Abstract
Antioxidant testing of natural products has attracted increasing interest in recent years, mainly due to the fact that an antioxidant-rich diet might provide health benefits. Activated macrophages are a major source of reactive oxygen species, reactive nitrogen species, and peroxynitrite generated through the so-called respiratory burst. Constitutively released proinflammatory cytokine, especially tumor necrosis factor-α, triggers nuclear factor-κB, and activator protein-1 translocation leading to the over production of reactive oxygen species and reactive nitrogen species in macrophages. Activation of transcription factors in the long-lived tissue-resident macrophages and/or monocyte-derived macrophages, trigger epigenetic modifications leading to the pathogenesis of chronic diseases. Nutraceuticals including lipid raft structure disruption agent, cholesterol depletion agent, farnesyltransferase inhibitor, nuclear factor-κB blocker (α,β-unsaturated carbonyl compounds), glucocorticoid receptor agonist, and peroxisome proliferator-activated receptor-γ agonist have long been used to inactive macrophage. The inhibition effects on the formation of nitric oxide, superoxide, and nitrite peroxide may be responsible for the anti-inflammatory functionalities. Activated macrophage models could be used to identify the active components for functional diets development through a multiple targets strategy.
Keywords: cell model, epigenetic, peroxynitrite, tissue resident macrophages
1. Oxidative stress and chronic diseases
The operational definition of oxidative stress is given by Lushchak [1] as “a situation when steady-state reactive oxygen species (ROS) concentration is transiently or chronically enhanced, disturbing cellular metabolism and its regulation, and damaging cellular constituents.” Reactive free radicals, including superoxide, hydroxyl radical, and peroxyl radical, generally result in degradation of protein, lipid peroxidation, and oxidation of DNA, which have possible linkage with many chronic diseases, such as diabetes, cancers, and atherosclerosis. ROS play an important role related to the degenerative or pathological processes of various serious diseases, such as age-related diseases, coronary heart disease, Alzheimer’s disease, neurodegenerative disorders, cataracts, and inflammation [2].
1.1. Reactive oxygen species
ROS is a collective term that includes both oxygen radicals and certain nonradicals that are oxidizing agents. In physiological conditions, mitochondria are the major source of intracellular ROS. Hyperglycemic conditions increase electron flux through the respiratory chain in mitochondria stimulating the formation of ROS. The key enzyme for ROS production in cells is NADPH oxidase. NADPH oxidase is a multisubunit enzyme comprising membrane and cytosolic component, which responses to environmental and micronutrient stimulants. The NADPH oxidase-mediated release of ROS in macrophages, also called respiratory burst (sometimes called oxidative burst), leads to the elimination of invading microorganisms [3]. Under unstimulated conditions, the multidomain regulatory subunits, p40phox, p47phox, and p67phox, exist as a complex in the cytosol. Upon stimulation, p47phox undergoes phosphorylation, translocates to the membrane to activate NADPH oxidase and produces superoxide [4]. In human monocytes and murine macrophages PI3K and protein kinase C (PKC) pathways are involved in NADPH oxidase stimulation. This mechanism triggers ERK1/2, p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB, which terminates in the activation of monocytes and proliferation of macrophages [5].
1.2. Reactive nitrogen species
Reactive nitrogen species oxidize proteins and nucleic acids. In addition to producing ROS, the mitochondrial respiratory chain is capable of producing nitric oxide (NO) [6]. Inducible NO synthase (iNOS) is a key enzyme in the macrophage that is potently induced in response to proinflammatory stimuli. Macrophages are activated by interferon-γ and microbial products such as lipopolysaccharides (LPS), leading to production of proinflammatory cytokines and high levels of NO. NO is a potent molecule involved in critical macrophages functions such as cytotoxicity against intracellular pathogens, viruses and tumors, and immune regulation [7].
1.3. Reactive carbonyl species increase nitric oxide and superoxide generation
An increase in steady-state level of reactive carbonyl species (RCS) may cause carbonyl stress. Methylglyoxal (MG) is one of the most studied RCS formed during glucose, protein and fatty acid metabolism and is increased especially in hyperglycemia conditions. An excess of MG formation can increase ROS formation and advanced glycation end products, and cause oxidative stress. RCS including MG and advanced glycation end products are also associated with the age-related diseases such as cardiovascular complications of diabetes, neurodegenerative diseases, and connective tissue disorders through increasing oxidative stress [8].
1.4. Macrophages are the major sources of oxidative stress
In macrophages NO is synthesized by iNOS; while superoxide is mainly produced by NADPH oxidase. The reaction of superoxide with NO leads to the formation of peroxynitrite in vivo. This iNOS-derived peroxynitrite results in nitrotyrosine formation, increased antibacterial activity, and cytotoxic actions of macrophages [9]. Recent evidence indicates that peroxynitrite contributes most of the cytotoxicity of resident macrophages. Peroxynitrite interacts with lipids, DNA, and proteins via direct oxidative reactions or indirect radical-mediated mechanisms. These reactions trigger cellular responses ranging from subtle modulations of cell signaling to overwhelming oxidative injury. In vivo, peroxynitrite generation has been attributed to inflammatory diseases such as stroke, myocardial infarction, chronic heart failure, diabetes, circulatory shock, cancer, and neurodegenerative disorders [10].
2. Epigenetic mechanism for disease pathogenesis
2.1. Tissue-resident macrophages
Tissue-resident macrophages are nonspecific killer cells that eliminate bacteria, foreign bodies, dead cells, and debris and recruit monocyte/macrophages in response to inflammatory signals. Specialized tissue-resident macrophages include osteoclasts (bone), alveolar macrophages (lung), histiocytes (interstitial connective tissue), Kupffer cells (liver), Langerhans (skin), microglia (brain), and mesangial cells (kidneys). Tissue-resident macrophages may initiate the inflammatory response depending on the nature of the insult and its magnitude [11].
2.2. Macrophage activation and inflammation
Macrophages are differentiated into phagocytes with immune and homeostatic functions. Macrophages can either be classically (M1) or alternatively (M2) activated dependent on the stimulus and the resulting phenotype of the cell. Classical activation (M1) of macrophages by LPS through Toll-like receptor 4 (TLR4) has been well characterized. An alternative pathway of macrophage activation by the TH2-type cytokines interleukin-4 (IL-4) and IL-13 account for a distinctive macrophage phenotype with a different role in humoral immunity [12]. Inflammatory stimuli are often sensed by macrophage receptors, resulting in the activation of downstream signaling cascades. Monocytes are recruited to sites of inflammation and terminally differentiate into macrophages. These monocyte-derived macrophages are the key constituent of inflammatory environments [13].
2.3. Role of macrophage in chronic diseases
2.3.1. Skeletal system: osteoclasts
The deregulation of bone remodeling, mediated by bone-forming osteoblasts and bone-resorbing osteoclasts may cause skeletal pathologies. Skeletal deformities are mostly reported in the bones, joints, and teeth. Most of the skeletal anomalies originate from aberrant cartilage and bone development. Primary inflammatory processes, including osteoporosis, rheumatoid arthritis, and periodontitis, secret proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1β, and NO in the extracellular matrix of cartilage and bone. These cytokines are produced by the activated osteoclasts [14].
2.3.1.1. Osteoporosis
Osteoclasts are bone-resorptive cells that adhere to the bone surface and secret protons by the vacuolar H+-ATPase pump. The bone minerals dissolve in the acidic environment of the extracellular space, paving the way for lysosomal proteases to degrade the organic components of the bone matrix [15]. Increases in mitogen-activated protein kinase NF-κB ligand (RANKL) mRNA expression and protein production increase the RANKL/OPG ratio and stimulate the differentiation of macrophage precursor cells into osteoclasts. They also stimulate the maturation and survival of the osteoclast, leading to bone loss. The differentiation of bone macrophages, osteoblasts and osteoclasts is accompanied by profound changes in gene expression. Histone deacetylation and DNA methylation are found to downregulate the expression of several genes that are closely associated with osteoblast differentiation [16].
2.3.1.2. Periodontitis
Periodontitis is an inflammatory disease characterized by periodontal pocket formation and alveolar bone resorption. Periodontal bone resorption is induced by RANKL, which is a central regulator of osteoclast development and function. The osteoclast, the principal bone resorptive cell, differentiates from monocyte/macrophage precursors under the regulation of the critical cytokines macrophage colony stimulating factor, RANKL, and osteoprotegerin. Macrophages promote bone loss through upregulated production of proinflammatory mediators and activation of the RANKL expression pathways [17].
2.3.1.3. Rheumatoid arthritis
Rheumatoid arthritis is a common autoimmune chronic inflammatory joint disease, characterized by macrophage infiltration, proliferation of synovial fibroblasts, and joint destruction. These cells of the innate immune system possess broad proinflammatory, destructive and remodeling capacities, and considerably contribute to inflammation and joint destruction both in the acute and chronic phases of rheumatoid arthritis [18]. Overexpression of major histocompatibility complex class II, granulocyte–macrophage colony-stimulating factor, proinflammatory cytokines and chemokines, metalloproteinases, and neopterin were frequently observed in the activated osteoclast [19].
2.3.2. Brain: microglia
Resident microglia and infiltrating monocytes play the vital role in central nervous system (CNS) autoimmune diseases [20]. The infiltrating monocytes are critical for the effector phase of autoimmune CNS inflammation, whereas microglia activation is required for monocyte recruitment to the CNS. Upon prolonged inflammation, microglia change their morphology towards an amoeboid shape. In addition to the morphological differentiation, several surface markers, such as F4/80 or Mac-1, which is typical for activated macrophages, are upregulated [21]. Interferon (IFN)-γ and LPS polarize microglia towards the activated state and increase the expression of proinflammatory factors such as iNOS leading to the pathogenesis.
2.3.3. Liver: Kupffer cells
Kupffer cells are resident macrophages of the liver and play an important role in the acute and chronic responses of the liver to toxic compounds. Activation of Kupffer cells directly or indirectly by toxic agents results in the release of inflammatory mediators, growth factors, and reactive oxygen species. This activation appears to modulate acute hepatocyte injury [22]. Kupffer cells may act both as effector cells in the destruction of hepatocytes by producing harmful soluble mediators as well as antigen presenting cells during viral infections of the liver. Their role in fibrosis is well established as they are one of the main sources of transforming growth factor β1 production, which leads to the transformation of stellate cells into myofibroblasts [22].
2.3.4. Pancreatic islet: resident macrophages
The classically activated macrophages that respond to intra-cellular pathogens by secreting proinflammatory cytokines and ROS, which initiate insulitis and pancreatic cell death during type 1 diabetes. Macrophages are involved in the final stage of autoimmune-mediated β-cell destruction. Classically activated macrophages are induced by stimulation with Th1-cell-derived IFN-γ and LPS. They respond to microbial infection with an enhanced phagocytic microbicidal capability through the expression of the cell adhesion molecules (CAMs) marker and iNOS, which catalyzes the conversion of L-arginine into ROS, such as NO [23].
2.3.5. Adipose tissue: macrophages
2.3.5.1. Insulin resistance
Obesity has been associated with several health disorders, such as type 2 diabetes, atherosclerosis, fatty liver disease, and certain forms of cancer. Among them, type 2 diabetes and atherosclerosis are well discussed. Abdominal visceral adipose tissue is correlated with insulin resistance. Proinflammatory cytokines such as TNFα, IL-1β, and monocyte chemoattractant protein-1 as well as free fatty acids have been reported to impair insulin signaling. The enlarged adipocytes of obese individuals recruit macrophages and promote inflammation and the release of a range of factors that predispose toward insulin resistance [24]. The adipose tissue enlarges significantly during the development of obesity and induces hypoxia in inner part of the adipose tissue. Prolonged exposure of the adipocyte to hypoxia might lead to cell death, recruitment of inflammatory monocyte-derived macrophages, and release of proinflammatory cytokines. In obesity, muscle inflammation is induced by increased production of TNFα, IL-1β, and IL-6 secreted from accumulated intramuscular adipose tissue and thus contributing to the development of insulin resistance [25].
2.3.5.2. Atherosclerosis
It is postulated that foam cells in the early atherosclerotic lesion are derived from the monocyte-derived macrophages in the artery wall. Evidence supports that over-activated macrophage in blood vessel is the major and direct cause of atherosclerosis. These inflammatory macrophages secrete proatherosclerotic cytokines (such as IL-6 and IL-12), ROS and reactive nitrogen species that exacerbate oxidative stress in the plaque formation [26].
3. Targets for natural products in epigenetic diets
It is well known that the lipid composition of cell membranes and lipid raft can be modified by various dietary lipids. n-3 Polyunsaturated fatty acids (PUFA) may regulate gene expression through interacting with nuclear receptors and transcription factors. The incorporation of n-3 PUFA into cell membranes can change the structure of lipid rafts and intra-cellular signaling processes [27]. An n-3 PUFA (docosahexaenoic acid) was reported to inhibit NF-kB activity through mediating signaling pathways that control the transcriptional activation of genes. All these data indicate that foods are important determinants of epigenetic functions and can exert variable effects on endogenous regulatory mechanisms [28].
3.1. Lipid raft disruption
3.1.1. Lipid raft structure disruption agent
Lipid rafts are microdomains of the plasma membrane enriched in cholesterol and sphingolipids, and play an important role in the initiation of many pharmaceutical agent-induced signaling pathways. Disruption of lipid rafts impairs the production of nitric oxide and TNF-α production in lipopolysaccharide-stimulated murine RAW264.7 macrophages [29]. Depletion of cholesterol can result in lipid rafts disruption and deactivate raft-associated proteins, such as death receptor proteins, protein kinases, and calcium channels. Lipid raft disintegration with a cholesterol-depleting agent, methyl-β-cyclodextrin results in dysregulated MAPK signaling through ERK 1/2, and attenuated production of TNF-α production in macrophage THP-1 cells [30].
3.1.1.1. Lycopene
Evidence suggests that lycopene may reduce intracellular levels of cholesterol in murine macrophage cell line J774.1 and human macrophage cell line THP-1 by inhibiting HMG-CoA reductase activity and expression. Lycopene was found to inhibit LPS-induced productions of NO and IL-6 in murine RAW264.7 macrophages by mechanisms related to inhibition of ERK and NF-κB. Zou et al [31] found that pretreatment of RAW264.7 cells with lycopene may inhibit LPS-induced recruitment of TLR4 into lipid raft. TLR4 are recruited into lipid rafts in response to LPS stimulation to cause NF-κB activation.
3.1.1.2. Resveratrol
Resveratrol(trans-3,4′,5-trihydroxystilbene), a polyphenolic phytoalexin found in grapes, fruits, and root extracts of the weed Polygonum cuspidatum, exhibits anti-inflammatory, cell growth-modulatory, and anticarcinogenic effects. Resveratrol downregulated the expression of iNOS and the secretion of IL-6 in LPS-stimulated RAW264.7 cells through the inhibition of the translocation of NF-κB p65 from the cytoplasm to the nucleus. Resveratrol also suppressed TNF-induced phosphorylation and nuclear translocation of the p65 subunit of NF-κB, and NF-κB-dependent reporter gene transcription. Endocytosis of resveratrol via lipid rafts is responsible for the above-mentioned activation of downstream signaling pathways [32].
3.1.2. Cholesterol depletion
The 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, statins, are potent inhibitors of cholesterol synthesis and have wide therapeutic use in cardiovascular diseases. HMG-CoA reductase is the key enzyme for cholesterol synthesis in mevalonate pathway in oxidized low-density lipoprotein-induced macrophage [33]. Monacolin k, an important metabolite of Monascus sp., was shown to be able to inhibit the synthesis of cholesterol. The critical reaction in the pathway of cholesterol synthesis is the formation of mevalonic acid from HMG-CoA by HMG-CoA reductase. Monacolin k is structurally similar to HMG-CoA and plays a role as a competitive inhibitor, which competes with HMG-CoA and reduces the synthesis of cholesterol [34].
It is well established that stimulation of macrophages with lipopolysaccharide (LPS) and/or IFN-γ results in the over expression of iNOS and excess NO formation. However, recent evidence suggests that monacolin k inhibits formation of NO in murine RAW 264.7 cells. Andrographolide, a labdane diterpenoid contains a γ-lactone ring, is the main active constituent of the plant, Andrographis paniculata [35]. Andrographolide shows a potent anti-inflammatory effect in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-kB pathway. Andrographolide has spatial structural similarity with monacolin K, and therefore may inhibit cholesterol biosynthesis by the same mechanisms.
3.1.3. Farnesyltransferase inhibitors
Farnesylation of Ras proteins is required for the activation of their downstream oncogenic proteins, including MAPKs (ERK, JNK, and p38) and Akt signaling leading to the regulation of osteoclast precursor proliferation. Farnesyltransferase transfers a farnesyl moiety from farnesyl pyrophosphate onto a C-terminal cysteine residue of Ras proteins [36]. As farnesyltransferase (FTase) is the key enzyme for the Ras proteins farnesylation, the inhibition of its activity could specifically stop Ras-mediated cellular proliferation. It is well established that NF-κB ligand (RANKL) is essential for inducing osteoclast differentiation through the MAPK activation. Bisphosphonates, typical FTase inhibitors, were found to inhibit protein farnesylation leading to an inactivation of downstream signaling pathways in J774 cells and osteoclast-like cells [37].
FTase inhibitors have mainly been used in cancer therapy and can be used for the treatment of other diseases. Many FTase inhibitors have been isolated from natural products, which possessed inhibitory activity against FTase. Dudakovic et al [38] reported that inhibition of the mevalonate pathway by statins and tocotrienols suppresses the prenylation of GTPase binding proteins, inhibits the activity of osteoclasts. Certain farnesyl pyrophosphate analogues, such as 3-allylfarnesol, geraniol, farnesol, and geranylgeraniol, are also potent inhibitors of mammalian FTase.
3.2. NF-κB blockage
3.2.1. α,β-unsaturated carbonyl compounds
Phytochemicals with a reactive α,β-unsaturated carbonyl group have been linked with antioxidant, anti-inflammatory, anticancer, antiviral, antibacterial, and antidiabetic properties. Numbers of phytochemicals which contain α,β-unsaturated carbonyl moiety or diketone groups were found to show potent anti-inflammation activities. Natural active components such as curcumin, okanin 2α-hydroxyl-3β-angeloylcinnamolide and helenalin were found to inhibit NF-κB translocation in macrophage. Helenalin inhibits the activation of NF-κB through the alkylation of the p65 subunit of NF-κB [39].
3.2.2. Glucocorticoid receptor agonist
Evidence from tissue culture, animal, and clinical studies suggests that hundreds of triterpenoid-rich plants in nature have the potential to be applied for inflammatory diseases. Pentacyclic triterpenes, including avicins, betulinic acid, boswellic acid, celastrol, diosgenin, madecassic acid, maslinic acid, momordin, saikosaponins, platycodon, pristimerin, ursolic acid, and withanolide belong to the cyclosqualenoid family, are widely used in Asian medicine [40]. Based on the structural similarity to glucocorticoid, these triterpenes can bind to the glucocorticoid receptor (GR), and induce its nuclear translocation and cause inhibition of GR-driven NF-kB activity. A pentacyclic terpene could be classified as nonsteroidal selective GR modulator [41].
3.2.3. Peroxisome proliferator-activated receptor-γ ligands
Ligand-mediated activation of peroxisome proliferator-activated receptor (PPAR)-γ has been linked to anti-inflammatory responses in macrophages and a positive regulator of differentiation into foam cell associated with atherogenesis. 15-Deoxy-δ-12,14-prostaglandin J2 (15d-PGJ2) suppresses the lipopolysaccharide (LPS)-induced expression of COX-2 in the macrophage-like differentiated U937 cells through blocking both activator protein (AP)-1 and NFkB-mediated gene expression. 15d-PGJ2 suppresses COX-2 promoter activity by interfering with the NF-κB signaling pathway in phorbol 12-myristate 13-acetate (PMA)-treated macrophages. Naturally occurring compounds such as fatty acids and 15d-PGJ2 bind to PPAR-γ and stimulate transcription of target genes. PPAR-γ is markedly upregulated in activated macrophages. PPAR-γ inhibits gene expression in part by antagonizing the activities of the transcription factors AP-1, STAT, and NF-κB. 15d-PGJ2, covalently bind to a cysteine residue in the PPAR-γ ligand binding pocket through a Michael addition reaction by an α,β-unsaturated ketone [42]. Thiazolidinedione is one of the most commonly prescribed medications for type 2 diabetes. Thiazolidinedione, 15d-PGJ2, and other PPAR-γ agonists inhibit the expression of a variety of proinflammatory proteins including cyclooxygenase-2, iNOS, and cytokines. These effects appear to be mediated by inhibitory effects on transcription factors, including NF-κB, AP-1, and STAT. The reactive cyclopentenone ring of 15d-PGJ2 reacts with cysteinyl thiol groups of PPAR-γ proteins through Michael addition reactions [43].
4. Assays of functionalities
4.1. In vitro methods
ROS may attack biological macromolecules, causing cell protein, lipid, and DNA damage, and resulting in oxidative stress-originated aging, diseases, and cancer. The most frequently encountered free radicals in cell are the hydroxyl radical, the superoxide radical, the nitric oxide radical, and the lipid peroxyl radical. The main methods for antioxidative activity evaluation are superoxide radicals scavenging; hypochlorous acid scavenging; hydroxyl radical scavenging, and peroxyl radical scavenging. Among them, 2,2′-azobis (2-amidinopropane) dihydrochloride (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and oxygen-radical absorbing capacity (ORAC) assays are well established and frequently used to estimate antioxidant capacities [44].
Generation of the stable ABTS radical cation and DPPH radical are the key principles employed for ABTS and DPPH assays respectively. The reaction between both electron and hydrogen donors with cation, resulting in reduction in color, forms the basis of the spectrophotometric methods that have been applied to the measurement of the total antioxidant activity. In specification, the ABTS assay is based on the generation of a blue/green ABTS radical cation that can be reduced by antioxidants; whereas the DPPH assay is based on the reduction of the purple DPPH radical to 1,1-diphenyl-2-picryl hydrazine [45].
A method of quantitating the ORAC of antioxidants in serum is developed. In this assay system, 2,2′-azobis(2-amidino-propane) dihydrochloride is used as a peroxyl radical generator, and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, a water-soluble vitamin E analogue) as a control standard. The uniqueness of this assay is that total antioxidant capacity of a sample is estimated by taking the oxidation reaction to completion. A modified ORAC assay depends on the free radical damage to a fluorescent probe, such as fluorescein. The degree of a downward change of fluorescent intensity is indicative of the amount of radical damage [46].
4.2. Cell models to evaluate the functionalities
4.2.1. 12-O-Tetradecanoylphorbol-13-acetate-treated macrophage model
A simple, rapid and semiautomated technique for the measurement of superoxide and H2O2 production by 12-O-tetradecanoylphorbol-13-acetate (TPA)-treated mouse alveolar macrophages was developed. The levels of superoxide and hydrogen peroxide were quantified by the cytochrome c assay and phenol red method, respectively. This is a very popular technique permitting the use of few cells and low reagent quantities for the kinetic analysis and the assay of a large number of samples [47]. TPA, a kind of phorbol ester, has been routinely used as an inducer for endogenous superoxide production. By a similar TPA-treated RAW264.7 cell model, eriodictyol was found to reduce the activation of PKC by blocking the phorbol ester binding site leading to the inhibition of superoxide generation.
4.2.2. LPS-treated macrophage model
LPS, lipoglycans, and endotoxins of Gram-negative bacteria, is another widely used stimulant to activate macrophage. LPS induces the uncontrolled release of proinflammatory mediators, especially TNF-α, several interleukins (IL-1β, IL-6, IL-8), NO, and ROS from monocytes and macrophages. LPS triggers the secretion of a variety of inflammatory products such as TNF-α and IL-6, as well as excessive amounts of NO via iNOS, which contribute to the pathological process of various acute and chronic inflammatory conditions. Inflammatory factors (TPA/PMA, LPS, and TNF-α) activate NF-κB translocation and trigger a respiratory burst [48]. NF-κB is the key transcription factor increases the expression of proinflammatory genes NADPH oxidase and inducible nitric oxide synthase leading to ROS and NO production in RAW264.7 cells. Binding of LPS to TLR4, the adaptor protein MyD88 is recruited to the receptor, triggers a signaling cascade leading to the activation of transcription factor NF-κB and MAPKs including ERK1/2, p38, and JNK. Many stimuli that trigger the respiratory burst in phagocytes induce the activation of the ERK. Thus, ERK activation may be a consequence of the burst or may play a role in the assembly of the NADPH oxidase. LPS-treated RAW264.7 cell model have been widely used to evaluate the anti-inflammation activity of various nutraceuticals [49].
4.2.3. TPA plus LPS-treated macrophage model
In TPA-treated RAW264.7 cells p47phox was found to be phosphorylated by kinases including PKC, extracellular-signal-regulated kinase ½, and p38 kinase. Cytosolic components (p47phox, p67phox and p40phox) translocase to the membrane to complex with membrane-bound components (gp91phox, and p22phox) leading to the formation of active NADPH oxidase assembly. Induction of iNOS was found to upregulate the TPA-induced assembly of NADPH oxidase and leads to the enhanced production of superoxide via a PKC-dependent mechanism [35]. LPS treated murine macrophages generate both nitric oxide and superoxide that interact to form the potent oxidant peroxynitrite, which enhances nitrotyrosine formation, cell membrane damage and contributes to cytotoxic actions of macrophages [9]. Peroxynitrite interacts with lipids, DNA, and proteins and plays a crucial pathogenic mechanism in chronic inflammatory diseases such as stroke, myocardial infarction, chronic heart failure, diabetes, circulatory shock, cancer, and neurodegenerative disorders [10]. Combination effect of LPS and PMA can generate endogenous peroxynitrite that is close to the real physiological condition.
5. Conclusion
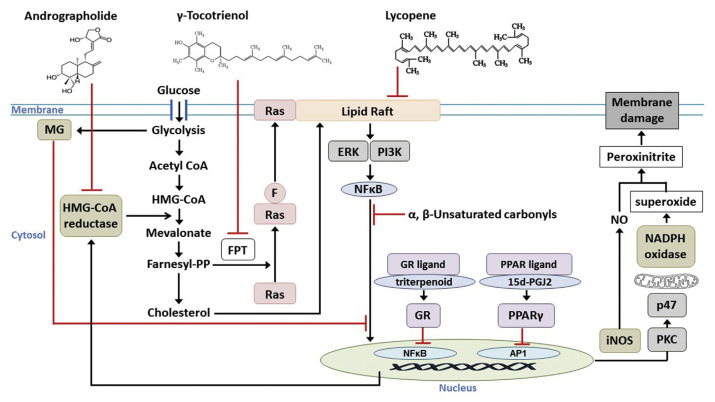
Chemical-based methods have been widely applied to evaluate the antioxidant potential of foods; however, it is important to note that the DPPH and ABTS tests only recognize free-radical scavenging capacities. Testing the benefits of dietary antioxidants with a macrophage model can take into account the key biological parameters that are needed to reflect the real physiological conditions. Activated macrophages are recognized as the major drivers in the pathogenesis of various chronic diseases. A multiple target pathway (Figure 1) was proposed to elucidate the mechanistic role of the active components in functional diets.
Figure 1.
Multiple targets in macrophage attacked by nutraceuticals. FPT, farnesyl prenyl transferase; MG, methylglyoxal; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A; Farnesyl-PP, farnesyl pyrophosphate; F, farnesyl group; ERK, extracellular signal-regulated kinase; PI3K, phosphoinositide 3-kinase; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; GR, glucocorticoid receptor; PPARγ, peroxisome proliferator-activated receptor gamma; AP1, activator protein 1; iNOS, inducible nitric oxide synthase; NO, nitric oxide; PKC, protein kinase C.
Footnotes
Conflicts of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1. Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–75. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 2. Rahman K. Studies on free radicals, antioxidants, and cofactors. Clin Interv Aging. 2007;2:219–36. [PMC free article] [PubMed] [Google Scholar]
- 3. Franchini AM, Hunt D, Melendez JA, Drake JR. FcγR-driven release of IL-6 by macrophages requires NOX2-dependent production of reactive oxygen species. J Biol Chem. 2013;288:25098–108. doi: 10.1074/jbc.M113.474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jialal I, Kaur H, Devaraj S. Human C-reactive protein accentuates macrophage activity in biobreeding diabetic rats. J Diabetes Complications. 2013;27:23–8. doi: 10.1016/j.jdiacomp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. San Jose G, Bidegain J, Robador PA, Diez J, Fortuno A, Zalba G. Insulin-induced NADPH oxidase activation promotes proliferation and matrix metalloproteinase activation in monocytes/macrophages. Free Radical Biol Med. 2009;46:1058–67. doi: 10.1016/j.freeradbiomed.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 6. Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–87. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 7. McNeill E, Crabtree MJ, Sahgal N, Patel J, Chuaiphichai S, Iqbal AJ, Hale AB, Greaves DR, Channon KM. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radical Biol Med. 2015;79:206–16. doi: 10.1016/j.freeradbiomed.2014.10.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desai KM, Chang T, Wang H, Banigesh A, Dhar A, Liu J, Untereiner A, Wu L. Oxidative stress and aging: is methylglyoxal the hidden enemy? Can J Physiol Pharmacol. 2010;88:273–84. doi: 10.1139/Y10-001. [DOI] [PubMed] [Google Scholar]
- 9. Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci U S A. 1997;94:6954–8. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanitakis J, Petruzzo P, Dubernard JM. Turnover of epidermal Langerhans’ cells. N Engl J Med. 2004;351:2661–2. doi: 10.1056/NEJM200412163512523. [DOI] [PubMed] [Google Scholar]
- 12. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 13. Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4:1886. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simonaro CM, D’Angelo M, Haskins ME, Schuchman EH. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr Res. 2005;57:701–7. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- 15. Monroy MA, Ross FP, Teitelbaum SL, Sands MS. Abnormal osteoclast morphology and bone remodeling in a murine model of a lysosomal storage disease. Bone. 2002;30:352–9. doi: 10.1016/s8756-3282(01)00679-2. [DOI] [PubMed] [Google Scholar]
- 16. Vrtačnik P, Marc J, Ostanek B. Epigenetic mechanisms in bone. Clin Chem Lab Med. 2014;52:589–608. doi: 10.1515/cclm-2013-0770. [DOI] [PubMed] [Google Scholar]
- 17. Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. 2015;2015:615486. doi: 10.1155/2015/615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma Y, Pope RM. The role of macrophages in rheumatoid arthritis. Curr Pharmaceut Design. 2005;11:569–80. doi: 10.2174/1381612053381927. [DOI] [PubMed] [Google Scholar]
- 19. Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999;26:717–9. [PubMed] [Google Scholar]
- 20. Rothhammer V, Quintana FJ. Role of astrocytes and microglia in central nervous system inflammation. Sem Immunopathol. 2015;37:575–6. doi: 10.1007/s00281-015-0521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Djukic M, Mildner A, Schmidt H, Czesnik D, Bruck W, Priller J, Nau R, Prinz M. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 2006;129:2394–403. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- 22. Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci. 2007;96:2–15. doi: 10.1093/toxsci/kfl173. [DOI] [PubMed] [Google Scholar]
- 23. Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, Cooke A. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–49. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 24. Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59:879–94. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–85. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 26. Adamson S, Leitinger N. Phenotypic modulation of macrophages in response to plaque lipids. Curr Opin Lipidol. 2011;22:335–42. doi: 10.1097/MOL.0b013e32834a97e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88:43–7. doi: 10.1016/j.plefa.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szarc vel Szic K, Declerck K, Vidakovic M, Vanden Berghe W. From inflammaging to healthy aging by dietary lifestyle choices: is epigenetics the key to personalized nutrition? Clin Epigenetics. 2015;7:33. doi: 10.1186/s13148-015-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang S, Jin H, Zhou J, Wei T. Disruption of lipid rafts impairs the production of nitric oxide in lipopolysaccharide-stimulated murine RAW264.7 macrophages. Res Chem Intermed. 2006;32:847–56. [Google Scholar]
- 30. Cuschieri J. Implications of lipid raft disintegration: enhanced anti-inflammatory macrophage phenotype. Surgery. 2004;136:169–75. doi: 10.1016/j.surg.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 31. Zou J, Feng D, Ling WH, Duan RD. Lycopene suppresses proinflammatory response in lipopolysaccharide-stimulated macrophages by inhibiting ROS-induced trafficking of TLR4 to lipid raft-like domains. J Nutr Biochem. 2013;24:1117–22. doi: 10.1016/j.jnutbio.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 32. Delmas D, Aires V, Colin DJ, Limagne E, Scagliarini A, Cotte AK, Ghiringhelli F. Importance of lipid microdomains, rafts, in absorption, delivery, and biological effects of resveratrol. Ann New York Acad Sci. 2013;1290:90–7. doi: 10.1111/nyas.12177. [DOI] [PubMed] [Google Scholar]
- 33. Sakai M, Kobori S, Matsumura T, Biwa T, Sato Y, Takemura T, Kakamata H, Horiuchi S, Shichiri M. HMG-CoA reductase inhibitors suppress macrophage growth induced by oxidized low density lipoprotein. Atherosclerosis. 1997;133:51–9. doi: 10.1016/s0021-9150(97)00118-4. [DOI] [PubMed] [Google Scholar]
- 34. Arunachalam C, Narmadhapriya D. Monascus fermented rice and its beneficial aspects: a new review. Asian J Pharmaceut Clin Res. 2011;4:29–31. [Google Scholar]
- 35. Zhao K, Huang Z, Lu H, Zhou J, Wei T. Induction of inducible nitric oxide synthase increases the production of reactive oxygen species in RAW264. 7 macrophages. Biosci Rep. 2010;30:233–41. doi: 10.1042/BSR20090048. [DOI] [PubMed] [Google Scholar]
- 36. Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunolog Rev. 2009;231:241–56. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 37. Dunford JE, Rogers MJ, Ebetino FH, Phipps RJ, Coxon FP. Inhibition of protein prenylation by bisphosphonates causes sustained activation of Rac, Cdc42, and Rho GTPases. J Bone Miner Res. 2006;21:684–94. doi: 10.1359/jbmr.060118. [DOI] [PubMed] [Google Scholar]
- 38. Dudakovic A, Wiemer AJ, Lamb KM, Vonnahme LA, Dietz SE, Hohl RJ. Inhibition of geranylgeranyl diphosphate synthase induces apoptosis through multiple mechanisms and displays synergy with inhibition of other isoprenoid biosynthetic enzymes. J Pharmacol Exp Ther. 2008;324:1028–36. doi: 10.1124/jpet.107.132217. [DOI] [PubMed] [Google Scholar]
- 39. Lyß G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-κB by directly targeting p65. J Biol Chem. 1998;273:33508–16. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- 40. Phillips DR, Rasbery JM, Bartel B, Matsuda SP. Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol. 2006;9:305–14. doi: 10.1016/j.pbi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 41. Haridas V, Xu Z-X, Kitchen D, Jiang A, Michels P, Gutterman JU. The anticancer plant triterpenoid, avicin d, regulates glucocorticoid receptor signaling: implications for cellular metabolism. PloS One. 2011;6:e28037. doi: 10.1371/journal.pone.0028037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes role of free fatty acids and tumor necrosis factor α. Arterioscler Thromb Vasc Biol. 2005;25:2062–8. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 43. Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang C-H, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-Deoxy-Δ(12,14)-prostaglandin J(2) inhibits multiple steps in the NF-κB signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–9. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Apak R, Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report) Pure Appl Chem. 2013;85:957–98. [Google Scholar]
- 45. Xie J, Schaich KM. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J Agric Food Chem. 2014;62:4251–60. doi: 10.1021/jf500180u. [DOI] [PubMed] [Google Scholar]
- 46. Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–26. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 47. Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Meth. 1981;46:211–26. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- 48. Veres B, Radnai B, Gallyas F, Varbiro G, Berente Z, Osz E, Sumegi B. Regulation of kinase cascades and transcription factors by a poly (ADP-ribose) polymerase-1 inhibitor, 4-hydroxyquinazoline, in lipopolysaccharide-induced inflammation in mice. J Pharmacol Exp Ther. 2004;310:247–55. doi: 10.1124/jpet.104.065151. [DOI] [PubMed] [Google Scholar]
- 49. Troutman TD, Bazan JF, Pasare C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle. 2012;11:3559–67. doi: 10.4161/cc.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]