Abstract
The anaerobic bacterium Syntrophus aciditrophicus metabolized benzoate in pure culture in the absence of hydrogen-utilizing partners or terminal electron acceptors. The pure culture of S. aciditrophicus produced approximately 0.5 mol of cyclohexane carboxylate and 1.5 mol of acetate per mol of benzoate, while a coculture of S. aciditrophicus with the hydrogen-using methanogen Methanospirillum hungatei produced 3 mol of acetate and 0.75 mol of methane per mol of benzoate. The growth yield of the S. aciditrophicus pure culture was 6.9 g (dry weight) per mol of benzoate metabolized, whereas the growth yield of the S. aciditrophicus-M. hungatei coculture was 11.8 g (dry weight) per mol of benzoate. Cyclohexane carboxylate was metabolized by S. aciditrophicus only in a coculture with a hydrogen user and was not metabolized by S. aciditrophicus pure cultures. Cyclohex-1-ene carboxylate was incompletely degraded by S. aciditrophicus pure cultures until a free energy change (ΔG′) of −9.2 kJ/mol was reached (−4.7 kJ/mol for the hydrogen-producing reaction). Cyclohex-1-ene carboxylate, pimelate, and glutarate transiently accumulated at micromolar levels during growth of an S. aciditrophicus pure culture with benzoate. High hydrogen (10.1 kPa) and acetate (60 mM) levels inhibited benzoate metabolism by S. aciditrophicus pure cultures. These results suggest that benzoate fermentation by S. aciditrophicus in the absence of hydrogen users proceeds via a dismutation reaction in which the reducing equivalents produced during oxidation of one benzoate molecule to acetate and carbon dioxide are used to reduce another benzoate molecule to cyclohexane carboxylate, which is not metabolized further. Benzoate fermentation to acetate, CO2, and cyclohexane carboxylate is thermodynamically favorable and can proceed at free energy values more positive than −20 kJ/mol, the postulated minimum free energy value for substrate metabolism.
Anaerobic metabolism of benzoate to acetate, CO2, and hydrogen or formate in the absence of light or terminal electron acceptors is thermodynamically unfavorable. Degradation proceeds only if the concentration of hydrogen produced during benzoate oxidation is continuously maintained at a low level by a hydrogen-using microorganism (12, 19, 24, 26). This kind of mutual cooperation between two species to degrade a single substrate via interspecies hydrogen transfer is called syntrophism. In syntrophic cocultures, hydrogen-utilizing bacteria are needed to maintain low hydrogen levels and are not directly involved in the metabolism of the original substrate. Also, many syntrophic microorganisms can grow in the absence of hydrogen-utilizing partners on unsaturated substrate analogues by using dismutation reactions in which part of the original substrate is used as an electron acceptor (24).
The syntrophic benzoate degrader Syntrophus aciditrophicus metabolizes benzoate to acetate, hydrogen, and CO2 in cocultures with a hydrogen-using methanogen or a sulfate reducer. S. aciditrophicus can also grow in pure culture on crotonate (11, 12). Recent studies of S. aciditrophicus-Methanospirillum hungatei cocultures showed that cyclohexane carboxylate transiently accumulated at a level that was 18% of the original benzoate concentration (8). The amount of methane produced per mole of benzoate consumed by S. aciditrophicus-M. hungatei cocultures was less than the theoretically predicted ratio (0.75) during the initial stages of benzoate metabolism. These two observations led us to hypothesize that a portion of the electrons produced during benzoate oxidation to acetate and CO2 is used to reduce benzoate to cyclohexane carboxylate (8). Benzoate reduction to cyclohexane carboxylate could provide an alternative to interspecies hydrogen transfer for the disposal of reducing equivalents produced during benzoate oxidation. Thermodynamic calculations indicate that benzoate oxidation to acetate, carbon dioxide, and hydrogen is thermodynamically unfavorable (Table 1, equation 1). However, benzoate reduction to cyclohexane carboxylate is an exergonic reaction (Table 1, equation 2), which makes the overall fermentation of benzoate to acetate, HCO3−, and cyclohexane carboxylate exergonic (ΔG0′, −12.0 kJ/mol) (Table 1, equation 3). We present evidence in this report that S. aciditrophicus is able to grow and metabolize benzoate in the absence of a hydrogen-utilizing partner. It grows in a monoculture by oxidizing about one half the benzoate to acetate and CO2 and reducing the other half to cyclohexane carboxylate (Table 1, equation 3).
TABLE 1.
ΔG0′ values for different oxidation-reduction reactions involved in benzoate, cyclohexane carboxylate, and cyclohex-1-ene carboxylate metabolisma
| Equation no. | Reaction | Equation | ΔG0′ (kJ/mol) |
|---|---|---|---|
| 1 | Benzoate oxidation | C7H5O2− + 7H2O → 3CH3COO− + HCO3− + 3H2 + 3H+ | 70.5 |
| 2 | Benzoate reduction | C7H5O2− + 3H2 → C7H11O2− | −94.5 |
| 3 | Benzoate fermentation | 2C7H5O2− + 7H2O → C7H11O2− + 3CH3COO− + HCO3− + 3H+ | −12.0 |
| 4 | Cyclohex-1-ene carboxylate oxidation | C7H9O2− + 7H2O → 3CH3COO− + HCO3− + 5H2 + 3H+ | 93.7 |
| 5 | Cyclohex-1-ene carboxylate reduction | C7H9O2− + H2 → C7H11O2− | −71.3 |
| 6 | Cyclohex-1-ene carboxylate fermentation | 6C7H9O2− + 7H2O → 3CH3COO− + HCO3− + 5C7H11O2− + 3H+ | −43.8 |
| 7 | Cyclohexane carboxylate oxidation | C7H11O2− + 7H2O → 3CH3COO− + HCO3− + 6H2 + 3H+ | 165.3 |
| 8 | Benzoate metabolism by S. aciditrophicus- M. hungatei coculture | C7H5O2− + 4.75H2O → 3CH3COO− + 0.25HCO3− + 0.75CH4 + 2.25H+ | −31.2 |
| 9 | Cyclohex-1-ene carboxylate metabolism by S. aciditrophicus-M. hungatei coculture | C7H9O2− + 3.25H2O + 0.25HCO3− → 3CH3COO− + 1.25CH4 + 1.75H+ | −75.5 |
| 10 | Cyclohexane carboxylate metabolism by S. aciditrophicus-M. hungatei coculture | C7H11O2− + 2.5H2O + 0.5HCO3− → 3CH3COO− + 1.5CH4 + 1.5H+ | −38.1 |
The ΔGf of benzoate (−225 kJ/mol) was obtained from reference 15. The ΔGf of cyclohexane carboxylate was estimated to be −319.5 kJ/mol based on the ΔGf of benzoate and the energy of benzene ring reduction (21). The ΔGf of cyclohex-1-ene carboxylate was estimated to be −248.2 kJ/mol based on the reported E0′ value of −350 mV for the benzoate–cyclohex-1-ene carboxylate redox pair (27). All other values were obtained from reference 29.
MATERIALS AND METHODS
Microorganisms and media.
S. aciditrophicus SBT (= ATCC 700169T) (12) and M. hungatei JF1 were obtained from our culture collection. All media and stock solutions were prepared anaerobically by using the techniques described by Balch and Wolfe (3). Pure cultures of S. aciditrophicus and M. hungatei were maintained in the basal medium lacking rumen fluid as described previously (8, 18). Cocultures of S. aciditrophicus and M. hungatei were established and grown in sulfate-free (<5 μM) benzoate basal medium as described previously (8). When S. aciditrophicus was tested for its ability to grow with aromatic or alicyclic substrates, basal medium containing the test substrate at a concentration of 1.2 to 1.7 mM was inoculated with crotonate-grown pure cultures in the stationary phase (inoculum size, 15 to 20%). All cultures were incubated at 37°C. Hydrogen-grown cultures of M. hungatei were incubated with shaking (100 rpm), while monocultures and cocultures of S. aciditrophicus and M. hungatei were incubated without shaking except when the effect of hydrogen on benzoate metabolism was studied. The latter cultures were incubated with shaking (100 rpm). The cultures were routinely checked for purity by microscopic observation, as well as by inoculation of thioglycolate medium. Methane production was routinely checked in pure cultures of S. aciditrophicus growing with benzoate, cyclohexane carboxylate, and cyclohex-1-ene carboxylate to ensure the absence of any methanogenic activity.
Analytical procedures.
Benzoate, cyclohexane carboxylate, and cyclohex-1-ene carboxylate were analyzed by high-performance liquid chromatography with a reverse-phase C18 Econosphere column (250 by 4.6 mm; particle size, 5 μm; Alltech Inc., Deerfield, Ill.). The isocratic mobile phase consisted of 75% phosphate buffer (25 mM sodium dihydrogen phosphate, pH 2.75) and 25% acetonitrile. A variable-wavelength UV absorbance detector set at 214 nm was used to detect substrates and metabolites. Benzoate was also occasionally analyzed with the same column by using detection at 254 nm and an isocratic mobile phase consisting of 70% 50 mM sodium acetate buffer (pH 4.5) and 30% acetonitrile (11). Gas chromatography-mass spectroscopy was used to identify and quantify metabolites produced at micromolar levels as described previously (8).
Acetate was quantified by ion chromatography using a Dionex system (Dionex, Sunnyvale, Calif.), an AS11A-SC column (particle size, 4 mm; Dionex), and 0.1% NaOH as the mobile phase. Butyrate and crotonate were analyzed by gas chromatography as described previously (12). Methane was analyzed by gas chromatography (14). Hydrogen contents were measured with a mercury vapor detector (12). Protein was quantified by the Bradford method (6) using commercially available kits (Pierce Chemical Co, Rockford, Ill.).
Thermodynamic calculations.
The ΔG′ values for the hydrogen-producing, overall syntrophic substrate degradation, and substrate fermentation reactions under experimental conditions were calculated according to Thauer et al. (29). Equations describing benzoate, cyclohexane carboxylate, and cyclohex-1-ene carboxylate fermentation or syntrophic degradation, as well as the ΔG0′ values used in these calculations, are given in Table 1. Most values are molar concentrations; the only exceptions are the hydrogen and methane values, which are expressed in atmospheres (1 atm = 101,325 Pa). The HCO3− concentration was not determined and was assumed to be 0.0365 M in all experiments.
Chemicals.
Sodium benzoate was purchased from Sigma Chemical Co. (St. Louis, Mo.), cyclohexane carboxylic acid was purchased from Acros Organics (Fair Lawn, N.J.), and cyclohex-1-ene carboxylic acid was purchased from Aldrich Chemical Co. (Milwaukee, Wis.). All other chemicals used in this study were obtained from Sigma, Aldrich, or Fisher (Pittsburgh, Pa.).
RESULTS
Metabolism of benzoate and cyclohexane carboxylate by S. aciditrophicus in the presence and absence of H2-utilizing microorganisms.
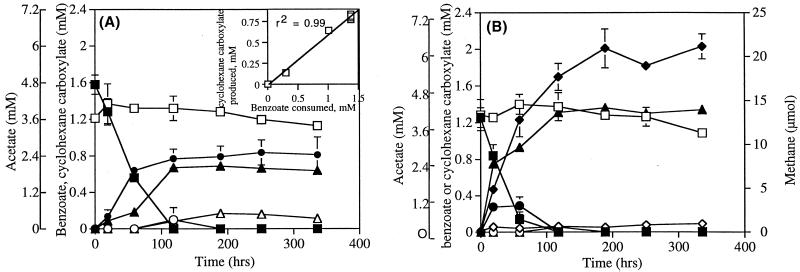
Pure cultures of S. aciditrophicus metabolized benzoate exponentially (Fig 1A) at a rate of 0.41 ± 0.04 day−1. S. aciditrophicus produced 0.53 mol of cyclohexane carboxylate and 1.44 mol of acetate per mol of benzoate degraded (Fig. 1A and Table 2). These values are close to those predicted by the fermentation reaction (Table 1, equation 3). There was a significant correlation (r2 = 0.99) between benzoate consumption and cyclohexane carboxylate production during the experiment (Fig. 1A, inset). The benzoate fermentation activity of monocultures of S. aciditrophicus could be maintained by repeated subculturing. No methane was detected during the experiment. Microscopic observation revealed the presence of only the S. aciditrophicus cell morphotype at the end of the experiment. The hydrogen concentration increased from 38.5 ± 9.5 Pa at the start of the experiment to 97.9 ± 7.2 Pa at the end of the experiment (Table 2), indicating that a small portion of the electrons produced during benzoate oxidation was used to reduce protons to hydrogen. The initial crotonate concentration was 120 μM due to carryover with the inoculum. The final crotonate concentration was 85 μM. Butyrate was not produced at detectable levels (<0.5 μM) by the end of the experiment, which indicated that crotonate did not act as a terminal electron acceptor in this experiment. The ΔG′ values for benzoate fermentation (Table 1, equation 3) and for hydrogen production from benzoate (Table 1, equation 1) were −15.4 and −3.3 kJ/mol, respectively. Analysis by gas chromatography-mass spectrometry showed that cyclohex-1-ene carboxylate, pimelate, and glutarate transiently accumulated at maximum concentrations of 24.2, 4.5, and 3.67 μM, respectively.
FIG. 1.
Metabolism of benzoate by pure cultures of S. aciditrophicus (A)and by S. aciditrophicus-M. hungatei cocultures (B). Symbols: ▪, benzoate; ●, cyclohexane carboxylate; ▴, acetate; ♦, methane; □, benzoate in autoclaved controls; ○, cyclohexane carboxylate in autoclaved controls; ▵, acetate in autoclaved controls; ⋄, methane in autoclaved controls. (Inset) Correlation between benzoate consumption and cyclohexane carboxylate production in S. aciditrophicus pure cultures. The data are averages ± standard deviations based on triplicate microcosms.
TABLE 2.
Stoichiometry of benzoate, cyclohex-1-ene carboxylate, and cyclohexane carboxylate metabolism by S. aciditrophicus in pure culture and in coculture with M. hungatei
| Culture | Original substrate concn (mM) | Final substrate concn (μM) | Cyclohexane carboxylate concn (mM) | Initial acetate concn (mM) | Final acetate concn (mM) | Amt of methane produced (μmol) | Final H2 concn (Pa) | Carbon recovery (%)a | Hydrogen recovery (%)b | ΔG′ of hydrogen-producing reaction (kJ/mol) | ΔG′ of overall reaction (kJ/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aciditrophicus with benzoate | 1.58 ± 0.10 | 5.1 ± 1.1 | 0.84 ± 0.14 | 1.67 ± 0.22 | 3.95 ± 0.39 | 97.9 ± 7.2 | 96.8 | 98.9 | −3.3 | −15.4 | |
| S. aciditrophicus-M. hungatei with benzoate | 1.25 ± 0.11 | 0.83 ± 0.13 | 2.18 ± 0.23 | 6.27 ± 0.41 | 21.1 | 2.32 ± 0.7 | 109.3 | 109.8 | −18.3 | −35.6 | |
| S. aciditrophicus with cyclohexane carboxylate | 1.65 ± 0.14 | 1,510 ± 160 | 1.87 ± 0.06 | 2.19 ± 0.23 | 96.2 ± 10.2 | NDd | ND | ND | ND | ||
| S. aciditrophicus-M. hungatei with cyclohexane carboxylate | 1.43 ± 0.05 | BDLc | 2.05 ± 0.18 | 6.73 ± 0.38 | 41.0 | 2.12 ± 0.5 | 105.6 | 104.7 | −13.2 | −44.4 | |
| S. aciditrophicus with cyclohex-1-ene carboxylate | 1.58 ± 0.01 | 590 ± 20 | 1.01 ± 0.22 | 1.95 ± 0.31 | 2.22 ± 0.18 | 839 ± 68.9 | 110.1 | 114.4 | −4.7 | −9.2 | |
| S. aciditrophicus-M. hungatei with cyclohex-1-ene carboxylate | 1.71 ± 0.08 | BDL | 1.82 ± 0.21 | 6.45 ± 0.78 | 44.3 | 2.6 ± 0.6 | 92.9 | 94.2 | −54.4 | −83.1 |
Carbon recovery values were calculated by excluding biomass. The concentration of HCO3− was not quantified, and the bicarbonate concentrations were calculated by assuming that 0.33 mol of HCO3 was made for every mol of acetate produced, corrected for the amount of HCO3− used to make CH4 in the methanogenic cocultures.
The hydrogen was the available hydrogen, as calculated by the method of Barker (4).
BDL, below detection limit. The detection limits were 5 μM for cyclohexane carboxylate and 1.5 μM for cyclohex-1-ene carboxylate. In both cases, a value of 1 μM was assumed for residual ΔG′ calculations.
ND, not determined.
The S. aciditrophicus-M. hungatei coculture metabolized benzoate at a slightly higher rate (0.53 ± 0.13 day−1) than S. aciditrophicus monocultures metabolized benzoate (Fig. 1B). The coculture produced 3.27 mol of acetate and 0.84 mol of methane per mol of benzoate metabolized (Table 2). These values were consistent with the theoretical stoichiometry (Table 1, equation 8).
Benzoate metabolism by monocultures of S. aciditrophicus was accompanied by a net increase of 3.26 μg of protein per μmol of benzoate metabolized. Assuming that 47% of the cell dry mass was protein (9), then the S. aciditrophicus cell yield was about 6.9 g/mol of benzoate. Therefore, about 0.66 mol of ATP (net) per mol of benzoate was produced during the fermentation (assuming a YATP value of 10.5 g of biomass/mol of substrate) (29). The S. aciditrophicus-M. hungatei coculture cell yield was 11.8 g/per mol of benzoate metabolized, indicating that about 1.1 mol of ATP (net) was produced per mol of benzoate metabolized.
S. aciditrophicus metabolized cyclohexane carboxylate only in a coculture with a hydrogen-using methanogen. The coculture produced 3.27 mol of acetate and 1.43 mol of methane per mol of cyclohexane carboxylate consumed (Table 2). These values are consistent with the theoretical stoichiometry for cyclohexane carboxylate degradation to 3 mol of acetate and 1.5 mol of methane per mol of cyclohexane carboxylate (Table1, equation 10).
Effects of hydrogen and acetate on benzoate metabolism by pure cultures of S. aciditrophicus.
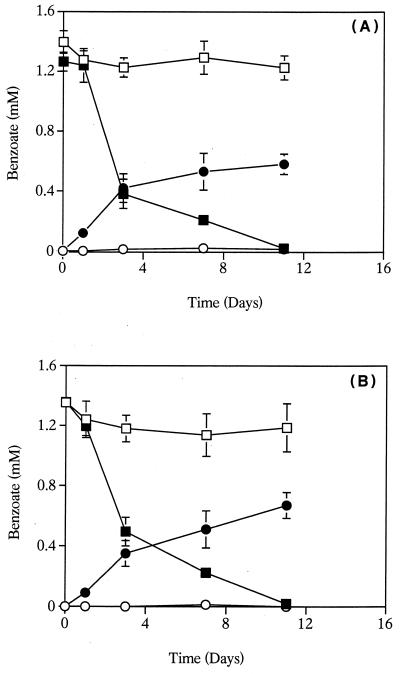
Benzoate degradation by S. aciditrophicus monoculture was inhibited by high levels of hydrogen (10.1 kPa) (Fig 2A). Benzoate consumption was not inhibited in controls that received an equal amount of nitrogen. Benzoate metabolism in pure cultures of S. aciditrophicus that received 60 mM sodium acetate was inhibited compared to controls that received 60 mM sodium chloride (Fig 2B).
FIG. 2.
(A) Effect of hydrogen on benzoate metabolism by S. aciditrophicus. Symbols: □ and ○, benzoate utilization and cyclohexane carboxylate production, respectively, in cultures receiving 10.1 kPa of hydrogen; ▪ and ●, benzoate production and cyclohexane carboxylate production, respectively, in cultures receiving 10.1 kPa of nitrogen. (B) Effect of acetate on benzoate metabolism by S. aciditrophicus. Symbols: □ and ○, benzoate utilization and cyclohexane carboxylate production, respectively, in cultures receiving 60 mM sodium acetate; ▪ and ●, benzoate production and cyclohexane carboxylate production, respectively, in cultures receiving 60 mM sodium chloride. The data are averages ± standard deviations based on triplicate microcosms.
Metabolism of cyclohex-1-ene carboxylate by pure cultures of S. aciditrophicus.
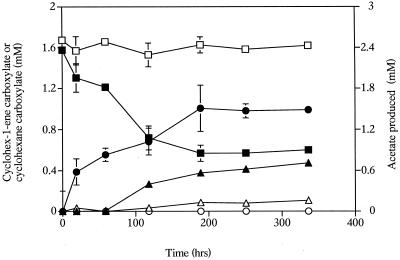
S. aciditrophicus partially metabolized cyclohex-1-ene carboxylate with concurrent production of cyclohexane carboxylate and small amounts of acetate (Fig 3). Only 63% of the initial amount of substrate was metabolized, and about 1 mol of cyclohexane carboxylate and 0.3 mol of acetate per mol of cyclohex-1-ene carboxylate were produced by S. aciditrophicus pure cultures (Table 2). The hydrogen level in cyclohex-1-ene carboxylate-grown monocultures increased from 51.7 ± 1.6 to 839 ± 68 Pa. The final value was about 320 times the value obtained for S. aciditrophicus-M. hungatei cocultures grown with cyclohex-1-ene carboxylate and 9 times the value obtained for S. aciditrophicus pure cultures grown with benzoate. The ΔG′ calculated for cyclohex-1-ene carboxylate oxidation (Table 1, equation 4) at the end of the experiment was −4.7 kJ, while the ΔG′ obtained for the overall cyclohex-1-ene carboxylate fermentation reaction (Table 1, equation 6) was −9.2 kJ/mol. S. aciditrophicus-M. hungatei cocultures completely metabolized cyclohex-1-ene carboxylate and produced 2.71 mol of acetate and 1.30 mol of methane per mol of cyclohex-1-ene carboxylate. These values are consistent with the theoretical stoichiometry for cyclohex-1-ene carboxylate metabolism by syntrophic cocultures (Table 1, equation 9).
FIG. 3.
Metabolism of cyclohex-1-ene carboxylate by S. aciditrophicus pure cultures. Symbols: ▪, cyclohex-1-ene carboxylate; ●, cyclohexane carboxylate; ▴, acetate; □, cyclohex-1-ene carboxylate in autoclaved controls; ○, cyclohexane carboxylate in autoclaved controls; ▵, acetate in autoclaved controls. The data are averages ± standard deviations based on triplicate microcosms.
DISCUSSION
An S. aciditrophicus monoculture metabolized benzoate to cyclohexane carboxylate, acetate, and CO2 (Table 1, equation 3). This reaction supported the growth of S. aciditrophicus, as indicated by our ability to successfully transfer the activity and by the results of the growth yield experiments which indicated that 6.9 g (dry weight) of cells was made per mol of benzoate. The redox potential of the benzoate-cyclohexane carboxylate electron pair (−244 mV) makes benzoate reduction to cyclohexane carboxylate exergonic if hydrogen (−410 mV), NADH (−320 mV), or reduced flavin adenine dinucleotide (−220 mV) is the electron donor. Our results add benzoate to the growing list of compounds, such as crotonate (2, 5, 27, 28, 31), unsaturated short-chain volatile fatty acids (1), pyruvate, fumarate (10, 30), acetoin, acetaldehyde (7), and acetylene (23), that support the growth of microorganisms once believed to be obligate syntrophs. It is clear that many of these organisms have diverse modes of metabolism. This may make assignment of physiological function based on molecular identification methods difficult.
Fermentation of other aromatic compounds has also been reported. Sporotomaculum hydroxybenzoicum degrades 3-hydroxybenzoate in the absence of H2-using microorganisms (20) by using the crotonyl coenzyme A produced during substrate degradation as an electron acceptor, which results in the production of butyrate, acetate, and HCO3− as end products. Karlsson et al. (16) recently described an enrichment that is capable of fermenting phenol by using the reducing equivalents to reduce phenol to benzoate via reductive elimination of the hydroxyl group. Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum has been reported to ferment benzoate (22). These recent findings suggest that fermentation, in addition to syntrophic metabolism, must be considered a possible mechanism for aromatic compound degradation in methanogenic systems.
The ability of S. aciditrophicus to ferment benzoate may have ecological significance since benzoate (or benzoyl coenzyme A) is a central intermediate in anaerobic degradation of many natural and xenobiotic aromatic compounds. Transient accumulation of cyclohexane carboxylate (maximum concentration, 140 μM [representing 28% of the original substrate concentration]) was observed in methanogenic phenol enrichments from landfill sediments (R. Jones and J. M. Suflita, unpublished data). Also, Kleerebezem et al. (17) observed accumulation of cyclohexane carboxylate in anaerobic sewage sludge enrichments amended with benzoate and one of the three phthalate isomers when methanogenesis in these enrichments was inhibited by bromoethanesulfonic acid. This observation implies that cyclohexane carboxylate production could be a mechanism for hydrogen removal in anaerobic ecosystems. Benzoate reduction to cyclohexane carboxylate may be regarded as a survival mechanism used by syntrophic microorganisms in the absence of interspecies hydrogen transfer.
Pure cultures of S. aciditrophicus grown with benzoate accumulated hydrogen at pressures up to 97.9 Pa, compared to the value of 2.3 Pa observed for S. aciditrophicus-M. hungatei cocultures. The higher hydrogen pressure values observed for S. aciditrophicus pure cultures were probably responsible for the higher final benzoate concentrations in these cultures than in a coculture (Table 2). Previous studies showed that hydrogen levels influence the extent of benzoate degradation in syntrophic cocultures, with benzoate metabolism ceasing at higher thresholds in cultures exposed to higher hydrogen levels (11, 13, 25, 32). High levels of hydrogen inhibited benzoate fermentation by S. aciditrophicus pure cultures growing on benzoate (Fig 3) in a manner similar to that observed in S. aciditrophicus cocultures with a hydrogen user. The inhibition of benzoate degradation by high hydrogen levels and the accumulation of hydrogen during benzoate fermentation suggest that hydrogen is produced as a free intermediate during benzoate oxidation by S. aciditrophicus pure cultures.
S. aciditrophicus monoculture was able to metabolize cyclohex-1-ene carboxylate. While only 1 mol of benzoate was reduced per mol of benzoate oxidized to acetate and CO2, 5 mol of cyclohex-1-ene carboxylate was needed to account for all of the reducing equivalents produced during oxidation of 1 mol of cyclohex-1-ene carboxylate to acetate and CO2. Therefore, much less energy was available from cyclohex-1-ene carboxylate fermentation than from benzoate fermentation to support growth. Cyclohex-1-ene carboxylate fermentation stopped after only 62.7% of the initial cyclohex-1-ene carboxylate was degraded. This may have been due to accumulation of high levels of hydrogen (839 Pa), which may have inhibited further oxidation of cyclohex-1-ene carboxylate to acetate and CO2. The ΔG′ values for benzoate and cyclohex-1-ene carboxylate metabolism observed in this study (Table 2) indicate that biological reactions can proceed at values close to thermodynamic equilibrium (ΔG′ = 0 kJ/mol) rather than at the previously suggested value, −20 kJ/mol (24).
ACKNOWLEDGMENTS
This work was supported by DOE grant DE-FG03-96-ER-20214/A003.
We thank Neil Q. Wofford and Luis A. Rios Hernandez for helpful discussions during this work.
REFERENCES
- 1.Amos D A, McInerney M J. Growth of Syntrophomonas wolfei on unsaturated short chain fatty acids. Arch Microbiol. 1990;154:31–36. [Google Scholar]
- 2.Auburger G, Winter J. Isolation and physiological characterization of Syntrophus buswellii strain GA from a syntrophic benzoate-degrading, strictly anaerobic coculture. Appl Microbiol Biotechnol. 1995;44:241–248. [Google Scholar]
- 3.Balch W E, Wolfe R S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol. 1976;32:781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker H A. On the fermentation of some dibasic C4 acids by Aerobacter aerogenes. K Akad Wetenschappen Amsterdam Proc. 1936;39:674–683. [Google Scholar]
- 5.Beaty P S, McInerney M J. Growth of Syntrophomonas wolfei in pure culture on crotonate. Arch Microbiol. 1987;147:389–393. [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Eichler B, Schink B. Fermentation of primary alcohols and diols, and pure cultures of syntrophically alcohol-oxidizing anaerobes. Arch Microbiol. 1986;143:60–66. [Google Scholar]
- 8.Elshahed M S, Bhupathiraju V K, Wofford N Q, Nanny M A, McInerney M J. Metabolism of benzoate, cyclohex-1-ene carboxylate, and cyclohexane carboxylate by Syntrophus aciditrophicus strain SB in syntrophic association with H2-using microorganisms. Appl Environ Microbiol. 2001;67:1728–1738. doi: 10.1128/AEM.67.4.1728-1738.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology Press; 1994. pp. 227–248. [Google Scholar]
- 10.Harmsen H J, Van Kuijk B L, Plugge C M, Akkermans A D, De Vos W M, Stams A J. Syntrophobacter fumaroxidans sp. nov., a syntrophic propionate-degrading sulfate-reducing bacterium. Int J Syst Bacteriol. 1998;48:1383–1387. doi: 10.1099/00207713-48-4-1383. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins B T, McInerney M J, Warikoo V. Evidence for anaerobic syntrophic benzoate degradation threshold and isolation of the syntrophic benzoate degrader. Appl Environ Microbiol. 1995;61:526–530. doi: 10.1128/aem.61.2.526-530.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson B E, Bhupathiraju V K, Tanner R S, Woese C R, McInerney M J. Syntrophus aciditrophicus sp. nov., a new anaerobic bacterium that degrades fatty acids and benzoate in syntrophic association with hydrogen-using microorganisms. Arch Microbiol. 1999;171:107–114. doi: 10.1007/s002030050685. [DOI] [PubMed] [Google Scholar]
- 13.Jackson B E. Bioenergetic perspectives of syntrophic substrate degradation. Ph.D. dissertation. Norman: University of Oklahoma; 1999. [Google Scholar]
- 14.Jenneman G E, McInerney M J, Knapp R M. Effect of nitrate on biogenic sulfide production. Appl Environ Microbiol. 1986;56:1205–1211. doi: 10.1128/aem.51.6.1205-1211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser J P, Hanselmann K W. Fermentative metabolism of substituted monoaromatic compounds by a bacterial community from anaerobic sediments. Arch Microbiol. 1982;133:185–194. [Google Scholar]
- 16.Karlsson A, Ejlertsson J, Svensson B H. CO2-dependent fermentation of phenol to acetate, butyrate and benzoate by an anaerobic, pasteurized culture. Arch Microbiol. 2000;173:398–402. doi: 10.1007/s002030000160. [DOI] [PubMed] [Google Scholar]
- 17.Kleerebezem R, Hulshoff L W, Lettinga G. Energetics of product formation during anaerobic degradation of phthalate isomers and benzoate. FEMS Microbiol Ecol. 1999;29:273–282. [Google Scholar]
- 18.McInerney M J, Bryant M P, Pfenning N. Anaerobic bacterium that degrades fatty acids in syntrophic association with methanogens. Arch Microbiol. 1979;122:129–135. doi: 10.1007/s002030050685. [DOI] [PubMed] [Google Scholar]
- 19.Mountfort D O, Bryant M P. Isolation and characterization of an anaerobic benzoate-degrading bacterium from sewage sludge. Arch Microbiol. 1982;133:249–256. [Google Scholar]
- 20.Müller J A, Schink B. Initial steps in the fermentation of 3-hydroxybenzoate by Sporotomaculum hydroxybenzoicum. Arch Microbiol. 2000;173:288–295. doi: 10.1007/s002030000148. [DOI] [PubMed] [Google Scholar]
- 21.Parks G S, Huffman H M. The free energies of some organic compounds. American Chemical Society Monograph Series no. 60. New York, N.Y.: The Chemical Catalog Company Inc.; 1932. [Google Scholar]
- 22.Plugge, C. M., M. Balke, and A. J. Stams.Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum, a thermophilic syntrophic propionate-oxidizing spore-forming bacterium. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 23.Schink B. Fermentation of acetylene by an obligate anaerobe, Pelobacter acetylenicus sp. nov. Arch Microbiol. 1985;142:295–301. [Google Scholar]
- 24.Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schöcke L, Schink B. Energetics of methanogenic benzoate degradation by Syntrophus gentianae in syntrophic coculture. Microbiology. 1997;143:2345–2351. doi: 10.1099/00221287-143-7-2345. [DOI] [PubMed] [Google Scholar]
- 26.Schöcke L, Schink B. Biochemistry of benzoate fermentation by S. gentinae. Arch Microbiol. 1999;77:100–110. [Google Scholar]
- 27.Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H. Syntrophothermus lipocalidus gen. nov., sp. nov., a novel thermophilic, syntrophic, fatty-acid-oxidizing anaerobe which utilizes isobutyrate. Int J Syst Evol Microbiol. 2000;50:771–779. doi: 10.1099/00207713-50-2-771. [DOI] [PubMed] [Google Scholar]
- 28.Svetlitshnyi V, Rainey F, Wiegel J. Thermosyntropha lipolytica gen. nov., sp. nov., a lipolytic, anaerobic, alkalitolerant, thermophilic bacterium utilizing short- and long-chain fatty acids in syntrophic coculture with a methanogenic archaeum. Int J Syst Bacteriol. 1996;46:1131–1137. doi: 10.1099/00207713-46-4-1131. [DOI] [PubMed] [Google Scholar]
- 29.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Kuijk B L, Schlosser E, Stams A J. Investigation of the fumarate metabolism of the syntrophic propionate-oxidizing bacterium strain MPOB. Arch Microbiol. 1998;169:346–352. doi: 10.1007/s002030050581. [DOI] [PubMed] [Google Scholar]
- 31.Wallrabenstein C, Gorny N, Springer N, Ludwig W, Schink B. Pure cultures of Syntrophus buswellii, definition of its phylogenetic status, and description of Syntrophus gentianae sp. nov. Syst Appl Microbiol. 1995;18:62–66. [Google Scholar]
- 32.Warikoo V, McInerney M J, Robinson J A, Suflita J M. Interspecies acetate transfer influences the extent of anaerobic benzoate degradation by syntrophic consortia. Appl Environ Microbiol. 1996;62:26–32. doi: 10.1128/aem.62.1.26-32.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]