flavonols, flavones are not distributed widely, with significant occurrences being reported in only celery, parsley, and some herbs.
Owing to the saturated C3 element, flavan-3-ols are nonplanar and are the most structurally complex subclass of flavonoids. The simple monomers of flavan-3-ols include (+)-catechin and its isomer (−)-epicatechin. The simple monomers can be hydroxylated to form gallocatechins and also undergo esterification with gallic acid, leading to complex structures such as the oligomeric EGCG and polymeric proanthocyanidins. The green tea catechins include (−)-epi-catechin 3-gallate, (−)-epigallocatechin, (−)-epicatechin, and EGCG. The chiral center in the structure of flavan-3-ols leads to complexity and diversity in their structure. At C2 and C3 positions of the flavan-3-ols, the two chiral centers produce four isomers for each level of B-ring hydroxylation. In the oligomeric and polymeric proanthocyanidins, there is an additional chiral center at C4 of each additional flavan-3-ol unit.
Type B proanthocyanidins originate from (+)-catechin and (−)-epicatechin with oxidative coupling taking place between the C4 of the heterocycle and the adjacent unit at the C6 or C8 positions to create oligomers or polymers. In comparison with type B proanthocyanidins, an additional ether bond between C2 and C7 occurs in type A proanthocyanidins. Proanthocya-nidins can occur as polymers with up to 50 units. Procyanidins are the most abundant type of proanthocyanidins in plants and consist exclusively of epicatechin units. Many condensed tannins contain more than one monomer. Flavan-3-ol monomers are extensively transformed during the traditional processing of wines, cocoa, and black tea—in the latter case, yielding theaflavins, theacitrins, and thearubigins.
Being similar to flavan-3-ols to a certain degree, the flavanones are nonplanar and have a chiral center at C2. The structure character of flavanone is that ring C is attached to B ring at C2 in the α-configuration in the majority of naturally occurring flavanones. Flavanones are present in especially high concentrations in citrus fruits.
For isoflavones, the B ring is attached at C3 rather than C2. They are found almost exclusively in leguminous plants, with the highest concentrations occurring in soy bean (Glycine max). These isoflavonoids appear to mimic the steroidal hormone estradiol.
2.2. Dietary nonflavonoids
The significant dietary nonflavonoids are the C6–C1 phenolic acids and the polyphenolic C6–C2–C6 stilbene. The most notable phenolic acid and edible stilbene are gallic acid and resveratrol, respectively. Gallic acid is the most common phenolic acid, which is the biosynthetic precursor of hydrolysable tannins, the C6–C3 hydroxycinammates and their conjugated derivatives. The main dietary source of stilbenes is resveratrol (Figure 1B), which can be found in red wine, and also in peanuts (Arachis hypogaea) [25], berries, and red cabbage (Brassica oleracea). Resveratrol occurs as cis and trans isomers.
Curcumin (Figure 1C) is also a natural nonflavonoid phenol that belongs to the group of curcuminoids, and is responsible for turmeric's yellow color. The curcuminoids are a mixture of curcumin, chemically a diferuloylmethane [1,7-bis (4-hydroxy-3-methoxyphenyl)-hepta-1,6-diene-3,5-dione] mixed with its two derivates, demethoxy-curcumin [4-hydroxycinnamoyl-(4-hydroxy-3-methoxycinnamoyl) methane] and bis-demethoxy-curcumin [bis-(4-hydroxy cinnamoyl) methane] [26].
3. Factors impacting the bioavailability of diet polyphenols
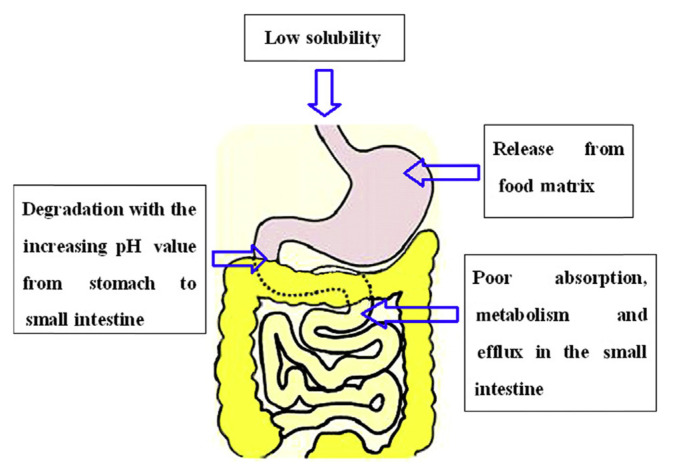
Bioavailability can be defined as the fraction of a nutrient or nonnutrient that is available for the human body for physiological functions and/or storage [27]. In other words, the term bioavailability refers to the fraction of a dose that is available at the site of action in the body [14]. For most oral doses, this definition is interpreted as the fraction of the dose that enters the bloodstream. In the case of polyphenols, this principally involves the following digestive processes: solubility of polyphenols in the GI environments; release of polyphenols from the food matrix; degradation of polyphenols during gastric/small-intestinal digestion; cellular uptake of polyphenols by enterocytes; Phase I/II enzyme modifications that occur upon uptake (mainly in the small intestine); final transport into the bloodstream and subsequent tissue redistribution. All of these steps play important roles in the absorption and metabolism of polyphenol. In this review, we focus on the factors during GI digestion, prior to transport into the bloodstream and tissue distribution. And the reasons for the low bioavailability of polyphenols are briefly summarized in Figure 3.
Figure 3.
Summarization of the factors accounting for the low bioavailability of polyphenols.
3.1. Solubility of polyphenols
Solubility of polyphenols is an essential physicochemical property that influences their bioavailability. Polyphenols' solubility has certain relationship with their bioavailability. However, there might be a misleading notion that the compound with high solubility could have a considerable high bioavailability, which mainly ignores the important role of intestinal cell membrane permeability of the polyphenol ingredients. Generally, polyphenols can be classified into three categories: (1) high solubility but poor cell membrane permeability; (2) low solubility and poor cell membrane permeability; and (3) low solubility but high cell membrane permeability. The selected polyphenol compounds in the present study—EGCG, curcumin, and resveratrol—belong to types 1, 2, and 3 polyphenols, respectively [14,26,28]. Therefore, for polyphenols such as curcumin and resveratrol, an effort should be first made to increase their solubility.
3.2. Effects of food matrices
Studies have shown that polyphenols from foods in combinations can have very different bioaccessibilities. Green et al [29] found that the addition of bovine, soy and rice milks, ascorbic acid, or citrus juices could increase the bioaccessibility of green tea catechins, which might be related to their stabilization and protection from auto-oxidation at alkaline pH. The bioaccessibility of EGC and EGCG in green tea was enhanced after association with sucrose and ascorbic acid simultaneously, which was further related to the increase of uptake in Caco-2 cells and bioavailability in rats [30]. In the model of codigestion with blueberries and milk, the recovery of total anthocyanins and total phenols was found to be reduced by milk [31]. Studies with raspberry juice showed that the addition of ice cream markedly reduced the recovery of total anthocyanins [32], whereas a wheat-based breakfast cereal did not influence recovery. Xie et al [33] reported that the uptake of catechins by Caco-2 cells was increased after addition of skimmed milk, although the recovery of catechins was decreased, which might be caused by the binding between catechins and the milk proteins. In their study, van het Hof et al [34] also found that no significant difference in serum/plasma bioavailability of tea catechins could be found when the participants were given tea with or without milk. It could be concluded from these studies that the polyphenols bound with proteins can also be available for the absorption in the small intestine. Dietary fats can increase polyphenol bioavailability in humans by increasing absorption, possibly by enhancing micellarization in the small intestine [35,36].
3.3. Release of polyphenols from the food matrix during GI digestion
During the digestion process, the majority of polyphenols appear to be released during the gastric phase. The gastric phase is usually the site where food stuffs are mainly dissolved. A finely ground digesta of the food stuffs, with increasingly decreased particle size, is caused by the combination effects of pepsin digestion, peristaltic movements, and low pH [37]. The low pH in the range of 2–4 in the stomach may favor the stability of polyphenols, which could foster their transition from the matrix into the aqueous phase because of reduced ionic interactions. As digesta passes from the stomach to the small intestine, the pH usually increases from around 2–4 to approximately 7. In the small intestine, there are several enzymes and biosurfactants secreted by the pancreas and bile, such as phospholipase, sterol esterase, amylase, carboxypeptidase, trypsinogen, chymotrypsinogen, lipase, and bile salts. Lipase and bile salts are essentially involved in the digestion of the more apolar food compounds such as lipids, apolar micronutrients, and phytochemicals, resulting in the formation of water-soluble mixed micelles [38–42].
3.4. Degradation of food polyphenols in GI tract
Generally, polyphenols are stable in the acidic gastric environment, but are, however, degraded under the neutral and weak alkalescent environment in the small intestine. The degradation of anthocyanins occurs mostly in the small intestine, which contributes to a low overall uptake into serum [43]. EGCG quickly degrades in intestinal juice neutral and alkaline environments, which is mainly attributed to the auto-oxidation of EGCG, forming its homodimers [44]. In acidic pH, trans-resveratrol is stable, whereas its degradation starts to increase exponentially above pH 6.8 [45]. Curcumin is stable under high pH values (>11.7), but degrades rapidly about pH 7.4 [46].
3.5. Uptake of polyphenols by enterocytes
The small intestine is thought to be the site for the absorption of the majority of polyphenols [47,48]. Passive diffusion is likely to constitute the major absorptive pathway for low-weight polyphenols based on Caco-2 cell trials [49]. Transcellular transport of resveratrol appeared to occur through a rapid passive direct-independent diffusion mechanism [50]. It was shown that curcumin permeated across the Caco-2 cell monolayers also via the permeation mechanism of passive diffusion [51].
Higher lipophilicity appears to facilitate epithelial uptake. Murota et al [52] reported a correlation between lipophilicity and enterocyte permeability based on Caco-2 cell trials, which follows the sequence genistin = daidzin < daidzein < genistein < flavonoid aglycones, corresponding to their lipophilic properties. About 5–8 times larger absorption through Caco-2 cells and more slowly glucuronated and sulfated in human liver cells were reported for methylated flavones compared with those of nonmethylated ones, which might indicate good bioavailability [53]. Cellular uptake of polyphenols also appeared to be substantially influenced by their polymerization degree. For example, the absorption of the dimers of procyanidins (<1%) was reported to be much lower than that of monomers such as epicatechin (~45%) [54,55]. Polymeric procyanidins, theaflavin, thearubigins, and tannins could not even be detected after oral uptake in an animal study [56,57].
Sodium-glucose transport proteins were suggested to be active in the transport of glycoside polyphenols, especially the sodium glucose-linked transporter 1 [58,59]. However, there is still some controversy about the role of polyphenol uptake, as not all studies with flavonoid glycosides could confirm its participation.
3.6. Metabolism and reconjugation in enterocytes
The intestine constitutes the first place for phase I/II metabolism [60]. For curcumin, the four double bonds of the heptadiene-3,5-dione system are successively reduced by phase I metabolism. Tetrahydrocurcumin and hexahydrocurcumin are the major products observed in most studies, whereas dihydrocurcumin and hexahydrocurcuminol usually represent minor products. Furthermore, the reduced curcumin undergo glucuronidation to C-glucuronide, dihydro-C-glucuronide, tetrahydrocurcumin-glucuronide, as well as sulfation to C-sulfate [61]. Two metabolites, resveratrol-3-sulfate and resveratrol-3-glucuronide, were detected as phase II biotransformation products [50], and sulfate conjugation was the major pathway for resveratrol in Caco-2 cell model. Methylation has also been observed, for instance, of green tea polyphenols [62] and quercetin [63], possibly via catechol-O-methyltransferase.
The active excretion of the metabolite of the polyphenols from enterocyte back into the gut lumen may occur via the ATP-binding cassette transporters [64]. Other transporters, including multidrug resistance proteins (MRPs) and monocarboxylate transporters may play a role in transport to the vascular side [65,66]. MRP2 and P-glycoprotein were also reported to play a role in the efflux uptake of metabolized polyphenols in a Caco-2 cell model [60].
4. Food macromolecule nanoparticles for encapsulation and delivery of polyphenols
4.1. Food protein nanoparticles
Food proteins are abundant renewable raw materials for developing nanocarriers for delivery of drug or nutraceuticals, which is mainly attributed to their exceptional characteristic of extraordinary binding capacity of various drugs or nutraceuticals. In addition, food proteins are biodegradable and nonantigenic, and have high nutritional value. Furthermore, protein nanoparticles can be easily prepared and scaled up during manufacture [67,68]. Several widely used food grade proteins for fabrication of nanoparticle delivery systems for encapsulation of diet polyphenols are summarized and discussed below.
4.1.1. Whey protein (mainly β-lactoglobulin) nanoparticles
Whey proteins, especially β-lactoglobulin (β-Lg), have attracted the lion's share of attention in preparation of food protein nanoparticles as carriers for polyphenols. EGCG was coassociated with the thermally modified β-Lg to form coassembled nanovehicles with particle size of about 50 nm, which still maintained excellent transparency, enabling their application in clear beverages. A 33-fold lower initial degradation rate and a 3.2-fold slower degradation over 8 days were observed for the β-Lg nanoentrapped EGCG compared with free EGCG [69]. Lestringant et al [70] found that desolvated β-Lg nanoparticles showed the highest binding affinity for EGCG, and the native β-Lg–EGCG complexes showed comparable stability and binding efficacy with those of the heated β-Lg nanoparticles. Similar to that of free ECGC, all β-Lg nanoparticle formulations from different processing proteins showed an inhibition effect in cellular proliferation [70]. Li et al [71] systematically studied the effects of fabrication parameters on the physicochemical properties of the EGCG loaded thermal modified β-Lg nanoparticles, including pH (2.5–7.0), heating temperature of β-Lg (30–85°C), molar ratio of β-Lg to EGCG (1:2–1:32), and β-Lg concentration (1–10 mg/mL) on the properties of β-Lg–EGCG complexes. A stable and clear solution system could be obtained at pH 6.4–7.0. The highest protection of EGCG antioxidant activity was obtained with β-Lg heated at 85°C and the molar ratio of 1:2 (β-Lg/EGCG) [71]. Native and thermally modified lactoferrin (LF) were also used as the carrier for EGCG. The interaction between EGCG and the protein was found to be pH dependent. The EGCG–protein nanoparticles were prepared at pH 3.5 and 5.0, whereas submicrometer particles appeared under pH 6.5 [72].
The water solubility and pH stability of curcumin were significantly increased after binding with β-Lg, which was treated with ultrasonic. Moreover, curcumin–β-Lg nanocomplexes were found to be resistant to pepsin digestion but sensitive to trypsin. In the Caco-2 cell model, the digested curcumin–β-Lg nanocomplexes significantly improved the permeation rate of curcumin [73].
Liang et al [74] reported that resveratrol interacted with β-LG to form the nanocomplexes. The β-LG–resveratrol interaction inhibited the self-association of both the polyphenol and the protein, respectively. Complexing with β-LG provided a slight increase in the photostability of resveratrol and a significant increase in its hydrosolubility [74]. Hemar et al [75] found that resveratrol could interact with whey proteins to form nanocomplexes. Furthermore, proteins selected in the fabrication of nanocomplexes included LF, holo-LF, apo-LF, whey protein isolate, and the β-LG- and α-lactalbumin-rich fractions of whey protein isolate. It was found that interaction between whey proteins and resveratrol did not affect the secondary structure of the protein [75]. Resveratrol–bovine serum albumin nanoparticles (RES–BSANP) exhibited chemotherapeutic properties via triggering apoptosis. Apoptotic body, nuclear condensation, and fragmentation were observed simultaneously following treatment of the cancer cells with RES–BSANP [76].
4.1.2. Casein nanoparticles
The caseins are proline-rich, open-structured rheomorphic proteins, which have distinct hydrophobic and hydrophilic domains. Caseins (95%) consist of αs1-, αs2-, β-, and κ-caseins and are naturally self-assembled into casein micelles that are spherical colloidal particles with diameters in the range of 50–500 nm (average 150 nm). Encapsulation of curcumin in camel β-casein micelle increased the solubility of curcumin at least 2500-fold, which was predominantly driven by hydrophobic interactions. The antioxidant activity of curcumin loaded with β-casein micelle was higher than that of both free β-casein and curcumin. And the encapsulated curcumin appeared to have higher cytotoxicity in human leukemia cell line K-562 compared with that of free curcumin [77]. Pan et al [78] reported a novel encapsulation method by spray-drying a warm aqueous ethanol solution with codissolved sodium caseinate and curcumin, resulting in curcumin-loaded casein nanoparticles. The curcumin encapsulated in casein nanoparticles showed higher antioxidant activity and cytotoxicity against cancer cells, compared with pristine curcumin. Furthermore, Pan et al [79] also reported a low-cost, low-energy, and organic solvent-free (without ethanol solution) encapsulation technology based on the pH-dependent solubility properties of curcumin and self-assembly properties of sodium caseinate. The neutralization of curcumin and caseinate at pH 12 and 21°C enabled the encapsulation of curcumin in self-assembled casein nanoparticles. The curcumin encapsulated in casein nanoparticles showed significantly improved antiproliferation activity against human colorectal and pancreatic cancer cells [79]. Sneharani et al [80] found that the stability of curcumin in solution at pH 7.2 was enhanced on binding with casein. Moreover, the ability of curcumin to protect erythrocytes against hemolysis was not affected because of the curcumin–casein interaction [80].
4.1.3. Gelatin nanoparticles
Gelatin is a denatured protein obtained from collagen by acid and alkaline hydrolysis. It is considered a generally recognized as safe material by the Food and Drug Administration and has been safely used for a long time in pharmaceuticals, cosmetics, as well as food products. Shutava et al [81] found that EGCG encapsulated in gelatin-based nanoparticles retained its biological activity for blocking hepatocyte growth factor-induced intracellular signaling in the breast cancer cell line MBA-MD-231 as potently as free EGCG. Karthikeyan et al [82] found that the coculture of resveratrol loaded gelatin nanoparticles with high loading efficiency induced cell death through alteration in expression of p53, p21, caspase-3, Bax, Bcl-2, and NF-κB.
4.1.4. Food prolamine based nanoparticles
Prolamins, a group of plant storage proteins with a high proline content, are found in seeds of cereal grains: wheat (gliadin), barley (hordein), rye (secalin), corn (zein), sorghum (kafirin), and as a minor protein, avenin, in oats. They are characterized by a high glutamine and proline content and are generally soluble only in strong alcohol solutions. Prolamins are excellent materials for preparation of food grade nanoparticles. Zein nanoparticles ranging from 175 to 900 nm were obtained with varying protein concentrations from 2.5% to 15% (w/w) using the electrohydrodynamic atomization method. After nanoencapsulation, curcumin presented good dispersion in an aqueous food matrix, such as semiskimmed milk [83]. Resveratrol was loaded in hordein nanoparticles through self-assembly by the liquid–liquid dispersion method, to improve its stability by 26% compared with that of free resveratrol after ultraviolet irradiation for 18 hours. Controlled release of resveratrol from the nanoparticles under simulated GI environment conditions was achieved. Both chemical and cellular assay results demonstrated that loading with hordein nanoparticles enhanced the antioxidant capability of resveratrol compared with the free one [84]. Penalva et al [85] also reported a resveratrol nanoparticle formulation based on zein, which provided high and prolonged plasma levels of the polyphenol for at least 48 hours. Furthermore, nanoparticles administered daily for 7 days at 15 mg/kg were able to diminish the endotoxic symptoms induced in mice via the intraperitoneal administration of lipopolysaccharide [85].
4.2. Food polysaccharide nanoparticles
Polysaccharides are a significant fraction of various foods, accounting for many of their texture, sensory properties, and caloric value. Polysaccharides are composed of monosaccharides joined by glycosidic bonds. The most attractive advantage of polysaccharides in fabrication of food nanoparticles is their bioadhesion property, especially for mucosal surfaces, which has been used for targeting specific organs or cells and prolonging the polyphenol residence time in the intestine. Among them, chitosan is the most widely used polysaccharide in oral delivery nanoparticle systems. Chitosan is the deacetylated form of chitin and composed of glucosamine, known as 2-amino-2-deoxy-(1→4)-β-d-glucopyranan. It is considered to be the most widely distributed biopolymer as a cationic, nontoxic, biodegradable, and biocompatible polyelectrolyte with an oral LD50 in mice of more than 16 g/kg [86], and has been approved for dietary applications in Japan, Italy, and Finland. It is considered beneficial for improving the intestinal absorption of active ingredients, especially for compounds, such as EGCG, that are soluble in water but have low permeability in the small intestine. Furthermore, chitosan is facilitated to interact with negatively charged polymers and to be modified with other functional groups to endow the nanoparticles with targeting properties.
Park et al [87] prepared the alginate–chitosan electronic complex nanoparticles induced by times recycle of high pressure, for encapsulation of EGCG with the highest encapsulation efficiency of 80.1%, which also showed the highest 2,2-diphenyl-1-picrylhydrazyl radical scavenging activities of 81.8% and 69.3% at pH 2.6 and pH 6.9, respectively [87]. Siddiqui et al [88] developed a chitosan nanoparticle based oral delivery system to encapsulate and deliver EGCG, which demonstrated an eightfold dose advantage over native free EGCG. Furthermore, nano-EGCG treated cells showed marked induction of apoptosis and cell cycle inhibition along with the growth of tumor xenograft mice. Nano-EGCG also inhibited proliferation and induced apoptosis in tumors harvested from the treated mice [88]. Lin et al [89] prepared potentially gastric cancer cell target-activated nanocomplexes comprising a fucose conjugated chitosan associated with gelatin containing encapsulated EGCG. In vitro results demonstrated that a controlled release of EGCG from the nanocomplexes inhibited gastric cancer cell growth, induced cell apoptosis, and reduced vascular endothelial growth factor protein expression. Furthermore, in vivo assay results indicated that the prepared EGCG-loaded nanoparticles significantly affected gastric tumor activity and reduced gastric and liver tissue inflammatory reaction in an orthotopic gastric tumor mouse model [89]. EGCG was also first incorporated as inclusion complexes in sulfobutylether-β-cyclodextrin sodium, and then ionotropically crosslinked with chitosan hydrochloride into nanoparticles, which could prevent the decrease in antioxidants of free EGCG with increasing pH, storage time, and temperature [90].
Chuah et al [91] prepared the curcumin-containing chitosan nanoparticles with mucoadhesive properties, which could be taken up by colon cancer cells and exerted anticancer effects. Chitosan–alginate nanoparticles for encapsulation of curcumin diethyl disuccinate (CDD) were prepared by oil-in-water emulsification and ionotropic gelation. The CDD nanoparticles were stable in storage at 4°C for 3 months. Furthermore, an in vitro cellular internalization study demonstrated that CDD nanoparticles had significantly higher cellular uptake in Caco-2 cells compared with free CDD [92]. Polyelectrolyte complexation between chitosan and gum arabic as novel delivery systems for curcumin was fabricated with the average diameter in the range of 250–290 nm; the curcumin encapsulation efficiency exceeded 90%, the loading content was more than 3.8%, and the retention rate was higher than 85% during storage. Besides the significantly elevated antioxidant activities of curcumin by nanoencapsulation, the chitosan–gum arabic nanoparticles improved the stability and delayed the release of curcumin in a simulated GI environment [93]. Carboxymethyl chitosan nanoparticles were fabricated via the emulsion crosslinking method to encapsulate and deliver resveratrol, which substantially improved the antioxidant activity of resveratrol. Furthermore, the nanoparticle encapsulated resveratrol exhibited increased in vivo absorption, prolonged duration of action, and increased relative bioavailability compared with the raw resveratrol [94].
The nanoparticles composed with hydroxypropyl methyl-cellulose as the stabilizer for trans-resveratrol were prepared using the temperature-controlled antisolvent precipitation method, which enhanced the bioavailability of trans-resveratrol [95].
4.3. Food protein–polysaccharide conjugate (complex) nanoparticles
4.3.1. Protein–polysaccharide conjugate nanoparticles
Polysaccharide glycosylated proteins prepared through the Maillard reaction are reported to inhibit the precipitation of the corresponding proteins caused by high concentration or interaction with polyphenols, which are materials for encapsulation of polyphenols. Tea polyphenols were loaded in the protein core of the gelatin–dextran conjugate nanoparticles, which were synthesized using the Maillard reaction. Under optimal conditions, the conjugate nanoparticles loaded with EGCG showed an average of 86 nm in diameter with narrow distribution. The encapsulation efficiency of EGCG is pH-independent, but the loading capacity is controllable and as high as 360 wt.% (weight/weight of protein). The encapsulated EGCG showed comparable or even stronger cytotoxicity against MCF-7 cells than free EGCG in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [96]. EGCG was also encapsulated in the dextran glycosylated casein nanoparticles with strong encapsulating and retaining capacity to EGCG, and high colloid stability of the nanoparticles in a wide concentration range during storage. The glycosylated casein could effectively protect EGCG from degradation in alkaline pH and displayed a slow and sustained release in intestinal fluid [97]. The glycosylation of protein was also prepared by conjugating dextran to bovine serum albumin through the Maillard reaction for delivery of EGCG [98].
4.3.2. Protein–polysaccharide complex nanoparticles
Biopolymer-based nanoparticles can be rationally engineered through the controlled self-assembly of protein and polysaccharides, mainly through electrostatic interactions [99]. One of the most commonly used methods of assembling proteins and polysaccharides into functional biopolymer particles is based on electrostatic attraction between oppositely charged groups under appropriate solution conditions [100,101]. Hydrogel particles have been prepared by heating the mixture solutions of β-Lg and the food polysaccharide such as chitosan [102,103] or pectin [104,105], which formed through their interactions.
Caseinophosphopeptide (CPP), a bioactive peptide isolated from the digestion products of milk casein protein, was applied not only to crosslink chitosan forming the nanoparticles, but also to bind EGCG for encapsulation within the nanoparticles [106]. It provides a new strategy to encapsulate EGCG, with the encapsulation efficiency much higher than that in the chitosan–tripolyphosphate nanoparticles [107,108]. In addition, chitosan–CPP nanoparticles appear to have a much higher biocompatibility than that of chitosan–-tripolyphosphate nanopartciles, with significantly lower cytotoxicity [106]. Furthermore, encapsulation of EGCG in chitosan–CPP nanoparticles can significantly elevate the cellular antioxidant activity of EGCG [109] and enhance its permeation rate through the Caco-2 cell monolayer membrane [106]. The EGCG loaded chitosan–CPP nanoparticles were found to be uptaken by the Caco-2 cell [107].
4.4. Food lipid based nanoparticles
Food lipid nanoparticles, especially solid lipid nanoparticles (SLN), show advantages in improving the solubility and bioavailability of lipid-soluble polyphenols. Guri et al [110] evaluated the capabilities of SLN to deliver curcumin in a coculture system of absorptive Caco-2 and mucus secreting HT29-MTX cells. The encapsulation of curcumin in SLN caused enhanced delivery compared with unencapsulated curcumin, without influence on the integrity of the cellular junctions [110]. Sun et al [111] reported that curcumin-loaded SLNs could prolong in vitro antitumor activity and cellular uptake, and improve the in vivo bioavailability of the loaded curcumin [111]. Neves et al [112] prepared two types of SLN for loading and encapsulation of resveratrol. The presence of resveratrol induced a disorder in the crystal structure of nanoparticles, suggesting a favoring of its entrapment. The in vitro simulation of the GI transit showed that resveratrol remained mostly associated with lipid nanoparticles after their incubation in digestive fluids [112]. Jose et al [113] fabricated the glyceryl behenate-based solid SLNs for encapsulation and delivery of resveratrol. The cytotoxicity assay showed that resveratrol loaded SLNs were equally effective as free resveratrol, being an antitumor agent. The in vivo bio-distribution study using Wistar rats demonstrated that SLNs could significantly (p < 0.001) increase the brain concentration of resveratrol [113]. Resveratrol loaded stearic acid based SLNs coated with poloxamer 188 were produced successfully using the solvent diffusion–solvent evaporation method, which showed prolonged drug release in vitro up to ~120 hours. The lipid formulation produced a significant (about eightfold) improvement in the oral bioavailability of resveratrol as compared with the resveratrol suspension [114]. Resveratrol solubility, stability, and intracellular delivery were all increased after loading into SLNs. SLNs below 180 nm loading with or without resveratrol moved promptly through the cell membrane, distributed throughout the cytosol, moved successively among different cellular levels, and localized in the perinuclear region without inducing cytotoxicity. The cytostatic effect of SLN–resveratrol was much more expressed than that of resveratrol in solution. Delivery of resveratrol by SLNs contributes to the effectiveness of resveratrol on decreasing cell proliferation, with potential benefits for prevention of skin cancer [115].
SLNs were also applied in encapsulation and delivery of water-soluble polyphenols. Zhang et al [116] have successfully synthesized EGCG encapsulated nanostructured lipid carriers (NLCE) and chitosan coated NLCE using natural lipids, surfactant, chitosan, and EGCG. And it is found that the nano-encapsulated EGCG may have a potential to inhibit atherosclerotic lesion development [116].
4.5. Food hybrid nanoparticles
The hybrid nanoparticles, usually coated with polysaccharides, endow multifunctions to the delivery system. Chitosan coated SLNs were prepared to encapsulate curcumin, which showed prolonged physical stability under room and refrigerated temperature conditions. Moreover, curcumin encapsulated in the chitosan coated SLNs has been shown to increase the bioavailability of curcumin above that of curcumin suspensions after oral administration [117]. The mixed colloidal dispersions of curcumin encapsulated zein nanoparticles and digestible lipid nanoparticles were fabricated, which could significantly enhance the bioaccessibility of curcumin [118]. D'Souza and Devarajan [119] investigated the bioenhancement of oral curcumin bioavailability from the curcumin–Gantrez nanoparticles that were adsorbed by different polysaccharides including galactose polysaccharides arabinogalactan and kappa-carrageenan, glucose polysaccharide pullulan. And the results showed that galactose polysaccharides could play an important role in enhancing absorption of drugs through galectin-mediated absorption. An absolute bioavailability of greater than 25% seen with the galactose polysaccharides arabinogalactan and kappa-carrageenan was extremely promising. Resveratrol was bound to caseinate or caseinate–dextran to form the biopolymer complexes. Then, the resveratrol bound caseinate or caseinate–dextran was coated to the surface of the zein core, forming the biopolymer nanoparticles. Both the biopolymer nanoparticles and complexes protected trans-resveratrol from isomerization when exposed to ultra violet light, with the nanoparticles being more effective. Nanoparticles coated by caseinate–dextran were more stable to aggregation under simulated GI conditions than those coated by caseinate. The bioaccessibility of resveratrol was enhanced when it was encapsulated in both biopolymer nanoparticles and biopolymer complexes [120].
5. Perspectives
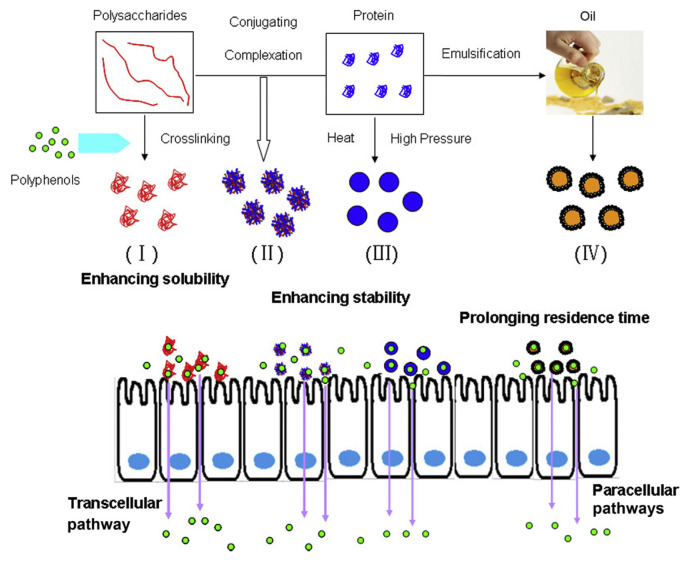
It can be concluded that food macromolecule based nanoparticles have potential in increasing the bioavailability of polyphenols, such as EGCG, curcumin, and resveratrol, mainly through enhancing their solubility, preventing their degradation in the intestinal environment, elevating the permeation rate in the small intestine, and even increasing their contents in the bloodstream (Figure 4). The bioactivities of polyphenols, such as anticancer, antioxidant, and antiinflammatory activities, have been reported to be enhanced after encapsulation with food macromolecule nanoparticles, also demonstrating the enhanced polyphenol bioavailability. For the oral administration route, the food macromolecular nanoparticles must first withstand the harsh pH and digestive enzyme environment in the GI tract, hold the loading polyphenols, reach the drug absorption site in the small intestine; otherwise, the nanoparticle-based oral delivery will be unachievable. Efforts should be made to enhance the stability of nanocarriers in the GI tract environment and characterize their structure behaviors [121]. Furthermore, the effects of nanoencapsulation on the metabolism of polyphenols and the influence on the corresponding metabolism enzymes should be given due attention to further illustrate the mechanism for enhanced bioavailability after nanoencapsulation.
Figure 4.
Formation of food macromolecule nanoparticles for enhancing the bioavailability of polyphenols (I, polysaccharide nanocomplexes; II, protein–polysaccharide complex or conjugate nanoparticles; III, protein nanoparticles; IV, solid lipid nanoparticles).
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 31501488), Natural Science Foundation of Jiangsu Province–Outstanding Youth Foundation (BK20160075), Fundamental Research Funds for the Central Universities (KJQN201648, KYLH201601), Open Foundation of Hubei Key Laboratory of Edible Wild Plants Conservation and Utilization, Grant No: EWPL201504.
Funding Statement
This work was supported by National Natural Science Foundation of China (No. 31501488), Natural Science Foundation of Jiangsu Province–Outstanding Youth Foundation (BK20160075), Fundamental Research Funds for the Central Universities (KJQN201648, KYLH201601), Open Foundation of Hubei Key Laboratory of Edible Wild Plants Conservation and Utilization, Grant No: EWPL201504.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1. Sargeant LA, Khaw KT, Bingham S, Day NE, Luben RN, Oakes S, Welch A, Wareham NJ. Fruit and vegetable intake and population glycosylated haemoglobin levels: the EPIC-Norfolk Study. Eur J Clin Nutr. 2001;55:342–8. doi: 10.1038/sj.ejcn.1601162. [DOI] [PubMed] [Google Scholar]
- 2. von Ruesten A, Feller S, Bergmann MM, Boeing H. Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr. 2013;67:412–9. doi: 10.1038/ejcn.2013.7. [DOI] [PubMed] [Google Scholar]
- 3. Masala G, Assedi M, Bendinelli B, Ermini I, Sieri S, Grioni S, Sacerdote C, Ricceri F, Panico S, Mattiello A, Tumino R, Giurdanella MC, Berrino F, Saieva C, Palli D. Fruit and vegetables consumption and breast cancer risk: the EPIC Italy study. Breast Cancer Res Treat. 2012;132:1127–36. doi: 10.1007/s10549-011-1939-7. [DOI] [PubMed] [Google Scholar]
- 4. Lunet N, Lacerda-Vieira A, Barros H. Fruit and vegetables consumption and gastric cancer: a systematic review and meta-analysis of cohort studies. Nutr Cancer. 2005;53:1–10. doi: 10.1207/s15327914nc5301_1. [DOI] [PubMed] [Google Scholar]
- 5. Suganya N, Bhakkiyalakshmi E, Sarada DVL, Ramkumar KM. Reversibility of endothelial dysfunction in diabetes: role of polyphenols. Br J Nutr. 2016;116:223–46. doi: 10.1017/S0007114516001884. [DOI] [PubMed] [Google Scholar]
- 6. Pan M-H, Ho C-T. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev. 2008;37:2558–74. doi: 10.1039/b801558a. [DOI] [PubMed] [Google Scholar]
- 7. Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–21. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation; diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol. 2015;83:111–24. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 9. Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus—systematic review and meta-analysis. Mol Nutr Food Res. 2015;59:147–59. doi: 10.1002/mnfr.201400173. [DOI] [PubMed] [Google Scholar]
- 10. Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, Ahmad N, Cui HD, Mousa SA, Mukhtar H. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heleno SA, Martins A, Queiroz MJRP, Ferreira ICFR. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chem. 2015;173:501–13. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 12. Li Z, Jiang H, Xu CM, Gu LW. A review: using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015;43:153–64. [Google Scholar]
- 13. Wang S, Su R, Nie SF, Sun M, Zhang J, Wu DY, Moustaid-Moussa N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25:363–76. doi: 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr Opin Colloid Interface. 2009;14:3–15. [Google Scholar]
- 15. des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat VJ. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 16. Mezzenga R, Schurtenberger P, Burbidge A, Michel M. Understanding foods as soft materials. Nat Mater. 2005;4:729–40. doi: 10.1038/nmat1496. [DOI] [PubMed] [Google Scholar]
- 17. Ubbink J, Burbidge A, Mezzenga R. Food structure and functionality: a soft matter perspective. Soft Matter. 2008;4:1569–81. doi: 10.1039/b802183j. [DOI] [PubMed] [Google Scholar]
- 18. Huang Q, Yu H, Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75:R50–7. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- 19. McClements DJ. Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter. 2011;7:2297–316. [Google Scholar]
- 20. Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–46. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsao R, McCallum J. Chemistry of flavonoids. In: de la Rosa LA, Alvarez-Parrilla E, Gonzalez-Aguilar G, editors. Fruit and vegetable phytochemicals: chemistry, nutritional value and stability. Chapter 5. Ames, IA, USA: Blackwell Publishing; 2009. pp. 131–53. [Google Scholar]
- 22.Valant-Vetschera KM, Wallenweber E. Flavones and flavonols. In: Anderson OM, Markham KR, editors. Flavonoids: chemistry, biochemistry and applications. Boca Raton, FL, USA: CRC Press/Taylor & Francis Group; 2006. pp. 618–748. [Google Scholar]
- 23.Williams CA. Flavone and flavonol O-glycosides. In: Anderson OM, Markham KR, editors. Flavonoids: chemistry, biochemistry and applications. Boca Raton, FL, USA: CRC Press/Taylor & Francis Group; 2006. pp. 749–856. [Google Scholar]
- 24. Manthey JA, Cesar TB, Jackson E, Mertens-Talcott S. Pharmacokinetic study of nobiletin and tangeretin in rat serum by high-performance liquid chromatography–electrospray ionization–mass spectrometry. J Agric Food Chem. 2011;59:145–51. doi: 10.1021/jf1033224. [DOI] [PubMed] [Google Scholar]
- 25. Burns J, Yokota T, Ashihara H, Lean MEJ, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–40. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 26. Siviero A, Gallo E, Maggini V, Gori L, Mugelli A, Firenzuoli F, Vannacci A. Curcumin, a golden spice with a low bioavailability. J Herb Med. 2015;5:57–70. [Google Scholar]
- 27. Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr Rev. 2016;72:429–52. doi: 10.1111/nure.12114. [DOI] [PubMed] [Google Scholar]
- 28. Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 29. Green RJ, Murphy AS, Schulz B, Watkins BA, Ferruzzi MG. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol Nutr Food Res. 2007;51:1152–62. doi: 10.1002/mnfr.200700086. [DOI] [PubMed] [Google Scholar]
- 30. Peters CM, Green RJ, Janle EM, Ferruzzi MG. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res Int. 2010;43:95–102. doi: 10.1016/j.foodres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cebeci F, Sahin-Yesilcubuk N. The matrix effect of blueberry, oat meal and milk on polyphenols, antioxidant activity and potential bioavailability. Int J Food Sci Nutr. 2014;65:69–78. doi: 10.3109/09637486.2013.825699. [DOI] [PubMed] [Google Scholar]
- 32. McDougall GJ, Dobson P, Smith P, Blake A, Stewart D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J Agric Food Chem. 2005;53:5896–904. doi: 10.1021/jf050131p. [DOI] [PubMed] [Google Scholar]
- 33. Xie Y, Kosinska A, Xu H, Andlauer W. Milk enhances intestinal absorption of green tea catechins in in vitro digestion/Caco-2 cells model. Food Res Int. 2013;53:793–800. [Google Scholar]
- 34. van het Hof KH, Kivits GA, Weststrate JA, Tijburg LB. Bioavailability of catechins from tea: the effect of milk. Eur J Clin Nutr. 1998;52:356–9. doi: 10.1038/sj.ejcn.1600568. [DOI] [PubMed] [Google Scholar]
- 35. Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, Pinto MD. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol. 2013;75:588–602. doi: 10.1111/j.1365-2125.2012.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tapiero H, Townsend DM, Tew KD. Organosulfur compounds from alliaceae in the prevention of human pathologies. Biomed Pharmacother. 2004;58:183–93. doi: 10.1016/j.biopha.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meyer JH. Gastric emptying of ordinary food: effect of antrum on particle size. Am J Physiol. 1980;239:G133–5. doi: 10.1152/ajpgi.1980.239.3.G133. [DOI] [PubMed] [Google Scholar]
- 38. Singh H, Ye A, Horne D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog Lipid Res. 2009;48:92–100. doi: 10.1016/j.plipres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 39. Wilde PJ, Chu BS. Interfacial & colloidal aspects of lipid digestion. Adv Colloid Interface Sci. 2011;165:14–22. doi: 10.1016/j.cis.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 40. Maldonado-Valderrama J, Woodward NC, Gunning AP, Ridout MJ, Husband FA, Mackie AR, Morris VJ, Wilde PJ. Interfacial characterization of β-lactoglobulin networks: displacement by bile salts. Langmuir. 2008;24:6759–67. doi: 10.1021/la800551u. [DOI] [PubMed] [Google Scholar]
- 41. Maldonado-Valderrama J, Miller R, Fainerman VB, Wilde PJ, Morris VJ. Effect of gastric conditions on β-lactoglobulin interfacial networks: influence of the oil phase on protein structure. Langmuir. 2010;26:15901–8. doi: 10.1021/la102294u. [DOI] [PubMed] [Google Scholar]
- 42. Euston SR, Baird WG, Campbell L, Kuhns M. Competitive adsorption of dihydroxy and trihydroxy bile salts with whey protein and casein in oil-in-water emulsions. Biomacromolecules. 2013;14:1850–8. doi: 10.1021/bm4002443. [DOI] [PubMed] [Google Scholar]
- 43. Fernandes I, Faria A, Calhau C, de Freitas V, Mateus N. Bioavailability of anthocyanins and derivatives. J Funct Foods. 2014;7:54–66. [Google Scholar]
- 44. Yoshino K, Suzuki M, Sasaki K, Miyase T, Sano M. Formation of antioxidants from (−)-epigallocatechin gallate in mild alkaline fluids, such as authentic intestinal juice and mouse plasma. J Nut Biochem. 1999;10:223–9. doi: 10.1016/s0955-2863(98)00103-x. [DOI] [PubMed] [Google Scholar]
- 45. Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: what formulation solutions to bioavailability limitations? J Control Release. 2012;158:182–93. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 46. Bernabé-Pineda M, Ramírez-Silva MT, Romero-Romo M, González-Vergara E, Rojas-Hernández A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60:1091–7. doi: 10.1016/S1386-1425(03)00342-1. [DOI] [PubMed] [Google Scholar]
- 47. Kahle K, Kraus M, Scheppach W, Richling E. Colonic availability of apple polyphenols–a study in ileostomy subjects. Mol Nutr Food Res. 2005;49:1143–50. doi: 10.1002/mnfr.200500132. [DOI] [PubMed] [Google Scholar]
- 48. Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJB, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62:1276–82. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- 49. Barrington R, Williamson G, Bennett RN, Davis BD, Brodbelt JS, Kroon PA. Absorption, conjugation and efflux of the flavonoids, kaempferol and galangin, using the intestinal CaCo-2/TC7 cell model. J Funct Foods. 2009;1:74–87. doi: 10.1016/j.jff.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaldas MI, Walle UK, Walle T. Resveratrol transport and metabolism by human intestinal Caco-2 cells. J Pharm Pharmacol. 2003;55:307–12. doi: 10.1211/002235702612. [DOI] [PubMed] [Google Scholar]
- 51. Yu HL, Huang QR. Investigation of the absorption mechanism of solubilized curcumin using Caco-2 cell monolayers. J Agric Food Chem. 2011;59:9120–6. doi: 10.1021/jf201451m. [DOI] [PubMed] [Google Scholar]
- 52. Murota K, Shimizu S, Miyamoto S, Izumi T, Obata A, Kikuchi M, Terao J. Unique uptake and transport of isoflavone aglycones by human intestinal Caco-2 cells: comparison of isoflavonoids and flavonoids. J Nutr. 2002;132:1956–61. doi: 10.1093/jn/132.7.1956. [DOI] [PubMed] [Google Scholar]
- 53. Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos. 2006;34:1786–92. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 54. Actis-Goretta L, Leveques A, Rein M, Teml A, Schafer C, Hofmann U, Li HQ, Schwab M, Eichelbaum M, Williamson G. Intestinal absorption, metabolism, and excretion of (−)-epicatechin in healthy humans assessed by using an intestinal perfusion technique. Am J Clin Nutr. 2013;98:924–33. doi: 10.3945/ajcn.113.065789. [DOI] [PubMed] [Google Scholar]
- 55. Appeldoorn MM, Vincken JP, Gruppen H, Hollman PCH. Procyanidin dimers A1, A2, and B2 are absorbed without conjugation or methylation from the small intestine of rats. J Nutr. 2009;139:1469–73. doi: 10.3945/jn.109.106765. [DOI] [PubMed] [Google Scholar]
- 56. Urpi-Sarda M, Monagas M, Khan N, Lamuela-Raventos RM, Santos-Buelga C, Sacanella E, Castell M, Permanyer J, Andres-Lacueva C. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal Bioanal Chem. 2009;394:1545–56. doi: 10.1007/s00216-009-2676-1. [DOI] [PubMed] [Google Scholar]
- 57. Gonthier MP, Donovan JL, Texier O, Felgines C, Remesy C, Scalbert A. Metabolism of dietary procyanidins in rats. Free Radic Biol Med. 2003;35:837–44. doi: 10.1016/s0891-5849(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 58. Wolffram S, Block M, Ader P. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J Nutr. 2002;132:630–5. doi: 10.1093/jn/132.4.630. [DOI] [PubMed] [Google Scholar]
- 59. Walle T, Walle UK. The β-d-glucoside and sodium-dependent glucose transporter 1 (SGLT1)-inhibitor phloridzin is transported by both SGLT1 and multidrug resistance-associated proteins 1/2. Drug Metab Dispos. 2003;31:1288–91. doi: 10.1124/dmd.31.11.1288. [DOI] [PubMed] [Google Scholar]
- 60. Teng Z, Yuan C, Zhang F, Huan ML, Cao WD, Li KC, Yang JY, Cao DY, Zhou SY, Mei QB. Intestinal absorption and first-pass metabolism of polyphenol compounds in rat and their transport dynamics in Caco-2 cells. PLoS ONE. 2012;7:e29647. doi: 10.1371/journal.pone.0029647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–94. [PubMed] [Google Scholar]
- 62. Sang SM, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 63. De Santi C, Pietrabissa A, Mosca F, Pacifici GM. Methylation of quercetin and fisetin, flavonoids widely distributed in edible vegetables, fruits and wine, by human liver. Int J Clin Pharm Ther. 2002;40:207–12. doi: 10.5414/cpp40207. [DOI] [PubMed] [Google Scholar]
- 64. Murakami T, Takano M. Intestinal efflux transporters and drug absorption. Expert Opin Drug Metab Toxicol. 2008;4:923–39. doi: 10.1517/17425255.4.7.923. [DOI] [PubMed] [Google Scholar]
- 65. Gill RK, Saksena S, Alrefai WA, Alrefai WA, Saksena S, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol. 2005;289:C846–52. doi: 10.1152/ajpcell.00112.2005. [DOI] [PubMed] [Google Scholar]
- 66. Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–6. [PubMed] [Google Scholar]
- 67. Elzoghby AO. Gelatin-based nanoparticles as drug and gene delivery systems: reviewing three decades of research. J Control Release. 2013;172:1075–91. doi: 10.1016/j.jconrel.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 68. Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. J Control Release. 2012;161:38–49. doi: 10.1016/j.jconrel.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 69. Shpigelman A, Israeli G, Livney YD. Thermally-induced protein polyphenol co-assemblies: beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010;24:735–43. [Google Scholar]
- 70. Lestringant P, Guri A, Gülseren İ, Relkin P, Corredig M. Effect of processing on physicochemical characteristics and bioefficacy of β-lactoglobulin–epigallocatechin-3-gallate complexes. J Agric Food Chem. 2014;62:8357–64. doi: 10.1021/jf5029834. [DOI] [PubMed] [Google Scholar]
- 71. Li B, Du WK, Jin JC, Du QZ. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J Agric Food Chem. 2012;60:3477–84. doi: 10.1021/jf300307t. [DOI] [PubMed] [Google Scholar]
- 72. Yang W, Xu CQ, Liu FG, Yuan F, Gao YX. Native and thermally modified protein–polyphenol coassemblies: lactoferrin-based nanoparticles and submicrometer particles as protective vehicles for (−)-epigallocatechin-3-gallate. J Agric Food Chem. 2014;62:10816–27. doi: 10.1021/jf5038147. [DOI] [PubMed] [Google Scholar]
- 73. Li M, Cui J, Ngadi MO, Ma Y. Absorption mechanism of whey-protein-delivered curcumin using Caco-2 cell monolayers. Food Chem. 2015;180:48–54. doi: 10.1016/j.foodchem.2015.01.132. [DOI] [PubMed] [Google Scholar]
- 74. Liang L, Tajmir-Riahi HA, Subirade M. Interaction of β-lactoglobulin with resveratrol and its biological implications. Biomacromolecules. 2008;9:50–6. doi: 10.1021/bm700728k. [DOI] [PubMed] [Google Scholar]
- 75. Hemar Y, Gerbeaud M, Oliver CM, Augustin MA. Investigation into the interaction between resveratrol and whey proteins using fluorescence spectroscopy. J Food Sci Technol. 2011;46:2137–44. [Google Scholar]
- 76. Guo LY, Peng Y, Li YL, Yao JP, Zhang GM, Chen J, Wand JG, Sui LH. Cell death pathway induced by resveratrol-bovine serum albumin nanoparticles in a human ovarian cell line. Oncol Lett. 2015;9:1359–63. doi: 10.3892/ol.2015.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Esmaili M, Ghaffari SM, Moosavi-Movahedi Z, Atri MS, Sharifizadeh A, Farhadi M, Yousefi R, Chobert JM, Haertlé T, Moosavi-Movahedi AA. β-Casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT-Food Sci Technol. 2011;44:2166–72. [Google Scholar]
- 78. Pan K, Zhong QX, Baek SJ. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J Agric Food Chem. 2013;61:6036–43. doi: 10.1021/jf400752a. [DOI] [PubMed] [Google Scholar]
- 79. Pan K, Luo YC, Gan YD, Baek SJ, Zhong QX. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter. 2014;10:6820–30. doi: 10.1039/c4sm00239c. [DOI] [PubMed] [Google Scholar]
- 80. Sneharani AH, Singh SA, Rao AGA. Interaction of rS1-casein with curcumin and its biological implications. J Agric Food Chem. 2009;57:10386–91. doi: 10.1021/jf902464p. [DOI] [PubMed] [Google Scholar]
- 81. Shutava TG, Balkundi SS, Vangala P, Steffan JJ, Bigelow RL, Cardelli JA, O'Neal DP, Lvov YM. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano. 2009;3:1877–85. doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- 82. Karthikeyan S, Hoti SL, Prasad NR. Resveratrol loaded gelatin nanoparticles synergistically inhibits cell cycle progression and constitutive NF-kappaB activation, and induces apoptosis in non-small cell lung cancer cells. Biomed Pharmacother. 2015;70:274–82. doi: 10.1016/j.biopha.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 83. Gomez-Estaca J, Balaguer MP, Gavara R, Hernandez-Munoz P. Formation of zein nanoparticles by electrohydrodynamic atomization: effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocoll. 2012;28:82–91. [Google Scholar]
- 84. Guan X, Yin T, Han F. Light stability, controlled-release and antioxidation of resveratrol–hordein composite nanoparticles. Chem J Chin Univ. 2015;36:1707–12. [Google Scholar]
- 85. Penalva R, Esparza I, Larraneta E, González-Navarro CJ, Gamazo C, Irache JM. Zein-based nanoparticles improve the oral bioavailability of resveratrol and its anti-inflammatory effects in a mouse model of endotoxic shock. J Agric Food Chem. 2015;63:5603–11. doi: 10.1021/jf505694e. [DOI] [PubMed] [Google Scholar]
- 86. Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan—a versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. 2011;36:981–1014. [Google Scholar]
- 87. Park SJ, Garcia CV, Shin GH, Kim JT. Fabrication and optimization of EGCG-loaded nanoparticles by high pressure homogenization. J Appl Polym Sci. 2016;133:43269. [Google Scholar]
- 88. Siddiqui IA, Bharali DJ, Nihal M, Adhami VM, Khan N, Chamcheu JC, Khan MI, Shaban S, Mous SA, Mukhtar H. Excellent anti-proliferative and pro-apoptotic effects of (−)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine NBM. 2014;10:1619–26. doi: 10.1016/j.nano.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 89. Lin Y-H, Chen Z-R, Lai C-H, Hsieh C-H, Feng C-L. Active targeted nanoparticles for oral administration of gastric cancer therapy. Biomacromolecules. 2015;16:3021–32. doi: 10.1021/acs.biomac.5b00907. [DOI] [PubMed] [Google Scholar]
- 90. Liu F, Majeed H, Antoniou J, Li Y, Ma Y, Yokoyama W, Ma JG, Zhong F. pH and temperature stability of (−)-epigallocatechin-3-gallate–β-cyclodextrin inclusion complex-loaded chitosan nanoparticles. Carbohydr Polym. 2016;149:340–7. doi: 10.1016/j.carbpol.2016.04.100. [DOI] [PubMed] [Google Scholar]
- 91. Chuah LH, Roberts CJ, Billa N, Abdullah S, Rosli R. Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloids Surf B Biointerfaces. 2014;116:228–36. doi: 10.1016/j.colsurfb.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 92. Bhunchu S, Rojsitthisak P, Rojsitthisak P. Effects of preparation parameters on the characteristics of chitosan–alginate nanoparticles containing curcumin diethyl disuccinate. J Drug Deliv Sci Technol. 2015;28:64–72. [Google Scholar]
- 93. Tan C, Xie JH, Zhang XM, Cai JB, Xia SQ. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016;57:236–45. [Google Scholar]
- 94. Zu YG, Zhang Y, Wang WG, Zhao XH, Han X, Wang KL, Ge YL. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016;23:981–91. doi: 10.3109/10717544.2014.924167. [DOI] [PubMed] [Google Scholar]
- 95. Kim S, Ng WK, Dong YC, Das S, Tan RBH. Preparation and physicochemical characterization of trans-resveratrol nanoparticles by temperature-controlled antisolvent precipitation. J Food Eng. 2012;108:37–42. [Google Scholar]
- 96. Zhou HH, Sun XY, Zhang LL, Zhang P, Li J, Liu Y-N. Fabrication of biopolymeric complex coacervation core micelles for efficient tea polyphenol delivery via a green process. Langmuir. 2012;28:14553–61. doi: 10.1021/la303062j. [DOI] [PubMed] [Google Scholar]
- 97. Xue J, Tan C, Zhang XM, Feng B, Xia SQ. Fabrication of epigallocatechin-3-gallate nanocarrier based on glycosylated casein: stability and interaction mechanism. J Agric Food Chem. 2014;62:4677–84. doi: 10.1021/jf405157x. [DOI] [PubMed] [Google Scholar]
- 98. Xia SQ, Li YQ, Xia QY, Zhang XM, Huang QR. Glycosylation of bovine serum albumin via Maillard reaction prevents epigallocatechin-3-gallate-induced protein aggregation. Food Hydrocoll. 2015;43:228–35. [Google Scholar]
- 99. Jones OG, McClements DJ. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein–polysaccharide complexes. Adv Colloid Interface Sci. 2011;167:49–62. doi: 10.1016/j.cis.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 100. Spada JC, Marczak LDF, Tessaro IC, Cardozo NSM. Interactions between soy protein from water-soluble soy extract and polysaccharides in solutions with polydextrose. Carbohydr Polym. 2015;134:119–27. doi: 10.1016/j.carbpol.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 101. Norton IT, Frith WJ. Microstructure design in mixed biopolymer composites. Food Hydrocoll. 2001;15:543–53. [Google Scholar]
- 102. Hong YH, McClements DJ. Formation of hydrogel particles by thermal treatment of beta-lactoglobulin–chitosan complexes. J Agric Food Chem. 2007;55:5653–60. doi: 10.1021/jf070564n. [DOI] [PubMed] [Google Scholar]
- 103. Lee AC, Hong YH. Coacervate formation of alpha-lactalbumin–chitosan and beta-lactoglobulin–chitosan complexes. Food Res Int. 2009;42:733–8. [Google Scholar]
- 104. Jones OG, Decker EA, McClements DJ. Formation of biopolymer particles by thermal treatment of beta-lactoglobulin–pectin complexes. Food Hydrocoll. 2009;23:1312–21. [Google Scholar]
- 105. Zimet P, Livney YD. Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for omega-3 polyunsaturated fatty acids. Food Hydrocoll. 2009;23:1120–6. [Google Scholar]
- 106. Hu B, Ting YW, Yang XQ, Tang WP, Zeng XX, Huang QR. Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem Commun. 2012;48:2421–3. doi: 10.1039/c2cc17295j. [DOI] [PubMed] [Google Scholar]
- 107. Hu B, Ting YW, Zeng XX, Huang QR. Cellular uptake and cytotoxicity of chitosan-caseinophosphopeptides nanocomplexes loaded with epigallocatechin gallate. Carbohydr Polym. 2012;89:362–70. doi: 10.1016/j.carbpol.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 108. Hu B, Pan CL, Sun Y, Ye H, Hou ZY, Ye H, Zeng XX. Optimization of fabrication parameters to produce chitosan–tripolyphosphate nanoparticles for delivery of tea catechins. J Agric Food Chem. 2008;56:7451–8. doi: 10.1021/jf801111c. [DOI] [PubMed] [Google Scholar]
- 109. Hu B, Ting YW, Zeng XX, Huang QR. Bioactive peptides/chitosan nanoparticles enhance cellular antioxidant activity of (−)-epigallocatechin-3-gallate. J Agric Food Chem. 2013;61:875–81. doi: 10.1021/jf304821k. [DOI] [PubMed] [Google Scholar]
- 110. Guri A, Gülseren I, Corredig M. Utilization of solid lipid nanoparticles for enhanced delivery of curcumin in cocultures of HT29-MTX and Caco-2 cells. Food Funct. 2013;4:1410–9. doi: 10.1039/c3fo60180c. [DOI] [PubMed] [Google Scholar]
- 111. Sun JB, Bi C, Chan HM, Sun SP, Zhang QW, Zhen Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf B Biointerfaces. 2013;111:367–75. doi: 10.1016/j.colsurfb.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 112. Neves AR, Lúcio M, Martins S, Lima JLC, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int J Nanomed. 2013;8:177–87. doi: 10.2147/IJN.S37840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jose S, Anju SS, Cinu TA, Aleykutty NA, Thomas S, Souto EB. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int J Pharm. 2014;474:6–13. doi: 10.1016/j.ijpharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 114. Pandita D, Kumar S, Poonia N, Lather V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res Int. 2014;62:1165–74. [Google Scholar]
- 115. Teskac K, Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int J Pharm. 2010;390:61–9. doi: 10.1016/j.ijpharm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 116. Zhang J, Nie SF, Wang S. Nanoencapsulation enhances epigallocatechin-3-gallate stability and its antiatherogenic bioactivities in macrophages. J Agric Food Chem. 2013;61:9200–9. doi: 10.1021/jf4023004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ramalingam P, Yoo SW, Ko YT. Nanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcumin. Food Res Int. 2016;84:113–9. [Google Scholar]
- 118. Zou LQ, Zheng BJ, Zhang RJ, Zhang ZP, Liu W, Liu CM, Xiao H, McClements DJ. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res Int. 2016;81:74–82. [Google Scholar]
- 119. D'Souza AA, Devarajan PV. Bioenhanced oral curcumin nanoparticles: role of carbohydrates. Carbohydr Polym. 2016;136:1251–8. doi: 10.1016/j.carbpol.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 120. Davidov-Pardo G, Pérez-Ciordia S, Marín-Arroyo MR, McClements DJ. Improving resveratrol bioaccessibility using biopolymer nanoparticles and complexes: impact of protein–carbohydrate Maillard conjugation. J Agric Food Chem. 2015;63:3915–23. doi: 10.1021/acs.jafc.5b00777. [DOI] [PubMed] [Google Scholar]
- 121. Hu B, Xie MH, Zhang C, Zeng XX. Genipin-structured peptide–polysaccharide nanoparticles with significantly improved resistance to harsh gastrointestinal environments and their potential for oral delivery of polyphenols. J Agric Food Chem. 2014;62:12443–52. doi: 10.1021/jf5046766. [DOI] [PubMed] [Google Scholar]