Abstract
Microsensors, including a recently developed NO3− biosensor, were applied to measure O2 and NO3− profiles in marine sediments from the upwelling area off central Chile and to investigate the influence of Thioploca spp. on the sedimentary nitrogen metabolism. The studies were performed in undisturbed sediment cores incubated in a small laboratory flume to simulate the environmental conditions of low O2, high NO3−, and bottom water current. On addition of NO3− and NO2−, Thioploca spp. exhibited positive chemotaxis and stretched out of the sediment into the flume water. In a core densely populated with Thioploca, the penetration depth of NO3− was only 0.5 mm and a sharp maximum of NO3− uptake was observed 0.5 mm above the sediment surface. In sediments with only few Thioploca spp., NO3− was detectable down to a depth of 2 mm and the maximum consumption rates were observed within the sediment. No chemotaxis toward nitrous oxide (N2O) was observed, which is consistent with the observation that Thioploca does not denitrify but reduces intracellular NO3− to NH4+. Measurements of the intracellular NO3− and S0 pools in Thioploca filaments from various depths in the sediment gave insights into possible differences in the migration behavior between the different species. Living filaments containing significant amounts of intracellular NO3− were found to a depth of at least 13 cm, providing final proof for the vertical shuttling of Thioploca spp. and nitrate transport into the sediment.
Although the ability of microorganisms to oxidize reduced sulfur compounds with nitrate as the electron acceptor has been known for about one hundred years (1) and several pure cultures have been obtained and studied (see, e.g., references 39 and 42), only a little is known about the ecological significance of this type of metabolism. For instance, the first report showing a clear coupling between the sulfur and nitrogen cycles in the marine environment was sulfide-driven denitrification at the oxic-anoxic interface in the water column of the Central Baltic Sea (3).
It was recently discovered that sulfide-oxidizing bacteria of the genus Thioploca possess large nitrate-filled vacuoles (10). Thioploca is highly abundant in the shelf sediments along Peru and Chile (11, 12, 14, 29), and it was therefore suspected that these organisms play a major role in coupling the biogeochemical cycles of nitrogen and sulfur in upwelling areas (10). The observation of benthic Thioploca filaments in the upwelling area of the Arabian Sea (20) and the finding of both Thioploca and the spherical, nitrate-storing bacterium Thiomargarita off Namibia (34) support this conclusion. Nitrate-storing sulfide-oxidizing bacteria have also been observed at hydrothermal vents and cold seeps and in organic-rich sediments (23, 24, 44).
Currently, none of the nitrate-storing sulfur bacteria is in pure culture, and alternative methods have to be applied to study their physiology and ecology. Enzyme preparations from partially purified Beggiatoa samples showed high nitrate reductase and ribulose-1,5-bisphosphate carboxylase-oxygenase activity and provide the first biochemical evidence for the use of nitrate as a electron acceptor for sulfide oxidation and chemoautotrophic growth (23). Incubation experiments with partially purified Thioploca filaments revealed that sulfide (H2S) was first rapidly oxidized to [S0], which was then further oxidized to sulfate (SO42−) in a second independent step. Intravacuolar [NO3−] served as the electron acceptor and was reduced to ammonium (NH4+) (25). Radiolabeled bicarbonate (H14CO3−) and [2-14C]acetate were assimilated, indicating that Thioploca is a facultative chemolithoautotroph capable of mixotrophic growth (22, 25). Whole-core incubations in small laboratory flumes have helped to unveil the chemotactic behavior of Thioploca under changing environmental conditions. Thioploca showed positive chemotaxis toward nitrate and low sulfide concentrations (<100 μM) but a phobic reaction toward oxygen and high sulfide concentrations (17). These observations and the finding of mostly vertically oriented living filaments several centimeters deep in the sediment led to the suggestion that Thioploca shuttles up and down between NO3−-rich bottom water and H2S-containing sediment (10, 17).
The samples for this study were collected at stations within and off the Bay of Concepción in central Chile, where the species composition and annual dynamics of the Thioploca population are known from a previous study (36). On the shelf, the community was composed mainly of T. araucae, T. chileae, and a yet undescribed form of Thioploca with much shorter cells. This so-called short-cell morphotype (SCM) (35, 36) is usually found in deeper sediment layers than the two other species and is characterized by rounded cells and a cell length/diameter ratio of ≤0.48. The SCM is closely related but not identical to the known Thioploca species as revealed by partial 16S rDNA analysis (35). Within the Bay of Concepción, large vacuolated filaments cover the sediment surface during part of the year. They are, apart from the lack of sheaths, phenotypically and phylogenetically almost identical to T. araucae (36, 40).
The aim of this study was to gain information about the ecology of Thioploca spp. and the influence of these organisms on the nitrogen and sulfur cycles in the habitat. By the use of microsensors, we show that the nitrate uptake of the sediment is strongly influenced by Thioploca. Chemotaxis toward inorganic nitrogen compounds was studied, and measurements of [S0] and [NO3−] concentrations were used to test the concept of a vertical shuttling between the nitrate-rich bottom water and the deeper sediment layers. We also demonstrate that nitrate is transported by Thioploca down to a sediment depth of at least 13 cm.
MATERIALS AND METHODS
Abbreviations.
Nitrate and elemental sulfur are generally abbreviated by NO3− and S0, respectively. For the intracellular pools [NO3−] and [S0] are used.
Study area.
The continental shelf region off the Concepción Bay (central Chile) is characterized by intense seasonal upwelling. Between austral late spring and early fall, southern and southwestern winds prevail and the northward-flowing Sub-Antarctic Surface water is forced off the coast, leading to upwelling of Equatorial Subsurface water from the Poleward Undercurrent at 100 to 400 m (37). The Equatorial Subsurface water is characterized by high salinity (34.4 to 34.8%), low temperature (8.5 to 10.5°C), low oxygen concentrations (<20 μM), but high nitrate (about 25 μM) and nutrient concentrations (37). Upwelling off central Chile is intermittent and usually lasts between 2 and 7 days (16). Primary and secondary productivity greatly increase when the nutrient-rich water is transported up into the euphotic zone. For the coastal upwelling area off central Chile, a primary production of 9.6 g of C m−2 day−1 has been reported (10), which is one of the highest observed in marine environments. Due to the lack of oxygen and sufficient amounts of alternative electron acceptors, sedimentary organic matter is almost exclusively degraded by sulfate-reducing bacteria (41). The sulfate reduction rates reported for this area (170 to 4,670 nmol cm−3 day−1 [(8)]) are among the highest observed in coastal margins, but free sulfide concentrations in these sediments are surprisingly low, indicating an efficient reoxidation of sulfide (8; J. Zopfi, M. E. Böttcher, and B. B. Jørgensen, submitted for publication).
Sampling and site description.
During January and February 1997, we repeatedly sampled sediment from three stations within and off the Bay of Concepción (Fig. 1). The sediment was collected from the research vessel Kay Kay of the University of Concepción (Concepción, Chile) by means of a small gravity corer. The cores were stored onboard at 4°C in a refrigerator and were transported on the day of sampling to the Marine Biological Station of the University Concepción in Dichato, where all experiments were performed.
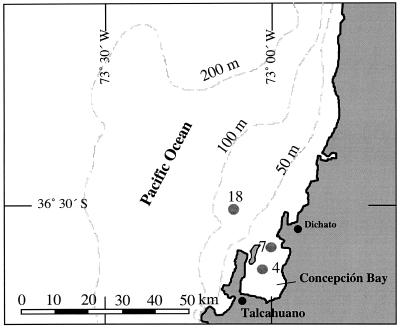
FIG. 1.
Sampling sites within the Bay of Concepción and on the adjacent continental shelf off central Chile.
Station 4 (36° 38′ 08" S; 073° 02′ 03" W) was 24 m deep and located within the bay (Fig. 1). The sediment at this station was highly sulfidic (up to 1,200 μM at 7 cm deep [Zopfi et al., submitted]) and was uniformly black below the brownish uppermost 3 to 4 mm. The top 4.5 to 5 cm of the sediment was a flocculent ooze, with mass accumulation of Beggiatoa spp. The sediment of Station 7 (36° 36′ 05" S; 073° 00′ 06" W; 32 m deep), at the mouth of the bay, was covered with a brown, spongy layer 1.5 cm thick, which was densely populated by Thioploca spp. Below this layer was a 0.4-cm-thick band of black iron sulfide, followed by gray-brownish sediment. Burrows of sediment-dwelling organisms were observed. The sediment of Station 18 (35° 30′ 08" S; 073° 07′ 06" W; 88 m deep) had a similar structure, with a spongy layer 0.5 to 1 cm thick, a thin black layer (0.2 to 0.3 cm thick), and then gray sediment below. Thioploca, however, was much less abundant, and no animal burrows were observed. The concentration of free sulfide was <6 μM down to a depth of 20 cm at Stations 7 and 18. During the sampling period, the bottom-water concentration of O2 was <2 to 7 μM at all stations. The NO3− concentrations were about 6 μM at Station 4, and 7 to 24 μM at Stations 7 and 18 (36).
Microsensor measurements and flux calculations.
A Clark-type O2 microsensor with a guard cathode was used to measure oxygen microprofiles (28). Nitrate was measured with a microbiosensor consisting of an electrochemical N2O microsensor surrounded by an outer casing (19). A 100 to 200-μm-long reaction chamber was formed between the tip of the internal N2O sensor and the ion-permeable membrane at the tip of the outer casing. An N2O reductase-deficient culture of Agrobacterium immobilized in the reaction chamber transformed NO3− and NO2− to N2O, which was detected by the N2O microsensor. Due to this design, the microsensor measured the combined NO3−, NO2−, and N2O concentrations in a sample. The N2O microsensor was constructed like the O2 microsensor (28) but the cathode was plated with silver and the electrolyte consisted of 0.5 M NaOH and 0.5 M KCl. The N2O microsensor was polarized at −1.2 V against an Ag/AgCl anode immersed in the electrolyte. The current from the microsensor was measured with a custom-made pA meter and recorded on a strip chart recorder. The active silver surface of the N2O sensing cathode is also sensitive toward H2S. However, the concentrations of free sulfide in the top centimeters of the Station 7 and 18 sediment was usually <1 μM, and hence no interference with H2S was anticipated. Calibrations and measurements were done at the same temperature. The detection limit of the sensor was about 3 μM NO3−, and the linear range was 3 to 70 μM NO3−. The sensitivity for N2O was about 1 μM, and the signal was linear up to least 1,000 μM. The response time for 90% signal intensity was about 50 s. A resting time of 1 min was used for each depth step in a profile. The position of the sediment surface was determined for each profile using a dissection microscope (magnification, ×10 to ×50).
Fluxes across the sediment-water interface were calculated from the upper linear part of the microsensor profiles by Fick's first law of diffusion, J = −D × dC/dx, where J is the flux (in micromoles per square centimeter per second), D is the diffusion coefficient (in square centimeters per second), and dC/dx is the concentration gradient (in micromoles per cubic centimeter per centimeter). The activity profiles were calculated as described in detail elsewhere (T. Kjær, L.-H. Larsen, and N. P. Revsbech, unpublished data). Tabulated diffusion coefficients were recalculated for the in situ temperature and salinity (4, 21). Since the sediment was highly porous in the top 1 cm, the same diffusion coefficients of 1.8 × 10−5 and 1.41 × 10−5 cm2 s−1 for O2 and NO3− respectively, were used for the sediment and water phase.
Experimental setup.
To simulate the environmental conditions prevailing off the coast, measurements were done in a small flow chamber where deaerated surface water from Station 7 was circulated. A small aquarium pump was used to create flows of approximately 4 to 6 cm s−1 about 2 cm above the sediment surface. The flow chamber consisted of two horizontal Plexiglas plates that were separated with a spacer. Both Plexiglas plates contained a central hole (50 cm2). A sediment core was brought into the flow through the hole in the bottom plate, and the microsensors were introduced through the hole in the top plate. A dissolved-O2 concentration of about 5 μM was maintained in the circulating water by adjusting the area of air-exposed seawater at the top hole, and the water was kept at the in situ temperature of 12°C by a thermostatted circulating cooler. For the chemotaxis experiments, NO3−, NO2−, or N2O was added to nitrate-depleted flume water and the behavior of Thioploca was observed from above through a dissection microscope. The number of filaments emerging from their sheaths was determined by setting the focus plane at about 2 mm above the sediment surface and by counting the filaments penetrating the plane. The length of a filament was determined with a measuring eyepiece and by focusing down from the filament tip to the sediment surface.
Extraction and analysis of [NO3−] and [S0] in Thioploca.
Bundles of Thioploca filaments from different sediment depths of Station 7 were picked out and aligned in a film of seawater on a microscope slide. Forceps and needles were used to rip the sheath apart so that intact single filaments could be isolated.
The length (l) and diameter (d) of each filament were determined, and the biovolume (V) was calculated (V = πld2/4). According to the cell diameter and the cell-length-to-diameter ratio, the organism was identified as T. araucae, T. chileae, or SCM (36). With the same formula, the volume ratio between the cytoplasm and vacuole was determined for each species by using the following cell dimensions (length, diameter): T. araucae, 14.4 μm, 15.4 μm; T. chileae, 35.5 μm, 24.2 μm; SCM, 35.5 μm, 9.4 μm. For all three species, a mean cytoplasm thickness of 1 μm was used (36; H. Schulz, personal communication).
A single filament was then picked up on the tip of a purpose-made glass needle and left to dry in air for a few minutes, so that the filaments died and the cells cracked. Nitrate was extracted from the filament by dipping the glass needle for 5 s into a droplet of 20 μl of demineralized water.
Nitrate was analyzed by the cadmium reduction method (13), with some adaptations to small amounts. The cadmium column typically consisted of a 3-cm-long glass tube (inner diameter, 1.1 mm) with a slightly coiled, 1-mm-wide cadmium rod inside. A bent glass capillary with a pointed tip was mounted on the upper end of the column, and a straight capillary was mounted on the bottom. The total system could contain about 10 μl of liquid, and once activated the column was always kept filled with buffer solution. The column was held almost vertical, and when the upper tip was dipped in liquid, gravity created a water flow of about 30 μl min−1 through the column. Capillary forces in the pointed tip prevented intrusion of air when the tip was out of water. The 20-μl sample with the extracted nitrate was sucked up, immediately followed by 40 μl of buffer solution. Below the reduction column the sample and buffer solution was collected in a 300-μl well of a microplate. Aliquots (20 μl) of reagents were added by the standard procedure, and 200 μl of demineralized water was mixed in as well. The color intensity was read at 670 nm, and corrections for turbidity, if any, were made by reading at 405 nm. NO3− standard solutions (20 μl each) were processed parallel with the filament extracts and used for calibration. Linearity was observed up to 250 μM. The reduction efficiency was checked by using NO2− standards, and the cadmium column was reactivated or renewed whenever required. The detection limit of the NO3− assay was about 20 pmol. We analyzed filaments with biovolumes from 0.0006 to 0.0154 mm3; the detection limit was therefore equivalent to an [NO3−] concentration of 33 mM for the smallest filament and 2 mM for the largest filament. No NO3− was detected when empty sheaths were analyzed or when a filament was dried and extracted a second time, thus confirming that no significant contamination or loss of nitrate occurred in the procedure.
After extraction of nitrate, the filament was air dried again and immersed in 50 μl of methanol for extraction of [S0]. The complete dissolution of sulfur globules was verified by extraction time series and light microscopy. Elemental sulfur in the extract was quantified as cyclo-octasulfur (S8) by high-performance liquid chromatography. Separation was done on a Zorbax ODS column (125 by 4 mm, 5 μm; Knauer, Germany) with methanol (100%; high-performance liquid chromatography grade) as the eluent at a flow rate of 1 ml min−1. S8 eluted after 3.5 min and was detected at 265 nm. The detection limit was 43 pmol and was equivalent to an S0 concentration of 72 mM for the smallest filament and 3 mM for the largest filament. Repeated measurements of [S0] in filaments of the same species inhabiting a common sheath showed a variability of <10% relative standard deviation. No loss of S0 was observed during the preceding [NO3−] extraction.
Elemental sulfur in the bulk sediment was extracted from Zn-preserved samples with pure methanol for 16 h on a rotary shaker; the sediment-to-extractant ratio was about 1:20 (wet wt/vol). S0 in the filtered (0.45-μm-pore-size filter) extracts was quantitated as described above. The variability within triplicate S0 extractions was <14%.
Statistical treatment.
The correlation between [NO3−] and [S0] was determined and tested for significance by the method of Spearman (30). The [NO3−]/[S0] ratios of filaments from different depth intervals were tested for similarity with the H test of Kruskal and Wallis (30).
RESULTS AND DISCUSSION
Influence of Thioploca on NO3− profiles and uptake rates.
Nitrate has been measured in biofilms and lake sediments by liquid ion exchanger (LIX)-based microsensors (7, 32, 38), but similar measurements in marine environments were not possible due to the interference of Cl− ions. In this study we used a recently developed NO3− biosensor that allowed us to measure microprofiles in sediments from the upwelling system off central Chile and to study the influence of Thioploca on the NO3− uptake. Huettel et al. (17) suggested that filament protrusion may be a strategy to overcome the diffusion limitation to NO3− uptake imposed by the boundary layer and that Thioploca may thereby outcompete NO3−-consuming bacteria in the sediment. To test this hypothesis, we incubated sediment from two different stations in the the flume under similar conditions to those described above and measured the oxygen and nitrate microprofiles.
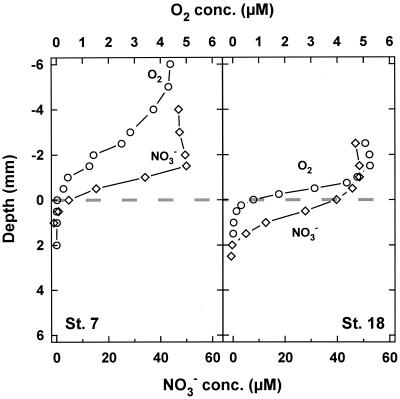
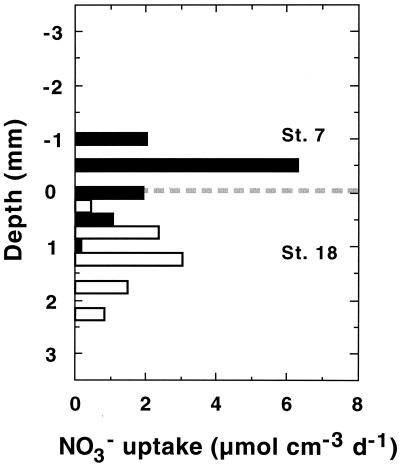
At the time of sampling, the sediment of Station 7 was densely populated by Thioploca spp., with a total wet biomass of 44 g m−2 (36) and the filaments stretched out of the sediment when nitrate was present in the flume water. Thioploca was much less abundant in the second core from Station 18, where the wet biomass was only 9 g m−2 (36). Protruding filaments were only sporadically observed in the core studied. This difference in Thioploca spp. abundance was clearly reflected in the O2 and NO3− profiles (Fig. 2). The profiles from Station 7 measured in the vicinity of Thioploca filaments were oddly shaped, and O2 and NO3− hardly penetrated to the sediment surface. Since the filaments protruded into the flume water, maximum NO3− uptake rates occured above the sediment surface (Fig. 3) and the NO3− penetration was only 0.5 mm. Station 18 showed an O2 microprofile normal for marine sediments, with a diffusive boundary layer thickness of about 1 mm and a maximal O2 penetration depth of 1 mm. Nitrate penetrated about 2 mm into the sediment, and the linear range of the NO3− gradient (Fig. 2) and maximal uptake rates were both within the sediment (Fig. 3). The profile structure and penetration depth were very similar to those found in organic-rich lake sediments (19, 38).
FIG. 2.
Laboratory flume measurements of O2 and NO3− concentrations in sediments with a high (Station 7) and very low (Station 18) density of Thioploca spp. The broken line indicates the sediment-water interface.
FIG. 3.
Vertical distribution of NO3− uptake rates in sediment with a high (Station 7) and low (Station 18) density of Thioploca filaments. The broken line indicates the sediment-water interface.
Interestingly, the diffusive boundary layer at Station 7 was >1.5 mm, considerably thicker than at Station 18 (Fig. 2). A likely explanation for this could be that Thioploca filaments protruding from the sediment impede the water flow, which leads to a thickening of the boundary layer and thus to a lower diffusive exchange across the sediment-water interface. Thus, the oxygen uptake at Station 7 was only 0.26 ± 0.11 mmol m−2 day−1 (n = 6) compared to 0.74 ± 0.13 mmol m−2 day−1 (n = 6) at Station 18. The calculated diffusive NO3− uptake at Station 7 was 5.45 ± 1.17 mmol m−2 day−1 (n = 4), about 55% higher than at Station 18 (3.53 ± 0.76 mmol m−2 day−1; n = 5). In summary, Thioploca spp. have a major influence on the sedimentary NO3− metabolism. They penetrate up through the diffusive boundary layer, cause a reduced NO3− penetration depth, move the maximum NO3− uptake upward, and increase the areal NO3− uptake rate.
An interesting effect of the Thioploca community might be that nitrate-reducing bacteria in the sediment are outcompeted for NO3− and that denitrification consequently plays a minor role in sediments densely inhabited by Thioploca spp. The situation, however, is probably more complex, as indicated by denitrification measurements. Despite the presence of Thioploca spp., denitrification rates of 4.5 and 9 mmol m−2 day−1 were determined for Station 7 by adding 100 μM 15NO3− to the flume water (L. P. Nielsen, unpublished data). These values are slightly higher than usually observed in normal coastal sediments at similar NO3− concentrations (references 2 and 15 and references therein) and suggest only an incomplete suppression of denitrification. Nitrate for denitrification may be supplied by leakage from Thioploca filaments or via advective transport of bottom water into the sediment. Advective transport becomes progressively more important with increasing flow velocities (9) and may indeed have been an important process, because the top 1 to 2 cm of the sediment had a spongy consistency and was very porous due to the Thioploca mat (26). Furthermore, the in situ flow velocity of the bottom water near the sediment may well exceed the 5 cm s−1 that we have used in our experiments.
Response of Thioploca filaments to NO2− and N2O.
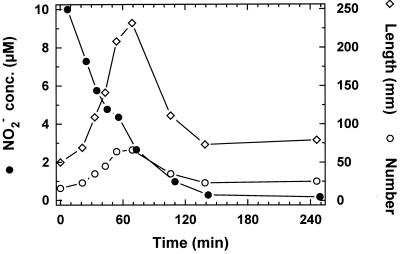
The chemotactic behavior of Thioploca spp. toward O2, NO3−, and H2S was studied by Huettel et al. (17). However, NO2− and N2O are also intermediates and by-products of nitrification and nitrate reduction processes (15, 18) and can be found in the oxygen minimum zone of upwelling areas (5, 6). We studied whether Thioploca spp. show chemotaxis toward NO2− and N2O and whether they can utilize them as electron acceptors by using cores from Station 7 and adding NO2− or N2O to nitrate-free flume water. On addition of 10 μM NO2−, the number and length of protruding filaments rapidly increased and reached a maximum after 1.2 h (Fig. 4). Single bundles were observed through the dissection microscope, and before the NO2− addition the filament tips were moving in and out of the sheath with frequent reversals just at the sediment-water interface. About 30 s after the NO2− addition, the reversals stopped and all the filaments moved upward, protruding from the sheath. When the NO2− concentration dropped below 2.6 μM, the filaments began to retreat until the initial positions were reestablished (Fig. 4). A similar sequence was observed when NO3− was added (data not shown). It cannot completely be ruled out that the real tactic trigger was NO3− produced by nitrifying bacteria. However, the fast reaction of Thioploca and the low O2 concentrations limiting nitrification in the setup do support a direct response to NO2−.
FIG. 4.
Response of Thioploca spp. to addition of 10 μM NO2− to the flume water as indicated by the number of filaments protruding >2 mm out of the sediment and by the total length of all filaments exposed to flume water.
In contrast to NO3− and NO2−, Thioploca filaments did not show chemotaxis toward N2O, suggesting that nitrous oxide may not be used as an electron acceptor for sulfide oxidation. Nitrous oxide is the obligate precursor for dinitrogen formation during denitrification and can be used by most, although not all, denitrifying bacteria as an electron acceptor (45). Reduction of N2O to ammonium has never been reported. The absence of a response to N2O therefore supports the finding of Otte et al. (25) that ammonium rather than dinitrogen, as previously assumed (10), is the terminal product of nitrate reduction.
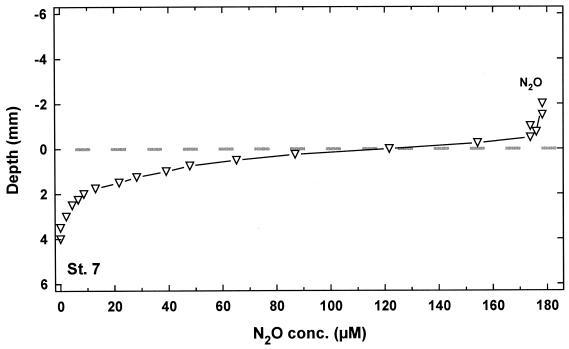
Microprofiles of N2O were measured during the chemotaxis experiment, and an average profile (n = 3) is depicted in Fig. 5. Since Thioploca did not stretch out from the sediment, the N2O profile exhibited a regular diffusive boundary layer of about 0.5 mm. However, even without the contribution of Thioploca spp., N2O was rapidly (15.1 ± 1.5 mmol m−2 day−1 consumed within the first 3.5 mm of the sediment, demonstrating the potential for sedimentary dinitrogen formation.
FIG. 5.
Microprofile of N2O in sediment from Station 7. Nitrous oxide was added to the flume water after the depletion of nitrate.
Internal [S0] and [NO3−] concentrations.
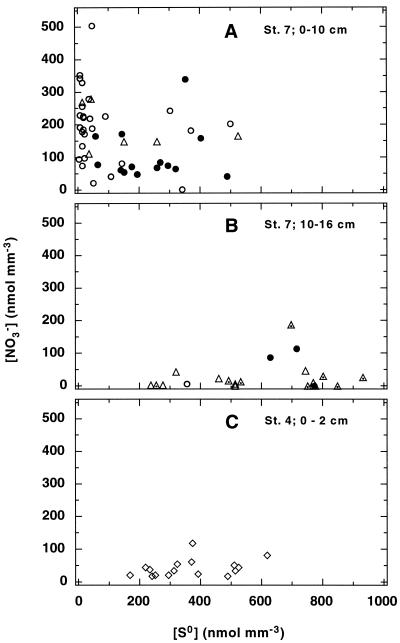
A concentration range of 150 to 500 nmol mm−3 has been reported for the [NO3−] content of Thioploca cells (10), but to date nothing is known about the variability within the different species or about the [NO3]/[S0] ratio within individual filaments and whether it changes with depth. Additionally, Thioploca filaments have been found down to 26 cm deep (36), but it was not clear whether they were still alive and contained [NO3−]. Therefore, Thioploca filaments were collected from different depths of a core from Station 7 and the concentrations of [NO3−] and [S0] were determined. The statistical analysis of the results from the depth intervals 0 to 1 cm, 1 to 2 cm, 2 to 3 cm, 3 to 4 cm, 4 to 7 cm, and 7 to 10 cm did not indicate significant differences in the [NO3−]/[S0] ratios between the different sections (P > 0.1). By the same statistical method, it was shown that the two lowest intervals from 10 to 13 cm and 13 to 16 cm, although not different from each other, were different from the first six intervals, with a high probability (P < 0.001). Based on these results, the measurements of [NO3−] and [S0] from 0 to 10 cm and from 10 to 16 cm deep, respectively, are grouped together (Fig. 6A and B).
FIG. 6.
(A and B) Concentrations of [NO3−] and [S0] in single Thioploca filaments collected at different sediment depths from Station 7. (C) Storage concentration in sheathless sulfur bacteria filaments from the sediment surface (0 to 2 cm) at Station 4. Open circles, T. araucae; solid circles, T. chileae; open triangles, SCM; dotted symbols, filaments from the deepest sediment section (13 to 16 cm); open diamonds, sheathless filaments.
In the upper part (0 to 10 cm) of the core, T. araucae (57%), and T. chileae (30%) were the dominant mat-forming species (Fig. 6A), with the rest being SCM thioplocas. The SCM were more abundant (81%) in the deeper section. Similarly, Schulz et al. (36) found the maximum T. araucae and T. chileae biomass close to the sediment-water interface whereas the SCM were most abundant below 7 cm deep. The difference in the species composition was also reflected in the [NO3−] and [S0] concentrations. Whereas [NO3−] and [S0] varied over similar concentration ranges (up to ∼500 nmol mm−3) in the upper section (Fig. 6A), the values were significantly shifted toward lower [NO3−] and higher [S0] concentrations in the 10- to 16-cm-deep section. Both the SCM and T. chileae stored [S0] up to ∼800 nmol mm−3. However, the maximum [S0] concentration determined for T. araucae was only 355 nmol mm−3 (Fig. 6B), which nicely corresponded to a lower volume ratio between cytoplasm and vacuole. Whereas SCM and T. chileae typically have a C/V ratio of 0.47, it is only 0.23 in T. araucae.
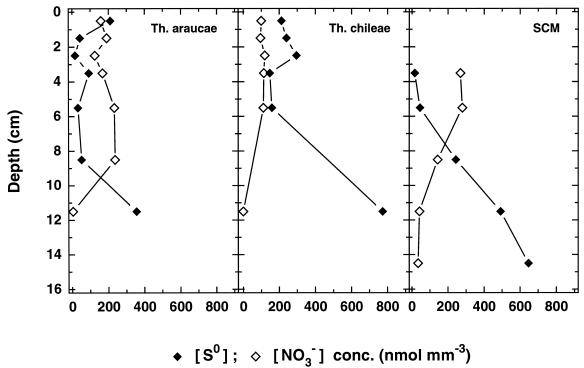
Based on results from chemotaxis experiments, it was concluded that Thioploca spp. fill their vacuoles with nitrate at the sediment surface. Then they migrate into deeper sediment layers, where they oxidize sulfide to [S0] and SO42− until low [NO3−] concentrations are reached and the upward movement is induced again (10, 17). Such a behavior would imply that (i) there exists a negative relationship between the two storage compounds in a filament and (ii) filaments with a high [NO3−]/[S0] ratio are found predominantly at the sediment surface whereas filaments rich in [S0] but depleted of [NO3−] are found in deeper sections. This, however, seems to be valid only for SCM thioplocas, where a negative correlation between [S0] and [NO3−] was found (r = −0.44; n = 24; P < 0.05; Spearman test) and a decreasing [NO3−]/[S0] ratio correlated with the sediment depth (r2 = 0.71). No significant correlations (P > 0.05) were found in T. araucae or in T. chileae, which suggests that they migrate in a different manner. A reason for the lack of correlation was found when the average [NO3−] and [S0] concentrations of all individuals of a species in a given depth interval were calculated (Fig. 7). Despite the variability in the [NO3−]/[S0] ratios, the average concentrations were surprisingly constant down several centimeters deep. This is probably best explained by a continuous and rapid shuttling of Thioploca filaments relative to the metabolic rate. A further consequence of the continuous shuttling is that the filaments frequently reach the sediment surface, where they can recharge their [NO3−] storage. Ample supply of nitrate, on the other hand, could allow Thioploca to oxidize H2S directly to SO42− instead of forming [S0] first. If this is true, it also explains why no clear correlation between [S0] and [NO3−] was detected in T. araucae and T. chileae, respectively. However, final proof for a vertical shuttling of Thioploca spp. and nitrate transport into the sediment comes from the finding of living filaments with substantial [NO3−] concentrations (31 ± 59 nmol mm−3; n = 9 [Fig. 6B]) in the 13- to 16-cm-deep sediment section. Calculations based on the turnover time of [NO3−] (8 to 10 days [25]) and a migration velocity of 5 mm h−1 (17) showed that the internal reservoir of electron acceptor in Thioploca cells is sufficient to reach such depths.
FIG. 7.
Vertical distribution of the mean [NO3−] and [S0] concentrations in the different Thioploca spp. from Station 7.
For comparison, [NO3−] and [S0] concentrations were also measured in the free filaments living at the sediment surface of Station 4 (Fig. 6C). Their [NO3−] content was 42 ± 27 nmol mm−3, i.e., much lower and less variable than in the 0- to 10-cm-deep section of Station 7 (Fig. 6A). It was also considerably lower than reported for Beggiatoa spp. from Monterey Canyon and Guaymas Basin (23). In general, [NO3−] and [S0] concentrations of the Station 4 filaments were similar to those of Thioploca spp. found below 10 cm at Station 7. This was probably due to the high H2S content (up to 1,200 μM) in this sediment. Based on the finding of a phobic response to sulfide concentrations of >500 μM (17) and the high morphological and phylogenetic similarity to T. araucae, it was even suggested that these filaments might actually be thioplocas that had moved out of their sheaths or did not produce them under the prevailing environmental conditions (40).
Intracellular versus extracellular pools of S0 and NO3−.
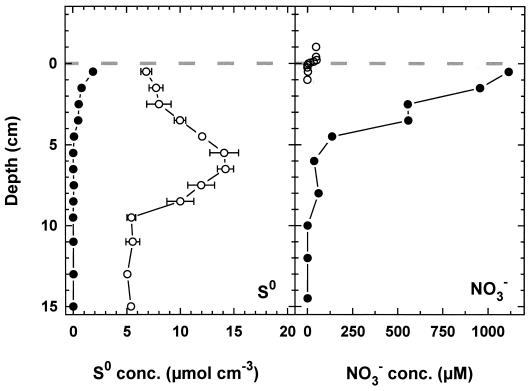
In sediments of a Danish fjord and in the Santa Barbara basin, it was observed that elemental sulfur was associated primarily with Beggiatoa filaments (31, 43). To quantify the contribution of [S0] to the total pool of S0 in the sediment, we manually collected all Thioploca filaments present in a core from Station 7 and determined the [S0] and S0 concentrations in the remaining bulk sediment (Fig. 8). In contrast to the two other studies, we found that [S0] made up maximally 27% of the total S0 pool. Furthermore, the two sulfur pools exhibited different distribution patterns. Whereas the [S0] corresponded to the distribution of Thioploca spp. (33) and decreased gradually with depth, the S0 was maximal at a depth of 5 cm. Because the turnover times of the two sulfur pools may be different, one cannot draw quantitative conclusions about the relative significance of Thioploca-associated and chemical sulfide oxidation, but it clearly demonstrates that other processes contribute to sulfide oxidation and sedimentary S0 formation. The distribution of chemical species (H2S, Fe2+, and S2O32−) in the pore water suggests that reducible iron oxides may also be an important oxidant for pore water H2S (41; Zopfi et al., submitted).
FIG. 8.
Intracellular (solid symbols) and extracellular (open symbols) pools of S0 and NO3− in sediment of Station 7. The broken line indicates the sediment surface.
From the known amount of [S0] and the average [NO3]/[S0]- ratio in a given depth interval, one can calculate the amount of nitrate being accumulated by Thioploca cells and transported into the sediment. If the [NO3−] were completely released from the cells, it would lead to pore water concentrations of about 1 mM (Fig. 8). There is evidence in the published literature that some measurements of pore water NO3− were influenced by [NO3−] released from vacuolated sulfur bacteria during pore water sampling. For example Henrichs and Farrington (14) noted that the NO3− pore water concentrations in Thioploca-containing sediment were higher than in the overlying seawater. In sediments from the Peru upwelling area, NO3− was found in significant concentrations down to an unusual depth of 10 cm and the maximum concentrations (up to 102 μM) were conspicuously high (11). In later publications where unusually high nitrate concentrations were observed, it was already suspected that they were affected by NO3− released from disrupted cells (27, 41).
Over recent years, a variety of approaches have been applied to estimate the contribution of Thioploca spp. to sulfide oxidation. Although the reported values vary from 3 to 91% (8, 10, 25, 41), most estimates fall in the range of 20 to 30%. However, even if Thioploca spp. were not the dominant player in sulfide oxidation in these sediments, they are most significant for the sedimentary nitrogen cycling. Organic matter in the sediments off Concepción Bay is almost exclusively degraded via sulfate reduction (equation 1) (41).
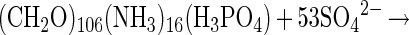 |
1 |
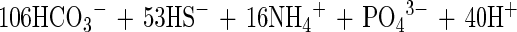 |
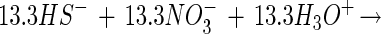 |
2 |
 |
If only 25% of the formed sulfide is oxidized by Thioploca spp. according to equation 2, this would lead to an 83% increase of the sedimentary ammonium production. Since ammonium is not lost from the environment, in contrast to N2, and since nitrogen tends to be the limiting factor for phytoplankton in the marine environment, this form of nutrient regeneration could have considerable consequences for the primary productivity in the area.
ACKNOWLEDGMENTS
The staff of Dichato, the crew of R/V Kay Kay, and all members of the Thioploca '97 expedition, V. A. Gallardo, J. G. Kuenen, S. Otte, H. Schulz, B. Strotmann, and A. Teske are thanked for their help and cooperation. A. Rusch is acknowledged for help with the statistical analysis, and T. Ferdelman and two anonymous reviewers are thanked for valuable comments on the manuscript.
This work was supported by the German Ministry for Education, Science, Research and Technology (to J.Z.) and the Max-Planck Society.
REFERENCES
- 1.Beijerinck M W. Phénomènes de réduction produits par les microbes. Arch Nederl Sci Exactes Nat. 1904;9:131–157. [Google Scholar]
- 2.Binnerup S J, Jensen K, Revsbech N P, Jensen M H, Sørensen J. Denitrification, dissimilatory reduction of nitrate to ammonium, and nitrification in a bioturbated estuarine sediment as measured with 15N and microsensor techniques. Appl Environ Microbiol. 1992;58:303–313. doi: 10.1128/aem.58.1.303-313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brettar I, Rheinheimer G. Denitrification in the central Baltic: evidence for H2S-oxidation as a motor of denitrification at the oxic anoxic interface. Mar Ecol Prog Ser. 1991;77:157–169. [Google Scholar]
- 4.Broecker W S, Peng T H. Gas exchange rates between air and sea. Tellus. 1974;26:21–35. [Google Scholar]
- 5.Codispoti L A, Friedrich G E, Packard T T, Glover H E, Kelly P J, Spinrad R W, Barber R T, Elkins J W, Ward B B, Lipschultz F, Lostaunau N. High nitrite levels off Northern Peru: a signal of instability in the marine denitrification rate. Science. 1986;233:1200–1202. doi: 10.1126/science.233.4769.1200. [DOI] [PubMed] [Google Scholar]
- 6.Copin-Montégut C, Raimbault P. The peruvian upwelling near 15°S in August 1986. Results of continuous measurements of physical and chemical properties between 0 and 220 m depth. Deep-Sea Res. 1994;41:439–467. [Google Scholar]
- 7.de Beer D, Sweerts J-P R A. Measurement of nitrate gradients with an ion-selective microelectrode. Anal Chim Acta. 1989;219:351–356. [Google Scholar]
- 8.Ferdelman T G, Lee C, Pantoja S, Harder J, Bebout B, Fossing H. Sulfate reduction and methanogenesis in a Thioploca-dominated sediment off the coast of Chile. Geochim Cosmochim Acta. 1997;61:3065–3079. [Google Scholar]
- 9.Forster S, Huettel M, Ziebis W. Impact of boundary layer flow velocity on oxygen utilization in coastal sediments. Mar Ecol Prog Ser. 1996;143:173–185. [Google Scholar]
- 10.Fossing H, Gallardo V A, Jørgensen B B, Huettel M, Nielsen L P, Schulz H, Canfield D, Foster S, Glud R N, Gundersen J K, Kuever J, Ramsing N B, Teske A, Thamdrup B, Ulloa O. Concentration and transport of nitrate by the mat-forming sulphur bacterium Thioploca. Nature. 1995;374:713–715. [Google Scholar]
- 11.Froelich P N, Arthur M A, Burnett W C, Deakin M, Hensley V, Jahnke R, Kaul L, Kim K-H, Roe K, Soutar A, Vathakanon C. Early diagenesis of organic matter in Peru continental margin sediments: phosphorite precipitation. Mar Geol. 1988;80:309–343. [Google Scholar]
- 12.Gallardo V A. Large benthic microbial communities in sulphide biota under Peru-Chile subsurface countercurrent. Nature. 1977;268:331–332. [Google Scholar]
- 13.Grasshoff K, Erhardt M, Kremling K. Methods of sea water analysis. Weinheim, Germany: Verlag Chemie; 1983. [Google Scholar]
- 14.Henrichs S M, Farrington J W. Peru upwelling region sediments near 15°S. 1. Remineralization and accumulation of organic matter. Limnol Oceanogr. 1984;29:1–19. [Google Scholar]
- 15.Herbert R A. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev. 1999;23:563–590. doi: 10.1111/j.1574-6976.1999.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 16.Hill A E, Hickey B M, Shillington F A, Strub P T, Brink K H, Barton E D, Thomas A C. Eastern ocean boundaries coastal segment (E) In: Robinson A R, Brink K H, editors. The sea. Vol. 11. New York, N.Y: John Wiley & Sons, Inc.; 1998. pp. 29–67. [Google Scholar]
- 17.Huettel M, Forster S, Klöser S, Fossing H. Vertical migration in the sediment dwelling sulfur bacteria Thioploca spp. in overcoming diffusion limitations. Appl Environ Microbiol. 1996;62:1863–1872. doi: 10.1128/aem.62.6.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowles R. Denitrification. Microbiol Rev. 1982;46:43–70. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen L H, Kjær T, Revsbech N P. A microscale NO3− biosensor for environmental applications. Anal Chem. 1997;69:3527–3531. doi: 10.1021/ac9700890. [DOI] [PubMed] [Google Scholar]
- 20.Levin L, Gage J, Lamont P, Cammidge L, Martin C, Patience A, Crooks J. Proceedings of the 30th Marine Biology Symposium. 1997. Infaunal community structure in a low-oxygen, organic rich habitat on the Oman continental slope, NW Arabian Sea; pp. 223–230. [Google Scholar]
- 21.Li Y H, Gregory S. Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta. 1974;38:703–714. [Google Scholar]
- 22.Maier S, Gallardo V A. Nutritional characteristics of two marine thioplocas determined by autoradiography. Arch Microbiol. 1984;139:218–220. [Google Scholar]
- 23.McHatton S C, Barry J P, Jannasch H W, Nelson D C. High nitrate concentrations in vacuolate, autotrophic marine Beggiatoa spp. Appl Environ Microbiol. 1996;62:954–958. doi: 10.1128/aem.62.3.954-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Namasaraev B B, Dulov L E, Dubinina G A, Zemskaya T I, Granina L Z, Karabanov E V. Bacterial synthesis and destruction of organic matter in microbial mats of Lake Baikal. Microbiology. 1994;63:193–197. [Google Scholar]
- 25.Otte S, Kuenen J G, Nielsen L P, Pearl H W, Zopfi J, Schulz H N, Teske A, Strotmann B, Gallardo V A, Jørgensen B B. Nitrogen, carbon and sulfur metabolism in natural Thioploca samples. Appl Environ Microbiol. 1999;65:3148–3157. doi: 10.1128/aem.65.7.3148-3157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimers C E. Organic matter in anoxic sediments off Central Peru: relations of porosity, microbial decomposition and deformation properties. Mar Geol. 1982;46:175–197. [Google Scholar]
- 27.Reimers C E, Ruttenberg K C, Canfield D E, Christiansen M B, Martin J B. Porewater pH and authigenic phases formed in the uppermost sediments of Santa Barbara Basin. Geochim Cosmochim Acta. 1996;60:4037–4057. [Google Scholar]
- 28.Revsbech N P. An oxygen microelectrode with a guard cathode. Limnol Oceanogr. 1989;34:472–476. [Google Scholar]
- 29.Rosenberg R, Arntz W E, de Flores E C, Flores L A, Carabajal G, Finger I, Tarazona J. Benthos biomass and oxygen deficiency in the upwelling system off Peru. J Mar Res. 1983;41:263–279. [Google Scholar]
- 30.Sachs L. Angewandte Statistik. 8th ed. Berlin, Germany: Springer-Verlag; 1997. [Google Scholar]
- 31.Schimmelmann A, Kastner M. Evolutionary changes over the last 1000 years of reduced sulfur phases and organic carbon in varved sediments of the Santa Barbara Basin, California. Geochim Cosmochim Acta. 1993;57:67–78. [Google Scholar]
- 32.Schramm A, Larsen L H, Revsbech N P, Ramsing N B, Amann R, Schleifer K-H. Structure and function of a nitrifying biofilm as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol. 1996;62:4641–4647. doi: 10.1128/aem.62.12.4641-4647.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulz H N, Jørgensen B B, Fossing H A, Ramsing N B. Community structure of filamentous, sheath-building sulfur bacteria, Thioploca spp. off the coast of Chile. Appl Environ Microbiol. 1996;62:1855–1862. doi: 10.1128/aem.62.6.1855-1862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz H N, Brinkhoff T, Ferdelman T G, Hernández Mariné M, Teske A, Jørgensen B B. Dense populations of a giant sulfur bacterium in Namibian shelf sediments. Science. 1999;284:389–544. doi: 10.1126/science.284.5413.493. [DOI] [PubMed] [Google Scholar]
- 35.Schulz H N. Nitrate-storing sulfur bacteria in sediments of coastal upwelling. Ph.D. thesis. Bremen, Germany: University of Bremen; 1999. [Google Scholar]
- 36.Schulz H N, Strotmann B, Gallardo V A, Jørgensen B B. Population study of filamentous sulfur bacteria Thioploca spp. off the Bay of Concepción, Chile. Mar Ecol Prog Ser. 2000;200:117–126. [Google Scholar]
- 37.Strub P T, Mesías J M, Montecino V, Rutlland J, Salinas J. Coastal ocean circulation off western south America. In: Robinson A R, Brink K H, editors. The sea. Vol. 11. New York, N.Y: John Wiley & Sons, Inc.; 1998. pp. 273–313. [Google Scholar]
- 38.Sweerts, J. P. R. A., and D. de Beer. Microelectrode measurements of nitrate gradients in the littoral and profundal sediments of a meso-eutrophic lake (Lake Vechten, The Netherlands). Appl. Environ. Microbiol. 55:754–757. [DOI] [PMC free article] [PubMed]
- 39.Taylor B F, Hoare D S, Hoare S L. Thiobacillus denitrificans as an obligate chemolithotroph. Isolation and growth studies. Arch Microbiol. 1971;78:193–204. doi: 10.1007/BF00424893. [DOI] [PubMed] [Google Scholar]
- 40.Teske A, Sogin M L, Nielsen L P, Jannasch H W. Phylogenetic relationships of large marine Beggiatoa. Syst Appl Microbiol. 1999;22:39–44. doi: 10.1016/S0723-2020(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 41.Thamdrup B, Canfield D E. Pathways of carbon oxidation in continental margin sediments off central Chile. Limnol Oceanogr. 1996;41:1629–1650. doi: 10.4319/lo.1996.41.8.1629. [DOI] [PubMed] [Google Scholar]
- 42.Timmer ten Hoor A. A new type of thiosulphate oxidizing, nitrate reducing microorganism: Thiomicrospira denitrificans sp. nov. Neth J Sea Res. 1975;9:343–351. [Google Scholar]
- 43.Troelsen H, Jørgensen B B. Seasonal dynamics of elemental sulfur in two coastal sediments. Esturine Coastal Shelf Sci. 1982;15:255–266. [Google Scholar]
- 44.Zemskaya T I, Namsaraev B B, Dultseva N M, Khanaeva T A, Golobokova L P, Dubinina G A, Dulov L E, Wada E. Ecophysiological characteristics of the mat forming bacterium Thioploca in bottom sediments of the Frolikha Bay, northern Baikal. Microbiology. 2001;70:335–341. [Google Scholar]
- 45.Zumft W G, Kroneck P M H. Metabolism of nitrous oxide. In: Revsbech N P, Sørensen J, editors. Denitrification in soil and sediments. New York, N.Y: Plenum Press; 1990. pp. 37–55. [Google Scholar]