Abstract
Glycyrrhizic acid and its primary metabolite glycyrrhetinic acid, are the main active ingredients in the licorice roots (glycyrrhiza species), which are widely used in several countries of the world, especially in east asian countries (China, Japan). These ingredients and their derivatives play an important role in treating many diseases, especially infectious diseases such as COVID-19 and hepatic infections. This review aims to summarize the different ways of synthesising the amide derivatives of glycyrrhizic acid and the main ways to synthesize the glycyrrhitinic acid derivatives. Also, to determine the main biological and pharmacological activity for these compounds from the previous studies to provide essential data to researchers for future studies.
Supplementary Information
The online version contains supplementary material available at 10.1134/S1068162022050132.
Keywords: glycyrrhizic acid, glycyrrhitinic acid, anti-viral activity, anti-bacterial activity, anti-coronavirus
INTRODUCTION
1. GENERAL METHODS OF PREPARING GLYCYRRHIZIC ACID DERIVATIVES
2. METHODS OF SYNTHESIS OF GLYCYRRHETINIC ACID (GA) DERIVATIVES
3. THE PHARMACOLOGICAL AND BIOLOGICAL ACTIVITY OF GLYCYRRHIZIC ACID AND GLYCYRRHITIC ACID DERIVATIVES
3.1. Anti-Coronavirus Activity
3.2. Anti-Influenza Activity
3.3. Anti-HIV Activity
3.4. Anti-Hepatic Viruses Activity
3.4.1 Suppression and inhibition of liver fibrosis
3.4.2. Anti-viral effects
3.4.3. Anti-inflammatory effect
3.4.4. Inhibition of hepatic apoptosis and necrosis
3.5. Antibacterial Effects of Glycyrrhizic Acid, Glycyrrhetinic Acid, and their Derivatives
CONCLUSIONS
REFERENCES
INTRODUCTION
Glycyrrhizic acid (GL) is a triterpene compound primarily obtained from perennial plants of glycyrrhiza species, especially from the root part. Several positions in the chemical structure of GL appear to be suitable for generating a variety of active pharmacological derivatives. These positions are the configuration of the 18 C atom, the glucuronic acid part, and free carboxylic acid. GL has been used with other drugs to improve activity and reduce side effects. Due to its synergistic action, which increases efficacy and reduces the toxicity of other drugs, glycyrrhizic acid has been used as a new drug delivery system and drug activity enhancer [1]. GL has a wide range of pharmaco-biological activities, including anti-inflammatory properties, inhibition of coronavirus replication, and reduction of virus pro-inflammatory cytokine production [2, 3]. The pentacyclic triterpenoid glycyrrhetinic acid (GA) is found in the roots of Glycyrrhiza glabra; it is the primary metabolite of glycyrrhizic acid. The scientific community is quite interested in GA compound because of the chemical structure of this type of triterpenoid, which has a basic chemical structure with five rings. This great interest in GA and its derivatives is also due to their wide range of biological and pharmacological activity, including anticancer, anti-inflammatory, anti-viral, anti-bacterial, anti-ulcer, hepatoprotective, cardioprotective, and neuroprotective properties. On the other hand, glycyrrhetinic acid has some unwanted properties, such as deficient lipophilicity, low water solubility, and poor bioavailability, which dramatically decrease the absorption of this compound. Fortunately, there are various permeability improvement techniques for enhancing its bioavailability [4]. In recent years, different methods of synthesis of glycyrrhizic acid and glycyrrhetinic acid have been widely used to synthesize many derivatives for many pharmacological applications, especially anti-viral and anti-bacterial uses. This article reviews the main methods of synthesising glycyrrhizic acid and glycyrrhetinic acid derivatives and their pharmacological applications to provide basic information for further studies in the future.
1 GENERAL METHODS OF PREPARING GLYCYRRHIZIC ACID DERIVATIVES
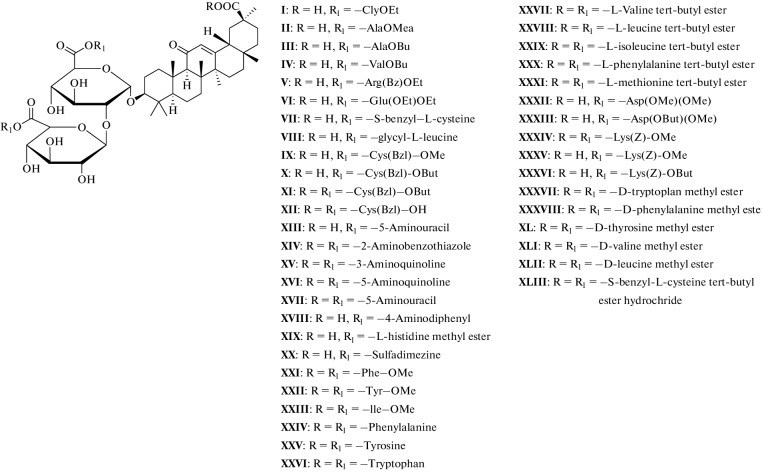
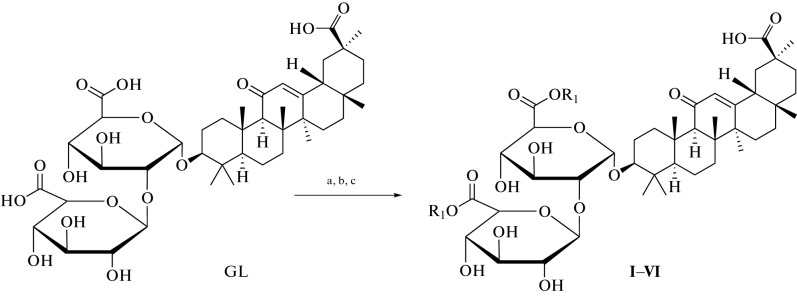
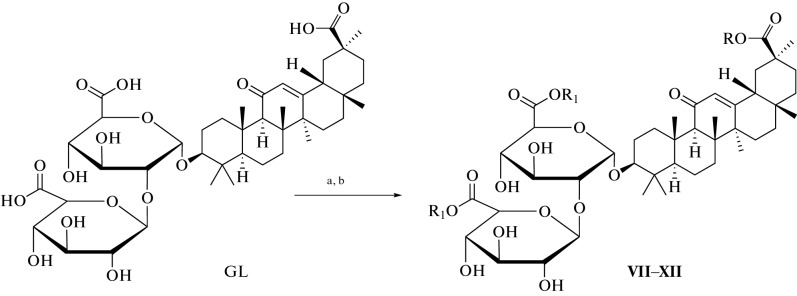
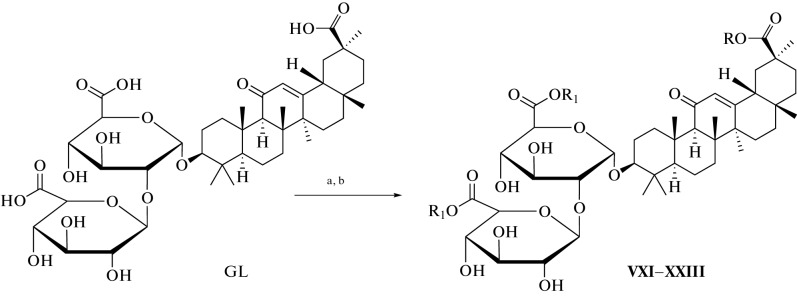
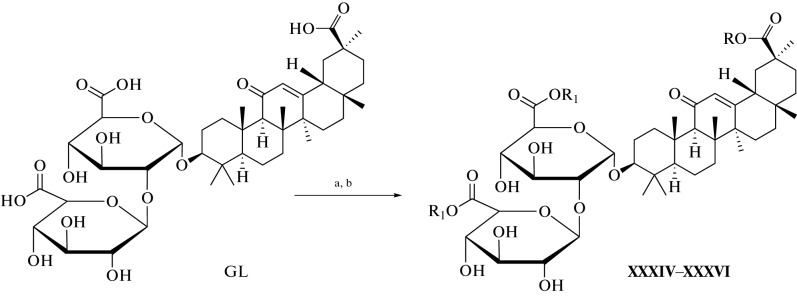
Many methods have been used to develop and synthesize new glycyrrhizic acid derivatives; most of these derivatives are amino acid derivatives, as shown in Fig. 1. The glycyrrhizic acid derivatives (I–VI) were synthesized via the condensation reaction of glycyrrhizic acid with the amino acid, and the “N-Hydroxysuccimide” was used as a coupling additive, and “N,N′-dicyclohexylcarbodiimide (DCC) as a coupling reagent, and tetrahydrofuran (THF) as a solvent [5], as shown in Fig. 2. GL derivatives of (VII–XII) were also prepared by the condensation reaction of GL with amino acids in the presence of N-hydroxysuccinimide as a coupling additive, DCC as a coupling reagent, and triethylamine (Et3N) as the reaction base, as shown in Fig. 3 [6, 7]. The derivatives (XIII–XVII) were synthesized by condensing Gl with amino acid in a mixture of dimethylformamide (DMF), pyridine, and DCC [8, 9]. Moreover, the derivatives (XVIII–XIX) were prepared by coupling the GL with the amino acids by the HoSu/DCC method in the presence of pyridine [10]. The derivatives (XX–XXIII) were obtained by GL coupling with amino acids by the DCC or HoBt/DCC methods in the presence of pyridine, and the process of the reaction was as in Fig. 4 [11]. Another method for producing glycyrrhizic acid derivatives (XXIV–XXVI) was to dissolve glycyrrhizic acid in THF at temperatures ranging from 0 to 5°C. Then DCC, HoBt, and amino acids were added to the solution at the same condition, and the process of the reaction was completed, as shown in the reference [12]. The derivatives (XXVII–XXXI) were prepared by coupling GL with the amino acids by the DMF/EDCI/triethylamine or THF/HoPt/DCC methods [13, 14]. Also, the GL derivatives (XXXII, XXXIII) were prepared by using the GL/HoSu/DCC method. The solvent in this method is DMF, or dioxane or THF [15]. As shown in Fig. 5, the derivatives (XXXIV–XXXVI) were synthesized via a condensation reaction between glycyrrhizic acid and [Lys(Z)-OMeCF3COOH] in the presence of hydroxybenzotriazole (HoBt) and DCC [16]. The derivatives (XXXVII–XLII) were prepared by coupling the GL with amino acids via dioxane/HoBt/DCC or dioxane/N-HONSu/DCC [17]. The derivative (XLIII) was prepared by coupling reaction with the amino acid by the DMF/HoBt/EDCI method in the presence of Et3N [18]. In conclusion, in the synthesis of amine derivatives of glycyrrhizic acid, glycyrrhizic acid can react with different amino acid types; the reaction was performed using different types of solvents, such as DMF, dioxane, and THF, as well as one type of coupling additive (HoBt, HoSu). In some methods, coupling reagents (DCC, EDCI) are also used in this reaction. Moreover, bases such as (N,N'-diisopropylethylamine (DIPEA), Triethylamine) have been used.
Fig. 1.
The amino derivatives of glycyrrhizic acid.
Fig. 2.
Reagents and conditions used in the synthesis of I–VI derivatives: (a) THF, HoSu, DCC; (b) 3 h at 0°C, 12 h in the refrigerator; (c) Et3N and amino acid, 24 h at rt.
Fig. 3.
Reagents and conditions used in the synthesis of VII–XII derivatives: (a) THF, HoSu, DCC, 2 h at 0°C; (b) Et3N and amino acid, 1 h at 0°C, 20 h at rt.
Fig. 4.
Reagents and conditions used in the synthesis of XXI–XXIII derivatives: (a) dioxane, pyridine, HoBt, DCC, 1 h at 0°C, 6 h at rt; (b) amino acid, N-ethylmorphaline, 12 h at rt.
Fig. 5.
Reagents and conditions used in the synthesis of XXXIV–XXXVI derivatives: (a) dioxane/HoBt/DCC or dioxane/HoSu/DCC; (b) (Lys-(Z) OMe. CF3COOH, DMF, Et3N, 24 h, rt.
2 METHODS OF SYNTHESIS OF GLYCYRRHETINIC ACID (GA) DERIVATIVES
Glycyrrhetinic acid is a hydrolytic triterpenoid product of the glycyrrhizic acid with a chemical structure as in Fig. 6. Four significant locations in the structure of GA have attracted scientists to study the chemical modification at these sites to make several derivatives using different methods discussed in this section. Glycyrrhetinic acid derivatives are more commonly synthesized by modifying the hydroxyl group at position 3 of the molecule, reducing the compound’s polarity and improving its pharmacologically and biologically effectiveness [19, 20]. Also, many derivatives were prepared by modification at position C-11 or by esterification at C-30 in the presence of DCC, HoBt, DIPEA [21, 22]. The modifications at the C3-OH group of 18β-glycyrrhetinic acid are identified to be relatively common and effective. The modification of the C3-OH group, altering the molecular polarity of 18β-glycyrrhetinic acid, may be an advantage in achieving better cytotoxicity or antiproliferative activity. For instance, the hydroxy group can be converted into an oxime, acyloxyimino, alkoxyimino, alkoxy [23]. As a proteasome inhibitor, glycyrrhetinic acid 3‑O-isophthalate, one derivative of the GA suppresses the chymotrypsin-like activity of the proteasome in MT4 cells with an IC50 of 0.22 μM, nearly 100-fold more potent than 18β-glycyrrhetinic acid [24]. Also, there are many derivatives of GA that have a strong action against arbovirus (Zika virus); one of those derivatives is 3-O-acetyl-30-aminopyridine GA, which was prepared by the following method: A heterocyclic amine 1.5 mmol was added to a solution of 3‑acetoxy-GA chloride 1 mmol in methylene chloride 20 mL and stirred for 8 hours at room temperature with TLC monitoring. The reaction mixture was diluted with methylene chloride 20 mL and rinsed with 5% aqueous NaHCO3 solution, saturated NaCl solution, water, and MgSO4 before drying. The crude product was purified using benzene as the eluent in column chromatography and recrystallized from aqueous ethanol [25]. There are a lot of articles about the ways of preparing the derivatives of GA. We will not discuss these methods, but we will mention the activity of these derivatives in the next chapter.
Fig. 6.
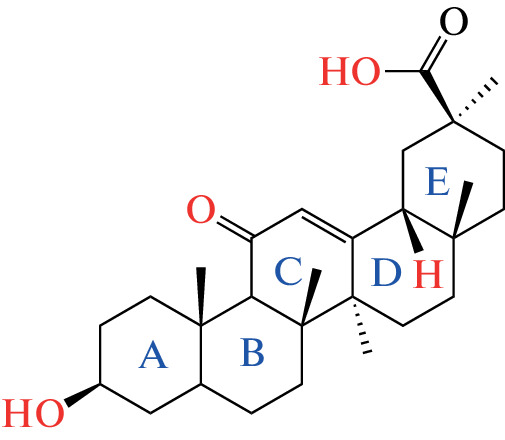
Structure of GA with the main position of synthesis modification.
3 THE PHARMACOLOGICAL AND BIOLOGICAL ACTIVITY OF GLYCYRRHIZIC ACID AND GLYCYRRHITIC ACID DERIVATIVES
Many derivatives of glycyrrhizic acid and glycyrrhetinic acid have a broad pharmacological spectrum because these derivatives have been used as anti-coronavirus, anti-influenza, hepatoprotective, and anti-bacterial agents.
3.1 Anti-Coronavirus Activity
A virus is a small infectious agent that contains only one type of nucleic acid; the virus only replicates inside the organism’s living cells; it can infect all types of living cells. Since the first anti-viral medication, idoxuridine, was authorized in 1963, 90 anti-viral medicines have been officially approved to treat viral diseases. Besides these drugs, traditional Chinese medicine has also been used as an anti-viral medication [26]. Glycyrrhizic acid and its amino acid derivatives prepared by coupling reaction have been used against severe acute respiratory syndrome (SARS) coronavirus in vitro using vero cell models. Glycyrrhizic acid at (365 μM) concentration can inhibit 50% of SARS-CoV growth; moreover, the derivatives compounds of glycyrrhizic acid showed more potent inhibition activity than GL. The anti-viral activity occurs for several reasons, such as the free 30-COOH in some derivatives, which is essential for activity, and the increasing hydrophilic properties of these derivatives by conjugation in the carbohydrate part. These characteristics let compounds easily interfere with the S-protein, which is responsible for entering the virus into the host cells; this interaction will reduce the activity of viruses [6, 27]. Also, by influencing SARS-coronavirus replication, glycyrrhizic acid showed great activity against SARS in Vero cells. There was a complete replication inhibition occurs at a 4000 mg/L concentration [28]. Because of its ability to interact with angiotensin-converting enzyme 2 (ACE2), glycyrrhizic acid has been employed as an anti-coronaviruses; this competition in the contact between GL and the virus leads to decreased viral activity [29]. Glycyrrhizin has a significant inhibitory effect on SARS-CoV replication and affects virus adsorption and membrane penetration in the initial phase of virus replication. To establish this effect, researchers compared the anti-viral efficacy of a variety of medications, including ribavirin, pyrazofuran, 6-azaguanosine mycophenolate acetate, and glycyrrhizin, in two patients who had been exposed to the SARS virus. The half-maximal effective concentration (EC50) for glycyrrhizin was 300–600 μg/mL, and the 50% cytotoxic concentration (CC50) was >20 000 μg/mL; this makes it a special medication for the treatment of coronavirus disease 2019 (COVID-19) [30]. Glycyrrhizin can be used as a combination therapy in the treatment of COVID-19 with chloroquine and tenofovir because it shows a critical role in controlling high mobility group box 1 (HMGB1) expression and decreases HMGB1 levels in the serum [31]. A combination of glycyrrhizic acid and vitamin C can also be used to treat COVID-19 by raising immunity, suppressing inflammatory stress and activating the signaling pathway of the T cell receptor, resulting in control of Fc gamma R-mediated phagocytosis [32]. According to the in vitro study and the molecular docking results, glycyrrhetinic acid is one of the promising compounds which can be used against the S and Mpro receptor pockets of SARS-CoV-2 [33]. Diammonium glycyrrhizinate is also one of the promising derivatives that can be used with vitamin C as an alternative drug to relieve the symptoms of COVID-19 [34]. At high concentrations, glycyrrhizin reduced SARS-CoV-2 replication in Vero E6 cells without causing cytotoxicity; this action occurs via stopping virus replication [35]. Furthermore, glycyrrhizin was reported to suppress the early stages of the virus’s reproduction cycle [36].
3.2 Anti-Influenza Activity
Influenza is a pandemic disease that can affect the human body. Influenza viruses can be divided into four classes depending on the different antigens determined by the matrix protein and nucleoprotein. The influenza virus A infects mucosal epithelial cells in the host respiratory tract and causes acute respiratory disease. After binding to isolated receptors on the host cell surface, influenza virus A is absorbed into the endosomal compartment; the acidic endosome environment also causes a conformation shift in influenza virus A, allowing the virus to combine with the endosome membrane and penetrate into the cytoplasm [37]. GA also has a defensive effect against influenza viruses by stimulating the development of interferon-gamma (IFN-gamma) by T cells [38]. The synthesis of glycyrrhizic acid derivatives by conjugating glycyrrhizic acid with ethyl and methyl esters of D-amino acid plays a principal role in treating influenza viruses depending on the ester group and chemical structure of the amino acid residue. The anti-influenza action of these derivatives was determined by using several types of cell lines, such as Madin–Darby canine kidney (MDCK) cells or MA-104 cells [39]. The conjugation of glycyrrhizic acid with many types of amino acids or the glycyrrhizic methyl ester can modify the function of glycyrrhizic acid against influenza viruses in MDCK cell models. The cytotoxicity of the substance in MDCK cell models was determined using a cell viability test. Considering the pandemic (H1N1pdm09 influenza A virus), the introduction of phenylalanine or cysteine amino acid into the carbohydrate portion of glycyrrhizic acid was found to be the most effective in terms of anti-viral activity when compared to the control group. This finding supports or is consistent with previously reported information about the glycyrrhizic acid derivative’s anti-viral activity, which also suggests that the presence of a free carboxylic functional group at position 30 is more important for the anti-viral efficacy of these derivatives in general [11]. Glycyrrhizin has been used in combination therapy with ribavirin to increase ribavirin’s anti-viral activity by significantly lowering the development of pro-inflammatory cytokine IL-6 (P) lung virus titer (P) and reducing ribavirin’s side effects. According to the findings, using these two drugs together has a synergistic effect on the survival of diseased mice [40]. Also, the combination of glycyrrhizic acid and dipeptide (glutamyl-tryptophan) has shown positive effects against the influenza virus in an animal model; the result showed an increase in the titer of interferon, which plays an important role in protecting the body against the virus. Additionally, the study showed an improvement in the lung tissue in the infected mice after they were given this combination treatment [41]. The approved parenteral glycyrrhizin preparation can also play a critical role in treating H5N1 of the influenza virus via reducing virus multiplication and pro-inflammatory gene expression in cell models [42]. Glycyrrhizin showed effectiveness in protecting cells from being infected by the influenza virus via the interaction between this compound and the cell membrane, which led to reduced endocytotic activity that inhibits the virus uptake into the cells [43]. Moreover, the thiol derivatives of GA have essential activity against influenza viruses. That action was determined in the MDCK cells infected by influenza A H3N2 virus [44].
3.3 Anti-HIV Activity
The modification of the structure of glycyrrhizic acid by synthesizing many derivatives that have activity against HIV was determined by using the MT-4 infected cell mod [45]. Glycyrrhizin also has the potential to inhibit HIV replication in vitro; that activity occurs due to the ability of glycyrrhizin to induce the production of beta-chemokines [46]. New 18 glycyrrhizic acid conjugates containing two L-aspartic acid or methyl esters in the carbohydrate portion were found to have activity against HIV via the minimization of the DNA polymerase enzyme of the virus reverse transcriptase (RT) activity, which is responsible for transcribed RNA to DNA and inhibition of virus antigen accumulation [15]. Moreover, there are also other derivatives of glycyrrhizic acid that are prepared by the conjugation of glycyrrhizic acid with many types of L-amino acids; these derivatives were found to increase the amount of agglutinins and hemolysins in the blood of mice. These derivatives also have anti-HIV activity in the cultures of MT-4 cells model [47]. Also, the synthesis of S-benzyl-L-cysteine derivative of glycyrrhizic acid by using EDCI, which improves this conjugation, has been found to play a critical role in treating HIV. The action of this compound against HIV was determined by using MT-4 cell culture, so the results show this compound inhibits the accumulation of specific virus protein in the MT-4 cell model [18]. The Penta-o-nicotinate of glycyrrhizic acid also has ani-HIV activity via suppressing the replication of HIV and inhibiting the reverse transcriptase (RT) enzyme. These results were determined in vitro using infected MT-4 cell culture [48]. Also, there are many derivatives of glycyrrhizic acid, as the carbocyclic, heteroaromatic amine and glucosamines derivatives of GL have activity against HIV [10, 49].
3.4 Anti-Hepatic Viruses Activity
Hepatitis B virus (HBV) and hepatitis C virus are the most serious viruses that can attack humans. Therefore, the researchers are particularly interested in hepatitis virus treatment; about 400 million individuals worldwide have an infection (HBV). Chronic hepatitis and progressive-phase liver diseases, like cirrhosis, fibrosis, and even hepatocellular carcinoma, may be caused by HBV infection; HBV treatment has several guidelines. The use of glycyrrhizic acid and its derivatives has promising roles in this field. Glycyrrhizic acid, or glycyrretinic acid, and its derivatives are considered to be some of the most promising compounds that play an essential role as hepatoprotective agents in several ways. According to the previous studies, we are going to summarize the hepatoprotective activity of these products via one of the following mechanisms:
3.4.1. Suppression and inhibition of liver fibrosis. Liver fibrosis refers to the exaggerated collection of extracellular matrix proteins present in the most advanced stage of chronic liver diseases. Cirrhosis, portal hypertension, liver failure are all the symptoms of advanced liver fibrosis, so this problem remains one of the most critical problems facing scientists and researchers today, which often requires liver transplantation. Glycyrrhizic acid and its derivatives showed inhibition of collagen type I alpha 2 (COL1A2) gene promoter and progression of liver fibrosis in the hepatic stellate cell model induced via CCl 4 injection; the inhibition activity of these compounds arises due to inhibiting the accumulation of Smad3 gene [50]. The combination between glycyrrhizic acid and aspartate aminotransferase (AST ) significantly decreased Smad3, mRNA expression level, as well as p-Smad2/3, Smad 3, and TGF-1 protein levels in the cells due to the inhibition action of this combination on the Smads/TGF-β1 singling pathway. These results were determined by using the stimulating fibrosis rats model [51]. The most important feature of liver fibrosis is inflammation, characterized by an aggregation of inflammatory cells in the malicious position. Due to the ability of glycyrrhizic acid to act as an anti-inflammatory agent, it can be used in liver fibrosis to inhibit disease progression [52, 53]. 18α-GL also plays a critical role in treating liver fibrosis by blocking the transportation of NF-κB into the nucleus, which is responsible for several liver diseases via the nuclear factor kappa B (NF- κB )pathway [54]. Moreover, glycyrrhizin showed reducing in the degree of liver damage via reducing the levels of the oxidative marker stress (MDA) and reactive oxygen radicals (ROS), Fe2+, which is an indicator of liver damage. Furthermore, glycyrrhizin also showed a relative increase in the level of glutathione (GSH), which has an important role in the regulation of cellular metabolic function and acts as an anti-oxidant defence agent [55]. Glycyrrhizin has also been used as a treatment for systemic sclerosis; this action was determined by examining the activity of glycyrrhizin in the animal model; the glycyrrhizin inhibits fibroblast activation and suppresses the activation of dermal fibroblasts induced by TGF-beta [56]. Additionally, glycyrrhizin plays an important role in CD4+T cell responses in liver fibrogenesis by inhibiting the infiltration of T helper (Th) cell type 1, Th2, Th17, and regulatory T cells, as well as by regulating the Th1/Th2 and Treg/Th17 balances to a relative dominance of Th1 and Treg lineages in livers [57].
3.4.2. Anti-viral effects. The liver can be affected by many types of viruses as a part of generalized host infection, such as hepatitis A, hepatitis C, hepatitis B, and hepatitis E viruses, which lead to liver damage [58]. Glycyrrhiza species are used as anti-viral agents because of its anti-viral activities that can occur via different mechanisms, such as reducing virus transport to the membrane and sialylation of the hepatitis B virus surface antigen, suppression of fusion of the HIV-1 viral membrane with the cell due to reduced membrane fluidity, activation of interferon-gamma in T-cells [59]. Glycyrrhizin can be used as an anti-viral agent against hepatitis C virus because it inhibits HCV full-length viral particles and has a synergistic effect with interferon, or it contributes to reducing the total bilirubin and AST and ALT levels two weeks later after IV treatment with glycyrrhizin [60, 61]. Combination therapy between kurarinol and diammonium glycyrrhizinate may further increase the amount of HBV-specific CD4+ and CTL, Th1 in chronic hepatitis B (CHB) patients, because CD4 is a master regulator of the adaptive immune response to HBV, cytotoxic T lymphocytes may also be involved in the immune clearance of HBV-infected cells and the pathogenesis of hepatitis B hepatocellular injury [62]. Glycyrrhizic Acid and its derivatives have also been used to treat hepatitis B by affecting the secretion of hepatitis B surface antigen (HBsAg) and transport through the Golgi region inside the cell [63]. Also, glycyrrhizin enhances the immune system of hepatitis B patients by eliminating sialic acid from the surface of HBsAg, leading to an improvement in hepatitis A surface antigen (HAsAg) antigenicity [64]. Moreover, the Anurag Tandon study investigates that GA can improve the anti-HBV effect of drugs such as lamivudine [65]. GL also inhibits the seroprevalence of Kaposi’s sarcoma-associated replication of herpesvirus (KSHV) in patients with chronic hepatitis B [66]. GL can also be combined with ursodeoxycholic acid to significantly decrease hepatitis C virus (HCV) patients’ ALT, gamma-glutamyl transferase (GGT), and AST; this action was determined by the clinical trial study in 170 patients. This combination therapy can also be used as an alternative to INF for patients who fail to respond to INF [67]. Glycyrrhizin has assured that combination therapy with ribavirin plays an essential role in the pharmacokinetic parameters of ribavirin by modifying the systemic availability of ribavirin and its main metabolite so that it can be used in the management of chronic hepatitis C [68].
3.4.3. Anti-inflammatory effect. Inflammation in the liver occurs when the liver cells are attacked by a disease-causing microbe or toxin or when the liver cells are attacked by an autoimmune disorder, which leads to activates pro-inflammatory cytokines interleukin-1β and IL-18 [69]. Glycyrrhizin certainly suppresses the outer membrane protein of E.coli, which stimulates the release of inflammatory cytokines; this action leads to a reduction of the following cytokines: TNF-α, IL-1β, NO, and induce prostaglandin E2 (PGE2) production [70]. Also, glycyrrhizic acid has been shown to inhibit the production of inflammatory mediators such as IFN-, HMGB1, TNF-, IL-6, and IL-17 [56]. When administered to an animal model of acute hepatitis, glycyrrhizin has a preventive and curative effect against LPS/D-GaIN-induced hepatotoxicity [71]. Glycyrrhizin can enhance the T helper lymphocyte proliferation and activity, promote lymphocytes cytokines IL-2, INF-γ, IL-1, inhibit IL-4, IL-10, IL-8, which plays a critical mechanistic role in many forms of liver injury; glycyrrhizin also has anti-inflammatory reactions by MAPK expression and the blockage of the p38 and JNK pathways [72–74]. Glycyrrhizin has been used as a glucocorticoid-like effect agent on TNF- and IL-1-induced IL-8 production via a mechanism distinct from glucocorticoids [75]. Glycyrrhizic acid nanoparticles can also be used as a novel product as anti-inflammatory agents because its activity to reduce the production of the pro-inflammatory cytokines [2]. Magnesium isoglycyrrhizinate is one of the derivatives of glycyrrhizic acid that has an anti-inflammatory effect; these effects occur due to the inhibition of the phospholipase A2/arachidonic acid pathway [76]. The lytic cycle is also commonly referred to as the “reproductive cycle” of the bacteriophage. The six stages of this cycle are attachment, penetration, transcription, biosynthesis, maturation, and lysis. When glycyrrhizin is administered to patients with viral hepatitis, it inhibits the lytic pathway of complement, which may explain its anti-inflammatory action on liver cells [77].
3.4.4. Inhibition of hepatic apoptosis and necrosis. Cell death in liver disease occurs mainly by apoptosis or necrosis, making apoptosis and necrosis the main challenges for researchers working in this field [78]. Two molecular pathways can trigger hepatocellular apoptosis: an extrinsic pathway mediated by death receptors on the cell surface and an intrinsic pathway, triggered at the mitochondrial level [79]. GL significantly decreases the number of TUNEL-labeled cells, the main indicator of identifying and quantifying apoptotic cells in acute hepatitis induced with LPS/ D-GalN-treatment [80]. Glycyrrhizin inhibits LPS/ D-galactosamine-induced liver injury by inhibiting IL-18 production and inflammatory responses [81]. GL decreases the number of apoptotic hepatocytes labeled with the TUNEL-method; also, there are significantly down-regulated serum levels of ALT, AST, and HMGB1 [82]. Matrix metalloproteinase (MMP-9) plays a role in developing lipopolysaccharide (LPS)/D-galactosamine (GalN)-induced mouse liver injury. The use of glycyrrhizin leads to the down-regulation of MMP-9 in the mice model [83]. GL also inhibits hepatocyte apoptosis via interference with TNFα-induced apoptotic hepatocyte death [84]. Glycyrrhizin has also been used to down-regulae the expression of caspase-3 and inhibit the release of cytochrome C from mitochondria into the cytoplasm, so it is used to suppress hepatocyte apoptosis [85]. GL exhibits pro-apoptotic properties by reducing glycochenodeoxycholic acid (GCDC)-dependent reactive oxygen species generation. In contrast, GA is a potent inhibitor of bile acid-induced apoptosis and necrosis in a manner consistent with its antioxidative effect [86]. Fas ligand is a type II membrane protein, which is a significant inducer of apoptosis; glycyrrhizin also acts as anti -apoptosis by inhibiting anti-fas antibody-induced hepatitis by working upstream of CPP32-like protease activation [87]. Additionally, glycyrrhizin has been used as an anti-apoptosis agent because it inhibits JNK1/2 and p38 MAPK phosphorylation and inactivates the CHOP protein, reducing endoplasmic reticulum stress [88]. GA can inhibit CCl4-induced hepatocyte apoptosis via a p53-depend mitochondrial pathway to retard the progress of liver fibrosis in rats [89]. Glycyrrhizin inhibits liver I/R, which promotes GSDMD-mediated pyroptotic cell death of Kupffer cells [90]. From the previous studies, we can summarize the effectiveness of the GA, and it is derivatives against several types of viruses in Table 1.
Table 1.
The mechanism of anti-viral action of some glycyrrhizic acid, glycyrrhetinic acid, and their derivatives
| Component | Mechanism of anti-viral action | Viral type |
|---|---|---|
| Glycyrrhizic Acid (GA) | Affects the extracellular secretion of HBsAg (hepatitis B surface antigen), which increases liver dysfunction in patients with chronic hepatitis B | HBV |
| Glycyrrhizin (GL) | Inhibits the secretion of HBsAg by inhibiting the transport of HBsAg within the cell through the Golgi region | HBV |
| Glycyrrhizin (GL) |
Removes sialic acid from the surface of HBsAg, which leads to an increase in the antigenicity of the HBsAg. Improves the anti-HBV influence of other drugs such as Lamivudine and ETV |
HBV |
|
Glycyrrhizic acid (GA) derivatives |
Potentiate γ-interferon production in vitro and in vivo | DENV and yellow fever viruses |
| Glycyrrhizin (GL) |
Stimulation of the development of IFN-gamma by T cells. Reduction of the endocytotic and virus uptake |
Influenza virus |
| Glycyrrhetinic acid derivatives | Inhibits VZV (varicella-zoster virus) replication in an initial cycle stage of replication | (HSV-1) |
| Glycyrrhizin (GL) | Improves the action of famciclovir in recurrent genital herpes | Genital herpes virus |
| Glycyrrhizic acid (GA) |
Affects the stimulation of β-chemokine development by competing with chemokine receptor-mediated cellular HIV infection. Affects a variety of signaling pathways, including casein kinase II, protein kinase II, and transcription factors |
HIV |
| Glycyrrhizin (GL) | Inhibits viral particles in full-length HCV and core gene expression of HCV | HCV |
3.5 Antibacterial Effects of Glycyrrhizic Acid, Glycyrrhetinic Acid, and Their Derivatives
Bacterial resistance to many drugs is the biggest challenge in treating bacterial diseases, so understanding the cellular structure of bacteria and knowing the mechanism of drug resistance has opened the way for researchers to treat many types of bacteria. Moreover, glycyrrhizic acid and its derivatives play an important role in treating various kinds of microorganisms, such as Staphylococcus aureus, Bacillus subtilis, Candida albicans, Pseudomonas aeruginosa, Escherichia coli [91]. Glycyrrhizic acid can prevent the growth and acid production of Streptococcus mutans; this action was determined in vitro by detecting the bacterial suspension optical absorption spectrum and the medium PH value during cultivation at definite times [92, 93]. Glycyrrhizic acid also inhibits the growth of Pseudomonas aeruginosa bacteria because of its activity on the permeability of the cell membrane, biofilm formation, and efflux activity. Moreover, glycyrrhizic acid has shown a minimum bactericidal concentration (MBC) and a minimum inhibitory concentration (MIC) of 400 and 100 µg mL–1, respectively. Additionally, GL decreases multidrug resistance by altering bacterial parameters, including viability and efflux pump activity. In vivo, it increases the effectiveness of ciprofloxacin, reducing ocular disease, platelet count, and myeloperoxidase activity [94, 95]. There were significant positive results for glycyrrhetinic acid and its derivatives as active compounds that play a vital role in the anti-bacterial activities against various Staphylococcus aureus strains, including 18 methicillin-resistant strains, by the inhibition of several pathways involved in amino acid and carbohydrate metabolism [96]. Also, there was a synergism effect between glycyrrhizic acid and gentamicin, which led to a reduction in bacterial resistance to gentamicin. This action was observed in vitro because the MIC of gentamicin was reduced in the presence of glycyrrhizic acid [97]. Additionally, 18β-glycyrrhetinic acid can be used to enhance the activity of aminoglycoside antibiotics against some types of bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), by using checkerboard assays. In vitro airway MRSA infection models are also used to determine the effect of 18β-GA on the MIC of different antibiotics against MRSA [98]. Disodium succinyl glycyrrhizinate, a glycyrrhetinic acid derivative, has anti-bacterial activity against streptococcus mutans strains by inhibiting sugar uptake and metabolism, causing the inhibition of streptococcus mutans growth, or by inhibiting acid manufacture in a dosedependent manner. According to these results, disodium succinyl glycyrrhizinate has an action against tooth decay and periodontitis [99]. Glycyrrhizic acid also plays an essential role as an anti-helicobacter pylori by inhibition of arylamine N-acetyltransferase activity, which leads to inhibition of the growth of H. pylori [100]. The hydroxylated derivatives of glycyrrhetinic acid have considerable activity against drug-resistant Enterococcus facialis [101]. 18β-Glycyrrhetinic acid or its derivatives also have potent synergism action against mycobacterium bovis when used with the first-line Mycobacterium bovis drugs [102]. Furthermore, the essential oil of Glycyrrhiza glabra leaves displays anti-microbial activities against Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, and Staphylococcus aureus [103]. The anti-microbial activity of Glycyrrhiza glabra root extract against gram-positive and gram-negative bacteria, such as Salmonella enteritidis, Escherichia coli, Bacillus cereus, and Staphylococcus aureus, was estimated by using a microdilution technique enzyme-linked immunosorbent assay (ELISA) [104]. A flavonoid-rich extract of Glycyrrhiza glabra (GutGards) has anti-bacterial action against Helicobacter pylori by inhibiting protein synthesis, DNA gyrase, and dihydrofolate reductase [105]. Also, there are many derivatives of 18β glycyrrhetinic acid that have inhibitory activity against some gram-positive and gram-negative bacteria such as Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Staphylococcus typhimurium [106]. From previous studies, we can summarize the effectiveness of glycyrrhizic acid, glycyrrhetinic acid, and their derivatives against several types of bacteria in Table 2.
Table 2.
The mechanism of anti-microbial action of some glycyrrhizic acid, glycyrrhetinic acid, and their derivatives
| Component | Mechanism of anti-microbial action | Microbial type |
|---|---|---|
|
Glycyrrhetinic acid, and its derivatives as GR-K and GR-S |
Several carbohydrates and amino acid metabolism pathways are inhibited | S. aureus |
| Glycyrrhizic acid | Improves the action gentamicin against | Enterococcus strains |
| Glycyrrhizic acid | Inhibits the membrane efflux activity and alteration of the membrane permeability and biofilm | Pseudomonas aeruginosa |
| 18β-Glycyrrhetinic acid | Enhances of the activity of aminoglycosides antibiotics | MRSA |
| Disodium succinyl glycyrrhizinate | Inhibits the sugar uptake and metabolism, causing the inhibition of S. mutans growth | S. mutans strains |
| Glycyrrhizic acid | Inhibits the arylamine NAT activity which leads to inhibition of growth of H. pylori | Helicobacter pylori |
| 18β-Glycyrrhetinic acid | Improves the action of the first-line drugs, which can be used against Mycobacterium bovis | Mycobacterium bovis |
| Flavonoid of Glycyrrhiza glabra (GutGards) | Inhibits the protein synthesis, DNA gyrase, and dihydrofolate reductase | Helicobacter pylori |
CONCLUSIONS
Glycyrrhizic acid and glycyrrhetinic acid and their derivatives are the most important compounds that have been synthesized by different chemical reactions for several pharmacological applications. This review summarizes the various synthesis methods of the glycyrrhizic acid derivatives and the main methods for synthesising glycyrrhetinic acid derivatives. Moreover, this review summarizes the pharmacological activity of those derivatives; according to the literature reports, those derivatives have antiviral activity against many types of viruses, such as coronaviruses, influenza viruses, HIV, and hepatic viruses. The antiviral activity of those derivatives occurs by different mechanisms according to the kind of virus or the chemical structure of the derivatives. Also, those derivatives have anti-inflammatory activity, hepatoprotective activity, and antibacterial activity against different bacterial organisms. This review summarizes different clinical studies that support the pharmacological activity of those compounds.
Supplementary Information
Conflict of Interest
The authors declare that they have no conflicts of interest.
COMPLIANCE WITH EHICAL STANDARDS
This article does not contain any studies involving human participants performed by any authors and does not contain any studies involving animals performed by any of these authors.
Footnotes
Abbreviations: GL, glycyrrhizic acid; NF-kB, nuclear factor-kB; PI3K, phosphoinositide 3-kinase; GA,glycyrrhetinic Acid; DCC, N,N'-dicyclohexylcarbodiimide; THF, tetrahydrofuran; Et3N, triethylamine; HoSu, N-hydroxysuccinimide; DMF, dimethylformamide; EDCI, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; HoPt, N-hydroxyphthalimide; HoBt, hydroxybenzotriazole; DIPEA, N,N-diisopropylethylamine; SARS, severe acute respiratory syndrome; ACE2, angiotensin-converting enzyme 2; EC50, half maximal effective concentration; CC50, the 50% cytotoxic concentration; COVID-19, coronavirus disease 2019; HMGB1, high mobility group box 1; INF-γ, interferon-gamma; MDCK, Madin–Darby canine kidney; HIV, human immunodeficiency virus; DNA, deoxyribonucleic acid; RT, reverse transcriptase; RNA, ribonucleic acid; HBV, hepatitis B virus; AST, aspartate aminotransferase; NF-κB, nuclear factor kappa B; GSH, glutathione; ALT, alanine aminotransferase; IL, interleukin; PGE2, prostaglandin E2; CHB, chronic hepatitis B; HBsAg, hepatitis B surface antigen; KSHV, Kaposi’s sarcoma herpesvirus; HCV, hepatitis C virus; GGT, gamma-glutamyl transferase; MMP-9, matrix metalloproteinase; GCDC, glycochenodeoxycholic acid; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; ELISA, enzyme-linked immunosorbent assay; MRSA, methicillin-resistant Staphylococcus aureus.
REFERENCES
- 1.Polyakov N., Leshina T. Open Conf. Proc. J. 2011;2:64–72. doi: 10.2174/2210289201102010064. [DOI] [Google Scholar]
- 2.Zhao Z., Xiao Y., Xu L., Liu Y., Jiang G., Wang W., Li B., Zhu T., Tan Q., Tang L., Zhou H., Huang X., Shan H. ACS Appl. Mat. Int. 2021;13:20995–21006. doi: 10.1021/acsami.1c02755. [DOI] [PubMed] [Google Scholar]
- 3.Wang C.-Y., Kao T.-C., Lo W.-H., Yen G.-C. J. Agricult. Food Chem. 2011;59:7726–7733. doi: 10.1021/jf2013265. [DOI] [PubMed] [Google Scholar]
- 4.Kowalska A., Kalinowska-Lis U. Int. J. Cosmetic Sci. 2019;41:325–331. doi: 10.1111/ics.12548. [DOI] [PubMed] [Google Scholar]
- 5.Kondratenko R.M., Baltina L.A., Vasil’eva E.V., Nasyrov Kh.M., Kireeva R.M., Baschenko N.Zh., Fridman S.M., Baltina L.A., Tolstikov G. Russ. J. Bioorg. Chem. 2004;30:148–153. doi: 10.1023/b:rubi.0000023100.52170.6b. [DOI] [PubMed] [Google Scholar]
- 6.Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., Doerr H.W., Cinatl J. J. Med. Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 7.Kondratenko R., Baltina L., Vasil’eva E., Ismagilova A., Nasyrov K. M., Baschenko N. Z., Kireeva R., Fridman S., Tolstikov G. Russ. J. Bioorg. Chem. 2004;30:53–59. doi: 10.1023/B:RUBI.0000015774.09619.80. [DOI] [PubMed] [Google Scholar]
- 8.Baltina L.A., Kondratenko R.M., Baltina L.A., Plyasunova O.A., Pokrovskii A.G., Tolstikov G.A. Pharm. Chem. J. 2009;43:539–548. doi: 10.1007/s11094-010-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baltina L., Vasil’eva E., Davydova V., Ismagilova A., Zarudii F., Tolstikov G. Pharm. Chem. J. 1996;30:503–506. doi: 10.1007/BF02334634. [DOI] [Google Scholar]
- 10.Baltina L.A., Kondratenko R., Baltina L.A., Plyasunova O.A., Pokrovskii A., Tolstikov G. Pharm. Chem. J. 2009;43:539–548. doi: 10.1007/s11094-010-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baltina L.A., Zarubaev V.V., Baltina L.A., Orshanskaya I.A., Fairushina A.I., Kiselev O.I., Yunusov M.S. Bioorg. Med. Chem. Lett. 2015;25:1742–1746. doi: 10.1016/j.bmcl.2015.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baltina L.A, Fairushina A.I., Eropkin M.Yu., Konovalova N.I, Petrova P.A., Eropkina E.M. Chem. Nat. Comp. 2017;53:1096–1100. doi: 10.1007/s10600-017-2209-7. [DOI] [Google Scholar]
- 13.Baltina L.A., Baltina L.A., Kondratenko R., Petrova S. Chem. Nat. Comp. 2020;56:569–571. doi: 10.1007/s10600-020-03095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baltina L.A., Fairushina A., Baltina L.A. Russ. J. Gen. Chem. 2015;85:2735–2738. doi: 10.1134/S1070363215120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baltina L.A., Chistoedova E.S., Baltina L.A., Kondratenko R.M., Plyasunova O.A. Chem. Nat. Comp. 2012;48:262–266. doi: 10.1007/s10600-012-0217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baltina L.A., Kondratenko R.M., Baltina L.A., Plyasunova O.A., Galin F.Z., Tolstikov G.A. Chem. Nat. Comp. 2006;42:543–548. doi: 10.1007/s10600-006-0210-7. [DOI] [Google Scholar]
- 17.Fayrushina A.I., Baltina L.A., Baltina L.A., Konovalova N.I., Petrova P.A., Eropkin M.Yu. Russ. J. Bioorg. Chem. 2017;43:456–462. doi: 10.1134/S1068162017040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baltina L.A., Kondratenko R.M., Baltina L.A., Plyasunova O.A. Pharm. Chem. J. 2021;55:224–227. doi: 10.1007/s11094-021-02402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csuk R., Schwarz S., Siewert B., Kluge R., Ströhl D. Eur. J. Med. Chem. 2011;46:5356–5369. doi: 10.1016/j.ejmech.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q., Mohammed E.A.H., Wang Y., Bai Z., Zhao Q., He D., Wang Z. Bioorg. Chem. 2020;99:103804. doi: 10.1016/j.bioorg.2020.103804. [DOI] [PubMed] [Google Scholar]
- 21.Lallemand B., Chaix F., Bury M., Bruyère C., Ghostin J., Becker J.P., Delporte C., Gelbcke M., Mathieu V., Dubois J., Prévost M., Jabin I., Kiss R. J. Med. Chem. 2011;54:6501–6513. doi: 10.1021/jm200285z. [DOI] [PubMed] [Google Scholar]
- 22.Alho D.P.S., Salvador J.A.R., Cascante M., Marin S. Molecules. 2019;24:2938. doi: 10.3390/molecules24162938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai D., Zhang Z., Meng Y., Zhu K., Chen L., Yu C., Yu C., Fu Z., Yang D., Gong Y. Beilstein J. Org. Chem. 2020;16:798–808. doi: 10.3762/bjoc.16.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L., Yu D., Ho P., Qian K., Lee K.-H., Chen C.-H. Bioorg. Med. Chem. 2008;16:6696–6701. doi: 10.1016/j.bmc.2008.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baltina L.A., Lai H.-C., Liu Y.-C., Huang S.-H., Hour M.-J., Baltina L.A., Nugumanov T.R., Borisevich S.S., Khalilov L.M., Petrova S.F. Bioorg. Med. Chem. 2021;41:116204. doi: 10.1016/j.bmc.2021.116204. [DOI] [PubMed] [Google Scholar]
- 26.De Clercq E., Li G. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/cmr.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baltina L.A., Kondratenko R.M., Baltina L.A., Plyasunova O.A., Pokrovskii A.G., Tolstikov G.A. Pharm. Chem. J. 2009;43:539–548. doi: 10.1007/s11094-010-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H. Lancet. 2003;361:2045–2046. doi: 10.1016/s0140-6736(03)13615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L., Hu C., Hood M., Zhang X., Zhang L., Kan J., Du J. Nutrients. 2020;12:1193. doi: 10.3390/nu12041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X., Jiang Y., Zhao Y., Xi H., Liu C., Qu F., Feng X. Eur. J. Clinic. Microbiol. Infect. Dis. 2020;39:1209–1220. doi: 10.1007/s10096-020-03897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailly C., Vergoten G. Pharm. Therapeutics. 2020;214:107618. doi: 10.1016/j.pharmthera.2020.107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R., Wu K., Li Y., Liang X., Lai K. P., Chen J. Briefings Bioinf. 2021;22:1161–1174. doi: 10.1093/bib/bbaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elebeedy D., Elkhatib W.F., Kandeil A., Ghanem A., Kutkat O., Alnajjar R., Saleh M.A., Abd El Maksoud A.I., Badawy I., Al-Karmalawy A.A. RSC Adv. 2021;11:29267–29286. doi: 10.1039/D1RA05268C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding H., Deng W., Ding L., Ye X., Yin S., Huang W. J. Med. Virol. 2020;92:2200–2204. doi: 10.1002/jmv.26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gowda P., Patrick S., Joshi S.D., Kumawat R.K., Sen E. Cytokine. 2021;142:155496. doi: 10.1016/j.cyto.2021.155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F., Chan K.H., Jiang Y., Kao R.Y., Lu H.T., Fan K.W., Cheng V.C., Tsui W.H., Hung I.F., Lee T.S., Guan Y., Peiris J.S., Yuen K.Y. J. Clin. Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burster T., Giffon T., Dahl M.E., Björck P., Bogyo M., Weber E., Mahmood K., Lewis D.B., Mellins E.D. Int. Immunol. 2007;19:645–655. doi: 10.1093/intimm/dxm030. [DOI] [PubMed] [Google Scholar]
- 38.Utsunomiya T., Kobayashi M., Pollard R.B., Suzuki F. Antimicrob. Agents Chemother. 1997;41:551–556. doi: 10.1128/aac.41.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fayrushina A.I., Baltina L.A., Baltina L.A., Konovalova N.I., Petrova P.A, Eropkin M.Yu. Russ. J. Bioorg. Chem. 2017;43:456–462. doi: 10.1134/s1068162017040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X., Zhou H., Qi W., Ning Z., Ma Y., Li Y., Wang G., Chen J. Yao Xue Xue Bao. 2015;50:966–972. [PubMed] [Google Scholar]
- 41.Smirnov V.S., Garshinina A.V., Shtro A.A., Anikin V.B., Galochkina A.V., Beliavskaia S.V., Zarubaev V.V. Vopr. Virusol. 2014;59:31–38. [PubMed] [Google Scholar]
- 42.Michaelis M., Geiler J., Naczk P., Sithisarn P., Leutz A., Doerr H.W., Cinatl J. PLoS One. 2011;6:e19705. doi: 10.1371/journal.pone.0019705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolkerstorfer A., Kurz H., Bachhofner N., Szolar O.H. Antiviral Res. 2009;83:171–178. doi: 10.1016/j.antiviral.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanetty C., Wolkerstorfer A., Amer H., Hofinger A., Jordis U., Claßen-Houben D., Kosma P. Beilstein J. Org. Chem. 2012;8:705–711. doi: 10.3762/bjoc.8.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baltina L.A., Stolyarova O.V., Baltina L.A., Kondratenko R.M., Plyasunova O.A., Pokrovskii A.G. Pharm. Chem. J. 2010;44:299–302. doi: 10.1007/s11094-010-0454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki H., Takei M., Kobayashi M., Pollard R.B., Suzuki F. Pathobiol. 2002;70:229–236. doi: 10.1159/000069334. [DOI] [PubMed] [Google Scholar]
- 47.Baltina L.A., Kondratenko R.M., Baltina L.A., Baschenko N.Z., Pliasunova O.A. Russ. J. Bioorg. Chem. 2009;35:563–571. doi: 10.1134/s1068162009040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pliasunova O.A. Il’ina, T.V., Kiseleva, Ya.Yu., Fediuk, N.V., Baltina, L.A., Tolstikov, G.A., and Pokrovskiĭ, A.G. Vestn. Ross. Akad. Med. Nauk. 2004;11:42–46. [PubMed] [Google Scholar]
- 49.Kondratenko R.M., Baltina L.A., Mustafina S.R., Vasil’eva E.V., Pompei R., Deidda D., Plyasunova O.A., Pokrovskii A.G., Tolstikov G.A. Russ. J. Bioorg. Chem. 2004;30:275–282. doi: 10.1023/B:RUBI.0000030135.97089.37. [DOI] [PubMed] [Google Scholar]
- 50.Moro T., Shimoyama Y., Kushida M., Hong Y.Y., Nakao S., Higashiyama R., Sugioka Y., Inoue H., Okazaki I., Inagaki Y. Life Sci. 2008;83:531–539. doi: 10.1016/j.lfs.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Y., Tong X., Ren S., Wang X., Chen J., Mu Y., Sun M., Chen G., Zhang H., Liu P. J. Ethnopharm. 2016;190:83–90. doi: 10.1016/j.jep.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Ikeda K., Kawamura Y., Kobayashi M., Fukushima T., Sezaki H., Hosaka T., Akuta N., Saitoh S., Suzuki F., Suzuki Y., Arase Y., Kumada H. Oncology. 2014;86:295–302. doi: 10.1159/000357713. [DOI] [PubMed] [Google Scholar]
- 53.Duval F., Moreno-Cuevas J.E., González-Garza M.T., Maldonado-Bernal C., Cruz-Vega D.E. Int. J. Inflammat. 2015;2015:943497. doi: 10.1155/2015/943497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qu Y., Chen W.-H., Zong L., Xu M.-Y., Lu L.-G. Med. Sci. Monit. 2012;18:BR24. doi: 10.12659/msm.882196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y., Chen Q., Shi C., Jiao F., Gong Z. Mol. Med. Rep. 2019;20:4081–4090. doi: 10.3892/mmr.2019.10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamashita T., Asano Y., Taniguchi T., Nakamura K., Saigusa R., Miura S., Toyama T., Takahashi T., Ichimura Y., Yoshizaki A. J. Inv. Dermatol. 2017;137:631–640. doi: 10.1016/j.jid.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tu C.-t., Li J., Wang F.-p., Li L., Wang J.-y., Jiang W. Int. Immunopharm. 2012;14:410–421. doi: 10.1016/j.intimp.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Adams D.H., Hubscher S.G. American J. Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiore C., Eisenhut M., Krausse R., Ragazzi E., Pellati D., Armanini D., Bielenberg J. Phytother. Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashfaq U.A., Masoud M.S., Nawaz Z., Riazuddin S. J. Transl. Med. 2011;9:112. doi: 10.1186/1479-5876-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X., Sun R., Liu R. Pharm. Res. 2019;144:210–226. doi: 10.1016/j.phrs.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 62.Gu X.B., Yang X.J., Jiang X.H., Hua Z., Lu Z.H., Zhang B., Zhu Y.F., Wu H.Y., Jiang Y.M., Chen H.K., Pei H., Zhou Y.L. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2012;26:108–110. [PubMed] [Google Scholar]
- 63.Takahara T., Watanabe A., Shiraki K. J. Hepatol. 1994;21:601–609. doi: 10.1016/s0168-8278(94)80108-8. [DOI] [PubMed] [Google Scholar]
- 64.Sato H., Goto W., Yamamura J.-I., Kurokawa M., Kageyama S., Takahara T., Watanabe A., Shiraki K. Antivir. Res. 1996;30:171–177. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- 65.Tandon A., Tandon B., Bhujwala R. Hepatol. Res. 2001;20:1–8. doi: 10.1016/s1386-6346(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 66.Xie Y., Ruan B., Chen Y., Wu N., Hu M., Zhu B. J. Med. Virol. 2011;83:879–883. doi: 10.1002/jmv.22001. [DOI] [PubMed] [Google Scholar]
- 67.Tsubota A., Kumada H., Arase Y., Chayama K., Saitoh S., Ikeda K., Kobayashi M., Suzuki Y., Murashima N. Eur. J. Gastroenterol. Hepatol. 1999;11:1077–1083. doi: 10.1097/00042737-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Liao S., Jin X., Li J., Zhang T., Zhang W., Shi W., Fan S., Wang X., Wang J., Zhong B., Zhang Z. Phytother. Res. 2016;30:618–626. doi: 10.1002/ptr.5567. [DOI] [PubMed] [Google Scholar]
- 69.Del Campo J.A., Gallego P., Grande L. World J. Hepatol. 2018;10:1–7. doi: 10.4254/wjh.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X.-r., Hao H.-g., Chu L. Micr. Pathogen. 2017;109:110–113. doi: 10.1016/j.micpath.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 71.El-Tahawy, N.F., Ali, A.H., Saied, S.R., and Abdel-Wahab, Z., Egyptian J. Histol., 2011, vol. 34, pp. 518–527. 10.1097/EHX.0000399701.81302.e1
- 72.Liu, L.-P., Ren, C.-A., and Zhao, H.-Y., Chinese J. Exp. Tradit. Med. Formul., 2010, vol. 6. https://en.cnki. com.cn/Article_en/CJFDTotal-ZSFX201006089.htm
- 73.Cai X., Wang X., Li J., Chen S. Exp. Therap. Med. 2017;14:1219–1226. doi: 10.3892/etm.2017.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joshi-Barve S., Barve S.S., Butt W., Klein J., McClain C.J. Hepatology. 2003;38:1178–1187. doi: 10.1053/jhep.2003.50470. [DOI] [PubMed] [Google Scholar]
- 75.Takei H., Baba Y., Hisatsune A., Katsuki H., Miyata T., Yokomizo K., Isohama Y. J. Pharm. Sci. 2008;106:460–468. doi: 10.1254/jphs.fp0072378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie C., Li X., Wu J., Liang Z., Deng F., Xie W., Zhu M., Zhu J., Zhu W., Geng S., Zhong C. Inflammation. 2015;38:1639–1648. doi: 10.1007/s10753-015-0140-2. [DOI] [PubMed] [Google Scholar]
- 77.Fujisawa Y., Sakamoto M., Matsushita M., Fujita T., Nishioka K. Microbiol. Immunol. 2000;44:799–804. doi: 10.1111/j.1348-0421.2000.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 78.Guicciardi M.E., Malhi H., Mott J.L., Gores G.J. Comprehensive Physiol. 2013;3:977–1010. doi: 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sprick M.R., Walczak H. Biochim. Biophys. Acta Mol. Cell Res. 2004;1644:125–132. doi: 10.1016/j.bbamcr.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 80.Ikeda T., Abe K., Kuroda N., Kida Y., Inoue H., Wake K., Morito M., Sato T. Arch. Histol. Cytol. 2008;71:163–178. doi: 10.1679/aohc.71.163. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida T., Abe K., Ikeda T., Matsushita T., Wake K., Sato T., Sato T., Inoue H. Eur. J. Pharm. 2007;576:136–142. doi: 10.1016/j.ejphar.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 82.Kuroda N., Inoue K., Ikeda T., Hara Y., Wake K., Sato T. PLoS One. 2014;9:e92884. doi: 10.1371/journal.pone.0092884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abe K., Ikeda T., Wake K., Sato T., Sato T., Inoue H. J. Pharm. Pharmacol. 2008;60:91–97. doi: 10.1211/jpp.60.1.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan T., Wang H., Zhao M., Yagai T., Chai Y., Krausz K. W., Xie C., Cheng X., Zhang J., Che Y., Lo F., Wu Y., Brocker C.N., Gonzalez F.J., Wang G., Hao H. Drug Metabol. Dis. 2016;44:720–731. doi: 10.1124/dmd.116.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang B., Qiao H., Meng F., Sun X. Brazilian J. Med. Biol. Res. 2007;40:1637–1646. doi: 10.1590/s0100-879x2006005000173. [DOI] [PubMed] [Google Scholar]
- 86.Gumpricht E., Dahl R., Devereaux M.W., Sokol R.J. J. Biol. Chem. 2005;280:10556–10563. doi: 10.1074/jbc.m411673200. [DOI] [PubMed] [Google Scholar]
- 87.Okamoto T. Eur. J. Pharm. 2000;387:229–232. doi: 10.1016/s0014-2999(99)00807-9. [DOI] [PubMed] [Google Scholar]
- 88.Tsai J.-J., Kuo H.-C., Lee K.-F., Tsai T.-H. Int. J. Mol. Sci. 2013;14:12563–12580. doi: 10.3390/ijms140612563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo X.-L., Liang B., Wang X.-W., Fan F.-G., Jin J., Lan R., Yang J.-H., Wang X.-C., Jin L., Cao Q. World J. Gastroenterol. 2013;19:3781–3791. doi: 10.3748/wjg.v19.i24.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hua S., Ma M., Fei X., Zhang Y., Gong F., Fang M. Int. Immunopharm. 2019;68:145–155. doi: 10.1016/j.intimp.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 91.Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M.B.P. Phytother. Res. 2018;32:2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu, G., He, Y.-H., Zhang, F.-F., Kong, X.-L., Wen, Y.-L., Ma, Q.-R., Yang, Y.-M., and Wan, H.-C., Sichuan Da Xue Xue Bao Yi Xue Ban (J. Sichuan University Med. Sci. Ed.), 2010, vol. 41, pp. 634–637. [PubMed]
- 93.Lingnan Z., Yonghong H., Feifei Z., Tingyu T., Wei S., Huchun W. West China . J. Stomatol. 2012;30:594–597. doi: 10.3969/j.issn.1000-1182.2012.06.008. [DOI] [Google Scholar]
- 94.Chakotiya A.S., Tanwar A., Narula A., Sharma R.K. Microbial Pathogen. 2016;98:98–105. doi: 10.1016/j.micpath.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Hazlett L.D., Ekanayaka S.A., McClellan S.A., Francis R. Inv. Ophthalmol. Vis. Sci. 2019;60:2978–2989. doi: 10.1167/iovs.19-27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oyama K., Kawada-Matsuo M., Oogai Y., Hayashi T., Nakamura N., Komatsuzawa H. PLoS One. 2016;11:e0165831. doi: 10.1371/journal.pone.0165831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmidt S., Heymann K., Melzig M.F., Bereswill S., Heimesaat M.M. Planta Med. 2016;82:1540–1545. doi: 10.1055/s-0042-114781. [DOI] [PubMed] [Google Scholar]
- 98.de Breij A., Karnaoukh T.G., Schrumpf J., Hiemstra P.S., Nibbering P.H., van Dissel J.T., de Visser P.C. Eur. J. Clinical Microbiol. Infect. Dis. 2016;35:555–562. doi: 10.1007/s10096-015-2570-z. [DOI] [PubMed] [Google Scholar]
- 99.Yamashita T., Kawada-Matsuo M., Katsumata T., Watanabe A., Oogai Y., Nishitani Y., Miyawaki S., Komatsuzawa H. Microbiol. Immunol. 2019;63:251–260. doi: 10.1111/1348-0421.12717. [DOI] [PubMed] [Google Scholar]
- 100.Chung J.G. Drug Chem. Toxicol. 1998;21:355–370. doi: 10.3109/01480549809002210. [DOI] [PubMed] [Google Scholar]
- 101.Qin Y.-J., Feng B., Song X.-B., Zhou W.-B., Yu H.-S., Zhao L.-L., Yu L.Y. Chinese J. Nat. Med. 2010;8:373–381. doi: 10.1016/S1875-5364(10)60045-3. [DOI] [Google Scholar]
- 102.Zhou X., Zhao L., Liu X., Li X., Jia F., Zhang Y., Wang Y. Phytother. Res. 2012;26:253–258. doi: 10.1002/ptr.3536. [DOI] [PubMed] [Google Scholar]
- 103.Chouitah O., Meddah B., Aoues A., Sonnet P. J. Essential Oil Bearing Plants. 2011;14:284–288. doi: 10.1080/0972060X.2011.10643935. [DOI] [Google Scholar]
- 104.Karami Z., Mirzaei H., Emam-Djomeh Z., Mahoonak A.S., Khomeiri M. Int. Food Res. J. 2013;20:2951–2957. [Google Scholar]
- 105.Asha M.K., Debraj D., Edwin J.R., Srikanth H., Muruganantham N., Dethe S.M., Anirban B., Jaya B., Deepak M., Agarwal A. J. Ethnopharm. 2013;145:581–586. doi: 10.1016/j.jep.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 106.Yang Y., Zhu Q., Zhong Y., Cui X., Jiang Z., Wu P., Zheng X., Zhang K., Zhao S. Bioorg. Chem. 2020;101:103985. doi: 10.1016/j.bioorg.2020.103985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.