Abstract
Median arcuate ligament syndrome (MALS) is a rare constellation of neurogenic gastrointestinal (GI) symptoms resulting from compression of the celiac trunk and celiac plexus by the median arcuate ligament. MALS is characterized by nonspecific symptoms including nausea, vomiting, diarrhea, bloating, unintentional weight loss due to food aversion, and postprandial epigastric abdominal pain. We present a case of atypical, chronic MALS that confounded clinicians for over a decade and led to various misdiagnoses, including early-onset Parkinson’s disease. Of the constellation of symptoms that MALS may present with, postprandial epigastric pain is a classic symptom and increases the index of suspicion for the diagnosis; however, the absence of the classic symptom of postprandial epigastric pain and the predomination of nonspecific GI symptoms and syncope in our patient further clouded clinicians’ ability to diagnose MALS. Upon further investigation, we elucidated a link between gabapentin, which our patient was chronically prescribed, and its efficacy in decreasing neurogenic hypersensitivity in the GI tract. Our case and the implications of gabapentin use to decrease neurogenic pain from MALS represents a novel addition to the literature on MALS treatment modalities and elucidates new avenues for continued research in the use of gabapentin as a symptom-modifying agent in the nonoperative and preoperative treatment of MALS.
Keywords: dunbar syndrome, neurogenic, atypical mals, gabapentin, median arcuate ligament syndrome
Introduction
Median arcuate ligament syndrome (MALS), also known as celiac artery compression syndrome or Dunbar syndrome, is a rare constellation of gastrointestinal (GI) symptoms resulting from extrinsic compression of the celiac trunk and plexus/ganglion by the median arcuate ligament and diaphragmatic crura. Most seen in 30- to 50-year-old women with thin body habitus, MALS typically presents with varying degrees of nonspecific symptoms including nausea, vomiting, diarrhea, bloating, unintentional weight loss due to food aversion, and, most notably, postprandial abdominal pain (primarily epigastric) [1-3]. Given the nonspecific symptoms and resemblance to other more common abdominal disorders, the diagnosis of MALS is challenging and generally considered to be one of exclusion.
The median arcuate ligament is a fibrous band connecting the diaphragmatic crura anteriorly, forming the aortic hiatus superior to the celiac trunk. In the presence of an unusually superior origin of the celiac trunk and/or unusually inferior insertion of the diaphragmatic crura, the median arcuate ligament may subsequently overlap and compress the celiac trunk and plexus/ganglion [4,5]. Although first described anatomically in 1917 by Lipshutz, the pathophysiology of MALS symptoms remains unknown [6]. Classic theory suggests that epigastric pain results from foregut ischemia caused by compression of the celiac artery. However, it has also been suggested that vascular steal syndrome following celiac artery compression may lead to midgut ischemia [7,8]. In contrast, others argue that the pain is neuropathic in origin and results from chronic compression and subsequent overstimulation of the celiac plexus/ganglion. This leads to irritation of the sympathetic visceral pain fibers and/or chronic splanchnic vasoconstriction and ischemia [9]. Although classically considered a vascular disorder, MALS is now often viewed as primarily a neurogenic condition [8].
Owing to its rarity, nonspecific presentation, and unknown pathogenesis, best practices for the diagnosis and intervention of MALS have yet to be defined in the literature. Current evidence suggests that duplex abdominal ultrasound during inspiration and deep expiration should be used as an initial assessment for compression of the celiac trunk [10-12]. Additional studies that may be useful in diagnosis include computed tomography (CT) angiography and magnetic resonance angiography [4,13]. Once diagnosed, percutaneous celiac plexus/ganglion block should be used to simulate decompression and determine potential surgical candidates [2,14]. Those who do not experience relief following neural blockade have a lower likelihood of success with a surgical intervention [8]. First described by Harjola et al. in 1963, the open release of the median arcuate ligament with removal and neurolysis of the celiac plexus/ganglion is the conventional approach to surgical intervention for MALS [15,16]. In recent years, however, laparoscopic decompression and neurolysis have become the accepted standard of care [13]. Not surprisingly, robotic-assisted release and decompression with neurolysis are now showing excellent promise given the ability for enhanced microdissection at the base of the celiac trunk [17,18]. Even with current advancements in diagnostic and management techniques, surgical intervention is not always clinically successful. Nonetheless, it has been reported that 75% to 85% of patients experience resolution of symptoms following surgery [19].
Case presentation
A 33-year-old male presented to the vascular surgery clinic with a 13-year history of intermittent syncopal episodes associated with nausea, vomiting, and diffuse/poorly localized abdominal pain that was previously thought to be an irritable bowel syndrome. He reported that the episodes occurred intermittently, were not specifically associated with meals, and were accompanied by diffuse body aches. His medical history included a diagnosis of attention-deficit hyperactivity disorder (ADHD) since childhood and restless leg syndrome secondary to traumatic compartment syndrome and subsequent fasciotomy of the left lower extremity seven years prior. He also endorsed left lower extremity neuropathic pain controlled with gabapentin. His family history was significant for epilepsy in his mother. His remaining histories were unremarkable.
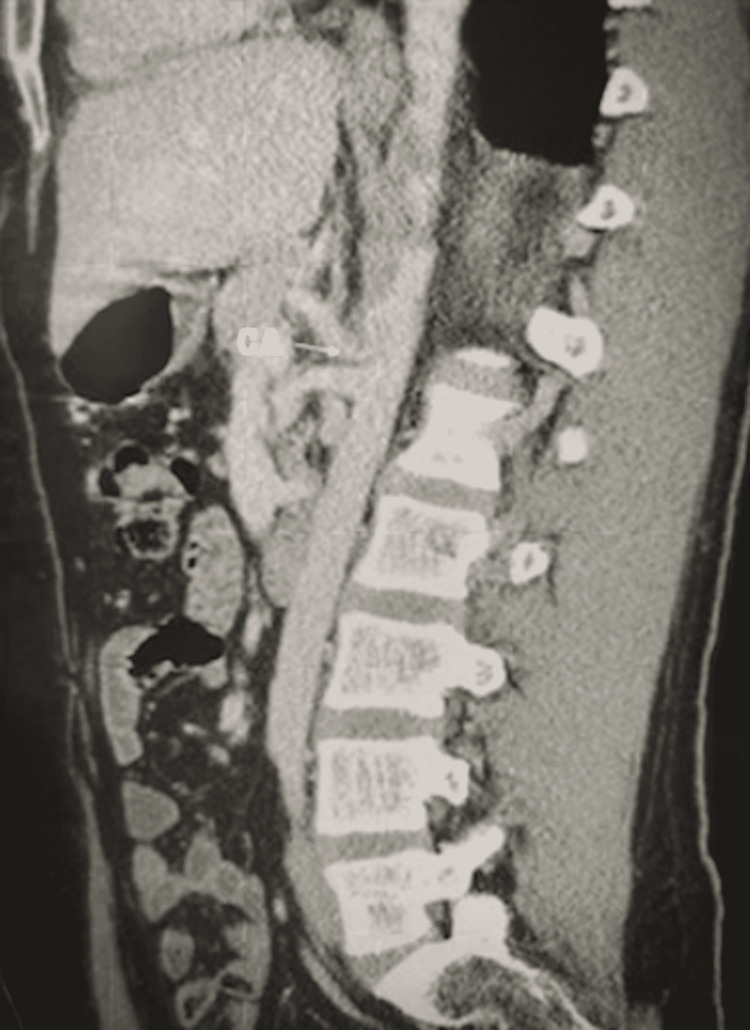
The sporadic nature of the episodes and variability of associated symptoms over the course of more than a decade led to a battery of diagnostic workups for multiple system atrophy by various specialists, including multiple neurologists and rheumatologists. However, these diagnostic workups were reported to be negative. Moreover, celiac plexus/ganglion blocks were attempted, and the results were unremarkable. At one point, he was diagnosed with early-onset Parkinson’s disease. Upon evaluation of the patient, the physical exam was unremarkable, but the patient stated that he had lost 20 lbs over the course of the previous few months due to decreased appetite and the development of an aversion to eating. MALS was suspected, and a vascular duplex ultrasound and CT angiography were performed, revealing more than 70% stenosis of the celiac artery and enlargement of the median arcuate ligament, resulting in significant compression of the celiac artery during inspiration (Figure 1).
Figure 1. Computed tomography angiography demonstrating 70% stenosis of the celiac artery upon inspiration.
CA = celiac artery
The decision was made to refer the patient to General Surgery for potential laparoscopic median arcuate ligament release after the patient was determined to be a poor candidate for open surgery. The patient was made aware of the risks and benefits of the procedure, especially due to the proximity of the aorta and the possibility of no symptom relief post-median arcuate ligament release. Consent was obtained and the decision was made to proceed with robotic-assisted celiac artery exposure, median arcuate ligament release, and robotic-assisted celiac neurolysis.
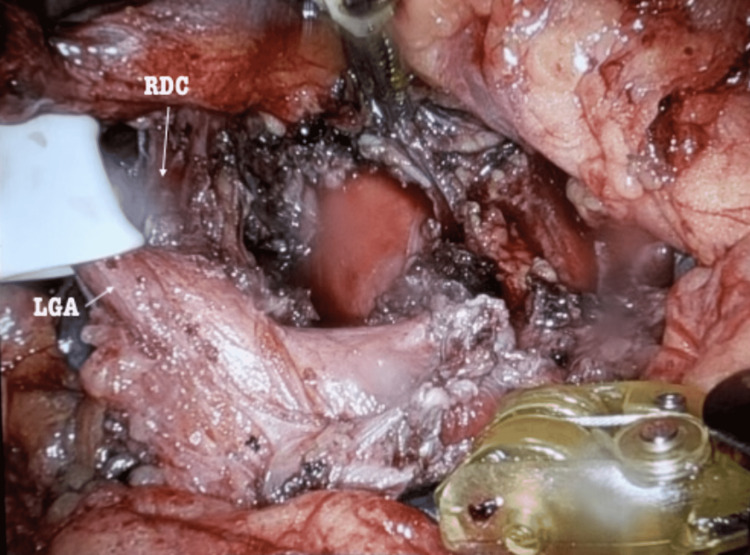
The patient was prepped appropriately for surgery and placed in the reverse left Trendelenburg position to allow for appropriate intraoperative exposure. Intraoperatively, the left gastric pedicle was retracted upward to expose the supraceliac area, with direct visualization of the fibers of the right diaphragmatic crus (Figure 2).
Figure 2. Intraoperative visualization of the fibers of the right diaphragmatic crus.
RDC = right diaphragmatic crus, LGA = left gastric artery
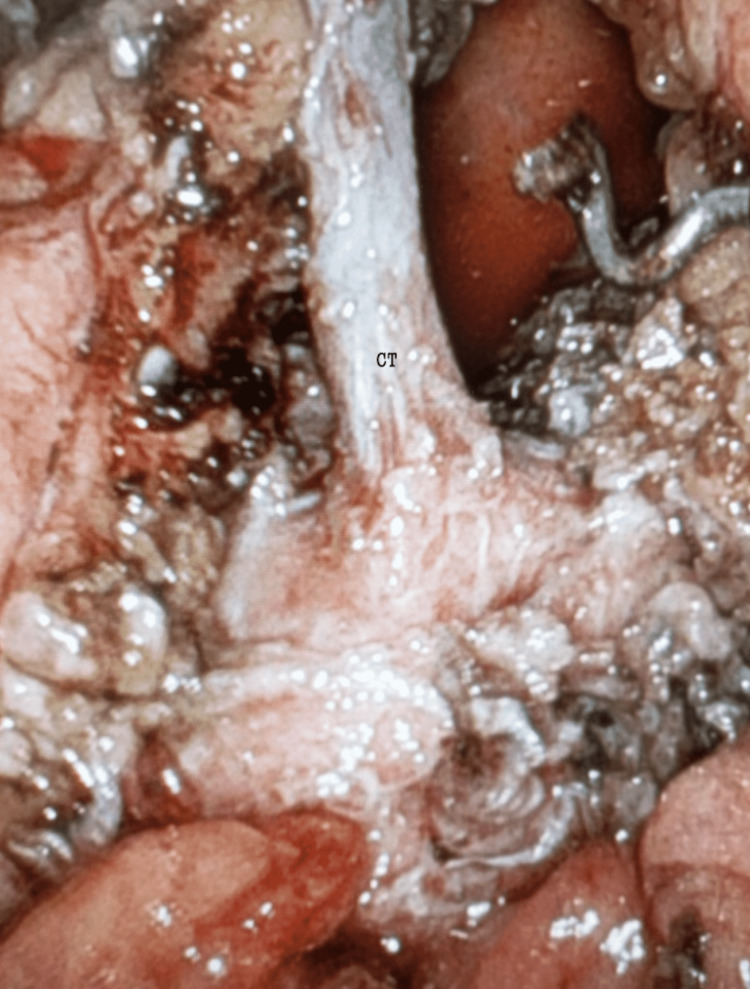
All three branches of the celiac trunk were identified, and attention was directed to the takedown and release of all neuromuscular fibers around the celiac trunk to complete the median arcuate ligament release and celiac neurolysis (Figure 3).
Figure 3. Median arcuate ligament release and celiac neurolysis.
CT = celiac trunk
The patient’s postoperative status was stable. He was seen two weeks and two months postoperatively in the clinic. The patient reported complete resolution of his symptoms from as early as his two-week follow-up and confirmed that he had developed no complications and continued to have complete resolution of his symptoms at his two-month follow-up.
Discussion
As previously indicated, MALS is a rare syndrome with variable and complex etiology and mechanism of disease [7-9]. Of the constellation of symptoms that MALS may present with, postprandial epigastric pain is a classic symptom and increases the index of suspicion for clinicians to engage in a diagnostic workup to assess the compressibility of the celiac trunk in various settings and especially upon inspiration [10-12]. Given that MALS is a diagnosis of exclusion, diagnosis is often delayed or missed altogether, and chronic manifestations of the disease often include progressive weight loss and food aversion [1-3].
In our patient’s case, his constellation of atypical symptoms, including the lack of consistent postprandial epigastric pain and the presence of diffuse, poorly localized abdominal pain and syncopal episodes, in addition to his personal history of ADHD, restless leg syndrome, peripheral neuropathic pain, and family history of epilepsy distracted from MALS as the cause of his presentation and led to a misdiagnosis of Parkinson’s disease. Moreover, the absence of the classic symptom of epigastric pain and the predomination of nonspecific GI symptoms and syncope in his presentation further clouded clinicians’ ability to diagnose MALS. Given that our patient was using gabapentin to treat his peripheral neuropathic pain, and the growing body of evidence supporting a neurogenic component of MALS presentation in some patients, we endeavored to better understand the intersection of our patient’s symptoms and the plausible etiology that resulted in his atypical, chronic MALS presentation [8,9].
Recent evidence presented in a double-blind clinical trial demonstrated the efficacy of gabapentin in reducing the severity of GI symptoms in patients with neurogenic irritable bowel syndrome symptoms, especially pain, reflux, and indigestion [20]. It is, therefore, reasonable to suspect that his use of gabapentin may have served as a symptomatic modifier as its effects on decreasing neurogenic hypersensitivity have been well documented in the literature [20]. This phenomenon may have contributed to the development of poorly localized, diffuse abdominal pain in our patient as neurogenic hypersensitivity in the GI tract has been shown to be mediated through δ2 receptors of calcium channels, which are targets of gabapentin inhibitory drug action [20].
Conclusions
The atypical and chronic presentation of MALS in our case, in conjunction with our patient’s use of gabapentin, the growing body of evidence presented in the literature supporting the neurogenic etiology of MALS, and recent evidence demonstrating the efficacy of gabapentin in decreasing neurogenic hypersensitivity of the GI tract, represents a novel addition to the literature on the treatment modalities of MALS and elucidates new avenues for continued research. Further research is needed to establish the use of gabapentin as a symptom-modifying agent in the nonoperative and preoperative treatment of MALS, and a reasonable index of suspicion for MALS must be maintained in patients on gabapentin presenting with nonspecific abdominal pain and weight loss.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Reversible gastroparesis: functional documentation of celiac axis compression syndrome and postoperative improvement. Trinidad-Hernandez M, Keith P, Habib I, White JV. Am Surg. 2006;72:339–344. [PubMed] [Google Scholar]
- 2.Treatment of celiac artery compression syndrome: does it really exist? Gloviczki P, Duncan AA. Perspect Vasc Surg Endovasc Ther. 2007;19:259–263. doi: 10.1177/1531003507305263. [DOI] [PubMed] [Google Scholar]
- 3.Management of median arcuate ligament syndrome: a new paradigm. Duffy AJ, Panait L, Eisenberg D, Bell RL, Roberts KE, Sumpio B. Ann Vasc Surg. 2009;23:778–784. doi: 10.1016/j.avsg.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Median arcuate ligament syndrome-review of this rare disease. Kim EN, Lamb K, Relles D, Moudgill N, DiMuzio PJ, Eisenberg JA. JAMA Surg. 2016;151:471–477. doi: 10.1001/jamasurg.2016.0002. [DOI] [PubMed] [Google Scholar]
- 5.Median arcuate ligament syndrome: evaluation with CT angiography. Horton KM, Talamini MA, Fishman EK. Radiographics. 2005;25:1177–1182. doi: 10.1148/rg.255055001. [DOI] [PubMed] [Google Scholar]
- 6.A composite study of the coeliac axis artery. Lipshutz B. Ann Surg. 1917;65:159–169. doi: 10.1097/00000658-191702000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical anatomy of celiac artery compression syndrome: a review. Loukas M, Pinyard J, Vaid S, Kinsella C, Tariq A, Tubbs RS. Clin Anat. 2007;20:612–617. doi: 10.1002/ca.20473. [DOI] [PubMed] [Google Scholar]
- 8.Median arcuate ligament syndrome is not a vascular disease. Weber JM, Boules M, Fong K, et al. Ann Vasc Surg. 2016;30:22–27. doi: 10.1016/j.avsg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Celiac artery compression syndromes. Bech FR. Surg Clin North Am. 1997;77:409–424. doi: 10.1016/s0039-6109(05)70558-2. [DOI] [PubMed] [Google Scholar]
- 10.Celiac artery compression syndrome in children, adolescents, and young adults: clinical and color duplex sonographic features in a series of 59 cases. Scholbach T. J Ultrasound Med. 2006;25:299–305. doi: 10.7863/jum.2006.25.3.299. [DOI] [PubMed] [Google Scholar]
- 11.Median arcuate ligament syndrome: a nonvascular, vascular diagnosis. Skeik N, Cooper LT, Duncan AA, Jabr FI. Vasc Endovascular Surg. 2011;45:433–437. doi: 10.1177/1538574411406453. [DOI] [PubMed] [Google Scholar]
- 12.Editor's choice - management of the diseases of mesenteric arteries and veins: clinical practice guidelines of the European Society of Vascular Surgery (ESVS) Björck M, Koelemay M, Acosta S, et al. Eur J Vasc Endovasc Surg. 2017;53:460–510. doi: 10.1016/j.ejvs.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Median arcuate ligament syndrome (Dunbar syndrome) Iqbal S, Chaudhary M. Cardiovasc Diagn Ther. 2021;11:1172–1176. doi: 10.21037/cdt-20-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Median arcuate ligament syndrome. Duncan AA. Curr Treat Options Cardiovasc Med. 2008;10:112–116. doi: 10.1007/s11936-008-0012-2. [DOI] [PubMed] [Google Scholar]
- 15.Late results following operative repair for celiac artery compression syndrome. Reilly LM, Ammar AD, Stoney RJ, Ehrenfeld WK. J Vasc Surg. 1985;2:79–91. [PubMed] [Google Scholar]
- 16.Treatment options and outcomes for celiac artery compression syndrome. Kohn GP, Bitar RS, Farber MA, Marston WA, Overby DW, Farrell TM. Surg Innov. 2011;18:338–343. doi: 10.1177/1553350610397383. [DOI] [PubMed] [Google Scholar]
- 17.Celiac artery compression syndrome: successful utilization of robotic-assisted laparoscopic approach. Jaik NP, Stawicki SP, Weger NS, Lukaszczyk JJ. https://pubmed.ncbi.nlm.nih.gov/17410294/ J Gastrointestin Liver Dis. 2007;16:93–96. [PubMed] [Google Scholar]
- 18.Robotic-assisted median arcuate ligament release. Relles D, Moudgill N, Rao A, Rosato F, DiMuzio P, Eisenberg J. J Vasc Surg. 2012;56:500–503. doi: 10.1016/j.jvs.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 19.Laparoscopic median arcuate ligament release: are we improving symptoms? El-Hayek KM, Titus J, Bui A, Mastracci T, Kroh M. J Am Coll Surg. 2013;216:272–279. doi: 10.1016/j.jamcollsurg.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Evaluating the effect of oral gabapentin on the improvement of gastrointestinal symptoms in patients with functional dyspepsia resistant to conventional treatments. Shafigh-Ardestani MH, Karami-Horestani M, Emami B, Arjmandpour A. Adv Biomed Res. 2019;8:53. doi: 10.4103/abr.abr_234_18. [DOI] [PMC free article] [PubMed] [Google Scholar]