Abstract
Background:
Refractory hypoxemia episodes are characteristic of obstructive sleep apnea (OSA). Patients with OSA suffer from oxidative stress in all systems. Atrial fibrillation (AF) is a type of arrhythmia that may be induced by OSA. In this study, we explored the dose-response relationship between OSA and AF. Our research provides the basis for a novel approach to AF prevention.
Methods:
We screened four databases (PubMed, Embase, the Cochrane Library, and Web of Science) for observational studies on OSA and AF. Studies were collected from database establishment to November 2020. We performed a traditional subgroup meta-analysis. Linear and spline dose-response models were applied to assess the association between the apnea-hypopnea index, an indicator of OSA severity, and the risk of AF. Review Manager version 5.3 software and Stata 16.0 were used for the analysis.
Results:
Sixteen observational studies were included in the study. We excluded a study from the conventional meta-analysis. In the subgroup analysis, the odds ratios for new onset AF for no obvious reason, new onset AF after surgical operations, such as coronary artery bypass grafting, and AF after ablation treatment were 1.71 (95% CI 1.37–2.13, P < .05), 2.65 (95% CI 2.32–3.01, P < .05), and 2.93 (95% CI 2.47–3.49, P < .05), respectively. Linear dose-response meta-analysis results revealed that the risk of AF increased with increasing apnea-hypopnea index value.
Conclusion:
Through dose-response meta-analysis, we found a potential dose-response relationship between OSA severity and the risk of AF. This relationship should be considered in interventions aimed at AF prevention in the future.
Keywords: AF, dose-response meta, meta, OSA
1. Introduction
Obstructive sleep apnea (OSA) is a common multifactorial disorder affecting both children and adults and is characterized by recurrent upper airway obstruction during sleep, leading to intermittent hypoxia.[1] All organs and systems are negatively affected by OSA, with cardiovascular consequences. However, OSA is an underdiagnosed condition with increasing prevalence, especially in men. According to Rojo-Sanchis et al[2] the prevalence of OSA is 5% to 25% in adults, 2% to 4% in males and 1% to 2% in females.
The apnea-hypopnea index (AHI) is an indicator of OSA severity and is used to determine whether to treat OSA.[3] The severity of OSA is classified into four categories based on the the AHI: no OSA (AHI < 5), mild OSA (5 ≤ AHI < 15), moderate OSA (15 ≤ AHI < 30) and severe OSA (AHI ≥ 30).[4] Moderate-severe OSA (AHI > 15) requires treatment with continuous positive airway pressure.[5] Intermittent hypoxemia activates the sympathetic nervous system, leading to myocardial remodeling and electrophysiological remodeling, suggesting a potential association between OSA and arrhythmia. The respiratory disturbance index, the total number of respiratory disturbances per hour, is another indicator used to evaluate OSA severity. In this research, we adopted the formula to estimate the AHI.
Atrial fibrillation (AF) is the most common arrhythmia observed in patients,[6] affecting 1% to 2% of the population worldwide,[7] and it is associated with increased cardiovascular mortality due to stroke and heart failure. AF has been proven to have an increasing prevalence with advancing age.[8] Qiu et al[9] reported that the morbidity of AF is 0.1% per year in patients aged 40 years and older. Previous studies found that AF and OSA were both present in high-risk groups.[10]
However, different types of AF have different characteristics. The fact that OSA was a risk factor for overall AF has been acknowledged. We wanted to determine the influence of OSA on different types of AF, which is more meaningful and applicable for clinical work. However, there seemed to be a lack of quantitative analyses on the relationship between OSA and AF. The AHI, as an indicator of OSA severity, is an independent variable that can be used for quantitative analysis. Here, we aimed to explore the relationship between the AHI and OSA. The results can be applied in the clinic and help physicians judge the risk of AF for OSA patients, which is advantageous for AF prevention. In the present research, 18 observational studies were included. We planned not only to explore the association between OSA and different subgroups of AF in a conventional meta-analysis but also to explore the dose-response relationship between AF and OSA severity in a dose-response meta-analysis. This study offers strong evidence and new thoughts on AF prevention.
2. Methods
We registered this systematic review and dose-response meta-analysis with the INPLASY registry (INPLASY2020120104). In addition, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for meta-analyses.
2.1. Study selection
In the present study, we included observational studies such as cohort studies, case-control studies and cross-sectional studies. The included studies all had clear outcomes and declared odds ratios (ORs), relative risks or hazard ratios and 95% confidence intervals for the association between OSA and AF. There were no restrictions on sex or age. We excluded studies in which indexes other than the AHI were used as indicators. Regarding the research type, case reports, fundamental studies and reviews were also excluded. The criteria for inclusion and exclusion are shown in Table 1.
Table 1.
The criteria for inclusion and exclusion.
| Criteria for inclusion | Criteria for exclusion |
|---|---|
| Patients with OSA or AF | All kinds of reviews, case reports, or fundamental researches |
| Researches provide accurate risk ratio or odds ratio and 95% confidence intervals | No clear outcome or selected other cardiovascular diseases as cases outcomes |
| All kinds of cross-section studies, cohort studies, or case-control studies | Without a control group |
| No integrated hazard ratio risk ratio or odds ratio |
2.2. Search strategy
We screened the PubMed, Embase, Cochrane Library and Web of Science databases. The retrieval time was from inception to November 2020. To meet the different demands of different databases, we modified the search terms and strategy. We screened all of the references of the included articles to ensure that we collected as many related studies as possible. Moreover, we communicated with senior specialists when possible to obtain additional references. The search strategy used in PubMed is shown in the supplementary materials, http://links.lww.com/MD/H163.
2.3. Study validation and data extraction
Two independent investigators (DZ, JX) extracted data from the included articles. Discrepancies were handled by consultation with and guidance from FY. Data regarding the baseline information of the participants, study design, and relevant statistics were extracted. No qualification was made to how AF severity was stratified.
We evaluated the included studies according to the Newcastle-Ottawa scale (NOS).[11] A quantitative scoring device proposed by the Cochrane Collaboration was adopted to assess the studies’ methodological quality. The NOS assesses three areas: subject selection, comparability between groups, and outcome measures. The most extreme values for each area are four, two, and three, respectively. The lower the total score, the worse the methodological quality of the article is.
2.4. Data synthesis and analysis
Two investigators (DZ, YM) completed the conventional meta-analyses with Cochrane Review Manager software (version 5.3) to assess the risks of specific outcomes.
Heterogeneity among studies was evaluated with the use of Q and I2. We adopted a P-value of < .1 to indicate statistical heterogeneity.[12]I2 describes the extent of variation due to heterogeneity rather than chance. The lower the I2, the less variation is present. I2 < 25% was considered to indicate little heterogeneity, 25% < I2 < 50%. I2 > 50% indicated that there was enough heterogeneity to select a random-effects model. With I2 < 50%, a fixed-effect model was employed.
For further analysis, we performed a dose-response meta-analysis using Stata version 16.0 software using a two-step method.[13] First, the correlation between the AHI and the risk of AF was evaluated with a spline model. In this spline model, we took the AHI as an independent variable and the OR/RR as a dependent variable. Second, we selected an appropriate merged model to merge the risk value for each study calculated in the first step due to heterogeneity.
2.5. Ethical approval
This study complied with the Declaration of Helsinki. Given that the study was a meta-analysis, no prior ethical approval was required. Informed consent was obtained from all subjects involved in each included study. In addition, written informed consent was obtained from the patients to publish the papers included in this meta-analysis.
3. Results
3.1. Selection of studies
The initial search identified 1940 articles according to the inclusion criteria and exclusion criteria. After reviewing the titles and abstracts, 42 articles remained, with ten extra articles identified from reference lists. Then, we screened whole texts, and 16 articles were selected. The studies were from America, Europe and Asia and had a variety of design types, including cross-sectional, cohort and case-control.
3.2. Characteristics and quality of the included studies
There were a total of 16 observational studies included in this research. Participants were involved. The included studies were different sizes (67 to 506,604), with different proportions of females (0–44%). A total of 3361 new cases of AF were diagnosed. The prevalence of AF in patients with OSA was approximately 34.61%. The baseline characteristics of each included study are listed in Table 2. The NOS score for each included study is shown in Table 3. The NOS evaluated the articles in three dimensions (selection quality, comparability and outcome/exposure quality) according to the corresponding criteria. Every asterisk represents one point, and we calculated the total number of points as the NOS outcome for each study. The NOS score remained high (mean score = 6.66), and every study scored more than 5 on the NOS, which means that the studies we selected had high enough quality for meta-analysis.
Table 2.
The baseline characters of the included studies.
| Study | Country | Participant | AF sample size | Prevalence of AF (%) | Study design | Female | Age range/mean age (yrs) |
|---|---|---|---|---|---|---|---|
| Uchôa 2015 | Brazil | 67 | 10 | 14.92537313 | Prospective study | 25% | 58 ± 8 |
| feng2019 | US | 506,604 | 183,075 | 36.13769335 | Retrospective cohort study | 31.80% | 66.2 ± 12.1 |
| gali2020 | US | 8612 | 2502 | 29.0524849 | Retrospective cohort study | 34.50% | 62.9 ± 13.8 |
| wong2015 | US | 545 | 226 | 41.46788991 | Retrospective cohort study | 13.21% | 66.15 |
| zhao2015 | Singapore | 160 | 35 | 21.875 | Prospective study | NR | 62.8 |
| chilukuri2009 | US | 210 | 45 | 21.42857143 | Prospective study | 20% | 58 ± 10 |
| chilukuri2010 | US | 109 | 34 | 31.19266055 | Prospective study | 21% | 60 ± 10 |
| jongnarangsin2008 | US | 324 | 127 | 39.19753086 | Prospective study | 24% | 57 ± 11 |
| neilan2013 | US | 720 | 720 | 100 | Retrospective cohort study | NR | NR |
| gami2007 | US | 3542 | 133 | 3.754940711 | Retrospective cohort study | 44% | 49 ± 14 |
| mehra2006 | US | 566 | 566 | 100 | Retrospective cohort study | NR | 69.4 |
| Mehra 2009 | US | 2911 | 138 | 4.740638956 | Cohort study | NR | 76.4 ± 5.4 |
| Selim 2016 | US | 697 | 38 | 5.451936872 | Cross-sectional analysis | 5.46% | 58.69 |
| Kendzerska 2018 | Canada | 8256 | 173 | 2.095445736 | Prospective study | 28% | 47 |
| Cadby 2015 | Australia | 6841 | 455 | 6.651074404 | Prospective study | 23% | 48.3 ± 12.5 |
| anzai2020 | US | 709 | 39 | 5.500705219 | Cross-sectional study | 0 | 83.2 ± 3.1 |
Table 3.
The NOS scale of the included studies.
| Study | Selection | Comparability | Exposure | Total |
|---|---|---|---|---|
| anzai2020 | ** | ** | ** | 6 |
| cadby2015 | *** | ** | ** | 7 |
| feng2019 | ** | ** | *** | 7 |
| gali2020 | ** | ** | ** | 6 |
| gami2007 | ** | * | *** | 6 |
| mehra2006 | ** | ** | *** | 7 |
| neilan2013 | ** | ** | ** | 6 |
| wong2015 | ** | ** | *** | 7 |
| zhao2015 | ** | ** | *** | 7 |
| Uchôa 2015 | *** | ** | ** | 7 |
| chilukuri2009 | ** | ** | *** | 7 |
| chilukuri2010 | ** | ** | *** | 7 |
| jongnarangsin2008 | ** | ** | *** | 7 |
| kendzerska2018 | ** | ** | *** | 7 |
| selim2016 | ** | ** | ** | 6 |
| Mehra2009 | ** | * | *** | 6 |
3.3. Meta-analysis results
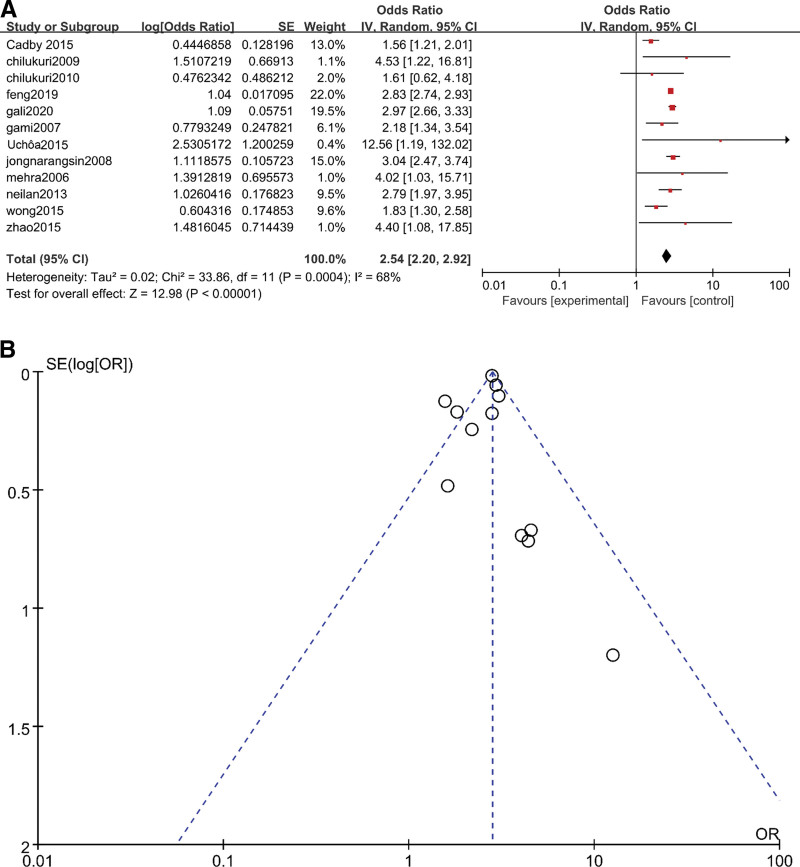
The included studies were divided into two groups, one for conventional meta-analysis and the other for dose-response meta-analysis. The studies included in the dose-response group all researched the correlation between AHI levels and the risk of AF occurrence. For the conventional group, the researchers only studied the association between OSA and AF. In the conventional meta-analysis, the result for overall AF was OR = 2.54 (95% CI 2.20–2.92, P < .05) (Fig. 1A). However, I2 = 68% (P = .004) (Fig. 1B) indicated heterogeneity in the analysis. To further explore the source of heterogeneity, the funnel plot indicated that the results from Cadby et al[14] and Wong et al[15] were outside of the 95% CI for overall AF. These were the main sources of heterogeneity.
Figure 1.
(A)Meta-analysis of OSA and risk of AF using random-effects models. (B) Funnel plots for assessment of publication bias among all included studies in the overall AF meta-analysis. AF = atrial fibrillation, OSA = obstructive sleep apnea.
The heterogeneity in the overall AF results showed that there existed a difference between each study and the others. Therefore, we performed a subgroup analysis on AF type. Briefly, the included studies were divided into three subgroups: new onset with no obvious reason, new onset after surgical operations, such as coronary artery bypass grafting (CABG), and AF after ablation treatment.
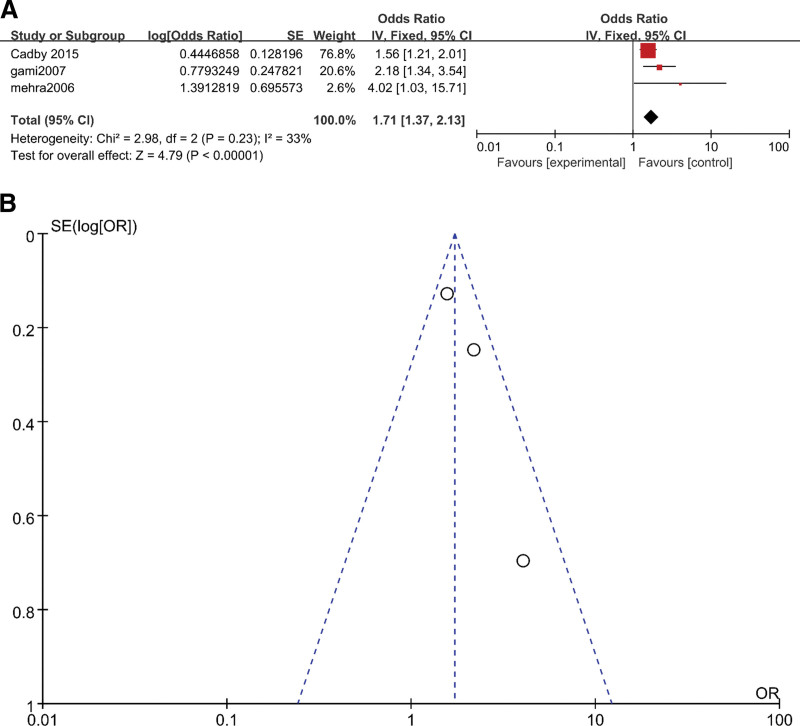
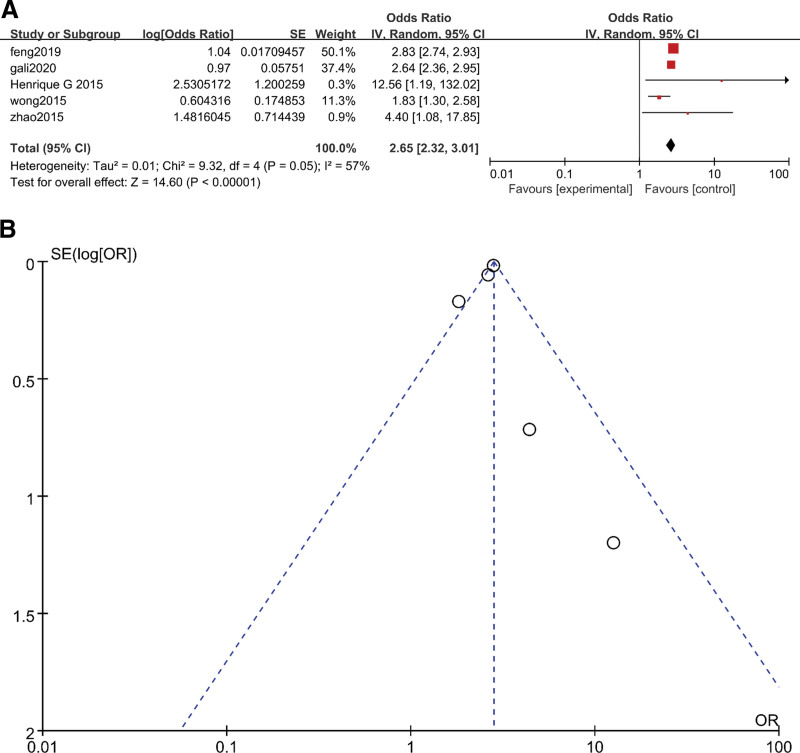
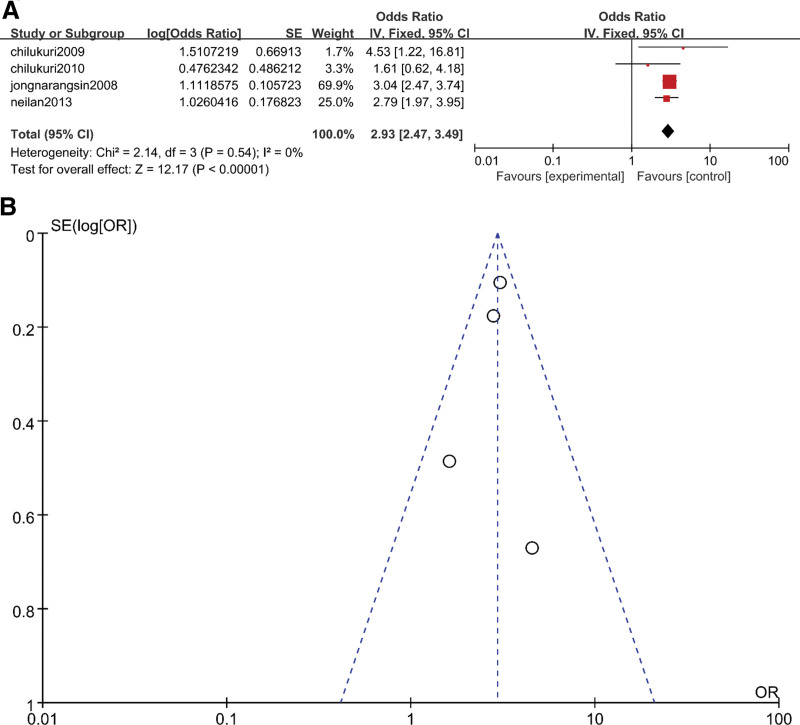
The results of the three subgroups were OR = 1.71 (95% CI 1.37–2.13, P < .05) (Fig. 2A), OR = 2.65 (95% CI 2.32–3.01, P < .05) (Fig. 3A) and OR = 2.93 (95% CI 2.47–3.49, P < .05), respectively (Fig. 4A). The heterogeneity results for these three subgroups were as follows: 33% (P = .23) (Fig. 2A), 57% (P = .05) (Fig. 3A) and 0 (P = .54) (Fig. 4A), respectively. We could judge from the funnel plots that there were no studies outside of the 95% CI for any subgroup (Fig. 2B, Fig. 3B and Fig. 4B). The results above revealed that subgroup analysis could significantly reduce the heterogeneity. The heterogeneity in the overall analysis was mainly due to the variety of AF types included in the overall analysis.
Figure 2.
(A) Meta-analysis of OSA and risk of new-onset AF using fixed-effects models. (B) Funnel plots for assessment of publication bias among all included studies in the new-onset AF subgroup meta-analysis. AF = atrial fibrillation, OSA = obstructive sleep apnea.
Figure 3.
(A) Meta-analysis of OSA and risk of AF after surgery using random-effects models. (B) Funnel plots for assessment of publication bias among all included studies in the AF after surgery subgroup meta-analysis. AF = atrial fibrillation, OSA = obstructive sleep apnea.
Figure 4.
(A) Meta-analysis of OSA and risk of recurrence AF using fixed-effects models. (B) Funnel plots for assessment of publication bias among all included studies in the recurrence AF subgroup meta-analysis. AF = atrial fibrillation, OSA = obstructive sleep apnea.
3.4. Dose-response meta-analysis
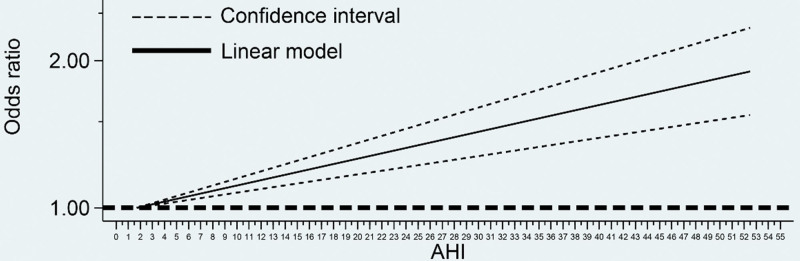
We performed a dose-response meta-analysis for new onset AF and different OSA severities to further explore the relationship between OSA and AF. We constructed linear and spline models. The spline analysis showed no association between the AHI and AF (P > .05). Therefore, we performed a restricted spline analysis to model the linear dose-response association, and the model indicated an increased risk of AF with increased OSA severity. The linear analysis revealed that the risk of AF occurrence increased by 1.26% (95% CI 0.86%–1.67%, P < .05) for each event per hour increase in AHI (OR = 1.013 95% CI 1.009–1.017, P < .05) (Fig. 5).
Figure 5.
Linear dose-response relationship between AHI and the risk of AF. The dashed line represents 95% CI. AF = atrial fibrillation, AHI = apnea-hypopnea index.
4. Discussion
In the present study, we performed a traditional meta-analysis and a dose-response meta-analysis to explore the correlation between OSA and the risk of AF in depth. Not all of the included primary studies concluded that OSA is a risk factor for the occurrence of AF. Moreover, the associations between different OSA severities and different AF types were not the same.
The conventional meta-analysis revealed that OSA was associated with the overall risk of AF (P < .05). However, heterogeneity reduced the reliability. Therefore, we performed a subgroup analysis. The results of the subgroup analysis revealed that OSA was associated with different AF types, including new-onset AF, AF after surgery and recurrent AF.
Mooe et al[16] observed that patients with OSA had a higher risk for postoperative AF (POAF) after CABG. However, they did not draw a clear conclusion regarding whether other variables played key roles in the development of POAF. In our research, after normalization of the original data, we were able to examine the influence of OSA on AF. The results indicated that OSA could be an independent risk factor for POAF.
In previous studies,[17,18] researchers proved that patients with OSA had a higher likelihood of AF recurrence and that continuous positive airway pressure benefited those patients. However, these studies defined AF recurrence as AF reappearing after successful treatment by antiarrhythmic drugs, electrical cardioversion, and catheter ablation. This limit the extension of their conclusions. In the present research, we focused on AF recurrence after ablation treatment. The results revealed that OSA significantly increased the risk of AF recurrence in patients who underwent AF ablation therapy.
Different researchers have reported different ORs for the association of OSA and new-onset AF, and in some cases, the discrepancies are significant. Due to data normalization, our results seem to be conservative. To perform a more precise analysis, we decided to explore the relationship between OSA severity and AF. In the dose-response meta-analysis, the results indicated a positive correlation between the AHI and the OR of new-onset AF.
4.1. Analysis of heterogeneity
In the meta-analysis of different AF groups, heterogeneity was present at a moderate level when merging the overall AF data from similar studies. In further subgroup analysis, the heterogeneity was effectively reduced. This revealed that the heterogeneity was mainly due to the different AF subtypes. The association between OSA and overall AF was reasonable but should be considered with caution. In the funnel plots, we verified that the primary sources of heterogeneity were Cadby et al[14] and Jongnarangsin et al[19] We speculated that the heterogeneity might be due to the following reasons. First, there were different proportions of age and sex among the participants in the included studies. According to Senaratna et al,[20] OSA prevalence and severity are higher in males and at older ages. We found that most of the included studies included more male than female patients. Briefly, the larger the male constituent ratio and the older the mean age, the higher the prevalence of AF will be. Furthermore, studies containing more women show a lower OR for AF. Sex-based differences introduced great heterogeneity to our research. Second, we did not match other factors. Some studies normalized their results, but others did not. For example, Chilukuri et al[21] used multivariate analysis, and Gami et al[22] selected univariate analysis. The multivariate analysis eliminated the errors from other factors, which led to a conservative result. This would surely introduce heterogeneity into the meta-analysis. Finally, the included studies had different study designs. Four were case-control studies, while the remaining six were cohort studies.
5. Dose-response meta-analysis
5.1. Comparison with similar studies
There have been no dose-response meta-analyses on the association between OSA and AF to date. Our research provided strong evidence that OSA is related to the risk of new-onset AF occurrence, and we analyzed the OR for AHI dose with linear and spline models. The results were consistent with previous traditional meta-analyses and other studies. Both models revealed that OSA was closely related to new-onset AF, especially at high AHI doses.
5.2. Possible mechanisms
OSA is characterized by intractable hypoxemia,[23] which leads to various pathologic conditions, including neural activation, systemic inflammation, oxidative stress loading, and hormone disorder.[24–27] There are many potential reasons for OSA leading to AF. Hypoxemia, pressure surges, and sympathetic activation are all potential mechanisms.[28] In addition, it has been proven that OSA increases the level of C-reactive protein, which is an independent predictive factor for the development of AF.[29,30] Moreover, intractable hypoxemia may cause mechanical and electrical changes in OSA patients.[31] These changes may lead to conditions conducive to AF. Another critical role in this process is an autonomic mechanism. Basic research studies have proven that cardiac autonomic ganglia ablation suppresses AF in a canine model of acute intermittent hypoxia.[32]
Our research has proven that OSA is a risk factor for AF. In addition, for different AF types, there are different ORs for OSA. OSA is a significant risk factor for patients who have undergone surgical operations such as CABG. Moreover, OSA is an essential factor in AF recurrence, which indicates that doctors should pay more attention to patients’ breathing during sleep after ablation treatment. For new-onset AF, the severity of OSA is positively related to the OR of AF. In this study, we constructed a dose-response model for AHI and new-onset AF. With the help of the model we built, physicians will have more evidence for clinical decision making regarding intervention timing for OSA patients to prevent AF.
6. Limitations
There are a few limitations to our research. First, some studies’ sample sizes were limited. Uchôa et al[33] only included 67 samples. Fewer subjects might yield a lower confidence level. This could be a reason for the heterogeneity. Second, when constructing the linear and spline models, we needed as much data as possible. The limited number of included studies introduced errors to the regression. Third, the measurement of OSA was inconsistent. The different measurement techniques might influence the AHI. In future studies, there may be challenges in selecting the timing of treatments for OSA. It will be necessary for doctors to choose appropriate opportunities to best benefit patients.
7. Conclusion
In summary, our study concluded that OSA was correlated with the risk of AF occurrence regardless of AF subtype. In the subsequent dose-response meta-analysis, we concluded that the AHI was positively related to new-onset AF. Further studies are needed to assess treatment timing for OSA patients according to their AHI for AF prevention.
Author contributions
Software: Dong Zhang.
Writing – original draft: Dong Zhang.
Abbreviations:
- AF =
- atrial fibrillation
- AHI =
- apnea-hypopnea index
- CABG =
- coronary artery bypass grafting
- NOS =
- Newcastle-Ottawa scale
- ORs =
- odds ratios
- OSA =
- obstructive sleep apnea
- POAF =
- postoperative AF
How to cite this article: Zhang D, Ma Y, Xu J, Yi F. Association between obstructive sleep apnea (OSA) and atrial fibrillation (AF): a dose-response meta-analysis. Medicine. 2022;101:30(e29443).
DZ and YM contributed equally to this work.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
AF = atrial fibrillation, OSA = obstructive sleep apnea.
AF = atrial fibrillation, NR = not reported.
NOS = Newcastle-Ottawa scale.
Contributor Information
Dong Zhang, Email: 466374026@qq.com.
Yibo Ma, Email: 1045508935@qq.com.
Jian Xu, Email: 397104841@qq.com.
References
- [1].Xu H, Wang J, Cai J, et al. Protective effect of Lactobacillus rhamnosus GG and its supernatant against myocardial dysfunction in obese mice exposed to intermittent hypoxia is associated with the activation of Nrf2 pathway. Int J Biol Sci 2019;15:2471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rojo-Sanchis C, Almerich-Silla JM, Paredes-Gallardo V, Montiel-Company JM, Bellot-Arcís C. Impact of bimaxillary advancement surgery on the upper airway and on obstructive sleep apnea syndrome: a meta-analysis. Sci Rep 2018;8:5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep 2012;35:17–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nikkonen S, Afara IO, Leppänen T, Töyräs J. Artificial neural network analysis of the oxygen saturation signal enables accurate diagnostics of sleep apnea. Sci Rep 2019;9:13200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Parrino L, Thomas RJ, Smerieri A, Spaggiari MC, Del Felice A, Terzano MG. Reorganization of sleep patterns in severe OSAS under prolonged CPAP treatment. Clin Neurophysiol 2005;116:2228–39. [DOI] [PubMed] [Google Scholar]
- [6].Calvo D, Filgueiras-Rama D, Jalife J. Mechanisms and drug development in atrial fibrillation. Pharmacol Rev 2018;70:505–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Choe WS, Kang JH, Choi EK, et al. A genetic risk score for atrial fibrillation predicts the response to catheter ablation. Korean Circ J 2019;49:338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cao W, Ma L. Curative effect of β-blocker on various ejection fractions of patients with atrial fibrillation. Exp Ther Med 2019;18:1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qiu R, Li M, Zhang X, Chen S, Li C, Shang H. Development of a core outcome set (COS) and selecting outcome measurement instruments (OMIs) for non-valvular atrial fibrillation in traditional Chinese medicine clinical trials: study protocol. Trials 2018;19:541–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lai S, Hua X, Gao R, et al. Combinational biomarkers for atrial fibrillation derived from atrial appendage and plasma metabolomics analysis. Sci Rep 2018;8:16930–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:1–173. iii–x. [DOI] [PubMed] [Google Scholar]
- [12].Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206. [DOI] [PubMed] [Google Scholar]
- [13].Xia W, Huang Y, Peng B, et al. Relationship between obstructive sleep apnoea syndrome and essential hypertension: a dose-response meta-analysis. Sleep Med 2018;47:11–8. [DOI] [PubMed] [Google Scholar]
- [14].Cadby G, McArdle N, Briffa T, et al. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest 2015;148:945–52. [DOI] [PubMed] [Google Scholar]
- [15].Wong JK, Maxwell BG, Kushida CA, et al. Obstructive sleep apnea is an independent predictor of postoperative atrial fibrillation in cardiac surgery. J Cardiothorac Vasc Anesth 2015;29:1140–7. [DOI] [PubMed] [Google Scholar]
- [16].Mooe T, Gullsby S, Rabben T, Eriksson P. Sleep-disordered breathing: a novel predictor of atrial fibrillation after coronary artery bypass surgery. Coronary Artery Disease 1996;7:475–8. [PubMed] [Google Scholar]
- [17].Goudis CA, Ketikoglou DG. Obstructive sleep and atrial fibrillation: Pathophysiological mechanisms and therapeutic implications. Int J Cardiol 2017;230:293–300. [DOI] [PubMed] [Google Scholar]
- [18].Yang Y, Ning Y, Wen W, et al. CPAP is associated with decreased risk of AF recurrence in patients with OSA, especially those younger and slimmer: a meta-analysis. J Intervent Card Electrophysiol 2020;58:369–79. [DOI] [PubMed] [Google Scholar]
- [19].Jongnarangsin K, Chugh A, Good E, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:668–72. [DOI] [PubMed] [Google Scholar]
- [20].Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- [21].Chilukuri K, Dalal D, Gadrey S, et al. A prospective study evaluating the role of obesity and obstructive sleep apnea for outcomes after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2010;21:521–5. [DOI] [PubMed] [Google Scholar]
- [22].Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110:364–7. [DOI] [PubMed] [Google Scholar]
- [23].Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015;147:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tahmasian M, Rosenzweig I, Eickhoff SB, et al. Structural and functional neural adaptations in obstructive sleep apnea: An activation likelihood estimation meta-analysis. Neurosci Biobehav Rev 2016;65:142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Laratta CR, Kendzerska T, Carlsten C, et al. Air pollution and systemic inflammation in patients with suspected OSA living in an urban residential area. Chest 2020;158:1713–22. [DOI] [PubMed] [Google Scholar]
- [26].Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest 2011;140:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Caretto M, Giannini A, Simoncini T. An integrated approach to diagnosing and managing sleep disorders in menopausal women. Maturitas 2019;128:1–3. [DOI] [PubMed] [Google Scholar]
- [28].Huang B, Liu H, Scherlag BJ, et al. Atrial fibrillation in obstructive sleep apnea: neural mechanisms and emerging therapies. Trends Cardiovasc Med 2021;31:127–32. [DOI] [PubMed] [Google Scholar]
- [29].Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019;24:429–35. [DOI] [PubMed] [Google Scholar]
- [30].Kwon CH, Kang JG, Lee HJ, et al. C-reactive protein and risk of atrial fibrillation in East Asians. Europace 2017;19:1643–9. [DOI] [PubMed] [Google Scholar]
- [31].Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol 2018;3:532–40. [DOI] [PubMed] [Google Scholar]
- [32].Yu X, Lu Z, He W, et al. Cardiac autonomic ganglia ablation suppresses atrial fibrillation in a canine model of acute intermittent hypoxia. Auton Neurosci 2017;205:26–32. [DOI] [PubMed] [Google Scholar]
- [33].Uchôa CHG, Danzi-Soares NJ, Nunes FS, et al. Impact of OSA on cardiovascular events after coronary artery bypass surgery. Chest 2015;147:1352–60. [DOI] [PubMed] [Google Scholar]