Abstract
Mycobacterium sp. strain AP1 grew with pyrene as a sole source of carbon and energy. The identification of metabolites accumulating during growth suggests that this strain initiates its attack on pyrene by either monooxygenation or dioxygenation at its C-4, C-5 positions to give trans- or cis-4,5-dihydroxy-4,5-dihydropyrene, respectively. Dehydrogenation of the latter, ortho cleavage of the resulting diol to form phenanthrene 4,5-dicarboxylic acid, and subsequent decarboxylation to phenanthrene 4-carboxylic acid lead to degradation of the phenanthrene 4-carboxylic acid via phthalate. A novel metabolite identified as 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid demonstrates a new branch in the pathway that involves the cleavage of both central rings of pyrene. In addition to pyrene, strain AP1 utilized hexadecane, phenanthrene, and fluoranthene for growth. Pyrene-grown cells oxidized the methylenic groups of fluorene and acenaphthene and catalyzed the dihydroxylation and ortho cleavage of one of the rings of naphthalene and phenanthrene to give 2-carboxycinnamic and diphenic acids, respectively. The catabolic versatility of strain AP1 and its use of ortho cleavage mechanisms during the degradation of polycyclic aromatic hydrocarbons (PAHs) give new insight into the role that pyrene-degrading bacterial strains may play in the environmental fate of PAH mixtures.
Polycyclic aromatic hydrocarbons (PAHs) are pollutants of concern because of their toxic and carcinogenic potentials (14). The combustion of organic materials is mainly responsible for their ubiquitous distribution in the atmosphere, surface waters, and sediments (8). PAHs are also constituents of crude oils and materials derived from coal (e.g., creosote and coal tar). Accidental spillage and improper disposal during the processing, transportation, and use of these materials have resulted in a number of contaminated sites presenting serious health and ecological risks. Bioremediation technologies have increasingly been proposed to decontaminate those sites. However, PAH-polluted sites frequently resist a fast and complete cleanup. Among the reasons suggested for the attenuation in the biodegradation rates are the accumulation of toxic metabolites and the fact that the residual concentrations comprise the components more resistant to degradation (18). Strategies to improve remediation tecnologies for PAH-contaminated soils require a broader understanding of the biochemical pathways involved in degradation and in the eventual formation of partially oxidized products.
The pathways for the biodegradation of PAHs containing two or three aromatic rings are well documented (3). However, only in the last 12 years have a number of bacteria capable of metabolizing PAHs with four rings been reported. Pyrene, one of the most abundant high-molecular-weight PAHs in environmental mixtures, is mainly degraded by actinomycetes such as Mycobacterium and Rhodococcus (15). Based on the identification of initial ring oxidation and ring cleavage metabolites produced during the growth of Mycobacterium PYR-1 on pyrene and low concentrations of organic nutrients, a pathway for pyrene degradation has been proposed (3, 13). This pathway, later confirmed in other microorganisms, including strains that use pyrene as a sole source of carbon and energy, is initiated by dioxygenation at the C-4 and C-5 positions followed by ortho cleavage to give phenanthrene 4,5-dicarboxylic acid (7, 15, 24, 25). Nevertheless, the complexity of the pyrene molecule and the identification of metabolites apparently resulting from dioxygenation at other positions by new strains (28) and of other metabolites (e.g., trans-dihydrodiols) whose subsequent metabolism has not been elucidated suggest that an understanding of the environmental routes for pyrene degradation requires further research.
Most studies on the microbial metabolism of PAHs have been performed with strains that use the compound under study as a growth substrate (3, 15). However, a number of degrading bacteria act on a variety of compounds that do not support their growth and produce partially oxidized products (10). This versatility is partly due to the broad substrate specificity of the degradative enzymes, as has been widely demonstrated for naphthalene and toluene dioxygenases (9, 27). Given that the proposed metabolic pathway for pyrene degradation includes atypical reactions in the degradation of PAHs, it is of interest to investigate the potential actions of pyrene-degrading bacteria on other PAHs.
We report the isolation of a new strain of Mycobacterium (AP1) that uses pyrene as a sole source of carbon and energy. In addition to the pathway previously proposed for other Mycobacterium isolates, strain AP1 degrades pyrene by an alternative branch that includes a novel metabolite resulting of two sequential ring cleavages. The strain also grows on hexadecane, phenanthrene, and fluoranthene and transforms several other PAHs. The actions of ring oxidation and ring cleavage for individual compounds are described.
MATERIALS AND METHODS
Chemicals.
PAHs and analogues were purchased from Aldrich Chemical Co., Inc., Milwaukee, Wis. Diazomethane was generated by alkaline decomposition of Diazald (N-methyl-N-nitroso-p-toluenesulfonamide) (2). Solvents were obtained from J. T. Baker, Deventer, The Netherlands. All chemicals and solvents were of the highest purity available.
Media and supply of hydrocarbons.
The mineral salts medium was described previously (12). When needed, mineral medium was supplemented with 5 g of Casamino Acids (Difco, Detroit, Mich.)/liter. To prepare mineral medium or Casamino Acids mineral medium with pyrene, this hydrocarbon in methylene chloride solution was added to empty flasks. The solvent was evaporated by gentle rotation, which resulted in the formation of a thin layer of pyrene on the bottom of the flasks. The liquid medium was added, and the flasks were sterilized in an autoclave. The final concentration of pyrene was 1 g/liter. An exception was made in the experiments demonstrating growth at the expense of pyrene; this compound in acetone solution (5%) was added to sterile mineral medium to give a final concentration of 0.2 g/liter. The flasks were shaken at 200 rpm and 30°C before inoculation to allow acetone removal. Solid medium with pyrene was prepared by adding the PAH in acetone solution to the sterile medium at 45°C to give a final concentration of 0.1 g/liter. Other aromatic compounds were added to the sterile liquid medium or to washed cell suspensions in acetone solution to reach a final concentration of 0.2 or 1.0 g/liter, respectively. Hexadecane was sterilized separately and added to sterile mineral medium.
Luria-Bertani medium (LB) was prepared with mineral salts and complemented with 2 g of glucose/liter.
Isolation and identification of the pyrene-degrading strain.
A sample of sand exposed to crude oil contamination was obtained from REPSOL S. A., Tarragona, Spain. Strain AP1 was isolated from an enrichment culture established in mineral medium with pyrene crystals (1 g/liter) using this sand as an inoculum. The strain grew on solid mineral medium with pyrene as a sole carbon source, forming colonies surrounded by a clearing zone that indicated pyrene degradation.
The partial 16S rRNA gene sequence (463 nucleotides) of the isolate was obtained at Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany) by direct sequencing of PCR-amplified 16S ribosomal DNA. Genomic DNA extraction, PCR-mediated amplification of the 16S ribosomal DNA, and purification of the PCR product were carried out as described by Rainey et al. (23). The purified PCR product was sequenced using an ABI PRISM dye terminator cycle sequencing ready reaction kit (Applied Biosystems, Foster City, Calif.) as directed in the protocol from the manufacturer. Sequence reaction mixtures were electrophoresed using an Applied Biosystems 373A DNA sequencer. The resulting data were entered into alignment editor ae2 (19) and aligned manually. The sequence was compared with the 16S rRNA gene sequences of representative organisms belonging to the Actinobacteria (19).
Utilization of pyrene as a sole source of carbon and energy.
Growth at the expense of pyrene was verified by demonstrating an increase in bacterial protein concentration concomitant with a decrease in pyrene concentration. Several colonies of strain AP1 grown on LB plates for 5 days were resuspended in mineral medium (A650, 0.7), and the suspension was used as an inoculum (0.5 ml). Replicate batch cultures were grown in 100-ml Erlenmeyer flasks containing 20 ml of mineral medium and pyrene (0.2 g/liter). Incubation was performed at 25°C with rotary shaking (200 rpm). Uninoculated flasks and flasks without pyrene served as controls. Pyrene and protein concentrations were measured at 48-h intervals over 14 days. Controls were analyzed at 0, 7, and 14 days. The protein concentration was measured using the entire flask contents of duplicate cultures by a modification of the Lowry method (6). The pyrene concentration was also determined using duplicate cultures by high-pressure liquid chromatography (HPLC) analysis of total organic extracts. Protein and pyrene concentrations at sampling intervals are expressed as the average of those obtained for duplicate flasks.
Isolation and identification of pyrene metabolites.
Pyrene metabolites were isolated from growing cultures of strain AP1 in mineral medium with pyrene (1 g/liter) as a sole source of carbon and energy. Colonies of strain AP1 grown on LB plates for 5 days at 25°C were resuspended in mineral medium (A650, 0.7) and used as an inoculum (1%) for 2-liter Erlenmeyer flasks containing 400 ml of medium. On the basis of previous time course experiments in which metabolite formation was monitored by HPLC analysis of culture fluids, cultures were incubated for 10 days at 25°C and 200 rpm. After this period, the cultures were filtered through glass wool and centrifuged (8,600 × g, 20 min) to remove cells and remaining pyrene crystals. Supernatants were extracted five times with 1 volume of ethyl acetate, acidified to pH 2 with 5 N HCl, and extracted again in the same manner. Extracts were dried over Na2SO4 and concentrated under vacuum. For initial detection and characterization of metabolites, aliquots of neutral and acidic extracts were dried under a gentle N2 stream, resuspended in methanol, and analyzed by HPLC. Portions of the extracts were treated with ethereal diazomethane and analyzed by gas chromatography (GC)-mass spectrometry (MS).
The metabolites were later isolated by preparative HPLC. Repeated injections of the extracts resulted in the separation of 60 fractions from each extract. The individual fractions were subjected to HPLC analysis, and those with similar compositions were combined and extracted with 5 volumes of methylene chloride. After concentration, the extracts from selected fractions were analyzed by GC-MS. Acidic compounds were analyzed after treatment with diazomethane, while neutral compounds were analyzed before and after derivatization. When the extract from a fraction contained a single compound (more than 95% in GC analysis), other fractions exhibiting the same compound combined with other products were discarded. For this reason, the amount reported for the purified metabolites corresponds to that used for identification, not to the total amount in the extracts. Compounds not previously reported in the literature were analyzed by 1H and 13C nuclear magnetic resonance (NMR) analyses.
Growth in the presence of or transformation of other hydrocarbons.
Growth in the presence of other hydrocarbons was screened using liquid mineral medium containing 0.2 g of substrate/liter. Erlenmeyer flasks (100 ml) containing 20 ml of mineral medium and the hydrocarbon were inoculated as described above for pyrene cultures and incubated at 25°C and 200 rpm for 20 days. Growth was considered positive if, after this time, the concentration of cell protein in cultures was fivefold that obtained for controls without the carbon source. In the case of volatile compounds such as naphthalene, biphenyl, and 1-methyl fluorene, the results were confirmed using mineral medium plates with crystals of the substrate on the lid.
Transformation of aromatic hydrocarbons by strain AP1 was demonstrated by metabolite formation in washed-cell suspensions incubated with the aromatic substrate. Cells were grown in mineral medium with Casamino Acids (5 g/liter) and pyrene (1 g/liter) until late exponential phase (10 days; A650, 0.7). Cultures were filtered to remove residual pyrene crystals and incubated for 30 min in order to consume any remaining pyrene. Cells were harvested by centrifugation (20 min at 9,000 × g), washed twice in 50 mM Na-K phosphate buffer, and resuspended in the same buffer at a fourfold concentration with respect to the cultures. Twenty-milliliter volumes of the cell suspension were incubated with each of the aromatic substrates for 72 h, and the suspension was filtered through glass wool and centrifuged. Cells and remaining crystals were discarded, and the supernatant was analyzed by HPLC for the detection of metabolites. The appearance of new peaks with respect to those for noninoculated controls was attributed to transformation of the substrates.
Analytical methods.
Reverse-phase HPLC was performed with a Hewlett-Packard model 1050 chromatograph equipped with a diode-array UV-visible detector set at 254 nm. For analytical purposes, separation was achieved using a Chromspher C18 (Chrompack) (25 cm by 4.6 mm [inside diameter]; 5-μm particle size) column and a linear gradient of methanol (10 to 95% [vol/vol] in 20 min) in acidified water (0.6% H3PO4). Flow was kept at 1 ml/min. The injection volume was 10 μl. Metabolites were isolated using a column with the same characteristics except for an internal diameter of 10 mm. Separation of the neutral extract was achieved under the conditions described above, while the acidic metabolites were purified using a linear gradient of methanol (40 to 80% [vol/vol] in 30 min) in acidified water (0.6% H3PO4) at a constant flow of 3 ml/min. The injection volume was 200 μl. Fractions were collected at 0.5-min intervals.
GC-MS analyses were performed with a Hewlett-Packard 5890 series II apparatus with a 5989 mass selective detector. Compounds were separated using an HP-5 capillary column (30 m by 0.25 mm [inside diameter]; 0.25-μm film thickness) and helium as the carrier gas. The column temperature was kept at 50°C for 1 min and then programmed to 310°C at a rate of 10°C/min. The mass spectrometer was operated at 70 eV of electron ionization energy. Injector and analyzer temperatures were set at 290 and 315°C, respectively.
1H NMR spectra were recorded on a Varian Gemini-200 (200-MHz) spectrometer, while 13C NMR analysis was performed on a Varian Unity 300 Plus (75.4-MHz) spectrometer. For both, the central signal of the solvent (methanol-d4, δ 3.30) was used as an internal reference.
RESULTS
Isolation and characterization of Mycobacterium sp. strain AP1.
Strain AP1 was isolated from an enrichment culture established in pyrene-mineral medium inoculated with crude oil-contaminated sand. After 30 days of incubation in pyrene-mineral medium plates, the isolate produced characteristic yellow colonies (about 1 mm) surrounded by clearing zones that indicated pyrene degradation. Similar colonies formed in LB medium (2% glucose) in 7 days.
Strain AP1 was a short rod-shaped, gram-positive, aerobic, nonmotile, and nonfermentative bacterium. The analysis of the partial 16S rRNA gene sequence located the strain within the genus Mycobacterium, showing 99.8% similarity to Mycobacterium sp. strain DSM 9673 and 99.3% similarity to Mycobacterium gilvum ATCC 43909.
Utilization of pyrene as a sole source of carbon and energy.
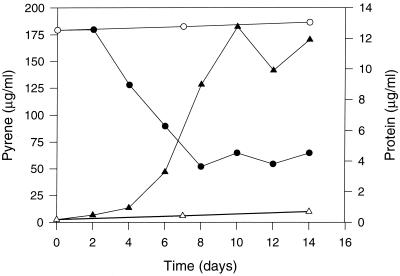
Utilization of pyrene as a sole source of carbon and energy by Mycobacterium sp. strain AP1 was confirmed by its removal from pyrene-mineral salts medium, with a corresponding increase in bacterial protein (Fig. 1). After the first 48 h of incubation, the concentration of pyrene recovered from cultures decreased dramatically over the next 6 days (from 180 to 50 μg/ml). Concomitantly, bacterial biomass increased from 0.46 μg/ml at the time of inoculation to a maximum of 12.7 μg/ml at day 10. These values indicate a conversion of approximately 130 μg of pyrene to 24.5 μg (dry weight) of cells (22). Given that carbon accounts for approximately 50% of cell dry weight, under these conditions there appears to be cellular assimilation of pyrene with an efficiency of about 10%. The fact that the substrate was not completely removed from the cultures is atypical and is discussed below.
FIG. 1.
Utilization of pyrene by Mycobacterium sp. strain AP1 in liquid mineral medium with pyrene as the sole source of carbon and energy at 25°C and 200 rpm. Growth is shown as an increase in the level of cell protein in cultures (▴) and in controls without a carbon source (▵). Pyrene concentrations were determined by HPLC analyses of organic extracts from cultures (●) and uninoculated controls (○).
Isolation and identification of pyrene metabolites.
HPLC analysis of supernatants removed at different times from cultures of strain AP1 in mineral medium containing an excess of pyrene (1.0 g/liter) showed the accumulation of a number of metabolites. The concentrations of two of the major metabolites (IV and VI) increased during incubation to maxima at the end of the exponential phase (10 days). On the basis of these results, a large-scale experiment was set up with 4.8 liters of mineral medium and 4.8 g of pyrene and incubation for 10 days.
The neutral extract of culture fluids after the removal of cells and residual pyrene crystals weighed 15 mg, while the weight of the acidic extract was 25 mg.
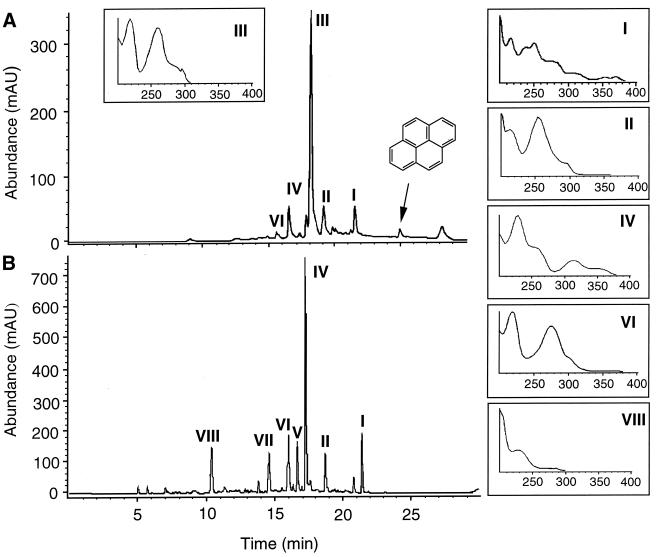
HPLC analysis of the neutral extracts (Fig. 2) revealed a major metabolite, III, at a retention time (Rt) of 18 min and four minor metabolites, I, II, IV, and VI, at Rts of 21.5, 19, 17.7, and 16.4 min, respectively. The HPLC elution profile of the acidic extracts revealed seven major products. Four of them corresponded to metabolites I, II, IV, and VI, previously detected in the neutral extracts. The higher concentrations of these products in the acidic extracts suggested that they were acidic metabolites that had been partially extracted at a neutral pH due to their relatively large nonpolar moieties. The acidic extracts showed three additional products: V (Rt, 16.7 min), VII (Rt, 14.8 min), and VIII (Rt, 10.5 min).
FIG. 2.
HPLC elution profiles of neutral (A) and acidic (B) extracts from cultures of strain AP1 in liquid mineral medium containing pyrene (1 g/liter). The UV-visible spectra of identified metabolites are displayed as insets. These metabolites were identified as follows: II, phenanthrene 4-carboxylic acid; III, 4,5-dihydroxy-4,5-dihydropyrene; IV, 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid; VI, phenanthrene 4,5-dicarboxylic acid; and VIII, phthalic acid. mAU, milliabsorbance unit.
Metabolites I, II, III, IV, VI, and VIII were purified from the extracts by preparative HPLC and subjected to GC-MS analyses (Table 1). Metabolites V and VII could not be purified and so could not be identified.
TABLE 1.
GC Rts and electron impact mass spectral properties of metabolites formed from pyrene by Mycobacterium sp. strain AP1
| Metabolite | Extracta | Rt (min) | Abundance (%) | m/z of fragment ions (% relative intensity) | Identificationb |
|---|---|---|---|---|---|
| I | A | 22.2 | 100 | 220 (M+, 100), 192 (M+ − CO, 22), 163 (48), 87 (12), 82 (14) | Not identified |
| II | A | 20.9 | 100 | 236 (89), 221 (M+ − CH3, 11), 205 (M+ − OCH3, 97), 177 (M+ − COOCH3, 100), 151 (28), 88 (68) | Phenanthrene 4-carboxylic acid (ME) |
| III | N | 23.7 | 94 | 236 (M+, 44), 218 (M+ − H2O, 100), 189 (78), 176 (16), 94 (33) | cis- and trans-4,5-Pyrene dihydrodiols |
| IV | A | 24.2 | 95 | 302 (M+, 33), 270 (M+ − CH3OH, 31), 243 (M+ − COOCH3, 45), 242 (M+ − COOCH3 − H+, 92), 241 (M+ − COOCH3 − H+ − H+, 45), 226 (M+ − COOCH3 − H+ − H+ − CH3, 100), 210 (M+ − COOCH3 − H+ − CH3OH, 13), 198 (M+ − COOCH3 − H+ − H+ − CH3 − CH3OH, 26), 184 (M+ − COOCH3 − COOCH3, 5), 183 (M+ − COOCH3 − H+ − COOCH3, 9), 182 (M+ − COOCH3 − H+ − H+ − COOCH3, 14), 170 (21), 139 (15) | 6,6′-Dihydroxy-2,2′-biphenyl dicarboxylic acid (diME) |
| VI | A | 23.3 | 100 | 294 (M+, 3), 263 (M+ − OCH3, 2), 235 (M+ − COOCH3, 100), 220 (M+ − COOCH3 − CH3, 57), 204 (6), 192 (16), 176 (M+ − COOCH3 − COOCH3, 12), 163 (15) | Phenanthrene 4,5-dicarboxylic acid (diME) |
| VIII | A | 12.9 | 78 | 194 (M+, 5), 163 (100), 135 (9), 105 (6), 92 (20), 77 (52) | Phthalic acid (diME) |
Extract from which the product was purified; A, acidic extract; N, neutral extract.
ME, methyl ester; diME, dimethyl ester. Acidic compounds were analyzed as methyl ester derivatives.
Metabolite I (0.4 mg) had a different mass spectrum from the pyrene metabolites previously described in the literature. Although this metabolite was more abundant in the acidic extracts, its mass spectrum showed no signs of derivatization after treatment with diazomethane. This finding suggests that this product could be a neutral derivative of an acidic metabolite formed during the extraction or the analytical procedures. Attempts to identify the compound by NMR analysis were unsuccessful due to the limited amount of material. The mass spectrum obtained for the methyl ester derivative of metabolite II (0.2 mg) was similar to that reported for a metabolite formed from pyrene by Mycobacterium PYR-1 and identified as phenanthrene 4-carboxylic acid by GC-MS, 1H NMR, and infrared analyses (13). Metabolite III (0.8 mg) showed a mass spectrum consistent with cis- and trans-4,5-pyrene dihydrodiols. Since both isomers are metabolites in the degradation of pyrene by strain PYR-1 (13), the fraction containing product III was subjected to further HPLC analysis in methanol-acidified H2O (55:45). In this system, product III was resolved in two peaks (Rts,10.5 and 11 min) with identical UV-visible spectra, a result which indicates the presence of both isomers of the dihydrodiol.
Metabolite IV was the most abundant product in the acidic extracts. After purification by HPLC (4.8 mg) and GC-MS analyses, the metabolite showed UV-visible and mass spectra suggestive of a structure not previously reported in the literature. The mass spectrum exhibited a molecular ion (m/z 302) and a fragmentation pattern (Table 1) compatible with a diaromatic structure with two carboxylic groups. The loss of methanol is frequent in molecules in which methoxylated carboxylic groups occupy vicinal positions with respect to hydroxyl groups, giving the corresponding lactones. These observations, together with easy deprotonation, are consistent with a biaryl structure containing two carboxylic and two hydroxylic groups.
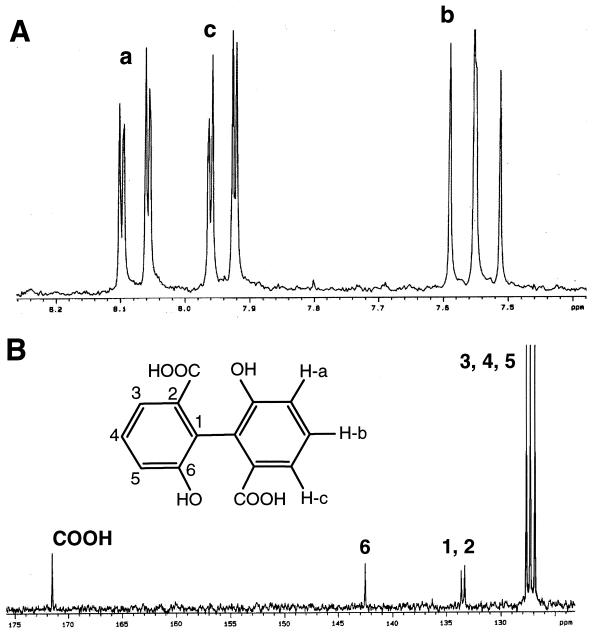
The amount of product IV isolated allowed its analysis by both 1H and 13C NMR techniques (Fig. 3). The solvent used was methanol-d4. The 1H-NMR spectrum of IV shows only three double doublets, all of them in the aromatic zone of the spectrum. The most shielded signal (δ 7.55) is coupled by coupling constants whose magnitude (7.4 and 8.0 Hz) corresponds to the coupling between protons in a 1,2 (ortho) situation in the aromatic ring. The two additional signals (δ 7.94 and δ 8.08) are coupled by one of the above-mentioned constants and a second, small constant (1.2 Hz) attributable to the coupling between protons in a 1,3 (meta) position. This coupling pattern corresponds to a nonsymmetrical 1,2,3-trisubstituted benzene ring. Moreover, the chemical shift of the two later signals seems to indicate the presence of deshielding groups on the ring. The 13C NMR spectrum shows seven absorption signals; six of them are in the aromatic zone, while the seventh is a carbonyl group corresponding to a carboxylic acid. Three of the aromatic singlets (δ 126.9, δ 127.3, and δ 127.7) correspond to CH groups, while the remaining absorption signals (δ 133.3, δ 133.6, and δ 142.5) belong to quaternary carbon atoms. This situation confirms the hypothesis of a trisubstituted benzene ring. According to these results and the mass spectrum of the methoxy derivative, metabolite IV was identified as 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid. The spectral characteristics reported here for this product are very similar to those reported previously by Rehmann et al. (24) for an unidentified compound that accumulated during the degradation of pyrene by Mycobacterium sp. strain KR2.
FIG. 3.
1H (A) and 13C (B) NMR spectra and chemical structure of metabolite IV.
The mass spectrum of the methyl ester of metabolite VI (0.3 mg) showed a fragmentation pattern indicative of phenanthrene 4,5-dicarboxylic acid. This product was previously proposed as an intermediate in the degradation of pyrene by other Mycobacterium strains (7, 24, 25) on the basis of a similar mass spectrum. In support of this identification was the detection of a product in one of the fractions of the neutral extract that showed a molecular ion at m/z 248 and a fragmentation pattern consistent with phenanthrene anhydride.
Subsequent analyses of the HPLC fractions corresponding to peak VIII showed a mixture of products. On the basis of its spectral characteristics and the results of coelution experiments with authentic material, the most abundant of those products (metabolite VIII) was identified as phthalic acid, which has also been reported as a metabolite in pyrene degradation (7, 24).
Growth and transformation of other hydrocarbons.
Mycobacterium sp. strain AP1 grew in mineral medium with phenanthrene, fluoranthene, or hexadecane as a sole source of carbon and energy. Acenaphthene, fluorene, and naphthalene did not support growth. However, HPLC and GC-MS analyses of the corresponding extracts demonstrated that washed cell suspensions of strain AP1 pregrown in the presence of pyrene transformed those compounds to a variety of products. Anthracene was not transformed.
Acenaphthene and fluorene underwent oxidation of the methylenic groups to give metabolites previously described in the literature (10). The neutral metabolite that accumulated from acenaphthene (Rt, 15.2 min; 100%) gave a mass spectrum [m/z 168 (85), 140 (100), 113 (10), 89 (9), 74 (6)] indistinguishable from that observed for authentic acenaphthenone, while the diazomethane-treated acidic extract showed two products (Rts, 18.9 and 19.8 min; 76 and 24%) with mass spectra [m/z 244 (28), 213 (100), 185 (34), 170 (45), 126 (18) and m/z 198 (58), 170 (4), 154 (100), 126 (99)] identical to those obtained previously for the dimethyl esters of 1,8-naphthalene dicarboxylic acid and naphthalic anhydride, respectively (10). Fluorene was transformed to 9-fluorenone (23% of the neutral extract) [m/z 180 (100), 152 (48), 126 (7)] and a mixture of nonidentified acidic metabolites.
HPLC analysis of the acidic extracts from naphthalene incubations yielded a single metabolite (Rt, 14.9 min; λmax at 226 and 274 nm). GC-MS analyses of the product treated with diazomethane (Rt, 14.6; 100%) showed a mass spectrum [molecular ion at m/z 220 (M+, 1) and major fragments at m/z 189 (M+ − CH3O, 7), 173 (12), 161 (M+ − COOCH3, 100), 145 (7), 129 (10), 118 (13), 102 (M+ − COOCH3 − COOCH3, 13), 76 (M+ − COOCH3 − COOCH3 − CH⩵CH, 9)] consistent with a dimethyl derivative of 2-carboxycinnamic acid. This identification was confirmed with the authentic dimethyl derivative of 2-carboxycinnamic acid, which showed identical UV-visible and mass spectra and coeluted with the product in HPLC and GC analyses.
Phenanthrene was also transformed to a single acidic metabolite eluting at 16.2 min in HPLC analysis and showing a UV-visible spectrum with λmax at 209 and 283 nm and a shoulder at 232 nm. After derivatization, the resulting compound exhibited a GC Rt of 17.9 min and a mass spectrum with a molecular ion at m/z 270 (M+, 2) and a fragmentation pattern [239 (M+ − OCH3, 3), 211 (M+ − COOCH3, 100), 196 (M+ − OCH3 − CH3, 18), 180 (M+ − COOCH3 − OCH3, 13), 152 (M+ − COOCH3 − COOCH3, 14), 104 (3), 76 (12)] consistent with a dimethyl derivative of diphenic acid. These characteristics were indistinguishable from those of authentic diphenic acid, which coeluted with the product in HPLC and GC analyses. The metabolism of fluoranthene and phenanthrene by growing cells is currently being investigated.
DISCUSSION
Mycobacterium sp. strain AP1 grew in mineral medium with pyrene as the sole source of carbon and energy, its growth yield suggesting an assimilation of about 10% of pyrene carbon. Growth in liquid mineral medium with pyrene (Fig. 1) did not result in complete removal of the substrate (0.06 mg/ml of the initial 0.2 mg/ml remained). However, since exponential growth of the strain continued for 2 days after pyrene removal had stopped, this finding cannot be attributed to the accumulation of toxic metabolites. Mulder et al. (21) reported that during the growth of Pseudomonas sp. strain 8909N in liquid medium with naphthalene, biofilm formation reduced the dissolution rate of the substrate by 90%, a result which had a dramatic effect on the biodegradation rates. Additionally, Wick et al. (30) demonstrated that the growth of Mycobacterium sp. strain LB501T was directly related to substrate dissolution from crystals and to the uptake of substrate from the solution by the microorganisms. Given that strain AP1 also forms biofilms on pyrene crystals, the observed attenuation in pyrene degradation could be due to biofilm formation and consequent prevention of pyrene dissolution, while previously accumulated intermediates would allow further cell growth.
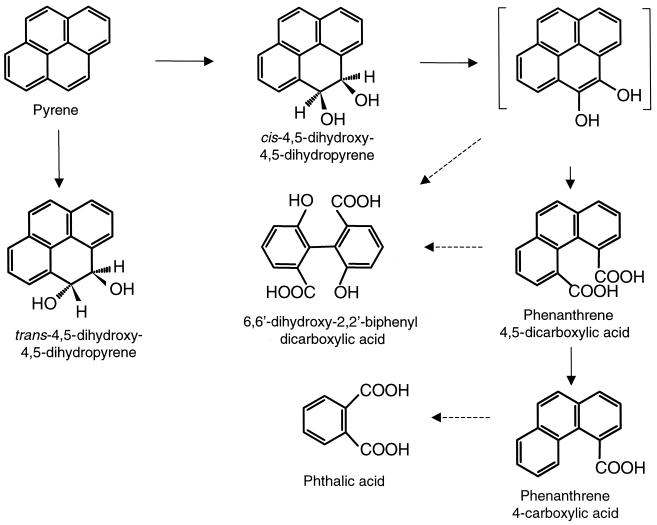
The identification of metabolites accumulating during growth indicates that Mycobacterium sp. strain AP1 initiates its attack on pyrene either by dioxygenation at C-4 and C-5 positions to give cis-4,5-dihydroxy-4,5-dihydropyrene or by monooxygenation at the same positions and subsequent hydrolysis to give trans-4,5-dihydroxy-4,5-dihydropyrene (Fig. 4). Dehydrogenation of the cis-dihydroxypyrene to the corresponding diol, followed by ortho cleavage of the oxidated ring, would yield the identified phenanthrene 4,5-dicarboxylic acid. Further metabolism of the latter would proceed by decarboxylation to phenanthrene 4-carboxylic acid, which could be degraded via phthalate by reactions previously described for the metabolism of PAHs. This degradation pathway was initially proposed by Cerniglia (3) for the degradation of pyrene by Mycobacterium sp. strain PYR-1 (13) and has also been proposed for Mycobacterium sp. strain RJG-II 135 (25), Mycobacterium flavescens (7), and Mycobacterium sp. strain KR2 (24). However, only the two latter strains use pyrene as a sole source of carbon and energy, as does strain AP1.
FIG. 4.
Schematic pathway proposed for the degradation of pyrene by Mycobacterium sp. strain AP1. The product in brackets has not been isolated. Dotted arrows indicate two or more successive reactions.
Cerniglia and Heitkamp (4) demonstrated that the formation of trans-4,5-dihydroxy-4,5-dihydropyrene by Mycobacterium sp. strain PYR-1 was due to the action of a P-450-dependent monooxygenase. More recently, the same researchers reported the isolation and characterization of a dioxygenase from the same strain that produces 4,5-dihydroxy-4,5-dihydropyrene from pyrene and a catalase-peroxidase whose role in pyrene catabolism is uncertain (17, 29). Nevertheless, the mechanisms for the ortho cleavage of the central ring of pyrene and the one-carbon excision from the K region of phenanthrene 4,5-dicarboxylic acid remain to be elucidated.
The identification of the novel metabolite 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid opens a new branch in the pathway. This metabolite is formed after dioxygenation on C-4 and C-5 positions and on C-9 and C-10 positions and cleavage of both central rings of pyrene. The positions of the substituents in the molecule and the possibility of rotation over the C1—C1′ bond offer a variety of possibilities, including meta and ortho cleavages of either ring, followed by one- or two-carbon excision. Therefore, the elucidation of the mechanisms and the order of the reactions requires further research. The fact that the concentration of this compound increases during incubation could suggest that it is a dead-end product. However, the excess of substrate and the fact that some bacterial strains cannot take up dicarboxylic acids once they have diffused into the medium leave open the possibility of further degradation of this compound. The detection of an unidentified product with similar spectral characteristics in the metabolism of pyrene by Mycobacterium sp. strain KR2 (24) suggests that this compound may be a common metabolite in mycobacterial routes for pyrene degradation.
None of the initial reactions proposed for the degradation of pyrene by strain AP1, except for the subsequent degradation of phenanthrene 4-carboxylic acid, produces energy for growth, which is consistent with the low yields observed. On the other hand, the possibility of additional oxidation routes whose metabolites are not accumulated under the conditions of study cannot be ruled out. Walter et al. (28) identified a product that was compatible with the recircularization of a meta-cleavage metabolite produced after either 1,2- or 4,5-dioxygenation and that accumulated during the growth of Rhodococcus sp. strain UW1 on pyrene. The 1,2-dioxygenation route has also been proposed to explain the accumulation of 4-hydroxyperinaphthenone by Mycobacterium sp. strain PYR-1 (13, 15).
Mycobacterium sp. strain AP1 is one of the few PAH-degrading strains able to grow on aliphatic compounds such as hexadecane. This ability has also been described for pyrene-degrading Mycobacterium sp. strain CH1 (5), DNA from which showed weak hybridization with the alkB gene of Pseudomonas oleovorans. The versatility of strain AP1 is also shown in its actions on PAHs. In addition to pyrene, it utilizes phenanthrene and fluoranthene as substrates for growth and transforms naphthalene, acenaphthene, and fluorene.
Pyrene-grown cells of strain AP1 monooxygenated the methylenic groups of fluorene and acenaphthene to give the corresponding ketones. Acenaphthenone was probably further oxygenated to give the quinone, which underwent hydrolysis to naphthalene 1,8-dicarboxylic acid, also detected as its anhydride. All these products accumulate in environmental samples (8). These monooxygenation reactions, described here for the first time for a pyrene-degrading strain, were previously observed during the degradation of creosote PAHs by a naphthalene- and fluorene-degrading strain of Bulkholderia (10), and they can be the result of the monooxygenase activity shown by naphthalene dioxygenase (9, 26). However, the possible presence of monooxygenases in strain AP1 (i.e., P-450-dependent monooxygenases), together with the lack of homology between the genes involved in pyrene degradation and nahAC (5), suggests that more research is needed in order to attribute a mechanism to ketone formation.
Naphthalene and phenanthrene were transformed to 2-carboxycinnamic and diphenic acids, respectively, indicating aromatic ring dioxygenation (in the case of phenanthrene, of the central ring) and ortho cleavage (Fig. 5). The degradation of phenanthrene and napththalene has been extensively documented, and it is generally accepted that bacteria initiate the metabolism of these PAHs by dioxygenation and meta cleavage of one of the external rings. Only a few recent publications have reported the bacterial oxidation of those compounds via ortho cleavage. 2-Carboxycinnamic acid has been identified as a metabolite of naphthalene in a strain of the thermophile Bacillus thermoleovorans (1), which apparently grows on naphthalene, degrading it by alternative meta- and ortho-cleavage pathways. As part of the ortho pathway, the authors proposed the further degradation of 2-carboxycinnamic acid via phthalate, but evidence was not provided. During growth on naphthalene of the pyrene-degrading strain Mycobacterium sp. strain PYR-1, ortho-cleavage metabolites were not detected (16).
FIG. 5.
Metabolites identified in washed-cell suspensions of Mycobacterium sp. strain AP1 incubated with PAHs. Reactions are grouped by type. Products in brackets have not been isolated.
Diphenic acid is a metabolite of phenanthrene in Mycobacterium sp. strain PYR-1, which also dioxygenates phenanthrene in 3,4-positions and degrades the resulting dihydrodiol via 1-hydroxy-2-naphthoic acid (20). In incubations of washed-cell suspensions of strain AP1 with phenanthrene, metabolites indicative of the latter pathway were not detected. However, since the production of diphenic acid does not provide energy, further degradation of this compound or alternative reactions must be expected during the growth of AP1 on phenanthrene. Research is in progress to clarify this point. Diphenic acid is also formed from phenanthrene 9,10-quinone during the oxidation of phenanthrene by the ligninolytic fungus Phanerochaete chrysosporium (11).
The reactions of dioxygenation and ortho cleavage of phenanthrene and naphthalene by strain AP1 are consistent with those proposed for the first steps of pyrene degradation. Given the scarcity of reports showing the presence of ortho-cleavage mechanisms in the degradation of those PAHs by nonpyrene degraders, the formation of 2-carboxycinnamic and diphenic acids by Mycobacterium sp. strain AP1 could be the result of the accommodation of phenanthrene and naphthalene by the initial enzymes of the pyrene pathway. As in the case of the newly identified pyrene metabolite 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid, the further metabolism of diphenic and 2-carboxycinnamic acids by strain AP1 remains to be elucidated. On the other hand, the degradation of these compounds by other bacterial strains has not been described.
The results presented here demonstrate that pyrene-degrading strains, such as Mycobacterium sp. strain AP1, may act on a number of components of hydrocarbon environmental mixtures, including aliphatic and aromatic compounds. The new metabolite described for pyrene metabolism and the transformation of the PAHs naphthalene and phenanthrene via ortho cleavage give new insight into the understanding of the biochemical processes that determine the environmental fate of PAHs and demand further biochemical and genetic studies.
ACKNOWLEDGMENTS
This research was funded by a grant from the Spanish Government's National Plan for Research (BIO98-0428).
We are grateful to Asunción Marín (Serveis Cientifico-Tècnics, University of Barcelona) for the acquisition of GC-MS data.
REFERENCES
- 1.Annweiler E, Richnow H H, Antranikian G, Hebenbrock S, Garms C, Franke S, Francke W, Michaelis W. Naphthalene degradation and incorporation of naphthalene-derived carbon into biomass by the thermophile Bacillus thermoleovorans. Appl Environ Microbiol. 2000;66:518–523. doi: 10.1128/aem.66.2.518-523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black T H. The preparation and reactions of diazomethane. Aldrichim Acta. 1983;16:39. [Google Scholar]
- 3.Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368. [Google Scholar]
- 4.Cerniglia C E, Heitkamp M A. Polycyclic aromatic hydrocarbon degradation by Mycobacterium. Methods Enzymol. 1990;188:148–153. doi: 10.1016/0076-6879(90)88027-8. [DOI] [PubMed] [Google Scholar]
- 5.Churchill S A, Harper J P, Churchill P F. Isolation and characterization of a Mycobacterium species capable of degrading three- and four-ring aromatic and aliphatic hydrocarbons. Appl Environ Microbiol. 1999;65:549–552. doi: 10.1128/aem.65.2.549-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels L, Handson R S, Phillips J A. Chemical analysis. In: Gerhardt A P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: ASM Press; 1994. p. 512. [Google Scholar]
- 7.Dean-Ross D, Cerniglia C E. Degradation of pyrene by Mycobacterium flavescens. Appl Microbiol Biotechnol. 1996;46:307–312. doi: 10.1007/s002530050822. [DOI] [PubMed] [Google Scholar]
- 8.Fernández P, Grifoll M, Solanas A M, Bayona J M, Albaigés J. Bioassay-directed chemical analysis of genotoxic components in coastal marine sediments. Environ Sci Technol. 1992;26:817–829. [Google Scholar]
- 9.Gibson D T, Resnick S M, Le K, Brand J M, Torok D S, Wackett L P, Schoken M J, Haigler B E. Desaturation, dioxygenation, and monooxygenation reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain 9816-4. J Bacteriol. 1995;177:2615–2621. doi: 10.1128/jb.177.10.2615-2621.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grifoll M, Selifonov S A, Gatlin C V, Chapman P J. Actions of a versatile fluorene-degrading bacterial isolate on polycyclic aromatic hydrocarbons. Appl Environ Microbiol. 1995;61:3711–3723. doi: 10.1128/aem.61.10.3711-3723.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammel K E, Gai W Z, Green B, Moen M A. Oxidative degradation of phenanthrene by the ligninolytic fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:1832–1838. doi: 10.1128/aem.58.6.1832-1838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hareland W A, Crawford R L, Chapman P J, Dagley S. Metabolic function and properties of 4-hydroxyphenyl acetic 1-hydroxylase fom Pseudomonas acidovorans. J Bacteriol. 1975;121:272–285. doi: 10.1128/jb.121.1.272-285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heitkamp M A, Freeman J P, Miller D W, Cerniglia C E. Pyrene degradation by a Mycobacterium sp.: identification of ring oxidation and ring fission products. Appl Environ Microbiol. 1988;54:2556–2565. doi: 10.1128/aem.54.10.2556-2565.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Association of Research Chemists. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Polynuclear aromatic compounds. Part 1. Int Assoc Res Chem Monogr. 1983;32:355–364. [Google Scholar]
- 15.Kanaly R A, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol. 2000;182:2059–2067. doi: 10.1128/jb.182.8.2059-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley I, Freeman J P, Cerniglia C E. Identification of metabolites from degradation of naphthalene by a Mycobacterium sp. Biodegradation. 1991;1:283–290. doi: 10.1007/BF00119765. [DOI] [PubMed] [Google Scholar]
- 17.Khan A A, Wang R-F, Cao W-W, Doerge D R, Wennerstrom D, Cerniglia C E. Molecular cloning, nucleotide sequence, and expression of genes encoding a polycyclic aromatic ring dioxygenase from Mycobacterium sp. strain PYR-1. Appl Environ Microbiol. 2001;67:3577–3585. doi: 10.1128/AEM.67.8.3577-3585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahro B. Bioavailability of contaminants. Bio/Technology. 2000;11b:61–88. [Google Scholar]
- 19.Maydak B L, Cole J R, Parker Jr C T, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overveek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moody J D, Freeman J P, Doerge D R, Cerniglia C E. Degradation of phenanthrene and anthracene by cell suspensions of Mycobacterium sp. strain PYR-1. Appl Environ Microbiol. 2001;67:1476–1483. doi: 10.1128/AEM.67.4.1476-1483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulder H, Breure A M, van Honschooten D, Grotenhuis J T C, van Andel J G, Rulkens W H. Effect of biofilm formation by Pseudomonas 8909N on the bioavailability of solid naphthalene. Appl Microbiol Biotechnol. 1998;50:277–283. [Google Scholar]
- 22.Neidhardt F C, Ingraham J L, Schaechter M. Physiology of the bacterial cell. A molecular approach. Sunderland, Mass: Sinauer Associates, Inc., Publishers; 1990. [Google Scholar]
- 23.Rainey F A, Ward-Rainey N, Kroppenstedt R M, Stackenbrandt E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage; proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 24.Rehmann K, Noll H P, Steinberg C E W, Kettrup A A. Pyrene degradation by Mycobacterium sp. strain KR2. Chemosphere. 1998;36:2977–2992. doi: 10.1016/s0045-6535(97)10240-5. [DOI] [PubMed] [Google Scholar]
- 25.Schneider J, Grosser R, Jayasimhulu K, Xue W, Warshawsky D. Degradation of pyrene, benz[a]anthracene, and benzo[a]pyrene by Mycobacterium sp. strain RJGII-135, isolated from a former coal gasification site. Appl Environ Microbiol. 1996;62:13–19. doi: 10.1128/aem.62.1.13-19.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selifonov S A, Grifoll M, Eaton R W, Chapman P J. Oxidation of naphthenoaromatic and methyl-substituted aromatic compounds by naphthalene 1,2-dioxygenase. Appl Environ Microbiol. 1996;62:507–514. doi: 10.1128/aem.62.2.507-514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wackett L P, Kwart L D, Gibson D T. Benzylic monooxygenation catalyzed by toluene dioxygenase from Pseudomonas putida. Biochemistry. 1988;27:1360–1367. doi: 10.1021/bi00404a041. [DOI] [PubMed] [Google Scholar]
- 28.Walter U, Beyer M, Klein J, Rehm H-J. Degradation of pyrene by Rhodococcus sp. UW1. Appl Microbiol Biotechnol. 1991;34:671–676. [Google Scholar]
- 29.Wang R-F, Wennerstrom D, Cao W-W, Khan A A, Cerniglia C E. Cloning, expression, and characterization of the katG gene, encoding catalase-peroxidase, from the polycyclic aromatic hydrocarbon-degrading bacterium Mycobacterium sp. strain PYR-1. Appl Environ Microbiol. 2000;66:4300–4304. doi: 10.1128/aem.66.10.4300-4304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wick L Y, Colangelo T, Harms H. Kinetics of mass transfer-limited bacterial growth on solid PAHs. Environ Sci Technol. 2001;35:354–361. doi: 10.1021/es001384w. [DOI] [PubMed] [Google Scholar]