Abstract
Cordyceps sinensis has various biological and pharmacological functions, and it has been claimed as a tonic supplement for sexual and reproductive dysfunctions for a long time in oriental society. In this article, the in vitro and in vivo effects of C. sinensis and cordycepin on mouse Leydig cell steroidogenesis are briefly described, the stimulatory mechanisms are summarized, and the recent findings related to the alternative substances regulating male reproductive functions are also discussed.
Keywords: cordycepin, Cordyceps sinensis, Leydig cell, male, reproduction, steroidogenesis, testosterone
1. Introduction
Cordyceps sinensis (CS) is a fungal parasite on the larvae of Lepidoptera. In late autumn, the fungus attacks the caterpillar and leisurely devours its host. By early summer of the following year, the fungal infestation has killed the caterpillar and the fruiting body protrudes from its head. Because of its particular life cycle, it is called the winter-worm, summer-plant in Chinese [1–3]. CS has long been used as a herbal tonic in traditional Chinese medicine to treat many illnesses in the oriental society. Nevertheless, the supply of CS is inadequate for the demand because of its low yield in a high-altitude area where it cannot be easily harvested. However, the mycelium of the fungus has been cultured and the dried powder of the mycelium is commercially available [1–3].
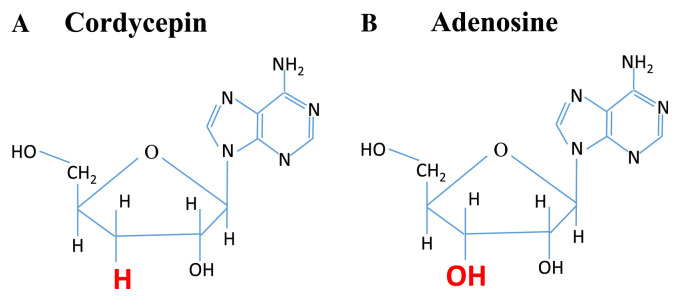
CS contains complex materials, including cordycepin [4–6], modified nucleosides [7,8], polysaccharides [9,10], and sterols [11,12]. Cordycepin, or 3′-deoxyadenosine, is a major bioactive component found in CS. Due to the absence of oxygen in the 3′ position of its ribose moiety and its similarity to adenosine (Figure 1) [6], some enzymes cannot discriminate between the two. Therefore, it can participate in certain biochemical reactions. For example, cordycepin could incorporate into an RNA molecule causing the premature termination of its synthesis, and has a wide range of biological effects in the regulation of inflammation and platelet aggregation [6,13,14]. Studies have demonstrated that CS has multiple pharmacological activities, including modulation of immune responses [15,16], inhibition of tumor growth [11,17,18], decrease of blood pressure [19,20], increase of hepatic energy metabolism and blood flow [21], improvement of bioenergy in liver [22], induction of cell apoptosis [23], and secretion of adrenal hormones [24].
Figure 1.
The difference in the chemical structures of bioactive compounds, (A) cordycepin and (B) adenosine [6].
2. Steroidogenic pathway related to male reproduction
In the male reproductive system, gonadotropin-releasing hormone from hypothalamus stimulates anterior lobe of pituitary gland to release luteinizing hormone (LH) [25]. It is well established that steroidogenesis in Leydig cells is regulated by LH/chorionic gonadotropin (CG). LH binds to its receptors in Leydig cells to activate G-proteins and, in turn, adenylate cyclase (AC), which can increase intracellular cyclic adenosine monophosphate (cAMP) formation [26]. The cAMP will then stimulate protein kinase A (PKA), which will phosphorylate some proteins and/or induce de novo synthesis of proteins, such as steroidogenic acute regulatory (StAR) protein [27]. StAR protein is considered as a rate-limiting step of steroid biosynthesis since it can facilitate the transfer of free cholesterol from cytoplasm into the inner membrane of mitochondria, where cytochrome P450 side-chain cleavage enzyme converts cholesterol to pregnenolone [28]. Pregnenolone will then be transported to the smooth endoplasmic reticulum, where contains 3β-hydroxysteroid dehydrogenase (HSD), 17α-hydroxylase, 20 α-hydroxylase, and 17β-HSD steroidogenic enzymes, to be processed to become testosterone, an essential steroid hormone for reproduction in men [25].
Although PKA-mediated protein phosphorylation is undoubtedly important in regulating steroid synthesis, other signaling systems have also been implicated. It has been shown that the activation of protein kinase C (PKC) signal pathway can strongly modulate Leydig cell steroidogenesis [29]. Likewise, evidence indicates that calcium is also involved in steroidogenesis. It has been shown that the removal of extracellular calcium, or the addition of calmodulin antagonist or calcium channel blocker blunts Leydig cell steroidogenesis [30,31].
3. CS stimulates mouse Leydig cell steroidogenesis
3.1. CS stimulates testosterone production in purified mouse Leydig cells
It is important to determine whether CS can stimulate testosterone production by mouse Leydig cells. Thus, the mouse Leydig cells are purified through Percoll gradient with purity reaching around 90%. These purified normal mouse Leydig cells are then treated with CS at different dosages for different time durations, and testosterone levels in media are evaluated. The results demonstrate that testosterone production is significantly elevated by the treatment of increasing dosages of CS (0.1–10 mg/mL), and reaches a maximum at 3 mg/mL CS in a 3-hour treatment as compared to control [32].
3.2. CS fractions stimulate testosterone production in purified mouse Leydig cells
In previous results, CS stimulated in vitro steroidogenesis in purified normal mouse Leydig cells [32]. What remains elusive, however, is which component of CS is responsible. Thus, a search for the components in CS with in vitro stimulatory effects on mouse Leydig cells is required. The purified mouse Leydig cells are treated with CS and its water-extracted fractions; F1 (water-soluble polysaccharide), F2 (water-soluble protein), and F3 (poorly water-soluble polysaccharide and protein); to determine the in vitro effects of CS fractions on steroidogenesis. In preparation of CS fractions, 100 g crude CS was extracted with 800 mL distilled water and shaken at 37°C for 72 hours. The solution was then centrifuged at 13,000× g at 4°C for 30 minutes to collect the pellet (F3). The supernatant was applied into G150 gel filtration column (3 cm × 100 cm) with 50mM acetonitrile buffer at pH 6.0. Two peaks were collected and the first peak was assigned as F1 and the second peak was assigned F2. The yield percentages of F1, F2, and F3 were 1.69%, 13.46%, and 84.85%, respectively. The results illustrate that F2 and F3 significantly stimulate in vitro testosterone production in purified mouse Leydig cells in dose- and time-dependent relationships with maximal responses at 3 mg/mL for 3 hours [33].
3.3. CS stimulates steroidogenesis in MA-10 mouse Leydig tumor cells
In purified normal mouse Leydig cell studies, many mice needed for sacrifice. Consequently, the MA-10 mouse Leydig tumor cell line is exploited. This well-studied mouse Leydig tumor cell line produces progesterone as the major steroid in response to both trophic hormones (LH and human CG, hCG) and cAMP analogues due to the low activity of 17α-hydroxylase [34,35]. MA-10 cells are treated with different concentrations of CS (2–10 mg/mL) for various time durations (6–24 hours), and progesterone productions are evaluated. Data show that progesterone production significantly increases with CS treatments in dose- and time-dependent relationships [36,37]. These results in MA-10 mouse Leydig tumor cell studies are consistent with the results in purified normal mouse Leydig cell studies.
3.4. CS fractions stimulate steroidogenesis in MA-10 mouse Leydig tumor cells
The effects of CS and three water-extracted fractions of CS (F1, F2, and F3) on progesterone production in MA-10 cells have been further studied. CS, F1, F2, or F3 at different dosages are added to cells for various durations. Data show that progesterone productions significantly increase in dose-and time-dependent relationships with the treatments of CS and F3 compared with the control. There is no difference in progesterone productions among control, F1 and F2 treatments in MA-10 cells [37]. These results in MA-10 cell studies are somewhat different from the results in purified normal mouse Leydig cell studies: F2 could stimulate steroidogenesis in normal mouse Leydig cells, but not in MA-10 cells.
3.5. CS stimulates plasma testosterone level in mouse
In previous results, CS stimulates in vitro steroidogenesis in normal mouse Leydig cells and in MA-10 mouse Leydig tumor cells [32,33,36,37]. What remains elusive, however, is whether CS has any in vivo effect on Leydig cell steroidogenesis. Thus, an investigation for CS with in vivo stimulatory effects on mouse Leydig cell steroidogenesis is essential, and CS has been further used to feed the mice to determine the in vivo effect on the levels of testosterone in plasma. Different dosages of CS (0.02 mg/g body weight or 0.2 mg/g body weight) are fed to immature (age, 5 weeks) or mature (age, 10 weeks) C57BL/6 (B6) mice for 1 day, 3 days, or 7 days. The plasma levels of testosterone are evaluated by radioimmunoassay. Results illustrate that CS significantly induces plasma testosterone levels both in immature and mature mice in 3- and 7-day treatments [33,38].
3.6. CS fractions stimulate plasma testosterone level in mouse
In previous results, CS significantly induced plasma testosterone levels both in immature and mature mice in 3- and 7-day treatments [33,38]. Which component of CS does the job to have positive in vivo effect on Leydig cell steroidogenesis remains unknown? Thus, an examination for the components in CS with in vivo stimulatory effects is executed. In normal mouse Leydig cell study treated by CS fractions, only F2 and F3 could induce testosterone production. Thus, F2 and F3 (0.02 mg/g body weight or 0.2 mg/g body weight) were fed to immature (age, 5 weeks) or mature (age, 10 weeks) B6 mice for 1 day, 3 days, or 7 days, respectively, and the changes of testosterone levels in plasma are examined. Results show that F2 and F3 at 0.02 mg/g body weight and/or 0.2 mg/g body weight for 3 days and/or 7 days could also significantly stimulate plasma testosterone levels both in immature and mature mice [33,38].
4. Cordycepin stimulates mouse Leydig cell steroidogenesis
CS fractions (F1, F2, and F3) have been used to examine whether these fractions could stimulate steroidogenesis in mouse Leydig cells, and the outcomes are that F2 and/or F3 could both induce steroidogenesis in vitro and in vivo [33,38]. Unfortunately, F2 and F3 still contain too many different substances. Thus, cordycepin, the major component in CS, is selected for further examination of the CS-stimulated effects on mouse Leydig steroidogenesis.
4.1. Cordycepin induces testosterone production in purified mouse Leydig cells
To verify if cordycepin directly stimulates testosterone production, the purified normal mouse Leydig cells were treated with various concentrations of cordycepin (10μM to 5mM) for 3 hours. Results show that testosterone production gradually rises as the concentration of cordycepin increases, and there is a significant three-fold increase under 1mM cordycepin treatment. In the temporal study, cordycepin (1mM) maximally stimulates testosterone production at 3 hours’ treatment. These results indicate that cordycepin stimulates normal mouse Leydig cell steroidogenesis in concentration-and time-dependent manners [39].
4.2. Cordycepin induces steroidogenesis in MA-10 mouse Leydig tumor cells
The well-studied MA-10 mouse Leydig tumor cell line produces progesterone as the major steroid in response to both trophic hormones (LH and hCG) and cAMP analogues due to the low activity of 17α-hydroxylase [34,35]. MA-10 cells are incubated with different dosages of cordycepin (1nM to 100μM) for different time durations (0 hours, 3 hours, 6 hours, 12 hours, and 24 hours). Results demonstrate that MA-10 cell steroidogenesis is significantly induced by 100μM cordycepin after 12- and 24-hour treatments. The progesterone production induced by 100μM cordycepin is 3-fold higher compared to control. These results illustrate that cordycepin stimulates MA-10 mouse Leydig tumor cell steroidogenesis in a concentration- and time-dependent manner [40,41].
4.3. Cordycepin induces plasma testosterone level in mouse
In previous results, cordycepin could stimulate in vitro steroidogenesis in normal mouse Leydig cells and in MA-10 mouse Leydig tumor cells [39–41]. Thus, cordycepin (20 mg/kg body weight or 40 mg/kg body weight) was further used to feed immature (age, 5 weeks) B6 mice for 7 days, and the changes of testosterone levels in plasma were examined. Results show that intraperitoneal administration of cordycepin (40 mg/kg) for 7 days significantly increases plasma testosterone concentration in mice, compared to the control group [39].
5. Mechanisms
We have demonstrated that CS and cordycepin could stimulate steroidogenesis in normal mouse Leydig cells and MA-10 mouse Leydig tumor cells, respectively [32,33,36–41]. We also found that CS and cordycepin could elevate plasma testosterone levels in B6 mice [33,38,39]. However, the mechanism whereby CS and cordycepin stimulate steroidogenesis in mouse Leydig cells remain elusive. Thus, the possible signal transduction pathways activated by CS and cordycepin in Leydig cells related to steroidogenesis have been investigated and are herein described.
5.1. CS stimulates steroidogenesis through cAMP-PKA pathway in normal mouse Leydig cells
CS could enhance steroidogenesis in purified normal mouse Leydig cells [32,33]. Thus, how CS activated signal pathways related to steroidogenesis in normal mouse Leydig cells is investigated and illustrated. Results show that CS significantly activates the PKA signal transduction pathway, but not the PKC signal pathway, to stimulate testosterone production in normal mouse Leydig cells [42].
5.2. CS stimulates steroidogenesis through PKA and PKC pathways in MA-10 cells
MA-10 cells were also used to examine the possible signal pathways activated by CS. Data demonstrated that CS significantly activates both PKA and PKC signal transduction pathways to stimulate MA-10 cell steroidogenesis [43]. Moreover, de novo protein synthesis is involved in CS stimulating steroidogenesis in MA-10 cells. StAR protein, mitochondrial electrochemical gradient, and calcium signal pathway also play important roles in this stimulation. However, P450 side-chain cleavage and 3β-HSD enzymes might not contribute to the stimulatory effect of CS in MA-10 cell steroidogenesis [44].
5.3. Cordycepin stimulates steroidogenesis through PKA and PKC pathways in normal mouse Leydig cells
Cordycepin, an important component in CS, was used to examine the possible mechanisms in mouse Leydig steroidogenesis. Data showed that cordycepin could activate cAMP-PKA signal transduction pathway to induce StAR protein expression, and then to enhance testosterone production in primary mouse Leydig cells. Additionally, cordycepin could possibly exert its effect through the association with adenosine receptor (AR) subtypes A1, A2a, and A3 [39].
5.4. Cordycepin stimulates steroidogenesis through PKA and PKC pathways in MA-10 cells
Cordycepin could stimulate the expressions of adenosine subtype receptors to induce MA-10 Leydig tumor cell steroidogenesis through PKA, PKC/phospholipase C (PLC) and mitogen-activated protein kinase (MAPK) signal pathways without increasing StAR protein expression. Interestingly, the stimulatory effect of PKA and PKC/PLC pathways and the inhibitory effect of ERK1/2 pathway activated by cordycepin might interact for the homeostasis status to stimulate steroidogenesis in MA-10 cells [40,41].
6. Discussion
In this review article, the in vitro and in vivo effects of CS, CS fractions, and cordycepin on steroidogenesis in mouse Leydig cells are summarily illustrated, and hope to correlate with the scientific evidence that CS and cordycepin do have the positive effects on Leydig cell steroidogenesis (Tables 1 and 2).
Table 1.
In vitro effect of Cordyceps sinensis (CS), CS fractions and cordycepin on steroidogenesis in normal mouse Leydig cells and MA-10 mouse Leydig tumor cells.
| CS | F1 | F2 | F3 | Cordycepin | |
|---|---|---|---|---|---|
| Normal Leydig cells →Testosterone | + | − | + | + | + |
| MA-10 cells →Progesterone | + | − | − | + | + |
+ = stimulation; − = no effect.
Table 2.
In vivo effect of Cordyceps sinensis (CS), CS fractions, and cordycepin on steroidogenesis in mouse.
| CS | F2 | F3 | Cordycepin | |||||
|---|---|---|---|---|---|---|---|---|
| Feeding days | 3 | 7 | 3 | 7 | 3 | 7 | 3 | 7 |
| Immature mouse | + | + | + | + | + | + | − | + |
| Mature mouse | + | + | + | + | + | + | − | − |
+ = stimulation; − = no effect.
6.1. In vitro effects of CS, CS fractions and cordycepin on mouse Leydig cells
In the in vitro study, the results of CS, F3, and cordycepin on steroidogenesis between normal mouse Leydig cells and MA-10 mouse Leydig tumor cells are consistent. F1 has no effect on either cell types. However, F2 can stimulate steroidogenesis in normal mouse Leydig cells, but not in MA-10 cells (Table 1). In the CS fraction preparations, CS is extracted by water, produces F1 (water soluble polysaccharide), F2 (water soluble protein), and F3 (poorly water soluble polysaccharide and protein). This preparation in F2 highly suggests that there are many water-soluble proteins that could associate with normal mouse Leydig cells to induce testosterone production. However, this F2 preparation may lack some ingredient(s) for inducing steroidogenesis in MA-10 tumor cells. Which protein(s) and/or compound(s) in CS provoking this different phenomenon between both cell types still need more scientific works. It is certain that, cordycepin does stimulate steroidogenesis both in normal mouse Leydig cells and MA-10 mouse Leydig tumor cells (Table 1).
6.2. In vivo effects of CS, CS fractions and cordycepin on mouse Leydig cells
Studies have demonstrated that Leydig cell function loss and sperm production decreases are due to the decrease of testosterone concentration in blood [45,46]. Thus, testosterone is indispensable to male reproduction. We have found that oral administration of CS and its fractions, F2 and F3, could increase plasma testosterone levels in both immature and mature B6 mice (Table 2). We further showed that cordycepin could stimulate immature B6 mouse plasma testosterone production accompanied by increasing testis weight, supporting that cordycepin is the bioactive pure material of CS regulating Leydig cell functions [39].
It is apparent that longer time application of cordycepin (7 days) is necessary in immature mouse for increasing plasma testosterone level. However, 3 days feeding of CS and its fractions (F2 and F3) is good enough to induce testosterone level in immature mice. This phenomenon indicates that there are some other factors in CS, F2, and F3 facilitating the efficacy of cordycepin on in vivo steroidogenesis. It will need more investigations to pinpoint what materials can facilitate cordycepin to stimulate testosterone production. Studies have shown that supplementation with Cordyceps mycelium improves sperm quality and quantity in subfertile boars and rats [47,48]. Another study also illustrates that long-term administration of cordycepin can counteract the decline of testicular function in middle-aged rats [49]. These three studies do not demonstrate whether Cordyceps and/or cordycepin could directly regulate testosterone production. However, functions of testosterone-dependent tissues and organs in the male reproductive system are improved. In addition, one study showed that supplementation of Cordyceps not only directly enhances the levels of serum LH and testosterone but also improves the sperm count and motility compared to the bisphenol A-treated rats [50]. Moreover, two papers have reported that CS contributes to help the penis trap blood for erection [51,52]. All these in vivo studies support the roles of Cordyceps and/or cordycepin in improving male sexual function.
6.3. Mechanisms of CS and cordycepin stimulating steroidogenesis on mouse Leydig cells
It is important to know the mechanisms by which CS and cordycepin can induce Leydig cell steroidogenesis, and part of the possible mechanisms is illustrated in Table 3. There are variations of the mechanisms how steroidogenesis is activated by CS and cordycepin between normal mouse Leydig cells and MA-10 mouse Leydig tumor cells, respectively. In normal mouse Leydig cells, both CS and cordycepin can stimulate cAMP-PKA pathway. However, StAR protein is activated by cordycepin, but not by CS. In addition, CS does not induce the PKC pathway, and whether cordycepin can activate the PKC pathway remains unknown. In MA-10 cells, both CS and cordycepin can stimulate cAMP-PKA, PKC, and PLC pathways. CS does stimulate StAR protein expression. However, cordycepin cannot induce StAR protein expression. Besides, cordycepin can induce MAPK-Erk activity to regulate cordycepin-induced steroidogenesis in MA-10 cells. Based on these observations (Table 3), there is a basic stimulation on PKA pathway by CS and cordycepin on both Leydig cell types.
Table 3.
Pathways activated by Cordyceps sinensis (CS) and cordycepin in normal mouse Leydig cells and MA-10 cells.
| Cells | Drugs | Pathways | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| PKA | StAR | PKC | PLC | ERK | AR | ||
| Normal Leydig cells | CS | + | N/A | − | N/A | N/A | N/A |
| Cordycepin | + | + | N/A | N/A | N/A | + | |
| MA-10 cells | CS | + | + | + | + | N/A | N/A |
| Cordycepin | + | − | + | + | + | + | |
+ = stimulation; − = no effect; N/A = not examined yet.
Several lines of evidence have illustrated that LH receptor mediating effects on Leydig cell steroidogenesis are mostly through the activation of the Gsα/AC/cAMP/PKA pathway [53]. PKA will then phosphorylate proteins and induce de novo synthesis of proteins to facilitate steroidogenesis [27,54]. Thus, that CS and cordycepin regulate cAMP-PKA signal transduction to induce steroidogenesis in mouse Leydig cells in our studies is not extraordinary. However, beside the PKA pathway, CS and cordycepin do activate a variety of pathways (PKC, PLC, and MAPK pathways, etc.) in normal mouse Leydig cells and MA-10 mouse Leydig tumor cells. Study has shown that different receptors can activate more than one type of G protein to induce different cellular functions [55]. Also, different ligands on the same receptor could differentially affect gene expression in cells with different reactions [56]. Thus, these dissimilar pathways activated by CS and cordycepin between normal and tumor Leydig cells are consequently expected. Certainly, more experiments will be needed to investigate comprehensively how various signal pathways are activated by CS and cordycepin on Leydig cell steroidogenesis.
It has been found that adenosine analogs can stimulate steroid production and adenylate cyclase activity in I-10 mouse Leydig tumor cells and rat adrenal cells [57,58]. However, the expression and characterization of ARs in Leydig cells remains unknown. We have illustrated the expression of four AR mRNAs (A1, A2a, A2b, and/or A3) in primary mouse Leydig cells and MA-10 cells, which is the first time that this has been demonstrated in reproductive tissue [39,40]. There are reports of coexisting AR subtypes in different combinations in a number of cells, such as A1 and A2a in glomerular and mesangial cells [59,60], and A1, A2a, and A2b [61] in porcine coronary artery smooth muscle cells. Nevertheless, mRNA expression of four AR subtypes in primary cultured rat adrenal cell has also been illustrated [62]. Thus, the coexpression of three or four AR subtypes in mouse Leydig cells with function-stimulating steroidogenesis in our study is not unprecedented [39,40].
6.4. CS stimulates steroidogenesis in other steriodogenic organs
In addition to the effect of CS on Leydig cell functions, the influences of CS on other steroidogenic tissues have also been investigated. Treatment of human granulosa–lutein cells with CS results in the increase of E2 production due, at least in part, to increase StAR and aromatase expressions. These data may help in the development of treatment regimens to improve the success rate of in vitro fertilization [63]. Moreover, plasma corticosterone levels can be significantly induced by F2 at 0.02 mg/g body weight with 7 days feeding in immature mice, and by CS at 0.02 mg/g body weight with 3 days feeding and F3 at 0.02 mg/g body weight for 7 days feeding in mature mice. Accordingly, CS and its fractions do stimulate mouse in vivo corticosterone production from adrenal gland [64]. Thus, CS can stimulate steroidogenesis among different steroidogenic organs (testis, ovary, and adrenal gland).
6.5. Different effects of CS on hCG-induced steroidogenesis between normal and tumor Leydig cells
It is well established that steroidogenesis in Leydig cells is regulated by LH/hCG. LH binds to its receptors in Leydig cells to activate G-proteins and, in turn, AC, which increases intracellular cAMP formation [26]. It should be noted that, in our study, hCG-stimulated testosterone production is suppressed by CS in a dose-dependent relationship in purified normal mouse Leydig cells [32]. CS also reduces dibutyryl cAMP-stimulated testosterone production, which indicates that CS affects signal transduction pathway of steroidogenesis after the formation of cyclic AMP [32]. However, in MA-10 cells, CS can induce more steroid production in hCG-treated MA-10 cells [37]. Therefore, the effects of CS plus hCG on steroidogenesis between normal and tumor mouse Leydig cells are totally opposite. It has been shown that one ligand can bind to different receptor subtypes to activate different cellular functions [65–67] between normal and tumor cells [35,68]. It is possible that binding sites for CS on normal and tumor mouse Leydig cells couple to different receptors, which lead to a contrary effect on hCG-stimulated steroid synthesis. These interesting phenomena should be investigated thoroughly, which could be helpful to distinguish the differences in cellular and molecular mechanisms between normal and tumor Leydig cells.
In the in vivo study, CS alone does induce testosterone production in immature (age, 5 weeks) and mature (age, 10 weeks) mice. However, CS with hCG treatment reduces plasma testosterone production in immature and mature mice (unpublished data), which is comparable to the in vitro results that CS suppresses hCG-stimulated testosterone productions in purified normal mouse Leydig cells [32]. In our study, the in vivo treatment of hCG in mouse is to mimic the sexually reproductive status, and CS feeding is to examine whether it will enhance more reproductive performance. These observations imply that CS might have unfavorable effect on reproductive performance in fully sexual individuals. Although 10-week-old mice in our study are considered as mature mice, those mice are actually in the status of producing low testosterone. Thus, these 5- and 10-week-old mice in our studies could be considered as sexual and reproductive dysfunctional individuals, and CS and cordycepin could increase serum testosterone production. Therefore, the application of CS and cordycepin in clinical practice related to male reproduction should be evaluated first upon the testosterone status among individuals and/or patients.
6.6. Alternative substances regulating reproductive functions
Physicians have treated men with insufficient testosterone secretion by modern medicines and techniques for decades, such as the injection of gonadotropin hormones and/or testosterone to restore reproductive functions [69–71]. By contrast, traditional healers have been using a wide variety of complementary medicines to improve sexual performance and fertility for millennia [72,73]. Alternative approaches, such as the intake of plants, fungi, and insects, or their extracts, have been practiced to enhance sexuality and ameliorate illness all around the world with notable successes [72–75]. However, the scientific evidence documenting the mechanisms and efficacy of these alternative medicines upon reproductive functions is both scarce and all too often unconvincing.
There are more than 400 different species of Cordyceps, and CS has been recognized as the most famous tonic herb in traditional Chinese medicine for centuries [6,76,77]. Most of the studies of Cordyceps have focused on the antitumor activity, immunomodulating effect, antioxidant activity, hypoglycemic activity, antifatigue activity, renal protection, and hepatoprotection [6,52,77]. Very few studies have tried to investigate the effect of CS on the reproductive system. Only one book chapter briefly summarizes our study of the positive in vitro and in vivo effect of CS and cordycepin on steroidogenesis [52]. In this review, the effects of CS and cordycepin upon in vitro and in vivo steroidogenesis related to male reproduction are first reported. Naturally, more investigations are needed to comprehend how CS and its pure compounds positively affect male reproductive system in cellular and molecular levels, which will enrich the knowledge to accelerate the application of traditional Chinese medicine in clinical practice, especially the CS and cordycepin.
7. Conclusion
CS and cordycepin can significantly stimulate in vitro and in vivo steroidogenesis in mouse Leydig cells through the activation, at least, of PKA pathway, highly suggesting functional enhancements in male reproduction.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Zhu JS, Halpern GM, Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: part I. J Altern Complement Med. 1998;4:289–303. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]
- 2. Zhu JS, Halpern GM, Jones K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: part II. J Altern Complement Med. 1998;4:429–57. doi: 10.1089/acm.1998.4.429. [DOI] [PubMed] [Google Scholar]
- 3. Xu J, Huang Y, Chen XX, Zheng SC, Chen P, Mo MH. The mechanisms of pharmacological activities of Ophiocordyceps sinensis fungi. Phytother Res. 2016;30:1572–83. doi: 10.1002/ptr.5673. [DOI] [PubMed] [Google Scholar]
- 4. Cunningham KG, Manson W, Spring FS, Hutchinson SA. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature. 1950;166:949. doi: 10.1038/166949a0. [DOI] [PubMed] [Google Scholar]
- 5. Leung PH, Wu JY. Effects of ammonium feeding on the production of bioactive metabolites (cordycepin and exopolysaccharides) in mycelial culture of a Cordyceps sinensis fungus. J Appl Microbiol. 2007;103:1942–9. doi: 10.1111/j.1365-2672.2007.03451.x. [DOI] [PubMed] [Google Scholar]
- 6. Liu Y, Wang J, Wang W, Zhang H, Zhang X, Han C. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid Based Complement Alternat Med. 2015;2015:575063. doi: 10.1155/2015/575063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li SP, Li P, Dong TT, Tsim KW. Determination of nucleosides in natural Cordyceps sinensis and cultured Cordyceps mycelia by capillary electrophoresis. Electrophoresis. 2001;22:144–50. doi: 10.1002/1522-2683(200101)22:1<144::AID-ELPS144>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8. Xiao JH, Qi Y, Xiong Q. Nucleosides, a valuable chemical marker for quality control in traditional Chinese medicine Cordyceps. Recent Pat Biotechol. 2013;7:153–66. doi: 10.2174/1872208311307020007. [DOI] [PubMed] [Google Scholar]
- 9. Li SP, Zhang GH, Zeng Q, Huang ZG, Wang YT, Dong TT, Tsim KW. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine. 2006;13:428–33. doi: 10.1016/j.phymed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 10. Ohta Y, Lee JB, Hayashi K, Fujita A, Park DK, Hayashi T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J Agric Food Chem. 2007;55:10194–9. doi: 10.1021/jf0721287. [DOI] [PubMed] [Google Scholar]
- 11. Bok JW, Lermer L, Chilton J, Klingeman HG, Towers GH. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry. 1999;51:891–8. doi: 10.1016/s0031-9422(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 12. Kuo YC, Weng SC, Chou CJ, Chang TT, Tsai WJ. Activation and proliferation signals in primary human T lymphocytes inhibited by ergosterol peroxide isolated from Cordyceps cicadae. Br J Pharmacol. 2003;140:895–906. doi: 10.1038/sj.bjp.0705500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho HJ, Cho JY, Rhee MH, Park HJ. Cordycepin (3′-deoxyadenosine) inhibits human platelet aggregation in a cyclic AMP- and cyclic GMP-dependent manner. Eur J Pharmacol. 2007;558:43–51. doi: 10.1016/j.ejphar.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 14. Jeong JW, Jin CY, Kim GY, Lee JD, Park C, Kim GD, Kim WJ, Jung WK, Seo SK, Choi IW, Choi YH. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int Immunopharmacol. 2010;10:1580–6. doi: 10.1016/j.intimp.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 15. Yang LY, Chen A, Kuo YC, Lin CY. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J Lab Clin. 1999;134:492–500. doi: 10.1016/s0022-2143(99)90171-3. [DOI] [PubMed] [Google Scholar]
- 16. Kuo YC, Tsai WJ, Wang JY, Chang SC, Lin CY, Shiao MS. Regulation of bronchoalveolar lavage fluids cell function by the immunomodulatory agents from Cordyceps sinensis. Life Sci. 2001;68:1067–82. doi: 10.1016/s0024-3205(00)01011-0. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida J, Takamura S, Yamaguchi N, Ren LJ, Chen H, Koshimura S, Suzuki S. Antitumor activity of an extract of Cordyceps sinensis (Berk.) Sacc. against murine tumor cell lines. Jpn J Exp Med. 1989;59:157–61. [PubMed] [Google Scholar]
- 18. Nakamura K, Shinozuka K, Yoshikawa N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J Pharmacol Sci. 2015;127:53–6. doi: 10.1016/j.jphs.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 19. Manabe N, Sugimoto M, Azuma Y, Taketomo N, Yamashita A, Tsuboi H, Tsunoo A, Kinjo N, Nian-Lai H, Miyamoto H. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism in the mouse. Jpn J Pharmacol. 1996;70:85–8. doi: 10.1254/jjp.70.85. [DOI] [PubMed] [Google Scholar]
- 20. Chiou WF, Chang PC, Chou CJ, Chen CF. Protein constituent contributes to the hypotensive and vasorelaxant activities of Cordyceps sinensis. Life Sci. 2000;66:1369–76. doi: 10.1016/s0024-3205(00)00445-8. [DOI] [PubMed] [Google Scholar]
- 21. Manabe N, Azuma Y, Sugimoto M, Uchio K, Miyamoto M, Taketomo N, Tsuchita H, Miyamoto H. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism and blood flow in dietary hypoferric anaemic mice. Br J Nutr. 2000;83:197–204. doi: 10.1017/s0007114500000258. [DOI] [PubMed] [Google Scholar]
- 22. Dai G, Bao T, Xu C, Cooper R, Zhu JS. CordyMax Cs-4 improves steady-state bioenergy status in mouse liver. J Altern Complement Med. 2001;7:231–40. doi: 10.1089/107555301300328106. [DOI] [PubMed] [Google Scholar]
- 23. Yang LY, Huang WJ, Hsieh HG, Lin CY. H1-A extracted from Cordyceps sinensis suppresses the proliferation of human mesangial cells and promotes apoptosis, probably by inhibiting the tyrosine phosphorylation of Bcl-2 and Bcl-XL. J Lab Clin Med. 2003;141:74–83. doi: 10.1067/mlc.2003.6. [DOI] [PubMed] [Google Scholar]
- 24. Wang SM, Lee LJ, Lin WW, Chang CM. Effects of a water-soluble extract of Cordyceps sinensis on steroidogenesis and capsular morphology of lipid droplets in cultured rat adrenocortical cells. J Cell Biochem. 1998;69:483–9. doi: 10.1002/(sici)1097-4644(19980615)69:4<483::aid-jcb9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25. Saez JM. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- 26. Richards JS. New signaling pathways for hormones and cyclic adenosine 3′, 5′-monophosphate action in endocrine cells. Mol Endocrinol. 2001;15:209–18. doi: 10.1210/mend.15.2.0606. [DOI] [PubMed] [Google Scholar]
- 27. Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–44. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 28. Stocco DM, Zhao AH, Tu LN, Morohaku K, Selvaraj V. A brief history of the search for the Protein(s) involved in the acute regulation of steroidogenesis. Mol Cell Endocrinol. 2016 doi: 10.1016/j.mce.2016.07.036. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirakawa T, Galet C, Ascoli M. MA-10 cells transfected with the human lutropin/choriogonadotropin receptor (hLHR): a novel experimental paradigm to study the functional properties of the hLHR. Endocrinology. 2002;143:1026–35. doi: 10.1210/endo.143.3.8702. [DOI] [PubMed] [Google Scholar]
- 30. Hall PF, Osawa S, Mrotek J. The influence of calmodulin on steroid synthesis in Leydig cells from rat testis. Endocrinology. 1981;109:1677–82. doi: 10.1210/endo-109-5-1677. [DOI] [PubMed] [Google Scholar]
- 31. Manna PR, Pakarinen P, El-hefnawy T, Huhtaniemi IT. Functional assessment of the calcium messenger system in cultured mouse Leydig tumor cells: regulation of human chorionic gonadotropin-induced expression of the steroidogenic acute regulatory protein. Endocrinology. 1999;140:1739–51. doi: 10.1210/endo.140.4.6650. [DOI] [PubMed] [Google Scholar]
- 32. Huang BM, Hsu CC, Tsai SJ, Sheu CC, Leu SF. Effects of Cordyceps sinensis on testosterone production in normal mouse Leydig cells. Life Sci. 2001;69:2593–602. doi: 10.1016/s0024-3205(01)01339-x. [DOI] [PubMed] [Google Scholar]
- 33. Hsu CC, Huang YL, Tsai SJ, Sheu CC, Huang BM. In vivo and in vitro stimulatory effects of Cordyceps sinensis on testosterone production in mouse Leydig cells. Life Sci. 2003;73:2127–36. doi: 10.1016/s0024-3205(03)00595-2. [DOI] [PubMed] [Google Scholar]
- 34. Ascoli M. Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptor and steroidogenic responses. Endocrinology. 1981;108:88–95. doi: 10.1210/endo-108-1-88. [DOI] [PubMed] [Google Scholar]
- 35. Huang BM, Stocco DM, Hutson JC, Norman RL. Corticotropin-releasing hormone stimulates steroidogenesis in mouse Leydig cells. Biol Reprod. 1995;53:620–6. doi: 10.1095/biolreprod53.3.620. [DOI] [PubMed] [Google Scholar]
- 36. Huang BM, Chuang YM, Chen CF, Leu SF. Effects of extracted Cordyceps sinensis on steroidogenesis in MA-10 mouse Leydig tumor cells. Biol Pharm Bull. 2000;23:1532–5. doi: 10.1248/bpb.23.1532. [DOI] [PubMed] [Google Scholar]
- 37. Huang BM, Ju SY, Wu CS, Chuang WJ, Sheu CC, Leu SF. Cordyceps sinensis and its fractions stimulate MA-10 mouse Leydig tumor cell steroidogenesis. J Androl. 2001;22:831–7. [PubMed] [Google Scholar]
- 38. Huang YL, Leu SF, Liu BC, Sheu CC, Huang BM. In vivo stimulatory effect of Cordyceps sinensis mycelium and its fractions on reproductive functions in male mouse. Life Sci. 2004;75:1051–62. doi: 10.1016/j.lfs.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 39. Leu SF, Poon SL, Pao HY, Huang BM. The in vivo and in vitro stimulatory effects of cordycepin on mouse Leydig cell steroidogenesis. Biosci Biotechnol Biochem. 2011;75:723–31. doi: 10.1271/bbb.100853. [DOI] [PubMed] [Google Scholar]
- 40. Pan BS, Lin CY, Huang BM. The effect of cordycepin on steroidogenesis and apoptosis in MA-10 mouse Leydig tumor cells. Evid Based Complement Alternat Med. 2011;2011:750468. doi: 10.1155/2011/750468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pao HY, Pan BS, Leu SF, Huang sBM. Cordycepin stimulated steroidogenesis in MA-10 mouse Leydig tumor cells through the protein kinase C Pathway. J Agric Food Chem. 2012;60:4905–13. doi: 10.1021/jf205091b. [DOI] [PubMed] [Google Scholar]
- 42. Hsu CC, Tsai SJ, Huang YL, Huang BM. Regulatory mechanism of Cordyceps sinensis mycelium on mouse Leydig cell steroidogenesis. FEBS Lett. 2003;543:140–3. doi: 10.1016/s0014-5793(03)00427-7. [DOI] [PubMed] [Google Scholar]
- 43. Chen YC, Huang YL, Huang BM. Cordyceps sinensis mycelium activates PKA and PKC signal pathways to stimulate steroidogenesis in MA-10 mouse Leydig tumor cells. Int J Biochem Cell Biol. 2005;37:214–23. doi: 10.1016/j.biocel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 44. Chen YC, Huang BM. Regulatory mechanisms of Cordyceps sinensis on steroidogenesis in MA-10 mouse Leydig tumor cells. Biosci Biotechnol Biochem. 2010;74:1855–9. doi: 10.1271/bbb.100262. [DOI] [PubMed] [Google Scholar]
- 45. Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15:551–7. [PubMed] [Google Scholar]
- 46. Pakarainen T, Zhang FP, Mäkelä S, Poutanen M, Huhtaniemi I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology. 2005;146:596–606. doi: 10.1210/en.2004-0913. [DOI] [PubMed] [Google Scholar]
- 47. Lin WH, Tsai MT, Chen YS, Hou RC, Hung HF, Li CH, Wang HK, Lai MN, Jeng KC. Improvement of sperm production in subfertile boars by Cordyceps militaris supplement. Am J Chin Med. 2007;35:631–41. doi: 10.1142/S0192415X07005120. [DOI] [PubMed] [Google Scholar]
- 48. Chang Y, Jeng KC, Huang KF, Lee YC, Hou CW, Chen KH, Cheng FY, Liao JW, Chen YS. Effect of Cordyceps militaris supplementation on sperm production, sperm motility and hormones in Sprague–Dawley rats. Am J Chin Med. 2008;36:849–59. doi: 10.1142/S0192415X08006296. [DOI] [PubMed] [Google Scholar]
- 49. Sohn SH, Lee SC, Hwang SY, Kim SW, Kim IW, Ye MB, Kim SK. Effect of long-term administration of cordycepin from Cordyceps militaris on testicular function in middle-aged rats. Planta Med. 2012;78:1620–5. doi: 10.1055/s-0032-1315212. [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Chen C, Jiang Z, Wang M, Jiang H, Zhang X. Protective effect of Cordyceps militaris extract against bisphenol A induced reproductive damage. Syst Biol Reprod Med. 2016;62:249–57. doi: 10.1080/19396368.2016.1182234. [DOI] [PubMed] [Google Scholar]
- 51. Drewes SE, George J, Khan F. Recent findings on natural products with erectile-dysfunction activity. Phytochemical. 2003;62:1019–25. doi: 10.1016/s0031-9422(02)00621-0. [DOI] [PubMed] [Google Scholar]
- 52.Lin B, Li S. Cordyceps as an herbal drug. In: Benzie IFF, Wachtel-Galor S, editors. Herbal medicine: biomolecular and clinical aspects. 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. [PubMed] [Google Scholar]
- 53. Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor. A 2002 perspective. Endocr Rev. 2002;23:141–74. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 54. Stocco DM. Clinical disorders associated with abnormal cholesterol transport: mutations in the steroidogenic acute regulatory protein. Mol Cell Endocrinol. 2002;191:19–25. doi: 10.1016/s0303-7207(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 55. Berlot CH. A highly effective dominant negative as construct containing mutations that affect distinct functions inhibits multiple Gs-coupled receptor signaling pathways. J Biol Chem. 2002;277:21080–5. doi: 10.1074/jbc.M201330200. [DOI] [PubMed] [Google Scholar]
- 56. Pandini G, Conte E, Medico E, Sciacca L, Vigneri R, Belfiore A. IGF-II binding to insulin receptor isoform A induces a partially different gene expression profile from insulin binding. Ann N Y Acad Sci. 2004;1028:450–6. doi: 10.1196/annals.1322.053. [DOI] [PubMed] [Google Scholar]
- 57. Wolff J, Cook GH. Activation of steroidogenesis and adenylate cyclase by adenosine in adrenal and Leydig tumor cells. J Biol Chem. 1977;252:687–93. [PubMed] [Google Scholar]
- 58. Chen YC, Chen Y, Huang SH, Wang SM. Protein kinase Cμ mediates adenosine-stimulated steroidogenesis in primary rat adrenal cells. FEBS Lett. 2010;584:4442–8. doi: 10.1016/j.febslet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 59. Olivera A, Lopez-Novoa JM. Effect of adenosine and adenosine analogues on cyclic AMP accumulation in cultured mesangial cells and isolated glomeruli of the rat. Br J Pharmacol. 1992;107:341–6. doi: 10.1111/j.1476-5381.1992.tb12748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramkumar V, Olah ME, Jacobson KA, Stiles GL. Distinct pathways of desensitization of A1- and A2-adenosine receptors in DDT1 MF-2 cells. Mol Pharmacol. 1991;40:639–47. [PMC free article] [PubMed] [Google Scholar]
- 61. Mills I, Gewirtz H. Cultured vascular smooth muscle cells from porcine coronary artery possess A1 and A2 adenosine receptor activity. Biochem Biophys Res Commun. 1990;168:1297–302. doi: 10.1016/0006-291x(90)91170-w. [DOI] [PubMed] [Google Scholar]
- 62. Chen YC, Huang SH, Wang SM. Adenosine-stimulated adrenal steroidogenesis involves the adenosine A2A and A2B receptors and the Janus kinase 2-mitogen-activated protein kinase kinase-extracellular signal-regulated kinase signaling pathway. Int J Biochem Cell Biol. 2008;40:2815–25. doi: 10.1016/j.biocel.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 63. Huang BM, Hsiao KY, Chuang PC, Wu MH, Pan HA, Tsai SJ. Upregulation of steroidogenic enzymes and ovarian 17beta-estradiol in human granulosa-lutein cells by Cordyceps sinensis mycelium. Biol Reprod. 2004;70:1358–64. doi: 10.1095/biolreprod.103.022855. [DOI] [PubMed] [Google Scholar]
- 64. Leu SF, Chien CH, Tseng CY, Kuo YM, Huang BM. The in vivo effect of Cordyceps sinensis mycelium on plasma corticosterone level in male mouse. Biol Pharm Bull. 2005;28:1722–5. doi: 10.1248/bpb.28.1722. [DOI] [PubMed] [Google Scholar]
- 65. Kasson BG, Adashi EY, Hsueh AJ. Arginine vasopressin in the testis: an intragonadal peptide control system. Endocr Rev. 1986;7:156–68. doi: 10.1210/edrv-7-2-156. [DOI] [PubMed] [Google Scholar]
- 66. Peroutka SJ. 5-Hydroxytryptamine receptor subtypes: molecular, biochemical and physiological characterization. Trends Neurosci. 1988;11:496–500. doi: 10.1016/0166-2236(88)90011-2. [DOI] [PubMed] [Google Scholar]
- 67. Zhong H, Minneman KP. Alpha1-adrenoceptor subtypes. Eur J Pharmacol. 1999;375:261–76. doi: 10.1016/s0014-2999(99)00222-8. [DOI] [PubMed] [Google Scholar]
- 68. Huang BM, Stocco DM, Norman RL. The cellular mechanisms of corticotropin-releasing hormone (CRH) stimulated steroidogenesis in mouse Leydig cells are similar to those for LH. J Androl. 1997;18:528–34. [PubMed] [Google Scholar]
- 69. Zitzmann M, Nieschlag E. Hormone substitution in male hypogonadism. Mol Cell Endocrinol. 2000;161:73–88. doi: 10.1016/s0303-7207(99)00227-0. [DOI] [PubMed] [Google Scholar]
- 70. Huff DS, Snyder HM, 3rd, Rusnack SL, Zderic SA, Carr MC, Canning DA. Hormonal therapy for the subfertility of cryptorchidism. Horm Res. 2001;55:38–40. doi: 10.1159/000049962. [DOI] [PubMed] [Google Scholar]
- 71. Bouloux P, Warne DW, Loumaye E. FSH Study Group in Men’s Infertility. Efficacy and safety of recombinant human follicle-stimulating hormone in men with isolated hypogonadotropic hypogonadism. Fertil Steril. 2002;77:270–3. doi: 10.1016/s0015-0282(01)02973-9. [DOI] [PubMed] [Google Scholar]
- 72. Crimmel AS, Conner CS, Monga M. Withered Yang: a review of traditional Chinese medical treatment of male infertility and erectile dysfunction. J Androl. 2001;22:173–82. [PubMed] [Google Scholar]
- 73. Chauhan NS, Sharma V, Dixit VK, Thakur M. A review on plants used for improvement of sexual performance and virility. Biomed Res Int. 2014;2014:868062. doi: 10.1155/2014/868062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rege NN, Date J, Kulkarni V, Prem AR, Punekar SV, Dahanukar SA. Effect of Y virilin on male infertility. J Postgrad Med. 1997;43:64–7. [PubMed] [Google Scholar]
- 75. Veal L. Complementary therapy and infertility: an Icelandic perspective. Complement Ther Nurs Midwifery. 1998;4:3–6. doi: 10.1016/s1353-6117(98)80003-6. [DOI] [PubMed] [Google Scholar]
- 76. Shrestha B, Sung JM. Notes on Cordyceps species collected from the central region of Nepal. Mycobiology. 2005;33:235–9. doi: 10.4489/MYCO.2005.33.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus Cordyceps: a chemical and pharmacological review. J Pharm Pharmacol. 2013;65:474–93. doi: 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]