Abstract
This srudy aimed to estimate the prevalence of trastuzumab-induced cardiotoxicity in Uruguayan women diagnosed with human epidermal growth factor receptor 2 (HER2)-positive breast cancer over a 10-year period, who were treated under the financial coverage of the National Resources Fund (Fondo Nacional de Recursos).
This was an observational, descriptive study based on the analysis of an anonymized database of Uruguayan women diagnosed with HER2-positive breast cancer who received adjuvant trastuzumab treatment from to 2006 to 2016, provided by the Fondo Nacional de Recursos. Statistical analysis was performed using SPSS Statistics version 25, and variables were assessed using measures of central tendency, dispersion, contingency tables, and proportions. The chi-square test was used to analyze the association between the different variables.
The study included 1401 patients diagnosed with stage I to III HER2-positive breast cancer. The mean age at diagnosis was 52 years. The prevalence of cardiotoxicity was 20.3%. Most patients who discontinued treatment owing to cardiotoxicity eventually resumed treatment (92.6%). Moreover, the prevalence of cardiotoxicity was similar among patients who received regimens with and without anthracyclines. No association was observed between prior cardiovascular events or trastuzumab administration (concurrent vs sequential) and the development of cardiotoxicity.
In the present study, the prevalence of cardiotoxicity was similar to that reported nationally and internationally. Most patients did not develop cardiotoxicity, while the ones who developed it remained asymptomatic and cardiotoxicity was reversible.
Keywords: breast cancer, cardio-oncology, cardiac monitoring;cardiotoxicity, trastuzumab
1. Introduction
Breast cancer (BC) is the most common cancer among women worldwide. Overall, 2.1 million new cases were registered worldwide in 2018, representing the leading cause of cancer-related deaths.[1] A similar epidemiological trend can be observed in Uruguay, where BC incidence and mortality rank first among women (excluding nonmelanoma skin cancer). Approximately 1926 new cases are diagnosed each year, with a mean annual mortality rate of 670.[2] The human epidermal growth factor receptor 2 (HER2)-positive (HER2+) BC has a worldwide incidence rate of 20% to 25%,[3] with a national study revealing a prevalence rate of 27%.[4] The HER2/neu receptor is known to activate various signaling pathways involved in cell growth and differentiation, and its expression in tumors is associated with an increased tumor growth rate, angiogenesis, and metastatic potential, thus constituting a poor prognosis.[5] Overexpression of the HER2 receptor is considered not only a prognostic factor but also a predictive factor of response to treatment, as there are therapies directed against the extracellular domain of the HER2 glycoprotein, including trastuzumab. Trastuzumab was approved in 1998 by the US Food and Drug Administration for the treatment of metastatic HER2+ BC and was subsequently approved for the treatment of early HER2+ BC. The addition of trastuzumab to chemotherapy regimens has shown a substantial improvement in disease-free survival and overall survival in HER2+ patients.[6–10] The Fondo Nacional de Recursos (FNR), a nongovernmental public institution created by law in Uruguay in 1979, is part of the National Integrated Health System and provides financial coverage for high-cost procedures and medications. In 2006, trastuzumab was incorporated into the coverage of the FNR for adjuvant treatment of BC and subsequently, in October 2008, for neoadjuvant therapy and for the treatment of advanced disease. The FNR coverage protocols established selection criteria and methods analogous to those included in pivotal trials.[11]
In a previous study, we assessed the survival rate in a real-life study of 19,044 patients treated with trastuzumab over a 10-year period under the financial coverage of the FNR and observed that our findings were similar to those observed in clinical studies. The patients included in the coverage regulations for adjuvant/neoadjuvant treatment are those with a diagnosis of node-positive or node-negative operable HER2+ BC with a primary tumor, with an invasive component of ≥1 cm; in our study, the overall survival rate at 5 years for patients with operable BC (stages I–IIIA) who received the adjuvant (with or without neoadjuvant) trastuzumab (n = 1233) was 86.4% (95% confidence interval, 84.0%–88.7%).[12]
Trastuzumab is used as a monotherapy and in combination with various drugs. The most commonly used combinations in adjuvant systemic treatment are those combining trastuzumab and taxanes concurrently after treatment with anthracyclines or those combining trastuzumab, docetaxel, and carboplatin. In adjuvant and/or neoadjuvant treatment of HER2+ BC, trastuzumab can be used concurrently with a taxane or a chemotherapy regimen with docetaxel and carboplatin, or sequentially after completion of all chemotherapy treatments. Furthermore, trastuzumab can be indicated in combination with radiotherapy, hormonal therapy, or other anti-HER2 agents such as pertuzumab. The standard duration of treatment in the early stages is 12 months, and it can be used as an adjuvant and/or neoadjuvant, administered weekly or every 3 weeks.[7–10] Most patients show an excellent tolerance to treatment. The main side effect is cardiotoxicity, most often manifested by an asymptomatic decrease in left ventricular ejection fraction (LVEF) and less often by symptoms of signs of clinical heart failure (HF) as new dyspnea on exertion, increased heart rate, edema, third heart sound (S3) gallop, or weight gain (≥2 kg in 1 week). Regarding other cardiovascular adverse effects: arrhythmias have been reported in 3% of the patients and there is no increased risk of arterial or venous thrombosis.
Other side effects that patients may experience with low frequency include diarrhea, pain, headache, insomnia, dizziness, asthenia, cough, dyspnea, and infusion-related symptoms (fever and chills), nausea and vomiting (with a low emetogenic potential).[13]
Although there is limited evidence to describe disease outcomes in patients with interruption and/or early discontinuation of adjuvant trastuzumab because of cardiotoxicity, a retrospective study shows that this has been associated with worse prognosis.[14]
Some factors reportedly increase the risk of developing trastuzumab cardiotoxicity, such as preexisting cardiac dysfunction (decreased LVEF or HF), use of anthracyclines before trastuzumab treatment, obesity, diabetes, age >65 years, and radiation therapy in the mediastinum or chest wall.[15] In phase III studies, the reported cardiotoxicity ranged from 4% to 34% for adjuvant treatment with trastuzumab and chemotherapy and a retrospective study shows nearly 20% of patients on trastuzumab may experience an interruption in trastuzumab therapy due to cardiac toxicity.[16,17] According to a previous national study, the reported frequency of cardiotoxicity was 27%, 20% of the patients were asymptomatic, and the remaining 7% presenting symptomatic HF.[18] Accumulated evidence has revealed that trastuzumab-induced cardiotoxicity is not dose-dependent and is reversible in most cases when trastuzumab administration is discontinued and/or cardioprotective treatment is initiated. The accepted cardiotoxicity criteria are a decrease in absolute values by <50% and/or a reduction in LVEF >10% to 20%, determining whether discontinuation of trastuzumab administration is temporary or permanent.
Another local study presented a case series in which 18% of the patients developed cardiotoxicity, with a mean decrease of 22.5% in LVEF after initiating trastuzumab, resulting in its temporary discontinuation in all cases and the initiation of treatment with drugs that block the renin–angiotensin–aldosterone system and beta-blockers. The mean time for recovery of baseline LVEF was 49.5 days. Subsequently, trastuzumab treatment was restarted while maintaining cardioprotective treatment.[19]
The present study included all patients treated with adjuvant trastuzumab, which was provided by the FNR over a 10-year period, thus reaching a significant number of patients in a longer follow-up period when compared with previous studies in Uruguay. Notably, the study included a real-life population, understood as patients with comorbidities and risk factors, which may not be represented in randomized clinical trials. This survey will provide significant data for understanding and analyzing trastuzumab-induced cardiotoxicity in Uruguayan patients. Furthermore, this information will be useful in routine clinical practice by offering health personnel the possibility of informing their patients on the use of this drug in a manner adjusted to the national population.
The objective of the study was to estimate the prevalence of trastuzumab-induced cardiotoxicity during treatment in Uruguayan women diagnosed with HER2+ BC over a 10-year period, treated under the financial coverage of the FNR. The additional objectives were as follows: to assess the association between the development of cardiotoxicity and prior cardiovascular disease and cardiovascular risk factors (CVRF), and to correlate variables inherent to the treatment (use or absence of anthracyclines and type of regimen used for drug administration) with the occurrence of cardiotoxicity.
2. Methodology
A descriptive and retrospective observational study was conducted by analyzing an anonymized database of Uruguayan women diagnosed with HER2+ BC and treated with adjuvant trastuzumab provided by the FNR from October 2006 to December 2016.
The criteria for patient inclusion in the study were the same as those for FNR coverage protocols for adjuvant trastuzumab treatment with exclusion of patients with uncontrolled high blood pressure, unstable arrhythmia, clinically significant valvular disease, history of myocardial infarction, cardiomyopathy, or LVEF decline to <50%. A complete list of inclusion and exclusion criteria is provided in Table 1. The variables analyzed to meet the objectives of this study were grouped into 3 categories: related to the patient, treatment, and safety of the treatment. The first included prior cardiac vascular disease, particularly arterial hypertension, arrhythmias, valvular heart disease, ischemic heart disease, HF, chronic venous insufficiency, deep vein thrombosis, chronic obstructive arteriopathy, and cardiovascular risk factors such as obesity, smoking, alcoholism, and diabetes mellitus. Treatment-related variables included weekly or 3 weekly regimens and previous use of anthracyclines or not. Regarding treatment safety, the development of cardiotoxicity was defined as a decrease in LVEF of >10% when compared with the previous value, an LVEF <50% at any time during treatment, or a diagnosis of HF, and the temporary or permanent discontinuation of treatment because of carditoxicity. The LVEF assessment was performed as recommended by international guidelines, prior to trastuzumab administration (and after anthracyclines if prior used), and during treatment at months 3, 6, 9, and 12 and then after the end of treatment. All data were collected by trained data managers from the application forms completed by the patient’s oncologists and from reports of paraclinical studies.
Table 1.
Criteria for trastuzumab treatment for operable breast cancer.[11]
| All criteria must be met: | |
|---|---|
| Inclusion criteria | Exclusion criteria |
| Anatomopathological diagnosis of breast adenocarcinoma | Locoregionally advanced or metastatic breast cancer |
| Positive axillary lymph nodes or negative axillary lymph nodes with a primary tumor whose invasive component is 1 cm or larger | Poor quality of life and/or poor life expectancy because of other comorbid situations |
| HER2 positive by immunohistochemistry with staining intensity scores 3+ or amplified by a situ hybridization technique if staining intensity scores 2+ | Uncontrolled high blood pressure, unstable arrhythmia, clinically significant valvular disease, history of myocardial infarction, cardiomyopathy, or LVEF decline to <50% (echocardiogram performed after anthracycline-based chemotherapy if previously received) |
| Age ≤70 yr (analyzed individually for older patients) | Severe psychiatric illness or drug dependence with psychological evaluation predicting nonadherence to treatment |
| Less than 3 mo elapsed after the end of adjuvant chemotherapy if not given concurrently with chemotherapy. | Liver disease is defined as increased bilirubin levels and serum aminotransferases >1.5 times the upper limit of normal |
| ECG and echocardiogram for determination of the LVEF that excludes the eventuality of structural heart disease | White blood cell levels <3000/mL or neutrophils <1500 or platelet count <100,000 previous to the beginning of treatment. |
| Pregnancy or lactation | |
Prior to the request and statistical analysis of the data for the target population, the protocol was submitted to the Research Ethics Committee of the Hospital de Clínicas “Dr. Manuel Quintela” for evaluation and approval. In this regard, patient confidentiality and privacy were protected by maintaining patient anonymity during statistical analysis. Moreover, with the Declaration of Helsinki of 2006, the Universal Declaration on Bioethics and Human Rights, and the National Decree No. 379/008, all patients signed an informed consent form, whereby they agreed to the use of information collected from their medical records by persons authorized by the FNR.
Data were analyzed using SPSS Statistics version 25 for Windows (IBM Corp., Armonk, NY). The variables were assessed using the summary measures and proportions. The chi-square test was used to analyze the correlation between different variables and the development of cardiotoxicity. The level of significance was set at 5%.
3. Results
In the present study, we included 1401 patients diagnosed with stage I to III HER2+ BC who received adjuvant trastuzumab, funded by the FNR, over a 10-year period, from October 2006 to December 2016. The mean age at diagnosis was 52.45 years, with a standard deviation of 11.05 years, and a peak incidence observed between 41 and 63 years. The most frequently observed stage was state II (748 patients, 53.4%), and most tumors were hormone receptor-positive (861 patients, 61.5%). The characteristics of the study population are summarized in Table 2. All patients received chemotherapy (with or without endocrine therapy, according to ER and/or PR status), with protocols selected from those validated by pivotal clinical trials with trastuzumab.
Table 2.
Baseline characteristics of the patients.
| Variable | N | % | Mean | Standard deviation |
|---|---|---|---|---|
| Age | 1401 | 100 | 52.45 years old | 11.05 years old |
| Stage | ||||
| I | 276 | 19.7 | ||
| IIA | 452 | 32.3 | ||
| IIB | 296 | 21.1 | ||
| IIIA | 188 | 13.4 | ||
| IIIB | 49 | 3.5 | ||
| IIIC | 86 | 6.1 | ||
| Unknown | 54 | 3.9 | ||
| Menopausal status | ||||
| Premenopausal | 548 | 39.1 | ||
| Postmenopausal | 758 | 54.1 | ||
| Unknown | 95 | 6.8 | ||
| ER and PR status | ||||
| ER+ PR+ | 669 | 47.8 | ||
| ER+ PR – | 166 | 11.8 | ||
| ER– PR+ | 26 | 1.9 | ||
| ER– PR– | 476 | 33.9 | ||
| Unknown | 64 | 4.6 | ||
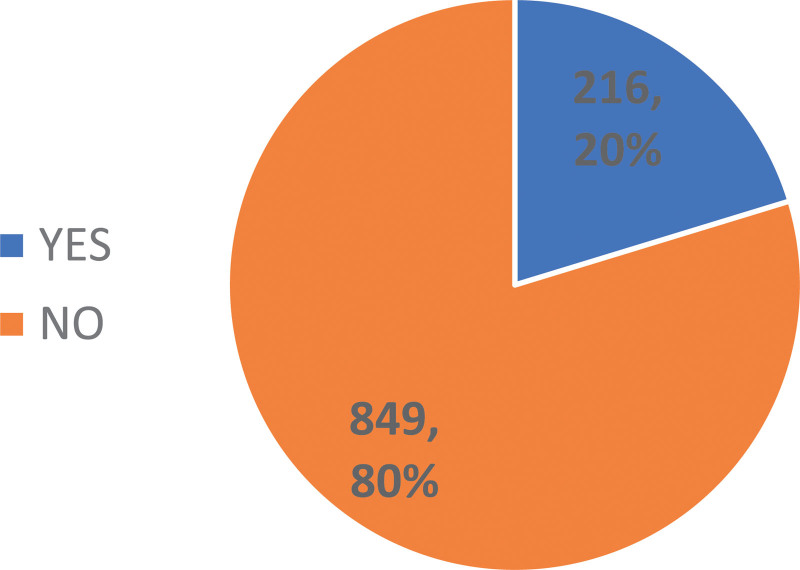
In the present study population, cardiotoxicity-related data were available for 1065 patients (76%). The prevalence of cardiotoxicity in this population was 20.3% (216 patients), data represented in Figure 1. Here, cardiotoxicity was determined by a decrease in LVEF of >10%, which was observed in 162 patients (75%), a diagnosis of symptomatic HF determined by the treating physician in 33 patients (15.3%), and an LVEF <50% observed during treatment in 21 patients (9.7%). The prevalence of symptomatic HF was 3% (33/1065 patients), considering those patients in whom cardiotoxicity could be assessed.
Figure 1.
Prevalence of cardiotoxicity.
Of the total number of patients who developed cardiotoxicity (216), 106 (49.1%) had prior cardiac vascular disease or CVRF. Table 3 shows the distribution of the number of prior cardiovascular disease or CVRF in these patients. Using the chi-square test, no statistically significant association was observed between prior cardiovascular disease or CVRF and the development of cardiotoxicity (P = .22).
Table 3.
Number of known prior cardiovascular disease or CVRF among patients who developed cardiotoxicity.
| Number of prior cardiovascular disease or CVRF | |
|---|---|
| 1 | 75 (70.7%) |
| 2 | 21 (18.8%) |
| 3 | 9 (8.5%) |
| 4 | 1 (1%) |
| 5 | 0 |
| Total | 106 |
The development of cardiotoxicity led to discontinuation of trastuzumab; however, 91.6% of these (198 patients) resumed treatment and completed the planned HER2-targeted therapy (18 cycles of treatment). No deaths were reported due to cardiotoxicity.
Regarding the treatment regimen, of the 1401 patients, 1138 (81.2%) received a chemotherapy regimen that included the prior use of anthracyclines and 161 (11.5%) received a chemotherapy regimen without anthracyclines, of whom 158 received chemotherapy with docetaxel and carboplatin (TCH); no available data were for the remaining 102 patients (7.3%). Of those who received anthracyclines, 172 patients (15.1%) developed cardiotoxicity, and of the 158 patients (11.3%) treated with the TCH regimen, 34 (21.5%) developed cardiotoxicity.
Considering the total number of patients with data available on the type of chemotherapy regimen (with prior anthracyclines versus without anthracyclines: 1138 and 161 patients, respectively), the correlation between the adopted regimen and development of cardiotoxicity was statistically analyzed using the chi-square test, and no significant association was detected between these variables (P = .05095). Furthermore, the odds ratio revealed that prior use of anthracyclines was not associated with an increased risk of developing cardiotoxicity when compared with chemotherapy regimens without anthracyclines (odds ratio, 0.6653; 95% confidence interval, 0.4436–1.015).
Regarding the treatment regimen, we further analyzed whether patients received trastuzumab concurrently or sequential to chemotherapy. Of the 1401 patients evaluated in this study, 450 (32.1%) received trastuzumab treatment sequential to chemotherapy, 849 (60.6%) received treatment concurrently, and there was no information available in the database for the remaining 102 patients (7.3%). Of the patients with available data who received sequential treatment, 71 developed cardiotoxicity (15.8%). Of those who received concurrent treatment, 135 developed cardiotoxicity (15.9%). No association was observed between the sequence of trastuzumab administration and the development of cardiotoxicity (P = .9538).
4. Discussion
The current study included 1401 women with operable HER2+ BC, with a mean age at diagnosis of 52 years and a peak incidence between 41 and 63 years, which was in contrast to the incidence of other BC subtypes, including HER2-negative luminal tumors observed in postmenopausal women or triple-negative tumors, which show peak incidence in premenopausal young women.[18–20] Regarding menopausal status, of the total number of patients, 758 (54.1%) were postmenopausal and 548 (39.1%) were premenopausal, with no data available for 95 patients (6.8%). It should be noted that HER2+ BC occurs in younger patients compared with BC in the general population. For example, in Uruguay, considering all biological subtypes of BC, 77.5% of patients are older than 50 years.[4] Regarding the stages, the diagnosis tended to occur at earlier stages, with a higher frequency of stage IIA (32.3%), similar to that reported in previous studies, such as Barrios and Garau.[4] Our study reported a cardiotoxicity prevalence (20.3%) similar to that reported in other previous retrospective studies performed in Uruguay: 27% in the study conducted by Camejo et al,[18] 18% reported by Gómez et al,[19] and also in United States.[17]
Of all patients who developed cardiotoxicity, 84.7% were asymptomatic, with decreased LVEF, and 15.3% presented with symptoms. The occurrence of symptomatic HF is low as symptomatic HF occurs in 3% of all patients, a prevalence that corroborates with that reported in the literature (2%–4%).[21] Regarding the reversibility of cardiotoxicity, it can be concluded that it is reversible in most cases after discontinuation of trastuzumab, with or without the need for additional cardiac interventions.[19,21]
The present study was unable to identify risk factors for the development of cardiotoxicity that would allow the use of suitable measures for prevention, which could be related to the coverage regulations used by the FNR, excluding patients with previous severe cardiac diseases such as uncontrolled arterial hypertension, unstable arrhythmia, clinically significant valvular heart disease, ischemic heart disease with previous infarction or angina, cardiomyopathy, or LVEF of <50%. Furthermore, no significant differences in the risk of cardiotoxicity were observed between patients who received trastuzumab treatment concurrently or sequentially to chemotherapy.
Although cautious interpretation is needed, as our results are derived from a nonrandomized retrospective analysis, the present study determined that prior exposure to anthracyclines did not increase the risk of cardiotoxicity in patients receiving adjuvant trastuzumab. Our findings are not consistent with those reported in the study by Slamon et al,[8] which revealed a statistically significant lower cardiotoxicity in patients randomized to receive TCH when compared with patients who were randomized to anthracyclines and taxanes. However, the interpretation of these results should consider that one of the main limitations is that the data were collected from forms completed by the physician at the time of requesting the medication, and not all requested clinical data was available in some cases.
5. Conclusions
Our study included a high number of patients treated in a real-world setting with adjuvant trastuzumab, showing a prevalence of cardiotoxicity similar to that reported in pivotal randomized clinical studies and consistent with reports from observational studies with fewer patients. Although 20% of the patients developed cardiotoxicity, it was reversible in most cases and allowed treatment continuation, with completion of 18 cycles. Moreover, the nonnegligible prevalence of cardiotoxicity reaffirms the need for accurate monitoring of trastuzumab treatment and referral to cardio-oncology when needed to optimize cardiovascular care and prevent interruptions for optimal treatment.
It was not possible to determine risk factors in our population, which would allow the identification of patients at greater risk of cardiotoxicity to prevent its development; however, there is a paucity of research in the area of cardiotoxicity prevention.[22]
Despite the previously mentioned methodological limitations, our results do not indicate a preferred chemotherapy regimen or sequence of trastuzumab administration (concurrent versus sequential to chemotherapy) associated with a lower risk of cardiotoxicity.
Acknowledgments
We thank the treating oncologists who provided the requested data in the FNR forms and the FNR team for their assistance in preparing the anonymized database of the requested data for this study.
Author contributions
CC, NC, NA, GP, LD contributed to the conception and design of the work; All authors were responsible for the acquisition the data. NC, CC, NA did the analysis of data and the draft of the article; LD critically revised the article; and all authors approved the final version of the article.
Abbreviations:
- BC =
- breast cancer
- CVRF =
- cardiovascular risk factor
- FNR =
- National Resources Fund (Fondo Nacional de Recursos)
- HER2 =
- human epidermal growth factor receptor 2
- HF =
- heart failure
- LVEF =
- left ventricular ejection fraction
- TCH =
- docetaxel, carboplatin, and trastuzumab.
The authors have no funding and conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
How to cite this article: Castillo C, Camejo N, Etcheverria C, Ferradaz J, Ferreira A, Fontan A, Gabin AS, Herrera G, Artagaveytia N, Parma G, Delgado L. Trastuzumab-induced cardiotoxicity in early breast cancer over a 10-year period in Uruguay. Medicine 2022;101:30(e29927).
ECG = electrocardiogram, HER2 = human epidermal growth factor receptor 2, LVEF = left ventricular ejection fraction.
ER = estrogen receptor, PR = progesterone receptor.
CVRF = cardiovascular risk factor.
Contributor Information
Natalia Camejo, Email: ncam3@yahoo.com.
Cristian Etcheverria, Email: Tycmultimedia@hotmial.com.
Jessica Ferradaz, Email: jferradazboeri@gmail.com.
Agustin Ferreira, Email: agusfb51502@gmail.com.
Analia Fontan, Email: anifontan15@gmail.com.
Ana Sofia Gabin, Email: anasofiagabin@gmail.com.
Guadalupe Herrera, Email: guadalupe.herrera2@gmail.com.
Nora Artagaveytia, Email: nartagave@gmail.com.
Gabriel Parma, Email: nrs30@adinet.com.uy.
Lucía Delgado, Email: ldelgadopebe@gmail.com.
References
- [1].Br Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Comision Honoraria de Lucha contra el Cancer. Annual report. Period 2012-2016. Available at: https://www.comisioncancer.org.uy/Ocultas/RESUMENES-ESTADISTICOS-Periodo-2012-2016-uc264 [access date January 10, 2021].
- [3].Baselga J, Perez EA, Pienkowski T, et al. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006;11(suppl 1):4–12. [DOI] [PubMed] [Google Scholar]
- [4].Barrios E, Garau M. Cáncer: magnitud del problema en el mundo y en Uruguay, aspectos epidemiológicos. Anfamed. 2017;4:04–66. [Google Scholar]
- [5].Conzen SD, Grushko TA, Olopade OI. Cancer of the breast. DeVita VT, Lawrence TS, Rosenberg S, eds. In: DeVita, Hellman & Rosenberg’s Cancer: Principles & Practices of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams & Wilkins, 2008:1595–1605. [Google Scholar]
- [6].Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. [DOI] [PubMed] [Google Scholar]
- [7].Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 3366;2011:3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. [DOI] [PubMed] [Google Scholar]
- [11].Uruguayan National Resource Fund Available at: http://www.fnr.gub.uy/sites/default/files/normativas/medicamentos/n_trat_canmama.pdf [access date October 6, 2020].
- [12].Camejo N, Castillo C, Alonso R, et al. Effectiveness of trastuzumab for human epidermal growth factor receptor 2-positive breast cancer in a real-life setting: one decade of experience under national treatment coverage regulations. JCO Glob Oncol. 2020;6:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jones RL, Smith IE. Efficacy and safety of trastuzumab. Expert Opin Drug Saf. 2004;3:317–27. [DOI] [PubMed] [Google Scholar]
- [14].Sardesai S, Sukumar J, Kassem M, et al. Clinical impact of interruption in adjuvant trastuzumab therapy in patients with operable HER-2 positive breast cancer. Cardiooncology. 2020;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Perez E, Morgan J. Cardiotoxicity of trastuzumab and other HER2-targeted agents. Available at: http://www.uptodate.com/contents/cardiotoxicity-of-trastuzumab-and-other-her2-targeted-agents [access date January 18 2020].
- [16].Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66:309–25. [DOI] [PubMed] [Google Scholar]
- [17].Yu AF, Yadav NU, Lung BY, et al. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res Treat. 2015;149:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gómez A, Américo C, Janssen B, et al. Cardiotoxicidad por trastuzumab en pacientes con cáncer de mama. Serie de casos. Rev Urug Cardiol. 2019; 34: 36–43. [Google Scholar]
- [18].Camejo N, Schiavone A, Díaz M, et al. Cardiotoxicidad inducida por trastuzumab en pacientes uruguayas portadoras de cáncer de mama HER positivo. Arch. Med Int. 2015; 37: 109–113. [Google Scholar]
- [20].Anderson WF, Chu KC, Chatterjee N, et al. Tumor variants by hormone receptor expression in white patients with node-negative breast cancer from the surveillance, epidemiology, and end results database. J Clin Oncol. 2001;19:18–27. [DOI] [PubMed] [Google Scholar]
- [21].Tocchetti CG, Ragone G, Coppola C, et al. Detection, monitoring, and management of trastuzumab-induced left ventricular dysfunction: an actual challenge. Eur J Heart Fail. 2012;14:130–7. [DOI] [PubMed] [Google Scholar]
- [22].Yasui Y, Potter JD. The shape of age-incidence curves of female breast cancer by hormone-receptor status. Cancer Causes Control. 1999;10:431–7. [DOI] [PubMed] [Google Scholar]