Abstract
Severe fever with thrombocytopenia syndrome (SFTS) has been acknowledged as an emerging infectious disease that is caused by the SFTS virus (SFTSV). The main clinical features of SFTS on presentation include fever, thrombocytopenia, leukocytopenia and gastrointestinal symptoms. The mortality rate is estimated to range between 5-30% in East Asia. However, SFTSV infection is increasing on an annual basis globally and is becoming a public health problem. The transmission cycle of SFTSV remains poorly understood, which is compounded by the pathogenesis of SFTS not being fully elucidated. Since the mechanism underlying the host immune response towards SFTSV is also unclear, there are no effective vaccines or specific therapeutic agents against SFTS, with supportive care being the only realistic option. Therefore, it is now crucial to understand all aspects of the host-virus interaction following SFTSV infection, including the antiviral states and viral evasion mechanisms. In the present review, recent research progress into the possible host immune responses against SFTSV was summarized, which may be useful in designing novel therapeutics against SFTS.
Keywords: immunological, severe fever with thrombocytopenia syndrome, severe fever with thrombocytopenia syndrome virus, innate immune response, adaptive immune response
1. Introduction
Severe fever with thrombocytopenia syndrome (SFTS) has been acknowledged to be an emerging infectious disease that is caused by the SFTS virus (SFTSV). This new virus has been isolated and identified in recent years. It belongs to the genus Bandavirus in the family Phenuiviride, order Bunyavirales (1,2). SFTSV was first isolated from a patient in 2009 in China (1), followed by Korea in 2010 (3), in 2013 in Japan (4), in 2017 in Vietnam (5), in 2018 in Myanmar (6), in 2019 in the Taiwan region (7), in 2020 in Thailand (8) and in Pakistan (9). In 2012, the United States isolated a virus similar to SFTSV and named it the 'Heartland Virus' (10). The primary features of SFTS on presentation include fever, thrombocytopenia, leukocytopenia and gastrointestinal symptoms (1,11). However, a minority of patients with severe forms of the disease may rapidly succumb to the symptoms stemming from multiple-organ failure (MOF), such as shock, respiratory failure and disseminated intravascular coagulation (DIC) (12). The mortality rate of SFTS currently ranges from 5-30% in East Asia (13).
According to previous studies, the tick is one of the primary host vectors for SFTSV (14,15). In addition, human-to-human transmission has also been found in some clusters and patients (16,17). SFTSV infection has been reported to be transmitted through blood contact, droplet contact (18) and aerosolized droplets (19). Consequently, exploring prevention and treatment strategies for SFTS is becoming a severe challenge that cannot be neglected. Supporting this, in 2017 the World Health Organization (WHO) ranked SFTS as the disease with the highest research priority (20).
In addition to the increasingly severe health issues associated with SFTSV, there is currently no effective treatment options for this virus, which contributes to the relatively high mortality rate. The optimal strategy to protect against being infected with SFTSV is to avoid tick bites. However, the pathogenesis of SFTS after infection remains poorly understood, particularly the interaction between the host immune response and SFTSV. SFTSV-induced modulation of host immunity involves immune cells [including dendritic cells (DCs), natural killer (NK) cells, macrophages, T cells and B cells], immunomodulatory cytokines and various signaling pathways [such as nuclear factor (NF)-κB and Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signaling]. Therefore, in the present review, recent research data regarding the host immune response to SFTSV and the pathogenesis of SFTS were summarized. The aim of this review was to provide a basis for the exploration of novel therapeutic targets to assist patients in coping with this disease.
2. Clinical manifestations and laboratory features of SFTS
The clinical symptoms of the SFTS include fever, anorexia, fatigue, nausea, abdominal pain or tenderness, vomiting, malaise, diarrhea, lymphadenopathy, myalgia, confusion, headache, throat congestion, cough, conjunctival congestion, petechiae, slurred speech and coma (1). The clinical manifestation of SFTS is heterogeneous, with fever and gastrointestinal symptoms being the most common. The laboratory findings of patients with SFTS, revealed thrombocytopenia, leukocytopenia, proteinuria, hematuria, fecal occult blood, and elevated levels of serum alanine aminotransferase, aspartate aminotransferase (AST), creatine kinase, lactate dehydrogenase (LDH), ferritin, and prolonged activated partial-thromboplastin time (APTT) (11). The most common laboratory abnormalities were thrombocytopenia (95%) and leukopenia (86%) (1). Patients with severe SFTS may succumb to this disease within 2 weeks due to MOF (11). Advanced age, altered mental status, high serum LDH and AST levels, prolonged APTT, and high viral RNA loads in the blood are indicators of poor prognosis of SFTS (12).
Hemophagocytic lymphohistiocytosis (HLH) is a an immune-mediated life-threatening clinical syndrome accompanied by excessive immune activation and affects multiple organ systems (21). Patients with unattributable, persistently high fever and signs of multiple organ involvement should be suspected of HLH (22), as the clinical symptoms are similar to SFTS. In fact, HLH associated with SFTS has been reported in China (23), Japan (24), and Korea (25). A previous study revealed that 33.3% of patients with SFTS had HLH, and the mortality rate was as high as 75%, indicating that patients with SFTS and HLH had a higher risk of poor prognosis (25).
3. Structure of SFTSV
SFTSV is spherical in shape with a diameter of 80-120 nm. It is a virus with a single negative strand of RNA that contains three RNA ring segments within its genome: Small (S), medium (M) and large (L) (1). The S segment contains 1,744 nucleotides, which primarily encodes the nucleocapsid protein (NP) and a nonstructural protein (NSs). The NP can encapsulate three RNA genome fragments of SFTSV and form ribonucleoprotein complexes with RNA-dependent RNA polymerase (RdRp), which protects the virus from nucleases and host immune system degradation. This suggests that the NP serves a key role in viral transcription and replication. By contrast, the NSs forms the main virulence factor of the SFTSV and has been previously shown to control the host innate immune response to promote virus replication (26). The M fragment is comprised of 3,378 nucleotides and has one open reading frame. It encodes the membrane precursor protein (Gp), which is then modified by an intracellular protease into two glycoproteins, Gn and Gc. Gn and Gc in turn mediate viral invasion, whereby Gn promotes the early infection of SFTSV by binding to non-muscle myosin heavy chain IIA on the cell surface (27). The L segment consists of 6,368 nucleotides and encodes RdRp, which triggers viral RNA replication and mRNA synthesis. A previous study revealed that the relative level of self-replication by the three fragments of Banyangvirus is M > L > S, which occurs in the host cell-matrix where transcription and translation take place simultaneously (28).
4. Epidemiology of SFTSV
SFTS was first identified in China in 2009 (1). Analysis of the epidemiological characteristics in mainland China from 2010 to 2019 yielded 13,824 patients with SFTS, including 8,899 lab-confirmed cases and 4,925 suspected cases (29). Although the number of patients with SFTS has shown a decreasing tendency over the past 3 years, this decline has been halted somewhat. The majority of the patients (99.3%) were distributed across seven provinces, namely Henan, Shandong, Anhui, Hubei, Liaoning, Zhejiang and Jiangsu. However, the regional distribution of the SFTS cases has expanded gradually from five provinces in 2010 to 25 in 2019, especially in the rural areas. With 713 cases of mortality, Zhejiang had the highest case fatality rate (CFR) of 11.5%, while Henan had the lowest CFR of 1.3% (25). However, this data may be biased, since according to a previous study, the CFR of Xinyang, a city in Henan province, was found to be 16.2% (30) whereas the average annual fatality rate was 5.2%, lower than the 2011-2017 CFR of 16.2% (13). The population with the highest CFR tended to be those of elderly age (≥85 years old), higher viral load, prolonged hospital admission delay, presence of diarrhea or dyspnea, development of hemorrhagic or neurological manifestations, elevated C-reactive protein levels, prolonged APTT and resident diagnosis and treatment levels (13,29). Reduction in the CFR may be associated with the optimization of diagnostic and treatment trials, professional experience of the doctors in question and the level of health education.
In South Korea, the first case of SFTS was identified in 2012 through the isolation of SFTSV from a stored blood sample collected shortly before the patient succumbed to the disease (3). From 2013, when the first case of SFTS was compiled, 1,373 patients have been identified as of December 2021 (31). Since then, the annual incidence has increased from 36 in 2013 to 81 cases in 2015, where the overall CFR was 32.6% (32). According to a previous retrospective study of patients with SFTS in South Korea, between 2013-2019, the overall CFR was 11.3% (33), which was lower compared with that aforementioned in the previous study (32).
In Japan, the first confirmed case of SFTS was reported at the end of 2012 (4). At present, 537 patients have been identified between 2013 and September 2021 (34). Kobayashi et al (35) previously conducted an epidemiological survey from 2013 to 2017, where 303 cases were described. Amongst this group, 133 patients (44%) were involved in the survey and the overall CFR was found to be 27%. In addition, a further epidemiological survey found that 64 patients (48%) had intimate contact with their pets within 2 weeks of disease initiation. In particular, two patients were in immediate contact with the saliva of an infected dog or cat, suggesting that infected animals may be the source of SFTSV infection.
5. Transmission cycle of SFTSV
Although the life cycle and continuous diffusion mechanism of SFTSV remain unclear, SFTSV is primarily transmitted between ticks and humans and certain animals through tick bites. The Asian long-horned tick, Haemaphysalis longicornis (H. longicornis), is one of the known primary vectors of SFTSV (14,36). A previous study revealed a prevalence of SFTSV in ticks, with an infection rate of ~11.1% (37). In terms of transmission, the animal-tick-human axis may be another form of propagation. A cohort investigation from China previously demonstrated that in domesticated animals that were naturally infected with SFTSV, sheep (69.5%), cattle (60.4%), dogs (37.9%) and chickens (47.4%) had the highest seroprevalence rates, whilst pigs (3.1%) had a low prevalence rate (38). By contrast, direct contact with SFTSV-infected cats has been reported to result in cat-to-human transmission (39). In addition, antigens and antibodies could be detected in wild animals, including wild deer (40), wild boars (41), wild badgers and masked palm civets (42). In terms of transmission between humans, those who were in close contact with infected body fluids, such as those with needlestick injuries and patients with SFTS, are particularly at risk (43,44). A previous meta-analysis revealed that from 1996 to 2019, China and South Korea published 40 publications regarding the clusters of human-to-human SFTSV transmission, of which there were 27, containing 138 cases in total (45). This meta-analysis also found that the clinical severity of the secondary cases was milder compared with that of the index cases, and the prognosis of secondary cases was improved (45). In addition, a recent study in Hefei, Anhui province, revealed that the overall seropositivity rate of SFTSV antibodies among healthy residents was 20.16% (46).
6. Innate immunity in SFTS
The host innate immune system serves as the first-line defense to immediately prevent virus invasion and replication. Furthermore, activation of innate immune cells is crucial for initiating adaptive immunity. Innate immune cells can detect viruses using pattern recognition receptors (PRRs) to recognize pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Following activation, expression of IFN-stimulated genes (ISGs) is increased to establish an antiviral state (47).
IFNs
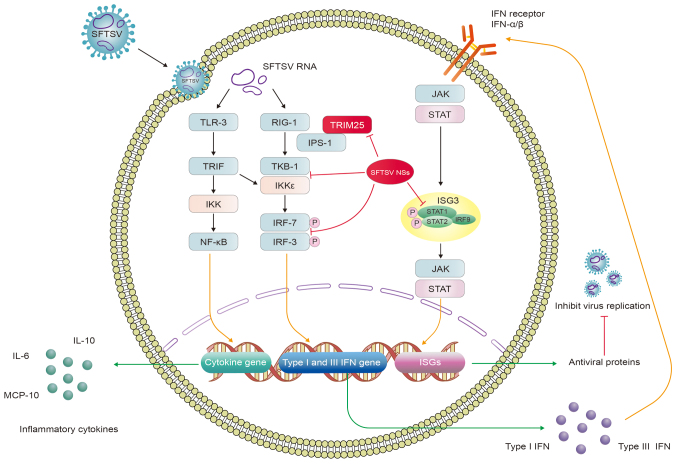
IFNs are puissant antiviral cytokines that induce various antiviral responses to suppress viral replication (48). IFN secretion contributes to the expression of ISG and promotes antiviral activity in infected cells after the body identifies specifically conserved molecular structures of pathogens, such as viral RNA or genomes, through different types of PRRs (49). The principal receptors that can serve this function include Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) and nucleoside acid-binding oligomeric receptors (NOD-like receptors or NLRs) (50). During the recognition process of SFTSV-infected cells, RIG-I has been reported to be the primary viral-RNA sensor molecule, which recruits IFN-β promoter stimulator 1 [IPS-1 or mitochondrial antiviral signaling protein (MAVS)]. IPS-1 then delivers the signal to TANK-binding kinase 1 (TBK1) and inhibitor of NF-κB kinase (IKK), which induces type I IFN secreted by activating the phosphorylation of IFN regulatory factor (IRF)-3 and IRF-7. In addition, cytosolic PRPs which recognize viral RNA products, including TLR3 and melanoma differentiation-associated protein 5 (MDA5) can also participate in the recognition process, however MDA5 is of lesser importance in this process (51,52). Nuclear scaffold attachment factor A (SAFA) is a novel viral-RNA sensor that can recognize SFTSV RNA by interacting with SFTSV NPs and mediate antiviral IFN and inflammatory responses in the cytoplasm (53). In particular, type I and III IFN can bind to its receptor and share a common signaling pathway that ultimately activates ISG factor 3 (ISGF3). ISGF3 in turn activates STAT1, the STAT2 heterodimer and IRF9 (54) to stimulate the JAK-STAT pathway to induce the expression of ISGs through autocrine and paracrine mechanisms. ISGs can be focused onto any stage of the viral life cycle by encoding various antiviral proteins, thereby establishing the antiviral status (52). Clinically, SFTSV infection can lead to an acute inflammatory response accompanied by the aberrant activation of proinflammatory cytokines in the serum of the patient, such as IL-6, IL-10, IFN-γ, IL-8 and monocyte chemotactic protein 1 (MCP-1) (55,56). Indeed, the increased concentration of IFN-α has also been reported to be associated with the severity of SFTS (Fig. 1) (57).
Figure 1.
SFTSV activates IFN production and cytokine storm to evade the innate immune response. SFTSV RNAs bind to TLR-3 and RIG-1 to activate TRIF, TBK1 and IKK, which in turn activate the NF-κB signaling pathway whilst phosphorylating IRF3 and IRF7. This induces the expression of cytokine, type I and type III IFN genes, promoting the production of inflammatory cytokines, type I and III IFNs. Type I and III IFNs can share the same JAK/STAT pathway by binding to their receptors, which activate the expression of ISGs and antiviral proteins that inhibit viral replication. SFTSV NSs can bind several host proteins to form inclusion bodies, such as RIG-I, TBK1, IKK, IRF3, IRF7, TRIM25, STAT1 and STAT2, leading to severe clinical implications. Red lines indicate inhibition. SFTSV, severe fever with thrombocytopenia syndrome virus; NSs, nonstructural protein; TLR, Toll-like receptor; RIG-I, retinoic acid-inducible gene I; TRIF, TIR-domain-containing adaptor inducing interferon-β; IKK, inhibitor of NF-κB kinase; IPS-1, IFN-β promoter stimulator 1; TBK, TANK-binding kinase 1; IRF, IFN regulatory factor; JAK, Janus kinase; ISGs, IFN-stimulated genes, TRIM, tripartite motif.
During this long-term struggle with the host, viruses have evolved various strategies to evade the innate immunity, such as avoiding host recognition, destroying IFN signal transduction, obstructing IFN production, regulating cell apoptosis and autophagy. In Vero cells (African green monkey kidney cells), SFTSV infection was found to accelerate the conversion of LC3-I to LC3-II, but treatment with lysosomal protease inhibitors E64d and pepstatin A had no impact on LC3-II accumulation, which avoided autophagic degradation during the viral replication stage as a possible mechanism (58). The unfolded protein response (UPR) is an evolutionarily conserved signaling system that is triggered by endoplasmic reticulum stress and it is associated with the viral life cycle in the host. SFTSV Gp has been found to initiate UPR, which can promote SFTSV replication (59). By contrast, SFTSV NSs can mediate the degradation of essential molecules in the host immune response pathway to avoid innate immune attack, such as RIG-I, TLR3, TNF-associated factors (TRAFs), STATs, TBK1 and MAVS (49).
Although SFTSV may block the IFN pathway at multiple steps, it is unclear how host cells can perceive SFTSV and trigger immune and inflammatory cytokine responses (50). Qu et al (60) previously attempted to dissect the host responses in monocytes and the viral immunological pathogenesis mechanisms in humans infected with SFTSV. Since the regulation of MAVS-mediated stimulation of IFN-β promoter activity requires the interaction between SFTSV NSs and TBK1, NSs can bind to TBK1 to impede the activation of IRF and NF-κB signaling downstream (60). This suggests that the interaction between SFTSV NSs and TBK1, which results in the inhibition of TBK1 autophosphorylation, is necessary to inhibit the MAVS-mediated activation of IFN-β promoter activity (61). Subsequently, a number of studies have proposed that RIG-I, TLR3, MDA5 and MAVS can participate in SFTSV recognition (51,62-64). SFTSV NSs mediate inhibitory effects on antiviral IFN generation and can interact with TBK1, IKKε and IRF3 either directly or indirectly to assist viral innate immune evasion (65-67). SFTSV NSs, according to Min et al (52), can also inhibit RLR antiviral signaling by RIG-I ubiquitination and activation. In addition, the specific interaction between NSs with the E3 ubiquitin ligase tripartite motif (TRIM)25 inhibits the TRMI25-mediated Lys-63 ubiquitination of RIG-I and activation of RIG-I, suppressing IFN excretion (52). Yoshikawa et al (68) previously reported that mice deficient in the gene encoding the α chain of the α- and β-IFN receptor (INFAR1−/− mice) and golden Syrian hamsters deficient in the gene encoding STAT2 (STAT2−/− hamsters) were highly susceptible to SFTSV infection (68). In addition, another study found that SFTSV NSs can also directly interact with and sequester IRF7 into inclusion bodies (IBs), which promotes IFN-β and in particular, IFN-α2 and -α4, to ensure effective evasion and suppression of innate immunity (68). It has also been previously found that NSs can sequester STAT2 into NSs-induced cytoplasmic IBs, thereby blocking type I IFN JAK/STAT signaling to evade innate immunity. However, NSs have minor physical and functional interactions with STAT1 (69). Overall, these observations suggest that SFTSV NSs are potent inhibitors of IFN production, by binding to several host molecules and sequester them into IBs, such as RIG-I, TBK1, IKK, IRF3, IRF7, TRIM25, STAT1 and STAT2. This results in severe clinical outcomes.
Cytokine storm
The 'cytokine storm' was first described in 1993 (70). Previous studies have shown that a cytokine storm syndrome of viral origin serves an essential role in the pathogenesis of viral infections, such as the Ebola virus (71), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (72), influenza A virus (IAVs) (73), severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) (74) and SFTSV (75). The cytokine storm appears to share common pathogenic characteristics, namely unbalanced immune responses with an exaggerated inflammatory cytokine reaction and T-cell depletion and functional exhaustion (70).
Although SFTS pathogenesis remains to be elucidated, there is little doubt in the literature that the severity of SFTS is associated with the cytokine storm (76). Cytokine storm-mediated immune activation and damage to the organs in the body is one of the most critical pathogenic mechanisms of SFTSV infection. Compared with those in healthy subjects, the expression of IL-6, IL-10, IL-8, IL-15, IL-1-RA, TNF-α, IFN-γ, IFN-γ-induced protein-10 (IP-10), macrophage inflammatory protein-1α, heat shock protein (HSP)-70, granulocyte colony-stimulating factors, granzyme B, MCP-1, caspase-8, C-C motif ligand (CCL)-7, silent information regulator 2, C-X-C motif ligand 9, signal transducing adaptor molecule binding protein, CCL20 and eukaryotic translation initiation factor 4E-binding protein 1 were markedly upregulated. Furthermore, TNF-α, CCL20 and CX3CL1 levels were found to be highly associated with fatality, whilst IL-6, IP-10, IFN-γ, HSP70 and TNF-α levels were positively correlated with the viral load and severity of patients with SFTS (12,66,77-79). IL-6 is a proinflammatory cytokine that is essential for accelerating the cell response to limit persistent viral infection. By contrast, IL-10 is an anti-inflammatory cytokine, the expression of which is significantly higher in patients with SFTS, especially in patients with fatal disease (12). The hypersecretion of IL-6 and IL-10 can generate a cytokine storm, which contributes to the pathology of SFTSV infection (55).
Other cytokine/chemokines were also found to be downregulated, such as tissue polypeptide antigen, platelet-derived growth factor-BB, growth-related oncogene, TGF-β and regulated on activation and normally T-cell expressed (RANTES), compared with those in healthy controls (66,77-79). In particular, RANTES was reported to be negatively correlated with viral load (80). However, the expression profile of cytokines in patients with SFTS remain contested. Deng et al (77) previously found that serum RANTES levels were increased, whilst the level of IFN-γ was decreased in patients with SFTS. However, another study found that IFN-γ levels are increased in fatal cases, whereas TNF-α levels were reduced in patients who succumbed to the disease or have recuperated (81).
A possible reason for two families of cytokines, such as TNF-α and IFN-γ, showing contradictory patterns in SFTS is because cytokine variation is dependent on the experimental protocol, such as sample size, cytokine detection time points, severity of the SFTS and intervention method. Furthermore, the mechanism of cytokine secretion is complex, such that the same cytokine can be secreted by several immune cells. TNF-α can be secreted by macrophages, DC cells and T cells, which leads to the inconsistent results of TNF-α reported in the literature. Cytokine secretion can also be regulated by a number of cell signaling pathways, such as JAK/STAT3, MAPK, NF-κB, mTOR and TLR4 signaling pathways (82). The crosstalk among these different signals can regulate the expression of cytokines.
However, the mechanism underlying cytokine storm occurrence remains unclear. Previous studies have shown that NSs can also mediate the induction of a cytokine storm after SFTSV infection. Khalil et al (83) revealed that TBK1 can suppress the NF-κB signaling pathway and cytokine/chemokine production through a kinase activation-dependent manner, whilst NSs can trap TBK1 to prevent it from inhibiting NF-κB, promoting the activation of NF-κB and its downstream cytokine/chemokine genes (83). Another study previously showed that NSs can induce IL-10 production through the tumor progression locus 2-binding inhibitor of NF-κB activation 2 (ABIN2)-p105 complex (84).
Cytokines can serve an essential role in regulating clinicopathological features. Monocytes, macrophages and T cells can all secrete TNF-α, leading to vasodilation and the induction of NO synthase activity, which increases endothelial permeability (77). In vitro analyses showed that SFTSV infection increased the vascular permeability of endothelial cells by activating tyrosine phosphorylation and the internalization of cadherin, the endothelial adhesion molecule that functions as the main component of endothelial integrity maintenance (85). These previous findings suggest that the cytokine/chemokine-mediated inflammatory response, which is exemplified by cytokine and chemokine expression imbalance, serves a vital role in the progression of SFTS.
DCs
DCs are one of the most important antigen-presenting cells (APC) and form part of a critical link between innate and adaptive immunity (86). In the periphery, DCs become activated in response to 'danger' signals provided directly by the microbial invaders (87), which are relayed through TLRs, RLRs and NLRs, receptors in the IFN secretion pathway. These DCs then migrate to the draining lymphoid tissues to present the acquired pathogen to appropriate adaptive T and B cells to mediate immune responses (88).
One previous study demonstrated persistent downregulation in the expression of the co-stimulatory molecule CD80/CD86 on the myeloid DCs (mDCs) in patients with SFTS (89). Reduced circulating mDCs can be measured as a valuable predictive biomarker, especially from day 9 following disease initiation, where disease severity is associated with TLR3 expression on mDCs. Furthermore, IL-6, IL-10, TNF-α and viral load were also found to be negatively associated with mDCs (89). Song et al (90) reported that the robust differentiation of mDCs in surviving patients and not those who succumbed to SFTS started in week 2 after the symptoms appeared, which continued until week 3 (90). It was also found that among the surviving patients, the expression of CD86 and the ratio of CD80+CD86+/mDCs in week 3 was significantly higher compared with that in the patients who succumbed to the disease in week 2 (90). SFTSV vaccines, such as human adenovirus type 5 (Ad5)-G-Gn (a recombinant replication-deficient Ad5 co-expressing rabies virus G and SFTSV Gn), have been reported to increase the production of neutralizing antibodies against SFTSV (91). This appeared to be mediated by the activation of additional DCs and B cells in lymph nodes to induce a Th1-/Th2-mediated immune response in splenocytes (91). This suggests that DCs can serve an antigen-presenting role in the function of SFTSV vaccines. These conclusions demonstrate that SFTSV infection can attenuate the antigen-presenting ability of mDCs to inhibit T-cell activation.
NK cells
NK cells are essential antiviral and anticancer immune cells that serve a crucial role in immune defense and surveillance (92). After recognizing infected cells, NK cells can rapidly secrete granzyme and perforin to lyse infected-cells. In addition, NK cells can also secrete proinflammatory factors, including IFN-γ and TNF-α, to induce a broader immune response to control the global viral load (93). Sun et al (94) previously observed that in individuals with severe SFTSV infection, the percentage of NK cells was increased during the acute phase. However, other studies reported that the number of NK cells was actually decreased in patients with SFTS during the first week, which was rapidly restored to normal levels after 6 months (95). In addition, another study found that the CD3−CD16+56+ NK cell count in patients with SFTS was lower compared with that in healthy controls, although there was no statistical difference (96). During the early stages of SFTSV infection, the frequency of CD56dimCD16+NK cells was significantly reduced, which was negatively correlated with the severity of the disease. Although the CD56dimCD16+NK cell population was depleted, the activation and functional enrichment suggest that their involvement can ward off early SFTSV infection (97). Therefore, the loss of NK cells may result in an upsurge in the viral load.
Monocytes and macrophages
Monocytes are widespread in the blood and form the first line of defense against microbial invasion. Following infection, monocytes change their cytokine/chemokine pattern, differentiate into long-lived macrophages (Mφ) and migrate into the tissue, becoming infected resident cells (98). Macrophages are important cells in the innate immune system and modulate the adaptive immune responses to pathogens through antigen processing and presentation (99). Following infection, macrophages differentiate into two distinct subsets: i) Classically activated or M1 macrophages, which are characterized by the secretion of proinflammatory cytokines and high levels of phagocytic activity (100); and ii) alternatively activated or M2 macrophages, which mainly manufacture anti-inflammatory cytokines and immunoregulatory molecules, leading to neutrophil, monocyte and T-lymphocyte recruitment (101).
A previous study revealed that SFTSV can replicate in human monocytes without inducing apoptosis by inhibiting the INF and NF-κB signaling pathways (102). In addition, the SFTSV can be harbored within splenic macrophages for long periods of time (103). However, the histopathological changes caused by SFTSV infection can be found in the spleen, lung, kidney and liver, where further study suggested that spleen and liver macrophages may be the primary target cells of SFTSV infection (85). A previous study by the authors also demonstrated that SFTSV can infect macrophages in vivo and elevated pro- and anti-inflammatory cytokines, as well as activation of CD69+ T cells (81). SFTSV infection of macrophages drives macrophage differentiation towards M2, which promotes viral shedding and transmission by targeting STAT1 (104). In addition, the integrated transcriptome of mRNAs and lncRNAs in THP-1 macrophages infected with SFTSV for 24 and 48 h was previously analyzed. It revealed 2,334 differentially expressed mRNAs and 154 differentially expressed lncRNAs. Amongst these, 577 mRNAs and 31 lncRNAs were commonly altered at 24 and 48 h, respectively. According to the analysis of differentially expressed mRNAs and transcription factors, they were mainly associated with innate immunity and cytokine signaling. In particular, IRF1, Salmonella pathogenicity island (SPI) 1, SPIB, E74-like ETS transcription factor 5 and FEV were significantly enriched following SFTSV infection (105). These results revealed how macrophages and SFTSV can interact in a complex manner.
γδT cells
γδT cells are another type of lymphocytes and they serve an essential role in the immune response and immunopathological processes, which is receiving particular attention (106). γδT cells are a critical component of innate immunity and serve an essential role in antiviral and antitumor activities. One study previously found that during the acute phase of SFTS, the number of Vδ2T cells fell considerably, which lasted for ~1 year. The Vδ2T cell population declined readily with the severity of SFTS. The possible mechanism of Vδ2T-cell depletion in SFTSV infection has also been previously associated with activation-induced cell death (107). To conclude, lymphopenia in patients with SFTS can affect the number of T-cell subsets, hindering the enhancement of the immune response and subsequent elimination of viral infections. However, the mechanism of T-lymphocyte depletion warrants further study.
Inflammasomes
Inflammasomes are mainly formed by multimeric protein complexes of sensor, adaptor and pro-caspase-1 components, such as NACHT, leucine-rich and pyrin domain-containing proteins (NALP), apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase 1 (CASP1) (108). Inflammasomes serve as receptors for innate immune cells, which detect circulating DAMPs and PAMP and activate caspase-1 to cleave pro-IL-1β and pro-IL-18 into IL-1β and IL-18, respectively (109). IL-1β recruits immune cells and causes the programmed death of cells by binding to IL-1R-expressing immune cells. Liu et al first reported that the secretion of IL-1β during SFTSV infection is mediated by the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome in a caspase-1-dependent manner (110). The use of short hairpin RNAs to knock down several NLRs in peripheral blood mononuclear cells revealed that the NLRP3 inflammasome is essential for the processing of pro-caspase-1 and pro-IL-1β (111). Further studies discovered that both wild-type SFTSV NSs and the 21/23A mutant of SFTSV NSs with alanine residues at positions 21 and 23 were able to reduce NLRP3 inflammasome-dependent IL-1β production (61). In addition, it was found that SFTSV infection can trigger the upregulation of BCL2 antagonist/killer 1 (BAK) expression and activation of BAK/BCL2-associated X (BAX), resulting in mitochondrial DNA (mtDNA) oxidation and subsequent cell membrane release (112). This mtDNA binding to NLRP3 leads to inflammasome activation and amplifies the inflammatory response (112). However, the exact composition and mechanism of inflammasomes are complex. Further studies are required to clarify the role of inflammasomes in SFTS.
7. Adaptive immunity in SFTS
The adaptive immune system consists of cellular immune responses mediated by T cells and a humoral immune response modulated by antibodies (113). Naïve CD4+T cells receive the major histocompatibility complex (MHC) II epitopes by APCs, such as DCs, macrophages and B cells, following which they differentiate into different cell types to regulate the adaptive immune response depending on the cytokine environment. CD8+T cells, also known as cytotoxic T lymphocytes or effector T cells, can directly kill virus-infected cells. By contrast, B cells are mainly involved in humoral immunity, where the body can generate specific antibodies to neutralize viruses through effector B cells (114).
T-cell-mediated immune response to SFTSV
T lymphocytes serve a primary role in antigen-specific immune responses. A number of studies previously demonstrated that CD3+, CD4+, and CD8+ T cells are diminished in patients with SFTS (94-96,115-119) and in experimental animal models (103). Sun et al (94) revealed a significant decline in CD3+ and CD4+ expression in the peripheral blood of patients with SFTS, where the average leukocyte count was 2.86±1.56×109/l. In patients with SFTS, not only were the number of T lymphocytes decreased, the expression of cell apoptotic indicators Annexin and CD95, proliferation and activation markers Ki-67, human leukocyte antigen DR and CD25, programmed death-1, granzyme B and IFN-γ (T-cell function markers), were significantly increased in the T lymphocytes (117). This suggests that T-cell function was enhanced. Furthermore, peripheral blood T cells were determined to be negatively correlated with SFTS severity. After 2 weeks, the number of T lymphocytes increased rapidly but returned to normal 6 months after onset (95). Another study previously revealed arginine deficiency in SFTS cases following the metabolomics analysis of two independent patient cohorts, which suggested that SFTSV infection and the eventual mortality resulted from nitric oxide synthase and arginase metabolism (120). Therefore, the lack of arginine was associated with the expansion of myeloid-derived suppressor cells (120), which may implicate a role in the impaired anti-SFTSV T-cell function.
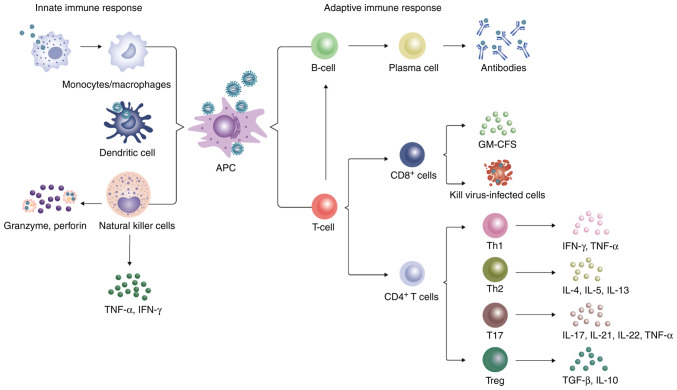
Naïve CD4+T cells can be divided into several subsets, including Th1, Th2, Th17 and regulatory T cells (Treg) based on their unique cytokine production profiles (114). Th1 cells mainly secrete IFN-γ and TNF-α cytokines and serve a key role in the immune response to viral infections (114). By contrast, Th2 cells are important for promoting immunity to helminth infection and allergic inflammation by emitting IL-4, IL-5 and IL-13 (114). T17 cells have been found to secrete cytokines, such as IL-17, IL-21, IL-22 and TNF-α, to induce immune damage in the presence of virus infection (121). Tregs mainly secrete anti-inflammatory cytokines, including TGF-β and IL-10, to limit the immune response to pathogens and control inflammation, contributing to immune homeostasis (Fig. 2) (122). The proportion of CD4+/total lymphocytes and CD4+CD25+/CD4+ cells noticeably declined in patients with SFTS compared with that in healthy individuals, but was higher compared with that in patients with severe disease. By contrast, the percentage of CD4+CD25+Foxp3+/CD4+CD25+ cells were markedly increased in the SFTS group but remained lower compared with that in the severe group (115). This suggests that the proportion of CD4+/total lymphocytes, CD4+CD25+/CD4+ cells and CD4+CD25+Foxp3+/CD4+CD25+ cells may be an important prognostic factor for patients with SFTS. Concurrently with CD4+ T-cell depletion, the number of Th1, Th2 and Treg cells was also found to be reduced in patients who succumbed to SFTS, whereas the population of Th17 cells showed no significant changes. In addition, the increase in the percentage of Th2 and Th17 cells in the CD4+ T-cell population resulted in abnormal Th1/Th2 and Th17/Treg ratios, which were positively correlated with disease severity (116).
Figure 2.
Innate immune response and the adaptive immune response against SFTSV. The antigen is presented to adaptive immune cells after recognizing the SFTSV by monocytes/macrophages, dendritic cells and NK cells. In addition, monocytes/macrophages can engulf the virus to induce apoptosis, whereas NK cells can release perforin and granzyme to lyse lean virus-infected cells. CD8+ T cells can directly kill virus-infected cells by removing cytotoxic particles. Naïve CD4+ T cells can differentiate into Th1, Th2, Th17 and Tregs under different cytokine environments. These helper T cells secrete various cytokines that provoke an inflammatory storm, leading to harmful outcomes and possibly to lymphopenia. T cells recognize virus-antigens presented by major histocompartibility complex II and stimulate the proliferation and differentiation of B cells by secreting cytokines. A portion of B cells differentiates into Ig-secreting plasma cells, which secrete anti-SFTSV antibodies. SFTSV, severe fever with thrombocytopenia syndrome virus; NK, natural killer.
B-cell-mediated immune response to SFTSV
B lymphocytes serve an integral role in the humoral immunity against viral infection. In general, the antigen can be recognized, endocytosed and/or degraded by the B-cell receptor and then presented on the cell surface by MHC II to search for explicitly differentiated CD4+T cells for the same antigen. A proportion of B cells can differentiate into plasma cells that secrete immunoglobulin (Ig) M, whilst others migrate to B-cell follicles and form germinal centers with the assistance of cytokines secreted by CD4+Th and follicular DCs (123). Following viral infection, antibodies bind to the virus to prevent it from attaching to and entering the target cell, which is then cleared by complement or antibody-dependent cell cytotoxicity (ADCC) (124).
Numerous studies have reported that compared with healthy subjects, the level of B cells was markedly elevated and positively correlated with the severity of SFTS disease (94,96,125,126). Further studies revealed that the peripheral blood mononuclear cells of patients with SFTS could induce propagation of atypical lymphocytes in vitro, and these transient atypical lymphocytes were activated B cells generated by stimuli other than virus particles, which were released by SFTSV-infected B cells, indicating that SFTSV-infected B cells release factors that cause B cells to differentiate into plasmablasts (118,126-128). By contrast, a previous cohort investigation study found that the number of B cells was markedly reduced during the first week of infection but quickly returned to normal levels in patients with SFTS (95). Further pathological examinations revealed that large numbers of activated mature plasmablasts were located in the secondary lymphoid organs from patients who succumbed to the disease (94). At the end of the lethal SFTSV infection, the B cells of the secondary lymphatic organs, which differentiate into plasmablasts and macrophages, are the target cells of fatal SFTSV infection. SFTSVs mainly infect B cell-lineage lymphocytes. Previous pathological observations support the notion that the SFTSV/B cell axis serves a key role in the pathogenesis of patients with SFTS (126). Further analysis of the B-cell subpopulations in the patient cohort revealed a meaningful difference between the survival group and the fatal group: Double-negative B-cells (CD27−IgD− cells) and plasma cells (CD27+IgD− cells) elevated in the fatal group. The marginal zone B cells (CD27+IgD+ cells) assessed in the survival group was lower compared with that in the fatal group (89). Additionally, the number of naïve B cells (CD27−IgD+ cells) in the survival and fatal groups was decreased overall, but the total number of naïve B cells in the survival groups was higher (89). In summary, these findings for detecting B-cell subpopulations suggest that patients who succumbed to SFTS have B-cell maturation disorders and subsequent dysregulation of the humoral immune response.
A study has been previously conducted to detect virus-specific antibodies in humans infected with SFTSV, including IgM, IgG and neutralizing antibodies (125,126). The SFTSV-specific IgM antibodies were detected between 4 and 21 days (median of 9 days) of onset, peaking by week 4 and lasting up to ≤6 months (95). SFTSV-specific IgG anti-bodies can be detected between 2 and 9 weeks (median of 6 weeks), where the maximum value was reached 6 months after infection. In addition, the majority of the patients remain positive after 3 years of illness (95). Initial levels of IgM and IgG antibodies were lower compared with those in patients with other underlying or compromised immune responses, such as the elderly and/or those with underlying diseases. The innate immune system may be severely suppressed in these subjects, leaving insufficient activation of adaptive immunity (95). Previous research also revealed that adaptive immunodeficiency caused by the disruption of humoral immunity mediated by B cells was a predictor of fatal SFTS. This cripples one's ability to mount a specific immune response from IgM or IgG to SFTSV NP and Gn, which is essential for neutralizing and eliminating the virus (89). Suzuki et al (129) examined the expression of immunoglobulins (IgM and IgG) in SFTV-infected B cells in the lymph nodes. Following germination, the majority of the infected B cells were transformed B cells, where the transformed B cells that were IgG-positive also infiltrated all organs of the body (129). These findings differed from those from previous studies, because various peripheral plasmablasts in patients with fatal SFTS do not express IgM and IgG, suggesting that activated and differentiated B cells cannot perform IgG conversion. These findings may explain the inadequate B-cell humoral response in patients who were deceased (129). Since the mechanism of B cells is unclear in individuals with SFTS, the role of B cells in the pathogenesis of SFTSV and its effects on SFTSV require further study.
8. Conclusion and future prospects
SFTSV infection is becoming an increasingly prevalent public health issue worldwide and its incidence is increasing annually. However, the transmission cycle of SFTSV remains poorly understood and its pathogenesis has not been elucidated, particularly its interaction with the host immune response. The immunopathogenesis of SFTSV infection is complex, which includes a cascade of reactions involving a wide range of immune cells, inflammatory mediators, inflammasomes and signaling pathways. Although studies have indicated the role of certain subsets of immune cells and NSs proteins in regulating the immune response during SFTSV infection, evasion of the immune system and the initiation of a proinflammatory response may serve a dual role. By contrast, the immune response can eliminate SFTSV; however, excessive immune activation can also lead to a hyperinflammatory response with clinical collateral damage. To designate strategies for the treatment of SFTSV, how innate immune dysregulation and proinflammatory molecules are generated, in addition to how to reveal and modulate critical signaling molecules must be studied. Damage to adaptive immunity in patients with fatal SFTS suggests that the severe immunological dysregulation caused by the SFTSV infection, along with the immunosuppressive milieu, leads to inadequate antigen presentation and subsequent class-switching B cells (130). SFTSV can also induce cellular damage through other mechanisms, such as mitochondrial dysfunction and ER stress. The cellular debris can trigger the immune defense through the positive feedback regulation of DAMPs and PAMPs, amplifying the inflammatory response and leading to further injury. Therefore, how an organism eliminates the virus using the immune response and restores homeostasis remains to be further investigated.
Previous studies have reported the role of exosomes in viral infection (131). The virus can complete its replication cycle in the host cell before the progeny virus is released. Viruses can hijack exosomes, utilize biogenesis systems and load their components to evade the host immune response and facilitate cell-to-cell diffusion (132). Exosomes, on the other hand, can be used by host cells to release antiviral substances and prevent viral infection. As a result, the interaction between exosomes generated by SFTSV-infected cells and the immune system of the host should further be studied. Exosomes are being employed as a next-generation drug delivery platform for a range of cargos, such that exosome-based therapies for SFTSV should be further explored. During the outbreak of coronavirus disease 2019, researchers and clinicians worldwide have been devoting considerable effort into researching coronavirus disease 2019 (COVID-19). Vaccination, effective antiviral therapy, modulation of the innate immune response and restoration of the adaptive immune response would improve the prognosis of patients with COVID-19 (133). Therefore, experience can be drawn from the treatment and management of COVID-19 to manage patients with SFTS, to improve their prognosis whilst lowering the mortality rate.
Overall, in the present review, recent research progress in host immune responses against SFTSV was summarized. In addition to strengthening public health education, it is necessary to accelerate research into the virus and its pathogenesis. Furthermore, a large-scale randomized controlled trial is required for the exploration of more particular treatment strategies and effective preventative measures to reduce the CFR. Clinicians need to actively exchange experience and continuously optimize diagnosis and treatment to cope with the SFTS disease jointly.
Acknowledgments
Not applicable.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 81871242).
Availability of data and materials
Data sharing does not apply to this article, as no datasets were generated or analyzed during the present study.
Authors' contributions
TY and HH performed the literature search and wrote the manuscript. JL and LJ supervised and revised the manuscript. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
References
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, et al. Fever with thrombocytopenia associated with a novel Bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhn JH, Adkins S, Agwanda BR, Al Kubrusli R, Alkhovsky SV, Amarasinghe GK, Avšič-Županc T, Ayllón MA, Bahl J, Balkema-Buschmann A, et al. 2021 Taxonomic update of phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch Virol. 2021;166:3513–3566. doi: 10.1007/s00705-021-05143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YR, Yun Y, Bae SG, Park D, Kim S, Lee JM, Cho NH, Kim YS, Lee KH. Severe Fever with Thrombocytopenia Syndrome Virus Infection, South Korea, 2010. Emerg Infect Dis. 2018;24:2103–2105. doi: 10.3201/eid2411.170756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis. 2014;209:816–827. doi: 10.1093/infdis/jit603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran XC, Yun Y, Van An L, Kim SH, Thao NTP, Man PKC, Yoo JR, Heo ST, Cho NH, Lee KH. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg Infect Dis. 2019;25:1029–1031. doi: 10.3201/eid2505.181463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Win AM, Nguyen YTH, Kim Y, Ha NY, Kang JG, Kim H, San B, Kyaw O, Htike WW, Choi DO, et al. Genotypic heterogeneity of orientia tsutsugamushi in scrub typhus patients and thrombocytopenia syndrome co-infection, Myanmar. Emerg Infect Dis. 2020;26:1878–1881. doi: 10.3201/eid2608.200135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng SH, Yang SL, Tang SE, Wang TC, Hsu TC, Su CL, Chen MY, Shimojima M, Yoshikawa T, Shu PY. Human case of severe fever with thrombocytopenia syndrome virus infection, Taiwan, 2019. Emerg Infect Dis. 2020;26:1612–1614. doi: 10.3201/eid2607.200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ongkittikul S, Watanawong R, Romopho P. Severe fever with thrombocytopenia syndrome virus: The first case report in Thailand. Bangkok Med J. 2020;16:204–206. doi: 10.31524/bkkmedj.2020.22.001. [DOI] [Google Scholar]
- 9.Zohaib A, Zhang J, Saqib M, Athar MA, Hussain MH, Chen J, Sial AU, Tayyab MH, Batool M, Khan S, et al. Serologic evidence of severe fever with thrombocytopenia syndrome virus and related viruses in Pakistan. Emerg Infect Dis. 2020;26:1513–1516. doi: 10.3201/eid2607.190611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albariño CG, Zaki SR, Rollin PE, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 11.Seo JW, Kim D, Yun N, Kim DM. Clinical update of severe fever with thrombocytopenia Syndrome. Viruses. 2021;13:1213. doi: 10.3390/v13071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo JR, Kim TJ, Heo ST, Hwang KA, Oh H, Ha T, Ko HK, Baek S, Kim JE, Kim JH, et al. IL-6 and IL-10 levels, rather than viral load and neutralizing antibody titers, determine the fate of patients with severe fever with thrombocytopenia syndrome virus infection in South Korea. Front Immunol. 2021;12:711847. doi: 10.3389/fimmu.2021.711847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Lu QB, Xing B, Zhang SF, Liu K, Du J, Li XK, Cui N, Yang ZD, Wang LY, et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011-17: A prospective observational study. Lancet Infect Dis. 2018;18:1127–1137. doi: 10.1016/S1473-3099(18)30293-7. [DOI] [PubMed] [Google Scholar]
- 14.Luo LM, Zhao L, Wen HL, Zhang ZT, Liu JW, Fang LZ, Xue ZF, Ma DQ, Zhang XS, Ding SJ, et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis. 2015;21:1770–1776. doi: 10.3201/eid2110.150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu YY, Zhuang L, Liu K, Sun Y, Dai K, Zhang XA, Zhang PH, Feng ZC, Li H, Liu W. Role of three tick species in the maintenance and transmission of severe fever with thrombocytopenia syndrome virus. PLoS Negl Trop Dis. 2020;14:e0008368. doi: 10.1371/journal.pntd.0008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Walker DH, Ren J, Wang Y, Yu XJ. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 2012;12:156–160. doi: 10.1089/vbz.2011.0758. [DOI] [PubMed] [Google Scholar]
- 17.Jung IY, Choi W, Kim J, Wang E, Park SW, Lee WJ, Choi JY, Kim HY, Uh Y, Kim YK. Nosocomial person-to-person transmission of severe fever with thrombocytopenia syndrome. Clin Microbiol Infect. 2019;25:633.e1–633.e4. doi: 10.1016/j.cmi.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Deng B, Zhang J, Cui W, Yao W, Liu P. Person-to-person asymptomatic infection of severe fever with thrombocytopenia syndrome virus through blood contact. Intern Med. 2014;53:903–906. doi: 10.2169/internalmedicine.53.1164. [DOI] [PubMed] [Google Scholar]
- 19.Gong Z, Gu S, Zhang Y, Sun J, Wu X, Ling F, Shi W, Zhang P, Li D, Mao H, et al. Probable aerosol transmission of severe fever with thrombocytopenia syndrome virus in southeastern China. Clin Microbiol Infect. 2015;21:1115–1120. doi: 10.1016/j.cmi.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) 2017 - First Annual review of diseases prioritized under the Research and Development Blueprint. WHO; Geneva: 2017. https://www.who.int/news-room/events/detail/2017/01/24/default-calendar/january-2017-first-annual-review-of-diseases-prioritized-under-the-research-and-development-blueprint. Accessed January 24, 2017. [Google Scholar]
- 21.Oh HS, Kim M, Lee JO, Kim H, Kim ES, Park KU, Kim HB, Song KH. Hemophagocytic lymphohistiocytosis associated with SFTS virus infection: A case report with literature review. Medicine (Baltimore) 2016;95:e4476. doi: 10.1097/MD.0000000000004476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 23.Weng Y, Chen N, Han Y, Xing Y, Li J. Clinical and laboratory characteristics of severe fever with thrombocytopenia syndrome in Chinese patients. Braz J Infect Dis. 2014;18:88–91. doi: 10.1016/j.bjid.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko M, Shikata H, Matsukage S, Maruta M, Shinomiya H, Suzuki T, Hasegawa H, Shimojima M, Saijo M. A patient with severe fever with thrombocytopenia syndrome and hemophagocytic lymphohistiocytosis-associated involvement of the central nervous system. J Infect Chemother. 2018;24:292–297. doi: 10.1016/j.jiac.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Jung IY, Ahn K, Kim J, Choi JY, Kim HY, Uh Y, Kim YK. Higher fatality for severe fever with thrombocytopenia syndrome complicated by hemophagocytic lymphohistiocytosis. Yonsei Med J. 2019;60:592–596. doi: 10.3349/ymj.2019.60.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalil J, Kato H, Fujita T. The role of non-structural protein NSs in the pathogenesis of severe fever with thrombocytopenia syndrome. Viruses. 2021;13:876. doi: 10.3390/v13050876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He CQ, Ding NZ. Discovery of severe fever with thrombocytopenia syndrome bunyavirus strains originating from intragenic recombination. J Virol. 2012;86:12426–12430. doi: 10.1128/JVI.01317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei XY, Liu MM, Yu XJ. Severe fever with thrombocytopenia syndrome and its pathogen SFTSV. Microbes Infect. 2015;17:149–154. doi: 10.1016/j.micinf.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Huang X, Li J, Li A, Wang S, Li D. Epidemiological characteristics of severe fever with thrombocytopenia syndrome from 2010 to 2019 in Mainland China. Int J Environ Res Public Health. 2021;18:3092. doi: 10.3390/ijerph18063092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu XJ. Risk factors for death in severe fever with thrombocytopenia syndrome. Lancet Infect Dis. 2018;18:1056–1057. doi: 10.1016/S1473-3099(18)30312-8. [DOI] [PubMed] [Google Scholar]
- 31.Korea Disease Control and Prevention Agency Ticks and Rodents Borne Infectious Diseases Guideline 2020. http://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&act=view&list_no=365644. Accessed December 30, 2021.
- 32.Choi SJ, Park SW, Bae IG, Kim SH, Ryu SY, Kim HA, Jang HC, Hur J, Jun JB, Jung Y, et al. Severe fever with thrombocytopenia syndrome in South Korea, 2013-2015. PLoS Negl Trop Dis. 2016;10:e0005264. doi: 10.1371/journal.pntd.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M, Heo ST, Oh H, Oh S, Lee KH, Yoo JR. Prognostic factors of severe fever with thrombocytopenia syndrome in South Korea. Viruses. 2021;13:10. doi: 10.3390/v13010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute of Infectious Diseases: Severe Fever with Thrombocytopenia Syndrome (SFTS) in Japan, as of February 2016. https://www.niid.go.jp/niid/en/iasr-vol33-e/865-iasr/6339-tpc433.html. Accessed September 26, 2021.
- 35.Kobayashi Y, Kato H, Yamagishi T, Shimada T, Matsui T, Yoshikawa T, Kurosu T, Shimojima M, Morikawa S, Hasegawa H, et al. Severe fever with thrombocytopenia syndrome, Japan, 2013-2017. Emerg Infect Dis. 2020;26:692–699. doi: 10.3201/eid2604.191011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L, Li J, Cui X, Jia N, Wei J, Xia L, Wang H, Zhou Y, Wang Q, Liu X, et al. Distribution of Haemaphysalis longicornis and associated pathogens: analysis of pooled data from a China field survey and global published data. Lancet Planet Health. 2020;4:e320–e329. doi: 10.1016/S2542-5196(20)30145-5. [DOI] [PubMed] [Google Scholar]
- 37.Yoo JR, Heo ST, Song SW, Bae SG, Lee S, Choi S, Lee C, Jeong S, Kim M, Sa W, et al. Severe fever with thrombocytopenia syndrome virus in ticks and SFTS incidence in humans, South Korea. Emerg Infect Dis. 2020;26:2292–2294. doi: 10.3201/eid2609.200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niu G, Li J, Liang M, Jiang X, Jiang M, Yin H, Wang Z, Li C, Zhang Q, Jin C, et al. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis. 2013;19:756–763. doi: 10.3201/eid1905.120245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kida K, Matsuoka Y, Shimoda T, Matsuoka H, Yamada H, Saito T, Imataki O, Kadowaki N, Noguchi K, Maeda K, et al. A Case of Cat-to-Human transmission of severe fever with thrombocytopenia syndrome virus. Jpn J Infect Dis. 2019;72:356–358. doi: 10.7883/yoken.JJID.2018.526. [DOI] [PubMed] [Google Scholar]
- 40.Kimura T, Fukuma A, Shimojima M, Yamashita Y, Mizota F, Yamashita M, Otsuka Y, Kan M, Fukushi S, Tani H, et al. Seroprevalence of severe fever with thrombocytopenia syndrome (SFTS) virus antibodies in humans and animals in Ehime prefecture, Japan, an endemic region of SFTS. J Infect Chemother. 2018;24:802–806. doi: 10.1016/j.jiac.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Rim JM, Han SW, Cho YK, Kang JG, Choi KS, Jeong H, Son K, Kim J, Choi Y, Kim WM, et al. Survey of severe fever with thrombocytopenia syndrome virus in wild boar in the Republic of Korea. Ticks Tick Borne Dis. 2021;12:101813. doi: 10.1016/j.ttbdis.2021.101813. [DOI] [PubMed] [Google Scholar]
- 42.Okada A, Hotta A, Kimura M, Park ES, Morikawa S, Inoshima Y. A retrospective survey of the seroprevalence of severe fever with thrombocytopenia syndrome virus in wild animals in Japan. Vet Med Sci. 2021;7:600–605. doi: 10.1002/vms3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Jia B, Huang R, Yan X, Xiong Y, Yong L, Chao W. Occupational severe fever with thrombocytopenia syndrome following needle-stick injury. Infect Control Hosp Epidemiol. 2017;38:760–762. doi: 10.1017/ice.2017.61. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Wu H, Gao J, Zhou X, Zhu R, Zhang C, Bai H, Abdullah AS, Pan H. Two confirmed cases of severe fever with thrombocytopenia syndrome with pneumonia: Implication for a family cluster in East China. BMC Infect Dis. 2017;17:537. doi: 10.1186/s12879-017-2645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang X, Hu J, Peng Z, Dai Q, Liu W, Liang S, Li Z, Zhang N, Bao C. Epidemiological and clinical characteristics of severe fever with thrombocytopenia syndrome bunyavirus human-to-human transmission. PLoS Negl Trop Dis. 2021;15:e0009037. doi: 10.1371/journal.pntd.0009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You E, Wang L, Zhang L, Wu J, Zhao K, Huang F. Epidemiological characteristics of severe fever with thrombocytopenia syndrome in Hefei of Anhui Province: A population-based surveillance study from 2011 to 2018. Eur J Clin Microbiol Infect Dis. 2021;40:929–939. doi: 10.1007/s10096-020-04098-x. [DOI] [PubMed] [Google Scholar]
- 47.Carty M, Guy C, Bowie AG. Detection of viral infections by innate immunity. Biochem Pharmacol. 2021;183:114316. doi: 10.1016/j.bcp.2020.114316. [DOI] [PubMed] [Google Scholar]
- 48.Park A, Iwasaki A. Type I and Type III interferons-induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoo JS, Kato H, Fujita T. Sensing viral invasion by RIG-I like receptors. Curr Opin Microbiol. 2014;20:131–138. doi: 10.1016/j.mib.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Chow KT, Gale M, Jr, Loo YM. RIG-I and Other RNA sensors in antiviral immunity. Annu Rev Immunol. 2018;36:667–694. doi: 10.1146/annurev-immunol-042617-053309. [DOI] [PubMed] [Google Scholar]
- 51.Yamada S, Shimojima M, Narita R, Tsukamoto Y, Kato H, Saijo M, Fujita T. RIG-I-like receptor and toll-like receptor signaling pathways cause aberrant production of inflammatory cytokines/chemokines in a severe fever with thrombocytopenia syndrome virus infection mouse model. J Virol. 2018;92:e02246–17. doi: 10.1128/JVI.02246-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Min YQ, Ning YJ, Wang H, Deng F. A RIG-I-like receptor directs antiviral responses to a bunyavirus and is antagonized by virus-induced blockade of TRIM25-mediated ubiquitination. J Biol Chem. 2020;295:9691–9711. doi: 10.1074/jbc.RA120.013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu BY, Yu XJ, Zhou CM. SAFA initiates innate immunity against cytoplasmic RNA virus SFTSV infection. PLoS Pathog. 2021;17:e1010070. doi: 10.1371/journal.ppat.1010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Onomoto K, Onoguchi K, Yoneyama M. Regulation of RIG-I-like receptor-mediated signaling: Interaction between host and viral factors. Cell Mol Immunol. 2021;18:539–555. doi: 10.1038/s41423-020-00602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y, Jin C, Zhan F, Wang X, Liang M, Zhang Q, Ding S, Guan X, Huo X, Li C, et al. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J Infect Dis. 2012;206:1085–1094. doi: 10.1093/infdis/jis452. [DOI] [PubMed] [Google Scholar]
- 56.Zhang YZ, He YW, Dai YA, Xiong Y, Zheng H, Zhou DJ, Li J, Sun Q, Luo XL, Cheng YL, et al. Hemorrhagic fever caused by a novel bunyavirus in China: Pathogenesis and correlates of fatal outcome. Clin Infect Dis. 2012;54:527–533. doi: 10.1093/cid/cir804. [DOI] [PubMed] [Google Scholar]
- 57.Liu MM, Lei XY, Yu H, Zhang JZ, Yu XJ. Correlation of cytokine level with the severity of severe fever with thrombocytopenia syndrome. Virol J. 2017;14:6. doi: 10.1186/s12985-016-0677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y, Liu MM, Lei XY, Yu XJ. SFTS phlebovirus promotes LC3-II accumulation and nonstructural protein of SFTS phlebovirus co-localizes with autophagy proteins. Sci Rep. 2018;8:5287. doi: 10.1038/s41598-018-23610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang LK, Wang B, Xin Q, Shang W, Shen S, Xiao G, Deng F, Wang H, Hu Z, Wang M. Quantitative proteomic analysis reveals unfolded-protein response involved in severe fever with thrombocytopenia syndrome virus infection. J Virol. 2019;93:e00308–19. doi: 10.1128/JVI.00308-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu B, Qi X, Wu X, Liang M, Li C, Cardona CJ, Xu W, Tang F, Li Z, Wu B, et al. Suppression of the interferon and NF-κB responses by severe fever with thrombocytopenia syndrome virus. J Virol. 2012;86:8388–8401. doi: 10.1128/JVI.00612-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moriyama M, Igarashi M, Koshiba T, Irie T, Takada A, Ichinohe T. Two conserved amino acids within the NSs of severe fever with thrombocytopenia syndrome phlebovirus are essential for anti-interferon activity. J Virol. 2018;92:e00706–18. doi: 10.1128/JVI.00706-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ning YJ, Wang M, Deng M, Shen S, Liu W, Cao WC, Deng F, Wang YY, Hu Z, Wang H. Viral suppression of innate immunity via spatial isolation of TBK1/IKKε from mitochondrial antiviral platform. J Mol Cell Biol. 2014;6:324–337. doi: 10.1093/jmcb/mju015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santiago FW, Covaleda LM, Sanchez-Aparicio MT, Silvas JA, Diaz-Vizarreta AC, Patel JR, Popov V, Yu XJ, García-Sastre A, Aguilar PV. Hijacking of RIG-I signaling proteins into virus-induced cytoplasmic structures correlates with the Inhibition of type I interferon responses. J Virol. 2014;88:4572–4585. doi: 10.1128/JVI.03021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ning YJ, Feng K, Min YQ, Cao WC, Wang M, Deng F, Hu Z, Wang H. Disruption of type I interferon signaling by the nonstructural protein of severe fever with thrombocytopenia syndrome virus via the Hijacking of STAT2 and STAT1 into Inclusion Bodies. J Virol. 2015;89:4227–4236. doi: 10.1128/JVI.00154-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong Y, Bai M, Qi X, Li C, Liang M, Li D, Cardona CJ, Xing Z. Suppression of the IFN-α and -β Induction through Sequestering IRF7 into viral inclusion bodies by nonstructural protein NSs in severe fever with thrombocytopenia syndrome bunyavirus infection. J Immunol. 2019;202:841–856. doi: 10.4049/jimmunol.1800576. [DOI] [PubMed] [Google Scholar]
- 66.Song P, Zheng N, Zhang L, Liu Y, Chen T, Bao C, Li Z, Yong W, Zhang Y, Wu C, Wu Z. Downregulation of Interferon-β and Inhibition of TLR3 expression are associated with fatal outcome of severe fever with thrombocytopenia syndrome. Sci Rep. 2017;7:6532. doi: 10.1038/s41598-017-06921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee JK, Shin OS. Nonstructural protein of severe fever with thrombocytopenia syndrome phlebovirus inhibits TBK1 to evade interferon-mediated response. J Microbiol Biotechnol. 2021;31:226–232. doi: 10.4014/jmb.2008.08048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshikawa R, Sakabe S, Urata S, Yasuda J. Species-Specific pathogenicity of severe fever with thrombocytopenia syndrome virus is determined by Anti-STAT2 Activity of NSs. J Virol. 2019;93:e02226–18. doi: 10.1128/JVI.02226-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitagawa Y, Sakai M, Shimojima M, Saijo M, Itoh M, Gotoh B. Nonstructural protein of severe fever with thrombocytopenia syndrome phlebovirus targets STAT2 and not STAT1 to inhibit type I interferon-stimulated JAK-STAT signaling. Microbes Infect. 2018;20:360–368. doi: 10.1016/j.micinf.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Ferrara JL, Abhyankar S, Gilliland DG. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc. 1993;25:1216–1217. [PubMed] [Google Scholar]
- 71.Teijaro JR. Cytokine storms in infectious diseases. Semin Immunopathol. 2017;39:501–503. doi: 10.1007/s00281-017-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu Y, Hsu AC, Pang Z, Pan H, Zuo X, Wang G, Zheng J, Wang F. Role of the innate cytokine storm induced by the influenza a virus. Viral Immunol. 2019;32:244–251. doi: 10.1089/vim.2019.0032. [DOI] [PubMed] [Google Scholar]
- 74.Ryabkova VA, Churilov LP, Shoenfeld Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm-The common denominator and the lessons to be learned. Clin Immunol. 2021;223:108652. doi: 10.1016/j.clim.2020.108652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bopp NE, Kaiser JA, Strother AE, Barrett ADT, Beasley DWC, Benassi V, Milligan GN, Preziosi MP, Reece LM. Baseline mapping of severe fever with thrombocytopenia syndrome virology, epidemiology and vaccine research and development. NPJ Vaccines. 2020;5:111. doi: 10.1038/s41541-020-00257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou C, Yu X. Unraveling the Underlying interaction mechanism between dabie bandavirus and innate immune response. Front Immunol. 2021;12:676861. doi: 10.3389/fimmu.2021.676861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deng B, Zhang S, Geng Y, Zhang Y, Wang Y, Yao W, Wen Y, Cui W, Zhou Y, Gu Q, et al. Cytokine and chemokine levels in patients with severe fever with thrombocytopenia syndrome virus. PLoS One. 2012;7:e41365. doi: 10.1371/journal.pone.0041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding YP, Liang MF, Ye JB, Liu QH, Xiong CH, Long B, Lin WB, Cui N, Zou ZQ, Song YL, et al. Prognostic value of clinical and immunological markers in acute phase of SFTS virus infection. Clin Microbiol Infect. 2014;20:O870–O878. doi: 10.1111/1469-0691.12636. [DOI] [PubMed] [Google Scholar]
- 79.Park A, Park SJ, Jung KL, Kim SM, Kim EH, Kim YI, Foo SS, Kim S, Kim SG, Yu KM, et al. Molecular signatures of inflammatory profile and B-Cell function in patients with severe fever with thrombocytopenia syndrome. mBio. 2021;12:e02583–20. doi: 10.1128/mBio.02583-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Z, Wang B, Li Y, Hu K, Yi Z, Ma H, Li X, Guo W, Xu B, Huang X. Changes in peripheral blood cytokines in patients with severe fever with thrombocytopenia syndrome. J Med Virol. 2021;93:4704–4713. doi: 10.1002/jmv.26877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J, Han Y, Xing Y, Li S, Kong L, Zhang Y, Zhang L, Liu N, Wang Q, Wang S, et al. Concurrent measurement of dynamic changes in viral load, serum enzymes, T cell subsets, and cytokines in patients with severe fever with thrombocytopenia syndrome. PLoS One. 2014;9:e91679. doi: 10.1371/journal.pone.0091679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khalil J, Yamada S, Tsukamoto Y, Abe H, Shimojima M, Kato H, Fujita T. The non-structural protein NSs of SFTSV causes a cytokine storm through the hyper-activation of NF-κB. Mol Cell Biol. 2021;41:e00542–20. doi: 10.1128/MCB.00542-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi Y, Park SJ, Sun Y, Yoo JS, Pudupakam RS, Foo SS, Shin WJ, Chen SB, Tsichlis PN, Lee WJ, et al. Severe fever with thrombocytopenia syndrome phlebovirus non-structural protein activates TPL2 signalling pathway for viral immunopathogenesis. Nat Microbiol. 2019;4:429–437. doi: 10.1038/s41564-018-0329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu S, Jiang N, Nawaz W, Liu B, Zhang F, Liu Y, Wu X, Wu Z. Infection of humanized mice with a novel phlebovirus presented pathogenic features of severe fever with thrombocytopenia syndrome. PLoS Pathog. 2021;17:e1009587. doi: 10.1371/journal.ppat.1009587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 87.Mailliard RB. Dendritic cells and antiviral defense. Viruses. 2020;12:1152. doi: 10.3390/v12101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 89.Zhang W, Li M, Xiong S, Wang H, Xiong Y, Li M, Lu M, Yang D, Peng C, Zheng X. Decreased myeloid dendritic cells indicate a poor prognosis in patients with severe fever with thrombocytopenia syndrome. Int J Infect Dis. 2017;54:113–120. doi: 10.1016/j.ijid.2016.11.418. [DOI] [PubMed] [Google Scholar]
- 90.Song P, Zheng N, Liu Y, Tian C, Wu X, Ma X, Chen D, Zou X, Wang G, Wang H, et al. Deficient humoral responses and disrupted B-cell immunity are associated with fatal SFTSV infection. Nat Commun. 2018;9:3328. doi: 10.1038/s41467-018-05746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao Z, Zheng W, Yan L, Sun P, Xu T, Zhu Y, Liu L, Tian L, He H, Wei Y, Zheng X. Recombinant human adenovirus type 5 Co-expressing RABV G and SFTSV Gn induces protective immunity against rabies virus and severe fever with thrombocytopenia syndrome virus in mice. Front Microbiol. 2020;11:1473. doi: 10.3389/fmicb.2020.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. 2019;19:282–290. doi: 10.1038/s41577-019-0139-2. [DOI] [PubMed] [Google Scholar]
- 93.Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 94.Sun L, Hu Y, Niyonsaba A, Tong Q, Lu L, Li H, Jie S. Detection and evaluation of immunofunction of patients with severe fever with thrombocytopenia syndrome. Clin Exp Med. 2014;14:389–395. doi: 10.1007/s10238-013-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu QB, Cui N, Hu JG, Chen WW, Xu W, Li H, Zhang XA, Ly H, Liu W, Cao WC. Characterization of immunological responses in patients with severe fever with thrombocytopenia syndrome: A cohort study in China. Vaccine. 2015;33:1250–1255. doi: 10.1016/j.vaccine.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 96.Liu J, Wang L, Feng Z, Geng D, Sun Y, Yuan G. Dynamic changes of laboratory parameters and peripheral blood lymphocyte subsets in severe fever with thrombocytopenia syndrome patients. Int J Infect Dis. 2017;58:45–51. doi: 10.1016/j.ijid.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 97.Li M, Xiong Y, Li M, Zhang W, Liu J, Zhang Y, Xiong S, Zou C, Liang B, Lu M, et al. Depletion but activation of CD56dimCD16+ NK Cells in acute infection with severe fever with thrombocytopenia syndrome virus. Virol Sin. 2020;35:588–598. doi: 10.1007/s12250-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nikitina E, Larionova I, Choinzonov E, Kzhyshkowska J. Monocytes and macrophages as viral targets and reservoirs. Int J Mol Sci. 2018;19:2821. doi: 10.3390/ijms19092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 101.Meidaninikjeh S, Sabouni N, Marzouni HZ, Bengar S, Khalili A, Jafari R. Monocytes and macrophages in COVID-19: Friends and foes. Life Sci. 2021;269:119010. doi: 10.1016/j.lfs.2020.119010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mendoza CA, Yamaoka S, Tsuda Y, Matsuno K, Weisend CM, Ebihara H. The NF-κB inhibitor, SC75741, is a novel antiviral against emerging tick-borne bandaviruses. Antiviral Res. 2021;185:104993. doi: 10.1016/j.antiviral.2020.104993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin C, Liang M, Ning J, Gu W, Jiang H, Wu W, Zhang F, Li C, Zhang Q, Zhu H, et al. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc Natl Acad Sci USA. 2012;109:10053–10058. doi: 10.1073/pnas.1120246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang L, Fu Y, Wang H, Guan Y, Zhu W, Guo M, Zheng N, Wu Z. Severe fever with thrombocytopenia syndrome virus-induced macrophage differentiation is regulated by miR-146. Front Immunol. 2019;10:1095. doi: 10.3389/fimmu.2019.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan JM, Zhang WK, Li F, Zhou CM, Yu XJ. Integrated transcriptome profiling in THP-1 macrophages infected with bunyavirus SFTSV. Virus Res. 2021;306:198594. doi: 10.1016/j.virusres.2021.198594. [DOI] [PubMed] [Google Scholar]
- 106.Kabelitz D. Gamma Delta T cells (γδ T Cells) in health and disease: In memory of professor wendy havran. Cells. 2020;9:2564. doi: 10.3390/cells9122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu W, Li XK, Lu QB, Yang ZD, Du J, Xing B, Cui N, Zhang XA, Zhang SF, Yang XX, et al. Association between peripheral γδ T cell subsets and disease progression of severe fever with thrombocytopenia syndrome virus infection. Pathog Dis. 2017;75 doi: 10.1093/femspd/ftx086. [DOI] [PubMed] [Google Scholar]
- 108.Broz P, Dixit VM. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 109.Martinon F, Burns K, Tschopp J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 110.Liu JW, Chu M, Jiao YJ, Zhou CM, Qi R, Yu XJ. SFTSV Infection Induced Interleukin-1β secretion through NLRP3 inflammasome activation. Front Immunol. 2021;12:595140. doi: 10.3389/fimmu.2021.595140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Z, Hu J, Bao C, Gao C, Zhang N, Cardona CJ, Xing Z. Activation of the NLRP3 inflammasome and elevation of interleukin-1β secretion in infection by sever fever with thrombocytopenia syndrome virus. Sci Rep. 2022;12:2573. doi: 10.1038/s41598-022-06229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li S, Li H, Zhang YL, Xin QL, Guan ZQ, Chen X, Zhang XA, Li XK, Xiao GF, Lozach PY, et al. SFTSV infection induces BAK/BAX-Dependent mitochondrial DNA release to Trigger NLRP3 inflammasome activation. Cell Rep. 2020;30:4370–4385.e7. doi: 10.1016/j.celrep.2020.02.105. [DOI] [PubMed] [Google Scholar]
- 113.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S33–S40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 114.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yi X, Li W, Li H, Jie S. Circulating regulatory T cells in patients with severe fever with thrombocytopenia syndrome. Infect Dis (Lond) 2015;47:294–301. doi: 10.3109/00365548.2014.987812. [DOI] [PubMed] [Google Scholar]
- 116.Li MM, Zhang WJ, Weng XF, Li MY, Liu J, Xiong Y, Xiong SE, Zou CC, Wang H, Lu MJ, et al. CD4 T cell loss and Th2 and Th17 bias are associated with the severity of severe fever with thrombocytopenia syndrome (SFTS) Clin Immunol. 2018;195:8–17. doi: 10.1016/j.clim.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li MM, Zhang WJ, Liu J, Li MY, Zhang YF, Xiong Y, Xiong SE, Zou CC, Xiong LQ, Liang BY, et al. Dynamic changes in the immunological characteristics of T lymphocytes in surviving patients with severe fever with thrombocytopenia syndrome (SFTS) Int J Infect Dis. 2018;70:72–80. doi: 10.1016/j.ijid.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 118.Takahashi T, Suzuki T, Hiroshige S, Nouno S, Matsumura T, Tominaga T, Yujiri T, Katano H, Sato Y, Hasegawa H. Transient appearance of plasmablasts in the peripheral blood of Japanese patients with severe fever with thrombocytopenia syndrome. J Infect Dis. 2019;220:23–27. doi: 10.1093/infdis/jiz054. [DOI] [PubMed] [Google Scholar]
- 119.Hu L, Kong Q, Yue C, Xu X, Xia L, Bian T, Liu Y, Zhang H, Ma X, Yin H, et al. Early-Warning immune predictors for invasive pulmonary aspergillosis in severe patients with severe fever with thrombocytopenia syndrome. Front Immunol. 2021;12:576640. doi: 10.3389/fimmu.2021.576640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li XK, Lu QB, Chen WW, Xu W, Liu R, Zhang SF, Du J, Li H, Yao K, Zhai D, et al. Arginine deficiency is involved in thrombocytopenia and immunosuppression in severe fever with thrombocytopenia syndrome. Sci Transl Med. 2018;10:eaat4162. doi: 10.1126/scitranslmed.aat4162. [DOI] [PubMed] [Google Scholar]
- 121.Aghbash PS, Hemmat N, Nahand JS, Shamekh A, Memar MY, Babaei A, Baghi HB. The role of Th17 cells in viral infections. Int Immunopharmacol. 2021;91:107331. doi: 10.1016/j.intimp.2020.107331. [DOI] [PubMed] [Google Scholar]
- 122.Kervevan J, Chakrabarti LA. Role of CD4+ T cells in the control of viral infections: Recent advances and open questions. Int J Mol Sci. 2021;22:523. doi: 10.3390/ijms22020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnett LG, Simkins HMA, Barnett BE, Korn LL, Johnson AL, Wherry EJ, Wu GF, Laufer TM. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol. 2014;192:3607–3617. doi: 10.4049/jimmunol.1301284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lam JH, Smith FL, Baumgarth N. B cell activation and response regulation during viral infections. Viral Immunol. 2020;33:294–306. doi: 10.1089/vim.2019.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takahashi T, Sano K, Suzuki T, Matsumura T, Sakai K, Tominaga T, Sato Y, Katano H, Hasegawa H. Virus-infected peripheral blood plasmablasts in a patient with severe fever with thrombocytopenia syndrome. Int J Hematol. 2021;113:436–440. doi: 10.1007/s12185-020-03040-3. [DOI] [PubMed] [Google Scholar]
- 126.Wada Y, Miyamoto S, Iida S, Sano K, Sato Y, Ainai A, Saito K, Katano H, Hasegawa H, Suzuki T. Propagation of activated B cells by in vitro severe fever with thrombocytopenia syndrome virus infection of human peripheral blood mononuclear cells. J Infect Dis. 2022;225:269–281. doi: 10.1093/infdis/jiab343. [DOI] [PubMed] [Google Scholar]
- 127.Wada T, Iwata Y, Kamikawa Y, Wada T, Yachie A. Peripheral blood plasmacytosis in severe fever with thrombocytopenia syndrome. Jpn J Infect Dis. 2017;70:470–471. doi: 10.7883/yoken.JJID.2016.575. [DOI] [PubMed] [Google Scholar]
- 128.Zhang J, Yan X, Li Y, Gao R, Wang P, Mo W. Reactive plasmacytosis mimicking multiple myeloma associated with SFTS virus infection: A report of two cases and literature review. BMC Infect Dis. 2018;18:528. doi: 10.1186/s12879-018-3431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suzuki T, Sato Y, Sano K, Arashiro T, Katano H, Nakajima N, Shimojima M, Kataoka M, Takahashi K, Wada Y, et al. Severe fever with thrombocytopenia syndrome virus targets B cells in lethal human infections. J Clin Invest. 2020;130:799–812. doi: 10.1172/JCI129171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mendoza CA, Ebihara A, Yamaoka S. Immune modulation and immune-mediated pathogenesis of emerging tickborne banyangviruses. Vaccines (Basel) 2019;7:125. doi: 10.3390/vaccines7040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caobi A, Nair M, Raymond AD. Extracellular vesicles in the pathogenesis of viral infections in humans. Viruses. 2020;12:1200. doi: 10.3390/v12101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saad MH, Badierah R, Redwan EM, El-Fakharany EM. A comprehensive insight into the role of exosomes in viral infection: Dual faces bearing different functions. Pharmaceutics. 2021;13:1405. doi: 10.3390/pharmaceutics13091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anka AU, Tahir MI, Abubakar SD, Alsabbagh M, Zian Z, Hamedifar H, Sabzevari A, Azizi G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand J Immunol. 2021;93:e12998. doi: 10.1111/sji.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article, as no datasets were generated or analyzed during the present study.