Abstract
The ABC transporter TliDEF was found to be an efficient secretory apparatus for extracellular lipase TliA in Pseudomonas fluorescens. For the enhanced secretion of the lipase, we tried to coexpress tliA and tliDEF in various Pseudomonas species. Whereas the coexpression of tliA and tliDEF was required for the lipase secretion in P. fragi, the expression of tliA was sufficient for the lipase secretion in P. fluorescens, P. syringae, and P. putida, indicating the existence of compatible ABC transporter in these species. However, P. fluorescens harboring tliDEFA secreted much more lipase than P. fluorescens harboring only tliA, but the tliDEF was functional only at temperatures below 30°C. The recombinant P. fluorescens overexpressing tliDEFA showed the highest secretion level, 217 U/ml · OD (optical density) (28 μg/ml · OD) of lipase in Luria-Bertani medium under microaerated conditions. With the increase of aeration, the lipase production was decreased and the lipase seemed to be degraded as the cells entered the cell death phase. These results demonstrate that P. fluorescens can be used as a host system for the secretory production of the lipase using the ABC transporter, thus producing lipase in over 14% of the total protein.
Pseudomonas lipases have a great potential in the areas of detergent additives, processing of fats or oils, organic synthesis, chiral resolution, and so on (20). P. fluorescens lipase (molecular mass, 50 kDa), in particular, has special applications in the chiral resolution of racemic mixtures (14, 31) and in the synthesis of polyunsaturated fatty acids (24, 38).
Recent understanding of the secretion mechanism in gram-negative bacteria has provided various approaches to the production of the Pseudomonas lipase. Pseudomonas lipases are secreted through two different secretion pathways. First, a general secretion pathway (GSP; type II pathway) is used by the signal sequence-containing lipases of P. glumae (11), P. alcaligenes (13), and P. aeruginosa (35). For correct folding and translocation, these lipases need molecular chaperones, which are located immediately downstream of the lipase structural genes (10, 17, 19, 37). Secondly, the lipase of P. fluorescens is secreted by the ABC pathway (7), which secretes the proteins, including toxins, proteases, and lipases, by a one-step mechanism (type I pathway). This secretion pathway involves only three membrane proteins, i.e., ABC protein, membrane fusion protein, and outer membrane protein, while the GSP (type II pathway) involves over 12 secretory proteins, in addition to sec proteins (36).
Many Pseudomonas lipases have been cloned and expressed in Escherichia coli (10, 19, 21, 30). However, they usually accumulate as an inactive inclusion body in the cell, and active lipase can be obtained only by refolding the inclusion body (1, 32). Lipases are also produced by chaperone-mediated folding and secretion in E. coli (15, 18, 30). On the other hand, lipase can be produced in homologous hosts by inserting a lipase gene into the original host. Pseudomonas lipases, especially those secreted by GSP, are produced by the recombinant Pseudomonas species harboring both lipase and its chaperone genes, as has been reported in the case of P. cepacia (16), Pseudomonas sp. KWI-56 (34), and P. alcaligenes (12).
Previously, we cloned an ABC transporter of the lipase from P. fluorescens SIK W1 and found that the lipase was secreted in recombinant E. coli with the aid of the ABC transporter gene (2). To enhance the production of extracellular lipase, as described in this report, we tried a homologous expression of lipase and ABC transporter genes in Pseudomonas. To our knowledge, there have been no other reports on the production of lipase in recombinant Pseudomonas with the aid of ABC transporter gene. Therefore, we report here the coexpression of lipase (tliA) and ABC transporter (tliDEF) genes in P. fluorescens, the original host. In addition, we compared the expressions of lipase and ABC transporter genes in other Pseudomonas species.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Various Pseudomonas species such as P. fluorescens SIK W1 (4), P. putida GM730 (23), P. acidovorans (recently reclassified as Comamonas acidovorans) KCTC1638, P. aeruginosa KCTC1750, P. syringae KCTC1832, and P. fragi KCTC2345 were used for the expression of tliDEF and tliA. E. coli XL1-Blue (Stratagene) and DH5α (33) were used as recipients for plasmid transformation and conjugation. Broad-host-range vectors, pDSK519 (IncQ; Kmr) and pRK415 (IncP; Tcr) were donated by N. T. Keen (22). Broad-host-range vector pBBR1MCS-4 (IncP-1; Apr) was donated by K. M. Peterson (25). Luria-Bertani (LB) medium was used for the growth of E. coli and various Pseudomonas species. P. fluorescens, P. putida, P. syringae, P. acidovorans, and P. fragi were grown at 25°C, and P. aeruginosa was grown at 37°C, unless otherwise described. The LAT plate (LB medium, 1.5% Bacto Agar, 0.5% tributyrin) was used to detect the lipase activity of recombinant E. coli and Pseudomonas. If required, ampicillin (50 μg/ml), chloramphenicol (170 μg/ml), kanamycin (50 μg/ml), and tetracycline (25 μg/ml) were included in the growth media.
Plasmid construction.
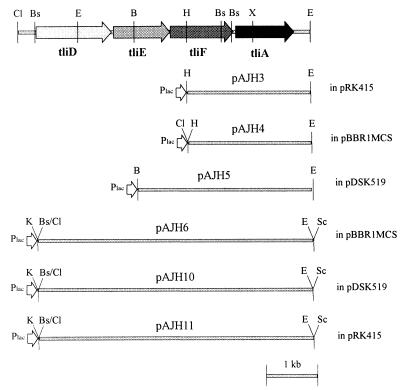
Figure 1 shows the plasmids used in this study. Plasmids pAJH3 and pAJH4 were constructed by inserting 2.9-kb HindIII-EcoRI fragments into pRK415 and pBBR1MCS-4, respectively. Plasmid pAJH5 was generated by inserting a 4-kb BamHI-EcoRI fragment into pDSK519. Plasmid pAJH6 was constructed by inserting a 3.5-kb BsrBI-HindIII fragment into the HindIII-ClaI (filled-in) site of pAJH4. The 6.3-kb KpnI-SacI fragment from pAJH6 was inserted into pDSK519 and pRK415, resulting in pAJH10 and pAJH11, respectively. Expression of these plasmids was constitutive, rather than influenced by isopropyl-β-d-thiogalactopyranoside (IPTG) induction, so IPTG was not used for induction.
FIG. 1.
Restriction endonuclease map of the various plasmids used. The relative positions of genes in P. fluorescens SIK W1 and restriction enzyme sites are depicted at the top. Inserts of subcloned plasmids are represented by the heavy line with pertinent enzyme sites of both ends. The names of subcloned plasmids and cloning vectors used are given above and on the right side of the heavy line, respectively. Plac is lac promoter. Restriction enzymes: B, BamHI; Bs, BsrBI; Cl, ClaI; E, EcoRI; H, HindIII; K, KpnI; X, XhoI; Ev, EcoRV; S, SacI.
Conjugal transfer of broad-host-range vector.
Triparental spot matings were performed essentially as described by Miller (29). Broad-host-range plasmids were mobilized by using helper plasmid pRK2013 (9). Late-log cultures of donor (E. coli), helper (E. coli/pRK2013), and recipient (Pseudomonas) cells were centrifuged, washed, and resuspended in one-third volume of 0.9% NaCl. Five microliters of recipient cells was spotted on an LB agar plate and allowed to dry, followed by spotting and drying of the same volume of helper and donor cells. Matings were allowed to proceed for 8 h at the growth temperatures appropriate for the various Pseudomonas species. The cells in the spots were resuspended in 0.9% NaCl and plated on selective LB media containing ampicillin, as well as other appropriate antibiotics. Because some Pseudomonas species did not grow on the Pseudomonas-isolating agar (Difco), selection was done by using ampicillin (50 μg/ml), to which all the Pseudomonas species tested were resistant. P. aeruginosa, P. acidovorans, and P. fragi were resistant to kanamycin (50 μg/ml), so these Pseudomonas species containing pDSK519 derivatives were plated and screened on LB agar containing 400 μg of kanamycin per ml. Plates were incubated at 37 or 25°C, and transconjugants usually appeared within 2 to 3 days.
SDS-PAGE and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with a 10% polyacrylamide gel as described by Laemmli (26). One milliliter of culture solution was harvested and centrifuged for 10 min at 13,000 × g. Cell pellets were dissolved in 200 μl of 0.1 M NaCl, 10 to 20 μl of the cell suspension was mixed with SDS sample buffer (5×), and then 15 μl was subjected to SDS-PAGE. The culture supernatant was obtained by centrifugation and mixed with SDS sample buffer (5×), and then 15 to 20 μl was subjected to SDS-PAGE. Proteins were stained with Coomassie brilliant blue R-250.
The antiserum against the P. fluorescens lipase used was described previously (2). Proteins in SDS-PAGE were transferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) by electrotransfer. The lipase was detected by immunoblotting with antilipase serum, followed by the binding of alkaline phosphatase-conjugated anti-rabbit immunoglobulin G (IgG), and then signals were detected with the reaction of alkaline phosphatase according to the manufacturer's instruction (Boehringer Mannheim).
Analytical methods.
Protein concentrations were determined by the Bradford assay (Bio-Rad) using bovine serum albumin as a standard. The concentration of extracellular lipase was estimated from the concentration of total extracellular proteins, and the percentage of extracellular lipase was estimated with the Imaging Densitometer (Model GS-700; Bio-Rad). The amount of cellular protein was estimated by determining the concentration of the cell extract obtained by centrifugation, resuspension, and sonication of cultured cells. The percentage of secreted lipase was calculated by dividing the amount of the secreted lipase by that of the total protein (cellular and extracellular). OD was measured at 600 nm (Ultraspec 2000 UV/visible spectrophotometer; Pharmacia).
The lipase activity was estimated by pH titration of fatty acids liberated from olive oil as described previously (27). One unit of lipase activity was defined as the amount of lipase necessary to release 1 μmol of fatty acids per min at 45°C and pH 8.5, conditions under which the P. fluorescens lipase shows optimal activity (29). The lipase activity was represented as specific activity and measured in units/milliliter · OD, indicating activity per culture supernatant added for reaction and per cell OD600.
RESULTS
Vector construction for the expression of tliDEFA in Pseudomonas
Previously, the lipase gene tliA (6) and the ABC transporter gene tliDEF (2) had been cloned. We expressed tliA and tliDEF in E. coli, which secreted lipase up to 5 U/ml · OD, while E. coli harboring only tliA secreted no lipase. To improve low heterologous expression in E. coli, we introduced tliA and tliDEF into P. fluorescens, from which tliA and tliDEF were cloned.
Broad-host-range vectors pDSK519, pRK415, and pBBR1MCS were used for the construction of expression vectors in Pseudomonas, resulting in six plasmids containing either tliA or tliDEFA as shown in Fig. 1. tliDEFA is organized as an operon where a promoter is upstream of tliD and a transcription terminator is downstream of tliA (2). The native promoter of tliD in the six plasmids was replaced with a lac promoter of the vectors. Among the six plasmids, pAJH3, pAJH5, pAJH10, and pAJH11, derived from pDSK519 and pRK415, were transferred from E. coli into P. fluorescens by triparental conjugation. However, pAJH4 and pAJH6, derived from pBBR1MCS, were not transferred for unknown reasons.
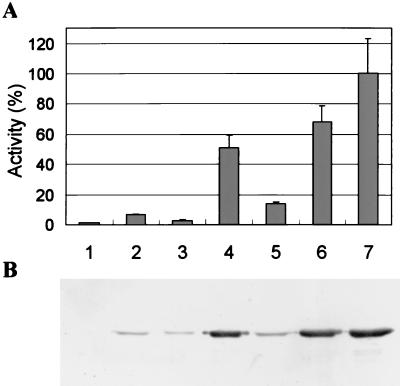
P. fluorescens organisms carrying each of the above four plasmids and sets of dual plasmids were grown at 25°C. The secreted lipase was detected by lipase activity assay (Fig. 2A) and Western blotting of each culture supernatant (Fig. 2B). No lipase was produced in the host P. fluorescens, although tliDEFA was intact in the host. Much more lipase was secreted by P. fluorescens supplemented by tliDEFA than by P. fluorescens supplemented only with tliA. P. fluorescens containing two plasmids (pAJH3 and pAJH10; pAJH11 and pAJH10) secreted more lipase than P. fluorescens containing one plasmid, indicating that the increased gene dosage of the ABC transporter and lipase enabled P. fluorescens to secrete more lipase. However, subsequent experiments proceeded with pAJH5 and pAJH10, which contained a selective marker gene for kanamycin. The reason for not using pRK415-derived plasmids was that the growth of P. fluorescens was retarded when tetracycline (the selective marker for pRK415) was present in the culture system.
FIG. 2.
Secretion of lipase by P. fluorescens harboring various plasmids. (A) The activity estimation of culture supernatant. P. fluorescens SIK W1 organisms harboring different plasmids were grown at 25°C in LB medium. The cells were harvested at the stationary phase when they reached an OD600 of around 3.0. Extracellular lipase activities were estimated by the pH titration method, and the activity is represented as relative value to the activity produced by P. fluorescens (pAJH11 pAJH10) (set as 100%). (B) Immunodetection of the lipase culture supernatant. Culture supernatant of 0.04 OD equivalent (12 μl of culture supernatant) was subjected to SDS-PAGE, transferred to nitrocellulose membrane, and analyzed by immunodetection. One OD equivalent corresponds to 1 ml of culture, OD600 = 1. Lanes: 1, no plasmid; 2, pAJH3; 3, pAJH5; 4, pAJH10; 5, pAJH11; 6, pAJH3 pAJH10; 7, pAJH11 pAJH10.
Temperature-dependent expression of tliDEFA in P. fluorescens
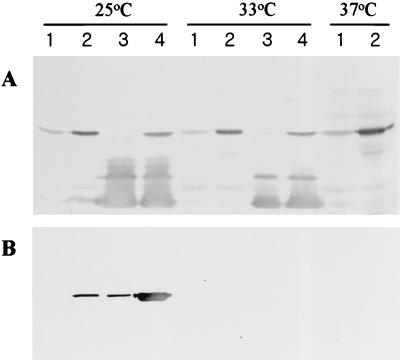
Previously, we reported that E. coli complemented by tliDEFA secretes the lipase only at temperatures below 30°C because tliDEF is functional at this temperature (2). We tested whether P. fluorescens also secreted the lipase in a temperature-dependent manner. E. coli and P. fluorescens harboring pAJH5 or pAJH10 were grown at different temperatures, and then the expression of lipase was determined inside the cell (Fig. 3A) and in the supernatant (Fig. 3B). P. fluorescens harboring tliA or tliDEFA secreted the lipase at temperatures below 30°C, demonstrating that the ABC transporter was also functional at low temperature in its original host. P. fluorescens harboring pAJH10 (lane 4) secreted much more lipase than E. coli harboring pAJH10 (lane 2) at 25°C. P. fluorescens harboring pAJH5 (lane 3) also secreted a small amount of lipase at 25°C by means of the chromosomal ABC transporter gene, although no lipase was secreted by E. coli harboring pAJH5 (lane 1).
FIG. 3.
Immunodetection of the lipase in cellular extracts (A) and culture supernatant (B) of E. coli and P. fluorescens carrying different plasmids. E. coli DH5α and P. fluorescens SIK W1 harboring different plasmids were grown at different temperatures in LB medium. P. fluorescens did not grow at 37°C, so there was no result. The cells were harvested at the stationary phase when they reached an OD600 of around 3.0. Cell extract and culture supernatant were prepared as described in Materials and Methods. Cell extract of 0.09 OD equivalent and culture supernatant of 0.04 OD equivalent were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and analyzed by immunodetection. Lanes: 1, E. coli (pAJH5); 2, E. coli (pAJH10); 3, P. fluorescens (pAJH5); 4, P. fluorescens (pAJH10).
We expected that the lipase would accumulate in the cell if the ABC transporter was absent or malfunctional at temperatures above 30°C. However, in the same host, the same amount of lipase was detected inside the cell at above 30°C, while less lipase was present in both E. coli and P. fluorescens supplemented with only tliA than in cells supplemented with tliDEFA (Fig. 3A).
Expression of tliDEFA in various Pseudomonas species.
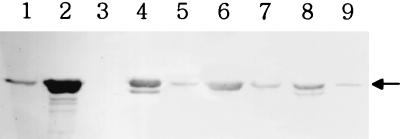
To see whether tliA and tliDEF could be expressed in other Pseudomonas species, pAJH5 and pAJH10 were introduced by conjugation into various Pseudomonas species, P. aeruginosa, P. fragi, P. putida, P. syringae, and P. acidovorans (“Comamonas acidovorans”). They were all grown in LB at 25°C for the expression of tliDEF, because tliDEF was functional at temperatures below 30°C. The lipase activities in the culture supernatants of the various Pseudomonas species were measured (Table 1), and the secreted lipase was detected by Western blotting (Fig. 4). All Pseudomonas species carrying no plasmid secreted no lipase (<0.3 U/ml · OD). However, the recombinant P. fluorescens, P. syringae, and P. putida, harboring pAJH5 containing tliA, secreted the lipase, although P. fragi secreted no lipase by complementing only tliA, indicating that these hosts had inherent ABC transporter for P. fluorescens lipase but P. fragi had not. Recombinant P. fluorescens, P. fragi, P. syringae, and P. putida secreted much more lipase by supplementing pAJH10 containing tliDEFA. Recombinant P. fluorescens harboring tliDEFA secreted 70 times more lipase (333 U/ml · OD) than P. fluorescens harboring tliA (4.7 U/ml · OD). P. aeruginosa and P. acidovorans did not secrete the lipase by supplementing pAJH5 or pAJH10. tliA or tliDEF seemed not to be functionally expressed in these hosts.
TABLE 1.
Secretion of lipase in various recombinant Pseudomonas and E. coli organismsa
| Strains | Activity (U/ml · OD)
|
||
|---|---|---|---|
| Original | pAJH5 | pAJH10 | |
| E. coli | <0.3 | <0.3 | 1.0 ± 0.2 |
| P. acidovorans | <0.3 | <0.3 | <0.3 |
| P. aeruginosa | <0.3 | <0.3 | <0.3 |
| P. fluorescens | <0.3 | 4.8 ± 1.6 | 333.1 ± 23.6 |
| P. fragi | <0.3 | <0.3 | 63.2 ± 6.2 |
| P. putida | <0.3 | 6.2 ± 1.0 | 14.8 ± 4.2 |
| P. syringae | <0.3 | 2.1 ± 1.2 | 81.0 ± 14.0 |
All the Pseudomonas species and E. coli were grown at 25°C in LB medium. The cells were harvested at the stationary phase when they reached an OD600 of around 3.0.
FIG. 4.
Immunodetection of the lipase in culture supernatant of high lipase producers. Culture supernatant of 0.04 OD equivalent was subjected to SDS-PAGE, transferred to nitrocellulose membrane, and analyzed by immunodetection. Lanes: 1, P. fluorescens (pAJH5); 2, P. fluorescens (pAJH10); 3, P. fragi (pAJH5); 4, P. fragi (pAJH10); 5, P. syringae (pAJH5); 6, P. syringae (pAJH10); 7, P. putida (pAJH5); 8, P. putida (pAJH10); 0.9, E. coli (pAJH10). The arrow indicates the expected band of lipase whose molecular mass is 49.9 kDa.
tliDEFA expression in P. fluorescens against cell growth.
P. fluorescens harboring pAJH10 secreted a considerable amount of lipase, but this result was reproducible neither in a flask nor in a fermentor but only in a test tube. This result led us to suspect that improved aeration, either in a flask or a fermentor, reduced the extracellular production of lipase, compared to the limited aeration in a test tube. Therefore, we designed several culture conditions to simulate various aeration rates. For this purpose, a 500-ml baffled flask containing 100 ml of LB medium and 500-ml unbaffled flasks containing 100 and 350 ml of LB medium were prepared to investigate the effect of aeration on the growth and lipase production for P. fluorescens harboring pAJH10.
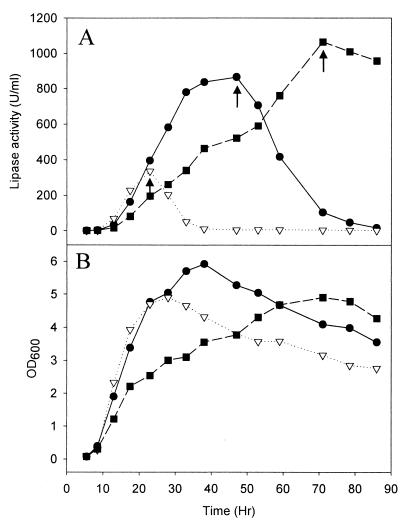
After inoculation with P. fluorescens (pAJH10), flasks were incubated at 25°C in an orbital shaker at 160 rpm. The extracellular lipase activity was monitored with culture supernatant (Fig. 5A) against bacterial cell growth (Fig. 5B). The growth of P. fluorescens was influenced not by the presence of pAJH10 but by aeration conditions (data not shown). P. fluorescens grown under well-aerated conditions (100-ml baffled flask) showed a yellow-white cell pellet after centrifugation but a red pellet under medium aeration (100-ml unbaffled flask) and microaeration (350-ml unbaffled flask), indicating that limited aeration induced P. fluorescens to produce red pigments. Although tliDEFA was under a lac promoter, the lipase was produced even without IPTG induction. In all of these cases, the lipase production in P. fluorescens (pAJH10) was growth associated, reaching a maximum at the end of the log phase, and the lipase disappeared after the cell death phase. Under microaeration, the cells grew slowly but secreted much more lipase than under medium aeration and well-aerated conditions. In addition, the lipase produced did not disappear during the prolonged incubation (longer than 7 days) after the culture harvest, whereas it did disappear under medium aeration and well-aerated conditions.
FIG. 5.
Lipase secretion against cell growth in different culture condition. (A) Lipase activity in the supernatant at different growth phases. Culture supernatant was prepared by centrifugation of culture solution and used for extracellular lipase activities by the pH-STAT method. (B) Cell growth curve. P. fluorescens harboring pAJH10 was grown in different conditions at 25°C in LB medium. ▿, 100-ml baffled flask; ●, 100-ml unbaffled flask; ▪, 350-ml unbaffled flask. Arrows indicate the sampling times indicated in the legend to Fig. 6.
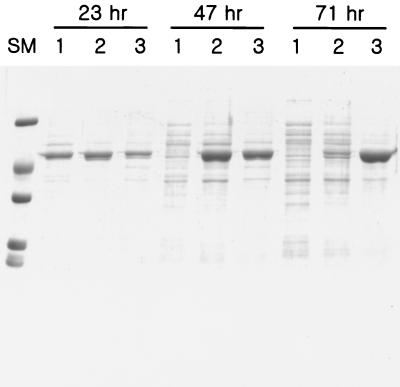
Figure 6 shows the SDS-PAGE for culture supernatants that were sampled at three cultivation times (23, 47, and 71 h) (Fig. 5A) for three different aeration conditions.
FIG. 6.
SDS-PAGE for culture supernatant of different culture conditions. The culture supernatants of three different conditions at three different times (23, 47, and 71 h) indicated by arrows in Fig. 5B were seen on SDS-PAGE. Each 16 μl of culture supernatant was subjected to SDS-PAGE. Lanes: SM, size marker; 1, well-aerated (100-ml baffle flask); 2, medium aeration (100-ml unbaffled flask); 3, microaerated conditions (350-ml unbaffled flask).
Under medium aeration and well-aerated conditions, there were some other proteins above and below the lipase band at each maximal production (lane 1 at 23 h; lane 2 at 47 h). The lipase band disappeared, and many other protein bands appeared, as the cell entered the cell death phase (lane 1 at 47 h; lanes 1 and 2 at 71 h). The degraded fragments of the lipase were, however, not detected by Western blotting (data not shown). Under microaerated conditions, the lipase was produced almost as a single band on SDS-PAGE. The secreted lipase concentration was 28 μg/ml · OD (217 U/ml · OD culture supernatant), which corresponded to 14.8% of the total protein. The amount of total protein was estimated from the amount of total cellular protein and extracellular protein as indicated in Materials and Methods. The secreted lipase was 8.0 and 3.8% of the total protein, respectively, at the maximum activity level of lipase under medium aeration and well-aerated conditions.
DISCUSSION
The ABC pathway is very simple, involving only three secretory components. In contrast, the GSP usually involves sec proteins for transport through the inner membrane and more than 12 secretory proteins for export through the outer membrane. Also, the ABC pathway is also efficient, because it is dedicated to transporting only some specific extracellular proteins, while the GSP secretes not only extracellular proteins but also various envelope proteins (8). In this report, we introduced a series of vectors carrying the ABC transporter and lipase genes into several Pseudomonas species, with the aim of achieving the extracellular production of lipase.
Although the secretion of the lipase was not observed above 30°C, the protein of tliA, organized as an operon behind tliDEF, was detected inside the cell at high temperature. Furthermore, more lipase was detected inside the cell in the presence of tliDEF, irrespective of the temperature. It was reported that substrate binding is required for assembly of three ABC transporter components (28). Thus, it could be speculated that, at high temperature, tliDEF proteins could interact with the lipase, thus protecting the lipase from the proteolytic degradation inside the cell, although they were not functional in lipase secretion. The secretion defect at high temperature was shown, both in the homologous host P. fluorescens and in the heterologous host E. coli, indicating that temperature dependency was derived from tliDEF itself, regardless of the host cells. The reason for this secretory defect at high temperature might be due to the disorder of the targeting and the assembling of the ABC transporter in the membrane at high temperature.
tliA and tliDEF were functionally expressed in all Pseudomonas species tested, except P. aeruginosa and P. acidovorans in liquid media. However, when recombinant P. aeruginosa was tested on LAT agar plate for the lipase activity, P. aeruginosa with pAJH5 containing only tliA showed an activity halo, whereas the original P. aeruginosa showed no halo. Previously, P. aeruginosa was reported to have an ABC transporter specific for alkaline protease, and the lipase of P. fluorescens was reported to be secreted by this ABC transporter (7). The recombinant P. aeruginosa harboring tliA seems to have secreted lipase using the endogenous ABC transporter on the solid medium. P. syringae and P. putida carrying only tliA secreted lipase, suggesting that they have an ABC transporter compatible with tliA, although there has been no report on the lipase secreted by ABC transporter or the presence of ABC transporter itself in these species.
Whereas the lipase was detected as a major single band on SDS-PAGE in the recombinant P. fluorescens under microaeration, it disappeared, and other proteins showed up in the culture supernatant with an increase of aeration. P. fluorescens lipase was strong enough to retain its activity after prolonged incubation or even after heat treatment at 100°C (3, 4), indicating that a decrease in the lipase band did not originate from autolysis. We suspected that proteases leaked during cell lysis might have degraded the secreted lipase under medium aeration and well-aerated conditions. We tested various protease inhibitors, such as EDTA, phenylmethylsulfonyl fluoride, pepstatin, E-64, or a protease inhibitor cocktail, to inhibit the degradation of lipase under well-aerated conditions. However, we could not find a specific inhibitor which blocked the degradation of the lipase. More investigation is needed to elucidate the mechanism for lipase disappearance under medium-aerated and well-aerated conditions.
While a small amount of the lipase was produced in P. fluorescens harboring only tliA, P. fluorescens harboring both tliA and tliDEF secreted up to 28 μg/ml · OD of lipase, which corresponded to about 14.8% of the total protein. Recently, Braun et al. attempted to use Pseudomonas as enzyme factories exploiting GSP for secretory production (5). However, our results strongly demonstrated the potential of Pseudomonas as a promising host for the extracellular production of lipase using the ABC pathway, which is more efficient than the GSP.
ACKNOWLEDGMENTS
We are grateful to E. S. Choi and H. A. Kang for reading the manuscript and providing us with helpful suggestions. We thank N. T. Keen for providing pDSK519 and pRK415 and K. M. Peterson for providing pBBR1MCS-4.
REFERENCES
- 1.Ahn J H, Lee Y P, Rhee J S. Investigation of refolding condition for Pseudomonas fluorescens lipase by response surface methodology. J Biotechnol. 1997;54:151–160. doi: 10.1016/s0168-1656(97)01693-3. [DOI] [PubMed] [Google Scholar]
- 2.Ahn J H, Pan J G, Rhee J S. Identification of the tliDEF ABC transporter specific for lipase in Pseudomonas fluorescens SIK W1. J Bacteriol. 1999;181:1847–1852. doi: 10.1128/jb.181.6.1847-1852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson R E. Microbial lipolysis at low temperatures. Appl Environ Microbiol. 1980;39:36–40. doi: 10.1128/aem.39.1.36-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson R E, Hedlund C B, Jonsson U. Thermal inactivation of a heat-resistant lipase produced by the psychotrophic bacterium Pseudomonas fluorescens. J Dairy Sci. 1979;62:361–367. doi: 10.3168/jds.s0022-0302(79)83252-x. [DOI] [PubMed] [Google Scholar]
- 5.Braun P, Gerritse G, van Dijl J M, Quax W J. Improving protein secretion by engineering components of the bacterial translocation machinery. Curr Opin Biotechnol. 1999;10:376–381. doi: 10.1016/s0958-1669(99)80068-8. [DOI] [PubMed] [Google Scholar]
- 6.Chung G H, Lee Y P, Jeohn G H, Yoo O J, Rhee J S. Cloning and nucleotide sequence of thermostable lipase gene from Pseudomonas fluorescens SIK W1. Agric Biol Chem. 1991;55:2359–2365. [PubMed] [Google Scholar]
- 7.Duong F, Soscia C, Lazdunski A, Murgier M. The Pseudomonas fluorescens lipase has a C-terminal secretion signal and is secreted by a three-component bacterial ABC-exporter system. Mol Microbiol. 1994;11:1117–1126. doi: 10.1111/j.1365-2958.1994.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 8.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frenken L G, Bos J W, Visser C, Muller W, Tommassen J, Verrips C T. An accessory gene, lipB, required for the production of active Pseudomonas glumae lipase. Mol Microbiol. 1993;9:579–589. doi: 10.1111/j.1365-2958.1993.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 11.Frenken L G, de Groot A, Tommassen J, Verrips C T. Role of the lipB gene product in the folding of the secreted lipase of Pseudomonas glumae. Mol Microbiol. 1993;9:591–599. doi: 10.1111/j.1365-2958.1993.tb01719.x. [DOI] [PubMed] [Google Scholar]
- 12.Gerritse G, Hommes R W, Quax W J. Development of a lipase fermentation process that uses a recombinant Pseudomonas alcaligenes strain. Appl Environ Microbiol. 1998;64:2644–2651. doi: 10.1128/aem.64.7.2644-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerritse G, Ure R, Bizoullier F, Quax W J. The phenotype enhancement method identifies the Xcp outer membrane secretion machinery from Pseudomonas alcaligenes as a bottleneck for lipase production. J Biotechnol. 1998;64:23–38. doi: 10.1016/s0168-1656(98)00101-1. [DOI] [PubMed] [Google Scholar]
- 14.Grisenti P, Ferraboschi P, Casati S, Santaniello E. Studies on the enantioselectivity of the transesterification of 2-methyl-1,4-butanediol and its derivatives catalyzed by Pseudomonas fluorescens lipase in organic solvents. Tetrahedron Asymmetry. 1993;4:997–1006. [Google Scholar]
- 15.Hobson A H, Buckley C M, Aamand J L, Jorgensen S T, Diderichsen B, McConnell D J. Activation of a bacterial lipase by its chaperone. Proc Natl Acad Sci USA. 1993;90:5682–5686. doi: 10.1073/pnas.90.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hom S S M, Scott E M, Atchison R E, Picataggio S, Mielenz J R. Characterization and over-expression of a cloned Pseudomonas lipase gene. GBF Monogr. 1991;16:267–270. [Google Scholar]
- 17.Ihara F, Okamoto I, Akao K, Nihira T, Yamada Y. Lipase modulator protein (LimL) of Pseudomonas sp. strain 109. J Bacteriol. 1995;177:1254–1258. doi: 10.1128/jb.177.5.1254-1258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ihara F, Okamoto I, Nihira T, Yamada Y. Requirement in trans of the downstream limL gene for activation of lactonizing lipase from Pseudomonas sp. 109. J Ferment Bioeng. 1992;73:337–342. [Google Scholar]
- 19.Iizumi T, Nakamura K, Shimada Y, Sugihara A, Tominaga Y, Fukase T. Cloning, nucleotide sequencing, and expression in Escherichia coli of a lipase and its activator genes from Pseudomonas sp. KWI-56. Agric Biol Chem. 1991;55:2349–2357. [PubMed] [Google Scholar]
- 20.Jaeger K E, Ransac S, Dijkstra B W, Colson C, van Heuvel M, Misset O. Bacterial lipases. FEMS Microbiol Rev. 1994;15:29–63. doi: 10.1111/j.1574-6976.1994.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen S, Skov K W, Diderichsen B. Cloning, sequence, and expression of a lipase gene from Pseudomonas cepacia: lipase production in heterologous hosts requires two Pseudomonas genes. J Bacteriol. 1991;173:559–567. doi: 10.1128/jb.173.2.559-567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim K, Lee S, Lee K, Lim D. Isolation and characterization of toluene-sensitive mutants from the toluene-resistant bacterium Pseudomonas putida GM73. J Bacteriol. 1998;180:3692–3696. doi: 10.1128/jb.180.14.3692-3696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosugi Y, Takahashi K, Lopez C. Large-scale immobilization of lipase from Pseudomonas fluorescens biotype I and an application for sardine oil hydrolysis. J Am Oil Chem Soc. 1995;72:1281–1285. [Google Scholar]
- 25.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop II R M, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y P, Chung G H, Rhee J S. Purification and characterization of Pseudomonas fluorescens SIK W1 lipase expressed in Escherichia coli. Biochim Biophys Acta. 1993;1169:156–164. doi: 10.1016/0005-2760(93)90200-s. [DOI] [PubMed] [Google Scholar]
- 28.Letoffe S, Delepelaire P, Wandersman C. Protein secretion in gram-negative bacteria: assembly of the three components of ABC protein-mediated exporters is ordered and promoted by substrate binding. EMBO J. 1996;15:5804–5811. [PMC free article] [PubMed] [Google Scholar]
- 29.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 30.Oshima-Hirayama N, Yoshikawa K, Nishioka T, Oda J. Lipase from Pseudomonas aeruginosa. Production in Escherichia coli and activation in vitro with a protein from the downstream gene. Eur J Biochem. 1993;215:239–246. doi: 10.1111/j.1432-1033.1993.tb18028.x. [DOI] [PubMed] [Google Scholar]
- 31.Patel R N, Howell J M, McNamee C G, Fortney K F, Szarka L J. Stereoselective enzymatic hydrolysis of alpha-((acetylthio)methyl)benzenepropanoic acid and 3-acetylthio-2-methylpropanoic acid. Biotechnol Appl Biochem. 1992;16:34–47. [Google Scholar]
- 32.Quyen D T, Schmidt-Dannert C, Schmid R D. High-level formation of active Pseudomonas cepacia lipase after heterologous expression of the encoding gene and its modified chaperone in Escherichia coli and rapid in vitro refolding. Appl Environ Microbiol. 1999;65:787–794. doi: 10.1128/aem.65.2.787-794.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Shimada Y, Sugihara A, Tominaga Y. Microbial lipase: structure and production. New York, N.Y: Marcel Dekker, Inc; 1994. [PubMed] [Google Scholar]
- 35.Tommassen J, Filloux A, Bally M, Murgier M, Lazdunski A. Protein secretion in Pseudomonas aeruginosa. FEMS Microbiol Rev. 1992;9:73–90. doi: 10.1016/0378-1097(92)90336-m. [DOI] [PubMed] [Google Scholar]
- 36.Wandersman C. Secretion across the bacterial outer membrane. Trends Genet. 1992;8:317–322. doi: 10.1016/0168-9525(92)90264-5. [DOI] [PubMed] [Google Scholar]
- 37.Wohlfarth S, Hoesche C, Strunk C, Winkler U K. Molecular genetics of the extracellular lipase of Pseudomonas aeruginosa PAO1. J Gen Microbiol. 1992;138:1325–1335. doi: 10.1099/00221287-138-7-1325. [DOI] [PubMed] [Google Scholar]
- 38.Yuzo, K., and S. Akira. 1997. Gene for lipase remarkably acting on highly unsaturated fatty acid ester and production of lipase with the same. Japanese patent JP08065430.