Abstract
Background:
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors. Surgical resection is often only possible in the early stages of HCC and among those with limited cirrhosis. Radiofrequency ablation and Microwave ablation are 2 main types of percutaneous thermal ablation for the treatment of HCC. The efficacy and safety between these 2 therapy methods are still under a debate.
Objective:
To compare the efficacy and safety of Radiofrequency ablation and Microwave ablation in treating HCC.
Methods:
PubMed, EMBASE, the Cochrane databases and Web of Science were systematically searched. We included randomized controlled trials and cohort studies comparing the efficacy and safety of Radiofrequency ablation and Microwave ablation in HCC patients. Outcome measures on local tumor progression, complete ablation, disease-free survival, overall survival, or major complications were compared between the 2 groups. The random effect model was used when there was significant heterogeneity between studies, otherwise the fixed effect model was used.
Results:
A total of 33 studies, involving a total of 4589 patients were identified, which included studies comprised 7 RCTs, 24 retrospective observational trials, and 2 prospective observational trial. Microwave ablation had a lower local tumor progression than Radiofrequency ablation in cohort studies (OR = 0.78, 95% CI 0.64–0.96, P = .02). Complete ablation rate of Microwave ablation was higher than that of Radiofrequency ablation in cohort studies (OR = 1.54, 95% CI 1.05–2.25, P = .03). There was no significant difference in overall survival and disease-free survival between the 2 groups. Meta-analysis showed that there was no significant difference in the main complications between Microwave ablation and Radiofrequency ablation.
Conclusions:
Microwave ablation has higher complete ablation and lower local tumor progression than Radiofrequency ablation in the ablation treatment of HCC nodules. There was no significant difference in overall survival between the 2 therapy methods.
Keywords: hepatocellular carcinoma, microwave ablation, meta-analysis, radiofrequency ablation
1. Introduction
Liver cancer is estimated to be ranked sixth on most currently diagnosed cancer as well as the fourth main reason of cancer death with about 841,000 new cases and 782,000 deaths occurred in 2018 worldwide.[1] Hepatocellular carcinoma (HCC) accounts for the majority of primary liver cancers, and surgical resection is considered the gold standard of treatment for curative intent but is often only possible in the early stages of HCC and among those with limited cirrhosis.[2] The multitude of available complimentary and additive locoregional therapies, which include trans arterial chemoembolization, percutaneous ethanol injection, Radiofrequency ablation (RFA), Microwave ablation (MWA), cryoablation, laser ablation, high-intensity focused ultrasound, and irreversible electroporation, encourage clinicians to implement a multidisciplinary treatment approach to improve the outcome of these patients.[3] RFA and MWA are 2 main types of percutaneous thermal ablation.[4] Despite several meta-analyses had compared MWA with RFA for the treatment of HCC,[5–7] the efficacy and safety between these 2 modalities are still under debate, and also some new published studies were not included. Therefore, we performed a systematic review and meta-analysis of all the available randomized and observational studies to compare the efficacy and safety of RFA and MWA in treating primary HCC.
2. Methods
2.1. Search strategy
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines.[8] We searched for medical literature in electronic databases including The Cochrane Library, PubMed, Embase and Web of Science without any language and the publication date was before 31 December 2020. The search strategy included the following mesh terms or free text: “Radiofrequency ablation,” “radiofrequency therapy,” “microwave therapy,” “microwave ablation,” “hepatocellular carcinoma,” “liver cancer,” “hepatic cancer.” In addition, the references included were searched manually to avoid omitting any studies that met the inclusion criteria. Ethical approval and patient consent were not required, as this study was done on published data.
2.2. Selection criteria
Studies meeting the following criteria were selected:
Randomized controlled trials (RCTs), prospective or retrospective cohort studies compared efficacy and safety of MWA and RFA in HCC patients.
Outcome measures on local tumor progression (LTP), complete ablation (CA), disease-free survival, overall survival, or major complications compared between MWA and RFA for HCC were provided.
The studies were limited in humans.
The most complete and recent report of the trial was used when the same investigator reported data obtained from the same patients.
Duplicate publications, reviews, case reports, animal or cell experiments, and trials with incomplete data were excluded.
In our study, LTP was defined as any new lesion inside or adjacent to the ablated zone. CA was defined as no enhancement in ablated areas after ablation. Disease-free survival was defined as the length of time that patients survived without any signs of HCC after ablation. OS was defined as the length of time from the start of ablation to the date of the death or the last follow-up. Major complications were defined as complications of grade 3 or higher according to the Clavien-Dindo Classification.[9]
2.3. Data extraction
Two reviewers (ZD and FL) independently performed the initial literature search and selected relevant studies based on the inclusion and exclusion criteria. Data were extracted independently by the 2 investigators. The data collection template was formulated in advance and the following was extracted: the first author, publication year, study design, country of origin, baseline characteristics of the patients (e.g., age, sample size of each group, Child-Pugh classification), mean tumor size, number of nodules, mean follow-up duration, and details about the outcome measures. Any discrepancies were resolved by discussion and consensus during the process of research selection and data extraction or by consulting the third investigator (LR) when necessary.
2.4. Quality assessment
The methodological quality of all the included studies was assessed by 2 reviewers (ZD and XS), with discrepancies resolved by consensus. The methodological quality of the RCTs was assessed by the Cochrane Collaboration tool for assessing the risk of bias (ROB). The total ROB of a study was considered “low” when more than 4 items associated with “low risk” by the Cochrane Collaboration ROB tool were considered applicable, “moderate” when 2 to 3 items were applicable, and “high” when fewer than 2 “low risk” items or more than 1 “high risk” item were considered applicable.[10] The Newcastle-Ottawa quality assessment Scale (NOS) was used to evaluate the quality of nonrandomized trials. The overall quality of a study was defined as “poor” if the total NOS score was less than 4, “fair” if the score was 4 to 6, and “good” with a score of 7 to 9.[11]
2.5. Statistical analysis
All statistical aspects of the meta-analysis were conducted in Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). Heterogeneity between included studies was assessed by means of Cochrane’s Chi-Squared test, with the significance threshold settled at 0.10, and I2 statistic, with a value of >50% being suggestive of significant heterogeneity.[12] The random effect model was used when there was significant heterogeneity between studies; otherwise, the fixed effect model was used. For dichotomous outcomes, the odds ratio (OR) and the corresponding 95% confidence intervals (CI) were calculated. Additionally, funnel plots were used to visually assess the publication bias of the enrolled studies. We applied unadjusted P values for the significance assessment in this study, which were set at the two-tailed .05 level for hypothesis testing.
3. Result
3.1. Study selection
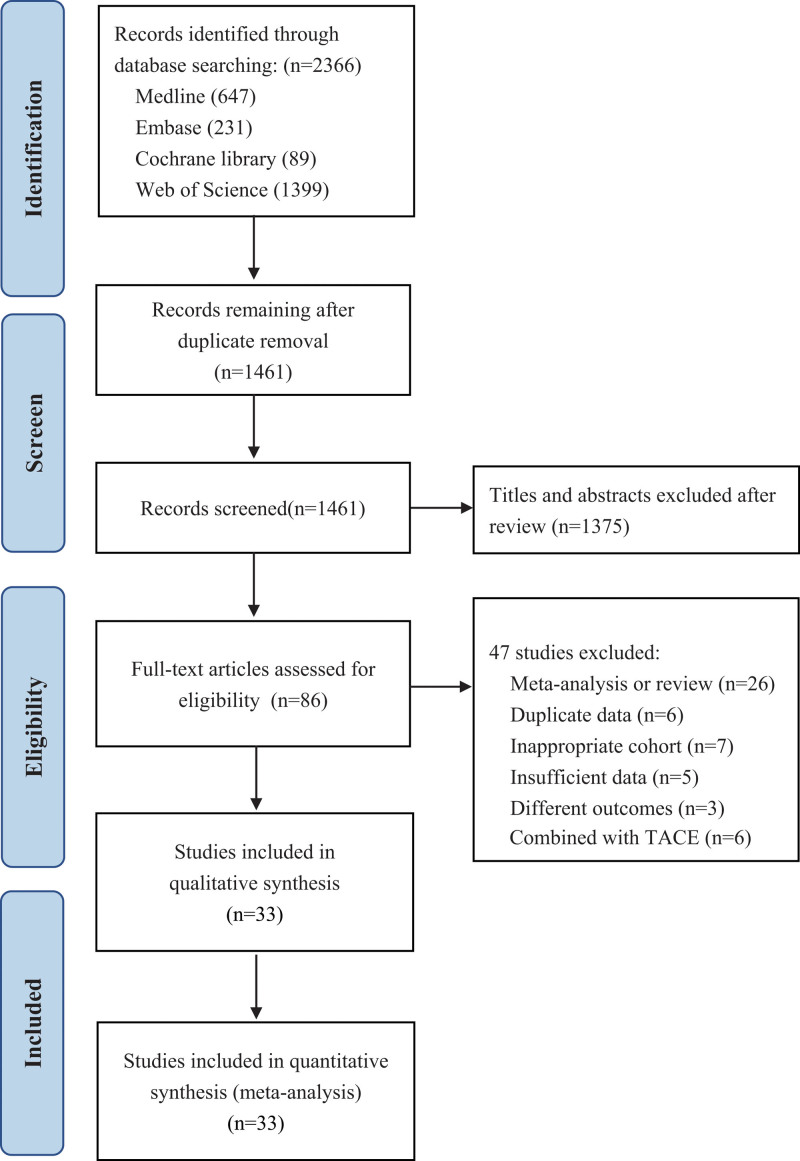
We present the entire search process and the reasons for excluding the ineligible studies in a flowchart. Our search strategy identified a total of 3015 studies, of which 1554 studies were excluded due to duplicate data. After reviewing the abstracts and titles, 1375 studies were excluded. Fifty two studies were excluded after a full-text screening. The remaining 33 studies with a total of 4589 patients (MWA = 2 044, RFA = 2 545) were included in our final analysis[13–45] (Fig. 1).
Figure 1.
The flowchart of the study selection process for the meta-analysis.
3.2. Characteristics of the studies and quality assessment
The 33 included studies comprised 7 RCTs,[13,17,20,33,36,38,44] 24 retrospective observational trials, and 2 prospective observational trial.[15,28] The sample sizes of each individual study range from 19 to 562 patients, whose age range from 50 to 69 years across studies, and male proportion rang from 38% to 94%. The recruitment period ranged from 2002 to 2019 and study follow-up range from 5 to 62 months. An overview of the characteristics of the included trials is presented in Table 1 and a summary of the risk of bias assessments of RCTs is presented in Figure S1 (Supplemental Digital Content, http://links.lww.com/MD2/B106) and Figure S2 (Supplemental Digital Content, http://links.lww.com/MD2/B107) and the quality scores of included cohort studies is presented in Table S1 (Supplemental Digital Content, http://links.lww.com/MD/G952). All the RCTs are considered low risk and all the cohort studies have a NOS score of good.
Table 1.
Characteristics of the 33 trials included in the meta-analysis.
| Groups | NP | No. of nodules | Size (mm) | CPC (A/B/C) | Follow-up | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO. | First author | Year | Country | Type | MWA | RFA | MWA | RFA | Ages(Y) | Male (%) | MWA | RFA | MWA | RFA | MWA | RFA | MWA | RFA |
| 1 | Abdelaziz | 2014 | Egypt | RCT | MWA | RFA | 66 | 45 | 55 | 71 | 76 | 52 | 29 (9.7) | 29.5 (10.3) | 25/41/0 | 24/21/0 | 40 | 40 |
| 2 | Chinnaratha | 2015 | Australia | Retrospective | MWA | RFA | 25 | 101 | 62 | 78 | 31 | 114 | NR | NR | NR | NR | 14 | 14 |
| 3 | Cillo U | 2014 | Italy | Prospective | MWA | RFA | 28 | 28 | 64 | 80 | NR | NR | 25 (15–53) | 27 (12–60) | NR | NR | 24 | 24 |
| 4 | Correa G | 2014 | USA | Retrospective | MWA | RFA | 67 | 67 | 55 | NR | 127 | 127 | NR | NR | NR | NR | 18 | 31 |
| 5 | Di Vece | 2014 | Italy | RCT | MWA | RFA | 20 | 20 | 61 | 73 | 20 | 20 | 36 (22–69) | 32 (23–64) | NR | NR | NR | NR |
| 6 | Ding J | 2013 | China | Retrospective | MWA | RFA | 113 | 85 | 59 | 77 | 131 | 98 | 25.5 (8.9) | 23.8 (8.1) | 75/38/0 | 49/36/0 | 18 | 28 |
| 7 | Hompes | 2010 | Belgium | Retrospective | MWA | RFA | 6 | 13 | 60 | 47 | 16 | 13 | NR | NR | NR | NR | 6 | NR |
| 8 | Kamal A | 2019 | Egypt | RCT | MWA | RFA | 28 | 28 | 55 | 77 | 34 | 34 | 32.5 (9.2) | 32.8 (9.1) | 22/6/0 | 22/6/0 | 12 | 12 |
| 9 | Kuang | 2011 | China | Retrospective | MWA | RFA | 19 | 31 | 55 | 94 | 19 | 31 | NR | NR | NR | NR | 45 | 45 |
| 10 | Lee KF | 2017 | China | Retrospective | MWA | RFA | 26 | 47 | 60 | 81 | 28 | 52 | 37.5 (20–60) | 31 (20–60) | 23/3/0 | 42/5/0 | 48 | 53 |
| 11 | Liu Y | 2013 | China | Retrospective | MWA | RFA | 35 | 54 | 53 | 61 | 62 | 70 | 23 (10) | 25 (10) | NR | NR | 32 | 32 |
| 12 | Liu W | 2018 | China | Retrospective | MWA | RFA | 126 | 436 | 56 | 90 | 162 | 482 | 22.5 (17–29) | 23 (18–30) | NR | NR | 37 | 34 |
| 13 | Lu M | 2005 | China | Retrospective | MWA | RFA | 49 | 53 | 52 | 85 | 98 | 72 | 25 (12) | 26 (12) | 22/27/0 | 47/6/0 | 25 | 25 |
| 14 | Ohmoto K | 2009 | Japan | Retrospective | MWA | RFA | 49 | 34 | 65 | 80 | 56 | 37 | 17 (8–20) | 16 (7–20) | 31/14/4 | 20/11/3 | 34 | 26 |
| 15 | Potrezzke | 2016 | USA | Retrospective | MWA | RFA | 99 | 55 | 61 | 79 | 136 | 69 | 22 (20–23) | 24 (22–26) | NR | NR | 24 | 31 |
| 16 | Qian | 2012 | China | Prospective | MWA | RFA | 22 | 20 | 54 | 93 | 22 | 20 | 21 (4) | 20 (5) | NR | NR | 5 | 5 |
| 17 | Sakaguchi | 2009 | Japan | Retrospective | MWA | RFA | 142 | 249 | 65 | 71 | 142 | 249 | 22.8 (7.4) | 24.8 (8.9) | 86/56/0 | 147/98/4 | NR | NR |
| 18 | Santambrogio R | 2017 | Italy | Retrospective | MWA | RFA | 60 | 94 | 69 | 73 | NR | NR | 21.5 (5.3) | 19.2 (5) | 60/0/0 | 94/0/0 | 27 | 27 |
| 19 | Sever IH | 2018 | Turkey | Retrospective | MWA | RFA | 20 | 20 | 64 | 70 | 30 | 25 | 28 (10) | 24 (11) | 14/4/2 | 11/4/5 | 6 | 6 |
| 20 | Shady | 2017 | USA | Retrospective | MWA | RFA | 48 | 62 | NR | 66 | 60 | 85 | 17 (7–37) | 18 (6–45) | NR | NR | 29 | 56 |
| 21 | Shibata T | 2002 | Japan | RCT | MWA | RFA | 36 | 36 | 63 | 69 | 46 | 48 | 22 (9–34) | 23 (10–37) | 19/17/0 | 21/15/0 | 18 | 18 |
| 22 | Simo KA | 2011 | USA | Retrospective | MWA | RFA | 13 | 22 | 59 | 74 | 15 | 27 | 23.1 (14–39) | 25.3 (12–44) | 7/6/0 | 12/7/3 | 7 | 19 |
| 23 | Sparchez Z | 2019 | Romania | Retrospective | MWA | RFA | 17 | 44 | 61 | 52 | 20 | 62 | 25.5 (15–33) | 25 (16.5–30) | NR | NR | NR | NR |
| 24 | Tian W | 2014 | China | RCT | MWA | RFA | 60 | 60 | 55 | 78 | 79 | 86 | 26 (13) | 22 (9) | NR | NR | NR | NR |
| 25 | van Tilborg | 2016 | Netherlands | Retrospective | MWA | RFA | 15 | 96 | 61 | 65 | 32 | 139 | 25 (4–65) | 24 (2–68) | NR | NR | 49 | NR |
| 26 | Vietti V | 2018 | Switzerland | RCT | MWA | RFA | 71 | 73 | 66 | 84 | 98 | 104 | 18 (6.5) | 18 (7.1) | 57/14/0 | 53/20/0 | 26 | 25 |
| 27 | Vogl TJ | 2015 | Germany | Retrospective | MWA | RFA | 28 | 25 | 59 | 79 | 36 | 32 | 36 (9–50) | 32 (8–45) | NR | NR | NR | NR |
| 28 | Xu HX | 2004 | China | Retrospective | MWA | RFA | 54 | 43 | 53 | 86 | 112 | 78 | 25 (11) | 26 (14) | NR | NR | NR | NR |
| 29 | Xu Y | 2017 | China | Retrospective | MWA | RFA | 301 | 159 | 54 | 80 | NR | NR | 17 (3) | 17 (3) | 278/23/0 | 140/19/0 | 53 | 62 |
| 30 | Yang B | 2017 | China | Retrospective | MWA | RFA | 71 | 108 | 50 | 65 | 121 | 188 | NR | NR | NR | NR | 39 | 39 |
| 31 | Yin X | 2009 | China | Retrospective | MWA | RFA | 50 | 59 | 53 | 87 | NR | NR | NR | NR | NR | NR | 22 | 22 |
| 32 | Yu J | 2017 | China | RCT | MWA | RFA | 203 | 200 | NR | NR | 265 | 251 | 27 (10) | 26 (10) | NR | NR | 35 | 35 |
| 33 | Zhang L | 2013 | China | Retrospective | MWA | RFA | 77 | 78 | 54 | 85 | 105 | 97 | 22 (4) | 23 (4) | 77/0/0 | 78/0/0 | 25 | 26 |
3.3. Local tumor progression
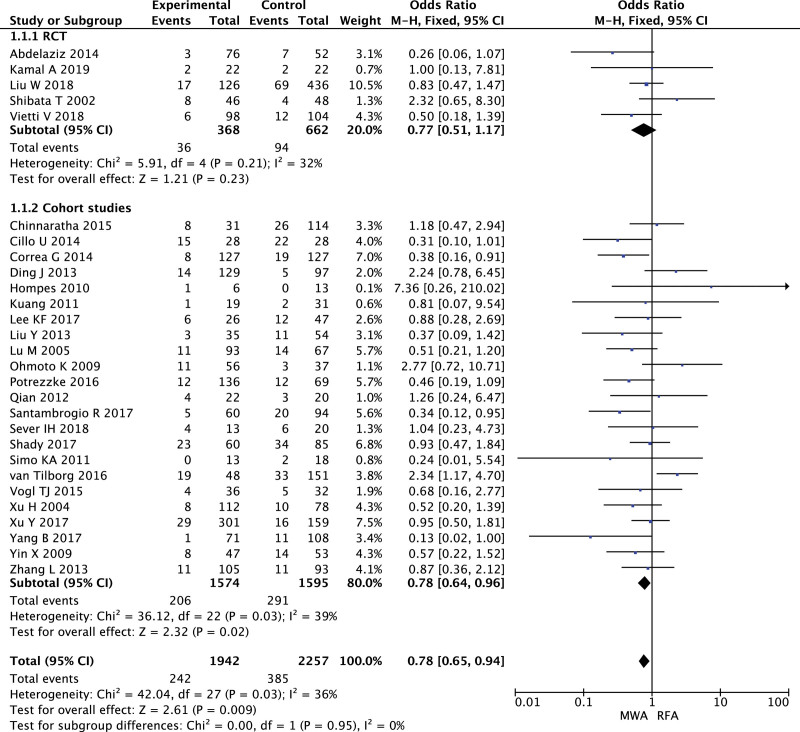
LTP were reported in 28 studies, in which 5 RCTs with 1030 patients and 23 cohorts with 3169 patients. MWA had a lower LTP than RFA in cohort studies (OR = 0.78, 95% CI 0.64–0.96, P = .02). There was no significant heterogeneity between RCTs (I2 = 32%), as did observational studies (I2 = 39%, Fig. 2), and visual inspection of a funnel plot suggested no evidence of publication bias Figure S3 (Supplemental Digital Content, http://links.lww.com/MD2/B108).
Figure 2.
Forest plot of meta-analysis comparing local tumor progression between MWA and RFA.
3.4. Complete ablation rate
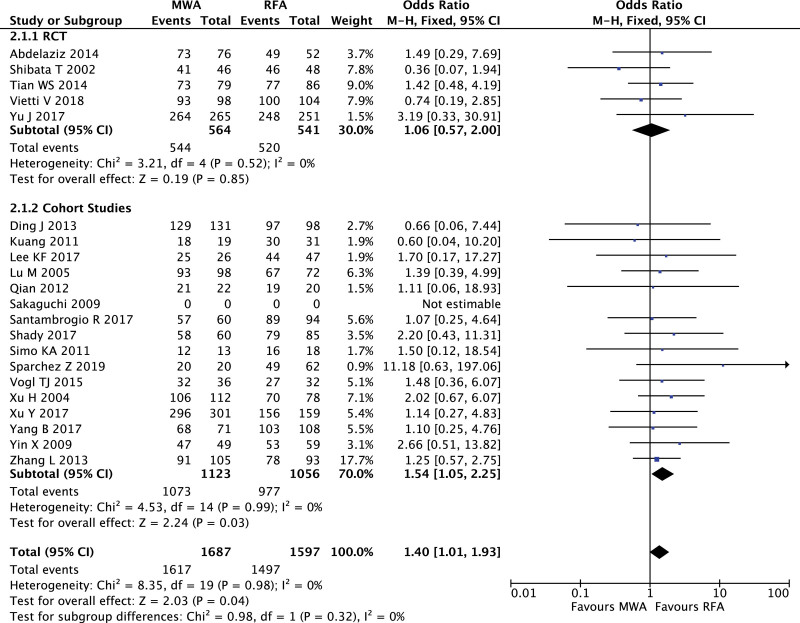
Five RCTs with 1105 patients and 16 cohort studies with 2179 patients reported CA rate, and heterogeneity and publication bias were not found in both of 2 groups (RCT: I2 = 0%; cohort studies I2 = 0%, Figure S4, Supplemental Digital Content, http://links.lww.com/MD2/B109). There was no significant difference of CA rate between MWA and RFA in RCTs (OR = 1.06, 95% CI 0.57–2.00, P = .85), but CA rate of MWA was higher than that of RFA in cohort studies (OR = 1.54, 95% CI 1.05–2.25, P = .03. Fig. 3).
Figure 3.
Forest plot of meta-analysis comparing complete ablation rate between MWA and RFA.
3.5. Overall survival
We compared the overall survival of 1-year, 3-year, and 5-year in RCTs and cohort studies respectively. In RCTs, there was no significant difference in overall survival rate between MWA and RFA (1-year: OR = 1.86, 95% CI 0.91–3.80, P = .09; 3-year: OR = 1.16, 95% CI 0.77–1.74, P = .49; 5-year: OR = 0.79, 95% CI 0.51–1.21, P = .27), and significant difference was also not found in cohort studies (1-year: OR = 0.97, 95% CI 0.69–1.36, P = .85; 3-year: OR = 0.92, 95% CI 0.75–1.13, P = .64; 5-year: OR = 1.12, 95% CI 0.93–1.36, P = .22). Low grade of intergroup heterogeneity was found in 1-year overall mortality of RCTs (I2 = 52%) and 3-year overall mortality of cohort studies (I2 = 64%) respectively (Supplemental Digital Content, Figure S5, http://links.lww.com/MD2/B110, Figure S6, http://links.lww.com/MD2/B111, Figure S7, http://links.lww.com/MD2/B112).
3.6. Disease-free survival
We also summarized the differences of disease-free survival of in RCTs and cohort studies separately. In RCTs, there was no significant difference in disease-free survival of 1-year (OR = 1.04, 95% CI 0.48–2.24, P = .92) and 3-year (OR = 3.00, 95% CI 0.91–9.87, P = .07) between MWA and RFA. Only one study in RCTs reported the disease-free survival of 5-year, which indicates that MWA was better than RFA (OR = 1.86, 95% CI 1.20–2.86, P = .005). For the cohort studies, the disease-free survival of 1-year (OR = 1.20, 95% CI 0.96–1.51, P = .11), 3-year (OR = 1.15, 95% CI 0.93–1.41, P = .20) and 5-year (OR = 0.84, 95% CI 0.67–1.05, P = 0.13) were not found any differences. No significant heterogeneity in all the studies (Supplemental Digital Content, Figure S8, http://links.lww.com/MD2/B113, Figure S9, http://links.lww.com/MD2/B114, Figure S10, http://links.lww.com/MD2/B115).
3.7. Major complication
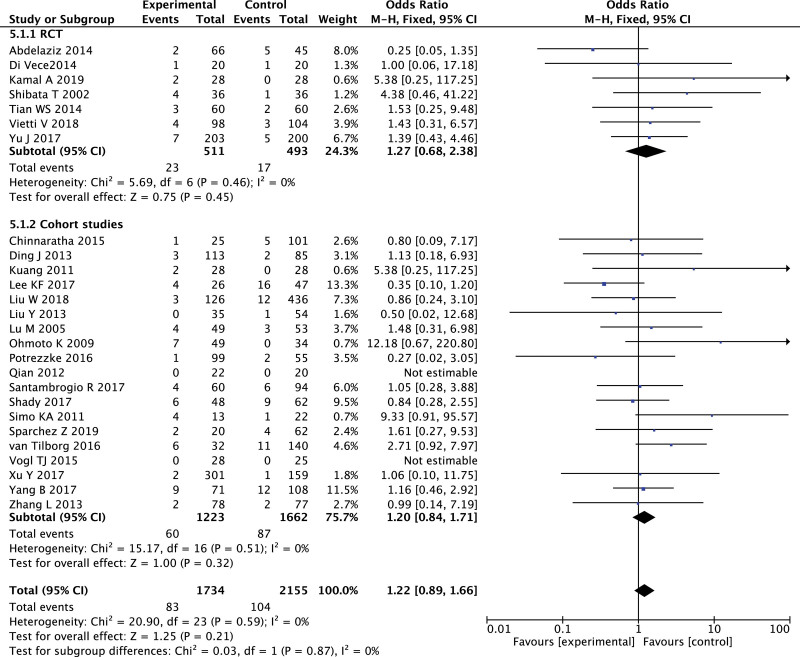
A total of 26 studies, including 3889 patients, reported major complications, with no significant heterogeneity and publication bias in each of 7 RCTs, and 19 cohort studies (Figure S11, Supplemental Digital Content, http://links.lww.com/MD2/B116). Meta-analysis showed that there was no significant difference in the main complications between MWA and RFA, whether in RCTs or in cohort studies (Fig. 4).
Figure 4.
Forest plot of meta-analysis comparing major complications between MWA and RFA.
4. Discussion
This study included 33 articles with a total of 4589 patients, which includes the largest number of patients and the latest literature on the research of Microwave ablation (MWA) and Radiofrequency ablation (RFA) in hepatocellular carcinoma (HCC). Our study found that the LTP after MWA treatment was lower than that of RFA, and the complete ablation (CA) rate of MWA was higher than that of RFA. There were no significant differences in the overall survival, disease-free survival, and major complications between the 2 kinds of ablation.
Ablation therapy is considered as the first choice of treatments for most of patients with small hepatocellular carcinoma nodules, or as an alternative treatment for patients who are not suitable for surgical resection or whose chemotherapy have failed.[46,47] The most commonly used ablation modalities in clinical practice are MWA and RFA.[48,49] RFA is performed by advancing an especially designed electrode into the lesion and radiofrequency energy emitted from the tip of the electrode is converted into heat to create a zone of thermal destruction that encompasses the tumor, but the result is also affected by the heat-sink effect.[50–52] RFA is considered the best therapeutic modality for very early and early-stage HCC according to BCLC staging when resection or liver transplantation is not indicated.[53–55] MWA uses electromagnetic energy to create an electromagnetic field that heats rapidly the target tissue and induces coagulation necrosis. In comparison with RFA, MWA is more homogenous and the heat-sink effect is reduced due to the higher temperatures and the faster heating that is produced by electromagnetic energy. On the other hand, the higher elevation of temperature in the MWA field can injure the adjacent structures.[56–58] Lloyd et al demonstrated rapid ablation and low morbidity in patients who underwent MWA.[59] Another recent study which enrolled 221 patients showed high technique effectiveness rate and well tolerance from patients.[60] Clinically, if the tumor nodule was less than 3 cm, both of the 2 methods may be considered. However, when the HCC diameter of nodules is larger than 3 cm, MWA can remove the nodules more effectively due to its higher temperature and faster heating.[61,62]
The treatment of patients with HCC is multidisciplinary, so nodules clearance alone cannot determine the prognosis of the patients.[2] It is the reason that why no difference in overall survival between the 2 groups was found in our study and previous studies only with the treatment of ablation. Local tumor progression responds to the clinical effects of MWA or RFA earlier and more accurately. However, no difference in 1-year disease-free survival may be related to the short postoperative period and delayed local invasion development.
The major complication rate of MWA and RFA remains controversial. Major complications of RFA include intraperitoneal bleeding, infections, liver failure, pneumothorax, organ injury, bile duct stenosis and tumor lysis syndrome, but the major complication rate and procedural mortality rate is significantly low.[63,64] The major complications of MWA are bleeding, peritoneal hemorrhage, liver abscess, hemothorax, colon perforation and bile duct stenosis.[65] Our study including 3889 patients found no difference in the main complications between MWA and RFA, whether in RCTs or in cohort studies, so as the previous studies.[5,6,66] Considering that MWA is less affected by the heat-sink effect, MWA can produce more larger tumor necrosis. However, these characteristics are in turn related to the increased risk of damaging neighboring organs, especially the structure of blood vessels. A larger ablation area might account for a higher complication rate.[67,68] A defect of MWA is high local development of tumor which may be caused by a larger applicator (5 mm in diameter) applied for tumor puncture increasing the risk of bleeding and subsequent tumor seeding.[69] It should be noted that RFA is performed by the guidance of ultrasound, computed tomography or magnetic resonance imaging, while MWA is performed under computed tomography or ultrasound guidance, so the types of devices and the experience of the operators would also affect the results, and high-quality evidence is needed to compare the complication rates of MWA and RFA.
5. Limitations
First, 24 of the 33 included studies were retrospective studies, in which the lack of randomization of patient grouping may affect the results of the study. However, the basic characteristics of each study included were not statistically different, so our results are still credible. Second, different types of generators and antennas were used in RFA and MWA in the included studies, while different stages, equipment, and experiences of operators may affect the treatment effects. Third, there are differences in the observation time points in the definition of CA and LTP. For example, complete tumor ablation should be assessed by imaging ideally 1 week to 1 month after the procedure and no later than 3 months afterwards in guidelines.[70] The evaluation of treatment effect at different observation times will inevitably lead to differences. Finally, most of the studies conducted in a single center, and the number of patients involved is small, which results in heterogeneity among some studies.
6. Conclusion
Our meta-analysis showed that Microwave ablation has higher complete ablation and lower local tumor progression than Radiofrequency ablation in the ablation treatment of hepatocellular carcinoma nodules. There was no significant difference in overall survival between the 2 treatments. Taking into account the differences in equipment and operator experience in the included studies, high-quality randomized controlled trials are needed to draw a conclusion on the pros and cons of Microwave ablation and Radiofrequency ablation.
Author contributions
Conceptualization: Zhimin Dou, Bin Li, Xun Li.
Data curation: Fei Lu, Zhimin Dou
Formal analysis: Fei Lu.
Funding acquisition: Zhimin Dou.
Investigation: Zhimin Dou, Fei Lu, Xiaojing Song, Xun Li.
Methodology: Longfei Ren, Xiaojing Song, Zhimin Dou.
Project administration: Zhimin Dou, Xun Li.
Software: Zhimin Dou, Fei Lu.
Supervision: Xun Li
Validation: Xiaojing Song
Visualization: Bin Li
Writing – original draft: Zhimin Dou, Bin Li, Fei Lu.
Writing – review & editing: Longfei Ren, Xiaojing Song, Xun Li, Zhimin Dou.
Supplementary Material
Abbreviations:
- CA =
- complete ablation
- HCC =
- hepatocellular carcinoma
- LTP =
- local tumor progression
- MWA =
- microwave ablation
- RFA =
- radiofrequency ablation
How to cite this article: Dou Z, Lu F, Ren L, Song X, Li B, Li X. Efficacy and safety of microwave ablation and radiofrequency ablation in the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Medicine. 2022;101:30(e29321).
This study was funded by the Natural Science Foundation of Gansu Province (Grant NO.: 20JR10RA710).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are publicly available.
Contributor Information
Zhimin Dou, Email: douzhm@126.com.
Fei Lu, Email: 28783666@qq.com.
Longfei Ren, Email: renlf2020@126.com.
Xiaojing Song, Email: 17739806658@126.com.
Bin Li, Email: lxdr_21@126.com.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019;380:1450–62. [DOI] [PubMed] [Google Scholar]
- [3].Lurje I, Czigany Z, Bednarsch J, et al. Treatment strategies for hepatocellular carcinoma (−) a multidisciplinary approach. Int J Mol Sci 2019;20: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].El-Serag HB. Advances in the management of hepatocellular carcinoma. Clin Adv Hematol Oncol 2017;15(Suppl 9):2–6. [PubMed] [Google Scholar]
- [5].Chinnaratha MA, Chuang MY, Fraser RJ, et al. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:294–301. [DOI] [PubMed] [Google Scholar]
- [6].Tan W, Deng Q, Lin S, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 2019;36:264–72. [DOI] [PubMed] [Google Scholar]
- [7].Facciorusso A, Abd El Aziz MA, Tartaglia N, et al. Microwave ablation versus radiofrequency ablation for treatment of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Cancers (Basel) 2020;12: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96. [DOI] [PubMed] [Google Scholar]
- [10].Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011. [Google Scholar]
- [11].Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta- analyses. 2019.
- [12].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc 2014;28:3429–34. [DOI] [PubMed] [Google Scholar]
- [14].Chinnaratha MA, Sathananthan D, Pateria P, et al. High local recurrence of early-stage hepatocellular carcinoma after percutaneous thermal ablation in routine clinical practice. Eur J Gastroenterol Hepatol 2015;27:349–54. [DOI] [PubMed] [Google Scholar]
- [15].Cillo U, Noaro G, Vitale A, et al. Laparoscopic microwave ablation in patients with hepatocellular carcinoma: a prospective cohort study. HPB (Oxford) 2014;16:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation versus radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol 2014;21:4278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Di Vece F, Tombesi P, Ermili F, et al. Coagulation areas produced by cool-tip radiofrequency ablation and microwave ablation using a device to decrease back-heating effects: a prospective pilot study. Cardiovasc Intervent Radiol 2014;37:723–9. [DOI] [PubMed] [Google Scholar]
- [18].Ding J, Jing X, Liu J, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol 2013;82:1379–84. [DOI] [PubMed] [Google Scholar]
- [19].Hompes R, Fieuws S, Aerts R, et al. Results of single-probe microwave ablation of metastatic liver cancer. Eur J Surg Oncol 2010;36:725–30. [DOI] [PubMed] [Google Scholar]
- [20].Kamal A, Elmoety AAA, Rostom YAM, et al. Percutaneous radiofrequency versus microwave ablation for management of hepatocellular carcinoma: a randomized controlled trial. J Gastrointest Oncol 2019;10:562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kuang M, Xie XY, Huang C, et al. Long-term outcome of percutaneous ablation in very early-stage hepatocellular carcinoma. J Gastrointest Surg 2011;15:2165–71. [DOI] [PubMed] [Google Scholar]
- [22].Lee KF, Wong J, Hui JW, et al. Long-term outcomes of microwave versus radiofrequency ablation for hepatocellular carcinoma by surgical approach: a retrospective comparative study. Asian J Surg 2017;40:301–8. [DOI] [PubMed] [Google Scholar]
- [23].Liu W, Zheng Y, He W, et al. Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Aliment Pharmacol Ther 2018;48:671–81. [DOI] [PubMed] [Google Scholar]
- [24].Liu Y, Li S, Wan X, et al. Efficacy and safety of thermal ablation in patients with liver metastases. Eur J Gastroenterol Hepatol 2013;25:442–6. [DOI] [PubMed] [Google Scholar]
- [25].Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009;24:223–7. [DOI] [PubMed] [Google Scholar]
- [26].Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol 2005;40:1054–60. [DOI] [PubMed] [Google Scholar]
- [27].Potretzke TA, Ziemlewicz TJ, Hinshaw JL, et al. Microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a comparison of efficacy at a single center. J Vasc Interv Radiol 2016;27:631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol 2012;22:1983–90. [DOI] [PubMed] [Google Scholar]
- [29].Sakaguchi H, Seki S, Tsuji K, et al. Endoscopic thermal ablation therapies for hepatocellular carcinoma: a multi-center study. Hepatol Res 2009;39:47–52. [DOI] [PubMed] [Google Scholar]
- [30].Santambrogio R, Chiang J, Barabino M, et al. Comparison of laparoscopic microwave to radiofrequency ablation of small hepatocellular carcinoma (</=3 cm). Ann Surg Oncol 2017;24:257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sever IH, Sucu M, Biyikli E. Radiofrequency and microwave ablation in the treatment of hepatocellular carcinoma. Iran J Radiol 2018;15:e62396. [Google Scholar]
- [32].Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol 2018;29:268–L 275.e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002;223:331–7. [DOI] [PubMed] [Google Scholar]
- [34].Simo KA, Sereika SE, Newton KN, et al. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol 2011;104:822–9. [DOI] [PubMed] [Google Scholar]
- [35].Sparchez Z, Mocan T, Hajjar NA, et al. Percutaneous ultrasound guided radiofrequency and microwave ablation in the treatment of hepatic metastases. A monocentric initial experience. Med Ultrason 2019;21:217–24. [DOI] [PubMed] [Google Scholar]
- [36].Tian W, Kuang M, Lv M, et al. A randomised comparative trial on liver tumors treated with ultrasound-guided percutaneous radiofrequency versus microwave ablation. Chin J Hepatobiliary Surg 2014;20:119–22. [Google Scholar]
- [37].van Tilborg AA, Scheffer HJ, de Jong MC, et al. MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient- and lesion-based analysis of two historical cohorts. Cardiovasc Intervent Radiol 2016;39:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol 2018;3:317–25. [DOI] [PubMed] [Google Scholar]
- [39].Vogl TJ, Farshid P, Naguib NN, et al. Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging 2015;40:1829–37. [DOI] [PubMed] [Google Scholar]
- [40].Xu HX, Xie XY, Lu MD, et al. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol 2004;59:53–61. [DOI] [PubMed] [Google Scholar]
- [41].Xu Y, Shen Q, Wang N, et al. Microwave ablation is as effective as radiofrequency ablation for very-early-stage hepatocellular carcinoma. Chin J Cancer 2017;36:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yang B, Li Y. A comparative study of laparoscopic microwave ablation with laparoscopic radiofrequency ablation for colorectal liver metastasis. J buon 2017;22:667–72. [PubMed] [Google Scholar]
- [43].Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer 2009;115:1914–23. [DOI] [PubMed] [Google Scholar]
- [44].Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut 2017;66:1172–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang L, Wang N, Shen Q, et al. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One 2013;8:e76119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Benson AB, 3rd, D’Angelica MI, Abbott DE, et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw 2017;15:563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer 2012;48:599–641. [DOI] [PubMed] [Google Scholar]
- [48].Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol 2013;16:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Iannuccilli JD, Dupuy DE. How to set up a successful tumor ablation practice. Tech Vasc Interv Radiol 2013;16:201–8. [DOI] [PubMed] [Google Scholar]
- [50].Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol 2017;15:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ryan MJ, Willatt J, Majdalany BS, et al. Ablation techniques for primary and metastatic liver tumors. World J Hepatol 2016;8:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421–30. [DOI] [PubMed] [Google Scholar]
- [54].Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol 2010;33:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Xu XL, Liu XD, Liang M, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology 2018;287:461–72. [DOI] [PubMed] [Google Scholar]
- [56].Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25:S69–83. [DOI] [PubMed] [Google Scholar]
- [57].Ward RC, Healey TT, Dupuy DE. Microwave ablation devices for interventional oncology. Expert Rev Med Devices 2013;10:225–38. [DOI] [PubMed] [Google Scholar]
- [58].Lopresto V, Pinto R, Farina L, et al. Treatment planning in microwave thermal ablation: clinical gaps and recent research advances. Int J Hyperthermia 2017;33:83–100. [DOI] [PubMed] [Google Scholar]
- [59].Lloyd DM, Lau KN, Welsh F, et al. International multicentre prospective study on microwave ablation of liver tumours: preliminary results. HPB (Oxford) 2011;13:579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang T, Lu XJ, Chi J, et al. Microwave ablation of hepatocellular carcinoma as first-line treatment: long term outcomes and prognostic factors in 221 patients. Sci Rep 2016;6:32728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shiina S, Sato K, Tateishi R, et al. Percutaneous ablation for hepatocellular carcinoma: comparison of various ablation techniques and surgery. Can J Gastroenterol Hepatol 2018;2018:4756147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ruiter SJS, Heerink WJ, de Jong KP. Liver microwave ablation: a systematic review of various FDA-approved systems. Eur Radiol 2019;29:4026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kwon HJ, Kim PN, Byun JH, et al. Various complications of percutaneous radiofrequency ablation for hepatic tumors: radiologic findings and technical tips. Acta Radiol 2014;55:1082–92. [DOI] [PubMed] [Google Scholar]
- [64].Gao J, Fan RF, Yang JY, et al. Radiofrequency ablation for hepatic hemangiomas: a consensus from a Chinese panel of experts. World J Gastroenterol 2017;23:7077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liang P, Yu J, Lu MD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol 2013;19:5430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia 2016;32:339–44. [DOI] [PubMed] [Google Scholar]
- [67].Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist 2019;24:e990–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Perrodin S, Lachenmayer A, Maurer M, et al. Percutaneous stereotactic image-guided microwave ablation for malignant liver lesions. Sci Rep 2019;9:13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Poulou LS, Botsa E, Thanou I, et al. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol 2015;7:1054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ahmed M. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update: supplement to the consensus document. J Vasc Interv Radiol 2014;25:1706–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.