Abstract
Background:
This study aimed to systematically review the existing literature on epithelioid trophoblastic tumors (ETTs), the rarest type of gestational trophoblastic neoplasia.
Methods:
A systematic review according to PRISMA guidelines was performed, using ScienceDirect, Web of Science, and Scopus databases. The only filter used was the English language. Eligibility/inclusion criteria: retrospective observational studies (case reports, case series) including full case description of epithelioid trophoblastic tumor lesions.
Results:
Seventy studies were assessed for synthesis, including 147 cases. 66.7% of patients with ETT presented with irregular vaginal bleeding. Pretreatment β-hCG levels ranged up to 1000 mIU/mL in 58.5% patients. Of most patients, 42.2% had stage I disease, 10.9% stage II, 25.2% stage III, and 21.8% of patients had stage IV. The most common sites of metastatic disease were the lungs, followed by the liver and brain. After treatment, complete remission was achieved in 75.5% of patients, partial remission in 10.2% of patients, and 14.3% of patients died. On univariate and multivariate analyses, stage IV disease was an independent prognostic factor for overall and disease-free survival.
Conclusions:
Hysterectomy and metastatic lesion resection are essential for controlling ETT. Investigational studies on molecules like EGFR, VEGF, PD-1, CD105, and LPCAT1 are potential therapeutic targets for metastatic ETT.
Keywords: cancer, epithelioid trophoblastic tumors, trophoblastic neoplasia
1. Introduction
Epithelioid trophoblastic tumor (ETT) is the rarest subtype of gestational trophoblastic neoplasia (GTN) accounting 1.0%–2.0% of all GTN cases.[1] Shih and Kurtmann first reported on the clinical and pathological features of ETT in 1998, and since 2003 the World Health Organization has accepted the classification of the tumor as a form of GTN.[2,3] ETT can arise from all types of pregnancy, including full-term delivery, miscarriage, ectopic pregnancy, or following a hydatidiform mole. The clinical presentations include mostly, vaginal bleeding and slightly elevated ß-serum chorionic gonadotropin (ß-hCG) levels.
Almost half of the cases occur in the cervix or the lower uterine segment forming a well-defined, expandable mass. The tumor appears as a discrete, nodular, expansile lesion with solid, cystic, and hemorrhagic components. The gross size varies from 0.5 to 5 cm.[4,5] Microscopically the growth pattern of ETT is nodular and well-circumscribed with focally infiltrative at the periphery.[6] The cells are relatively uniform, mononucleate arranged in nests and cords, the predominant cell type being chorionic-type intermediate trophoblast. Also, compared to placental trophoblastic tumors and choriocarcinoma, calcification is common in epithelioid trophoblastic tumors.[6] Mitoses are reported to range from 0-9/10 high-power fields with a mean of 2/10 and the Ki-67/MIB1 proliferative index ranges from 10%–25%.[4] Immunohistochemically, ETT has diffuse expression of HLA-G, p63, inhibin, cyclin E, HSD3B1, GATA3.[6] Other trophoblastic markers including Mel-CAM and hPL are focally positive.[6]
ETT has a high chemo-resistance to single-agent chemotherapy and the need to institute a multiagent treatment is required for poor prognosis and/or metastatic disease, with a mortality rate of 10% to 24%.[2] The current standard of care has focused on total hysterectomy with bilateral salpingo-oophorectomy and lymphadenectomy for early-stage disease and resection of residual disease sites (metastasectomy), following the multiagent chemotherapy in metastatic disease. The aims of this systematic review were: (1) to provide and summarize the literature on a rare event on which there is scant data, such as the epithelioid trophoblastic tumor; (2) to provide any information about patient characteristics, chemotherapy use, and outcomes over 23 years (1998–2021).
2. Materials and Methods
A systematic review was performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the ScienceDirect, Web of Science, and Scopus databases.
Ethical approval was not required for this study as all the research materials are derived from published studies.
2.1. Eligibility criteria
After an initial review of the literature we noted that no clinical trials have been conducted on patients with ETT. Due to the rarity of this pathology, most of the literature describes case reports which led us to include only these types of papers;
The present review assessed the following PICOs (Population, Intervention, Comparison, Outcomes) questions:
Population: We included case reports or case series of reproductive age and postmenopausal women diagnosed with epithelioid trophoblastic tumors;
Intervention: We considered all care interventions including surgery and chemotherapy use;
Comparison: no comparison expected;
Outcomes: Our primary outcomes measure was patients and disease characteristics. The secondary outcome was final follow-up health outcomes (alive, recurrence, death).
Eligibility/inclusion criteria: studies including full case description of epithelioid trophoblastic tumor lesions. Exclusion criteria: (1) review articles; (2) research articles without a full case description; (3) reports in a non-English language.
2.2. Information sources and search strategy
We conducted electronic searches for eligible reports within each of the following databases: ScienceDirect, Web of Science, and Scopus (Title/Abstract/Keywords). We searched using the search syntax: “epithelioid trophoblastic tumor” OR “Placental Trophoblastic Tumor” OR “Placental Trophoblastic Tumors” OR “Placental-Site Trophoblastic Tumor” OR “Trophoblastic Tumors”.
The process of developing the search strategy consisted of studying several relevant articles to identify records in the databases. Search terms were identified by examining words in titles, abstracts, keywords, and indexing by the subject of these records. A design of a search strategy was drafted using these terms and additional search terms were identified from the results of that strategy.
The only filter used was the English language. Relevant articles were obtained in full-text format and screened for additional references.
2.3. Study selection
Two independent reviewers (IMC and OMG) selected the studies using a 2-steps screening method. At first, the screening of titles and abstracts was performed to assess eligibility and inclusion criteria and exclude irrelevant studies. Afterward, the 2 reviewers evaluated full texts of included articles to assess study eligibility and inclusion criteria and avoid duplications of the included cases. Two other authors (DMS and MF) manually searched reference lists to search for additional relevant publications. CC, LT, and FG checked the data extracted.
2.4. Assessing the risk of bias
The inclusion of only case reports in this review presents a risk of bias. We assessed the methodological quality (risk of bias) of the papers included using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports which consists of 8 yes/no/unclear questions.[7] This is the only tool for assessing the methodological quality of case reports and case series.[8] Two reviewers (FG, LT) assessed the quality of the included studies. Disagreements between reviewers at different stages of the review were resolved by a third reviewer (OMG).
2.5. Data collection process/data items
Data collection was study-related (authors and year of study publication) and case-related (patient characteristics, signs/symptoms at clinical presentation, chemotherapy data, cancer lesion characteristics, treatment, and outcome).
Survival estimates were calculated using the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards regression model. All statistical analyses were conducted using the Statistical Package for the Social Sciences (software version 25; IBM Corp., Armonk, NY, USA). A 2-sided P value of < .05 was considered statistically significant.
3. Results
3.1. Reports selection
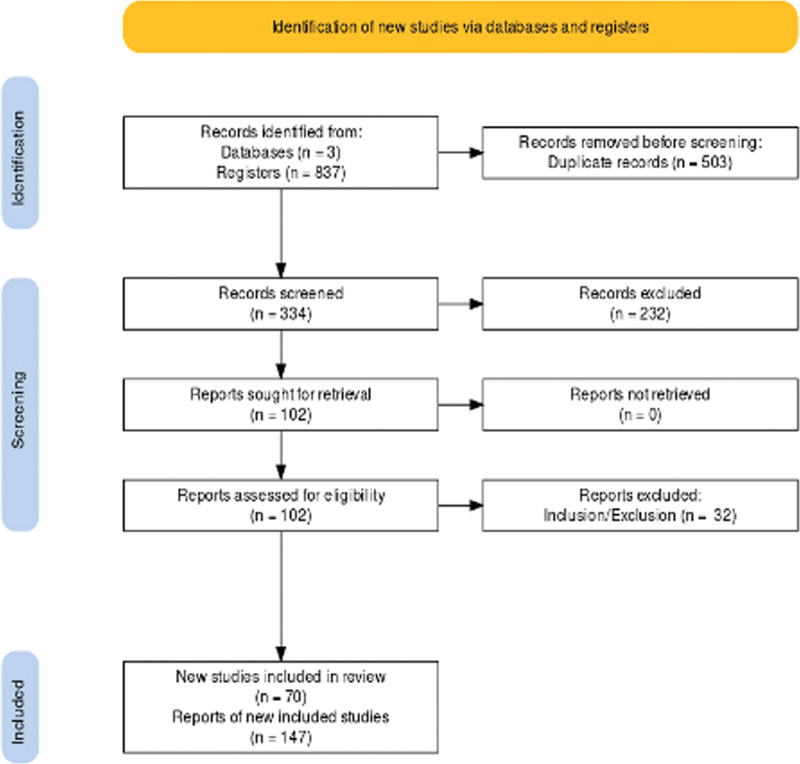
Figure 1 shows the literature review flowchart. We retrieved 375 articles on ScienceDirect, 187 articles on Web of Science and 275 papers on Scopus databases (accessed on 24 August 2021). After duplicates were removed, 334 articles were assessed. Based on the title and abstract, 232 records were excluded. Then, the full text of 102 papers was evaluated for eligibility. Based on inclusion and exclusion criteria, 32 articles were further removed. Finally, 70 studies were assessed for qualitative synthesis, including 147 cases.
Figure 1.
Flowchart of literature review.
3.2. Studies characteristics
The main characteristics of the included case reports were reported in Table 1.
Table 1.
Studies characteristics.
| Study ID | Year | Study design | Symptoms | Primary Tumor Location | Stage | Outcome |
|---|---|---|---|---|---|---|
| Fang et al | 2014 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Saeeda Almarzooqi et al | 2014 | Case series | Vaginal bleeding | Uterine | I | Remission |
| McGregor SM et al | 2020 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Mma E et al | 2015 | Case series | Vaginal bleeding (both cases) | Uterine (both cases) | I (1st case); IV (2nd case) | Remission (1st case); died of disease (2nd case) |
| Zhou F et al | 2017 | Case report | Asymptomatic | Uterine | I | Remission |
| Lo C et al | 2006 | Case report | Vaginal bleeding | Uterine | I | Remission |
| ZHabg Di et al | 2020 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Manoharan J et al | 2016 | Case report | Abdominal pain | Uterine | I | Remission |
| Moutte A et al | 2013 | Case series | Vaginal bleeding- 3 cases; abdominal pain: 2 cases; asymptomatic-2 cases | Uterine: 6 cases; vagina-1 case | I: 5 cases; II: 1 case; III: 1 case | Remission |
| Davis M et al | 2015 | Case series | Vaginal bleeding | Uterine | I: 1 case; III-2 cases; IV: 3 cases | Remission (4 cases); partial remission (2 cases-stage 4) |
| Qi Li et al | 2007 | Case series | Vaginal bleeding (all) | Uterine | I (2 cases); IV (1 case) | Remission (2 cases: stage 1); Died of disease (1 case: stage 4) |
| Shih M et al | 1999 | Case series | Vaginal bleeding (11 cases); abdominal pain (1 case); asymptomatic (2 cases) | Uterine (13 cases); bowel (1 case) | I (10 cases); III (3 cases); IV (1 case) | Remission -12 cases; partial remission: 2 cases (1 case: stage III, 1 case: stage iv) |
| Yang J et al | 2019 | Case series | Vaginal bleeding (14 cases); others (6 cases); asymptomatic (1 case) | Uterine (all) | I (8 cases); II (3 cases); III (6 cases); IV (4 cases) | Remission (15 cases); partial remission: 3 cases (2 cases: stage 3; 1 case: stage 4); died of disease: 3 cases (stage 4) |
| Trujilo A et al | 2017 | Case series | Vaginal bleeding (1 case); asymptomatic (1 case) | Uterine | I | Remission |
| Venken P et al | 2006 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Takekawa Y et al | 2010 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Coulson L et al | 2000 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Scott E et al | 2012 | Case report | Abdominal pain | Uterine | I | Remission |
| Ohya A et al | 2017 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Stanculescu R et al | 2016 | Case report | Asymptomatic | Uterine | I | Remission |
| Park JW et al | 2016 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Kurt S et al | 2021 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Sung WJ et al | 2013 | Case series | Vaginal bleeding | Uterine | I | Remission |
| Sobecki-Rausch J et al | 2018 | Case series | Vaginal bleeding (3 cases); asymptomatic (1 case); others (1 case) | Uterine (4 cases); lung (1 case) | I (1 case); III (3 cases); 4 (1 case) | Remission: 4 cases; died of disease: 1 case (stage4) |
| Zhang X et al | 2015 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Shen X et al | 2011 | Case series | Abdominal pain (3 cases); vaginal bleeding (5 cases); asymptomatic (1 case) | Uterine (all) | I (1 case); II (2 cases); III (2 cases); IV (4 cases) | Remission -4 cases (1: stage 1; 2: stage 2; 1 stage 3); died of disease -5 cases (1: stage 3; 4: stage 4) |
| Qian XQ et al | 2020 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Fadare O et al | 2006 | Case series | Vaginal bleeding (3 cases); asymptomatic (2 cases) | Uterine (2 cases); cervix (2 cases); ovarian (1 case) | I (1 case); II (2 cases); III (2 cases) | Remission (4 cases); partial remission (1 case: stage 2) |
| Li J et al | 2011 | Case series | Asymptomatic (2 cases); vaginal bleeding (5 cases) | Uterine | I (4 cases); III (2 cases); IV (1 case) | Remission (6 cases); died of disease (1 case: stage 3) |
| Jiang F et al | 2018 | Case report | Vaginal bleeding | Recto-uterine pouch | I | Remission |
| Li J et al | 2019 | Case report | Vaginal bleeding | Uterine | III | Remission |
| Meydanli MM | 2002 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Yigit S et al | 2020 | Case report | Vaginal bleeding | Uterine | I | Remission |
| Black K.A et al | 2021 | Case report | Vaginal bleeding | Uterine | II | Remission |
| Phippen NT et al | 2010 | Case report | Abdominal pain | Cervix | II | Remission |
| Vemula S et al | 2015 | Case report | Abdominal pain | Cervix | II | Remission |
| Kuo KT et al | 2004 | Case report | Vaginal bleeding | Uterine | II | Remission |
| Parker A et al | 2003 | Case report | Abdominal pain | Fallopian tube | II | Remission |
| Zhao J et al | 2013 | Case report | Asymptomatic | Vagina | II | Remission |
| Khunamornpong S et al | 2011 | Case report | Abdominal pain | Ovarian | II | Remission |
| Choi MC et al | 2019 | Case report | Asymptomatic | Uterine | II | Remission |
| Kavurmaci Ö et al | 2018 | Case report | Asymptomatic | Uterine | III | Remission |
| Kim JH et al | 2017 | Case report | Abdominal pain | Abdominal | III | Remission |
| Jiang L et al | 2017 | Case report | Vaginal bleeding | Fallopian tube | III | Remission |
| Lewin SN et al | 2009 | Case series | Asymptomatic (1 case); vaginal bleeding (2 cases) | Uterine (1 case); lung (2 cases) | III (all cases) | Remission (all cases) |
| Abrão FC et al | 2011 | Case report | Vaginal bleeding | Lung | III | Partial remission |
| Lei W | 2018 | Case report | Asymptomatic | Uterine | III | Remission |
| Okereke IC | 2014 | Case report | Vaginal bleeding | Uterine | III | Partial remission |
| Li JW et al | 2019 | Case report | Asymptomatic | Lung | III | Remission |
| Fénichel P et al | 2014 | Case report | Vaginal bleeding | Ovarian | III | Remission |
| Ahn HY et al | 2013 | Case report | Asymptomatic | Lung | III | Remission |
| Urabe S et al | 2007 | Case report | Others | Lung | III | Remission |
| Kageyama S et al | 2016 | Case report | Abdominal pain | Uterine | III | Remission |
| Luo R et al | 2016 | Case report | Asymptomatic | Vagina | III | Remission |
| Kim J et al | 2013 | Case report | Abdominal pain | Uterine | IV | Remission |
| Nakamura B et al | 2021 | Case report | Abdominal pain | Uterine | IV | Remission |
| Bell S et al | 2021 | Case report | Asymptomatic | Abdominal soft tissue | IV | Partial remission |
| NoH et al | 2008 | Case report | Abdominal pain | Uterine | IV | Partial remission |
| Andriani A et al | 2021 | Case report | Abdominal pain | Abdominal soft tissue | IV | Remission |
| Macdonald M et al | 2010 | Case series | Vaginal bleeding | Uterine | IV | Died of disease: case; partial remission: 1 case |
| Dobrosavljevic A et al | 2019 | Case report | Abdominal pain | Uterine | IV | Died of disease |
| Jordan S et al | 2011 | Case report | Abdominal pain | Cervix | IV | Died of disease |
| Chohan M et al | 2004 | Case report | Others | Spine | IV | Died of disease |
| Kodey PD et al | 2014 | Case report | Vaginal bleeding | Uterine | IV | Died of disease |
| Gil F et al | 2019 | Case report | Vaginal bleeding | Vulvar | IV | Died of disease |
| Shet T et al | 2008 | Case report | Vaginal bleeding | Uterine | IV | Died of disease |
| Madhu B et al | 2012 | Case report | Abdominal pain | Liver | IV | Partial remission |
| Hsiue EHC et al | 2017 | Case report | Abdominal pain | Uterine | IV | Died of disease |
| Mahmood H et al | 2016 | Case report | Vaginal bleeding | Ovarian | IV | Partial remission |
3.3. Risk of bias
Fifty-seven case reports scored “yes” for all 8-checklist questions, hence included case report was scored as high quality.
3.4. Clinical presentation
The most common presenting symptom was irregular vaginal bleeding (61.2%), and the antecedent obstetrical event before diagnosis was full-term pregnancy in 91 patients (61.9%) whereas 39 (26.5%) underwent an abortion. Pretreatment serum human chorionic gonadotropin (β-hCG) levels ranged up to 1000 mIU/mL in 86 patients (58.5%),and in 36 patients (24.5%) over 1000 mIU/mL. Most patients (42.2%) had stage I disease; 37 patients (25.2%) had lung metastases (FIGO stage III) and 32 patients (21.8%) had FIGO stage IV disease (Table 2). Most common sites of metastatic disease were the lungs (n = 39), followed by liver (n = 9) and brain (n = 7)
Table 2.
Characteristics of 147 patient diagnosed with epithelioid trophoblastic tumor (ETT).
| Parameters | No. of patients, N (%) |
|---|---|
| Age | |
| <40 y | 88 (59.9%) |
| >40 y | 59 (40.1%) |
| Symptoms | |
| Vaginal bleeding | 90 (61.2%) |
| Abdominal pain | 36 (24.5%) |
| Asymptomatic | 21 (14.3%) |
| AP outcome | |
| Full-term birth | 91 (61.9%) |
| Abortion | 39 (26.5%) |
| Molar pregnancy | 9 (6.1%) |
| Ectopic pregnancy | 1 (0.7%) |
| Interval to AP | |
| <48 months | 77 (52.4%) |
| ≥48 months | 60 (40.8%) |
| No data | 10 (6.8%) |
| ß-HCG | |
| <100 mIU/mL | 50 (34.0%) |
| 100-1000 mIU/mL | 36 (24.5%) |
| ≥1000 mIU/mL | 36 (24.5%) |
| No data | 25 (17.0%) |
| FIGO Stage | |
| I | 62 (42.2%) |
| II | 16 (10.9%) |
| III | 37 (25.2%) |
| IV | 32 (21.8%) |
3.5. Poor outcome prediction based on clinical characteristics
Binomial logistic regression was performed to ascertain the effects of clinical characteristics on the likelihood that participants died during follow-up.
The logistic regression model was statistically significant, χ2(4) = 27.40, P < .001.
3.6. Treatment and outcome
All 62 patients (100%) who were included in stage I, achieved complete remission (CR) after treatment. Surgery was used in 96.7% being combined with first-line chemotherapy in 33.9% of cases (21 patients), and the most common regimen used to be EMA/CO (42.7%) (Table 3).
Table 3.
Treatment and outcomes in 62 patients included in stage I of the disease.
| Treatment | Total no. of patients/% |
|---|---|
| Surgery | |
| TAH | 22/35.5% |
| TAH/BSO | 18/29% |
| TAH/BSO/LFN | 11/17.7% |
| TAH/BSO/MTS | 1/1.6% |
| TE | 8/12.9% |
| NOS | 2/3.2% |
| First-line chemotherapy | |
| EMA/CO | 9/14.5% |
| FAEV | 5/8.1% |
| TP/TE | 1/1.6% |
| EMA/EP | 2/3.2% |
| EP/EMA | 1/1.6% |
| MTX | 2/3.2% |
| MTX-FA | 1/1.6% |
| NO-CHEMO | 41/66.1% |
| Total with chemotherapy | 21/33.9% |
In stage II, 15 patients (93.8%) achieved complete remission (CR), and one patient (6.3%) with partial remission PR). Surgery was performed in all 16 cases. In 13 patients (81.2%) first-line chemotherapy was used combined with surgery and 3 patients (18.7%), needed second-line chemotherapy. The most frequent first-line chemotherapy used, was EMA/CO in 5 patients (31.3%) (Table 4).
Table 4.
Treatment and outcomes in 16 patients included in stage II of the disease.
| Treatment | Total no. of patients/% | Total remission no. of patients /% | Partial remission no. of patients/% |
|---|---|---|---|
| Surgery | |||
| TAH | 3/18.8% | 3/100% | – |
| TAH/BSO | 6/37.5% | 5/83.3% | 1/16.7% |
| TAH/BSO/LFN | 2/12.5% | 2/100% | – |
| TAH/BSO/MTS | 1/6.3% | 1/100% | – |
| TE | 3/18.8% | 3/100% | – |
| USO | 1/6.3% | 1/100% | – |
| First-line chemotherapy | |||
| EMA/CO | 5/31.3% | – | – |
| FAEV | 4/25.0% | – | – |
| EMA/EP | 2/12.5% | – | – |
| 5FU/MTX | 1/6.3% | – | – |
| BEP | 1/6.3% | – | 1/100% |
| Second-line chemotherapy | |||
| EMA/CO | 1/6.3% | 1/100% | – |
| TIP | 1/6.3% | 1/100% | – |
| Pembrolizumab | 1/6.3% | 1/100% | – |
In stage III, 30 patients (81.1%) achieved complete remission (CR), 5 patients (13.5%) with partial remission (PR), and 2 patients (5.4%), died of disease. Surgery was performed in 35 patients (94.6%), chemotherapy was used in 30 patients (81.1%), and most used regimens were EMA/CO (33.3%) and EP/EMA (26.6%). Second-line chemotherapy was used in 6 patients (16.2%), with a survey rate of 66.6% (Table 5).
Table 5.
Treatment and outcomes in 37 patients included in stage III of the disease.
| Treatment | Total no. of patients/% | Total remission no. of patients/% | Partial remission no. of patients/% | Death of disease no. of patients/% |
|---|---|---|---|---|
| Surgery | ||||
| TAH | 2/5.4% | 1/50.0% | – | 1/50.0% |
| TAH/BSO | 3/8.1% | 1/33.3% | 2/66.7% | – |
| TAH/BSO/LFN | 5/13.5% | 3/60.0% | 2/40.0% | – |
| NOS | 2/5.4% | 1/50.0% | 1/50.0% | – |
| MTS | 11/29.7% | 11/100% | – | – |
| TAH/BSO/MTS | 10/27.0% | 9/90.0% | – | 1/10% |
| TE/MTS | 3/8.1% | 3/100% | – | – |
| O/MTS | 1/2.7% | 1/100% | – | – |
| Total | 37/100% | 30/81.1% | 5/13.5% | 2/5.4% |
| First line chemotherapy | ||||
| EMA/CO | 10/27.0% | 8/80.0% | – | 2/20.0% |
| FAEV | 6/16.2% | 4/66.7% | 2/33.3% | – |
| TP/TE | 3/8.1% | 3/100% | – | – |
| EMA/EP | 1/2.7% | – | 1/100% | – |
| EP/EMA | 8/21.6% | 7/87.5% | 1/12.5% | – |
| MTX | 1/2.7% | 1/100% | – | – |
| BEP | 1/2.7% | 1/100% | – | – |
| NO-CHEMO | 7/18.9% | 6/85.7% | 1/14.3% | – |
| Total | 37/100% | 30/81.1% | 5/13.5% | 2/5.4% |
| Second-line chemotherapy | ||||
| NO-CHEMO | 31/83.8% | 28/90.3% | 3/9.7% | – |
| EMA/CO | 2/5.4% | – | 2/100% | – |
| TIP | 1/2.7% | – | – | 1/100% |
| TP/TE | 1/2.7% | 1/100% | – | – |
| BEP | 1/2.7% | – | – | 1/100% |
| ICE | 1/2.7% | 1/100% | – | – |
In stage IV, 4 patients (12.5%) achieved complete remission (CR), 9 patients (28.1%) achieved partial remission, and 19 patients (59.4%), died of disease. Surgery was performed in 26 patients (81.25%), chemotherapy was used in 31 patients (96.9%), and the most used regimens were EMA/CO (40.6%) and FAEV (25%). Second-line chemotherapy was used in 14 patients (43.7%), with the most common regimen EMA/CO, used in 6 patients (42.8%), and 9 patients (64.2%) failed to survey second-line chemotherapy (Table 6).
Table 6.
Treatment and outcomes in 32 patients included in stage IV of the disease.
| Treatment | Total no. of patients/% | Total remission no. of patients/% | Partial remission no. of patients/% | Death of disease no. of patients/% |
|---|---|---|---|---|
| Surgery | ||||
| TAH | 5/15.6% | 1/20.0% | – | 4/80.0% |
| TAH/BSO | 7/21.9% | – | 2/ 28.6% | 5/71.4% |
| NOS | 6/18.8% | 0/0.0% | 1/16.7% | 5/83.3% |
| MTS | 3/9.4% | – | 2/66.7% | 1/33.3% |
| TAH/BSO/MTS | 9/28.1% | 3/ 33.3% | 3/33.3% | 3/33.3% |
| TE/MTS | 1/1/3.1% | – | 1/100% | – |
| SPS | 1/3.1% | – | – | 1/ 100% |
| Total | 32 | 4/ 12.5% | 9/ 28.1% | 19/ 59.4% |
| First-line chemotherapy | ||||
| EMA/CO | 13/40.6% | 1/7/7% | 3/23.1% | 9/69.2% |
| FAEV | 8/25.0% | – | 1/12.5% | 7/87.5% |
| EMA/EP | 5/15.6% | 1/20.0% | 4/80.0% | – |
| EP/EMA | 3/ 9.4% | 1/33.3% | 1/33.3% | 1/33.3% |
| NO-CHEMO | 1/3.4% | 1/100% | – | – |
| BEP | 1/3.1% | - | – | 1/100% |
| CEC | 1/3.1% | 1/100% | ||
| Second-line chemotherapy | ||||
| NO-CHEMO | 19/ 59.4% | 4/21.0% | 4/21.0% | 11/57.9% |
| EMA/CO | 6/18.8% | 1/16.7% | 5/83.3% | |
| TIP | 1/3.1% | – | – | 1/100% |
| Pembrolizumab | 2/ 6.3% | – | 2/100% | – |
| BEP | 1/3.1% | 1/100% | ||
| VIP | 1/3.1% | 1/100% | ||
| C/T | 2/6.3% | – | 2/100% |
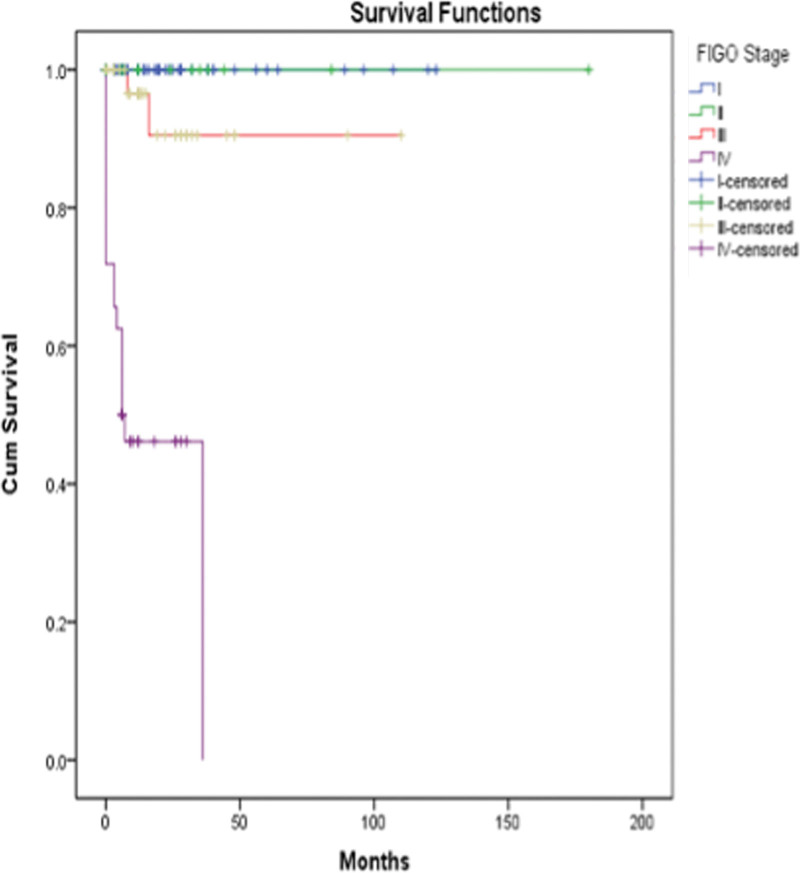
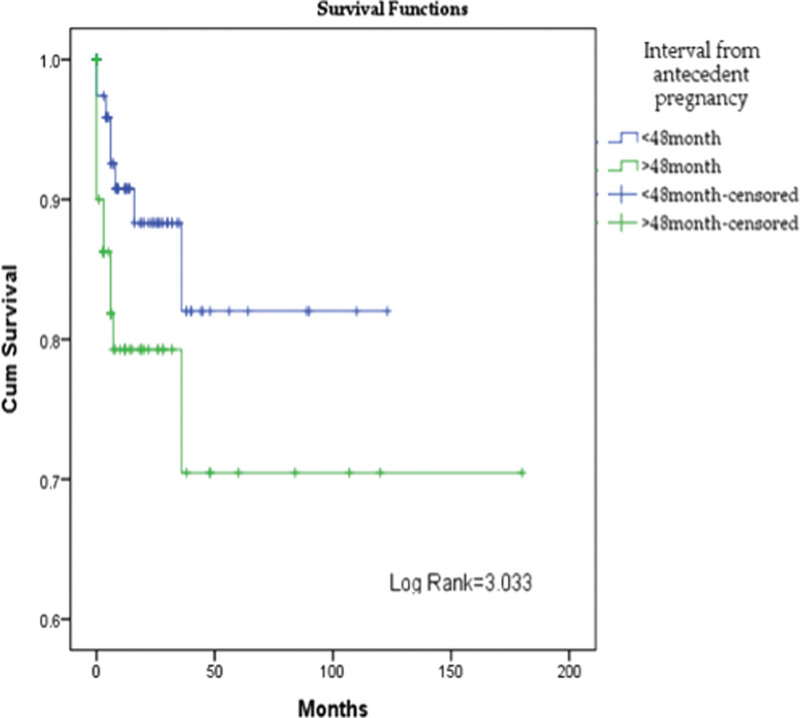
Regardless of the stage of the disease, patients with the onset of the disease more than 48 months after the previous pregnancy had a mortality of 20% (n = 12/48), compared with a mortality of 10.4% (n =8/77) in patients with disease’s onset at less than 48 months (chi-square value = 2.498, P = .114). No significant statistical difference in survival distribution was observed (log rank= 3.033, P = .08). A statistically significant difference was observed in cumulative survival between stages of the disease with a log rank= 76.384 (Figs. 2,3).
Figure 2.
Cumulative survival in women with ETT, depending on FIGO stage of the disease. ETT = epithelioid trophoblastic tumor.
Figure 3.
Cumulative survival in women with ETT, depending on the time between previous pregnancy and the onset of the disease. ETT = epithelioid trophoblastic tumor.
4. Discussion
Follow-up information was available in 119 patients. The mean follow-up was 20.6 months (median, 12 months; range, 1–180 months). Overall survival is 85.7%, with a 75.5% disease-free survival, and 21 patients died 14.3%. Stage IV appeared most important poor prognostic factor.
The reports included in this review showed a median age of patients with ETT of 30 years. Other studies showed that ETT occurs in women between 15 and 48 years of age with a mean of 36.1 years.[9] By dividing the patients into 2 age groups, according to the FIGO classification, we showed that the incidence of ETT is more frequent in women under 40 years of age compared to those over 40 years of age. Classically, it is considered that ETT occurs in women of childbearing age, usually after a normal pregnancy.[10] In addition, the time from gestation to presentation varies, reporting intervals from 1 year to 18 years.[4] However, recent reports describe cases of ETT, one with an aggressive spread in the abdomen and pelvis, in a 68-year-old woman, 30 years after the last birth, and one in a 53-year-old woman, 25 years after spontaneous delivery.[5,10]
In addition, we showed that an interval ≥48 months, from the last known/presumed pregnancy, is a predictor of poor outcome with 12 patients (20.0%) who died. Other studies demonstrated that an interval of ≥48 months from the antecedent pregnancy was associated with a 100% death rate, independent of the stage.
Diagnosis of ETT remains a challenge due to its rarity and diverse presentations, which can lead to potential mismanagement and delay in treatment. In line with previous studies, irregular vaginal bleeding was the most common symptom of ETT (59.9%), in our study.[11]
ETT can develop after any antecedent pregnancy event, in the present study, 61.9% of such events were full-term pregnancies, comparable to a mean of 65.0%, in the literature.[3,4]
In 80% of the patient with ETT is detectable mild to moderate elevation of serum hCG of less than 2500 mIU/mL.[5] In the present study, 86 patients (58.5%) had β-hCG levels ≤ 1000 IU/L. In previous studies, patients with ETT had slightly increased levels of β-hCG ≤ 1000 IU/L (55.2%).[12]
Approximately 30.0% of patients reportedly presented with metastatic disease at diagnosis.[3,11] The uterus is the most common primary site of ETT but may occur at extrauterine locations, including the fallopian tube, ovary, and pelvic peritoneum, while the lung is the most common extrauterine site of metastasis, accounting for 19.0% of cases.[5,13] In our review 72 patients (48.9%) presented with metastatic disease at diagnosis. The most common extra-uterine site of metastasis is the lung (26.5%), follow by the liver (6.1%), and brain (4.8%). Surgical resection is the primary treatment modality and is important for ETT present with non-metastatic disease1. Our reviewed data have shown that 41 patients (66.1%) were in stage I of the disease. Previous studies have shown that patients with diseases limited to the uterus could be cured by hysterectomy without adjuvant chemotherapy.[14] Surgery is also important for eradicating metastatic disease and drug-resistant residual disease.[14,15] Our results showed that 90.5% of patients with advanced-stage disease underwent multiple surgical procedures, including hysterectomy and resection of isolated tumors.
Regarding chemotherapy, it was used in 33.4% of patients in stage I, 81.3% of patients in stage II, 81% of patients with stage III, and 97% of patients in stage IV. Chemotherapy alone is inadequate for advanced-stage and metastatic or locally advanced disease, together with surgery plays an important role in the management of ETT patients. The most used regimens of first-line chemotherapy were EMA/CO (39%), FAEV (24.2%), EP/EMA (13%), and EMA/EP (10.5%). The first-line chemotherapy successful was complete remission (60%), partial remission (24.2%), and no response (15.8%). The most used regimen EMA/CO efficiency was complete remission (56.8%), partial remission (24.3%), and no response (18.9%). In stage III 2 patients died, the first-line chemotherapy used was EMA/CO, and 8 patients achieved complete remission. Stage IV is a poor prognostic factor, 19 patients (59.3%) died of the disease. The failure of first-line chemotherapy was 5 patients EMA/CO, 6 patients FAEV, 1 patient BEP, and one patient EMA/EP. Second-line chemotherapy was successfully for 6 patients 2 EMA/CO, one BEP, one VIP, and 2 pembrolizumab. First-line chemotherapy with complete remission was achieved by 3 patients, one EMA/CO, one EP/EMA, and one EMA/EP. The treatment of patients with stage IV or metastatic ETT remains a challenge. Ghorani et al found that pembrolizumab (a humanized monoclonal antibody against PD-1) was effective in patients with no response to chemotherapy.[16] Veras et al reported that 8 of 14 patients with ETT had detectable levels of PD-L1 in their tumors.[17] In our study, 3 patients received second-line chemotherapy with pembrolizumab, 1 in stage II who achieved complete remission, and 2 patients in stage IV, with partial remission, who are alive with disease. Cho et al in their study, analyzed 4 cases of ETT, for differential gene expression, pathway alteration, and PD-L1 expression level.[18] A variable high expression level of PD-L1, with PI3K-alternated pathways, was found in those tumors, which suggested pembrolizumab, an anti-PD-1 drug, is effective for resistant chemotherapy in epithelioid trophoblastic tumors. High levels of EGFR expressions have been detected in epithelioid trophoblastic tumors.[19] The anti-EGFR regimen has become a target-based treatment for various malignant diseases. Drugs that target EGFR, including cetuximab, and gefitinib, can produce benefits for patients with ETT, in combination with chemotherapy used. Mutational analyses of the EGFR and PI3K pathway, together with their gene-expresso levels, are essential for treatment development, and optimal therapeutic effects. Frijstein et a. report a conducted study on 32 patients and evaluated the effect of high-dose-chemotherapy (HDC) with peripheral blood stem cell support (PBSCS) on survival patients with refractory response to chemotherapy.[20] The results show that HDC can be beneficial for some patients who have failed adjuvant treatment for poor-prognosis ETT. Oliver et al, using RNA-sequentially analysis in 5 patients with ETT, found LPCAT1-TERT fusion proteins that can positively modulate cell proliferation.[21] These findings could have potential diagnostic, prognostic, and therapeutic relevance. New therapeutic regimens are needed to reduce the toxic effects associated with current chemotherapyThis study has a few limitations. First, due to the rarity of this pathology, we had to include only case report papers in the review, which increases the risk of bias. Also due to the small number of cases, we cannot make recommendations on the treatment of this pathology.
5. Conclusions
Surgery combined with chemotherapy is recommended for patients with metastatic disease. Despite the rarity of ETT with a small number of cases in analyses, it is difficult to establish a management strategy for patients with this disease. In our opinion, the future treatment of ETT could be by immunotherapy, investigational studies on biological agents targeting molecules like EGFR, VEGF, PD-1, PD-L2, CD-105, and LPCAT1 are required, especially in resistant chemotherapy disease.
Author contributions
Conceptualization: CC, FG, and AM; methodology: MF and OMG; software: LT and DMS; formal analysis: IMC; investigation: OMG, FM, and IMC; data curation: OMG and IMC; writing–original draft preparation: CC and LT; writing–review and editing: IS and FG.; visualization: IS; supervision: AM.
Abbreviations:
- 5-FU/MTX =
- 5-fluorouracil, methotrexate
- AP =
- antecedent pregnancy
- BSO =
- bilateral salpingo-oophorectomy
- C/T =
- carboplatin; paclitaxel
- CEC =
- cyclophosphamide, etoposide, cisplatin
- EMA/CO =
- etoposide, methotrexate, actinomycin-D/ cyclophos-phamide, vincristine
- EMA/EP =
- etoposide, methotrexate, actinomycin-D/etoposide and cisplatin
- EP/EMA =
- etoposide, cisplatin/etoposide, methotrexate, dactinomycin
- ETTs =
- epithelioid trophoblastic tumors
- FAEV =
- 5-fluorouracil, actinomycin-D, etoposide, vincristine
- GTN =
- gestational trophoblastic neoplasia
- ICE =
- ifosfamide; carboplatin, etoposide
- LFN =
- lymphadenectomy
- MTS =
- metastasectomy
- NO-CHEMO =
- no chemotherapy
- NOS =
- no surgery
- PICOs =
- Population, Intervention, Comparison, Outcomes
- SPN =
- spinal surgery
- TAH =
- total abdominal hysterectomy
- TE =
- tumor excision
- TIP =
- paclitaxel, ifosfamide, cisplatin
- TP/TE =
- paclitaxel, cisplatin/paclitaxel, etoposide
- USO =
- unilateral salpingo-oophorectomy
- VIP =
- etoposide, ifosfamide, cisplatin
- β-hCG =
- ß-serum chorionic gonadotropin
The authors have no funding and conflicts of interest to disclose.
Data availability statement: All data generated or analysed during this study are included in this published article.
How to cite this article: Gorun F, Tomescu L, Motoc A, Citu C, Sas I, Serban DM, Forga M, Citu IM, Gorun OM. Clinical features and management of trophoblastic epithelioid tumors: a systematic review. Medicine 2022;101:30(e29934).
Simple Summary: Epithelioid trophoblastic tumor (ETT) is an extremely rare subtype of gestational trophoblastic neoplasia (GTN), accounting for only 1.0–2.0% of all GTN cases. We investigated the clinical features, treatments, outcomes, and prognostic factors in patients with ETT, and explored potential therapeutic targets, over 23 years (1998–2021). The management of this rare pathology remains a challenge, 47% of patients from reviewed reports have metastases at the time of diagnosis. In this regard, a systematic literature review on the epithelioid trophoblastic tumor was performed to provide useful clinical information about patient characteristics, chemotherapy use, and outcomes.
AP = antecedent pregnancy, ß-hcg = ß human chorionic gonadotrotropin.
BSO = bilateral salpingo-oophorectomy, EMA/CO = etoposide, methotrexate, actinomycin-D/cyclophos-phamide, vincristine, EMA/EP = etoposide, methotrexate, actinomycin-D/etoposide and cisplatin, EP/EMA-etoposide = cisplatin/etoposide, methotrexate, dactinomycin, FAEV = 5-fluorouracil, actinomycin-D, etoposide, vincristine, LFN = lymphadenectomy, MTS = metastasectomy, NO-CHEMO = no chemotherapy, NOS-no surgery, TAH = total abdominal hysterectomy, TE = tumor excision, TP/TE = paclitaxel, cisplatin/paclitaxel, etoposide.
BSO = bilateral salpingo-oophorectomy, EMA/CO = etoposide, methotrexate, actinomycin-D/cyclophos-phamide, vincristine, EMA/EP = etoposide, methotrexate, actinomycin-D/etoposide and cisplatin, LFN = lymphadenectomy, NO-CHEMO = no chemotherapy, NOS-no surgery, TAH = total abdominal hysterectomy, TE = tumor excision, USO-unilateral salpingo-oophorectomy.
5 = FU/MTX -5-fluorouracil, methotrexate, BEP = bleomycin, etoposide, cisplatin, BSO = bilateral salpingo-oophorectomy, C/T = carboplatin, paclitaxel, CEC = cyclophosphamide, etoposide, cisplatin, EMA/CO = etoposide, methotrexate, actinomycin-D/cyclophosphamide, vincristine, EMA/EP = etoposide, methotrexate, actinomycin-D/etoposide and cisplatin, EP/EMA = etoposide, cisplatin/ etoposide, methotrexate, dactinomycin, FAEV = 5-fluorouracil, actinomycin-D, etoposide, vincristine, ICE = ifosfamide, carboplatin, etoposide, LFN = lymphadenectomy, MTS = metastasectomy, NO-CHEMO = no chemotherapy, NOS = no surgery, SPN = spinal surgery, TAH = total abdominal hysterectomy, TE = tumor excision, TIP = paclitaxel, ifosfamide, cisplatin, TP/TE = paclitaxel, cisplatin/paclitaxel, etoposide, USO = unilateral salpingo-oophorectomy, VIP = etoposide, ifosfamide, cisplatin.
5 = FU/MTX -5-fluorouracil, methotrexate, BEP = bleomycin, etoposide, cisplatin, BSO = bilateral salpingo-oophorectomy, C/T = carboplatin, paclitaxel, CEC = cyclophosphamide, etoposide, cisplatin, EMA/CO = etoposide, methotrexate, actinomycin-D/cyclophosphamide, vincristine, EMA/EP = etoposide, methotrexate, actinomycin-D/etoposide and cisplatin, EP/EMA = etoposide, cisplatin/ etoposide, methotrexate, dactinomycin, FAEV = 5-fluorouracil, actinomycin-D, etoposide, vincristine, ICE = ifosfamide, carboplatin, etoposide, LFN = lymphadenectomy, MTS = metastasectomy, NO-CHEMO = no chemotherapy, NOS = no surgery, SPN = spinal surgery, TAH = total abdominal hysterectomy, TE = tumor excision, TIP = paclitaxel, ifosfamide, cisplatin, TP/TE = paclitaxel, cisplatin/paclitaxel, etoposide, USO = unilateral salpingo-oophorectomy, VIP = etoposide, ifosfamide, cisplatin.
Contributor Information
Florin Gorun, Email: oanabalan@hotmail.com.
Andrei Motoc, Email: amotoc@umft.ro.
Cosmin Citu, Email: citu.ioana@umft.ro.
Ioan Sas, Email: ioansas58@yahoo.com.
Denis Mihai Serban, Email: denis.serban@umft.ro.
Marius Forga, Email: forga.marius@umft.ro.
Ioana Mihaela Citu, Email: citu.ioana@umft.ro.
Oana Maria Gorun, Email: oanabalan@hotmail.com.
References
- [1].Li J, Shi Y, Wan X, et al. Epithelioid trophoblastic tumor: a clinicopathological and immunohistochemical study of seven cases. Med Oncol. 2011;28:294–9. [DOI] [PubMed] [Google Scholar]
- [2].Lybol C, Thomas CM, Bulten J, et al. Increase in the incidence of gestational trophoblastic disease in the Netherands. Gynecol Oncol. 2011;121:334–8. [DOI] [PubMed] [Google Scholar]
- [3].Shih I-M, Kurman RJ. Epithelioid trophoblastic tumor: a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. Am J Surg Pathol. 1998;22:1393–403. [DOI] [PubMed] [Google Scholar]
- [4].Allison KH, Love JE, Garcia RL, et al. Epithelioid trophoblastic tumor: review of a rare neoplasm of the chorionic-type intermediate trophoblast. Arch Pathol Lab Med. 2006;130:1875–7. [DOI] [PubMed] [Google Scholar]
- [5].Narita F, Takeuchi K, Hamana S, et al. Epithelioid trophoblastic tumor (ett) initially interpreted as cervical cancer. Int J Gynecol Cancer. 2003;13:551–4. [DOI] [PubMed] [Google Scholar]
- [6].Lanjewar S, Gupta R. Epithelioid trophoblastic tumor. PathologyOutlines.com. Available at: https://www.pathologyoutlines.com/topic/placentaETT.html. [access date July 16, 2022].
- [7].Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020;18:2127–33. [DOI] [PubMed] [Google Scholar]
- [8].Ma L-L, Wang Y-Y, Yang Z-H, et al. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hui P. Gestational trophoblastic tumors: a timely review of diagnostic pathology. Arch Pathol Lab Med. 2019;143:65–74. [DOI] [PubMed] [Google Scholar]
- [10].McGregor SM, Furtado LV, Montag AG, et al. Epithelioid trophoblastic tumor: expanding the clinicopathologic spectrum of a rare malignancy. Int J Gynecol Pathol. 2020;39:8–18. [DOI] [PubMed] [Google Scholar]
- [11].Palmer JE, Macdonald M, Wells M, et al. Epithelioid trophoblastic tumor: a review of the literature. J Reprod Med. 2008;53:465–75. [PubMed] [Google Scholar]
- [12].Froeling FEM, Ramaswami R, Papanastasopoulos P, et al. Intensified therapies improve survival and identification of novel prognostic factors for placental-site and epithelioid trophoblastic tumours. Br J Cancer. 2019;120:587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang X, Lü W, Lü B. Epithelioid trophoblastic tumor: an outcome-based literature review of 78 reported cases. Int J Gynecol Cancer. 2013;23:1334–8. [DOI] [PubMed] [Google Scholar]
- [14].Davis MR, Howitt BE, Quade BJ, et al. Epithelioid trophoblastic tumor: a single institution case series at the new England trophoblastic disease center. Gynecol Oncol. 2015;137:456–61. [DOI] [PubMed] [Google Scholar]
- [15].Hancock BW, Tidy J. Placental site trophoblastic tumour and epithelioid trophoblastic tumour. Best Pract Res Clin Obstet Gynaecol. 2021;74:131–48. [DOI] [PubMed] [Google Scholar]
- [16].Ghorani E, Kaur B, Fisher RA, et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. 2017;390:2343–5. [DOI] [PubMed] [Google Scholar]
- [17].Veras E, Kurman RJ, Wang TL, et al. PD-L1 Expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol. 2017;36:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cho EJ, Chun SM, Park H, et al. Whole transcriptome analysis of gestational trophoblastic neoplasms reveals altered PI3K signaling pathway in epithelioid trophoblastic tumor. Gynecol Oncol. 2020;157:151–60. [DOI] [PubMed] [Google Scholar]
- [19].Tuncer ZS, Vegh GL, Fulop V, et al. Expression of epidermal growth factor receptor-related family products in gestational trophoblastic diseases and normal placenta and its relationship with development of postmolar tumor. Gynecol Oncol. 2000;77:389–93. [DOI] [PubMed] [Google Scholar]
- [20].Frijstein MM, Lok CAR, Short D, et al. The results of treatment with high-dose chemotherapy and peripheral blood stem cell support for gestational trophoblastic neoplasia. Eur J Cancer Oxf Engl 1990. 2019;109:162–71. [DOI] [PubMed] [Google Scholar]
- [21].Oliver GR, Marcano-Bonilla S, Quist J, et al. LPCAT1-TERT Fusions are uniquely recurrent in epithelioid trophoblastic tumors and positively regulate cell growth. Plos One. 2021;16:e0250518. [DOI] [PMC free article] [PubMed] [Google Scholar]