Abstract
Background:
The effects of omega-3 fatty acid on cardiovascular health obtained inconsistent results. A systematic review and meta-analysis were therefore conducted to assess the effects of omega-3 fatty acid supplementation for primary and secondary prevention strategies of major cardiovascular outcomes.
Methods:
The databases of PubMed, Embase, and the Cochrane library were systematically searched from their inception until September 2020. Relative risks (RRs) with 95% confidence intervals were used to assess effect estimates by using the random-effects model.
Results:
Twenty-eight randomized controlled trials involving 136,965 individuals were selected for the final meta-analysis. Omega-3 fatty acid was noted to be associated with a lower risk of major cardiovascular events (RR, 0.94; 95% CI, 0.89–1.00; P = .049) and cardiac death (RR, 0.92; 95% CI, 0.85–0.99; P = .022). However, no significant differences was noted between omega-3 fatty acid and the control for the risks of all-cause mortality (RR, 0.97; 95% CI, 0.92–1.03; P = .301), myocardial infarction (RR, 0.90; 95% CI, 0.80–1.01; P = .077), and stroke (RR, 1.02; 95% CI, 0.94–1.11; P = .694).
Conclusions:
Major cardiovascular events and cardiac death risks could be avoided with the use of omega-3 fatty acid. However, it has no significant effects on the risk of all-cause mortality, myocardial infarction, and stroke.
Keywords: omega-3 fatty acid, cardiovascular disease, meta-analysis
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death accounting for 179 million deaths annually worldwide. The incidence of CVD remains high although patients at high cardiovascular risk were treated with primary and secondary prevention strategies.[1–3] Patients still suffer substantial residual cardiovascular risk even if the CVD risk was significantly reduced in patients using appropriate treatment with statins.[4] An elevated triglyceride level was considered as an independent factor for the high residual risk on subsequent CVD.[5,6] Therefore, additional strategies should be applied to further reduce residual risk in patients.
Omega-3 fatty acids have already been approved by the US Food and Drug Administration to further reduce elevated triglyceride levels. However, studies found that long-chain omega-3 fatty acids, which including eicosapentaenoic (EPA) and docosahexaenoic acids (DHA), did not show CVD benefits, irrespective of primary or secondary prevention.[7,8] Moreover, the use of omega-3 fatty acid showed better tolerability and safety for preventing further CVD risk.[9] Furthermore, lowering of blood pressure, increasing plaque stability, and improving endothelial function are the potential benefits of omega-3 fatty acids.[10–12] Furthermore, the effects of omega-3 fatty acids on the risk of major cardiovascular outcomes obtained inconsistent results. Numerous randomized controlled trials (RCTs) have already been completed. Khan conducted a systematic review and found EPA and DHA reduced cardiovascular mortality and improved cardiovascular outcomes.[13] However, other omega-3 fatty acid (e.g., fish oils and α-linolenic acid) were not included in Khan’s study which also suggested favorable effect to cardiovascular outcomes.[26] Therefore, these data should be entered into the meta-analysis and the pooled conclusions updated. Therefore, a systematic review and meta-analysis of RCTs were conducted to evaluate the effects of omega-3 fatty acid supplementation on major cardiovascular outcomes. Moreover, the effects of omega-3 fatty acid according to the different characteristics of patients were also illustrated.
2. Methods
2.1. Ethical approvement and clinical registration
This study is a meta-analysis and does not contain any information of patients and ethical approvement and clinical registration are not applicable.
2.2. Data sources, search strategy, and selection criteria
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement was used to guide the performance and conduct of this systematic review and meta-analysis.[14] Included in this study were RCTs that investigated the effects of omega-3 fatty acid supplementation on major cardiovascular outcomes. However, the language of publication was restricted to English. The electronic databases of PubMed, Embase, and the Cochrane library were systematically searched for eligible studies using the following search terms: “omega-3 FA,” “omega-3 polyunsaturated fat,” “fish oils,” “ω-3 FA,” and “randomized controlled trial.” The publication data for the trials were from their inception until September 2020. The ongoing RCTs were also identified in https://clinicaltrials.gov/ which summarizes the trials that have already registered or have been completed but not yet published. The bibliographies of the retrieved trials were also manually reviewed for any new relevant trials.
Two reviewers independently performed the literature search and study selection. Inconsistencies between reviewers were resolved by group discussion. The trial was included if they met the following inclusion criteria: (1) participants (patients with cardiovascular disease (CVD) history or at high risk for CVD); (2) intervention (omega-3 fatty acid supplementation); (3) control (omega-6 fatty acid supplementation, placebo, or usual care); (4) outcome (the study should have reported at least one of the major cardiovascular events (MACEs), all-cause mortality, cardiac death, myocardial infarction (MI), and stroke); and (5) study design (the study had to have the RCT design).
2.3. Data collection and quality assessment
The data from the retrieved trials were independently abstracted by two reviewers. The collected data included the first author or the name of the study group, publication year, country, sample size, mean age, male gender (in percent), body mass index (BMI), smoking (in percent), hypertension (in percent), diabetes mellitus (DM), prevention, intervention, follow-up duration, and reported outcomes. The Jadad scale, which was based on randomization, concealment of the treatment allocation, blinding, completeness of follow-up, or the use of the intention-to-treat analysis, was used by two reviewers to independently assess the quality of the individual trial. The scale system ranged from 0–5.[15] Conflicts on data collection and quality assessment between reviewers were settled by an additional reviewer who referred to the original article.
2.4. Statistical analysis
The results of MACEs, all-cause mortality, cardiac death, MI, and stroke in each trial were assigned as dichotomous data. In addition, the individual relative risk (RR) with 95% confidence interval (CI) was calculated before data pooling. Furthermore, random-effects were applied to calculate the pooled effect estimates considering the underlying variations across the included trials.[16,17] The I2 and Q statistics were used to assess the heterogeneity across the included trials. Significant heterogeneity was defined as I2 > 50.0% or P < .10.[18,19] Sensitivity analysis was conducted to assess the stability of pooled conclusions by sequentially excluding individual trials.[20] Subgroup analyses were performed for MACEs, all-cause mortality, cardiac death, MI, and stroke according to sample size, mean age, male (in percent), BMI (in percent), smoking (in percent), hypertension (in percent), DM (in percent), prevention, follow-up, or study quality. Moreover, the interaction tests, which was based on Student’s t-distribution, was used to evaluate the differences between subgroups.[21] The qualitative (funnel plot) and quantitative methods (Egger and Begg tests) were also used to evaluate reported outcomes of publication biases.[22,23] The inspective level for pooled results is two-sided, and 0.05 was regarded as the cutoff. All statistical analyses in this study were conducted using the software STATA (version 10.0 StataCorp, College Station, TX).
3. Results
3.1. Search for published literature
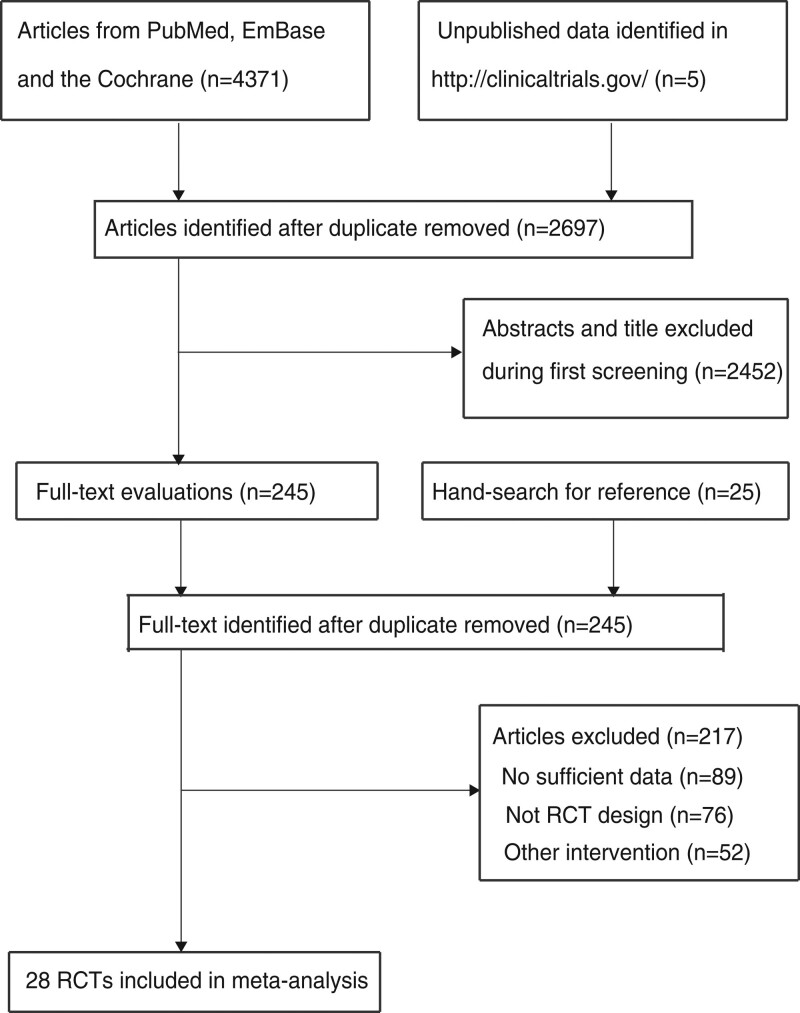
Initial electronic searches identified 4371 records, and 2697 articles were retained after the duplicates were removed. Identified for full-text evaluations were 245 articles, and 217 studies were excluded because of insufficient data (n = 89), absence of an RCT design (n = 76), and other intervention (n = 52). Reviewing the reference lists of the remaining trials yielded 25 potentially eligible trials. All of these trials were included in initial electronic searches. The remaining 28 RCTs were then selected for the final meta-analysis [24–51]. The details of the study selection are shown in Figure 1.
Figure 1.
PRISMA flowchart for the literature search and trial selection.
3.2. Characteristics of the included studies
Table 1 shows the baseline characteristics of the included studies and involved patients. Of the 28 included trials, 136,965 patients at high cardiovascular risk were recruited. The included trials were published between 1989 and 2019, and 101–25,871 patients were included in individual trials. Twelve and 18 trials applied omega-3 fatty acids as primary and secondary preventions, respectively. The mean follow-up duration ranged from 1–7.4 years, and the Jadad scale for the included trials ranged from 3–5. Twelve, ten, and six trials scored 5, 4, and 3, respectively. The trials that scored 4 or 5 in this study were considered as high quality.
Table 1.
The summary characteristics in eligible study and involved individuals.
| Study | Country | Sample size | Mean age (yr) | Male (%) | BMI (kg/m2) | Smoking (%) | Hypertension (%) | DM (%) | Prevention | Intervention | Follow-up | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burr 1989 [23] | UK | 2033 (1015/1018) | 56.5 | 100.0 | NA | 62.0 | 23.6 | NA | Secondary | N-3 EPA + DHA vs nil or oily fish advice (or capsule) vs not | 2.0 yr | 3 |
| Eritsland 1996 [24] | Norway | 610 (317/293) | 60.0 | 86.9 | 25.3 | 19.2 | 22.3 | 6.9 | Secondary | N-3 EPA + DHA vs nil | 1.0 yr | 4 |
| GISSI-P 1999 [25] | Italy | 11324 (5666/5658) | 59.4 | 85.3 | 26.5 | 42.4 | 35.6 | 14.8 | Secondary | N-3 EPA + DHA vs nil | 3.5 yr | 5 |
| Nilsen 2001 [26] | Norway | 300 (150/150) | 64.0 | 79.3 | 26.0 | 38.7 | 24.3 | 10.3 | Secondary | N-3 EPA + DHA vs corn oil | 2.0 yr | 3 |
| Bemelmans 2002 [27] | Netherlands | 266 (109/157) | 54.1 | 44.0 | NA | 49.2 | 48.5 | NA | Primary | a-linolenic acid vs omega-6 | 2.0 yr | 4 |
| Burr 2003 [28] | UK | 3114 (1571/1543) | 61.1 | 100.0 | 28.2 | 23.7 | 48.0 | 12.4 | Secondary | Oily fish or capsules n-3 EPA + DHA vs nil | 3.0–9.0 yr | 3 |
| Leaf 2005 [29] | USA | 402 (200/202) | 65.5 | 83.1 | NA | 12.2 | NA | NA | Secondary | N-3 EPA + DHA vs MUFA | 1.0 yr | 4 |
| Raitt 2005 [30] | USA | 200 (100/100) | 62.5 | 86.0 | NA | NA | 50.5 | 23.5 | Secondary | N-3 EPA + DHA vs MUFA | 2.0 yr | 4 |
| Brouwer 2006 [31] | Europe (8 countries) | 546 (273/273) | 61.5 | 84.1 | 26.9 | 12.3 | 50.7 | 15.9 | Secondary | N-3 EPA + DHA vs MUFA and n6 | 1.0 yr | 5 |
| Yokoyama 2007 [32] | Japan | 18645 (9326/9319) | 61.0 | 31.5 | 24.0 | 19.0 | 35.5 | 16.0 | Primary and secondary | EPA capsule vs nil | 5.0 yr | 4 |
| GISSI-HF 2008 [33] | Italy | 6975 (3494/3481) | 67.0 | 78.3 | 27.0 | 14.2 | 54.6 | 28.3 | Secondary | N-3 EPA + DHA vs MUFA | 3.9 yr | 5 |
| Tuttle 2008 [34] | USA | 101 (51/50) | 58.0 | 74.3 | 30.5 | 27.7 | 46.5 | 19.8 | Secondary | EPA + DHA vs MUFA | 2.0 yr | 4 |
| Quinn 2010 [35] | USA | 402 (238/164) | 76.0 | 47.8 | 26.0 | 23.4 | NA | NA | Primary | N-3 DHA vs n-6 LA | 1.5 yr | 5 |
| Kromhout 2010 [36] | Netherlands | 4837 (2404/2433) | 69.0 | 78.1 | 27.8 | 16.8 | 89.7 | 21.0 | Secondary | N-3 EPA + DHA vs nil | 3.3 yr | 5 |
| Einvik 2010 [37] | Norway | 563 (282/281) | 70.1 | 100.0 | 26.5 | 34.0 | 28.0 | 14.5 | Primary | N-3 DHA + EPA vs n-6 LA also dietary advice intervention | 3.0 yr | 4 |
| Rauch 2010 [38] | Germany | 3818 (1925/1893) | 64.0 | 74.4 | 27.5 | 36.7 | 66.5 | 27.0 | Secondary | Omega-3 vs olive oil | 1.0 yr | 5 |
| Galan 2010 [39] | France | 2501 (1253/1248) | 60.6 | 79.4 | 27.2 | 10.9 | NA | NA | Primary | N-3 omega-3 vs paraffin (non-fat), also B vitamin comparison | 4.0 yr | 5 |
| ORIGIN 2012 [40] | 40 locations in Europe and the Americas | 12536 (6281/6255) | 63.5 | 65.0 | 29.8 | 12.3 | 79.5 | NA | Primary | N-3 omega-3 vs MUFA | 6.0 yr | 5 |
| Macchia 2013 [41] | Argentina | 586 (289/297) | 66.1 | 54.8 | NA | 7.6 | 91.4 | 12.9 | Secondary | N-3 EPA + DHA vs MUFA | 1.0 yr | 4 |
| Risk & Prevention 2013 [42] | Italy | 12513 (6244/6269) | 64.0 | 61.5 | NA | 21.8 | 84.6 | 59.9 | Primary | N-3 omega-3 vs olive oil | 5.0 yr | 4 |
| Nigam 2014 [43] | Canada | 316 (153/163) | 61.0 | 66.8 | 29.0 | NA | 43.4 | 8.2 | Secondary | N-3 EPA + DHA vs n-6 | 1.0 yr | 3 |
| AREDS2 2014 [44] | USA | 4203 (2147/2056) | 74.3 | 43.2 | NA | 56.6 | NA | 13.0 | Primary | N-3 EPA + DHA vs nil | 5.0 yr | 5 |
| Doi 2014 [45] | Japan | 115 (57/58) | 70.0 | 74.8 | 24.0 | 34.8 | 68.7 | 37.4 | Secondary | N-3 EPA vs nil | 1.0 yr | 3 |
| Alfaddagh 2017 [46] | USA | 240 (126/114) | 63.0 | 85.0 | 30.7 | NA | 83.3 | 28.3 | Secondary | N-3 omega-3 vs nil | 2.5 yr | 3 |
| ASCEND 2018 [47] | UK | 15480 (7740/7740) | 63.3 | 62.6 | 30.8 | 8.3 | NA | 100.0 | Primary | N-3 EPA + DHA vs MUFA | 7.4 yr | 5 |
| Pahor 2019 [48] | USA | 289 (148/141) | 77.6 | 52.6 | 31.4 | NA | 69.2 | 23.5 | Primary | N-3 vs PUFA plus or minus losartan | 1.0 yr | 4 |
| Bhatt 2019 [49] | 11 Countries in Westernised, Eastern Europe, Asia Pacific | 8179 (4089/4090) | 64.0 | 71.2 | 30.8 | NA | NA | 58.5 | Primary and secondary | N-3 omega-3 vs paraffin oil | 4.9 yr | 5 |
| Manson 2019 [50] | USA | 25871 (12933/12938) | 67.1 | 49.4 | 28.1 | 7.2 | 49.8 | 13.7 | Primary | N-3 omega-3 vs MUFA | 5.3 yr | 5 |
3.3. Major cardiovascular events
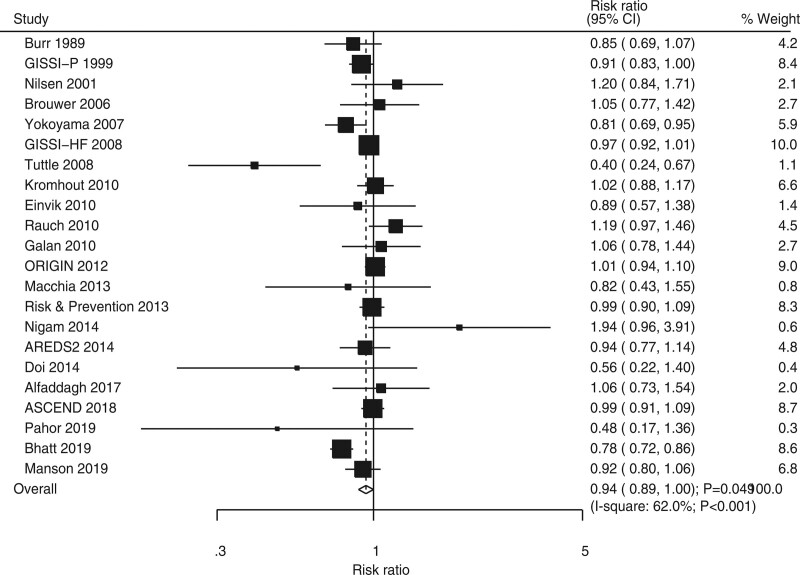
Twenty-two RCTs showed the effect of omega-3 fatty acids on the risk of MACEs. Omega-3 fatty acids was associated with a reduced risk of MACEs (RR, 0.94; 95% CI, 0.89–1.00; P = .049; Fig. 2). In addition, significant heterogeneity was seen across included trials (I2 = 62.0%; P < 0.001). The pooled conclusion for MACEs was variable after sequentially excluding individual trials because of the marginal 95% CI (Supplemental Digital Content 1, http://links.lww.com/MD2/B85). Subgroup analysis suggested that the beneficial effect of omega-3 fatty acids on MACEs risk was mainly observed in the groups with a sample size of ≥1,000, a male proportion of ≥80.0%, omega-3 fatty acids used as primary prevention, follow-up duration of ≥3 years, and trials of high quality (Table 2). Moreover, the differences among subgroups based on smoking (P < .001) and hypertension proportions (P = .002) were associated with statistical significance. No significant publication bias for MACEs was observed (P-value for Egger, 0.648; P value for Begg, 0.236; Supplemental Digital Content 2, http://links.lww.com/MD2/B86).
Figure 2.
Forest plot for the effects of omega-3 fatty acids on the risk of major cardiovascular events.
Table 2.
Subgroup analyses.
| Outcomes | Variables | Group | RR and 95% CI | P value | Heterogeneity (%) | P value for heterogeneity | P value between subgroups |
|---|---|---|---|---|---|---|---|
| Major cardiovascular events | Sample size | = 1000 | 0.94 (0.89–1.00) | .038 | 65.3 | .001 | 1.000 |
| < 1000 | 0.89 (0.68–1.17) | .406 | 61.4 | .008 | |||
| Mean age (yr) | = 60.0 | 0.96 (0.91–1.02) | .184 | 57.6 | .001 | .080 | |
| < 60.0 | 0.76 (0.57–1.02) | .066 | 79.5 | .008 | |||
| Male proportion (%) | = 80.0 | 0.92 (0.85–0.99) | .036 | 0.0 | .781 | .399 | |
| < 80.0 | 0.95 (0.88–1.01) | .122 | 69.7 | ||||
| BMI (kg/m2) | = 28.0 | 0.90 (0.79–1.04) | .158 | 81.6 | .479 | ||
| < 28.0 | 0.97 (0.90–1.03) | .323 | 36.2 | .119 | |||
| Not reported | 0.95 (0.89–1.04) | .295 | 0.0 | .637 | |||
| Smoking (%) | = 30.0 | 0.96 (0.86–1.07) | .479 | 34.4 | .165 | ||
| < 30.0 | 0.96 (0.91–1.02) | .161 | 50.0 | .029 | |||
| Not reported | 0.96 (0.65–1.40) | .819 | 68.2 | .024 | |||
| Hypertension (%) | = 50.0 | 0.99 (0.95–1.02) | .486 | 0.0 | .494 | .002 | |
| < 50.0 | 0.89 (0.78–1.01) | .076 | 63.0 | .008 | |||
| Not reported | 0.92 (0.79–1.08) | .296 | 81.0 | .001 | |||
| DM (%) | = 20.0 | 0.96 (0.88–1.05) | .346 | 72.5 | .134 | ||
| < 20.0 | 0.91 (0.81–1.02) | .108 | 54.9 | .018 | |||
| Not reported | 0.99 (0.91–1.08) | .851 | 8.5 | .335 | |||
| Prevention | Primary | 0.92 (0.85–1.00) | .050 | 68.0 | .001 | .237 | |
| Secondary | 0.97 (0.88–1.07) | .540 | 57.3 | .007 | |||
| Follow-up (yr) | = 3.0 | 0.94 (0.89–1.00) | .040 | 65.1 | .001 | .877 | |
| < 3.0 | 0.94 (0.80–1.11) | .474 | 62.5 | .003 | |||
| Study quality | High | 0.93 (0.88–1.00) | .037 | 70.0 | .619 | ||
| Low | 1.00 (0.86–1.15) | .949 | 28.4 | .212 | |||
| All-cause mortality | Sample size | = 1000 | 0.98 (0.93–1.03) | .421 | 47.6 | .029 | .158 |
| < 1000 | 0.77 (0.56–1.07) | .121 | 7.7 | .371 | |||
| Mean age (yr) | = 60.0 | 0.99 (0.95–1.04) | .751 | 16.0 | .255 | .004 | |
| < 60.0 | 0.79 (0.63–0.99) | .042 | 38.3 | .182 | |||
| Male proportion (%) | = 80.0 | 0.86 (0.70–1.05) | .135 | 61.0 | .012 | .155 | |
| < 80.0 | 0.98 (0.94–1.02) | .358 | 4.7 | .400 | |||
| BMI (kg/m2) | = 28.0 | 0.99 (0.91–1.07) | .750 | 48.2 | .085 | .621 | |
| < 28.0 | 0.97 (0.89–1.07) | .593 | 33.4 | .123 | |||
| Not reported | 0.86 (0.67–1.11) | .258 | 41.9 | .126 | |||
| Smoking (%) | = 30.0 | 0.86 (0.71–1.04) | .130 | 38.9 | .133 | .017 | |
| < 30.0 | 1.00 (0.95–1.04) | .919 | 12.0 | .319 | |||
| Not reported | 0.73 (0.37–1.42) | .353 | 46.5 | .171 | |||
| Hypertension (%) | = 50.0 | 0.98 (0.92–1.04) | .504 | 13.6 | .321 | .763 | |
| < 50.0 | 0.95 (0.83–1.09) | .492 | 61.8 | .005 | |||
| Not reported | 0.94 (0.87–1.02) | .135 | 0.0 | .667 | |||
| DM (%) | = 20.0 | 0.96 (0.90–1.03) | .244 | 20.8 | .265 | .670 | |
| < 20.0 | 0.99 (0.86–1.13) | .835 | 52.9 | .024 | |||
| Not reported | 0.92 (0.79–1.09) | .334 | 28.5 | .221 | |||
| Prevention | Primary | 0.99 (0.94–1.04) | .618 | 10.5 | .346 | .239 | |
| Secondary | 0.95 (0.85–1.06) | .336 | 46.5 | .029 | |||
| Follow-up (yr) | = 3.0 | 0.99 (0.94–1.04) | .682 | 27.7 | .181 | .024 | |
| < 3.0 | 0.88 (0.73–1.06) | .178 | 28.6 | .157 | |||
| Study quality | High | 0.97 (0.92–1.02) | .233 | 23.5 | .171 | .714 | |
| Low | 0.86 (0.61–1.23) | .415 | 66.8 | .017 | |||
| Cardiac death | Sample size | = 1000 | 0.92 (0.85–1.00) | .050 | 48.7 | .029 | .205 |
| < 1000 | 0.70 (0.45–1.08) | .105 | 0.0 | .702 | |||
| Mean age (yr) | = 60.0 | 0.95 (0.88–1.02) | .146 | 20.6 | .224 | .015 | |
| < 60.0 | 0.78 (0.67–0.92) | .003 | 9.2 | .347 | |||
| Male proportion (%) | = 80.0 | 0.83 (0.63–1.09) | .189 | 68.5 | .004 | .518 | |
| < 80.0 | 0.93 (0.88–0.99) | .013 | 0.0 | .768 | |||
| BMI (kg/m2) | = 28.0 | 0.95 (0.82–1.10) | .492 | 64.8 | .014 | .431 | |
| < 28.0 | 0.90 (0.84–0.97) | .007 | 0.0 | .755 | |||
| Not reported | 0.85 (0.62–1.15) | .279 | 40.7 | .150 | |||
| Smoking (%) | = 30.0 | 0.80 (0.71–0.91) | .001 | 0.0 | .721 | .016 | |
| < 30.0 | 0.97 (0.89–1.05) | .462 | 33.1 | .134 | |||
| Not reported | 0.81 (0.67–0.98) | .032 | 0.0 | .390 | |||
| Hypertension (%) | = 50.0 | 0.95 (0.89–1.02) | .151 | 0.0 | .594 | .135 | |
| < 50.0 | 0.91 (0.75–1.10) | .306 | 55.6 | .021 | |||
| Not reported | 0.82 (0.72–0.94) | .004 | 0.0 | .901 | |||
| DM (%) | = 20.0 | 0.91 (0.85–0.97) | .006 | 0.0 | .516 | .774 | |
| < 20.0 | 0.94 (0.76–1.15) | .518 | 52.3 | .040 | |||
| Not reported | 0.86 (0.65–1.14) | .289 | 53.3 | .092 | |||
| Prevention | Primary | 0.93 (0.86–1.00) | .053 | 0.0 | .506 | .838 | |
| Secondary | 0.91 (0.79–1.04) | .173 | 51.3 | .025 | |||
| Follow-up (yr) | = 3.0 | 0.95 (0.88–1.03) | .245 | 37.1 | .112 | .012 | |
| < 3.0 | 0.80 (0.70–0.90) | < .001 | 0.0 | .626 | |||
| Study quality | High | 0.92 (0.87–0.97) | .001 | 0.0 | .708 | .430 | |
| Low | 0.85 (0.52–1.37) | .500 | 80.7 | .001 | |||
| Myocardial infarction | Sample size | = 1000 | 0.90 (0.79–1.02) | .091 | 63.4 | .002 | .818 |
| < 1000 | 0.97 (0.59–1.59) | .890 | 0.0 | .431 | |||
| Mean age (yr) | = 60.0 | 0.87 (0.77–0.99) | .028 | 47.9 | .023 | .101 | |
| < 60.0 | 1.03 (0.68–1.55) | .889 | 46.8 | .130 | |||
| Male proportion (%) | = 80.0 | 1.07 (0.73–1.59) | .723 | 39.1 | .177 | .082 | |
| < 80.0 | 0.87 (0.76–0.98) | .026 | 48.7 | .021 | |||
| BMI (kg/m2) | = 28.0 | 0.84 (0.68–1.04) | .102 | 79.5 | .001 | .424 | |
| < 28.0 | 0.91 (0.79–1.04) | .165 | 0.6 | .419 | |||
| Not reported | 1.01 (0.76–1.33) | .956 | 17.0 | .304 | |||
| Smoking (%) | = 30.0 | 1.09 (0.88–1.36) | .441 | 12.4 | .336 | .001 | |
| < 30.0 | 0.89 (0.78–1.00) | .055 | 36.4 | .117 | |||
| Not reported | 0.70 (0.60–0.82) | 0.0 | .515 | ||||
| Hypertension (%) | = 50.0 | 0.98 (0.87–1.11) | .762 | 2.4 | .407 | .051 | |
| < 50.0 | 0.92 (0.72–1.17) | .501 | 58.1 | .026 | |||
| Not reported | 0.85 (0.69–1.05) | .140 | 56.4 | .076 | |||
| DM (%) | = 20.0 | 0.82 (0.71–0.93) | .003 | 24.7 | .249 | ||
| < 20.0 | 0.83 (0.72–0.97) | .017 | 9.2 | .359 | |||
| Not reported | 1.13 (0.97–1.31) | .127 | 3.9 | .373 | |||
| Prevention | Primary | 0.86 (0.74–1.00) | .045 | 62.7 | .006 | .190 | |
| Secondary | 0.99 (0.80–1.23) | .948 | 21.0 | .256 | |||
| Follow-up (yr) | = 3.0 | 0.86 (0.75–0.98) | .022 | 61.3 | .008 | .053 | |
| < 3.0 | 1.07 (0.83–1.38) | .588 | 10.2 | .350 | |||
| Study quality | High | 0.86 (0.76–0.97) | .013 | 50.1 | .020 | .022 | |
| Low | 1.23 (0.92–1.64) | .167 | 0.4 | .404 | |||
| Stroke | Sample size | = 1000 | 1.03 (0.93–1.13) | .616 | 32.7 | .146 | .861 |
| < 1000 | 0.92 (0.36–2.35) | .861 | 0.0 | .736 | |||
| Mean age (yr) | = 60.0 | 1.00 (0.92–1.09) | .976 | 10.7 | .340 | .171 | |
| < 60.0 | 1.23 (0.92–1.64) | .163 | 0.0 | .545 | |||
| Male proportion (%) | = 80.0 | 1.23 (0.91–1.64) | .174 | - | - | .183 | |
| < 80.0 | 1.00 (0.92–1.09) | 1.000 | 4.7 | .400 | |||
| BMI (kg/m2) | = 28.0 | 0.94 (0.84–1.05) | .279 | 18.0 | .297 | .047 | |
| < 28.0 | 1.11 (0.97–1.27) | .145 | 0.0 | .771 | |||
| Not reported | 1.23 (0.95–1.59) | .112 | 0.0 | .710 | |||
| Smoking (%) | = 30.0 | 1.17 (0.92–1.48) | .193 | 0.0 | .671 | .019 | |
| < 30.0 | 1.03 (0.95–1.12) | .511 | 0.0 | .663 | |||
| Not reported | 0.73 (0.56–0.94) | .015 | 0.0 | .796 | |||
| Hypertension (%) | = 50.0 | 1.08 (0.90–1.29) | .436 | 29.6 | .224 | .372 | |
| < 50.0 | 1.07 (0.93–1.23) | .322 | 0.0 | .758 | |||
| Not reported | 0.94 (0.77–1.14) | .512 | 41.4 | .163 | |||
| DM (%) | = 20.0 | 1.02 (0.82–1.28) | .851 | 62.5 | .031 | .354 | |
| < 20.0 | 1.08 (0.95–1.23) | .252 | 0.0 | .926 | |||
| Not reported | 0.94 (0.81–1.08) | .365 | 0.0 | .695 | |||
| Prevention | Primary | 0.99 (0.89–1.09) | .795 | 23.5 | .234 | .063 | |
| Secondary | 1.19 (0.99–1.44) | .065 | 0.0 | .916 | |||
| Follow-up (yr) | = 3.0 | 1.01 (0.91–1.11) | .872 | 31.1 | .169 | .226 | |
| < 3.0 | 1.19 (0.90–1.58) | .213 | 0.0 | .803 | |||
| Study quality | High | 1.02 (0.93–1.12) | .684 | 23.8 | .210 | .757 | |
| Low | 1.08 (0.72–1.61) | .719 | 0.0 | .645 |
3.4. All-cause mortality
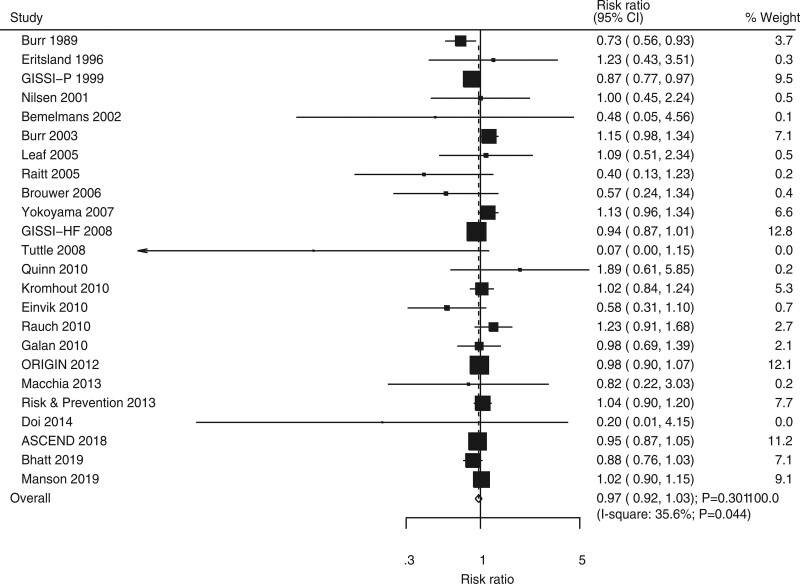
Twenty-four RCTs showed the effect of omega-3 fatty acids on the risk of all-cause mortality. No significant difference was noted between omega-3 fatty acids and control for the risks of all-cause mortality (RR, 0.97; 95% CI, 0.92–1.03; P = .301; Fig. 3). Potential significant heterogeneity was detected across included trials (I2 = 35.6%; P = .044). The pooled conclusion was robustness and was not changed when a sensitivity analysis was conducted (Supplemental Digital Content 1, http://links.lww.com/MD2/B85). Subgroup analysis suggested that omega-3 fatty acids could protect against all-cause mortality risk when the mean age of individuals was <60 years (Table 2). Moreover, the effects of omega-3 fatty acids on the risk of all-cause mortality could be affected by mean age (P = .004), smoking proportion (P = .017), and follow-up duration (P = .024). No significant publication bias was noted for all-cause mortality (P value for Egger, 0.337; P value for Begg, 0.309; Supplemental Digital Content 2, http://links.lww.com/MD2/B86).
Figure 3.
Forest plot for the effects of omega-3 fatty acids on the risk of all-cause mortality.
3.5. Cardiac death
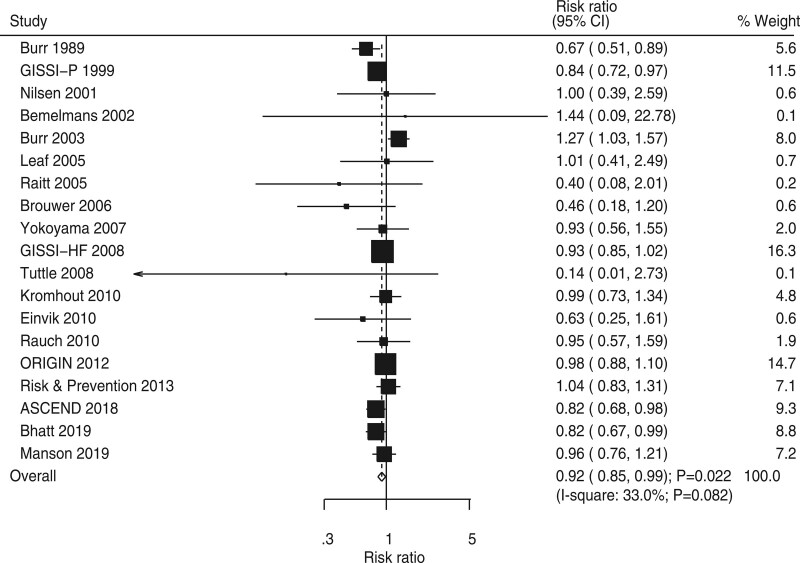
Nineteen RCTs showed the effect of omega-3 fatty acids on the risk of cardiac death. The pooled RR indicated that omega-3 fatty acids could protect against cardiac death risk (RR, 0.92; 95% CI, 0.85–0.99; P = .022; Fig. 4) and potential heterogeneity among included trials (I2 = 33.0%; P = .082). The pooled conclusion for cardiac death risk was variable owing to the marginal 95% CI (Supplemental Digital Content 1, http://links.lww.com/MD2/B85). Subgroup analysis found that the beneficial effects of omega-3 fatty acids on cardiac death were mainly observed in the groups with a sample size of ≥1,000, mean age of <60 years, a male proportion of <80%, BMI of <28 kg m−2, the smoking proportion of ≥30% or trials that did not report smoking proportion, trials that did not report hypertension proportion, DM proportion of ≥20%, follow-up duration of <3 years, and trials of high quality (Table 2). Moreover, the risk of cardiac death for the use of omega-3 fatty acids could be affected by mean age (P = .015), smoking proportion (P = .016), and follow-up duration (P = .012). Moreover, no significant publication bias for cardiac death was detected (P value for Egger, .282; P value for Begg, 0.576; Supplemental Digital Content 2, http://links.lww.com/MD2/B86).
Figure 4.
Forest plot for the effects of omega-3 fatty acids on the risk of cardiac death.
3.6. Myocardial infarction
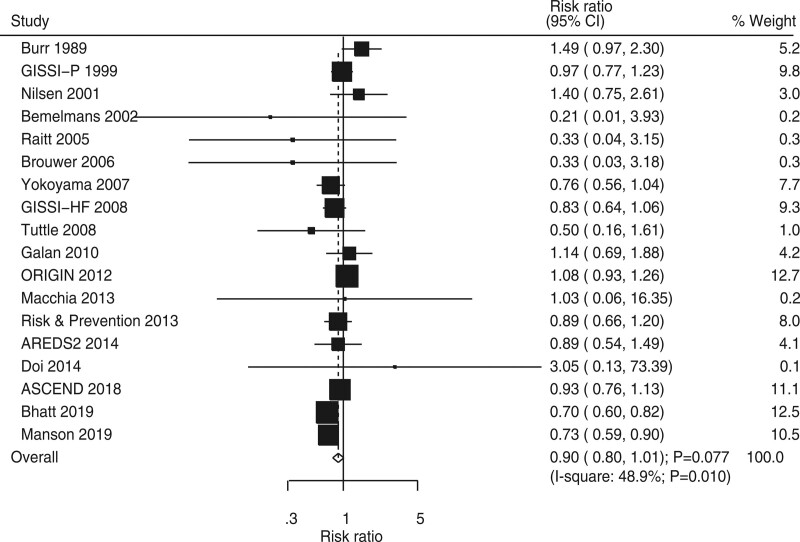
Eighteen RCTs showed the effect of omega-3 fatty acids on the risk of MI. Omega-3 fatty acids was noted to not be associated with a reduced risk of MI (RR, 0.90; 95% CI, 0.80–1.01; P = .077; Fig. 5), and significant heterogeneity was detected across included trials (I2 = 48.9%; P = .010). Sensitivity analysis indicated that the risk of MI may be reduced by sequentially excluding individual trials (Supplemental Digital Content 1, http://links.lww.com/MD2/B85). Subgroup analysis suggested that omega-3 fatty acids significantly reduced the risk of MI when the mean age was ≥60 years, the male proportion was <80%, trials on smoking proportion were not reported, DM proportion was ≥20% or <20%, omega-3 fatty acids were used as primary prevention, follow-up duration was ≥3 years, and trials were of high quality (Table 2). Moreover, smoking proportion (P = .001), DM proportion (P <.001), and study quality (P = .022) could affect the effects of omega-3 fatty acids on the risk of MI. No significant publication bias exists for the risk of MI (P value for Egger, .979; P value for Begg, .880; Supplemental Digital Content 2, http://links.lww.com/MD2/B86).
Figure 5.
Forest plot for the effects of omega-3 fatty acids on the risk of myocardial infarction.
3.7. Stroke
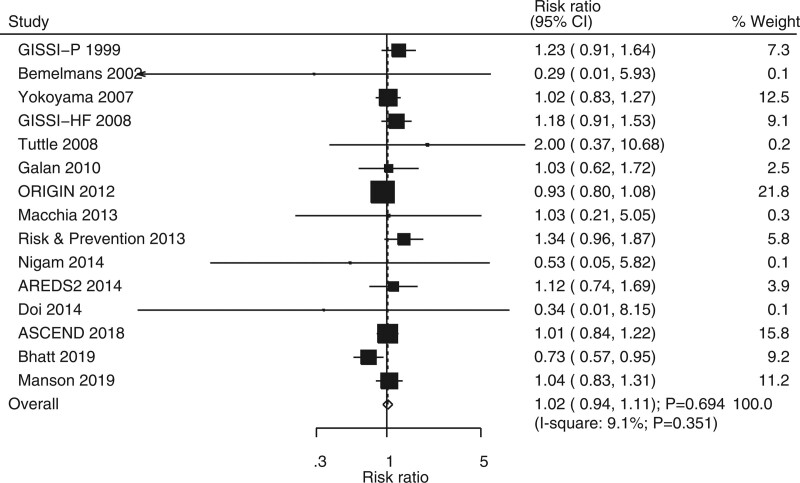
Fifteen RCTs showed the effect of omega-3 fatty acids on the risk of stroke. No significant differences were noted between omega-3 fatty acids and control for the risk of stroke (RR, 1.02; 95% CI, 0.94–1.11; P = .694; Fig. 6). In addition, unimportant heterogeneity was seen among the included trials (I2 = 9.1%; P = .351). The pooled conclusion was robustness and was not altered by sequentially excluding individual trials (Supplemental Digital Content 1, http://links.lww.com/MD2/B85). Subgroup analysis suggested that omega-3 fatty acids could protect against stroke risk when pooled trials did not report smoking proportion (Table 2). Moreover, the effects of omega-3 fatty acids on the risk of stroke could be affected by BMI (P = .047) and smoking proportion (P = .019). No significant publication bias was detected for the risk of stroke (P value for Egger, .893; P value for Begg, .767; Supplemental Digital Content 2, http://links.lww.com/MD2/B86).
Figure 6.
Forest plot for the effects of omega-3 fatty acids on the risk of stroke.
4. Discussion
An observational study initially reported the potential role of omega-3 fatty acids for in preventing the risks of major cardiovascular outcomes.[52] However, this effect lacks further intervention RCTs confirmed to date. The current study included RCTs and assessed the effects of omega-3 fatty acids on the outcomes of MACEs, all-cause mortality, cardiac death, MI, and stroke. This comprehensive, quantitative meta-analysis involved 136,965 individuals from 28 trials across a wide range of characteristics. Furthermore, this study suggested that omega-3 fatty acids could protect against the risk of MACEs and cardiac death. However, omega-3 fatty acids were not associated with the risk of all-cause mortality, MI, and stroke. The effects of omega-3 fatty acids could be affected by mean age, BMI, smoking proportion, hypertension proportion, DM proportion, follow-up duration, and study quality as found in the results of subgroup analysis.
The role of omega-3 fatty acids on major cardiovascular outcomes have already been illustrated in several systematic reviews and meta-analyses. A meta-analysis conducted by Marik et al contained 11 RCTs and found that dietary supplementation with omega-3 fatty acids could reduce the risk of nonfatal MACEs, cardiac death, sudden cardiac death, and all-cause mortality. Thus, it should be applied as a secondary prevention for major cardiovascular outcomes.[53] On the one hand, Filion et al conducted a meta-analysis of 29 RCTs and found that omega-3 fatty acids did not yield significant benefits on the risk of all-cause mortality and restenosis for patients at high cardiovascular risk.[54] On the other hand, Kwak et al performed a meta-analysis of 14 RCTs and found that the use of omega-3 fatty acids as secondary prevention did not contribute sufficient effects on MACEs for patients with CVD history.[55] Moreover, a meta-analysis conducted by Rizos et al included 20 RCTs and found that the use of omega-3 fatty acids did not yield significant benefits for cardiovascular outcomes.[56] Furthermore, Casula et al conducted a meta-analysis of 11 RCTs to assess the effects of long-term omega-3 fatty acids for the secondary prevention of major cardiovascular outcomes and found the protective role of long-term high-dose omega-3 fatty acids on the risk of cardiac death, sudden death, and MI for patients with CVD history.[57] In addition, a meta-analysis conducted by Wen et al included 14 RCTs and found that omega-3 fatty acids have no significant effect on the risk of MACEs while it could reduce the risk of all-cause mortality, cardiac death, and sudden cardiac death for patients with coronary heart disease.[58] Moreover, Aung et al conducted a meta-analysis of 10 RCTs and found that omega-3 fatty acids were not associated with the risk of fatal or nonfatal coronary heart disease or MACEs.[59] Furthermore, Popoff et al conducted a meta-analysis of 10 RCTs and found that omega-3 fatty acids did not provide significant benefits on cardiovascular health for patients after acute MI.[60] However, several new published RCTs should be included and the pooled conclusions needed to be updated. Therefore, the current systematic review and meta-analysis were conducted to assess the effects of omega-3 fatty acids on major cardiovascular outcomes.
In summary, the results suggested that omega-3 fatty acids could protect against the risk of MACEs. Most of the included trials did not find significant differences between omega-3 fatty acids and control, while four trials reported a similar conclusion.[26,33,35,50] The GISSI-Prevenzione trial found that dietary supplementation with omega-3 fatty acids could yield significant benefits on MACEs (all-cause mortality, nonfatal MI, and nonfatal stroke).[26] The Japan EPA Lipid Intervention Study trial suggested that the use of eicosapentaenoic acid should be considered as a promising strategy for the prevention of MACEs for hypercholesterolemic patients.[33] The THIS-DIET trial found active intervention with the Mediterranean-style diet and could provide significant benefits on cardiovascular health in patients after MI.[35] The REDUCE-IT trial found that the risk for MACEs was significantly reduced for patients with elevated triglyceride levels applied with 2 g of omega-3 fatty acids.[50] The potential reason for this could be that omega-3 fatty acids have antiarrhythmic effects.[61,62] Moreover, the use of omega-3 fatty acids could reduce platelet aggregation,[63,64] vasodilation,[65,66] antiproliferation,[67] plaque stabilization,[68] and reduction in lipid action.[69,70]
The use of omega-3 fatty acids was noted to prevent the risk of cardiac death. However, it has no significant effects on the risk of all-cause mortality, MI, and stroke. The protective role of omega-3 fatty acids on cardiac death could be explained by the low dose of omega-3 fatty acids that could prevent sudden cardiac death through an antiarrhythmic effect.[71] Sensitivity analysis found that omega-3 fatty acids may play a beneficial effect on the risk of all-cause mortality. This result could be explained by the high proportion of death caused by cardiac reasons. Furthermore, the use of omega-3 fatty acids did not affect the risk of MI and stroke. These results could be affected by the dose and duration of omega-3 fatty acid supplementation.
Significant heterogeneity exists for several major cardiovascular outcomes, and subgroup analysis was performed to assess the role of omega-3 fatty acids in patients with specific characteristics. Mean age, BMI, smoking proportion, hypertension proportion, DM proportion, follow-up duration, and study quality were noted to affect the effects of omega-3 fatty acids on major cardiovascular outcomes. Several reasons could explain these results. First, cardiovascular risk could be affected by the mean age of the patients, and the proportion of comorbidity across patients is different, which could affect the progression of major cardiovascular outcomes. Second, the role of omega-3 fatty acids may be more evident for patients at low cardiovascular risk, including the characteristics of BMI, smoking, hypertension, and DM proportion. (3) Third, the follow-up duration is significantly correlated with the duration of the use of omega-3 fatty acids and the events of interest outcome. (4) Lastly, the quality of the trials was related to the evidence level and the reliability of the pooled conclusions.
Several limitations of this study should be mentioned. First, the type of omega-3 fatty acids may affect the progression of major cardiovascular outcomes. Second, the treatment effect between the omega-3 fatty acids and control could be affected by the background intake of omega-3 fatty acids and other treatment strategies. Third, the definition of MACEs is different across the included trials, and the risk of MACEs for individuals using omega-3 fatty acids could be affected. Fourth, the subgroup analyses according to background therapies were not conducted because the stratified data according to the specific treatment strategy were not available. Lastly, inherent limitations exist for meta-analysis based on pooled data, including inevitable publication bias and restricted detailed analyses.
In conclusion, this study found that the use of omega-3 fatty acids could significantly reduce the risk of MACEs and cardiac death. However, no significant differences were found between omega-3 fatty acids and control for the risk of all-cause mortality, MI, and stroke. Further large-scale RCT should be conducted to assess the effects of omega-3 fatty acids on major cardiovascular outcomes. In addition, a cumulative meta-analysis should be conducted to assess the pooled effect estimates in clinical practice.
Author contributions
Conceptualization: Fangyu Yu, Shun Qi.
Data curation: Ruokui Cao, Shaohong Fang, Shun Qi, Xizhi Wang, Yanan Ji.
Formal analysis: Fangyu Yu, Yanan Ji.
Methodology: Fangyu Yu.
Project administration: Fangyu Yu.
Writing – original draft: Fangyu Yu, Ruokui Cao, Shaohong Fang, Shun Qi, Xizhi Wang, Yanan Ji.
Writing – review & editing: Ruokui Cao, Shaohong Fang, Shun Qi, Xizhi Wang.
Abbreviations:
- BMI =
- body mass index
- CVD =
- cardiovascular disease
- DM =
- diabetes mellitus
- MACEs =
- major cardiovascular events
- MI =
- myocardial infarction
- RCTs =
- randomized controlled trials
- RRs =
- relative risks
How to cite this article: Yu F, Qi S, Ji Y, Wang X, Fang S, Cao R. Effects of omega-3 fatty acid on major cardiovascular outcomes: a systematic review and meta-analysis. Medicine. 2022;101:30(e29556).
Funding information was not available.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
DM = diabetes mellitus.
BMI = body mass index, CI = confidence interval, DM = diabetes mellitus.
Contributor Information
Shun Qi, Email: qishun0001@qq.com.
Yanan Ji, Email: 1401120933@qq.com.
Xizhi Wang, Email: 315076373@qq.com.
Shaohong Fang, Email: 148132054@qq.com.
Ruokui Cao, Email: ruokui@sina.com.
References
- [1].Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–7. [DOI] [PubMed] [Google Scholar]
- [2].Nambi V, Bhatt DL. Primary prevention of atherosclerosis: time to take a selfie? J Am Coll Cardiol. 2017;70:2992–4. [DOI] [PubMed] [Google Scholar]
- [3].Vaduganathan M, Venkataramani AS, Bhatt DL. Moving toward global primordial prevention in cardiovascular disease: the heart of the matter. J Am Coll Cardiol. 2015;66:1535–7. [DOI] [PubMed] [Google Scholar]
- [4].Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Klempfner R, Erez A, Sagit BZ, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the bezafibrate infarction prevention study and registry. Circ Cardiovasc Qual Outcomes. 2016;9:100–8. [DOI] [PubMed] [Google Scholar]
- [6].Toth PP, Granowitz C, Hull M, et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7:e008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J. 2015;36:774–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72:330–43. [DOI] [PubMed] [Google Scholar]
- [9].Siscovick DS, Barringer TA, Fretts AM, et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135:e867–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].British Nutrition Foundation. n-3 Fatty acids and health. London: British Nutrition Foundation, 1999; Available at: https://www.nutrition.org.uk/attachments/156_n-3%20Fatty%20acids%20and%20health%20summary.pdf. [Google Scholar]
- [11].Bhatnagar D, Durrington PN. Omega-3 fatty acids: their role in the prevention and treatment of atherosclerosis related risk factors and complications. Int J Clin Pract. 2003;57:305–14. [PubMed] [Google Scholar]
- [12].Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–85. [DOI] [PubMed] [Google Scholar]
- [13].Khan SU, Lone AN, Khan MS, et al. Effect of omega-3 fatty acids on cardiovascular outcomes: a systematic review and meta-analysis. EClinicalMedicine. 2021;38:100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- [16].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [17].Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25:646–54. [DOI] [PubMed] [Google Scholar]
- [18].Deeks JJ, Higgins J, Altman DG. Higgins J, Green S. Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions UK: Oxford, 2008;243–96. [Google Scholar]
- [19].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Technical Bulletin. 1999;8:7526–9. [Google Scholar]
- [21].Altman DG. Statistics notes: interaction revisited: the difference between two estimates. BMJ. 2003;326:219–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- [24].Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989;2:757–61. [DOI] [PubMed] [Google Scholar]
- [25].Eritsland J, Arnesen H, Gronseth K, et al. Effect of dietary supplementation with n-3 fatty acids on coronary artery bypass graft patency. Am J Cardiol. 1996;77:31–6. [DOI] [PubMed] [Google Scholar]
- [26].GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- [27].Nilsen DW, Albrektsen G, Landmark K, et al. Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am J Clin Nutr. 2001;74:50–6. [DOI] [PubMed] [Google Scholar]
- [28].Bemelmans WJ, Broer J, Feskens EJ, et al. Effect of an increased intake of alpha-linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean Alpha-linolenic Enriched Groningen Dietary Intervention (MARGARIN) study. Am J Clin Nutr. 2002;75:221–7. [DOI] [PubMed] [Google Scholar]
- [29].Burr ML, Ashfield-Watt PA, Dunstan FD, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57:193–200. [DOI] [PubMed] [Google Scholar]
- [30].Leaf A, Albert CM, Josephson M, et al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–8. [DOI] [PubMed] [Google Scholar]
- [31].Raitt MH, Connor WE, Morris C, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators. JAMA. 2005;293:2884–91. [DOI] [PubMed] [Google Scholar]
- [32].Brouwer IA, Zock PL, Camm AJ, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006;295:2613–9. [DOI] [PubMed] [Google Scholar]
- [33].Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. [DOI] [PubMed] [Google Scholar]
- [34].investigators G-H. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–30. [DOI] [PubMed] [Google Scholar]
- [35].Tuttle KR, Shuler LA, Packard DP, et al. Comparison of low-fat versus Mediterranean-style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial). Am J Cardiol. 2008;101:1523–30. [DOI] [PubMed] [Google Scholar]
- [36].Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kromhout D, Giltay EJ, Geleijnse JM, et al. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–26. [DOI] [PubMed] [Google Scholar]
- [38].Einvik G, Ole Klemsdal T, Sandvik L, et al. A randomized clinical trial on n-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. Eur J Cardiovasc Prev Rehabil. 2010;17:588–92. [DOI] [PubMed] [Google Scholar]
- [39].Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–9. [DOI] [PubMed] [Google Scholar]
- [40].Galan P, Kesse-Guyot E, Czernichow S, et al. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bosch J, Gerstein HC, et al.; Origin Trial Investigators. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–18. [DOI] [PubMed] [Google Scholar]
- [42].Macchia A, Grancelli H, Varini S, et al. Omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: results of the FORWARD (Randomized Trial to Assess Efficacy of PUFA for the Maintenance of Sinus Rhythm in Persistent Atrial Fibrillation) trial. J Am Coll Cardiol. 2013;61:463–8. [DOI] [PubMed] [Google Scholar]
- [43].Roncaglioni MC, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–8. [DOI] [PubMed] [Google Scholar]
- [44].Nigam A, Talajic M, Roy D, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. J Am Coll Cardiol. 2014;64:1441–8. [DOI] [PubMed] [Google Scholar]
- [45].Bonds DE, Harrington M, et al.; Writing Group for the ARG. Effect of long-chain omega-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Intern Med. 2014;174:763–71. [DOI] [PubMed] [Google Scholar]
- [46].Doi M, Nosaka K, Miyoshi T, et al. Early eicosapentaenoic acid treatment after percutaneous coronary intervention reduces acute inflammatory responses and ventricular arrhythmias in patients with acute myocardial infarction: a randomized, controlled study. Int J Cardiol. 2014;176:577–82. [DOI] [PubMed] [Google Scholar]
- [47].Alfaddagh A, Elajami TK, Ashfaque H, et al. Effect of eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease: a randomized clinical trial. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Group ASC, Bowman L, Mafham M, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540–50. [DOI] [PubMed] [Google Scholar]
- [49].Pahor M, Anton SD, Beavers DP, et al. Effect of losartan and fish oil on plasma IL-6 and mobility in older persons. the ENRGISE pilot randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2019;74:1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- [51].Manson JE, Cook NR, Lee IM, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–67. [DOI] [PubMed] [Google Scholar]
- [53].Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Filion KB, El Khoury F, Bielinski M, et al. Omega-3 fatty acids in high-risk cardiovascular patients: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2010;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kwak SM, Myung SK, Lee YJ, et al. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012;172:686–94. [DOI] [PubMed] [Google Scholar]
- [56].Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–33. [DOI] [PubMed] [Google Scholar]
- [57].Casula M, Soranna D, Catapano AL, et al. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: a meta-analysis of randomized, placebo controlled trials [corrected]. Atheroscler Suppl. 2013;14:243–51. [DOI] [PubMed] [Google Scholar]
- [58].Wen YT, Dai JH, Gao Q. Effects of Omega-3 fatty acid on major cardiovascular events and mortality in patients with coronary heart disease: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:470–5. [DOI] [PubMed] [Google Scholar]
- [59].Aung T, Halsey J, Kromhout D, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Popoff F, Balaciano G, Bardach A, et al. Omega 3 fatty acid supplementation after myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2019;19:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Harris WS, Park Y, Isley WL. Cardiovascular disease and long-chain omega-3 fatty acids. Curr Opin Lipidol. 2003;14:9–14. [DOI] [PubMed] [Google Scholar]
- [62].Din JN, Newby DE, Flapan AD. Omega 3 fatty acids and cardiovascular disease--fishing for a natural treatment. BMJ. 2004;328:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hirai A, Terano T, Hamazaki T, et al. The effects of the oral administration of fish oil concentrate on the release and the metabolism of [14C]arachidonic acid and [14C]eicosapentaenoic acid by human platelets. Thromb Res. 1982;28:285–98. [DOI] [PubMed] [Google Scholar]
- [64].Tamura Y, Hirai A, Terano T, et al. Clinical and epidemiological studies of eicosapentaenoic acid (EPA) in Japan. Prog Lipid Res. 1986;25:461–6. [DOI] [PubMed] [Google Scholar]
- [65].Hamazaki T, Hirai A, Terano T, et al. Effects of orally administered ethyl ester of eicosapentaenoic acid (EPA; C20:5, ω-3) on PGI2-like substance production by rat aorta. Prostaglandins. 1982;23:557–67. [DOI] [PubMed] [Google Scholar]
- [66].Okuda Y, Kawashima K, Sawada T, et al. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun. 1997;232:487–91. [DOI] [PubMed] [Google Scholar]
- [67].Terano T, Shiina T, Tamura Y. Eicosapentaenoic acid suppressed the proliferation of vascular smooth muscle cells through modulation of various steps of growth signals. Lipids. 1996;31(Suppl):S301–4. [DOI] [PubMed] [Google Scholar]
- [68].Kawano H, Yano T, Mizuguchi K, et al. Changes in aspects such as the collagenous fiber density and foam cell size of atherosclerotic lesions composed of foam cells, smooth muscle cells and fibrous components in rabbits caused by all-cis-5, 8, 11, 14, 17-icosapentaenoic acid. J Atheroscler Thromb. 2002;9:170–7. [DOI] [PubMed] [Google Scholar]
- [69].Nozaki S, Matsuzawa Y, Hirano K, et al. Effects of purified eicosapentaenoic acid ethyl ester on plasma lipoproteins in primary hypercholesterolemia. Int J Vitam Nutr Res. 1992;62:256–60. [PubMed] [Google Scholar]
- [70].Ando M, Sanaka T, Nihei H. Eicosapentanoic acid reduces plasma levels of remnant lipoproteins and prevents in vivo peroxidation of LDL in dialysis patients. J Am Soc Nephrol. 1999;10:2177–84. [DOI] [PubMed] [Google Scholar]
- [71].Kang JX, Leaf A. Prevention of fatal cardiac arrhythmias by polyunsaturated fatty acids. Am J Clin Nutr. 2000;71:202S–7S. [DOI] [PubMed] [Google Scholar]