Abstract
Gardenia jasminoides, grown in multiple regions in China, was commonly used as a natural yellow dye but has been one of the popular traditional Chinese medicines since the discovery of its biological property a few decades ago. It has been reported that G. jasminoides possess multiple biological activities, such as antioxidant properties, hypoglycemic effect, inhibition of inflammation, antidepression activity, and improved sleeping quality. In this review, our aim was to have a comprehensive summary of its phytochemistry including the extraction, isolation, and characterization of volatiles and bioactive molecules in G. jasminoides, focusing on the two major phytochemicals, genipin and crocin, which possess potent medicinal properties. Furthermore, this study attempted to establish a structure–activity relationship between the two major series of molecules with two pharmcophores and their biological activities, which would serve further exploration of the properties of phytocompounds in G. jasminoides as potential functional foods and medicines.
Keywords: crocin, Gardenia jasminoides, geniposide, genipin, iridiod
1. Introduction
Gardenia jasminoides, an evergreen tree that belongs to the Rubiaceae family, is cultivated in multiple areas in China, with a Chinese name of Zhi Zi. It grows in many temperate regions and has fragrant white flowers [1]. It is not only used as natural yellow dyes for many years [2,3], but also has various biological activities, such as antidiabetic [4], anti-inflammatory [5], antidepression [6], and antioxidant properties [7], and improvement of the quality of sleep [8]. It is commonly used in traditional Chinese medicine. The chemical analysis of G. jasminoides has been mainly focused on extraction technologies in recent years. Obtained extracts have exerted certain biological activities both in vitro and in vivo. Recent research showed that the oil extract from the G. jasminoides had antidepressant activity [6], and other new techniques to extract the oil and the complex biological activity have also been discussed. Herein, we reviewed the chemical components and biological activities of G. jasminoides as well as new techniques to extract and isolate the natural compounds from G. jasminoides.
2. Chemistry
A number of chemical components of G. jasminoides have been isolated and characterized, including iridoids, iridoid glucosides, triterpenoids, organic acids, and volatile compounds. Geniposide, genipin, gardenoside, crocin, and iridiod are the major bioactive compounds found in G. jasminoides. For instance, the yield of geniposide reached 10.9% under certain extraction conditions [9].
2.1. Volatiles in G. jasminoides
The major volatile compounds in essential oil of G. jasminoides are aliphatic acids, ketones, aldehydes, esters, alcohols, and aromatic derivatives [6,10]. Because of the different temperature and duration of processing, the essential oil from G. jasminoides contains varied contents and proportions of volatile compounds. In addition, unstable components such as iridoids may be partially converted to volatile components during high temperature processing [6].
Gas chromatography–mass spectrometry (GC/MS) was the major technique used to identify volatile components from G. jasminoides [6]. He et al [11] reported that the oil of the fruits of G. jasminoides was extracted by supercritical fluid CO2, in which 16 major components of the oil extract were revealed by GC/MS. Myristic acid (15.3%) had the highest relative content, whereas the lowest one was caproic acid (0.24%) [12]. Because of the pharmacological activities exerted by G. jasminoides oil and the availability of modern extraction techniques, many efforts were invested in the extraction of the G. jasminoides, in order to find the optimal extraction method. The extraction methods of volatile oil from G. jasminoides are listed in Table 1.
Table 1.
Extraction method of volatile oil from Gardenia jasminoides.
| Extraction method | Parameters of extraction | Results (%) | Refs |
|---|---|---|---|
| Supercritical fluid extraction (SFE) | Extraction pressure: 36.8 MPa Temperature: 65°C CO2 flow rate: 15 kg/h |
Linoleic acid, 44; palmitic acid, 26.4; oleic acid, 24.6 | [12] |
| Temperature: 49.94°C Pressure: 29.89 MPa Time: 93.82 min |
16 major components of the oil extract were characterized | [6] | |
| Pressure: 30 MPa Temperature: 55°C CO2 flow rate: 15 kg/h |
Linoleic acid, 44.38; oleic acid, 24.96; palmitic acid, 24.83; stearic acid, 2.55; linolenic acid, 1.31 | [13] | |
| Pressure: 12 MPa or 25 MPa Temperature: 45°C |
Oil yield, 12 | [13] | |
| Subcritical fluid extraction | Subcritical butane | Fatty acids, 77.6 | [14] |
| Ultrasound-assisted extraction | 28 kHz & 100 W Material/solvent ratio: 1:10 (g/mL) |
Oil yield, 16.49 | [15] |
| Steam distillation | Distilled water | Oil yield, 0.12 | [14] |
2.2. Iridoids and iridoid glycoside
Iridoids and iridoid glycoside are rich in G. jasminoides. The iridoids and iridiod glycosides include genipin, geniposide, and gardenoside. Many researchers have found multiple positive health effect of geniposide, including anti-inflammation [16], antidepression [16], antidiabetic properties [4], antithrombotic activities [18], as well as protection against lipopolysaccharide (LPS)-induced apoptotic liver damage [19]. The content of iridoid glycosides may vary from different regions at about 5–6% [20]. A study quantified the content of geniposide, gardenoside, geniposidic acid, and chlorogenic acid as 56.37 ± 26.24 μg/mg, 49.57 ± 18.78 μg/mg, 3.15 ± 3.27 μg/mg, and 0.69 ± 0.39 μg/mg, respectively, measured in 68 samples from different regions in China and Korea [21].
2.2.1. Extraction of iridoids
Because of the pharmacological effects of G. jasminoides and the application of modern extraction technology, many efforts have been invested in the preparation of different G. jasminoides extracts, in an attempt to find a potent ingredient that can have significant effects on diseases, such as high blood pressure, hyperglycemia, cancer, hyperlipidemia, and Alzheimer’s disease (AD) [22]. Certain positive results have been obtained with specified extracts, but in the future, further fractionation and evaluation of the active components of this plant should be a better direction. With the emergence of several new extraction methods, several minor components with potent biological activity may be found in G. jasminoides. Extraction methods such as solvent extraction, as well ultrasound-and microwave-assisted extraction (MAE) have been used to extract iridoids.
2.2.2. Solvent extraction method
Most solvent extraction methods require heating. The optimal solvent extraction parameters of G. jasminoides were 51.3% of ethanol/water mixture with an extraction temperature of 70.4°C for 28.6 minutes. Under this condition, the yield of geniposide and total phenolic compounds 10.9% and 2.497%, respectively [9].
2.2.3. Ultrasound- and microwave-assisted extraction
Many advanced extraction techniques have been introduced in recent years including ultrasound- and microwave-assisted extraction. Ultrasonic extraction was conducted by ultrasonic-assisted solvent extraction; with this technique, the sound waves can produce strong shear force and agitation to disrupt plant cells, which allows the solvent to penetrate into the cells, thereby shortening the extraction time and increasing the extraction yield. It has been found that the optimum conditions that obtain the highest yields of geniposide from G. jasminoides using ultrasound-assisted extraction were as follows: water with a solid/liquid ratio of 1:30 at a temperature of 70°C for 30 minutes, yielding geniposide of 4.1% [23].
2.2.4. Isolation of iridoids
The isolation of iridoids includes solvent partition separation, classic column chromatography, preparative high-performance liquid chromatography(prep-HPLC), high-speed countercurrent chromatography (HSCCC), and other isolation methods. At least 15 iridoids were reported to be isolated and identified, including iridoids, iridiod glucosides, secoiridoids, and secoiridoid glucosides. Table 2 summarizes the reported iridoids isolated from G. jasminoides.
Table 2.
Iridoids and iridoid glycosides isolated from Gardenia jasminoides.
| No. | Name | Structure | Extraction & isolation | Refs |
|---|---|---|---|---|
| 1 | Geniposide |
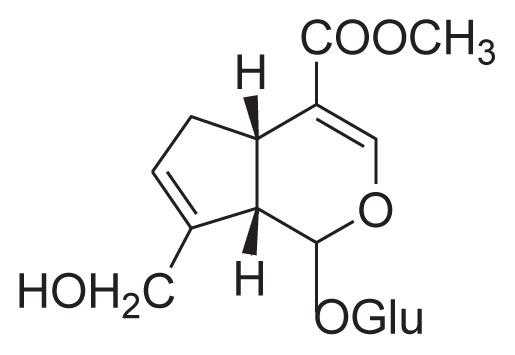
|
Reflux with 50% ethanol, HSCCC, MSPD | [29] [30] [33] |
| 2 | 6β-Hydroxy geniposide |
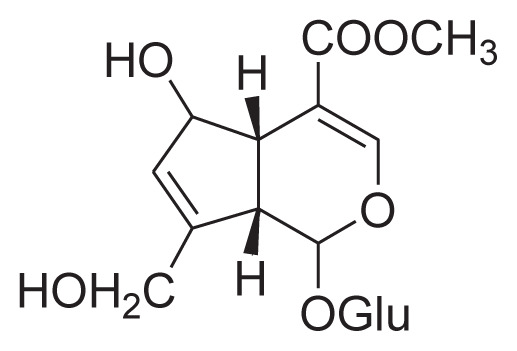
|
Cold percolate with 40% ethanol, macroporous resin HSCCC | [30] |
| 3 | Geniposidic acid |
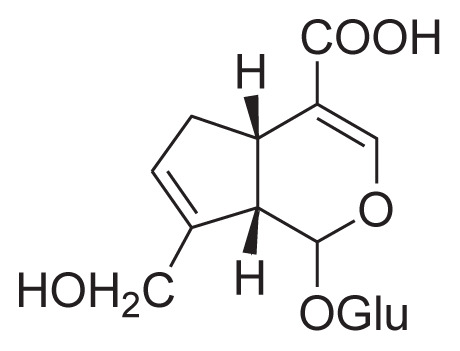
|
Cold percolate with 40% ethanol Macroporous resin HSCCC |
[30] |
| 4 | Gardenoside |
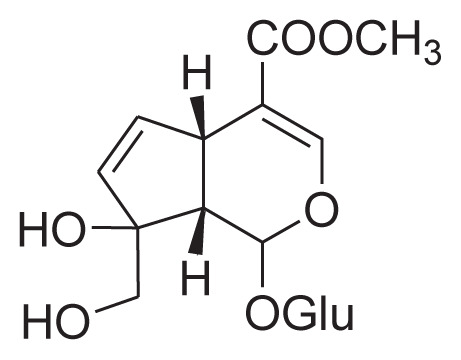
|
Cold percolate with 40% ethanol, macroporous resin, HSCCC | [30] [25] |
| 5 | 6α-Hydroxy geniposide |
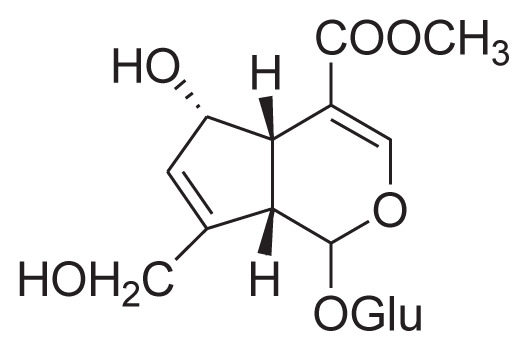
|
Reflux with 60% ethanol, Silica gel column, C18 column, preparative HPLC | [25] |
| 6 | 6-O-Methylscandoside methyl ester |
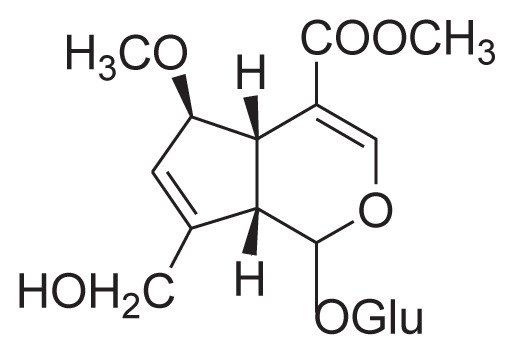
|
||
| 7 | 6-O-Methyldeacetylasperulosidic acid methyl ester |
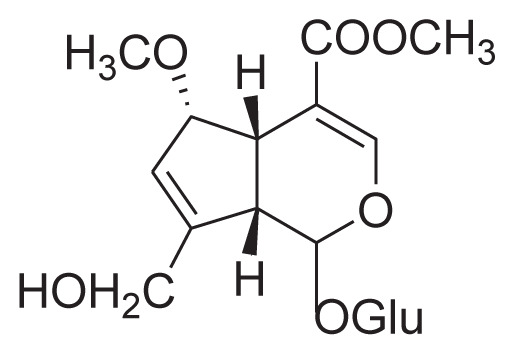
|
||
| 8 | 8-O-Methylmonotropein methyl ester |
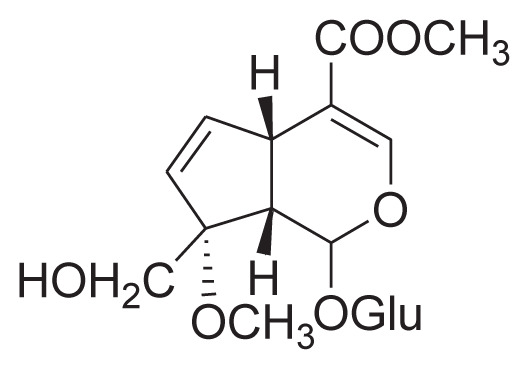
|
||
| 9 | Shanzhiside |
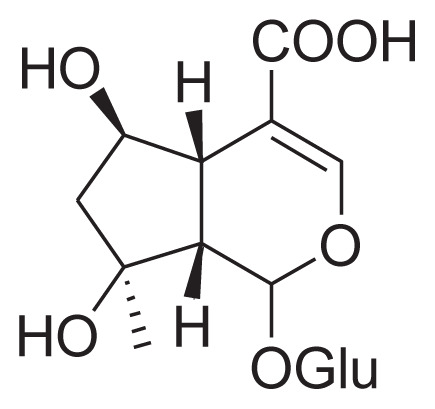
|
||
| 10 | Gardoside |
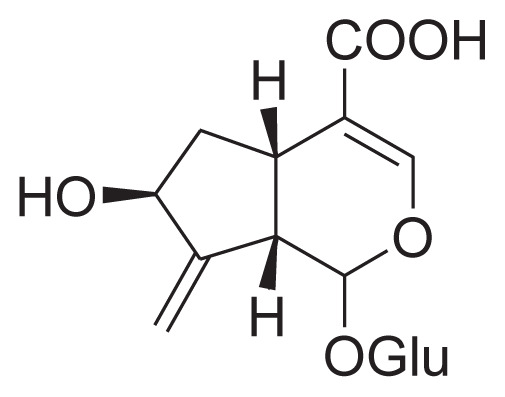
|
||
| 11 | 10-O-trans-Sinapoylgeniposide |
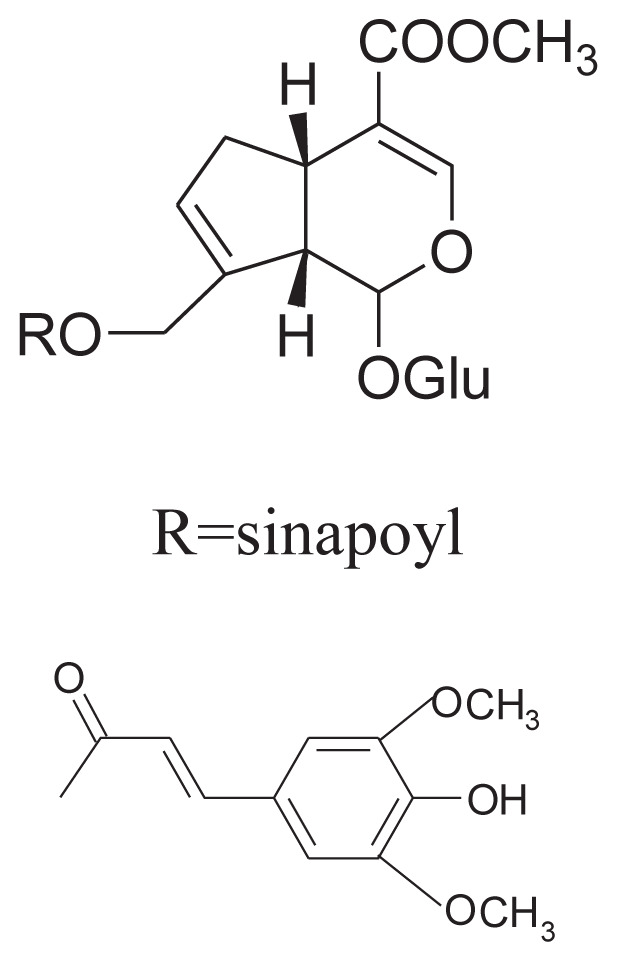
|
||
| 12 | 6″-O-trans-Sinapoylgenipin gentiobioside |
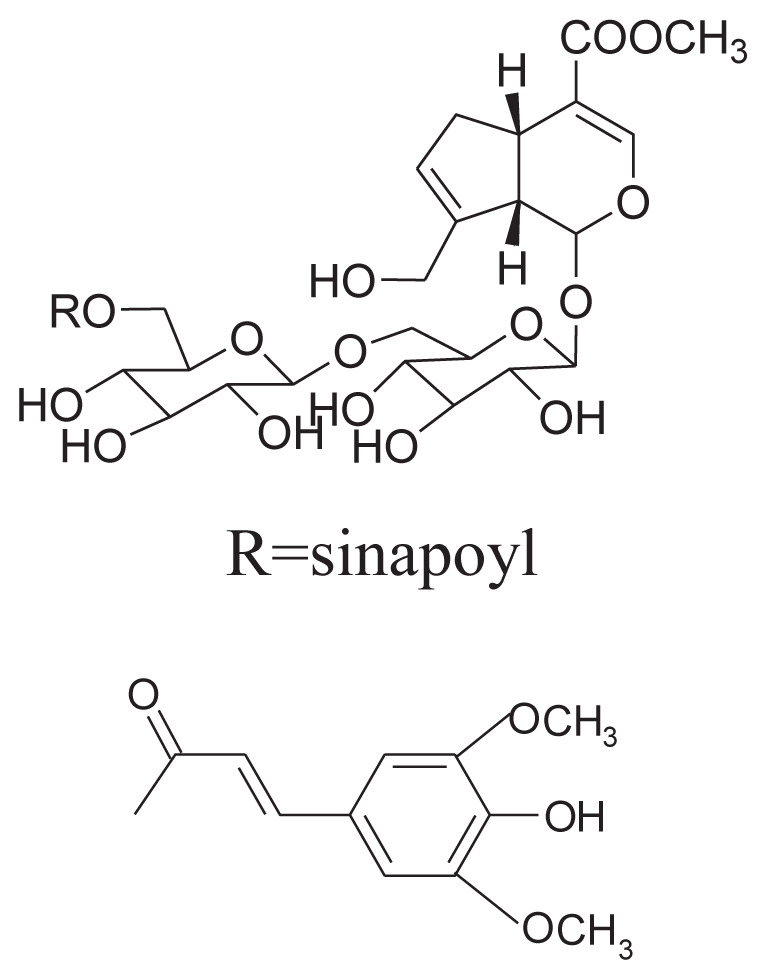
|
||
| 13 | 6″-O-trans-p-Coumaroylgenipin gentiobioside |
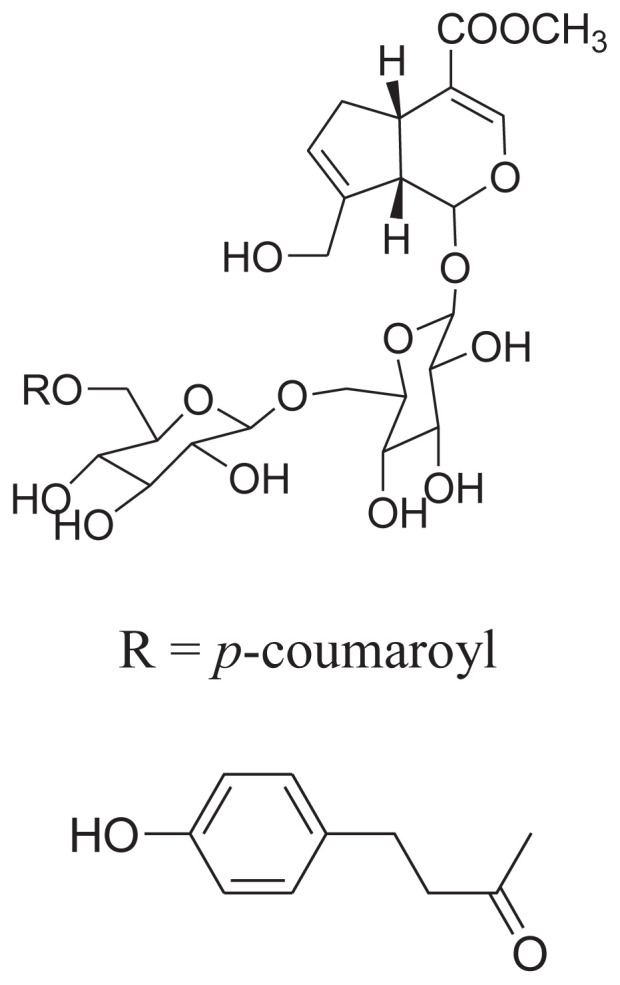
|
||
| 14 | 6′-O-Sinapoylgeniposide |
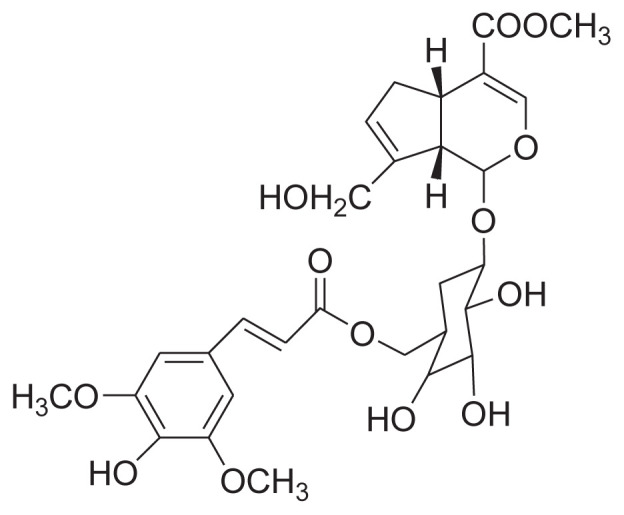
|
Reflux with 90%, 70% EtOH Silica gel & Sephadex column |
[34] |
| 15 | 6″-O-Caffeoylgenipin gentiobioside |
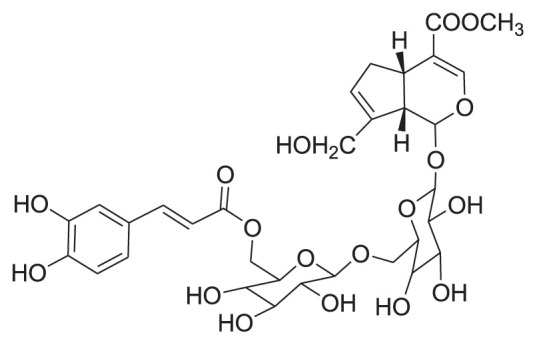
|
60% EtOH Silica gel, & preparative HPLC |
[35] |
| 16 | Genipin 1-O-β-d-apiofuranosyl (1→6)-β-d-Glucopyranoside |
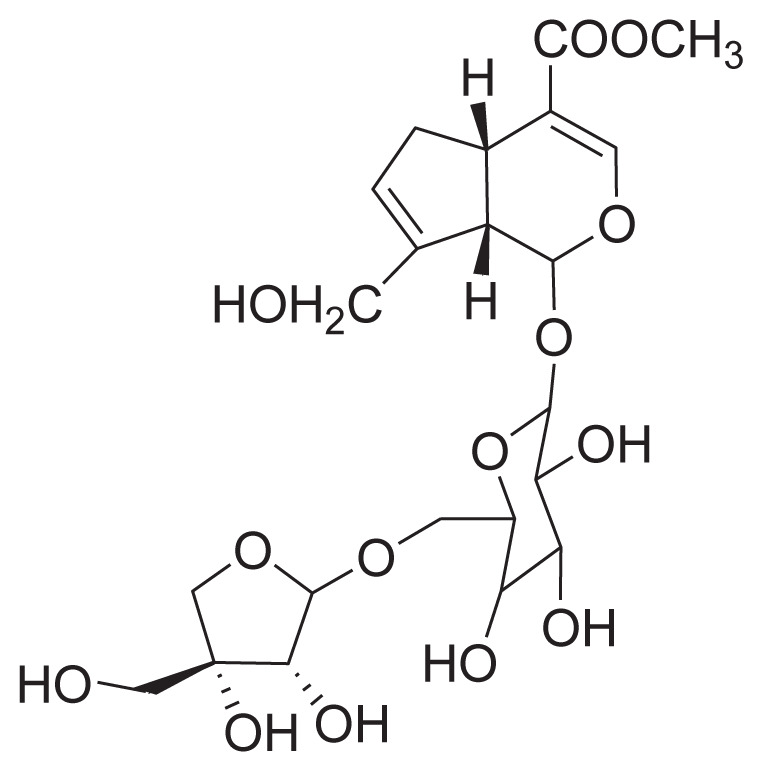
|
||
| 17 | Genipin 1-O-α-d-xylopyranosyl (1→6)-β-d-glucopyranoside |
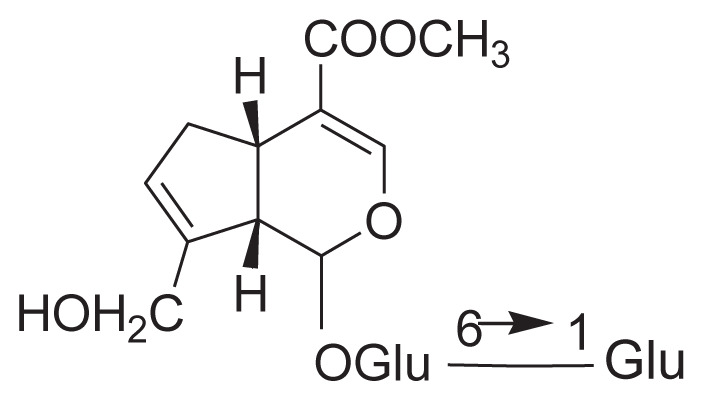
|
||
| 18 | 6β-Hydroxy genipin |
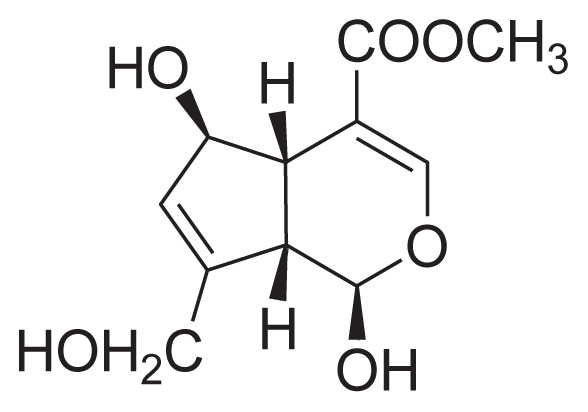
|
||
| 19 | Genipin |
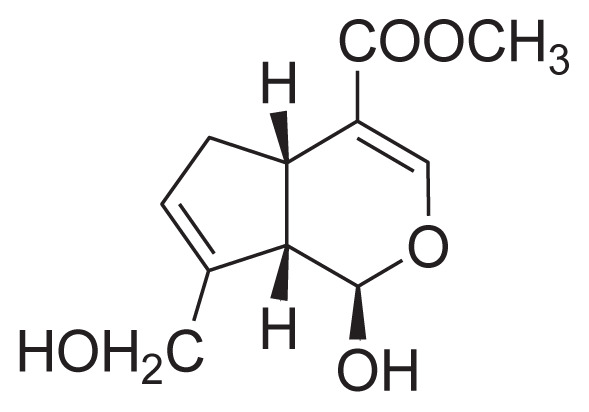
|
||
| 20 | Gardenoside |
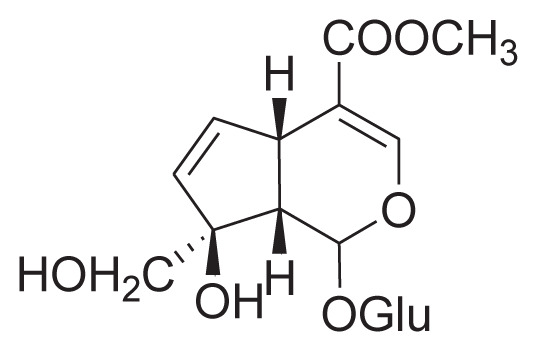
|
HPLC–MS | [28] |
| 21 | Deacetylasperulosidic acid methyl ester |
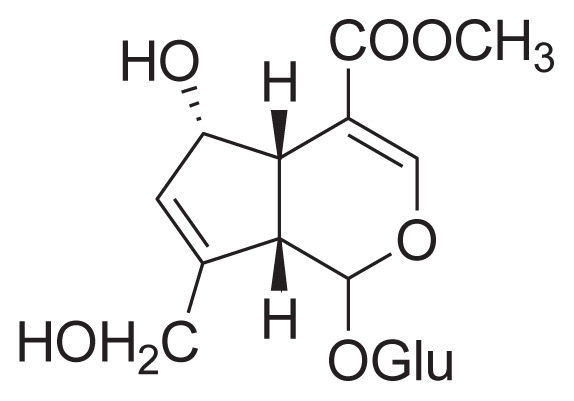
|
||
| 22 | Scandoside methyl ester |
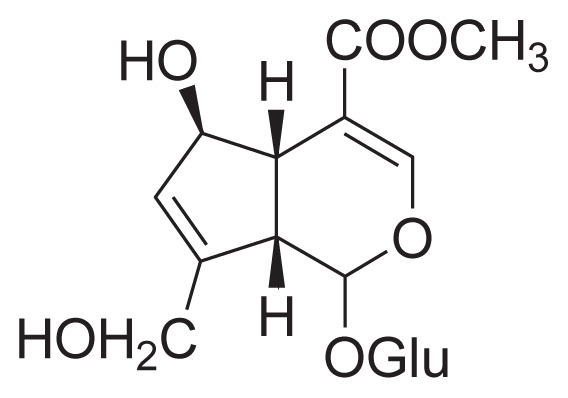
|
||
| 23 | 4″-O-[(E)-p-Coumaroyl] gentiobiosylgenipin |
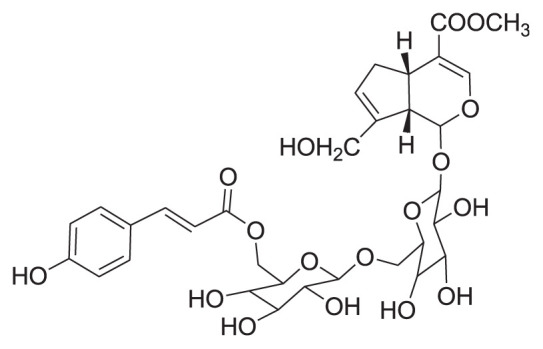
|
80% EtOH Silica gel column |
[36] |
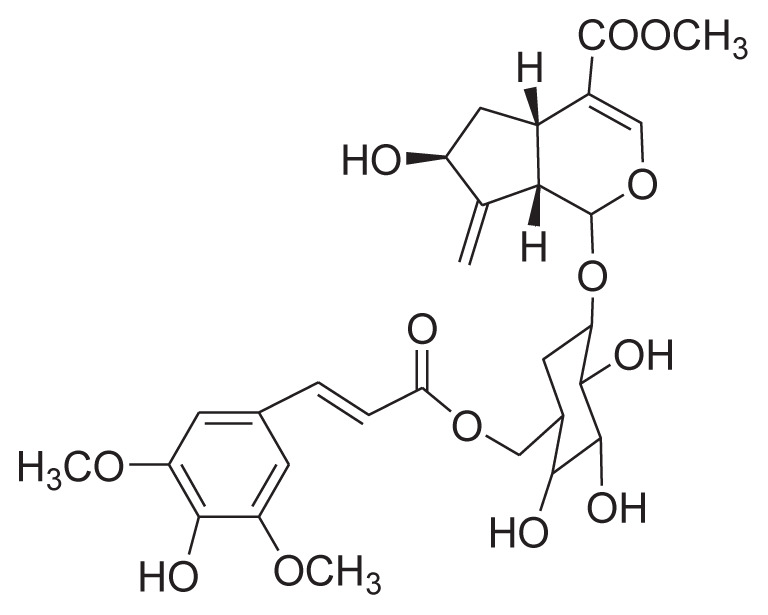
| 24 | 6′-O-[(E)-Sinapoyl]gardoside |
|
||
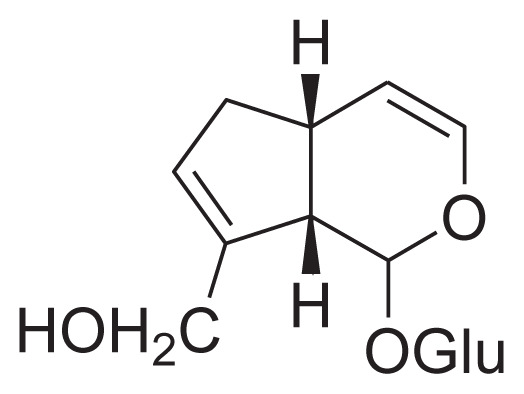
| 25 | Bartsioside |
|
||
| 26 | Gardenal-I |
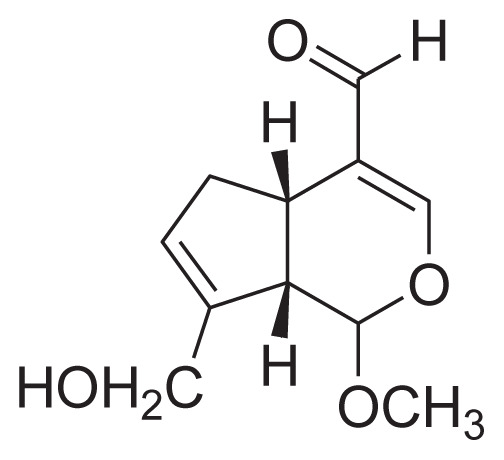
|
EtOH/H2O (9:1) at room temperature C18 column |
[37] |
| 27 | Gardenal-II |
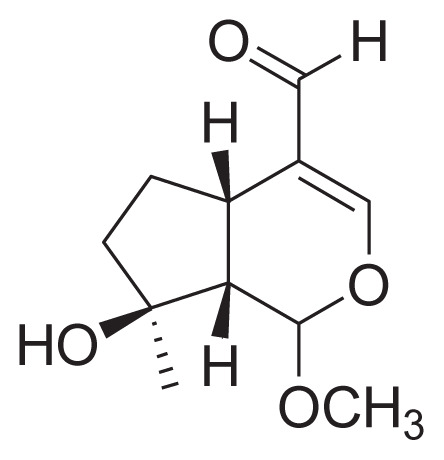
|
||
| 28 | Gardenal-III |
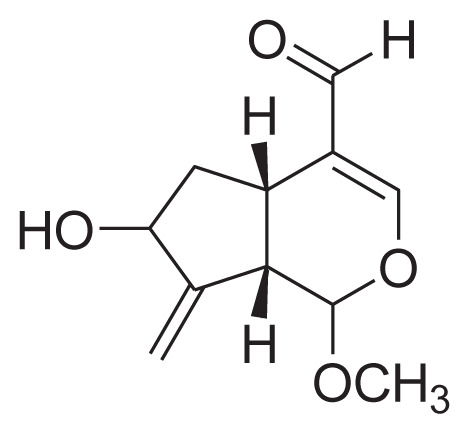
|
||
| 29 | Ixoroside |
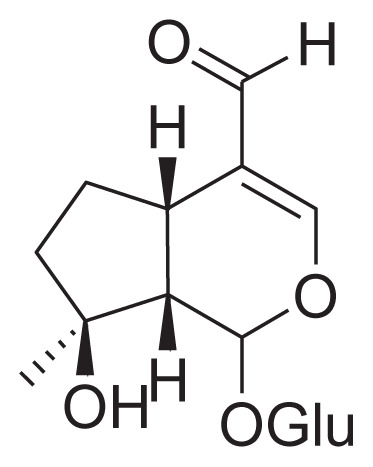
|
Reflux with 50% aqueous ethanol Macroporous Resin column |
[38] |
| 30 | (+)-(7S,8R,8′R)-Lyoniresinol 9-O-β-d-(6″-O-trans-sinapoyl) glucopyranoside |
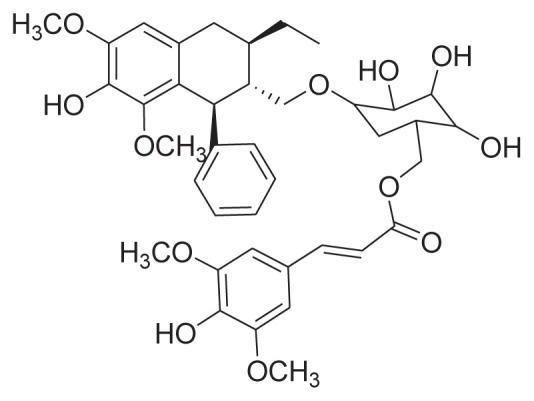
|
Reflux with 60% EtOH Prep-HPLC |
[27] |
| 31 | 10-O-trans-Sinpoylgeniposide |
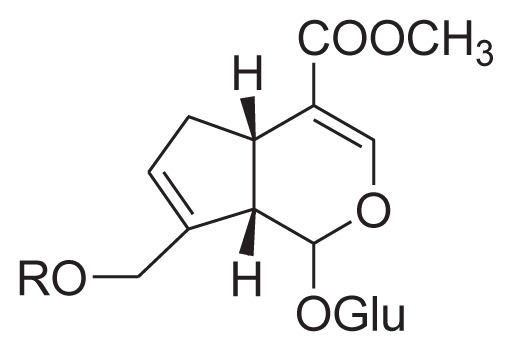 R = sinapoyl |
||
| 32 | Shanzhiside methyl ester (I) |
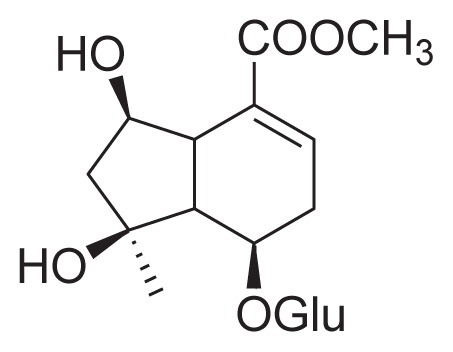
|
Reflux with 65% EtOH HSCCC |
[31] |
| 33 | Phloyoside (II) |
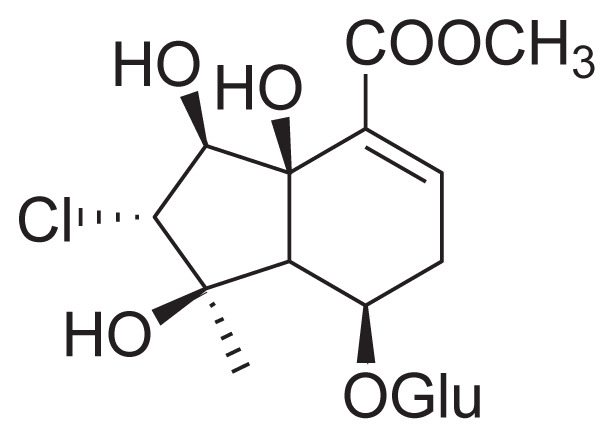
|
||
| 34 | Chlorotuberside (III) |
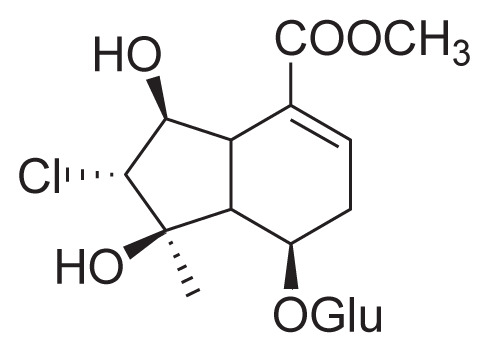
|
||
| 35 | Penstemonoside (IV) |
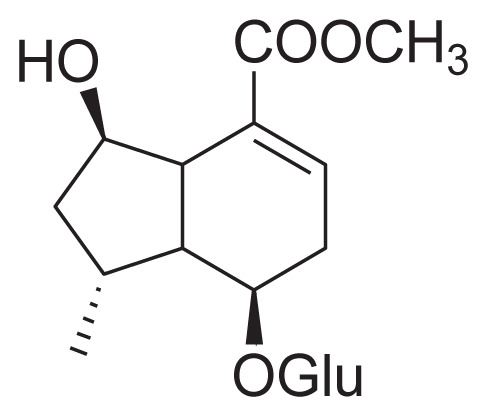
|
2.2.5. Solvent partition
As an example of solvent extraction, dried fruit (8.0 kg) of G. jasminoides was powdered and extracted with 60% ethanol solution (64 L) for 2 hours under reflux. The extraction process was repeated three times. The combined extract (1000 g) after concentration under vacuum was suspended in 3 L of water and partitioned with cyclohexane, ethyl acetate, and n-butanol, respectively [25]. The compounds in cyclohexane fraction were dominated with volatile oil and some fat-soluble pigments. The major components in ethyl acetate fraction were iridoids and iridoid glycosides. The compounds found in n-butanol fraction are mostly iridoid glycosides. The substances in the water fraction were glycosides and water-soluble pigments.
2.2.6. Column chromatography
The different extract fractions from the plant can be subjected to different kinds of column chromatography. Column chromatography has been classified as normal phase (silica gel) column chromatography, reversed-phase (C4, C8, C18, etc.) column chromatography, macroporous resin column chromatography, polyamide column chromatography, or Sephadex column chromatography, depending on the packing material used. The separation mechanism and the solvents used to elute the column are different for different packing materials. For example, the ethyl acetate fraction of G. jasminoides was subjected to silica gel column chromatography eluting with a gradient mixture of chloroform and methanol and yielded nine fractions, which were respectively subjected to different kinds of column and prep-HPLC when needed. The structures of the compounds were identified by nuclear magnetic resonance [25]. In another example, dried G. jasminoides (6.0 kg) were extracted by 95% ethanol/water (v/v), and the filtration was concentrated under reduced pressure. Then the extract (1.2 kg) was suspended in water and extracted with petroleum ether and chloroform. The chloroform fraction (210.3 g) was subjected to silica gel column chromatography eluting with petroleum ether–ethyl acetate mixture, and six fractions were obtained. The six fractions were respectively subjected to repeated silica gel column chromatography, C18 column chromatography, macroporous resin (D101) column, and prep-HPLC eluting with methanol–water (v/v 25:75). The obtained compounds were iridoids and iridoid glucosides [26]. In some cases, there was no solvent extraction prior to the classical column chromatography. For example, G. jasminoides (8.0 kg) was refluxed with 60% of aqueous ethanol. The extract was concentrated, suspended with water, and subjected to macroporous resin (D101) column eluted with an ethanol/water gradient. The 50% ethanol elute was subjected to silica gel eluting with chloroform/methanol. The fraction was further subjected to prep-HPLC, to obtain two new compounds—a new lignan glucoside and a new iridoid glucoside [27]. In another example, six iridoid glycosides were isolated and purified from G. jasminoides by two-dimensional prep-HPLC [28].
2.2.7. High-speed countercurrent chromatography
HSCCC is a liquid–liquid separation technology. It has been reported that HSCCC successfully isolated geniposide from G. jasminoides. Zhou et al [29] showed that 389 mg of geniposide was recovered from 1 g of partially purified sample from macroporous resin column. In another research, after HSCCC isolation, 151.1 mg of gardenoside, 52.2 mg of 6β-hydroxy geniposide, 24.5 mg of geniposidic acid, 587.2 mg of geniposide, 246.2 mg of crocin-1, 34.2 mg of crocin-2, 24.4 mg of crocin-3, and 24.7 mg of crocin-4 were extracted and isolated from 2 kg of G. jasminoides after macroporous resin (HPD-100) column separation and HSCCC with purities ranging from 91.7% to 98.9% [30]. Four new water-soluble iridoid glucosides were isolated by HSCCC. They are shanzhiside methyl ester (I), phloyoside (II), chlorotuberside (III), and penstemonoside (IV) [31].
2.2.8. Other isolation methods
It has been reported that water-soluble residue from G. jasminoides was treated with sodium carbonate and extracted with n-butanol several times. Then, the n-butanol extracts are treated with activated granular charcoal. At least 70 g of geniposide with 98% purity was obtained from 10 kg of G. jasminoides [32]. Another isolation method is matrix solid phase dispersion (MSPD); the optimal conditions that can isolate the geniposide by MSPD is 0.5 g of fruit of G. jasminoides, 0.75 g of Celite (diatomaceous earth) as the dispersing sorbent, and a volume of 25 mL of 70% of methanol solution as the elution solvent [33].
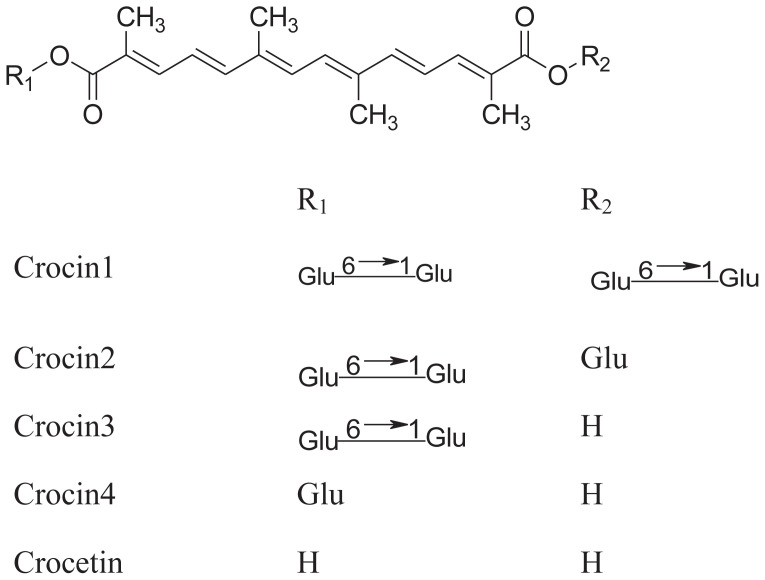
2.3. Crocin and its derivatives in G. jasminoides
Crocin and its derivatives extracted from G. jasminoides have been characterized as low toxicity, low allergy, and eco-friendly compared with saffron [3]. Crocin and crocetin were initially found in saffron, which was the dried stigma of the flower of Crocus sativus L. Saffron has a wide range of uses especially in the dye and pharmaceutical industry. It also has medicinal effects in certain conditions such as weight loss, sexual dysfunction, and premenstrual syndrome. These medicinal properties of saffron are likely attributable to a number of compounds it contains, including crocetin, crocins, and safrana [39].
2.3.1. Extraction and isolation of crocin and its derivatives
G. jasminoides was extracted using a homogenate extraction technology under the condition of 50% ethanol/water solution, with a liquid/material ratio of 15:1 (v/w), and particle size of material 1.7 mm with an extraction time of 41 seconds [40]. In another report, the extraction condition of G. jasminoides used the following conditions: ethanol/water (40:60, v/v) and cold percolation without damaging percolation [41]. By using the MAE system, the extraction yield of the edible yellow pigment from G. jasminoides was 50% higher than that obtained with the conventional extraction method [24].
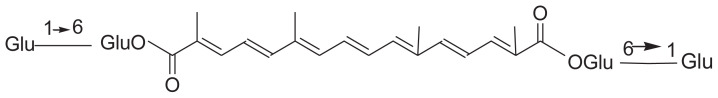
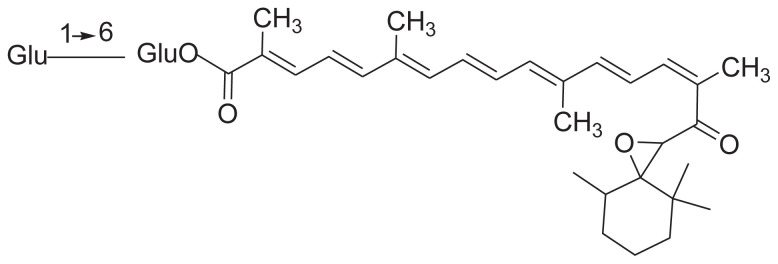
The isolation of crocin and its chain derivatives is similar to that of iridoids (Table 3). However, the polarity of solvents in the isolation of crocin is slightly higher than that in iridoids (Table 4). The extracts can be suspended in water, then partitioned with ethyl acetate. The ethyl acetate extract was successively subjected to silica gel column, Sephadex (LH-20) column, and medium pressure preparative liquid chromatography, and yielded gardecin. The water extract was successively subjected to macroporous resin (HPD-100), silica gel, and C18 column, and crocin was obtained [41].
Table 3.
Crocin and its chain derivatives in Gardenia jasminoides.
Table 4.
Isolation methods of crocin and its chain derivatives.
| Name | Extraction & isolation methods | Refs |
|---|---|---|
| Crocin1 | Temperature 25°C; Time: 1 h; solvent: methanol, ethanol, 1-propanol, 2-propanol | [3] |
| Temperature: 60°C; Time: 2 h; Solvent: water | [42] | |
| Temperature: 70.4°C; Time: 28.6 min; Solvent: 51.3% ethanol | [9] | |
| Crocin1, crocin 2, crocin 3, crocin 4 | Ethanol–water (40%) by cold percolation macroporous resin (HPD-100); HSCCC | [30] |
| Crocin1, crocin 2, crocin 3, crocin 4 | Ethanol–water (40%) cold percolation; silica gel & C18 column; macroporous resin (HPD-100) | [43] |
| Crocin1, crocin 2, crocin 3, crocin 4; cis-crocin1, gardecin | Ethanol/water (40:60); cold percolation; silica gel column; macroporous resin (HPD-100) | [41] |
| Crocetin | Ethanol–water (40%) cold percolation; silica gel; macroporous resin (HPD-100) | [43] |
| Crocin1, crocin 2, crocin 3, crocetin | Macroporous resin; C18 | [40] |
| Crocin 1, crocin 3, crocetin | 70% ethanol | [44] |
2.4. Terpenoids in G. jasminoides
Terpenoids in G. jasminoides include secoiridoids and monoterpenoid. Iridoids mentioned above belong to secoiridoids. Here, we summarize monoterpenoids and their glycosides. Some terpenoids, especially the small number of carbon atoms, exist in volatile oil. The extraction and isolation methods of terpenoids are mostly the same as in iridoids. In recent years, many terpenoids in G. jasminoides have been found. Their structures are listed in Table 5.
Table 5.
Structures of monoterpenoids in Gardenia jasminoides.
| No. | Name | Structures | Refs |
|---|---|---|---|
| 1 | 6′-O-trans-Sinapoyljasminoside C |
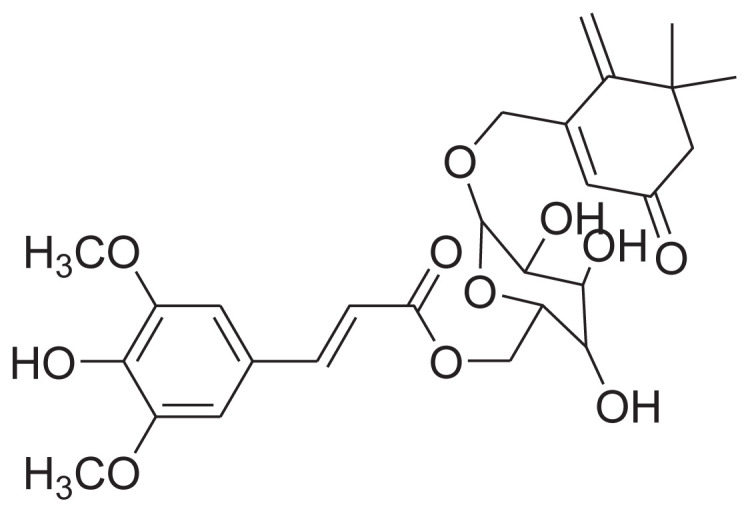
|
[25,27] |
| 2 | 6′-O-trans-Sinapoyljasminoside A |
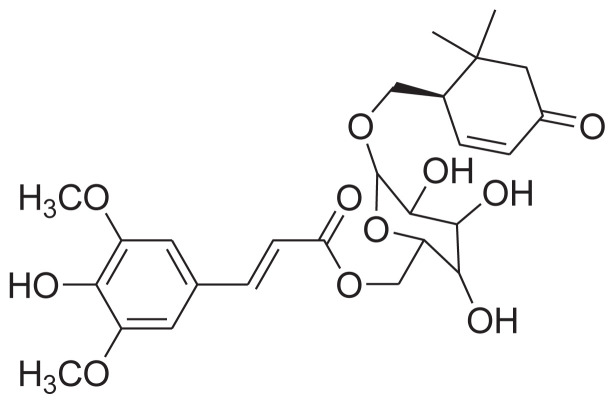
|
|
| 3 | Rehmapicrogenin |
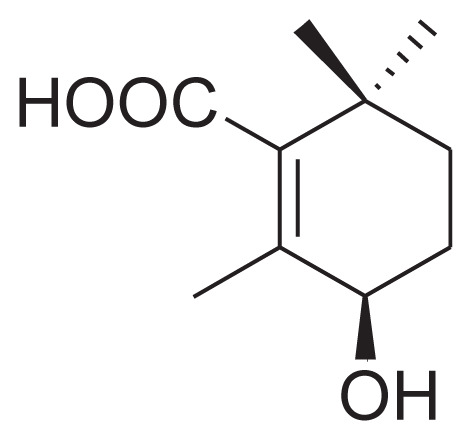
|
[25] |
| 4 | Jasminoside C |
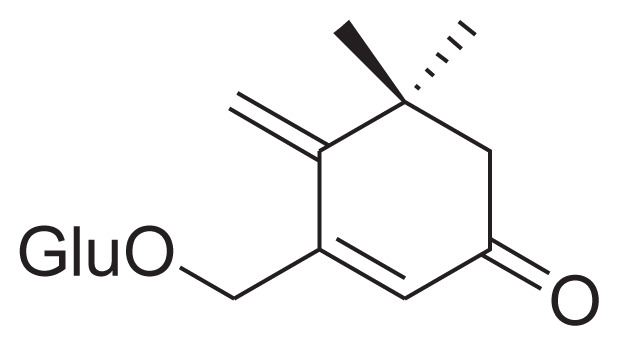
|
|
| 5 | Jasminoside B |
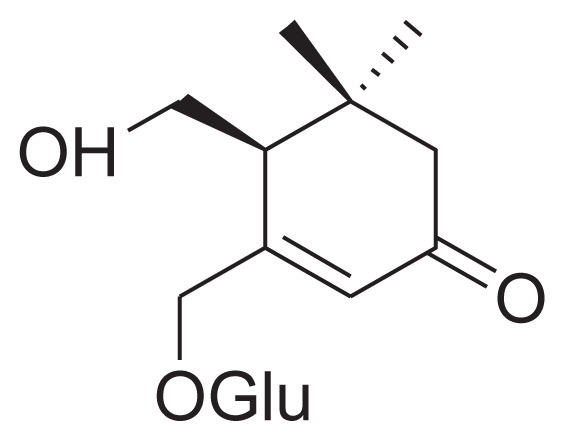
|
|
| 6 | Jasminoside G |
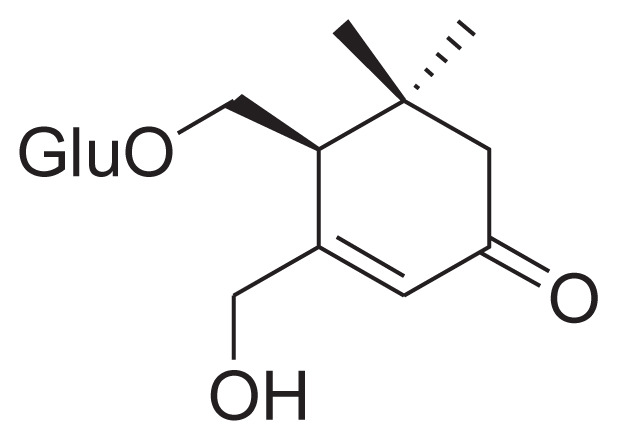
|
|
| 7 | Jasminoside K |
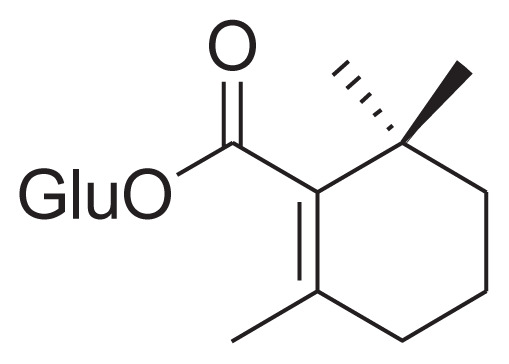
|
[25,44] |
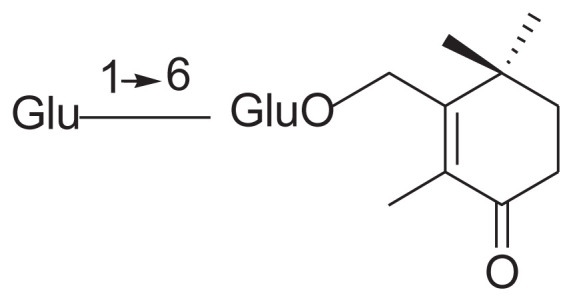
| 8 | Jasminoside I |
|
|
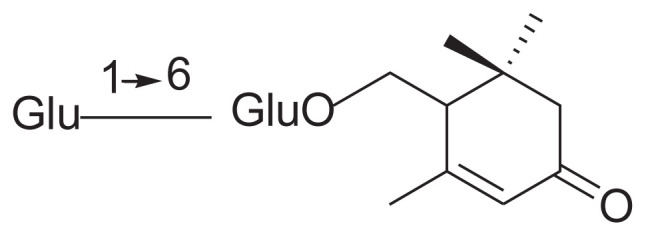
| 9 | Jasminoside H |
|
|
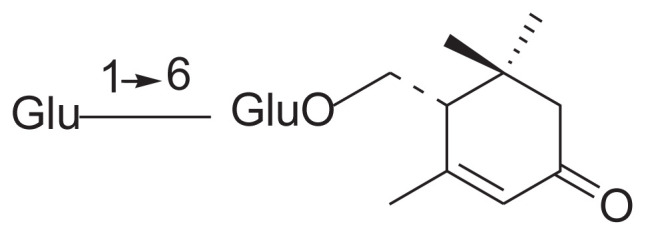
| 10 | Epi-jasminoside H |
|
|
| 11 | 6′-O-trans-Sinapoyljasminoside L |
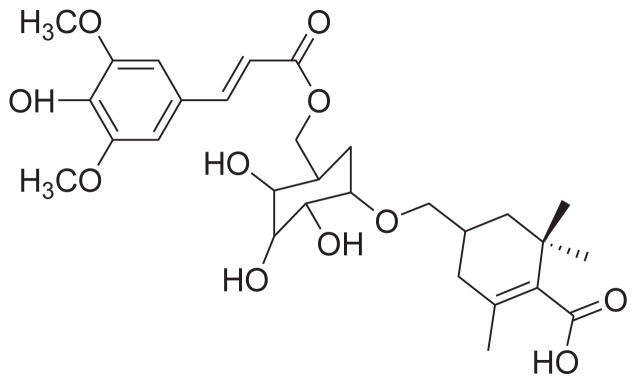
|
|
| 12 | Jasminoside S |
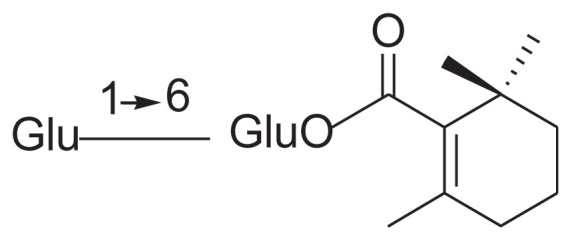
|
[35] |
| 13 | Jasminoside J |

|
[44] |
| 14 | 6′-O-trans-Sinapoyljasminoside B |
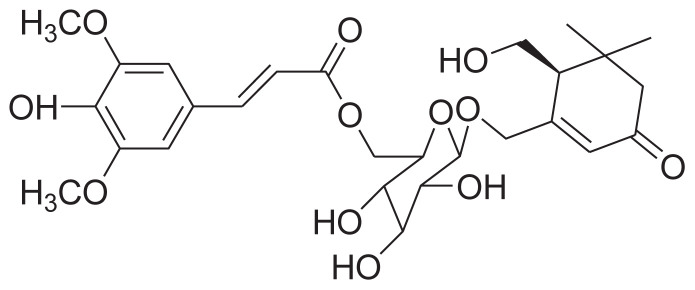
|
|
| 15 | 6′-O-trans-Sinapoyljasminoside L |
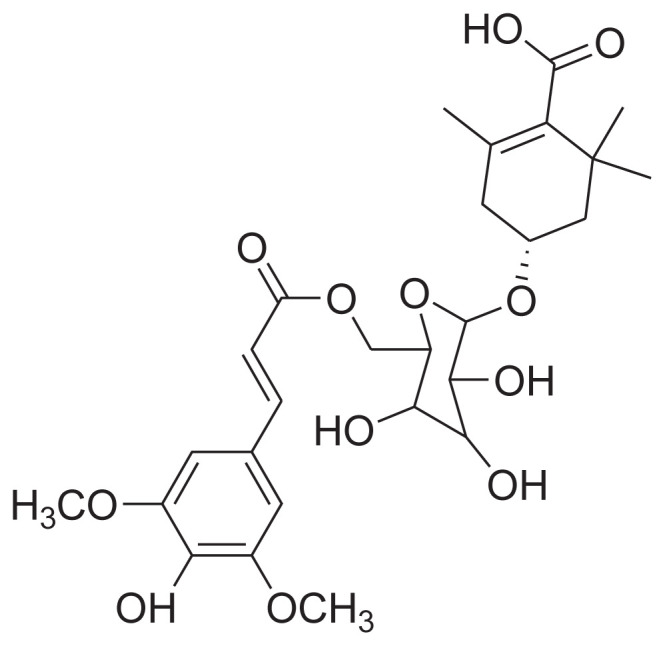
|
|
| 16 | Jasminoside M |
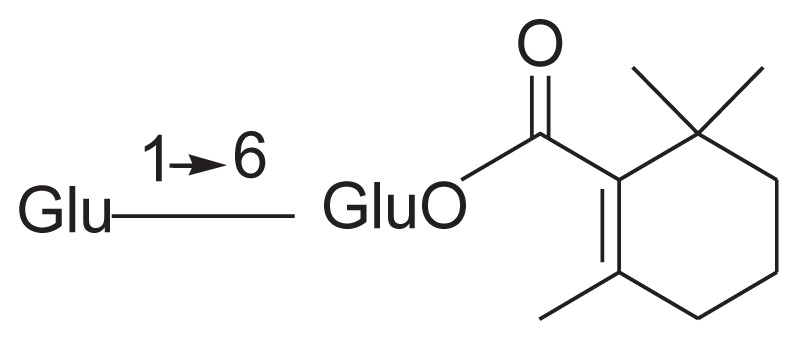
|
|
| 17 | Jasminoside N |
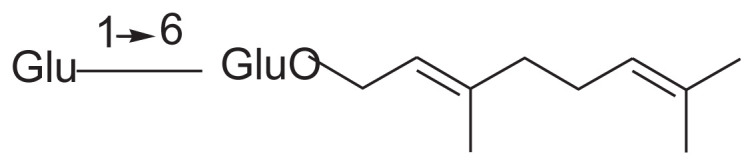
|
|
| 18 | 6-O-β-d-Xylopyranosyl-β-d-glucopyranosyl (2E)-3, 7-dimethylocta-2,6-dienoate |
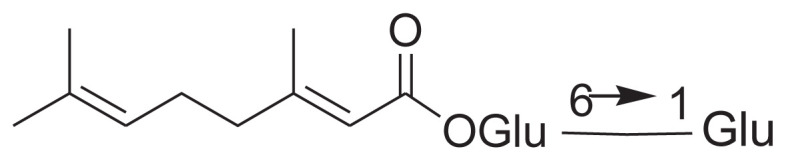
|
|
| 19 | 6-O-β-d-Glucopyranosyl-β-d-glucopyranosyl (2E)-3, 7-Dimethylocta-2,6-dienoate |
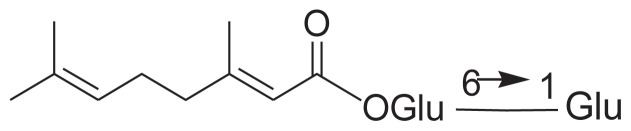
|
|
| 20 | Jasminoside C |
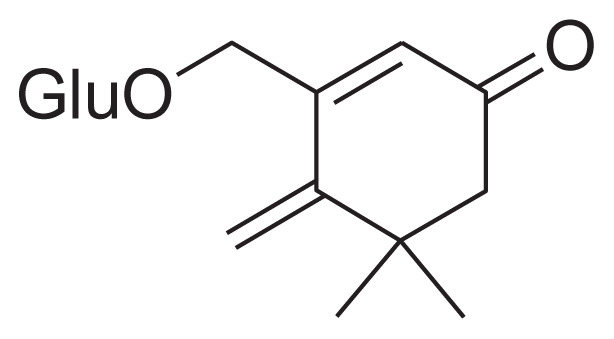
|
|
| 21 | Jasminoside E |
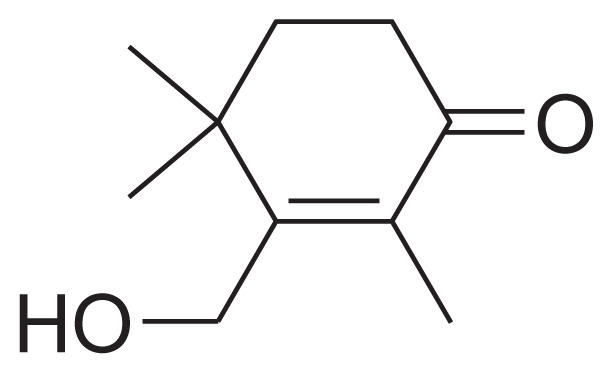
|
|
| 22 | Sacranoside B |
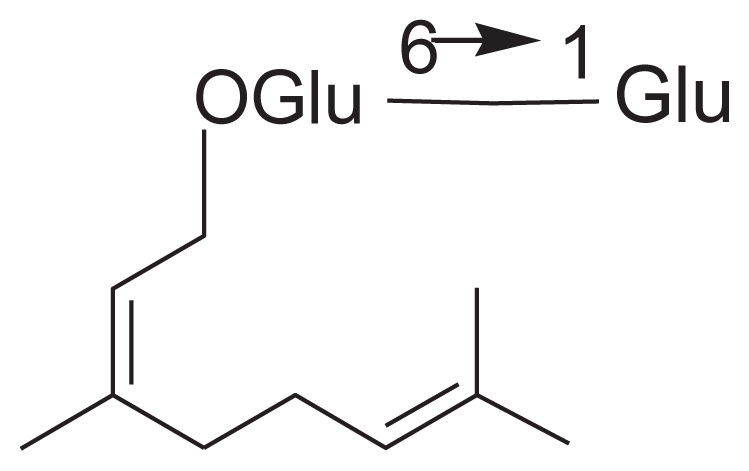
|
|
| 23 | Jasminodiol |
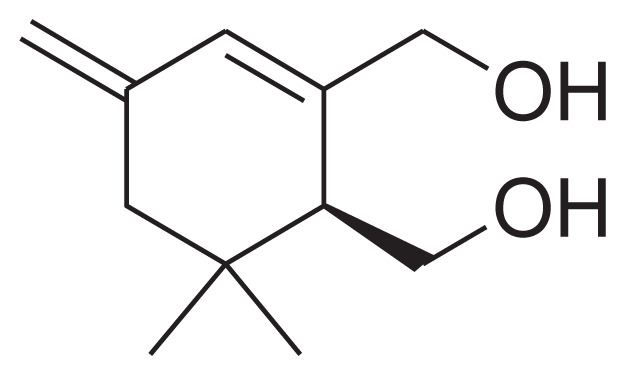
|
[45] |
| 24 | Jasminoside H |
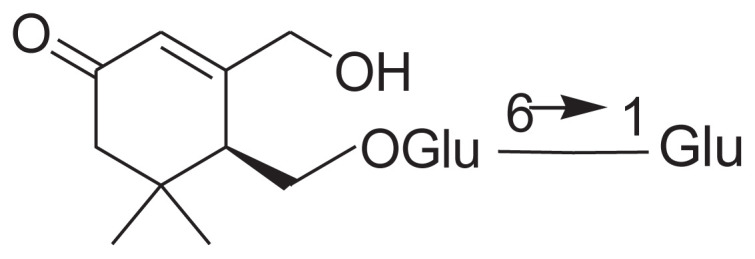
|
|
| 25 | 6′-O-Sinapoyljasminoside A |
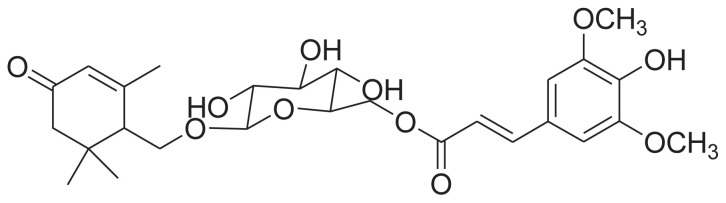
|
|
| 26 | 6′-O-Sinapoyljasminoside C |
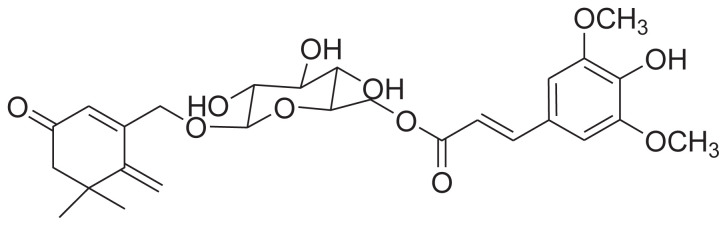
|
|
| 27 | Jasminoside I |
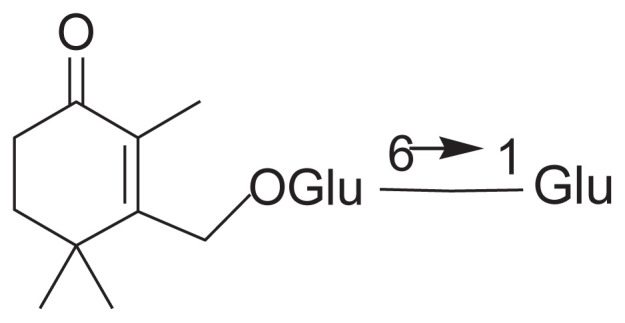
|
2.5. Phenolic compounds
Several phenolic acids have been identified in G. jasminoides, such as 3,5-di-O-caffeoyl-4-O-(3-hydroxy-3-methyl) glutaroylquinic acid, 3,5-di-O-caffeoylquinic acid, 4-O-sinapoyl-5-O-caffeoyl-quinic acid [27], and chlorogenic acid [46]. One new lignin glucoside, (+)-(7S,8R,8′R)-lyoniresinol 9-O-β-d-(6″-Otrans-sinapoyl)glucopyranoside, has been found in G. jasminoides. The extraction and isolation of the new lignin glucoside were as follows: G. jasminoides (8 kg) was cut into small pieces and refluxed with 60% of EtOH (v/v). The 60% EtOH was concentrated and produced a dark brown residue, which was suspended with H2O and subjected to column chromatography eluted with different proportions of EtOH and H2O. The 50% EtOH elute (105 g) was subjected to column chromatography (silica gel), eluting with increasing MeOH in CHCl3. Fraction G was further separated by column chromatography over octadecylsilyl (ODS), eluting with increasing MeOH in H2O to give G1–G7. G5 was purified by Toyopearl HW-40 and prep RP-HPLC to obtain the new lignin glucoside [27]. Its structure is shown in Figure 1.
Figure 1.
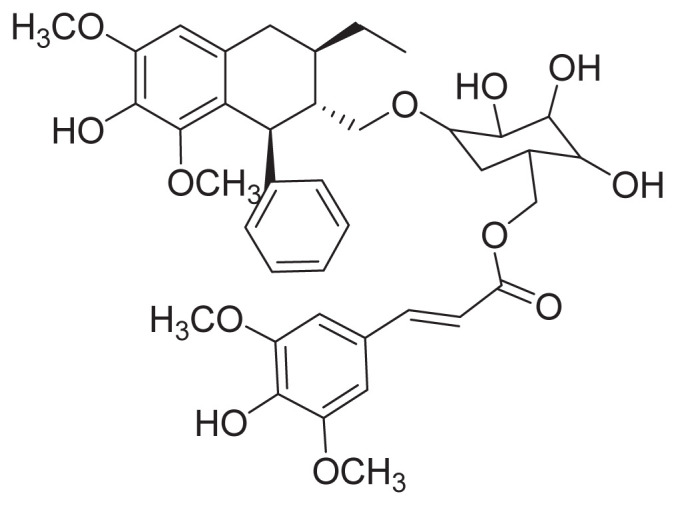
Chemical structure of (+)-(7S,8R,8′R)-lyoniresinol 9-O-β-d-(6″-O-trans-sinapoyl) glucopyranoside.
3. Biological activities
G. jasminoides has been reported to possess various biological activities and positive effects on human health, which are summarized in Table 6.
Table 6.
Different extracts from Gardenia jasminoides and their bioactivities.
| G. jasminoides | Bioactivities | Model | Proposed mechanism | Refs |
|---|---|---|---|---|
| Water extracts | Antioxidant activity | In vitro | [7] | |
| Improvement of insulin sensitivity | In vivo mouse | Exert a peroxisome proliferator activated receptor | [48] | |
| Anti-inflammation | In vitro | Reduce JNK1/2, & p38 MAPKs phosphorylation, & slightly reduce cyclooxygenase (COX)-2 expression in BV-2 cells | [53] | |
| Prevention of arteriosclerosis & thrombosis | In vivo | The hot water extracts of G. jasminoides did not stimulate the proliferation of cultured vascular smooth muscle cells | [60] | |
| Oil | Antidepressant activity | In vivo | [54] | |
| Ethanol extract | Antidepressant activity | In vivo | Associated with the elevated expression of brain-derived neurotrophic factor in the hippocampus | [57] |
| Antigastritic activity | In vivo | [65] | ||
| n-Butanol fraction | Antiangiogenic activity | In vivo | [61] | |
| Genipin | Reduce insulin resistance | In vivo | A close relationship with the improvement of hepatic oxidative stress, mitochondrial dysfunction & insulin signal impairment | [49] |
| Antidepressant activity | In vivo | Regulating the glycolysis/gluconeogenesis, TCA cycle & lipid metabolism of liver | [58] | |
| Protection of liver damage | In vivo | Antioxidative, antiapoptotic activities, & inhibition of NF-κB nuclear translocation & nuclear p-c-Jun expression | [19] | |
| Inhibit gastric lesions | In vivo | Was relevant with the antioxidant activities, acid-neutralizing capacities, & anti-Helicobacter pylori action | [65] | |
| Antithrombotic effect | In vivo | Inhibition of PLA2 activity | [18] | |
| Genotoxicity | In vitro | Damage of DNA in rec assay using V79 cells | [78] | |
| Geniposide | Antidiabetes | In vivo | Inhibited the adhesion of monocytes to HUVECs & the expression of CAMs induced by high glucose | [51] |
| Anti-inflammatory | In vivo | Reducing the expression of TLR4 by LPS | [16,55] | |
| Antiarthritis | In vivo | Downregulated the expression of p-JNK. | [68] | |
| In vivo | Decreased the expression level of TNF-α, IL-1, & IL-6, increasing the production of IL-10 & inhibiting the expression of phospho-p38 (pp38) related proteins in FLS | [69] | ||
| Antithrombotic & antiangiogenic | In vivo | Inhibited collagen-induced, but did not inhibit arachidonate-induced, mouse platelet aggregation | [62] | |
| Antidepressant activity | In vivo | Increased the levels of serotonin in striatum & hippocampus in mice | [17] | |
| Antidepressant activity | In vivo | Monoamine oxidase-B | [59] | |
| Effects on AD & PD | In vivo | Increased growth factor signaling & decreases apoptosis | [71] | |
| Crocin | Antioxidant | In vitro | [47] | |
| Anti-inflammatory | In vivo | Inhibited COX-1 & COX-2 enzymes | [56] | |
| Protective of the injured liver | In vivo | [66] [67] |
||
| Antihyperlipidemic | In vivo | Selectively inhibited the activity of pancreatic lipase | [73] | |
| Crocetin | Antihypertensive & antithrombotic effects | In vivo | Related to the increase in bioavailable NO | [63] [64] |
| Prevent insulin resistance | In vivo | [52] | ||
| Inhibit retinal damage | In vivo | Inhibited increase in caspase-3 & -9 activities | [76] | |
| Alleviate renal dysfunction | In vivo | [76] | ||
| Improve the quality of sleep | In vivo adult men | [8] |
AD = Alzheimer’s disease; CAMs = cell adhesion molecules; FLS = Fixed lag; HUVECs = human umbilical vein endothelial cells; IL = interleukin; JNK1/2 = c-Jun N-terminal protein kinase 1 and 2; LPS = lipopolysaccharide; MAPK = mitogen-activated protein kinase; NF-κB = nuclear factor-kappa B; NO = nitric oxide; PD = Parkinson’s disease; PLA2 = phospholipase A2; TCA = tricarboxylic acid; TLR4 = Toll-like receptor 4; TNFα = tumor necrosis factor alpha.
3.1. Antioxidant activity
Both water and ethanol extracts from the fruit of G. jasminoides had been found to exert antioxidant activity. The IC50 (half-maximal inhibitory concentration) values of the water extracts from G. jasminoides for DPPH (1,1-diphenyl-2-picrylhydrazyl), ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt], hydroxyl, and superoxide radical-scavenging activities were 0.14 mg/mL, 0.21 mg/mL, 1.08 mg/mL, and 1.43 mg/mL, respectively, and those of ethanol extracts were 0.36 mg/mL, 0.39 mg/mL, 1.56 mg/mL, and 1.99 mg/mL, respectively. Hence, water extract had a higher antioxidant activity than the ethanol extract. Purified crocin at concentrations of up to 40 ppm showed potent antioxidative activity, which was evaluated using the thiocyanate method and the thiobarbituric acid method. At a concentration of 20 ppm, crocin’s antioxidative activity was comparable to that of butylated hydroxyanisole (BHA) [47].
3.2. Improving insulin sensitivity and antidiabetes
Insulin resistance leads to type 2 diabetes. Water extracts from G. Jasminoides improve insulin sensitivity in steroid-induced insulin-resistant rats with an optimal dose of G. jasminoides water extract of 200 mg/kg [48]. Genipin ameliorated age-related insulin resistance, which had a close relationship with the improvement of hepatic oxidative stress and mitochondria dysfunction and insulin signal impairment [49]. Geniposide alleviated abnormal glucose tolerance and hyperinsulinemia, which are recognized in genetic type 2 diabetes patients caused by visceral fat accumulation [50] Geniposide (200 mg/kg and 400mg/kg) was shown to be an effective hypoglycemic agent in diabetic mice that significantly decreased blood glucose, insulin, and triglyceride levels in diabeticmice in a dose-dependent manner [4]. Geniposide also demonstrated beneficial effects on diabetic vascular injury by inhibiting the adhesion of monocytes to human umbilical vein endothelial cells and the expression of cell adhesion molecules induced by high glucose [51]. It was suggested that crocetin might prevent dexamethasone-induced insulin resistance by lowering serum insulin, free fatty acids, and blood triglyceride [52].
3.3. Anti-inflammatory activity
Water extracts of G. jasminoides exhibited anti-inflammatory properties by significantly reducing JNK2/1 (c-Jun N-terminal protein kinase) and p38 MAPKs (mitogen-activated protein kinase) phosphorylation, and decreasing COX-2 (cyclooxygenase-2) expression in LPS-induced BV-2 cells. When the water extracts of G. jasminoides was used on LPS-induced hepatic injury of rats, the liver pathology was substantially reduced [53]. Geniposide was shown to alleviate inflammation by suppressing MeCP2 (methyl cytosine binding protein-2) in mice with CCl4-induced acute liver injury and LPS-treated THP-1 cells [54]. Geniposide had an anti-inflammatory effect by reducing the expression of Toll-like receptor 4 upregulated by LPS, which inactivated the downstream NF-κB (nuclear factor-κB) and MAPK signaling pathways. Geniposide may be a potential anti-inflammatory drug to treat acute liver injury, acute lung injury, and mastitis [55]. Crocin could inhibit COX-1 and COX-2 activities, and production of prostaglandin E2, and inhibited xylene-induced ear edema inmice and carrageenan-induced paw edema in rats [56].
3.4. Antidepressant activity
The oil extracted from G. jasminoides by supercritical fluid extraction and geniposide showed antidepressant activity, which was evaluated by tail suspension test and forced swim test [6]. G. jasminoides showed an antidepressant effect in the tail suspension test at 24 hours [57]. Genipin play an antidepressant role through regulating the glycolysis/gluconeogenesis TCA cycle and lipid metabolism of liver [58]. The mechanism of antidepressive in geniposide may be linked to the increase of serotonin level in the striatum and hippocampus of mice and monoamine oxidase B [17,59].
3.5. Effects of blood circulation
The hot water extracts of G. jasminoides did not stimulate the proliferation of cultured vascular smooth muscle cells, but selectively stimulated the endothelial cell proliferation, which might prevent arteriosclerosis and thrombosis [60].The n-butanol fraction of 70% ethanol extract of G. jasminoides showed effective antiangiogenic activity by using CAM (chick chorioallantoic membrane) assay [61]. Geniposide was also an active antiangiogenic agent that inhibited the growth of the transformed N1H3T3 cell line in the range of 25–100 μM [62]. Both genipin and geniposide had antithrombotic effect in vivo via the suppression of platelet aggregation by inhibiting of PLA2 activity, which is different from that of aspirin (which exerts its effect by inhibiting arachidonate-induced platelet aggregation) [18].
Crocetin (25 mg/kg/d and 50mg/kg/d, 3 weeks) significantly moderated the systolic blood pressure, decreasing thrombogenesis and increasing antioxidant activity and urinary nitric oxide (NO) metabolite levels. It was concluded that crocetin had antihypertensive and antithrombotic effects, which might be related to the increase in bioavailable NO [63]. Because crocetin can increase endothelial NO synthase activity, it was believed to have an antihypercholesterolemic effect [64].
3.6. Other biological activities
3.6.1. Antigastritic activity
G. jasminoides-ethanolic extracts had a protective effect against potential gastric diseases. This action may attributable to the ursolic acid and genipin in G. jasminoides-ethanolic extracts, which inhibit AGS and SUN638 gastric cancer cells [65].
3.6.2. Effects on liver
Genipin (25 mg/kg, 50 mg/kg, 100 mg/kg, and 200 mg/kg) was injected on mice 1 hour prior to d-galactosamine N (GalN) (700mg/kg)/LPS (10 μg/kg) administration. The death rate in the genipin ingestion group was significantly reduced. Therefore, genipin offered remarkable hepatoprotection against damage induced by GalN/LPS because of its antioxidative and anti-apoptotic activities, and inhibition of NF-κB nuclear translocation and nuclear p-c-Jun expression [19]. The level of malondialdehyde decreased remarkably when diazinon and 25 mg crocin were used in male Wistar rats (n = 6) for 4 weeks, which led to the conclusion that crocinmight reduce diazinon-induced hepatotoxicity [66]. In another study, male Wistar rats were given a control diet, high fat diet, or high fat diet plus crocin (25mg/kg/d, 50mg/kg/d, and 100mg/kg/d) for 4 weeks. In the crocin treatment group, it was shown to reduce liver injury caused by hepatic steatosis in rats fed with high fat diet [67].
3.6.3. Antiarthritis
Geniposide had positive effects on rats with adjuvant arthritis by downregulating the expression of p-JNK (phosphorylated c-Jun N-terminal kinases) [68]. Geniposide also suppressed arthritis in adjuvant-induced arthritis rats by decreasing the expression levels of tumor necrosis factor-α, interleukin (IL)-1β, and IL-6, increasing the production of IL-10, and inhibiting the expression of phospho-p38 (p-p38) related proteins in FLS (Fixed lag) [69].
3.6.4. AD and PD
Geniposide and its derivatives, which improved the short-term memory capacity at varying extent, might be a potential drug for AD [70]. Geniposide exhibits neuroprotective property by inhibiting alpha-synuclein expression, a common pathological symptom in Parkinson’s disease (PD) [79]. Geniposide has a neuroprotective effect in the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of PD [71].
3.6.5. Antihyperlipidemia
Crocin obtained from the water extracts of G. jasminoides was found to exhibit antihyperlipidemic effect. When crocin and crocetin were given to the tested mice, the triglyceride and total cholesterol were significantly decreased, and the high-density lipoprotein-cholesterol levels were significantly increased. Thus, it can be concluded that crocin has antihyperlipidemic effect [72]. The mechanism of crocin’s hypolipidemic effect in rats was that crocin could selectively inhibit the activity of pancreatic lipase [73]. The hypolipidemic effect of crocin fermented with lactic acid bacteria can be improved [74].
3.6.6. Inhibition of retinal damage
Crocetin (100 mg/kg, p.o.) alleviated retinal damage though their antioxidant properties and by downregulating caspase-3 and -9 activities after retinal damage [75,76].
3.6.7. Improvement of the quality of sleep
Crocetin was used on healthy adult men with mild sleep complaints by reducing the number of wakening episodes compared to that of the placebo (p = 0.025), which potentially improved the quality of sleep [8].
3.6.8. Renal dysfunction
Crocetin (50 mg/kg) at 2 hours after resuscitation significantly alleviated renal dysfunction caused by hemorrhage shock and resuscitation [77].
3.6.9. Genotoxicity
“Gardenia yellow,” containing crocetin, crocin geniposide, and genipin, was a natural colorant extracted by ethanol from G. jasminoides. It was reported that genipin possesses genotoxicity. Genipin caused damage of DNA in rec assay [78].
4. Conclusion
G. jasminoides is commonly used as herbal medicine in Asia. This review summarized the extraction and isolation methods of four major constituents in G. jasminoides—genipin, geniposide, crocin, and crocetin—and discussed their bioactivities. The main extraction methods of G. jasminoides are solvent extraction method, and ultrasound- and microwave-assisted extraction. The isolation methods of G. Jasminoides are solvent partition, column chromatography, and HSCCC. Genipin reduces insulin resistance, demonstrates antidepressive and antithrombotic effects, provides protection against liver damage, and inhibits gastric lesions. Geniposide possesses antidiabetes, anti-inflammatory, antiarthritis, antithrombotic, and antiangiogenic properties, demonstrates antidepressant activity, and has beneficial effects on AD and PD. Crocin has many medicinal effects such as antioxidant and anti-inflammatory activities, is antihyperlipidemic, and is protective of the injured liver. Crocetin has antihypertensive and antithrombotic effects, prevents insulin resistance, inhibits retinal damage, alleviates renal dysfunction, and improves sleep quality. Most importantly, oil extract from G. jasminoides demonstrates antidepressant activity. It is hoped that, in the near future, oil from G. jasminoides could be developed as a kind of therapeutic agent in fighting depression.
Acknowledgments
This work was supported by Enterprise project for young teachers in Hubei Province Education Department (XD2014310) and Program of studying abroad for young teachers in Hubei Province Education Department.
Funding Statement
This work was supported by Enterprise project for young teachers in Hubei Province Education Department (XD2014310) and Program of studying abroad for young teachers in Hubei Province Education Department.
Footnotes
Conflicts of interest
We declare that there is no conflict of interest with any parties involved in this article.
REFERENCES
- 1.Gilam EF. Dissertation. Gainesville, Florida, USA: University of Florida; 1999. Gardenia jasminoides. [Google Scholar]
- 2. Zhou Y, Zhang J, Tang R, Zhang J. Simultaneous dyeing and functionalization of silk with three natural yellow dyes. Indus Crops Prod. 2015;64:224–32. [Google Scholar]
- 3. Hong IK, Jeon H, Lee SB. Extraction of natural dye from Gardenia and chromaticity analysis according to chi parameter. J Ind Eng Chem. 2015;24:326–32. [Google Scholar]
- 4. Wu S, Wang G, Liu Z, Rao J, Lv L, Xu W, Wu S, Zhang J. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol Sin. 2009;30:202–8. doi: 10.1038/aps.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lim H, Park KR, Lee DU, Kim YS, Kim HP. Effects of the constituents of Gardenia Fructus on prostaglandin and NO reduction. Biomol Ther. 2008;16:82–6. [Google Scholar]
- 6. Tao W, Zhang H, Xue W, Ren L, Xia B, Zhou X, Wu H, Duan J, Chen G. Optimization of supercritical fluid extraction of oil from the Gardenia jasminoides and its antidepressant activity. Molecules. 2014;19:19350–60. doi: 10.3390/molecules191219350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Debnath T, Park PJ, Nath NCD, Samad NB, Park HW, Lim BO. Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chem. 2011;128:697–703. [Google Scholar]
- 8. Kuratsune H, Umigai N, Takeno R, Kajimoto Y, Nakano T. Effect of crocetin from Gardenia jasminoides Ellis on sleep: a pilot study. Phytomedicine. 2010;17:840–3. doi: 10.1016/j.phymed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 9. Yang B, Liu X, Gao Y. Extraction optimization of bioactive compounds (crocin, geniposide and total phenolic compounds) from Gardenia (Gardenia jasminoides Ellis) fruits with response surface methodology. Innov Food Sci Emerg Technol. 2009;10:610–5. [Google Scholar]
- 10. Liu H, Yao L, Chen J, Gu X, Ma Y, Chen Y, Li P, Zhang C. Analysis of volatile ingredients in Gardeniae Fructus and its processed products by GC-MS. J Tradit Chin Med. 2015;40:1732–7. [PubMed] [Google Scholar]
- 11. He W, Gao Y, Yuan F, Bao Y, Liu F, Dong J. Optimization of supercritical carbon dioxide extraction of Gardenia fruit oil and the analysis of functional components. J Am Oil Chem Soc. 2010;87:1071–9. [Google Scholar]
- 12. Bao Y, Dong J, Yuan F. Effect of supercritical carbon dioxide extraction conditions on fatty acid composition and antioxidant activity of Gardenia fruit oil. Food Sci. 2011;32:12–7. [Google Scholar]
- 13. Li B, Liu Y, Yuan B. Study of supercritical CO2 extraction technology on Gardenia oil. Med Theory Pract. 2008;6:232–3. [Google Scholar]
- 14.Li H, Wang F, Liu H, Wang Z, Yu W, Wang K, Han S. Proceedings of The Third Henan Province Conference on Chemistry. Pingdingshan, Henan Province; China: 2015. Chemical characteristics and compositions of gardenia fruit oils by using various extraction methods; pp. 1–5. [Google Scholar]
- 15. Yang J, Xu S, Hu L. Study on ultrasound-assisted extraction of gardenia jasminoides seed oil and its analysis of fatty acid composition. Food Sci. 2008;29:246–9. [Google Scholar]
- 16. Fu Y, Liu B, Liu J, Liu Z, Liang D, Li F, Li D, Cao Y, Zhang X, Zhang N, Yang Z. Geniposide, from Gardenia jasminoides Ellis, inhibits the inflammatory response in the primary mouse macrophages and mouse models. Int Immunopharmacol. 2012;14:792–8. doi: 10.1016/j.intimp.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 17. Liu S, Lin Y, Huang T, Huang S, Peng WH. Anti-depressive activity of Gardeniae fructus and geniposide in mouse models of depression. Afr J Pharm Pharmacol. 2011;5:1580–8. [Google Scholar]
- 18. Suzuki Y, Konda K, lkeda Y, Umemura K. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. 2001;67:807–10. doi: 10.1055/s-2001-18842. [DOI] [PubMed] [Google Scholar]
- 19. Kim SJ, Kim JK, Lee DU, Kwak JH, Lee SM. Genipin protects lipopolysaccharide-induced apoptotic liver damage in d-galactosamine-sensitized mice. Eur J Pharmacol. 2010;63:188–93. doi: 10.1016/j.ejphar.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 20. Zhao S, Yang Y, Liang D. Quantitative analysis of genioside in fructus gardenia and its different processed products. Chin J Chin Mater Med. 1994;19:601–2. [PubMed] [Google Scholar]
- 21. Lee EJ, Hong JK, Whang WK. Simultaneous determination of bioactive marker compounds from Gardeniae fructus by high performance liquid chromatography. Arch Pharm Res. 2014;37:992–1000. doi: 10.1007/s12272-013-0293-1. [DOI] [PubMed] [Google Scholar]
- 22. Jhansi LB, Jaganmohan RK. Phytochemical studies of Gardenia jasminoides. Int J BioSci Technol. 2012;5:54–8. [Google Scholar]
- 23. Wang X, Wu Y, Dai S, Chen R, Shao Y. Ultrasound-assisted extraction of geniposide from Gardenia jasminoides. Ultrasonics Sonochem. 2012;19:1155–9. doi: 10.1016/j.ultsonch.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 24. Jun SJ, Chun JK. Design of U-column microwave-assisted extraction system and its application to pigment extraction from food. Food Bioprod Proc. 1998;76:231–6. [Google Scholar]
- 25. Yang L, Peng K, Zhao S, Chen L, Qiu F. Monoterpenoids from the fruit of Gardenia jasminoides Ellis (Rubiaceae) Biochem Syst Ecol. 2013;50:435–7. [Google Scholar]
- 26. Jia L, Wang R, Yan P, Qi H. Iridoids from the flowers of Gardenia jasminoides Ellis and their chemotaxonomic significance. Biochem Syst Ecol. 2014;56:267–70. [Google Scholar]
- 27. Yang Y, Xiao L, Gao H, Xie Z, Dai Y, Xiao J, Kurihara H, Ye W, Zhong Y, Xin S. Chemical constituents from the fruits of Gardenia jasminoides Ellis. Fitoterapia. 2012;83:563–7. doi: 10.1016/j.fitote.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 28. Zhou T, Zhao W, Fan G, Chai Y, Wu Y. Isolation and purification of iridoid glycosides from Gardenia jasminoides Ellis by isocratic reversed-phase two-dimensional preparative high-performance liquid chromatography with column switch technology. J Chromatogr B. 2007;858:296–301. doi: 10.1016/j.jchromb.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 29. Zhou T, Fan GR, Hong Z, Chai Y, Wu Y. Large-scale isolation and purification of geniposide from the fruit of Gardenia jasminoides Ellis by high-speed counter-current chromatography. J Chromatogr A. 2005;1100:76–80. doi: 10.1016/j.chroma.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Chen Y, Deng L, Cai S, Liu J, Li W, Du L, Cui G, Xu X, Lu T, Chen P, Zhang H. Systematic separation and purification of iridoid glycosides and crocetin derivatives from Gardenia jasminoides Ellis by high-speed counter-current chromatography. Phytochem Anal. 2015;26:202–8. doi: 10.1002/pca.2553. [DOI] [PubMed] [Google Scholar]
- 31. Yue H, Zhao X, Wang Q, Tao Y. Separation and purification of water-soluble iridoid glucosides by high speed counter-current chromatography combined with macroporous resin column separation. J Chromatogr B. 2013;93:57–62. doi: 10.1016/j.jchromb.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 32. Zhou M, Zhuo J, Wei W, Zhu J, Ling X. Simple and effective large-scale preparation of geniposide from fruit of Gardenia jasminoides Ellis using a liquid–liquid two-phase extraction. Fitoterapia. 2012;83:1558–61. doi: 10.1016/j.fitote.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 33. Gao Y, Sun Y, Wang Y, Zhang J, Xu B, Zhang H, Song D. A practical and rapid method for the simultaneous isolation, purification and quantification of geniposide from the fruit of Gardenia jasminoides Ellis by MSPD extraction and UFLC analysis. Anal Methods. 2013;5:4112–8. [Google Scholar]
- 34. Zhou X, Bi Z, Li P, Tang D, Cai H. A new iridoid glycoside from Gardenia jasminoides. Chin Chem Lett. 2007;18:1221–3. [Google Scholar]
- 35. Peng K, Yang L, Zhao Z, Chen L, Zhao F, Qiu F. Chemical constituents from the fruit of Gardenia jasminoides and their inhibitory effects on nitric oxide production. Bioorg Med Chem Lett. 2013;23:1127–31. doi: 10.1016/j.bmcl.2012.11.099. [DOI] [PubMed] [Google Scholar]
- 36. Xiao M, Gui X, Wang Z. Iridoid glycosides from Gardenia jasminoides E. Helv Chim Acta. 2008;91:646–52. [Google Scholar]
- 37. Sridhar RA, Shankara CJ, Merugu R. Iridoids from Gardenia jasminoides Ellis. Int J ChemTech Res. 2013;5:418–21. [Google Scholar]
- 38. Wang Y, Liu H, Shen LF, Yao L, Ma Y, Yu D, Chen J, Li P, Chen Y, Zhang C. Isolation and purification of six iridoid glycosides from Gardenia jasminoides fruit bymedium-pressure liquid chromatography combined with macroporous resin chromatography. J Sep Sci. 2015;38:4119–26. doi: 10.1002/jssc.201500705. [DOI] [PubMed] [Google Scholar]
- 39. Hausenblas HA, Heekin K, Mutchie HL. Stephen Anton. A systematic review of randomized controlled trials examining the effectiveness of saffron (Crocus sativus L.) on psychological and behavioral outcomes. J Integr Med. 2015;13:231–40. doi: 10.1016/S2095-4964(15)60176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu X, Mang Y, Shen F, Su J. Homogenate extraction of gardenia yellow pigment from Gardenia jasminoides Ellis fruit using response surface methodology. Food Sci Technol. 2014;51:1575–81. doi: 10.1007/s13197-012-0683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen Y, Cai L, Zhao C, Xu H, Cao C, Liu Y, Lin J, Hong X, Chen C, Zhang H. Spectroscopic, stability and radical-scavenging properties of a novel pigment from gardenia. Food Chem. 2013;109:269–72. doi: 10.1016/j.foodchem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 42. Chen Y, Zhang H, Xi T, Zhao C, Cai L, Liu Y, Jia L, Hong X, Chen C. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides Ellis and Crocus sativus L.: a relationship investigation between antioxidant activity and crocin contents. Food Chem. 2008;109:484–92. [Google Scholar]
- 43. Pfister S, Meyer P, Steck A, Fander PF. Isolation and structure elucidation of carotenoid-glycosyl esters in Gardenia fruits (Gardenia jasminoides Ellis) and saffron (Crocus sativus Linne) Agric Food Chem. 1996;44:2612–5. [Google Scholar]
- 44. Yang Y, Gao H, Dai Y, Wang Y, Chen HR, Yao XS. Monoterpenoids from the fruit of Gardenia jasminoides. Helv Chim Acta. 2010;93:763–71. [Google Scholar]
- 45. Chen Q, Youn UJ, Min B, Bae KH. Pyronane monoterpenoids from the fruit of Gardenia jasminoides. J Nat Prod. 2008;71:995–9. doi: 10.1021/np800002z. [DOI] [PubMed] [Google Scholar]
- 46. He M, Cheng X, Chen J, Zhou T. Simultaneous determination of five major biologically active ingredients in different parts of Gardenia jasminoides fruits by HPLC with diode-array detection. Chromatographia. 2006;64:713–7. [Google Scholar]
- 47. Pham TQ, Cormier F, Farnworth E, Tong VH, Van Calsteren MR. Antioxidant properties of crocin from Gardenia jasminoides Ellis and study of the reactions of crocin with linoleic acid and crocin with oxygen. J Agric Food Chem. 2000;48:1455–61. doi: 10.1021/jf991263j. [DOI] [PubMed] [Google Scholar]
- 48. Chen YI, Cheng YW, Tzeng CY, Lee YC, Chang YN, Lee SC, Tsai CC, Chen JC, Cheng Tzen JZ, Chang SL. Peroxisome proliferator-activated receptor activating hypoglycemic effect of Gardenia jasminoides Ellis aqueous extract and improvement of insulin sensitivity in steroid induced insulin resistant rats. BMC Complement Altern Med. 2014;14:30. doi: 10.1186/1472-6882-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guan L, Feng H, Gong D, Zhao X, Cai L, Wu Q, Yuan B, Yang Y, Zhao J, Zou Y. Genipin ameliorates age-related insulin resistance through inhibiting hepatic oxidative stress and mitochondrial dysfunction. Exp Gerontol. 2013;48:1387–94. doi: 10.1016/j.exger.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 50. Kojima K, Tsutomu ST, Yasuhiro NY, Watanabe M, Junko I, Shizaki JI, Yoshimichi SY, Miyamoto KI, Aburada M. Preventive effect of geniposide on metabolic disease status in spontaneously obese type 2 diabetic mice and free fatty acid-treated HepG2 cells. Biol Pharm Bull. 2011;34:1613–8. doi: 10.1248/bpb.34.1613. [DOI] [PubMed] [Google Scholar]
- 51. Wang G, Wu S, Xu W, Jin H, Zheng G, Li Z, Yuan X, Zhang J, Rao J, Wu S. Geniposide inhibits high glucose-induced cell adhesion through the NF-κB signaling pathway in human umbilical vein endothelial cells. Acta Pharmacol Sin. 2010;31:953–62. doi: 10.1038/aps.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xi L, Qian Z, Shen X, Wen N, Zhang Y. Crocetin prevent dexamethasone-induced insulin resistance in rats. Planta Med. 2005;71:917–22. doi: 10.1055/s-2005-871248. [DOI] [PubMed] [Google Scholar]
- 53. Lin W, Kuo HH, Ho LH, Tseng ML, Siao AC, Hung CT, Jeng KC, Hou CW. Gardenia jasminoides extracts and gallic acid inhibit lipopolysaccharide-induced inflammation by suppression of JNK2/1 signaling pathways in BV-2 cells. Iranian J Basic Med Sci. 2015;18:555–62. [PMC free article] [PubMed] [Google Scholar]
- 54. Ma T, Li X, Li W, Yang Y, Huang C, Meng X, Zhang L, Li J. Geniposide alleviates inflammation by suppressing MeCP2 in mice with carbon tetrachloride-induced acute liver injury and LPS-treated THP-1 cells. Int Immunopharmacol. 2015;29:739–47. doi: 10.1016/j.intimp.2015.08.045. [DOI] [PubMed] [Google Scholar]
- 55. Xiao J, Zhang W, Wang T, Jiang H, Zhang Z, Fu Y, Yang Z, Cao Y, Zhang N. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation. 2014;37:1588–98. doi: 10.1007/s10753-014-9885-2. [DOI] [PubMed] [Google Scholar]
- 56. Xu G, Li G, Ma H, Zhong H, Liu F, Ao G. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J Agric Food Chem. 2009;57:8325–30. doi: 10.1021/jf901752f. [DOI] [PubMed] [Google Scholar]
- 57. Zhang H, Xue W, Wu R, Gong T, Tao W, Zhou X, Jiang J, Zhang J, Zhang N, Cui Y, Chen C, Chen G. Rapid antidepressant activity of ethanol extract of Gardenia jasminoides Ellis is associated with upregulation of BDNF expression in the hippocampus. J Evid Based Complement Altern Med. 2015:1–8. doi: 10.1155/2015/761238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen J, Shi B, Xiang H, Hou W, Qin X, Tian J, Du G. 1H NMR-based metabolic profiling of liver in chronic unpredictable mild stress rats with genipin treatment. J Pharm Biomed Anal. 2015;115:150–8. doi: 10.1016/j.jpba.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 59. Ho KJ, Hee KG, Hee HK. Monoamine oxidase and dopamine b-hydroxylase inhibitors from the fruits of Gardenia jasminoides. Biomol Ther. 2012;20:214–9. doi: 10.4062/biomolther.2012.20.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kaji T, Hayashi T, Nsimba M, Kaga K, Ejiri N, Sakuragawa N. Gardenia fruit extracts does not stimulate the proliferation of cultured vascular smooth muscle cells, A10. Chem Pharm Bull. 1991;39:1312–4. doi: 10.1248/cpb.39.1312. [DOI] [PubMed] [Google Scholar]
- 61. Park EH, Joo MH, Kim SH, Lim CJ. Antiangiogenic activity of Gardenia jasminoides fruit. Phytother Res. 2003;17:961–2. doi: 10.1002/ptr.1259. [DOI] [PubMed] [Google Scholar]
- 62. Koo HJ, Lee S, Shin KH, Kim BC, Lim CJ, Park EH. Geniposide, an anti-angiogenic compound from the fruit of Gardenia jasminoides. Lett Planta Med. 2004;70:467–9. doi: 10.1055/s-2004-818978. [DOI] [PubMed] [Google Scholar]
- 63. Higashino S, Sasaki Y, Giddings JC, Hyodo K, Sakata SF, Matsuda K, Horikawa Y, Yamamoto J. Crocetin, a carotenoid from Gardenia jasminoides Ellis, protects against hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Phytother Res. 2014;28:1315–9. doi: 10.1002/ptr.5130. [DOI] [PubMed] [Google Scholar]
- 64. Tang FT, Qian ZY, Liu PQ, Zheng SG, He SY, Bao LP, Huang HQ. Crocetin improves endothelium-dependent relaxation of thoracic aorta in hypercholesterolemic rabbit by increasing eNOS activity. Biochem Pharmacol. 2006;72:558–65. doi: 10.1016/j.bcp.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 65. Lee JH, Lee DU, Jeong CS. Gardenia jasminoides Ellis ethanol extract and its constituents reduce the risks of gastritis and reverse gastric lesions in rats. Food Chem Toxicol. 2009;47:1127–31. doi: 10.1016/j.fct.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 66. Lari P, Abnous K, Imenshahidi M, Rashedinia M, Razavi M, Hosseinzadeh H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol Ind Health. 2015;31:367–74. doi: 10.1177/0748233713475519. [DOI] [PubMed] [Google Scholar]
- 67. Mohajeri D, Nazeri M. Inhibitory effect of crocin on hepatic steatosis in the rats fed with high fat diet. J Anim Vet Adv. 2012;11:2073–379. [Google Scholar]
- 68. Dai M, Wu H, Li H, Chen J, Chen J, Hu S, Shen C. Effects and mechanisms of geniposide on rats with adjuvant arthritis. Int Immunopharmacol. 2014;20:46–53. doi: 10.1016/j.intimp.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 69. Chen J, Wu H, Li H, Hu S, Dai M, Chen J. Anti-inflammatory effects and pharmacokinetics study of geniposide on rats with adjuvant arthritis. Int Immunopharmacol. 2015;24:102–9. doi: 10.1016/j.intimp.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 70. Yu Y, Xie Z, Gao H, Ma W, Dai Y, Wang Y, Zhong Y, Yao X. Bioactive iridoid glucosides from the fruit of Gardenia jasminoides. J Nat Prod. 2009;72:1459–64. doi: 10.1021/np900176q. [DOI] [PubMed] [Google Scholar]
- 71. Chen Y, Zhang Y, Li L, Hölscher C. Neuroprotective effects of geniposide in the MPTP mouse model of Parkinson’s disease. Eur J Pharmacol. 2015;768:21–7. doi: 10.1016/j.ejphar.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 72. Lee IA, Lee JH, Baek NI, Kim DH. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biol Pharm Bull. 2005;28:2106–10. doi: 10.1248/bpb.28.2106. [DOI] [PubMed] [Google Scholar]
- 73. Sheng L, Qian Z, Zheng S, Xi L. Mechanism of hypolipidemic effect of crocin in rats: crocin inhibits pancreatic lipase. Eur J Pharmacol. 2006;543:116–22. doi: 10.1016/j.ejphar.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 74. Lee IA, Min SW, Kim DH. Lactic acid bacteria increases hypolipidemic effect of crocin isolated from fructus of Gardenia jasminoides. Microbiol Biotechnol. 2006;16:1084–9. [Google Scholar]
- 75. Yamauchi M, Tsuruma K, Imai S, Nakanishi T, Umigai N, Shimazawa M, Hara H. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur J Pharmacol. 2011;650:110–9. doi: 10.1016/j.ejphar.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 76. Ishizuka F, Shimazawa M, Umigai N, Ogishima H, Nakamura S, Tsuruma K, Hara H. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur J Pharmacol. 2013;703:1–10. doi: 10.1016/j.ejphar.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 77. Wang Y, Yan J, Xi L, Qian Z, Wang Z, Yang L. Protective effect of crocetin on hemorrhagic shock-induced acute renal failure in rats. Shock. 2012;38:63–7. doi: 10.1097/SHK.0b013e3182596ec4. [DOI] [PubMed] [Google Scholar]
- 78. Ozaki A, Kitano M, Furusawa N, Yamaguchi H, Kuroda K, Endo G. Genotoxicity of gardenia yellow and its components. Food Chem Toxicol. 2002;40:1603–10. doi: 10.1016/s0278-6915(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 79. Su C, Yang X, Lou J. Geniposide reduces-synuclein by blocking microRNA-21/lysosome-associated membrane protein 2A interaction in Parkinson disease models. Brain Res. 2016;1644:98–106. doi: 10.1016/j.brainres.2016.05.011. [DOI] [PubMed] [Google Scholar]