Abstract
FELASA and AALAS established a joint working group to advise on good practices for the exchange of fish for research. In a first manuscript, the working group made recommendations for health monitoring and reporting of monitoring results. The focus of this second related manuscript is biosecurity in fish facilities. First, we define the risk of contamination of personnel by zoonotic pathogens from fish or from system water, including human mycobacteriosis. Preventive measures are recommended, such as wearing task-specific personal protective equipment. Then we discuss biosecurity, highlighting the establishment of biosecurity barriers to preserve the health status of a facility. A functional biosecurity program relies on integration of the entire animal facility organization, including the flow of staff and animals, water treatments, and equipment sanitation. Finally, we propose 4 steps for introducing new fish colonies: consideration of international trade and national restrictions; assessing risk according to fish source and developmental stage; establishing quarantine barriers; and the triage, screening, and treatment of newly imported fish. We then provide 3 realistic sample scenarios to illustrate practical biosecurity risk assessments and mitigation measures based on considerations of health status and quarantine conditions.
Abbreviations and Acronyms: EU, epidemiologic unit; NNV, nervous necrosis virus; OIE, World Organization for Animal Health; PI, principal investigator; PPE, personal protective equipment; RO, reverse osmosis; SLOM, screen less often microorganisms; SMOP screen more often pathogens
Introduction
This FELASA-AALAS working group was established to address the challenges associated with the exchange of fish between laboratories. The group first produced recommendations for the monitoring of fish health, explaining how daily care and screening programs are essential for the detection of diseases that are potentially damaging to fish welfare and research.63 These recommendations led to the design of templates for use in reporting health status and describing fish facilities. These templates are intended to support communication when exchanging animals and to help with assessment of biosecurity risks linked to the introduction of new fish.
In 2018, we distributed an electronic survey to collect data on current health monitoring and biosecurity practices in institutions worldwide. A key finding of the survey was that a substantial number of institutions had questionable import and quarantine practices. The majority accepted all fish, regardless of information on health status. About 1 in 6 laboratories did not have quarantine facilities or procedures, and only 5% of facilities (out of 145 respondents) seemed to have a reliably secure quarantine. In light of these findings, the working group developed recommendations for the management of zoonotic hazards, biosecurity, preimportation risk assessment, and quarantine. These recommendations are intended to aid institutions in strengthening their own practices and to support assessment of the reliability of data from exporting institutions. Finally, we provide scenarios to illustrate application of the recommendations in different import situations based on consideration of health monitoring reports, import and biosecurity programs, and quarantine challenges.
Key Definitions
Some essential concepts are defined in our first document,63 which focused on health monitoring. The concept of Epidemiologic Unit (EU), as defined in,63 is key to our recommendations. The scenarios also use other concepts, including the pathogen panels Screen More Often Pathogens (SMOP) and Screen Less Often Microbes (SLOM). We define the biosecurity program as the set of procedures and barriers designed to protect the EU, and the facility in general, against biologic and chemical hazards. The list of bacteria, fungi, parasites, and viruses (that is, all agents inclusively referred to below as microbes or microorganisms) that are to be kept out of the EU constitutes the exclusion list. The EU is delimited by barriers, which are physical or conceptual obstacles designed to contain or mitigate biosecurity risks or contamination. These barriers can include measures governing import, quarantine procedures, and enforcement of the exclusion list. An import refers to the introduction of fish (or germplasm) into the animal facility from outside sources (local or international). Quarantine is the isolation and observation of imported animals before they are allowed transfer into the main EUs. To preserve the health status of the main EUs, the quarantine area must be a biocontainment area that constitutes an independent EU. To manage these many biosecurity challenges, all staff should be trained and understand the need to exchange fish safely and observe local rules when working in the facility and importing fish.
Prevent Zoonosis
Zoonoses, which are diseases caused by infectious agents derived from animals, can be transmitted to personnel by animals themselves or by contaminated water, biofilm, surfaces, or instruments (for example, nets or scissors). Fish culturing is generally a low-risk activity for immunocompetent individuals using sound standard operating procedures and personal protective equipment (PPE). However, when handling fish, aquatic facility personnel may be subject to skin punctures and cuts (from bones, teeth, scalpel-like scales, or equipment), envenomation, and electrocution. Exposure to zoonotic agents can occur through contact with fish water, biofilm, and infected fish. To prevent contamination, special care must be taken when performing activities with greater risk such as fish handling, mouth pipetting, and cleaning of equipment used for fish culture (for example, sumps, siphons, tanks, or gutters).2,43 Ingestion of water from a fish system must be avoided. We did not deem zoonosis related to consumption of fish to be a relevant topic for laboratory fish.
Zoonotic agents in the aquatic facility.
Organisms that are potentially zoonotic and that have been documented in fish or aquarium water and biofilms include bacteria (for example, Aeromonas spp., Clostridium spp., Klebsiella spp., Edwardsiella tarda, Erysipelothrix spp., Escherichia coli, Mycobacterium spp., Nocardia spp., Plesiomonas shigelloides, Pseudomonas spp., Salmonella spp., Staphylococcus spp., Streptococcus spp., Vibrio spp.) and protozoa like Cryptosporidium spp.10,33,40 These organisms may cause human infection through wounds, mucosal contamination, or ingestion of fish water, often resulting in gastroenteritis. For example, Aeromonas hydrophila is a facultative anaerobic Gram-negative rod that is sometimes found in normal intestinal microflora of healthy fish or in fish water systems. Infected humans may present with gastroenteritis or localized wound infections. Aeromonas hydrophila is more often associated with human disease in immunocompromised patients.10 The bacteria responsible for the infection may even have antibiotic resistance associated with the ornamental trade or aquaculture.11,85
Human mycobacteriosis.
Multiple mycobacterial species that are detected in aquatic facilities are considered zoonotic (for example, M. abscessus, M. chelonae, M. fortuitum, M. haemophilum, M. marinum).30 Mycobacteria are nonmotile acid-fast Gram-positive bacteria. Some mycobacteria are considered obligate or facultative pathogens, others are opportunistic and ubiquitous in the environment. Zoonotic infections by Mycobacterium marinum are a recognized hazard when caring for pet fish, and the infection in humans is sometimes called ‘fish TB’ (in reference to Mycobacterium tuberculosis), ‘fish tank granuloma,’ ‘fish handler’s disease,’ or ‘finger syndrome.’ Some mycobacterial infections may occur through direct injury from fish fins or bites, but infections are also acquired during aquarium handling (for example, cleaning or changing water).43,59 Personnel can be infected by contamination of damaged skin (for example, a preexisting wound) by organisms in water, biofilm, or fish. Lesions are formed at the site of infection in the weeks or months after contamination. Superficial cutaneous infections appear as crusted or ulcerated nodules or verrucous plaques. These may evolve to abscesses and granulomas or to nodular lymphangitis. Infections can even extend into deeper tissues, inducing arthritis, tenosynovitis, osteomyelitis, or bursitis. Infections may become systemic in immunocompromised individuals and/or in cases of delayed diagnosis. Treatment consists of several months of antibiotic therapy.43 In a case of M. marinum infecting staff and fish of a zebrafish facility, measures to control the outbreak and reduce fish mortality required the engagement of the whole establishment.59
Protect personnel against zoonosis.
Protection from zoonotic disease is an important consideration for animal facilities and research staff. Personnel must be informed of this risk and of the importance of mitigation measures. The risk of human contamination is first reduced by the use of routine hygiene. Cleaning and disinfection of equipment and facility surfaces and removal of biofilm and carcasses help reduce the load of zoonotic pathogens in the environment (that is, infection pressure).63 To avoid contact between fish or water and skin, personnel should wear waterproof gloves when handling fish, water, or potentially contaminated items, including anything wet or covered by biofilm. Long sleeve gloves should be worn if water will spread above the wrist, such as during sump sludge cleaning or sampling. Any open wound should be covered by a waterproof bandage to prevent contact with water or other hazards. Staff should wash their hands with soap and disinfect them (if soap and water may not be sufficient) when exiting aquatic facilities or after completing wet tasks. Personnel should never smoke, drink, or eat in animal rooms. Mouth pipetting should be discouraged, and contact of fish system water with unprotected skin and mucosa avoided. Eye protection (goggles) should be considered for specific tasks in which the risk of exposure to water droplets at head level is increased. Care should be taken to minimize time spent wearing wet clothing, and dedicated scrubs or waterproof overalls should be used for wet tasks. The possibility of cross-contamination of items like keyboards, computer mice, and doorknobs should be considered, although some agents may not live long outside a wet surface.53,59 These precautions may be most important to consider in quarantine areas because the health status of the fish may not be well documented. In general, immunocompetent individuals are more resistant to such infections, whereas immunocompromised individuals (that is, those receiving chemotherapy, immunosuppressive therapy (such as steroids), or with naturally immunosuppressive conditions) are more likely to develop infection from zoonotic agents in fish facilities.30,43 We recommend that such personnel identify themselves as soon as the condition is known, and duties linked to an increased risk of contamination should be performed by other staff members. Similarly, physicians should be aware that their patients work with fish to direct diagnostic investigations appropriately.
Prevent the Spread of Fish Microbes
Biosecurity is designed to prevent the introduction and dissemination of microbes within the facility. The introduction of new microorganisms to an EU may compromise fish health or models by inducing nonprotocol-related variation, such as changes in microbiota, immunity, or behavior.32,60,61,90 Microbes may be introduced by imported fish, supply water, incoming material, air, staff, and visitors. Biosecurity barriers should mitigate the risk of such contamination of the EU. Inside the facility, proper biosecurity practices can prevent cross- contamination between quarantine and main holding systems; isolate an outbreak or contamination of a colony in the EU; prevent contamination between different fish species, systems, or tanks with different microbiologic statuses; and protect bio-contained immunocompromised fish colonies. A careful assessment of workflow can identify critical points of increased risk, and preventive measures can then be applied to avert or mitigate hazards.76,109 The design or evaluation of a biosecurity plan is key to the demarcation of an EU. Indeed, in absence of strict internal barriers (for example, separated workflows, equipment, sinks, or working surfaces like benches and shelves), fish held in different systems, whether in the same room or not, should be deemed as being from the same EU. Therefore, the EU is ideally contained within a structural outer shell: walls, ceiling, and entry barrier.
Adapt biosecurity to health status.
Facilities should be designed to maximize biosecurity by isolation of quarantine areas, compartmentalization of EUs, separation of workflows, and prevention of cross-contamination. Some construction materials (for example, nonporous bench surfaces, antifungal paint) are useful for this purpose. The biosecurity program should also support the long-term aims of the animal facility. For example, a toxicology laboratory may want to make greater efforts against chemical pollution and so include a robustly filtered water supply for their flow-through systems. Alternatively, a breeding facility may choose to focus on minimization of microbiologic cross-contamination by investing in resource-demanding sterilization equipment (for example, an autoclave). Colony health status will be influenced by these decisions but, in the long term, will most likely be determined by the weakest point in the biosecurity plan. For example, there is little point in autoclaving tanks between quarantine and the main holding systems if quarantine is in the same room as the main holding systems and water cross-contamination is unavoidable. Another example is the use of HEPA-filtered ventilation and air pressure differentials to prevent aerosol cross-contamination between EUs or from quarantine.84,107 These are very resource-demanding systems and should only be considered after other risks of cross-contamination are controlled (for example, fish, water, staff, and equipment).
Mitigate risks due to exposure to wild natural elements.
Facilities relying on an untreated wild water supply cannot achieve a higher health status than their water supply and can only mitigate the risks for other contamination sources. When laboratory fish are exposed to wild natural elements, the external environment must be controlled appropriately to ensure a sustainable defined health status for the laboratory fishes, with recognition that ambitious biosecurity measures may be constrained by uncontrollable circumstances (such as birds, overflowing, rodents, or upstream and downstream fish). Fish facilities that are exposed to wild natural elements are at particular risk of microbial contamination. Water can facilitate the horizontal transmission of pathogens, even when fish are separated by a long distance.42 Microbes can also be introduced by animal vectors that actively spread contamination,68 that serve as intermediate or final hosts,63 or that carry microbes on their bodies1,75 or in their feces.106 Parasites can be vectors for viruses, bacteria, and other parasites.1,75 Outdoor facilities should design barriers to reduce such introductions (for example, wire mesh, net covering, and pool elevation). Fish transport trucks should be disinfected in a dedicated area to prevent fish system contamination.
Treat and filter water to reduce contamination.
To assess or develop a biosecurity program, it is critical to consider the presence of potential hazards in the supply and/or recirculated water. Contaminated and untreated water can distribute microbiologic or chemical contamination throughout a facility (including to taps to wash hands or tanks). Municipal water does not receive sufficient treatment to prevent contamination with all relevant microbes and chemicals.2,22 Methods used to address such contaminants include filtrations (for example, mechanical, carbon, reverse osmosis [RO], and zeolite) and treatments (for example, Ultra-Violet [UV], ozone, and deionization). Each type of system (for example, recirculating, flow-through, static, open circuit, or closed circuit) presents different biosecurity challenges, and water treatments can be placed in specific locations across a facility to serve different purposes. In all cases, treatment of the supply water is key to preventing the introduction of pathogens and pollutants. Inside the water recirculation loop, water treatment mitigates dissemination of microbiologic and/or chemical contamination by reduction of microbial load (for example, mechanical filtration, UV, or ozone treatment) or adsorption of molecules (that is, carbon filtration).2 To prevent water contamination, fish and equipment that fall and contact the floor should not reenter the system without a decontamination process (for example, euthanasia or isolation in quarantine for fish, cleaning and disinfection for equipment).88,102 Finally, wastewater released to the outside environment can be treated, as is required in some geographic locations to reduce the release of contaminants of any kind to the ecosystem. The main environmental risks are microbiologic contamination and chemical pollution by medicated water. The former can be reduced by heat or chemical treatment of wastewater before release. The latter can be mitigated by carbon filtration and other waste disposal methods. Local regulations may apply.
Sanitize equipment.
Cleaning and disinfection of equipment in contact with fish or fish water is necessary to reduce microbiologic cross-contamination between systems and tanks. This can be achieved manually or with a washing machine, as long as the machine is not shared between EUs or with quarantine. An alternative is to manually clean and then sterilize (that is, autoclave) items like tanks and nets before they reach communal washing devices, although not all equipment is autoclave-safe. Indeed, cleaning and disinfection processes must be adapted to equipment material and function. Chemical and biologic indicators are useful for monitoring the efficacy of disinfection and sterilization processes.3,45,64 Different disinfection protocols can be used in the main rooms and in quarantine, and they can also be adjusted to known threats and desired biosecurity levels. Sanitation of working benches is no exception, although care should be taken to avoid residue contamination of system water. For example, the use of 70% ethanol may be advisable in the main holding rooms while other chemicals may be preferable for use in quarantine. Depending on the presence or absence of sterilization processes, equipment dedicated to different EUs or quarantine may be best stored in different rooms.
Assess biosecurity risk for environmental enrichment.
The decision to introduce items for environmental enrichment should be based on scientific merit.93 Biosecurity risks should also be considered and resources allocated to mitigate the risks. Even seemingly inert items from the natural environment (for example, sand, rock, or wood) can carry living organisms. Some snails can transmit bacteria and parasites.12,44,57 Items added to the tank environment can support biofilm growth (for example, Mycobacterium spp. and Ichthyophthirius multifiliis).5,17,47,63,78,79 Therefore, inert items should be thoroughly cleaned and disinfected regularly. Rocks or living items, like plants or snails, should be quarantined until natural disappearance of contaminating developmental stages. Plants can be treated with potassium permanganate 5 mg/L for 5 min.105
Pathogens may also be introduced through feeds. Fish-based diets are a concern. Drying or freezing processes are not always sufficient to eradicate all contamination.35,86 Live feed (for example, shrimp or worms) present a risk as they can be a source of viral pathogens or constitute intermediate or paratenic hosts of parasites. Feed cultures (for example, shrimp, rotifer, or paramecia) can be a culture media for pathogens, including pathogenic bacteria, and every effort should be made to avoid contaminating them with microbes from other EUs or from quarantine.13,16,103 However, live feed may be necessary to stimulate natural prey-capture behavior and provide environmental enrichment, which are important for behavioral neuroscience reproducibility.2,29,34 Ideally, the microbial status of live feed should be controlled at the source prior to introduction to the animal facility. Feed cultures should be monitored routinely.63 The microbial status of the nutritional sources for live feeds should also be known.
Organize the animal facility.
Incubators and refrigerators for feed storage are sometimes weak points in a biosecurity plan. They should be dedicated to an EU or to quarantine and located in the area that they serve. Traffic paths from the feed processing room should be clearly separated between EUs and quarantine, and cross-contamination should be avoided when refilling feed distribution devices. Devices used in quarantine should stay in quarantine. Single-use or disinfected containers used to transport food to quarantine should not travel back to the main EUs without treatment (for example, sterilization) to remove contaminants. Other small consumables and devices are a significant risk of cross-contamination if they are not sufficiently available in each EU and quarantine. For example, water testing kits should be readily available where needed. Maintenance tools are likely to contact water-contaminated devices and should be dedicated to individual EUs. Phones and computers used to access databases and the internet must be distributed with regard to biosecurity barriers and risk of cross-contamination through keyboards.
Movement of dirtier (potentially contaminated) items and disposal of cadavers and waste must follow defined flows. All items coming from a dirtier area, like quarantine, should be double-bagged out for transfer to a communal area, like a corridor or a morgue. Cadavers should be frozen and biologic waste stored in freezers dedicated to their respective EU or quarantine, if possible. This should follow a one-way traffic flow, and such items should be incinerated to lower the risk of contamination of cleaner areas. Finally, having a means to restrict escape of fish of all life stages to the waste disposal system is essential to avoiding the introduction of genetic alterations, pathogens, or new and possibly invasive species to a geographical area. Check local regulations to assure compliance.
Manage staff and animal flows.
The control of fish and personnel traffic is key to the biosecurity plan. Personnel may include staff caring for the fish, facility managers, regulatory representatives, researchers, students, veterinary staff, maintenance staff, and visitors. Ideally, the facility will be equipped with microscopes and other equipment that allow embryo screening in areas dedicated to a respective EU or to quarantine. Fish that have been taken to a laboratory external to the animal facility or to an area shared with another EU should not be permitted to reenter their original EU (unless the original EU is quarantine or the EU of the lowest health status sharing the area). It is advisable to decline entry of visitors who have entered other aquatic animal facilities within a short timeframe (for example, 24 or 72 h). Personnel movement between EUs should also be restricted, as staff can be vectors or fomites for pathogens. The strength of an entry barrier seems often related to the number of staff members that cross it daily. Thus, it can be judicious to restrict access to the most hazardous (for example, quarantine) or sensitive (for example, gnotobiotic) rooms of the facility. Moreover, the ‘from clean to dirty’ rule should apply to staff flow between EUs, including quarantine. When entering an EU, staff should have access to dedicated space in which to wash and disinfect their hands and don appropriate PPE. The choice of the right protective equipment should be defined according to health and safety, microbiologic status of the fish, and staff comfort. To ensure compliance of all staff to the biosecurity rules, the boundaries of an EU should be physically marked so that staff must consciously pass a visible barrier. This point can be used for scientists to disinfect incoming equipment. Incoming goods and biologic materials (for example, cells, biomedia) that are potentially contaminated with fish pathogens should be screened before entry, and these materials should be carried and used in compliance with local rules that are accepted by the scientists. Similarly, the whole biosecurity plan should be discussed by all stakeholders as it is being designed and whenever it is changed to encourage buy-in of all personnel. All personnel must be well informed about the internal rules and the rationale behind them. Staff who are less accustomed to the local biosecurity rules (for example, maintenance personnel and visitors) should be escorted or given short and easy-to-follow directions, including graphics, if possible.
Recommendations for Biosecure Introduction of New Fish Colonies
Many times, elimination or eradication of fish pathogens within an EU is only achievable with sanitary voids/culling and disinfection of the associated life support system.59,104 Control measures may maintain an infection at an acceptable level for contexts outside research, but any level of infection may be less acceptable in research colonies due to potential impacts of disease on scientific data. Therefore, preventing the introduction of pathogens into an EU is crucial. The import process should follow a triage pattern in which regulations and risk assessments are made before further screening and biosecurity procedures occur in quarantine. When incoming fish have an unknown health status, the risk of contamination of the main stock must be minimized by the implementation of a more robust quarantine program and mitigation or treatment plans for microbes excluded from the main EU.
Notifiable diseases—Check local and national regulations.
The World Organization for Animal Health (OIE) is the intergovernmental organization responsible for improving animal health worldwide. Among the formal obligations of an OIE Member Country is the submission of information on its relevant animal disease situation—including the presence of any zoonosis—in the most timely and transparent way. The OIE lists fish pathogens to consider for international trade and proposes further information about the related diseases, diagnostic tests to identify them, and their management.96,97,99 National regulations are often based on the OIE list, with some adaptation to local situations. Some countries or zones may exclude and/or be deemed free of certain specific pathogens. Reports of detection of specific pathogens may be required by local authorities. Some regulations set specific permit, quarantine, and screening requirements based on fish species and pathogens.41 These are known as notifiable diseases,99 and the latest updates of specific national requirements are available on the links in Table 1. Before starting an import process, national regulations should be checked for specific restrictions and requirements along 3 themes: notifiable diseases for the relevant geographic areas, the susceptibility of the fish species to a listed pathogen, and the potential of the fish species to be a vector of a listed pathogen.18 Once the legal aspects of the import are cleared, an assessment of its biosecurity risk should be performed.
Table 1.
Links to latest updates for specific national requirements on notifiable diseases
Assess risk before importation.
Before agreeing to accept a shipment of fish, the responsible representative of the receiving facility should review the health reports and facility description of the exporting facility.63 Health monitoring reports provide useful information that allows an informed decision on triage and quarantining of newly imported fish to best reduce risk of importing unwanted microbes. This type of information should be readily available from most research laboratories and some aquaculture farms (for example, for notifiable diseases). Health monitoring information is less often available from pet shops or fish trade sources and is often not available for wild-caught fish, making these sources a last resort. Research establishments that are required to follow Directive 2010/63/EU, which concerns the protection of animals used for scientific purposes in the European Union, must import zebrafish only from establishments authorized to supply Danio rerio purpose-bred for research.26 Zebrafish is the only fish species mentioned in this European Directive regarding acquisition. This restriction seems to have reduced the risk of importing zebrafish that are contaminated with less common pathogens.44,49,89 We therefore strongly recommend that every effort be made for all facilities to source new fish stock solely from laboratories, research stock centers (for example, for zebrafish: Zebrafish International Resource Center and European Zebrafish Resource Center), and facilities deemed free of agents to be excluded.67,71 A fish shipment can be refused if it is potentially infected with a pathogen excluded at the receiving facility. Alternatively, the type of specimen imported may be dictated by the pathogen status of the exporting facility. For example, fish lines from a facility known to be contaminated with the intestinal nematode Pseudocapillaria tomentosa may be approved for importation only as surface-sanitized eggs or cryopreserved sperm.74 Geographical data on pathogen prevalence can be informative for risk linked with the origin of the fish. The OIE World Animal Health Information System provides geographic information on notifiable diseases.98 To avoid pathogen introduction, fish should be imported only from sources that share health reports and adhere to strict biosecurity processes. In the absence of quarantine, one option is to import fish only from sources validated as free of excluded pathogens. In this case, the importer relies entirely on the health reports and biosecurity program description provided by the exporter to determine a necessary risk management strategy. Alternatively, if a reliable quarantine is established, the importing facility may design a triage system to discriminate between sources with an acceptable health status for import and those that should be declined. In this case, quarantine is an extra step to confirm the health status of the imported fish before allowing them into the main EUs. Imports of fish potentially contaminated with excluded pathogens (for example, M. marinum) may be declined outright, even from entry into quarantine, regardless of the developmental stage or method of disinfection. Local rules may also require all imports to be received in quarantine as embryos to be egg surface sanitized at the place of origin or on site after arrival. Facilities often adopt a mix of the rules described here, based on local needs, facility design, and capacity.
Mitigate risk according to fish developmental stage.
An important factor to control at the start of the import process is the developmental stage that will be accepted for shipment. Species characteristics to consider include reproduction mode (oviparous or ovoviviparous), lifecycle (duration), and other reproductive factors (for example, seasonal fertility and ability to breed in captivity). In many situations in fish research facilities, importing embryos is not possible (for example, due to the species characteristics or the ability to perform egg surface sanitation). Imported adults in quarantine can be screened nonlethally and postmortem after they spawn or sperm is cryopreserved. Frozen gametes and other germplasm can also be transferred to establish new colonies. The freezing process may reduce the risk of contamination by killing some specific pathogens. However, many microbes resist cryopreservation, so frozen biologic material can remain a biosecurity hazard.74 Furthermore, wild-type lines and maternal-effect mutations cannot be propagated with the male genome alone.
Adult fish are more likely to be infected with a wide variety of pathogens. Therefore, when possible, some facilities reduce the biosecurity risk and welfare impairment associated with importation of adult fish by importing only embryos from surface-sanitized eggs. Fish may hatch out of chorions during shipment. In this case, the importing facility relies on the surface sanitation methods of the exporting facility, whose chemical disinfectants, exposure times, and sanitation efficacy may differ from those of the importing facility protocol. Surface sanitation aims at reducing gamete-associated transmission of microbes between generations. The process relies on chemical disinfection of the egg surface and physical cleaning of eggs by mechanical flushing in sieves. Egg surface sanitation methods are described for a large number of oviparous fishes (for example, Danio rerio, Dicentrarchus labrax, Diplodus sargus sargus, Gadus morhua, Nothobranchius furzeri, Oncorhynchus masou, Oncorhynchus mykiss, Oreochromis spp., Oryzias latipes, Salmo salar, Scophthalmus maximus, and Sparus aurata) using several disinfectants or other products (for example, bronopol, formaldehyde, glutaraldehyde, hydrogen peroxide, hypochlorite sodium, iodophors, ozone, or peracetic acid).8,15,20,46,52,80,94,95 This process does not result in sterilization as chemical disinfection may not eradicate all microbes on egg surfaces. Furthermore, it will not remove intraova microbes, which require other techniques to reduce vertical transmission (for example, antibiotic prophylaxis against Renibacterium spp.).8,9,28,49,70,77,81,87,101 Sanitation should be performed in an uncluttered and dedicated area in the facility, following the ‘clean to dirty’ rule, especially for transfer of lines from quarantine to main holding systems. Egg surface sanitation constitutes a weak barrier between quarantine and EUs.
Biocontain imported fish in quarantine.
Once the introduction of new fish is approved, all fish should be imported into a biocontained, physically isolated quarantine room. The quarantine room should not be a part of a main EU. Pathogens may be borne by vectors (for example, birds, air, water, biologic material, or fish); contamination can also occur due to unexpected contact between surfaces. Therefore, quarantine and the main holding systems cannot share the same room, water system, bench, sink, or other space or equipment. The quarantine area should be independent from other EUs and contain all necessary equipment (for example, refrigerators, incubators, feeding devices, microscopes, water testing kits, maintenance tools, etc.). Preferably, the quarantine water supply would be flow-through and always fitted with back-flow valves or otherwise constructed to prevent it from contaminating water resources of other EUs.
Most importantly, the barrier for exiting quarantine should be robust. For example, waste and dirty items should be double-bagged out for removal from the quarantine area. To do this, items are first placed in a bag in the quarantine area. As the first bag crosses the barrier to exit quarantine, it is enclosed in a second bag used to prevent contamination of corridors and other areas, as the outer layer has never been inside the quarantine area. Nonetheless, to avoid cross-contamination outside quarantine, tanks and nets should not cross paths with similar items from other EUs without prior sterilization. For example, tanks and nets should be cleaned in quarantine, double bagged out, and autoclaved before they are cleaned in communal machines that would disinfect but not sterilize. Staff should enter the quarantine room only after donning dedicated PPE. Quarantine is a high-risk area, sometimes housing fish of undetermined pathogen status, and PPE should protect personnel from potential zoonotic pathogens (for example, long sleeve gloves, coveralls, and disposable scrubs). PPE should be left in the quarantine area after use or disposed of as staff step out. To ease processes of entering and exiting quarantine, a lobby room can be set between the communal corridor and the quarantine room. More general details about mitigation for cross-contamination inside the animal facility are discussed above.
Personnel duties should be reduced in quarantine. Quarantine should be operated as a short-term breeding facility, with no long-term holding or experimental work other than that necessary for colony assessment and maintenance. Staff flow is controlled by restricting user access, dedicating staff to quarantine duties, and imposing a ‘clean to dirty’ rule so that staff perform any assigned animal work in the main holding rooms or related space before entering quarantine. Staff should not return to the main facility after working in quarantine without adequate sanitation (for example, showering and changing clothing). For instance, in some facilities, the manager is in charge of quarantine duties, providing a daily link with animal work without disturbing the routine in main holding rooms. Coverage of quarantine on weekends and during emergencies should also be scheduled. To ensure the sustainability of quarantine biosecurity, all quarantine procedures must be approved by the user community, ethics committees (or animal welfare bodies), and other relevant bodies, with universal understanding and acceptance of rules for new fish introduction.
Provide ample time for quarantine and acclimatization.
Upon arrival, fish should be observed in quarantine for a minimum of 2 wk.2 Depending on estimated risks of the shipment and local regulations,4 this observation period may require extension for up to or over 6 wk. In public aquaria, the recommended minimum duration of quarantine is 30 d; in aquaculture it is 30 to 60 d.23,25,37 If imported fish show signs of ill health, special care should be taken for disposal of potentially hazardous animal waste and system water (for example, cadaver incineration, heat or sodium hypochlorite treatment of wastewater before release). Importing fish in sequential batches rather than mixing import batches or species during quarantine is recommended if space allows. Preferably, the quarantine room would use an ‘all in to all out’ rotation, in which the entire quarantine area (that is, room, system, PPE, and equipment) is cleaned and disinfected after each batch. When necessary, quarantine sanitation can be followed by a dry-out period (that is, a period of drought to further reduce microbial survival) of 2 to 4 wk.38 Biomedia from the main holding rooms’ biofilters can then be transferred to restart the quarantine biofilters.108 Once fish are transferred from quarantine to a permanent holding room, an acclimatization period of a minimum of 2 wk should be permitted before initiating research use.14
Once the imported fish are deemed healthy enough to exit quarantine, the recommended route, in species such as zebrafish, is not to move adults but only to introduce embryos from surface-sanitized eggs produced in quarantine into the main holding rooms. This process is sometimes (for example, in higher risk imports) performed as a 2-step quarantine: first, fish are imported and reared in quarantine; then, embryos produced are sanitized and transferred to a second quarantine system; finally, fish raised in the second system produce embryos that are sanitized and introduced into the main system(s).58 The caveat is that prolonged time in quarantine may either delay researchers’ access to the line or compromise quarantine biocontainment if research and extra barrier crossings are permitted.
Screen imported fish for pathogens.
While fish are in quarantine, they should be monitored for obvious signs of disease or increased mortality and screened for pathogens. Some imported fish and/or quarantine system water can be tested for excluded microbes.63 The health monitoring of imported fish should be adapted to the current facility circumstances (that is, excluded pathogens, number of imports present, size of quarantine, and stages of development upon arrival). In larger species, clinical examinations can be an option, usually under sedation. Setting sentinels dedicated to each imported batch may be useful, particularly when viral serology is available for the species. Otherwise, a system-wide sentinel program is useful when overlapping batches enter quarantine, as this can provide information on the pathogens that are present without the need to independently test each incoming group. Other beneficial practices are screening the system environment, evaluating each import group directly, and assessing clinical cases.
When importing embryos, a portion of the batch may be tested by PCR upon arrival or a few weeks later once the yolk sac is exhausted.48 Similar testing can be performed on culture water if fish are not kept in a recirculating system, although this is not the most sensitive method.19 After imported embryos have developed for a few months, or when adult fish are imported, breeding devices can be placed in the holding tanks of some species to collect eggs, feces, and sludge for screening without culling fish.66 For even more confidence that pathogens are excluded, imported fish can be culled and tested.88,102 In some species, this can be done after spawning to produce embryos to be moved to the main facility—pending diagnostic results on the parents.72 This requires rapid turnaround for diagnostic results. Alternatively, some fish can be spawned in quarantine and a portion of the embryos tested by PCR. In this case, fish can be spawned multiple times until a ‘clean’ clutch is identified.48,65
If excluded pathogens are detected in quarantined lines, the options are (1) cull the colonies and disinfect the system(s) and the whole quarantine area as relevant;65,88,102 (2) treat fish if possible (for example, many endo- and ecto-parasites such as P. tomentosa and Ichthyophthirius multifiliis);47,50,51,56,79 (3) cryopreserve sperm before culling; or (4) spawn adults and treat and/or test a portion of the embryos.48,101 Publications from public aquarium-related scientific literature and aquaculture describe prophylactic and curative treatments for some infections.24,37,39,73,83,90,105 Fish can be treated and then rescreened to confirm treatment success. System disinfection should also be validated before restocking. Disinfection protocols for this purpose are described in the literature and can be followed by a dry-out period of 2 to 4 wk.38,39,54,55,59,82,104,109 Besides disinfectant efficacy, the selection of a disinfection protocol must consider health and safety of personnel, environmental contamination, and compatibility of chemicals with system material and equipment (for example, pipes, tanks, pumps, and probes).
Scenarios
This section illustrates how to apply the recommendations in our prior publication63 to develop a health monitoring program for a medium-sized facility (Scenario #1, home scenario) that is importing fish from either a small facility housing one aquatic species (Scenario #2) or from a larger facility housing multiple aquatic species (Scenario #3). Facility sketches and tables are included for further explanation. The scenarios only illustrate examples of applications. For instance, diagnostic assays should be adapted to local expertise,7,62 whereas the lists of assays are simplified in the scenarios.
Scenario #1: home scenario.
The home facility, which is the recipient facility in all 3 scenarios, has 3 holding rooms, including a separate quarantine room, all housing only zebrafish (Danio rerio).
Overview of home facility.
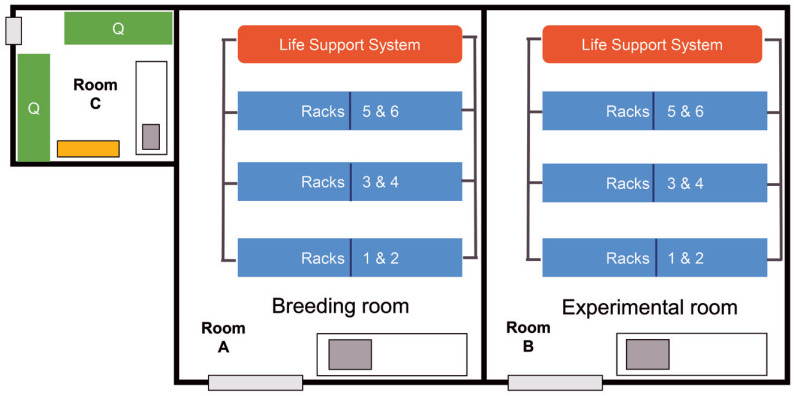
Rooms A and B each have a recirculating water system supplying 6 racks, with a capacity of 20,000 adult zebrafish per system. Room C is an independent quarantine room with 2 stand-alone recirculating racks. In this scenario, the biosecurity program is based on considering Rooms A and B as a single EU (EU1) and Room C as a separate EU (Q1). Please see facility sketch (Figure 1).
Figure 1.
Sketch scenario #1 - Home facility. Rooms A and B each have a recirculating water system supplying 6 racks. Room A is dedicated to housing fish for breeding and larviculture. Room B is dedicated to housing fish that are being used in experiments. Room C is an independent quarantine room with 2 stand-alone recirculating racks (Q).
Management of EU1.
Room A is dedicated to housing fish for breeding and larviculture. Room B is dedicated to housing fish that are being used in experiments. Fish required for experiments are sent from Room A to Room B and do not return to Room A. The separation of EU1 into 2 rooms as described serves 3 purposes: (1) Facility and research staff can more easily manage breeding and embryo collection outside the more crowded room in which experiments are performed; (2) Using a different feeding regimen for young fish is easier if they are housed in a single location rather than being distributed throughout 2 rooms; (3) Scientists can more easily organize their colonies.
The only separation between Rooms A and B in EU1 are walls and doors. Fish are bred and raised in Room A and then may be moved to Room B without restriction. Although Rooms A and B operate on separate recirculating water systems, fish are transported from A to B in water from system A, and therefore some of system A water will be added to system B. Despite fish movement being unidirectional, these 2 rooms are viewed as having the same health status. Microbial flora present in Room A will be transferred to Room B by fish, water, staff, and other means.
Staffing and equipment are shared within EU1 without restriction. Shared resources include the RO water source, tanks, tank accessories, nets, feeders, refrigerators, incubators, microscopes, water testing and maintenance kits, and storage spaces. Gloves and laboratory coats or scrubs are worn by all personnel. Each room has a dedicated system to convert RO water to fish-ready water and to recirculate and recondition the water used in the room; this is done for convenience and is not a barrier. This also provides a source of backup water between the rooms. Food preparation and distribution are performed for both rooms in common. The feed used includes a commercial dry diet and 2 live diets cultured inhouse (rotifers, Artemia spp.). Tanks are soaked and hand-scrubbed to remove algae build-up prior to sanitation in a dedicated tank washer. Between uses, nets are disinfected (that is, manually rinsed, soaked in a chlorine solution, heat-treated, and dried).
Management of Q1: Room C (Quarantine).
Imported adults or embryos are never accepted directly into EU1. They are only accepted into Q1 with dual permission from the facility veterinary staff and manager. Health surveillance information is reviewed by this team before imports are accepted, and imports from colonies contaminated by M. marinum or M. haemophilum are excluded. Only surface-sanitized eggs are permitted to enter EU1 from Q1, with the understanding that this practice may not exclude Pseudoloma neurophilia and some mycobacterial species. Personnel are dedicated to either EU1 or Q1 and do not enter EU1 after being in Q1 on the same day. Q1 has its own dedicated RO water supply and rooms for equipment, storage, and food preparation. Dedicated equipment includes tanks, tank accessories, nets, feeders, refrigerators, incubators, microscopes, and water testing and maintenance kits. Staff wear disposable coveralls over their street clothes, shoe covers, and dedicated gloves. PPE is doffed when leaving Q1 and is not worn into other areas. All items in this room are double bagged when leaving quarantine. Tanks are hand cleaned, autoclaved, and then machine washed in the same tank washer that supports EU1. Feed and nets are processed in a manner similar to EU1, but on a separate dedicated flow.
Health monitoring for EU1.
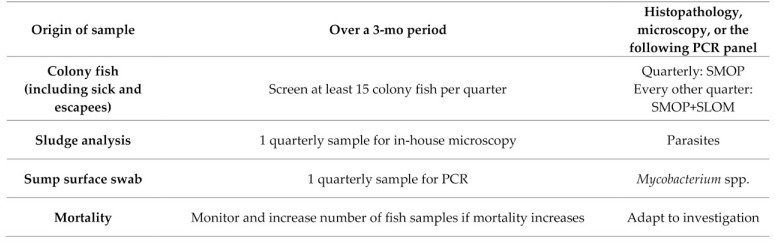
Both Rooms A and B have prefiltration sentinels placed on each system for at least 3 mo before testing. Samples from both rooms are pooled and submitted together, and the health statuses of each room are not discriminated. The health monitoring follows the recommendations in part 1 of our report (see the screening pattern in Figure 2, the list of SMOP and SLOM in Table S3 column D. rerio (isolated), and the health monitoring template Table S5 in63). Figure 2 shows the list of SMOP and SLOM, Figure 3 the screening pattern, and Figures 4, 5, and 6 some sections of the health monitoring report for scenario #1. EU1 is considered positive for P. neurophilia and picornavirus (ZfPV-1). Agents to be excluded from EU1 are the SMOP M. haemophilum, M. marinum, and P. tomentosa, and the SLOM Edwardsiella ictaluri, Flavobacterium columnare, Ichthyophthirius multifiliis, Piscinoodinium pillulare, and Pleistophora hyphessobryconis.
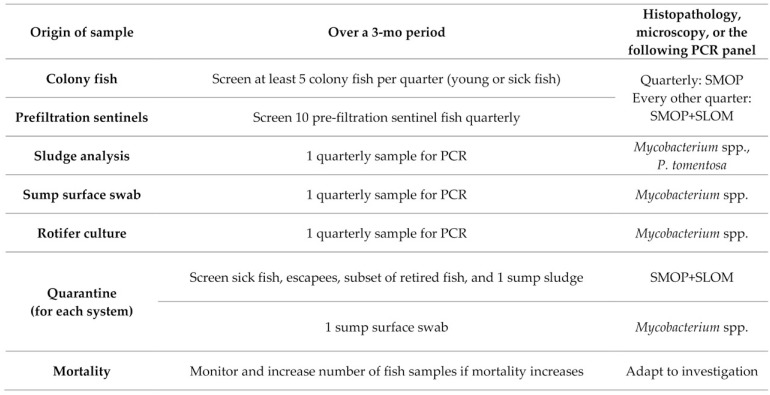
Figure 2.
List of SMOP and SLOM for the scenario EUs. For routine screening, SMOP are tested quarterly and SLOM twice a year. In quarantine, samples are tested quarterly, at or soon after arrival of fish, and samples are screened for both SMOP and SLOM of the corresponding EUs. The list of SMOP and SLOM are particularly useful when setting panels for pathogen-specific screening (for example, PCR, mycobacterial culture, or serology). Other techniques like histopathology, microscopy, and bacterial culture allow a broader and less specific detection of pathogens and diseases and should be used as the methods of choice for clinical investigation.
Figure 3.
Quarterly routine screening pattern for EU1 and Q1. All fish are imported in quarantine and screened for SMOP and SLOM by testing sick or surplus fish and tank sludge by PCR as soon as practical after arrival. Each quarantine system is tested quarterly with their dedicated samples. Environmental samples are routinely tested by PCR.
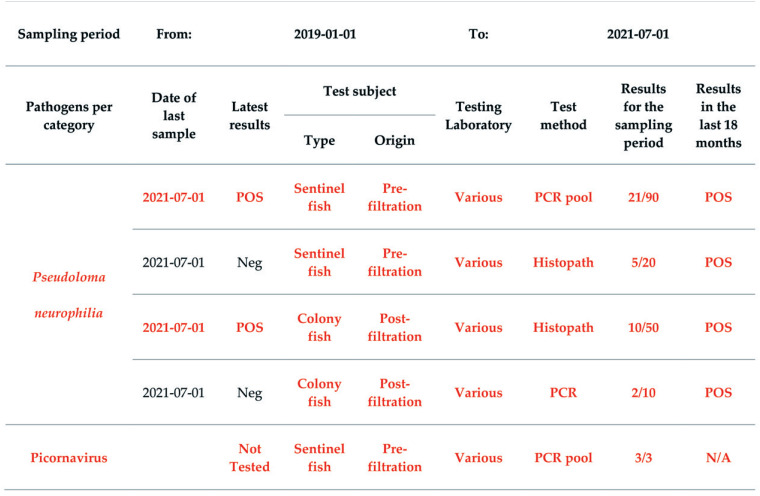
Figure 4.
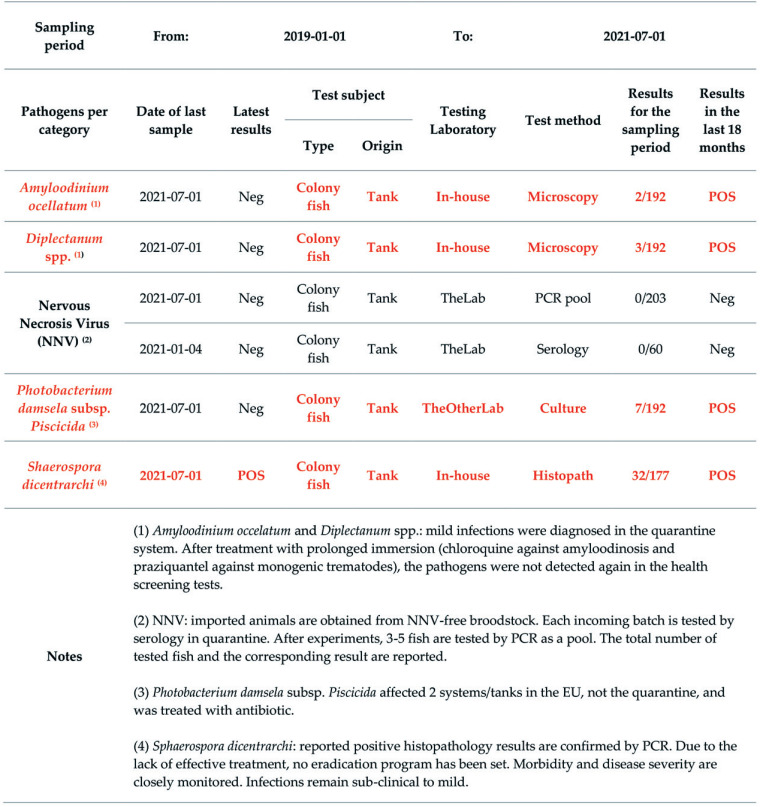
Health Monitoring Report for EU1 - Mycobacterium marinum section. For PCR pool, 3 to 5 fish are submitted in one pool. The total number of tested fish and the corresponding result are reported. Dates are indicated as yyyy-mm-dd.
Figure 5.
Health Monitoring Report for EU1 – Detected microbes section. For PCR pool, 3 to 5 fish are submitted in one pool. The total number of tested fish and the corresponding result are reported. Dates are indicated as yyyy-mm-dd.
Figure 6.
Health Monitoring Report for EU1 – Other mycobacteria section. For PCR pool, 3 to 5 fish are submitted in one pool. The total number of tested fish and the corresponding result are reported. Dates are indicated as yyyy-mm-dd.
Health monitoring for Q1.
All incoming fish are screened for SMOP and SLOM by testing sick or surplus fish and tank sludge by PCR as soon as practical after arrival (see Figure 3). If either fish or environmental samples from a tank are positive for M. marinum or M. haemophilum, the tank population is euthanized. According to the approved contingency plan, fish that are positive for P. tomentosa or other pathogens excluded from EU1 can be treated, euthanized, or kept under closer surveillance in Q1. Moreover, on a quarterly basis, sick and sump fish and retired fish are tested for SMOP and SLOM by PCR or fixed and submitted for histopathology. Environmental samples are tested quarterly for each quarantine system (that is, sump surface for Mycobacterium spp. and sump sludge for SMOP and SLOM). When mortality or morbidity rates exceed a defined threshold, further testing is performed to determine the cause.
Scenario #2: Import of potentially
P. tomentosa positive colonies.
A new principal investigator (PI) from a single species facility has been recruited. This researcher has multiple unique zebrafish lines to be imported to the scenario #1 facility. Description of the exporting facility context and recommendations for the protection of the recipient facility are made.
Overview of exporting facility.
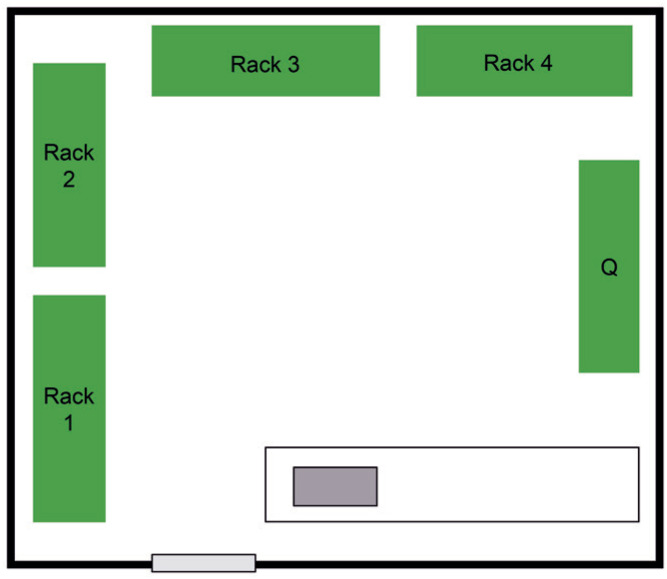
The colonies of the PI are housed in a single room containing 4 stand-alone housing racks and 1 stand-alone quarantine rack (Figure 7). Each rack can hold up to 750 adult zebrafish. The 4 housing racks constitute a single EU (designated EU2), and the quarantine rack is considered a separate EU (designated Q2). This is not strictly correct because all 5 racks share the same space, the same sink, and the same bench, and water cross-contamination between the quarantine rack and the housing racks seems unavoidable. Nevertheless, understanding internal barriers and flow described by the facility management and the weakest points of the biosecurity system, such as importation of fish onto the quarantine rack in the absence of physical barriers, is essential to developing the best plan for transferring fish from EU2 to the scenario #1 facility.
Figure 7.
Sketch scenario #2 - Small exporting facility. A single room containing 4 stand-alone housing racks and 1 stand-alone quarantine rack (Q), sharing the same space, the same sink, and the same bench.
Management of exporting facility.
EU2 and Q2 have no true barriers between them. The PI’s research staff performs all husbandry. Personnel are dedicated to either EU2 or Q2 daily. The staff member dedicated to Q2 does not work on EU2 racks on the same day. Gloves and laboratory coats are worn by all personnel. The 4 EU2 racks are considered to have identical health status, and all supplies and equipment for these racks are shared. Tanks, tank accessories, nets, and feeders for the quarantine rack are dedicated to that rack; these items are handled and disinfected separately from those used for the other racks. Feed for all racks consists of a commercial dry pellet and live food (Paramecium spp. and Artemia spp., cultured inhouse). All feed is stored in the same area, with 1 location for all live feed cultures. Live feed for Q2 is removed as an aliquot from the total amount cultured daily and fed to Q2 tanks with dedicated equipment. A single RO water maker is used to make source water for all racks. Separate tanks are used for the preparation and storage of fish-ready water for EU2 or Q2. Although water quality parameters are the same for all racks, personnel for Q2 only handle water from the storage barrel for Q2. A separate incubator accommodates quarantined embryos and larvae. Eggs destined to be moved to EU2 must be surface-sanitized before transfer from the Q2 incubator to the EU2 incubator. EU2 and Q2 are not otherwise separated, and shared equipment includes refrigerators, microscopes, maintenance equipment, and storage space.
Imported adult fish and eggs are only accepted onto the quarantine rack (Q2). Only surface-sanitized eggs produced after arrival from quarantined adults are moved from Q2 to EU2. Staff in the scenario #1 facility view the division in this facility into 2 EUs as somewhat artificial and they should take this into consideration when planning to import the fish.
Health monitoring for EU2.
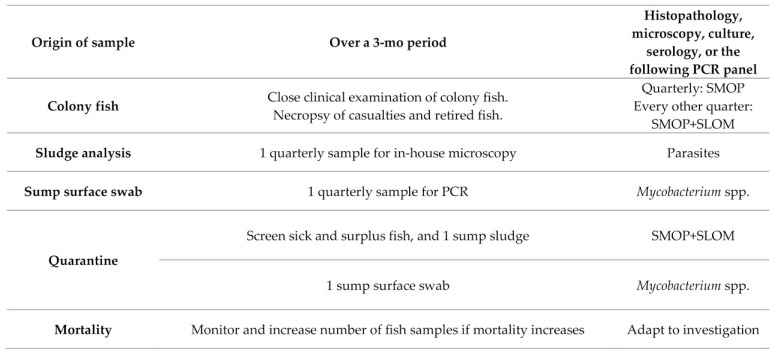
The health monitoring for EU2 follows the recommendations presented in part 1 of this series,63 with some adaptation due to the small size of the facility and the difficulty to maintain sentinels in this set-up. Figure 2 lists SMOP and SLOM and Figure 8 provides the screening pattern. Sentinel fish are not used. Instead, submitted samples include at least 15 fish that were sick, found dead, found in sumps (escapees), or randomly selected. Environmental samples are also included for PCR testing for Mycobacterium spp.; these include a pooled sump surface swab and a Paramecium spp. sample. A sludge sample from a few tanks or sumps is also examined quarterly inhouse by microscopy for detection of P. tomentosa eggs. EU2 is considered positive for P. neurophilia and picornavirus (ZfPV-1) and has the same exclusion list as EU1.
Figure 8.
Quarterly routine screening pattern for EU2 and Q2. All fish are imported in quarantine and screened for SMOP and SLOM by testing sick or surplus fish and tank sludge by PCR as soon as practical after arrival. Sump sludge is only tested by microscopy for P. tomentosa (including for quarantine sump samples).
Health monitoring for Q2.
Q2 is screened in a manner similar to Q1 in scenario #1, except that the Q2 sump sludge is submitted only for inhouse microscopy to detect parasites (P. tomentosa) due to limited budget. The contingency plan for Q2 is to euthanize fish that are positive for pathogens excluded from EU2, and to treat as necessary the Q2 water system and equipment. Care is taken when handling samples from Q2 to avoid contamination of EU2. Deaths are tracked daily, and if increased numbers of deaths occur, testing is performed to determine the cause.
Microbiologic status of exporting facility.
Historical data show that fish on the 4 housing racks (EU2) are positive for Mycobacterium chelonae and P. neurophilia. The most recent Q2 health report indicates that it is also positive for P. tomentosa. Treatment of fish and the water system in Q2 will be implemented. However, when planning to import from this facility, the lack of strict barriers between EU2 and Q2 support considering all fish, including those in EU2, as potentially positive for P. tomentosa.
Recommendations for importation from EU2.
Due to the risk of P. tomentosa transmission, only surface-sanitized eggs should be accepted from EU2 into quarantine in the recipient facility. However, in this scenario, the transfer will include both embryos and adult fish onto Q1 in scenario #1. Although a known risk exists for P. tomentosa, the risk of introducing other pathogens must be more completely assessed before approving the import. First, veterinary and management personnel of the recipient facility must assess the Zebrafish Health Monitoring report and the Fish Husbandry, Biosecurity and Health Monitoring Description (see templates in63) from the exporting facility. Then, the recipient facility should make specific queries regarding fish that have been imported into Q2 in the past year. Finally, recipient stakeholders should discuss and approve a standard protocol for surface sanitization of eggs and decide whether the embryos will be treated before or after shipment.
To assess P. tomentosa contamination in imported adult fish, personnel in the recipient facility can sample water and feces from the shipping bags and set aside some fish for postmortem screening, particularly those with lower body condition scores. Supplementary fish can be added to the imported cohort for euthanasia and screening at arrival and during quarantine. From 1 wk after arrival and at monthly intervals thereafter, both sludge from tanks and fish that are losing body condition can be tested. Actions (for example, treatment or euthanasia) to be taken in case of positive results must be agreed upon in advance with the PI.
Scenario #3: Importing from a multispecies facility.
A scenario #1 facility investigator is requesting importation of zebrafish embryos from a colleague who works in a multispecies facility at a different institution. The multispecies facility program is described, followed by recommendations for managing this importation to best protect the recipient facility.
Overview of multispecies facility.
The exporting facility has 4 aquatic animal holding rooms (EU3A to EU3D) and 2 dedicated quarantine rooms (Q3A and Q3B) (Figure 9). Room EU3A has 2 independent recirculating water systems with zebrafish on one and Japanese medaka (Oryzias latipes) on the other. Each system has a capacity of up to 20,000 adult fish. Room EU3B has 1 recirculating water system with a capacity for 500 adult African turquoise killifish (Nothobranchius furzeri). Room EU3C has 6 independent recirculating water systems, each with a capacity of 40 to 400 European seabass (Dicentrarchus labrax) weighing from 50 to 500 g each. Room EU3D has a single flow-through system with a capacity of up to 175 African clawed frogs (Xenopus laevis).
Figure 9.
Sketch scenario #3 - Multispecies facility. The exporting facility has 4 aquatic animal holding rooms (EU3A to EU3D) and 2 dedicated quarantine rooms (Q3A and Q3B). Room EU3A has 2 independent recirculating water systems with zebrafish on one and Japanese medaka on the other. Room EU3B has 1 recirculating water system holding African turquoise killifish. Room EU3C has 6 independent recirculating water systems for European seabass. Room EU3D has a single flow-through system with African clawed frogs. Quarantine room Q3A is dedicated to D. rerio, O. latipes and N. furzeri, with an independent recirculating water system (Q) for each species. Quarantine (Q) room Q3B is dedicated to X. laevis. Seabass are quarantined in a dedicated system (Q) located in the seabass holding room, EU3C. This room has a separate sink and counter area dedicated to work with quarantined seabass.
Quarantine room Q3A is dedicated to D. rerio, O. latipes and N. furzeri, with an independent recirculating water system for each species. Quarantine room Q3B is dedicated to X. laevis. Seabass are quarantined in a dedicated system located in the seabass holding room, EU3C. This room has a separate sink and counter area dedicated to work with quarantined seabass. EUs 3A, 3B, 3C, and 3D are in the same building; Q3A and Q3B are both located in a second building.
Management of multispecies facility.
Research staff from the laboratories perform all feeding and daily health checks in all 6 EUs. Personnel wear gloves and laboratory coats in all rooms. Although research staff are dedicated to each EU based in part on the species with which individuals work, the daily workflow of animal facility staff moves from the room with lowest to the room with highest risk. EU3A (D. rerio and O. latipes) and EU3B (N. furzeri) are considered to be of equal status (lowest risk). The seabass housing rack in EU3C is next, followed by the quarantine rack in the same room. Room Q3A (D. rerio, O. latipes, and N. furzeri) is next, followed by Room EU3D (X. laevis) and finally room Q3B (X. laevis). EU3D and Q3B both house amphibians, which can share parasites, bacteria, fungi-like pathogens, and viruses with fish (see Table S3 in63). These joint susceptibilities must be considered when determining the order in which to enter rooms to prevent these agents from crossing from amphibian into fish rooms. Staff do not return to any other fish or amphibian rooms on the same day after working in either quarantine room. All tanks, tank accessories, equipment, food, and supplies for each EU and quarantine area are dedicated and cleaned separately.
Dechlorinated municipal water is converted to RO water that is used to make culture-ready water for all species. Each room has its own RO water maker. Reserve water tanks for the storage of fish-ready water are present in each room. Natural seawater is not used in the seabass systems due to the risk of introducing marine pathogens. Reserve water for all 6 systems in the seabass room is kept in 1 reserve water tank. Feed for zebrafish, medaka, and killifish consists of a commercial dry pellet and live food (Paramecium spp. and Artemia spp. cultured inhouse). One live food prep room is present in the building with the main EUs, away from the quarantine building. Live food is transported from this room to each housing room daily. Each housing room has refrigerated storage for holding any additional dedicated diet (for example, frozen Tubifex spp., bloodworms for killifish, and beef heart for frogs).
Zebrafish and killifish are imported from other research facilities as surface-sanitized eggs, but adults are occasionally imported from approved sources. Medaka are wild-caught. If fish are imported as juveniles or adults, they are quarantined in isolated tanks that are separated from fish that were received as surface-sanitized eggs. Zebrafish, medaka, and killifish are transferred to the main EUs as surface-sanitized eggs. African clawed frogs are imported as adults or juveniles from resource centers, and seabass juveniles and adults are imported from aquaculture facilities.
Health monitoring of EUs and quarantines.
The health monitoring follows the recommendations described previously,63 with the adaptations necessary for multiple species. The SMOP and SLOM statuses are based on available literature and, in the absence of information, on the likelihood of infection of the animal species by the pathogen. For zebrafish and medaka in EU3A, the list of SMOP and SLOM are shown in Figure 2 of this manuscript and in the multispecies columns of Table S3 in63; the screening pattern is shown in Figure 10. One prefiltration zebrafish sentinel tank is detached from the recirculating systems and receives sump water from both racks. These fish are routinely tested by PCR. A collection of euthanized colony fish from both racks, with a majority of medaka, is screened by histopathology to ensure that SLOM not covered by PCR assays are monitored. Depending on the seabass health status, Nervous Necrosis Virus (NNV) is considered a SMOP or a SLOM and is tested accordingly.
Figure 10.
Quarterly routine screening pattern for EU3A and Q3A. Euthanized colony fish from both racks, with a majority of medaka, are screened by histopathology, to ensure SLOM not covered by PCR assays are monitored. All fish are imported in Q3A (quarantine for zebrafish, medaka, and killifish), and screened for SMOP and SLOM by testing sick or surplus fish and tank sludge by histopathology, microscopy, or PCR as soon as practical after arrival. On a quarterly routine pattern for Q3A, all sick, sump or retired fish are examined by histopathology (to cover SMOP and SLOM of the respective species) and/or by PCR (for Mycobacterium spp. and other specific pathogens when available). Q3A environmental samples are taken quarterly from each system: sump surface swabs are tested by PCR for Mycobacterium spp., and sump sludge samples are screened by microscopy for parasites.
For health monitoring of Q3A (quarantine for zebrafish, medaka, and killifish), shipments are tested within 1 wk of arrival. All sick, sump, or retired fish are tested quarterly by histopathology to assess SMOP and SLOM of the respective species or by PCR for Mycobacterium spp. and other specific pathogens, if available. Environmental samples are taken quarterly; sump surface swabs are tested by PCR for Mycobacterium spp., and sump sludge samples are screened by microscopy for parasites, using the same schedule as described for room EU3A.
For the killifish in EU3B, the screening pattern is described in Figure 11 and the list of SMOP and SLOM in Figure 2. Due to the short life span of this species, colony fish should be more available for postmortem screening. However, if PCR assays are not available, only euthanized and freshly fixed killifish can be used for screening because fish that are found dead are likely to be autolyzed and therefore unsuitable for histopathology.
Figure 11.
Quarterly routine screening pattern for EU3B. Live feed is already screened with EU3A. There is no quarantine and only killifish in this EU. Considering the short life span of this species, euthanized and freshly fixed colony killifish are analyzed by histopathology.
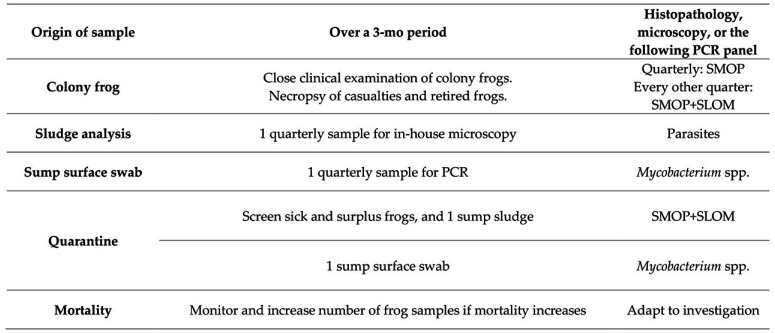
In EU3C, as described in Figures 2, 12, and 13, seabass are screened in quarantine and afterward by clinical examination under sedation. Wet mounts of cutaneous mucous and gill biopsies are examined by microscopy. Necropsies are performed on casualties along with microscopy, microbiologic cultures, histopathology, and PCR. Whenever possible, seabass importations are designed to contain surplus fish that can be euthanized for health and pathogen screening upon arrival and during and after quarantine. Extra animals are also requested for X. laevis imports. Screening for this amphibian is detailed in Figures 2 and 14.
Figure 12.
Quarterly routine screening pattern for EU3C. Healthy, sedated, sick, recently dead, and seabass that are retired from experiments during the year are analyzed. The aim is to screen at least 15 seabass per quarter (excluding quarantine screening). Wet mounts of cutaneous mucous and gill biopsies are examined by microscopy. Necropsies with complementary histopathology are completed, and bacterial cultures are performed when clinical signs or lesions point to a bacterial infection. Whenever possible, seabass imports contain surplus fish to be euthanized for health and pathogen screening upon arrival, during, and after quarantine. Each imported batch is tested for NNV by serology at arrival and by PCR after experiments.
Figure 13.
Health Monitoring Report for EU3C Seabass. For PCR pool, 3 to 5 fish are submitted in one pool. The total number of tested fish and the corresponding result are reported. Dates are indicated as yyyy-mm-dd.
Figure 14.
Quarterly routine screening pattern for EU3D and Q3B. Healthy, sick, recently dead, and frogs that are retired from experiments during the year are analyzed. The aim is to screen at least 15 frogs per quarter (excluding quarantine screening). Samples of cutaneous mucous are analyzed. Necropsies with complementary histopathology are completed, and bacterial cultures or PCR are performed for routine screening or when clinical signs or lesions point to a bacterial infection or a specific pathogen. Whenever possible, X. laevis imports contain surplus frogs to be euthanized for health and pathogen screening upon arrival, during, and after quarantine.
To mitigate acclimatization stress and decrease parasite load, killifish and seabass undergo quarantine treatment protocols (for example, salinity manipulation and other antiparasitic treatments). Also, as a prophylactic measure, snails are not permitted in the systems.
Microbiologic status of exporting facility.
In this scenario, zebrafish from the multispecies facility are requested to be imported onto the quarantine of the scenario #1 facility (Q1). The microbiologic status of each species in the multispecies facility depends on the reliability of the biosecurity barriers between EUs. Three fish species (Danio rerio, Oryzias latipes, and Nothobranchius furzeri) are quarantined in a single room, and another species (seabass) is quarantined in the same room with the main housing tanks for that species. This situation introduces the risk that pathogens adapted to a particular species may ultimately cross over to other species (see Table S3 in63 and eventual SLOM/SMOP classification for each fish species). This risk is influenced by the animal species present in the facility at a given time, even if they are in different EUs. For example, if African turquoise killifish of wild origin are quarantined in the same room with zebrafish and Japanese medaka, the risk of cross-contamination by pathogens like Piscinoodinium pillulare is increased because this pathogen is common in wild African turquoise killifish. Furthermore, because zebrafish share the same EU with Japanese medaka and are housed in close proximity to African turquoise killifish, the SLOM/SMOP classification should be more extensive for these zebrafish than for a facility housing only isolated zebrafish. Some pathogens may even change SLOM or SMOP status (for example, NNV will change status in zebrafish depending on its presence in seabass; see Figure 2). Therefore, SLOM/SMOP lists will be somewhat fluid, depending on the overall epidemiologic context at the time and previous screening results from the other species present in the facility. All of these possibilities should be considered when deciding how to best import fish from such a facility. The exporting facility should be asked to provide the Health Monitoring report for all 3 species housed in the zebrafish quarantine room. Ideally, the Health Monitoring report from the other 2 species (seabass and Xenopus spp.) should also be made available for inspection by the importing facility (See below for EU3C—seabass).
Recommendations for importation from this facility.
To help assess the biosecurity barriers, the Fish Husbandry, Biosecurity and Health Monitoring Description report should be requested at least for EU3A (holding room of zebrafish and medaka). Other useful information to request is the microbiologic status of animals imported in the past year. To finalize import processes, stakeholders should discuss and standardize the protocol for sanitization of egg surfaces and decide whether the embryos will be treated before or after shipment.
Health monitoring report for EU3C—European seabass.
The health monitoring report template (Table S5 in63) is adapted to the species. The list of pathogens, including mycobacteria, is different. For example, Mycobacterium pseudoshottsii is reported as a SMOP, and Mycobacterium hippocampi DL is reported in the “other Mycobacterium spp.” section.69,92 As the quarantine system is in the same room as the main holding systems, all systems in the room must be viewed as part of the same EU, despite any biosecurity containment measures. Quarantine screening data (for example, NNV serology on arrival) should thus be reported with other results from that room. The situation would be different if quarantine was contained in an isolated EU. Figure 13 shows a few entries of the health monitoring report, based on Figures 2 and 12. These examples concern SMOP that require further explanations in the “Notes” box.
Conclusion
Our recommendations are provided to help facilities improve quarantine and biosecurity processes, which are key for excluding specific pathogens. More generally, following good practices for quarantine and importation would significantly mitigate the risks associated with the introduction of new fish, and thus facilitate the exchange of animal models. This document completes and illustrates recommendations on health monitoring programs and reporting of health status.63 Safe exchange of fish should take place only after biosecurity risks are assessed. Our scenarios illustrate how quarantine processes and health status reports are the pillars of biosecurity risk assessment for import triage.
Other relevant points of discussion include how to mitigate outbreaks, eliminate fish contamination, and eradicate specific pathogens from aquatic systems while preserving equipment integrity and animal models. In the future, further focus on microbiologic status might be shifted toward the exhaustive identification of the existing microbiota, rather than confirming the absence of specific microbes.6,31
References
- 1.Ahne W, Bjorklund HV, Essbauer S, Fijan N, Kurath G, Winton JR. 2002. Spring viremia of carp (SVC). Dis Aquat Organ 52:261–272. 10.3354/dao052261. [DOI] [PubMed] [Google Scholar]
- 2.Aleström P, D’Angelo L, Midtlyng PJ, Schorderet DF, Schulte-Merker S, Sohm F, Warner S. 2020. Zebrafish: Housing and husbandry recommendations. Lab Anim 54:213–224. 10.1177/0023677219869037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbeitskreis Käfigaufbereitung (Working Group for Cage Processing). [Internet]. 2020. Fish tank processing in animal facilities. [Cited 29 December 2021]. Available at: https://www.tecniplast.it/usermedia/de/AKKAB/1st_ISSUE_AK_KAB_FISH_TANK_PROCE.pdf
- 4.Arthur JR, Bondad-Reantaso MG, Subasinghe RP.2008. Procedures for the quarantine of live aquatic animals. Rome (Italy): Food and Agriculture Organization of the United Nations. [Google Scholar]
- 5.Beran V, Matlova L, Dvorska L, Svastova P, Pavlik I. 2006. Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment. J Fish Dis 29:383–393. 10.1111/j.1365-2761.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 6.Bleich A, Fox JG. 2015. The Mammalian Microbiome and Its Importance in Laboratory Animal Research. ILAR J 56:153–158. 10.1093/ilar/ilv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borges AC, Pereira N, Franco M, Vale L, Pereira M, Cunha MV, Amaro A, Albuquerque T, Rebelo M. 2016. Implementation of a zebrafish health program in a research facility: A 4-year retrospective study. Zebrafish 13 Suppl 1:S115–S126. 10.1089/zeb.2015.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bovo G, Håstein T, Hill B, LaPatra S, Michel C, Olesen NJ, Shchelkunov I, Storset A, Wolffrom T, Midtlyng PJ. 2005. Work package 1 report: Hazard identification for vertical transfer of fish disease agents. VESO-1601:1–36. Available at: https://www.eurl-fish-crustacean.eu/fish/scientific-reports
- 9.Brown LL, Cox WT, Levine RP. 1997. Evidence that the causal agent of bacterial cold-water disease Flavobacterium Psychrophilum is transmitted within salmonid eggs. Dis Aquat Organ 29:213–218. 10.3354/dao029213. [DOI] [Google Scholar]
- 10.Byers KB, Matthews JL. 2002. Use of zebrafish and zoonoses. Appl Biosaf 7:117–119. 10.1177/153567600200700302. [DOI] [Google Scholar]
- 11.Cabello FC, Godfrey HP, Buschmann AH, Dölz HJ. 2016. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect Dis 16:e127–e133. 10.1016/S1473-3099(16)00100-6. [DOI] [PubMed] [Google Scholar]
- 12.Calhoun DM, Leslie K, Riepe T, Achatz T, McDevitt-Galles T, Tkach V, Johnson P. 2020. Patterns of Clinostomum marginatum infection in fishes and amphibians: Integration of field, genetic, and experimental approaches. J Helminthol 94:e44. 10.1017/S0022149X18001244. [DOI] [PubMed] [Google Scholar]
- 13.Cano I, Lopez-Jimena B, Garcia-Rosado E, Ortiz-Delgado JB, Alonso MC, Borrego JJ, Sarasquete C, Castro D. 2009. Detection and persistence of Lymphocystis disease virus (LCDV) in Artemia sp. Aquaculture 291:230–236. 10.1016/j.aquaculture.2009.03.018. [DOI] [Google Scholar]
- 14.Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE. editors. 2020. The zebrafish in biomedical research. San Diego (CA): Elsevier Inc. [Google Scholar]
- 15.Chang CT, Amack JD, Whipps CM. 2016. Zebrafish embryo disinfection with povidone–iodine: evaluating an alternative to chlorine bleach. Zebrafish 13 Suppl 1:S96–S101. 10.1089/zeb.2015.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CT, Benedict S, Whipps CM. 2019. Transmission of Mycobacterium chelonae and Mycobacterium marinum in laboratory zebrafish through live feeds. J Fish Dis 42:1425–1431. 10.1111/jfd.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CT, Lewis J, Whipps CM. 2019. Source or Sink: Examining the Role of Biofilms in Transmission of Mycobacterium spp. in Laboratory Zebrafish. Zebrafish 16:197–206. 10.1089/zeb.2018.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Council of the European Communities. 2008. Council Directive 2006/88/EC as regards conditions and certification requirements for the placing on the market and the import into the Community of aquaculture animals and products thereof and laying down a list of vector species. Off J Eur Commun L337:41–75. [Google Scholar]
- 19.Crim MJ, Lawrence C, Livingston RS, Rakitin A, Hurley SJ, Riley LK. 2017. Comparison of antemortem and environmental samples for zebrafish health monitoring and quarantine. J Am Assoc Lab Anim Sci 56:412–424. [PMC free article] [PubMed] [Google Scholar]
- 20.De Swaef E, Van Den Broeck W, Dierckens K, Decostere A. 2016. Disinfection of teleost eggs: a review. Rev Aquacult 8:321–341. 10.1111/raq.12096. [DOI] [Google Scholar]
- 21.Department of Agriculture, Water and the Environment. Australian Government. [Internet]. 2021. Australia’s National List of Reportable Diseases of Aquatic Animals. [Cited 29 December 2021]. Available at: https://www.awe.gov.au/agriculture-land/animal/aquatic/reporting/reportable-diseases#finfish
- 22.Donohue MJ, Vesper S, Mistry J, Donohue JM. 2019. Impact of Chlorine and Chloramine on the Detection and Quantification of Legionella pneumophila and Mycobacterium Species. Appl Environ Microbiol 85:e01942-19. 10.1128/AEM.01942-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvorak G. [Internet]. 2009. Biosecurity for Aquaculture Facilities in the North Central Region. NCRAC Extension Fact Sheets. 8. [Cited 29 December 2021]. Available at: http://lib.dr.iastate.edu/ncrac_factsheets/8
- 24.Erlacher-Reid CD. 2018. Considerations for treatment of large zoologic collections: fish. Vet Clin North Am Exot Anim Pract 21:311–325. 10.1016/j.cvex.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 25.European Commission. 2008. Commission Decision 2008/946/EC of 12 December 2008 implementing Council Directive 2006/88/EC as regards requirements for quarantine of aquaculture animals (notified under document number C(2008) 7905). Off J Eur Commun L337:94–101. [Google Scholar]
- 26.European Parliament, Council of the European Union. 2010. Directive 2010/63/EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off J Eur Union L 276:33–79. [Google Scholar]
- 27.European Parliament. 2021. Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law). Off J Eur Commun L84:1–208 – amended 02016R0429-21.04.2021-002.001. [Google Scholar]
- 28.Evelyn TPT, Ketcheson JE, Prosperi-Porta L. 1984. Further evidence for the presence of Renibacterium salmoninarum in salmonid eggs and for the failure of povidone-iodine to reduce the intra-ovum infection rate in water-hardened eggs. J Fish Dis 7:173–182. 10.1111/j.1365-2761.1984.tb00921.x. [DOI] [Google Scholar]
- 29.Filosa A, Barker AJ, Dal Maschio M, Baier H. 2016. Feeding state modulates behavioral choice and processing of prey stimuli in the zebrafish tectum. Neuron 90:596–608. 10.1016/j.neuron.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Forbes BA, Hall GS, Miller MB, Novak SM, Rowlinson MC, Salfinger M, Somoskövi A, Warshauer DM, Wilson ML. 2018. Practice guidelines for clinical microbiology laboratories: Mycobacteria. Clin Microbiol Rev 31:e00038-17. 10.1128/CMR.00038-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin CL, Ericsson AC. 2020. Complex Microbiota in Laboratory Rodents: Management Considerations. ILAR J 60:289–297. 10.1093/ilar/ilaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaulke CA, Martins ML, Watral VG, Humphreys IR, Spagnoli ST, Kent ML, Sharpton TJ. 2019. A longitudinal assessment of host-microbe-parasite interactions resolves the zebrafish gut microbiome’s link to Pseudocapillaria tomentosa infection and pathology. Microbiome 7:10. 10.1186/s40168-019-0622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gauthier DT. 2015. Bacterial zoonoses of fishes: A review and appraisal of evidence for linkages between fish and human infections. Vet J 203:27–35. 10.1016/j.tvjl.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Gerlai R. 2019. Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacol Biochem Behav 178:30–38. 10.1016/j.pbb.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Gomez KD, Mori K, Okinaka Y, Nakai T, Park SC. 2010. Trash fish can be a source of betanodaviruses for cultured marine fish. Aquaculture 302:158–163. 10.1016/j.aquaculture.2010.02.033. [DOI] [Google Scholar]
- 36.Government of Canada. [Internet]. 2019. Reportable diseases: Aquatic animals. [Cited 29 December 2021]. Available at: https://inspection.canada.ca/animal-health/aquatic-animals/diseases/reportable-diseases/eng/1322940971192/1322941111904
- 37.Hadfield C. 2012. Quarantine of fish and aquatic invertebrates in public display aquaria, ch 26 p 202–209. In: Miller ER, Fowler ME, editors. Fowler’s zoo and wild animal medicine, vol 7. Saint-Louis (MO): WB Saunders. [Google Scholar]
- 38.Hadfield CA, Clayton LA. 2011. Fish quarantine: Current practices in public zoos and aquaria. J Zoo Wildl Med 42:641–650. 10.1638/2011-0034.1. [DOI] [PubMed] [Google Scholar]
- 39.Hadfield CA, Clayton LA. editors. 2021. Clinical Guide to Fish Medicine. Hoboken (NJ): Wiley-Blackwell. 10.1002/9781119259886 [DOI] [Google Scholar]
- 40.Haenen OL, Evans JJ, Berthe F. 2013. Bacterial infections from aquatic species: Potential for and prevention of contact zoonoses. Rev Sci Tech 32:497–507. 10.20506/rst.32.2.2245. [DOI] [PubMed] [Google Scholar]
- 41.Hanwell D, Hutchinson SA, Collymore C, Bruce AE, Louis R, Ghalami A, Allison WT, Ekker M, Eames BF, Childs S, Kurrasch DM, Gerlai R, Thiele T, Scott I, Ciruna B, Dowling JJ, McFarlane S, Huang P, Wen XY, Akimenko MA, Waskiewicz AJ, Drapeau P, Babiuk LA, Dragon D, Smida A, Buret AG, O’Grady E, Wilson J, Sowden-Plunkett L, Robertson JV, Tropepe V. 2016. Restrictions on the Importation of Zebrafish into Canada Associated with Spring Viremia of Carp Virus. Zebrafish 13 Suppl 1:S153–S163. 10.1089/zeb.2016.1286. [DOI] [PubMed] [Google Scholar]
- 42.Haramoto E, Kitajima M, Katayama H, Ohgaki S. 2007. Detection of koi herpesvirus DNA in river water in Japan. J Fish Dis 30:59–61. 10.1111/j.1365-2761.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 43.Hashish E, Merwad A, Elgaml S, Amer A, Kamal H, Elsadek A, Marei A, Sitohy M. 2018. Mycobacterium marinum infection in fish and man: epidemiology, pathophysiology and management; a review. Vet Q 38:35–46. 10.1080/01652176.2018.1447171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iaria C, Migliore S, Macri D, Bivona M, Capparucci F, Gaglio G, Marino F. 2019. Evidence of Centrocestus formosanus (Nishigori, 1924) in Zebrafish (Danio rerio). Zebrafish 16:522–526. 10.1089/zeb.2019.1744. [DOI] [PubMed] [Google Scholar]
- 45.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 46.Jantrakajorn S, Wongtavatchai J. 2015. Egg surface decontamination with bronopol increases larval survival of Nile tilapia, Oreochromis niloticus. Czech J Anim Sci 60:436–442. 10.17221/8523-CJAS. [DOI] [Google Scholar]
- 47.Jørgensen LVG. 2017. The fish parasite Ichthyophthirius multifiliis - Host immunology, vaccines and novel treatments. Fish Shellfish Immunol 67:586–595. 10.1016/j.fsi.2017.06.044. [DOI] [PubMed] [Google Scholar]
- 48.Kent ML, Buchner C, Watral VG, Sanders JL, Ladu J, Peterson TS, Tanguay RL. 2011. Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Organ 95:73–79. 10.3354/dao02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kent ML, Sanders JL, Spagnoli S, Al-Samarrie CE, Murray KN. 2020. Review of diseases and health management in zebrafish Danio rerio (Hamilton 1822) in research facilities. J Fish Dis 43:637–650. 10.1111/jfd.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kent ML, Watral V, Gaulke CA, Sharpton TJ. 2019. Further evaluation of the efficacy of emamectin benzoate for treating Pseudocapillaria tomentosa (Dujardin 1843) in zebrafish Danio rerio (Hamilton 1822). J Fish Dis 42:1351–1357. 10.1111/jfd.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kent ML, Watral V, Villegas EN, Gaulke CA. 2019. Viability of Pseudocapillaria tomentosa eggs exposed to heat, ultraviolet light, chlorine, iodine, and desiccation. Zebrafish 16:460–468. 10.1089/zeb.2019.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinoshita M, Murata K, Naruse K, Tanaka M. 2009. Medaka: Biology, Management, and Experimental Protocols. Chichester (UK): John Wiley & Sons Ltd. [Google Scholar]
- 53.Kong FE, Deighton MA, Thurbon NA, Smith SR, Rouch DA. 2018. Cryptosporidium parvum decay during air drying and stockpiling of mesophilic anaerobically digested sewage sludge in a simulation experiment and oocyst counts in sludge collected from operational treatment lagoons in Victoria, Australia. J Water Health 16:435–448. 10.2166/wh.2018.018. [DOI] [PubMed] [Google Scholar]
- 54.Lazado CC, Good C. 2021. Survey findings of disinfection strategies at selected Norwegian and North American land-based RAS facilities: A comparative insight. Aquaculture 532:736038. 10.1016/j.aquaculture.2020.736038. [DOI] [Google Scholar]
- 55.Mainous ME, Smith SA. 2005. Efficacy of common disinfectants against Mycobacterium marinum. J Aquat Anim Health 17:284–288. 10.1577/H04-051.1. [DOI] [Google Scholar]
- 56.Maley D, Laird AS, Rinkwitz S, Becker TS. 2013. A simple and efficient protocol for the treatment of zebrafish colonies infected with parasitic nematodes. Zebrafish 10:447–450. 10.1089/zeb.2013.0868. [DOI] [PubMed] [Google Scholar]
- 57.Marsollier L, Sévérin T, Aubry J, Merritt RW, Saint André JP, Legras P, Manceau AL, Chauty A, Carbonnelle B, Cole ST. 2004. Aquatic snails, passive hosts of Mycobacterium ulcerans. Appl Environ Microbiol 70:6296–6298. 10.1128/AEM.70.10.6296-6298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martins S, Monteiro JF, Vito M, Weintraub D, Almeida J, Certal AC. 2016. Toward an integrated zebrafish health management program supporting cancer and neuroscience research. Zebrafish 13 Suppl 1:S47–S55. 10.1089/zeb.2015.1198. [DOI] [PubMed] [Google Scholar]
- 59.Mason T, Snell K, Mittge E, Melancon E, Montgomery R, McFadden M, Camoriano J, Kent ML, Whipps CM, Peirce J. 2016. Strategies to mitigate a Mycobacterium marinum outbreak in a zebrafish research facility. Zebrafish 13 Suppl 1:S77–S87. 10.1089/zeb.2015.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Midttun HLE, Vindas MA, Nadler LE, Øverli Ø, Johansen IB. 2020. Behavioural effects of the common brain-infecting parasite Pseudoloma neurophilia in laboratory zebrafish (Danio rerio). Sci Rep 10:8083. 10.1038/s41598-020-64948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Midttun HLE, Vindas MA, Whatmore PJ, Øverli Ø, Johansen IB. 2020. Effects of Pseudoloma neurophilia infection on the brain transcriptome in zebrafish (Danio rerio). J Fish Dis 43:863–875. 10.1111/jfd.13198. [DOI] [PubMed] [Google Scholar]
- 62.Mocho JP. 2016. Three-dimensional screen: A comprehensive approach to the health monitoring of zebrafish. Zebrafish 13 Suppl 1:S132–S137. 10.1089/zeb.2015.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mocho JP, Collymore C, Farmer SC, Leguay E, Murray KN, Pereira N. 2022. FELASA-AALAS recommendations for monitoring and reporting of laboratory fish diseases and health status, with an emphasis on zebrafish (Danio rerio). Comp Med 72:(Forthcoming). 10.30802/AALAS-CM-22-000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mocho JP, Coutot R, Douglas M, Szpiro L, Bouchami D, Durimel L, Moulès V, Hardy P. 2021. Assessment of microbial reduction by cage washing and thermal disinfection using quantitative biologic indicators for spores, viruses and vegetative bacteria. J Am Assoc Lab Anim Sci 60:529–538. 10.30802/AALAS-JAALAS-21-000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mocho JP, Lang F, Valentin G, Bedu S, McKimm R, Ramos J, Saavedra Torres Y, Wheatley SE, Higgins J, Millington ME, Rengtved Lundegaard P, Chamorro Valverde R, Jenčič V, von Krogh K. 2022. A Multi-Site Assessment of Anesthetic Overdose, Hypothermic Shock, and Electrical Stunning as Methods of Euthanasia for Zebrafish (Danio rerio) Embryos and Larvae. Biology (Basel) 11:546. 10.3390/biology11040546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mocho JP, Martin DJ, Millington ME, Saavedra Torres Y. 2017. Environmental screening of Aeromonas hydrophila, Mycobacterium spp., and Pseudocapillaria tomentosa in zebrafish systems. J Vis Exp 130. 10.3791/55306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mocho JP, Pereira N. 2021. Health monitoring, disease, and clinical pathology, p. 81–100. In: d’Angelo L, de Girolamo P, editors. Laboratory Fish in Biomedical Research. San Diego (CA): Academic Press. [Google Scholar]
- 68.Molloy SD, Pietrak MR, Bricknell I, Bouchard DA. 2013. Experimental transmission of infectious pancreatic necrosis virus from the blue mussel, Mytilus edulis, to cohabitating Atlantic Salmon (Salmo salar) smolts. Appl Environ Microbiol 79:5882–5890. 10.1128/AEM.01142-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mugetti D, Varello K, Gustinelli A, Pastorino P, Menconi V, Florio D, Fioravanti ML, Bozzetta E, Zoppi S, Dondo A, Prearo M. 2020. Mycobacterium pseudoshottsii in Mediterranean Fish Farms: New Trouble for European Aquaculture? Pathogens 9:610. 10.3390/pathogens9080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mulcahy D, Pascho RJ. 1985. vertical transmission of infectious haematopoietic necrosis virus in sockeye salmon, Oncorhynchus nerka (Walbaum): Isolation of virus from dead eggs and fry. J Fish Dis 8:393–396. 10.1111/j.1365-2761.1985.tb00962.x. [DOI] [Google Scholar]
- 71.Murray KN, Clark TS, Kebus MJ, Kent ML. 2022. Specific Pathogen Free - A review of strategies in agriculture, aquaculture, and laboratory mammals and how they inform new recommendations for laboratory zebrafish. Res Vet Sci 142:78–93. 10.1016/j.rvsc.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murray KN, Varga ZM, Kent ML. 2016. Biosecurity and health monitoring at the Zebrafish International Resource Center. Zebrafish 13 Suppl 1:S30–S38. 10.1089/zeb.2015.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noga EJ. 2010. Fish disease: diagnosis and treatment. Ames (IA): Wiley-Blackwell. 10.1002/9781118786758 [DOI] [Google Scholar]
- 74.Norris LJ, Watral V, Kent ML. 2018. Survival of bacterial and parasitic pathogens from zebrafish (Danio rerio) after cryopreservation and thawing. Zebrafish 15:188–201. 10.1089/zeb.2017.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Öktener A, Ali AH, Gustinelli A, Fioravanti ML. 2006. New host records for fish louse, Argulus foliaceus L., 1758 (Crustacea, Branchiura) in Turkey. Ittiopatologia 3:161–167. [Google Scholar]
- 76.Palic D, Scarfe AD. 2019. Biosecurity in aquaculture: Practical veterinary approaches for aquatic animal disease prevention, control, and potential eradication, Ch 19 p 497–519. In:Dewulf J, Van Immerseel F, editors. Biosecurity in animal production and veterinary medicine: From principles to practice. Boston (MA): CABI. [Google Scholar]
- 77.Phelps NB, Goodwin AE. 2008. Vertical transmission of Ovipleistophora ovariae (microspora) within the eggs of the golden shiner. J Aquat Anim Health 20:45–53. 10.1577/H07-029.1. [DOI] [PubMed] [Google Scholar]
- 78.Picón-Camacho SM, Leclercq E, Bron JE, Shinn AP. 2012. The potential utility of the leopard pleco (Glyptoperichthys gibbiceps) as a biological control of the ciliate protozoan Ichthyophthirius multifiliis. Pest Manag Sci 68:557–563. 10.1002/ps.2293. [DOI] [PubMed] [Google Scholar]
- 79.Picón-Camacho SM, Marcos-Lopez M, Bron JE, Shinn AP. 2012. An assessment of the use of drug and non-drug interventions in the treatment of Ichthyophthirius Multifiliis Fouquet, 1876, a protozoan parasite of freshwater fish. Parasitology 139:149–190. 10.1017/S0031182011001867. [DOI] [PubMed] [Google Scholar]
- 80.Polačik M, Blažek R, Reichard M. 2016. Laboratory breeding of the short-lived annual killifish Nothobranchius furzeri. Nat Protoc 11:1396–1413. 10.1038/nprot.2016.080. [DOI] [PubMed] [Google Scholar]
- 81.Pradeep PJ, Suebsing R, Sirithammajak S, Kampeera J, Turner W, Jeffs A, Kiatpathomchai W, Withyachumanarnkul B. 2017. Vertical transmission and concurrent infection of multiple bacterial pathogens in naturally infected red tilapia (Oreochromis spp.). Aquacult Res 48:2706–2717. 10.1111/are.13102. [DOI] [Google Scholar]
- 82.Rácz A, Dwyer T, Killen SS. 2019. Overview of a Disease Outbreak and Introduction of a Step-by-Step Protocol for the Eradication of Mycobacterium haemophilum in a Zebrafish System. Zebrafish 16:77–86. 10.1089/zeb.2018.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rigos G, Kogiannou D, Padrós F, Cristòfol C, Florio D, Fioravanti M, Zarza C. 2021. Best therapeutic practices for the use of antibacterial agents in finfish aquaculture: a particular view on European seabass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata) in Mediterranean aquaculture. Rev Aquacult 13:1285–1323. 10.1111/raq.12523. [DOI] [Google Scholar]
- 84.Roberts-Thomson A, Barnes A, Fielder DS, Lester RJG, Adlard RD. 2006. Aerosol dispersal of the fish pathogen, Amyloodinium ocellatum. Aquaculture 257:118–123. 10.1016/j.aquaculture.2006.02.058. [DOI] [Google Scholar]
- 85.Rose S, Hill R, Bermudez LE, Miller-Morgan T. 2013. Imported ornamental fish are colonized with antibiotic-resistant bacteria. J Fish Dis 36:533–542. 10.1111/jfd.12044. [DOI] [PubMed] [Google Scholar]
- 86.Ross G, Earp BJ, Wood JW. 1959. Mycobacterial infections in adult salmon and steelhead trout returning to the Columbia river basin and other areas in 1957. Spec Sci Rep–Fisheries no. 332. Washington (DC): U.S. Dept. of Interior, Fish and Wildlife Service.
- 87.Sanders JL, Watral V, Clarkson K, Kent ML. 2013. Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the Zebrafish, Danio rerio. PLoS One 8:e76064. 10.1371/journal.pone.0076064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schroeder P, Lloyd R, McKimm R, Metselaar M, Navarro J, O’Farrell M, Readman GD, Speilberg L, Mocho JP. 2021. Anaesthesia of laboratory, aquaculture and ornamental fish: Proceedings of the first LASA-FVS Symposium. Lab Anim 55:317–328. 10.1177/0023677221998403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silveira T, Kütter MT, Martins CMG, Marins LF, Boyle RT, Campos VF, Remião MH. 2021. First Record of Clinostomum sp. (Digenea: Clinostomidae) in Danio rerio (Actinopterygii: Cyprinidae) and the Implication of Using Zebrafish from Pet Stores on Research. Zebrafish 18:139–148. 10.1089/zeb.2020.1950. [DOI] [PubMed] [Google Scholar]
- 90.Smith SA. editor. 2019. Fish Diseases and Medicine. Boca Raton (FL): CRC Press. 10.1201/9780429195259 [DOI] [Google Scholar]
- 91.Spagnoli S, Sanders J, Kent ML. 2017. The common neural parasite Pseudoloma neurophilia causes altered shoaling behaviour in adult laboratory zebrafish (Danio rerio) and its implications for neurobehavioural research. J Fish Dis 40:443–446. 10.1111/jfd.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stathopoulou P, Asimakis E, Petropoulos Y, Tsiamis G. 2020. Draft Genome Sequence of Mycobacterium hippocampi DL, Isolated from European Sea Bass (Dicentrarchus labrax). Microbiol Resour Announc 9:e00816-20. 10.1128/MRA.00816-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stevens CH, Reed BT, Hawkins P. 2021. Enrichment for Laboratory Zebrafish—A Review of the Evidence and the Challenges. Animals (Basel) 11:698. 10.3390/ani11030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subasinghe RP, Sommerville C. 1985. Disinfection of Oreochromis mossambicus (Peters) eggs against commonly occurring potentially pathogenic bacteria and fungi under artificial hatchery conditions. Aquacult Res 16:121–127. 10.1111/j.1365-2109.1985.tb00301.x. [DOI] [Google Scholar]
- 95.The World Organisation for Animal Health (OIE). [Internet]. 2017. Recommendations for surface disinfection of salmonid eggs. [Cited 29 December 2021]. Available at: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/aquatic-code-online-access/?id=169&L=1&htmfile=chapitre_disinfection_eggs.htm
- 96.The World Organisation for Animal Health (OIE). [Internet]. 2021. Aquatic animal health code. [Cited 29 December 2021]. Available at: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/aquatic-code-online-access/
- 97.The World Organisation for Animal Health (OIE). [Internet]. 2021. Manual of Diagnostic Tests for Aquatic Animals. [Cited 29 December 2021]. Available at: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access/
- 98.The World Organisation for Animal Health (OIE). [Internet]. 2021. OIE World Animal Health Information System (OIE_WAHIS). [Cited 29 December 2021]. Available at: https://wahis.oie.int/#/dashboards/country-or-disease-dashboard
- 99.The World Organisation for Animal Health (OIE). [Internet]. 2021. OIE-Listed diseases, infections and infestations in force in 2021. [Cited 29 December 2021]. Available at: https://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2021/
- 100.United States Department of Agriculture. Animal and Plant Health Inspection Service. [Internet]. 2021. Aquatic Animal Diseases. [Cited 29 December 2021]. Available at: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/aquaculture/aquatic-animal-diseases/index
- 101.Ventura Fernandes BH, Caetano da Silva C, Bissegato D, Kent ML, Carvalho LR. 2022. Rederivation of a mutant line (prop 1) of zebrafish Danio rerio infected with Pseudoloma neurophilia using in vitro fertilization with eggs from pathogen-free wild-type (AB) females and sperm from prop 1 males. J Fish Dis 45:35–39. 10.1111/jfd.13529. [DOI] [PubMed] [Google Scholar]
- 102.von Krogh K, Higgins J, Saavedra Torres Y, Mocho JP. 2021. Screening of Anaesthetics in Adult Zebrafish (Danio rerio) for the Induction of Euthanasia by Overdose. Biology (Basel) 10:1133. 10.3390/biology10111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Watts SA, Lawrence C, Powell M, D’Abramo LR. 2016. The vital relationship between nutrition and health in zebrafish. Zebrafish 13 Suppl 1:S72–S76. 10.1089/zeb.2016.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whipps CM, Lieggi C, Wagner R. 2012. Mycobacteriosis in zebrafish colonies. ILAR J 53:95–105. 10.1093/ilar.53.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wildgoose WH. editor. 2001. BSAVA Manual of Ornamental Fish, 2nd edition. Gloucester (UK): British Small Animal Veterinary Association. [Google Scholar]
- 106.Willumsen B. 1989. Birds and wild fish as potential vectors of Yersinia ruckeri. J Fish Dis 12:275–277. 10.1111/j.1365-2761.1989.tb00313.x. [DOI] [Google Scholar]
- 107.Wooster GA, Bowser PR. 1996. The Aerobiological Pathway of a Fish Pathogen: Survival and Dissemination of Aeromonas salmonicida in Aerosols and its Implications in Fish Health Management. J World Aquacult Soc 27:7–14. 10.1111/j.1749-7345.1996.tb00588.x. [DOI] [Google Scholar]
- 108.Yanong RPE. 2004. Fish health management considerations in recirculating aquaculture systems-Part 1: introduction and general principles: Cir 120/FA099, 12/2003. EDIS 2004. 10.32473/edis-fa099-2003. [DOI]
- 109.Yanong RPE, Erlacher-Reid C. 2012. Biosecurity in aquaculture, part 1: An overview. SRAC Publication 4707.