Abstract
Betta splendens, also called Siamese fighting fish or ‘betta,’ are a popular species in the fishkeeping hobby. Native to Southeast Asia, betta have been selectively bred for their fighting ability for hundreds of years, which has resulted in the species’ characteristic male aggression. More recently, betta have been bred for a number of ornamental traits such as coloration, fin morphology, and body size. Betta have unique characteristics and an evolutionary history that make them a useful model for studies in the fields of behavior, endocrinology, neurobiology, genetics, development, and evolution. However, standard laboratory procedures for raising and keeping these fish are not well established, which has limited their use. Here, we briefly review the past and present use of betta in research, with a focus on their utility in behavioral, neurobiological, and evolutionary studies. We then describe effective husbandry practices for maintaining betta as a research colony.
Abbreviations: dpf, days post-fertilization
Introduction
Betta splendens, also called Siamese fighting fish, or simply ‘betta,’ are anabantoids, a suborder of fish that possesses a labyrinth organ that allows them to breathe air from the surface of the water.29,55 The Betta genus contains many different species that are endemic to parts of Southeast Asia. They are usually found in shallow, still bodies of water, including rice paddies and areas that flood during the rainy season.54,69 Betta are a unique potential model for evolutionary, neurobiologic, and behavioral studies. Although they have been studied scientifically for over one hundred years, a lack of standard and effective husbandry practices has hindered wider adoption of the species as a model system.
Betta splendens exhibit aggressive territorial behaviors, and in 18th and 19th century Thailand, pairing 2 opponent fish in staged fights became a national pastime.69 By the 19th century, both wild-caught fish and domesticated fish were used in these staged fights. Some written reports suggest domestication began around 1850,69 but other reports suggest the practice likely began over 600 y ago.5 Recent genetic analyses are consistent with domestication beginning at least 400 y ago.41 As a result of hundreds of years of selective breeding for fighting ability, domesticated betta are now highly aggressive as compared with wild betta.76
Variations in color and morphology also arose as a result of domestication (Figure 1). This diversification, along with importation of betta to Europe and the United States in the early 1900s, led to a boom in popularity. Ornamental strains of betta can now be purchased in most pet stores, and the wide variety of different colors, patterns, and fin types makes them a popular fish for aquarium hobbyists. Soon after their introduction into the aquarium hobby in the early 1900s, a scientific interest in betta emerged. The earliest studies on betta investigated the patterns of inheritance of certain morphologic traits,27,42,78 experimented with sex reversal,71 and described behaviors such as nesting9,11,28,59 and aggression.47,66,72
Figure 1.
Six varieties of Betta splendens, varying in fin type, color, and patterning. A) blue veiltail male ornamental, B) red crowntail male ornamental, C) mosaic short halfmoon male ornamental, D) yellow plakat male ornamental, E) plakat male ‘fighter’ strain raised in Thailand for betta fighting competitions, F) wild-caught male Betta splendens.
Here we review betta’s past and present use in scientific research and detail the effective, streamlined practices we have developed for keeping and propagating betta in the lab. Our goal in providing this information is to facilitate the adoption of betta as a model system, paving the way for future work on betta husbandry, genetics, development, evolution, behavior, and neurobiology.
Betta as a Model for Social Behaviors and Neurobiology
Betta are a unique model for studying complex social behaviors. Aggression in betta is highly robust and stereotyped.66 A male betta, when presented with an opponent male, will flare his fins, erect his gill covers, and perform lateral tail beating movements. Male fish will engage in this behavior even if they are in separate tanks. The male aggression response can also be elicited by allowing the fish to see their reflection in a mirror.47 While a number of model organisms are used to study aggressive behaviors (for example, mice and fruit flies), aggression in those species depends largely on olfactory cues. However, in betta, visual cues alone are sufficient to evoke an aggressive response, making them particularly suitable for the study of visually evoked aggression.
Betta are also useful as a model for paternal behavior. Betta splendens are bubble nesters; prior to mating, males create a bubble nest for developing offspring by blowing mucus-coated bubbles at the surface of water.9 Betta spawning takes an average of 5 h,59 during which eggs are released a few at a time by the female and are fertilized externally before falling down the water column. The male retrieves approximately 90% of the fallen eggs in his mouth,59 with limited help from the female, and places them into the bubble nest. Eggs and newly hatched larvae remain in the bubble nest for up to 3 d, and the male usually stays in the immediate area, picking up fallen eggs or larvae in his mouth and returning them to the bubble nest.12
Aggressive and paternal behaviors, and factors that modulate these behaviors, have been characterized in previous laboratory studies. Studies on betta aggression have examined the effects of pharmacological interventions on aggressive responses46,73 and how aggression is modulated by the environment,15,31,32 prior social experience,10,14,20 and opponent characteristics.3,8,47 Studies of nesting behavior have revealed the composition of bubble nests36 and how nest characteristics are altered by environmental conditions35 and competition for resources.11 This characterization of behavior, combined with an understanding of the brain regions involved in aggression13 and the availability of brain atlases45 provides a strong foundation for future investigation of the neurobiology and neural circuitry underlying these behaviors.
Betta as a Genetic Model for the Evolution of Development and Behavior
The evolutionary history of betta makes this species ideal for studying the evolution of behavior and morphology, and the genetic and genomic bases of betta traits. Some of the earliest studies on betta examined the Mendelian inheritance patterns underlying certain morphologic traits such as Cambodian patterning27,78 and blue coloration.42 Previous studies have also investigated the cellular architecture of pigmentation in betta,4,37,38 providing additional foundation for genetic analyses of coloration and patterning.
Annotated reference genomes for wild41 and domesticated22,57,79 betta are now available, enabling powerful genetic and genomic analyses. The B. splendens genome is only 440–450 Mb, one of the most compact vertebrate genomes, resulting in lower costs for sequencing-based studies. Recent work using genome-wide association studies and quantitative trait analyses have uncovered candidate genes involved in sex determination, coloration, and fin shape.41,79 Some studies have evaluated these candidate genes through induced mutations with CRISPR/Cas9,79 laying the groundwork for the future use of modern genetic tools to study betta.
Betta splendens, and the Betta genus in general, also have great potential as models for studying domestication and population genetics. Over 70 species of Betta are currently recognized, with new species, such as B. mahachaiensis, being described only recently.40 The mitochondrial genomes of many Betta species have recently been sequenced,2,56,67 and their genome-wide variation relative to B. splendens has been characterized,41 providing a strong basis for future phylogenetic analyses. Recent genomic analyses have revealed that betta were likely domesticated over 400 y ago.41 Genetic evidence also indicated that the modern domesticated Betta splendens has hybridized with other species in the B. splendens species complex: B. imbellis and B. mahachaiensis.41 Future studies could examine the behavioral, morphologic, and evolutionary consequences of this hybridization.
Housing And Water Parameters
Good water quality is critical for keeping and raising healthy betta. Like many aquarium species, betta are susceptible to illness and disease when kept in water of poor quality. Ammonia, a byproduct of the breakdown of fish waste and uneaten food, has detrimental effects on health (see “Health and Disease” below) at levels above 0.25 ppm. To maintain good water quality, we use a standalone recirculating housing system (Figure 2A) designed for housing zebrafish (Aquaneering, San Diego, CA), in which water moves continuously through tanks and passes through multiple filtration steps. In our system, water is mechanically filtered through a particulate filter mat, which captures large debris and uneaten food. The water is then directed through a biologic filter, which breaks down ammonia into less toxic nitrites and nitrates. In the third filtration step, the water flows through a set of carbon filters that remove chlorine and organic compounds. Finally, the water passes through UV light to kill pathogens. Clean water is then directed into individual tanks at a rate of approximately 150 mL per minute, displacing water from the back of the tanks, where it begins the filtration process. We replace 15% of the total water volume in our system daily with fresh reverse osmosis water; this replacement removes nitrites and nitrates that build up through the biologic filtration step described above. We use an aquarium water test kit (API Freshwater Master Test Kit, API/Mars Fishcare North America, Chalfont, PA) weekly to determine the ammonia, nitrite, and nitrate levels on our system. These processes reliably maintain nondetectable ammonia, nitrite, and nitrate levels in hundreds of tanks on our system.
Figure 2.
Betta housing system. A) Standalone recirculating housing system, with automated dosing system and rows supporting multiple individual tanks. B) Top: 2.8-L tanks housing multiple late larval fish; bottom: 1.4-L tanks housing single adult fish. White acrylic dividers are placed between tanks holding adult fish to prevent aggressive display. Individual tubing provides constant water flow to each tank, displacing water from the back of the tank where it begins the water filtration process. C) Side view of 1.4-L tank containing a single adult fish and suction cup plant enrichment item. Lids and baffles at the back of the tank prevent fish from escaping into the system.
pH and salinity.
Betta can tolerate a pH range63 of 5.0 to 9.0 and salinity levels81 of up to 9.35 mS/cm (6,000 ppm). Despite the range of values tolerated by betta, we aim to keep the pH and salinity of our system stable to minimize potential behavioral variation in our experiments. Our system is maintained at a pH of 7.0 (range 6.9 to 7.2), and a salinity of 1.0 mS/cm (range 0.9 to 1.1 mS/cm or 600 to 700 ppm). The addition of small amounts of salt to freshwater aquaria can reduce disease48,49,58 and improve fish growth.7 The daily 15% freshwater replacement introduces reverse osmosis water to the system, which lowers the salinity and pH. However, we maintain consistent pH and salinity levels through an automatic dosing system that adds, dropwise, a solution of sodium bicarbonate (65 g per 3 L water) or aquarium salt (Instant Ocean, Blacksburg, VA; 280 g per 3 L water; for composition see ref 34) until the desired pH or salinity is achieved. If it is necessary to reduce the pH or salinity of the system, we add more fresh reverse osmosis water.
Water temperature and light cycle.
Our system water temperature is kept at 28°C, which is ideal for bubble nest construction33 and ovarian development65 in betta, and is consistent with common betta husbandry practices.60,77 Furthermore, growth at lower temperatures (23 to 25°C) might lead to a biased sex ratio with a high incidence of females,80 whereas growth at higher temperatures (33°C) increases larval mortality and may lead to a higher incidence of males.24 At 28°C the sex ratio is usually about 50% of each sex. The 28°C system temperature also reduces the viability of some pathogens,50 aiding in disease prevention. We obtain a consistent water temperature of 28°C by setting the room to 26 to 27°C (the highest temperature that central heating in our building can provide) and using water heaters in the sumps of our housing system to supply additional heat. Our colony is maintained on a 14:10-hr light-dark cycle (using fluorescent bulbs for illumination), which mimics summer lighting conditions in betta’s natural geographic range. Published studies have not determined the optimal lighting conditions for betta reproduction. Our lighting conditions are based on the assumption that betta reproduce best during Thailand’s rainy season, which coincides with the summer months. Therefore, we use light-dark cycles that occur during the summer months.
Housing.
If good water quality is maintained, adult betta can be effectively housed in a variety of tank sizes.64 In our colony, adult fish are individually housed in 0.8-L or 1.4-L tanks (Figure 2B) on our recirculating system. Due to the aggressive behavior of male betta, adult males are individually housed. We place opaque, white, nonreflective, plastic dividers between tanks containing males to prevent aggressive display in response to neighboring fish. We also tend to individually house adult female betta, but we have had success cohousing them at a density of 2 fish per L, provided these tanks are carefully monitored for excessive aggression. If particularly aggressive females are identified in tanks with cohousing, the aggressive fish are removed and individually housed. We also cohouse betta while rearing larvae and juveniles, as described in further detail in the “Feeding and Rearing” section of this manuscript.
Wild betta and other strains tend to jump through the holes on the lid used to introduce food, or through small gaps between the lid and the tank. Therefore, lids must always be properly secured, and feeding holes in the tank lids can be covered using mesh tape (Duck Brand, product #282084, Avon, OH). The holes of the mesh tape are about 3 mm wide and are convenient for feeding, but are too small for fish to go through.
A build-up of algae may occur on tank walls after a few months, impeding the view into the tank. When this occurs, fish should be transferred to a clean tank. Aquaneering tanks are made of polycarbonate and can be washed in a standard facility tunnel or rack cage wash machine at 83°C without detergents. We do not recommend autoclaving the tanks as our attempts have caused warping and reduced the transparency of the plastic.
Enrichment.
Environmental enrichment may reduce abnormal fish behaviors,23 and studies have shown that enriched areas are preferred by tank inhabitants.70 For enrichment purposes, we provide each tank with one of 2 types of plastic plants (Imagitarium, a Petco brand, San Diego, CA). The first type of plant adheres to the side of the tank with a suction cup (Figure 2C). When this type of plant is placed within a few centimeters of the surface of the water, we often observe fish lying on this plant like a hammock. The second type of plant enrichment floats on the surface and hangs down into the water. Plant enrichment items should be carefully checked to assure that they are not impeding water flow in the tanks. Before introducing new plants to tanks, or when they become visibly dirty, we scrub them clean of organic materials and soak in a 5% bleach solution for 24 h before carefully dechlorinating them in a solution of 10 g/L sodium thiosulfate.
Breeding and Reproduction
Methods for setting up betta mating tanks vary widely in the hobbyist and commercial communities. We have had success using 5-L acrylic tanks (30 cm × 12 cm × 22 cm high) for mating (Figure 3A). We cover 3 sides of the tank with brown paper to prevent the breeding pairs from seeing into adjacent tanks or being disturbed by movement in our fish room. The mating tanks are filled with system water to a height of approximately 10 cm and are static (that is, they are not connected to our recirculating system). We place flat heat mats (VivoSun, Ontario, CA), designed for use under potted plants or reptile tanks below the tanks to keep the water at 28°C. Because Betta splendens are bubble nesters, we provide each tank with a floating substrate under which the male can build his nest. Hobby and research breeders use various materials for this purpose, such as small pieces of bubble wrap, Styrofoam cups cut in half lengthwise, and plastic container lids. We use a piece of dried Indian almond leaf (Terminalia catappa from SunGrow through Amazon.com; Figure 3B) as the bubble nest substrate. Indian almond leaves release tannins into the water, which slightly reduce the pH and may also decrease the risk of disease.43 Regardless of the potential health benefits, we have found that it is easier to harvest eggs from bubble nests built under Indian almond leaves than from bubble nests built under other substrates. We also add items in each tank to provide places in which fish can hide from aggressive, persistent partners (Figure 3A). Plastic cylinders, terracotta pots, and either silk or live plants can all provide shelter. Finally, we place acrylic covers over all tanks to maintain high levels of humidity (90% humidity in mating tanks, compared with approximately 25% in the fish room), which helps to keep the bubble nest intact.12
Figure 3.
Betta mating set up. A) 5-L static mating tank containing plants and stones for fish to hide. B) Male ornamental betta with bubble nest built under Indian almond leaf. C) Nongravid female betta. D) Gravid female betta; note the rounded belly (arrow).
Multiple methods can be used to introduce breeding pairs to the mating tanks. Many breeders start by placing the female behind a clear divider, such as inside a plastic cup, which allows the pair to see each other, but not physically interact; this can also allow the male to build a bubble nest without interruption. In this method, the divider is removed the next morning, giving the female access to the male. However, we have found that separating the pair is not necessary for successful mating; doing so increases the work required without improving mating success rates or reducing female injuries. However, variations on this method could be useful under certain circumstances, such as for observing mating behaviors or when precise timing of mating is important (for example, when planning to inject zygotes for genetic manipulation). In our routine mating method, we place the male and female together in the tank without a divider. We usually add mating pairs to the tanks 3 to 4 h before the lights are set to turn off and watch for mating the following morning. With these methods, we achieve an overall mating success rate (defined as the presence of eggs within the maximal 3-d period during which the pair is in the mating tank) of approximately 50% for all betta varieties (ornamentals, wild-caught, and fighters), consistent with success rates obtained with other laboratory methods.17
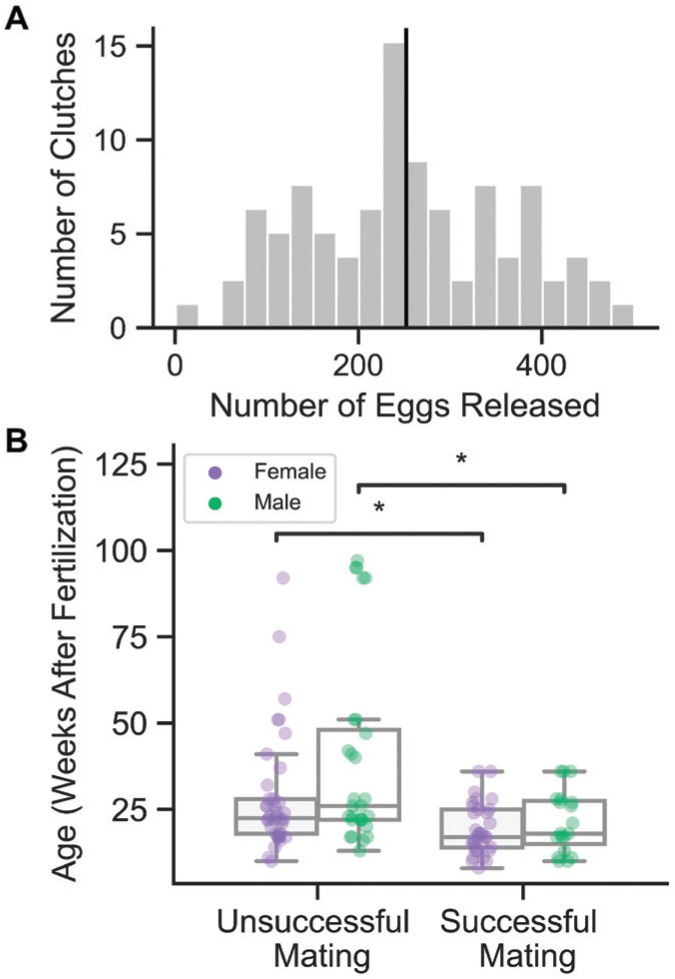
Most pairs begin mating between 1 and 5 h after light onset, and approximately 90% of our successful matings occur the day after introduction. During the mating process, the female will release a few eggs during each ‘embrace’ with the male, who fertilizes eggs every minute or so over the course of 1 to 4 h as they are being released. Mating is complete when the female retreats, often to a part of the tank where she can hide from the male. As soon as pairs finish mating, we remove the female and male with a net and place them back in their home tanks before collecting eggs from the bubble nest to be raised in vitro. The number of eggs released during mating ranges from 12 to 492, with a mean of 252 eggs (Figure 4A). This variation could be due, at least in part, to factors such as age, inbreeding, strain, and recency of previous matings.
Figure 4.
Clutch sizes and mating success. A) Distribution of clutch sizes. Black line at mean clutch size = 252 eggs (range 12 to 492 eggs; n = 79 matings). B) Age of fish of each sex at breeding according to outcome of mating (“success” means animals mate and release eggs). Comparisons were performed using a 2-sided Mann-Whitney test with Bonferroni correction (successful n = 56, unsuccessful n = 62; *, P ≤ 0.05).
With our routine mating method, serious mating-related injuries are rare. While minor nipping and biting is expected, fish should be observed after introduction to ensure that neither individual is excessively aggressive. If either individual experiences sustained biting, the mating pair should be separated, and mating reattempted at a later date. However, we have also found that some fish are repeatedly very aggressive during mating, regardless of whether we use our routine mating setup or the divider method; these fish may be unsuitable for mating. If fish sustain minor injuries during mating, we prevent infection by using a prophylactic 5-d course of methylene blue (Methylene Blue, Kordon, Hayward, CA), which has antifungal properties.16,74 We add methylene blue daily to the home tanks of injured fish at a final concentration of 3 ppm (0.0003%), and we remove these fish from the recirculating system during this treatment.
In our experience, a number of variables contribute to the successful mating of a breeding pair. Females that appear gravid (Figure 3C, D) and males that build bubble nests in their home tanks make ideal breeders, although the presence of either of these characteristics is not necessary for fish to successfully mate. Undernourished and underfed fish rarely mate successfully. When we tried feeding our adult colony only dried food, the mating success rate fell to around 30%, an observation backed up by the literature.6,44 Therefore, we feed our colony live, newly hatched brine shrimp (for more information on feeding protocols, see “Feeding and Rearing” below) at least once per day. In addition, we have not observed successful mating in any pair kept in a mating tank for longer than 3 d.
Age and the timing of previous matings are also important factors in mating success. In our experience, females require at least 4 wk between matings. Females that are mated within a shorter interval may not release any eggs, or will release eggs that are nonviable. Males can mate more frequently. We have had success after waiting only a week between matings of males, but we have not tried mating individual males more frequently than 2 wk in a row. Successful breeders tend to be younger, and we have not seen successful mating with fish older than 40 wk (Figure 4B) (the life span of betta is around 2 y under laboratory conditions). Fish raised under our conditions can successfully mate as early as 10 wk after fertilization if individually housed for at least 5 d starting at 9 wk after fertilization.
Feeding and Rearing
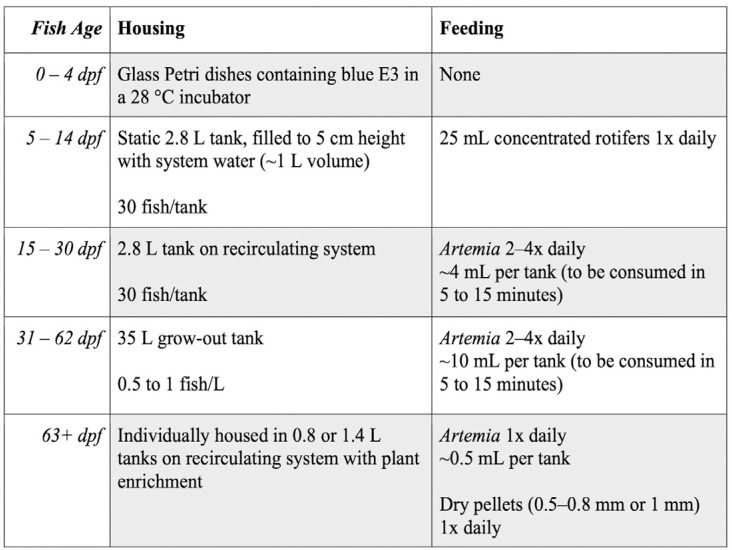
Hatchling stage (0 to 4 dpf).
We routinely raise developing embryos and newly hatched larvae from 0 to 4 d post-fertilization (dpf) in glass culture dishes; this allows us to monitor the number of eggs released and embryo survival rates. The chorion of developing betta sticks easily to plastic, so we use glass tools when handling eggs and unhatched, developing fish. Eggs are removed from the bubble nest shortly after mating, either by inverting the bubble nest substrate directly into a glass culture dish, or by using a spoon to scrape the eggs from the bubble nest. Once collected, the eggs are maintained in a glass culture dish containing freshly made E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, 0.0001% methylene blue) for 5 d. E3 contains methylene blue, which limits bacterial and fungal growth.26,39 Culture dishes containing developing fish are kept in an incubator (Heratherm, Thermo Scientific) set at 28°C. Our incubator has a glass door that allows the entry of ambient light from our main laboratory space. This lab space is lighted for roughly 12 h per day (from around 7:00-19:00) with white and yellow LEDs, and has windows to the outside of the building. Larval fish begin hatching between 29 and 44 h after fertilization19,30,75 and begin swimming by 72 h after fertilization.30 We use a glass transfer pipet daily to remove unfertilized eggs or embryos that are not developing or that are clearly malformed. Daily removal of nonviable embryos is necessary to prevent pathogen growth and an increase in ammonia. Yolk reserves are maintained in developing embryos and newly hatched larvae through 120 h after fertilization,30 so we do not feed fish while they are in culture dishes (Figure 5).
Figure 5.
Feeding and housing regimens for in-house raised fish of different developmental stages.
Early larval stage (5 to 14 dpf).
At 5 dpf, larvae are moved from culture dishes to 2.8-L tanks that are filled with 1 L of system water (that is, to a water height of about 5 cm). Because larval fish at this stage are very small (from approximately 2 mm at 5 dpf to 8 mm at 14 dpf), they are kept in relatively shallow, still water that gives them easy access to the surface. Access to the surface of the water prevents hypoxic conditions that are associated with reduced survival.6 In the age range of 5 to 14 dpf, the fish are too small to be kept on the recirculating system without being washed out of the tanks, and housing larval fish in tanks with static water allows food to remain in the tanks for longer periods of time. Each tank is stocked at a density of approximately 30 larvae per tank (range 20 to 50). Larval fish can be carefully transferred to these tanks using a glass transfer pipet, as they will stick to nets and mesh sieves.
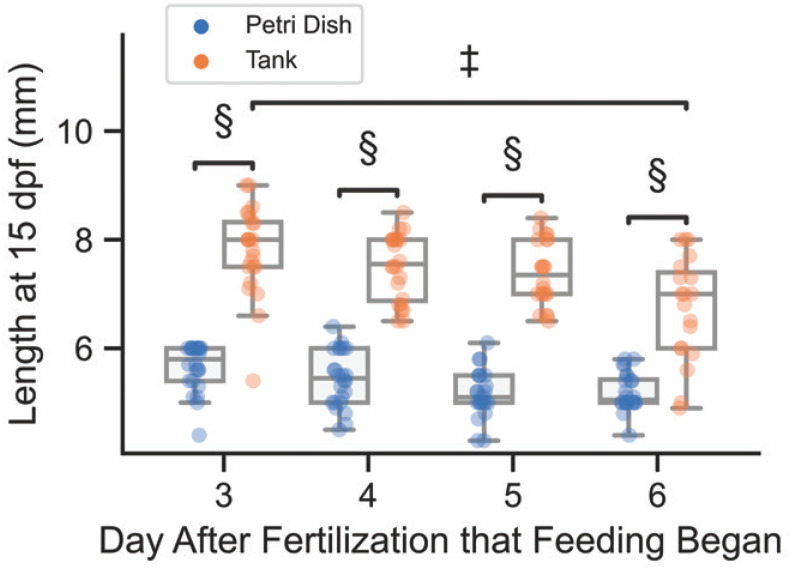
On days 5 to 14 after fertilization, we feed the larvae with Brachionus rotundiformis rotifers. Rotifers have been shown to increase early betta growth rates as compared with other live foods.53 We start to feed rotifers at 5 dpf, rather than 3 dpf, because there is no difference between these two start times in the size of the fish when they reach 15 dpf (Figure 6). However, larval size at 15 dpf appears to be lower if rotifer feeding starts at 6 dpf. Furthermore, starting rotifer feedings on 5 dpf yields survival rates of approximately 90% of larval fish at 15 dpf, as compared with approximately 75% survival rate when feeding begins on 6 dpf.
Figure 6.
Betta size at 15 dpf under different raising conditions. All fish were raised in culture dishes in a 28°C incubator from 0–4 dpf, then either moved to 2.8-L tanks for 5–15 dpf (orange points) or kept in culture dishes for 5–15 dpf (blue points). Daily feeding with rotifers for both housing conditions began on either day 3, 4, 5, or 6 (n≈24 individuals per treatment group). Between housing condition comparisons were performed using a 2-sided Mann-Whitney-Wilcoxon test with Bonferroni correction. Between feeding condition comparisons for tank-raised fish were performed using a Kruskal Wallis test, with posthoc analysis performed using Dunn’s test. Nonsignificant comparisons not marked. (‡, P ≤ 0.001; §, P ≤ 0.0001)
Rotifers can be cultured easily using a culture bucket, a heater to maintain temperature at 31°C, filter media, an air pump, and a peristaltic dosing machine to feed the rotifers suspended algae at a rate of 1 mL/hour (culture implements from Reed Mariculture, Campbell, CA). To feed, we harvest 4 L of our 15-L culture daily (27%) and condense these 4 L down to 250 mL by straining through a 41-µm sieve and resuspending in system water. As a cautionary note, if rotifers are being cultured alongside other food sources like brine shrimp, harvesting equipment for each culture should be kept separate and cleaned frequently, using bleach followed by sodium thiosulfate. Contamination of the rotifer culture with brine shrimp cysts can result in rogue brine shrimp hatching in the rotifer culture, and these individuals can consume enough rotifers to cause the culture to crash.
Larval fish are fed with 25 mL of the concentrated rotifer solution once daily. Well-established cultures have 200 to 350 rotifers per mL (before concentrating) and require little upkeep; our 4-L harvest gives us enough rotifers to feed up to 10 tanks of larval fish every day. Some rotifers survive in betta system water for at least 24 h, giving the larval fish access to food for the whole day. Ammonia levels in larval tanks receiving daily rotifers remain at 0 to 0.25 ppm from 5 to 14 dpf, so we do not perform any water changes during this period.
In developing our maintenance protocol, we experimented with feeding paramecium, which we found to be more difficult and time-consuming to culture than rotifers, and with dry powder food, which rapidly decreased water quality. We also tried feeding brine shrimp (Artemia nauplii) starting at 5 dpf, but found that because brine shrimp do not survive for more than an hour in system water, uneaten dead brine shrimp accumulated in the tanks. This accumulation led to high ammonia levels, which required frequent water changes and reduced larvae survival.
We achieve the best growth rates when early larval fish are moved to tanks at 5 dpf (that is, raised in culture dishes from 0 to 4 dpf, and then moved to tanks at 5 dpf; Figure 6), but they can also be maintained in culture dishes through 15 dpf. Keeping larval fish in culture dishes can be useful for some experiments. If we raise early larval stage fish in culture dishes, we keep them in the 28°C incubator during this time period, and feed rotifers daily at the same rate of 1 mL concentrated rotifer suspension per 40 mL water volume. Care must be taken to diligently remove dead fish and other debris using a transfer pipet to avoid poor water quality.
Late larval stage (15 to 30 dpf).
At 15 dpf, we add a 400-µm diameter mesh fry screen to each 2.8-L tank and move tanks to the recirculating system with a slow flow (100 mL per min) of water (Figure 2B). At this stage, larvae are fed newly hatched brine shrimp (Artemia nauplii) 2 to 4 times daily, consistent with feeding protocols in published betta literature.60 Brine shrimp are cultured daily in a 19-L acrylic hatching cone (Pentair, Cary, NC) at 28°C in 8 L of water with a salinity of 25 to 35 ppt, prepared with Instant Ocean aquarium salt. The culture must be well aerated with an air pump. We start a new culture each morning and harvest hatched brine shrimp 24 h later. The correct salinity and temperature are necessary for a successful brine shrimp hatch; with our methods, suboptimal hatching rates can almost always be traced to a malfunctioning water heater in the culture cone or to water salinity outside the specified range.
Brine shrimp cysts come in 2 varieties: intact (capsulated) and decapsulated. We highly recommend the use of intact cysts, as we have found that cultures started using decapsulated cysts often fail to hatch. We have never had a failed hatch using intact cysts. When using intact cysts to culture brine shrimp, care should be taken to remove the capsules before feeding the shrimp to the betta. Betta will eat the capsules, which leads to gut blockages and buoyancy issues. Capsules can be separated from hatched shrimp by stopping aeration and allowing the capsules to float to the surface, before harvesting the hatched shrimp from the lower portion of the culture cone using the spout at the bottom. We purchase shrimp with intact cysts that have been coated in a nontoxic layer of iron (Artemia International, Fairview, TX), allowing easy removal of the capsules with a strong magnet.
Each tank containing late larval stage fish receives an amount of brine shrimp that can be consumed between 5 and 15 min (that is, approximately 20 to 50 shrimp per fish, depending on age). If larvae are overfed, uneaten brine shrimp may form a layer on the bottom of the tank. On the recirculating system, we have not noticed any health issues or increases in ammonia caused by a buildup of uneaten food, but this layer can impede the view into the tank. We move fish to a clean tank if this occurs.
Juvenile stage (31 to 62 dpf).
A previous study showed that juvenile fish raised individually in small water volumes (150 mL) exhibit the most rapid growth.64 However, that study also found that ammonia levels were highest in the tanks with the smallest water volume. To keep ammonia levels low while housing more fish, we raise our juvenile fish in 35-L grow-out tanks. We move fish to these tanks around 30 dpf, or once fish reach approximately 1 cm in length. We keep fish at a density of 0.5 to 1 fish per L in these tanks, which is similar to the practices we observed in betta farms in Thailand. Stocking at a higher density leads to slower growth. Juveniles in our grow-out tanks are fed newly hatched brine shrimp 2 to 4 times daily, and tanks are fed an amount of shrimp that can be consumed within 5 to 15 min.
Our grow-out tanks contain rocks, terracotta pots, and silk and/or live plants (Figure 7). Live plants reduce ammonia and nitrite levels in the tanks, and plants and rocks provide places for the fish to hide. Each tank has a custom-cut lid to prevent excessive evaporation that can lead to increased salinity. These tanks are also hooked up to our recirculating system, with a water flow that replaces 100% of the water daily, eliminating the need for time-consuming daily manual water changes. To remove debris from the bottom of the grow-out tank, we siphon the bottom of each tank at least once per week. For tanks containing fish under 2 cm long, we add a piece of mesh to the end of our siphon to avoid accidentally siphoning up any tank inhabitants. Algae growth is normal in grow-out tanks. Excess algae can be removed by scraping the sides of the tank with a razor blade, taking care not to damage silicone sealant in the corners.
Figure 7.
Grow-out tanks for housing juvenile and adult betta raised in-house. Grow-out tanks are 35 L, connected to the recirculating system, contain numerous hiding places, and are stocked at 0.5–1 fish/L.
Adult stage (63+ dpf).
Under our growing conditions, ornamental betta can reach sexual maturity by 10 wk of age, which is 6 to 7 wk faster than is achieved with other methods.62 Fish in our grow-out tanks begin blowing bubble nests by 8 wk after fertilization, and around this time some fish can be sexed based on observation of a female ovipositor. Beginning at 8 wk, we individually house the largest fish and those that we can sex. To do this, we move individuals from their grow-out tank to a 0.8-L or a 1.4-L tank and add this tank to the main recirculating system. Adults placed on the system can breed 5 to 7 d after removal from the grow-out tanks. Adults on the recirculating system receive a mixed diet of newly hatched brine shrimp once daily and food pellets once daily (approximately 10 Golden Pearls 500- to 800-µm pellets [protein 54%, crude fat 9%, fiber 2%, moisture 9%] or 2 to 3 Ken’s 1-mm fish pellets [protein 47%, crude fat 17%, fiber 3%, moisture 8%; both from Ken’s Fish, Taunton, MA), consistent with published betta feeding protocols.60
Fish cohoused from birth can generally continue to be housed together indefinitely, regardless of their sex, but special care should be taken to monitor for particularly aggressive fish, which should be individually housed. We individually house adult fish on the recirculating system prior to use in breeding or behavioral experiments, or if they become too aggressive for the grow-out tanks. Once male fish have been individually housed, they cannot be reintroduced into a cohoused tank due to their high aggression.
Health and Disease
Unlike more widely used fish such as zebrafish and medaka, no laboratory strains or pathogen-free sources of betta are currently available. In addition, to study the wide variety of traits and genetics present in betta, we often import fish from the aquarium trade or hobbyist breeders into our colony, and betta in our colony may be exposed to diseases from these sources. To reduce the likelihood of pathogens spreading to our colony from outside sources, we quarantine all new arrivals for at least one week, with an additional week of quarantine for wild-caught fish. During the quarantine period, we house fish in static tanks with an added prophylactic dose of methylene blue (3 ppm, 5-d treatment course) and perform daily water changes by hand, replacing approximately 100% of the tank water with fresh water at system pH, salinity, and temperature. We visually inspect all individuals in quarantine daily for any signs of infection or illness. While our quarantine practice involves static tanks and hand water changes, the ideal quarantine program for betta has not yet been empirically determined, and others may choose to provide a separate recirculating system to house their quarantined fish. With that approach, sentinel fish could be used to alert staff to disease outbreaks.
While a quarantine procedure reduces the number of disease outbreaks,61 importing fish from multiple sources means that some pathogens will inevitably enter the system. Brine shrimp can also be a vector for the transmission of marine bacteria.18 Maintaining pristine water quality is crucial for preventing disease spread,1,21,68 as is recognizing and quickly isolating sick individuals. We perform a daily health check on all fish, carefully checking each tank for signs of disease. Poor health usually leads to recognizable changes in fish behavior. Betta that are ill often present with clamped fins (Figure 8A), lethargy, and disinterest in food. Any fish exhibiting signs of disease are removed from the recirculating system immediately to prevent spread to other tanks.
Figure 8.
Common signs of diseased betta. A) A fish with clamped fins, a nonspecific symptom that is present in many betta illnesses. B) Adult male fish with fin rot. Note the ‘wasted,’ jagged fins, and the pink necrosis present on the dorsal fin.
Common betta diseases are velvet, fin rot, Mycobacterium infection, and buoyancy disorders. Velvet is a disease caused by dinoflagellate parasites of the genus Oodinium.52 The easiest way to identify betta with velvet is by observing a coating that looks like gold dust on the fish’s body. Betta with velvet also usually have clamped fins, are lethargic, and may stop eating. If the condition is caught early, we have successfully treated velvet in our facility using a 5-d treatment course of Proform C (Pentair, Cary, NC) at a dose of 30 µL per L of tank volume, with daily 100% water changes. Our only experience with velvet occurred before the installation of our recirculating system. In the 3 y after installing our recirculating system, we have seen no cases of velvet in our colony.
Fin rot is a nonspecific term for lesions on fins caused by either bacteria or fungi.52 Betta with fin rot often have fins with jagged edges, and the fins may become more transparent and duller in color (Figure 8B). We have had some success treating mild cases of fin rot with a 4-d treatment course of the antibiotic Furan 2 (nitrofurazone) antibiotic51 at 46 mg/L, with daily 100% water changes. While rare in young individually housed fish on our recirculating system, we carefully monitor for this disease in grow-out tanks. If fish are nipped excessively by tank mates, fin wounds can become infected and fin rot can occur. Anecdotally, fish older than 1.5 y seem to be more susceptible.
Betta can also be infected with Mycobacteria spp. Clinical signs are nonspecific and can be difficult to distinguish from other illnesses. Wasting of fins, dull coloration, inactivity and clamped fins can all be signs of mycobacteriosis. Fish with clinical signs that cannot be linked to another disease may indicate a mycobacteriosis outbreak. In these instances, we euthanize 1 to 2 individual fish and send tissue samples to an animal diagnostic lab for evaluation for infection with Mycobacteria. Diagnosis is performed using Ziehl-Neelsen staining, culture, and/or PCR. All fish with potential mycobacteriosis should be isolated to prevent spread to other tanks. Few rigorous clinical studies have been performed regarding this condition,25 and we have had minimal success with attempted treatments.
Buoyancy disorders are another health issue we have experienced in our colony. Fish may either float toward the surface of the water, or their tails may sink to the bottom so that they swim vertically in the water. Sinking is more common in developing fish and is often linked to excessive feeding. As long as the fish can reach the surface to breathe, sinking issues in grow-out tanks resolve as fish grow. In our experience, floating fish show moderate loss of buoyancy control and a round, bloated appearance of the abdomen. Approximately half of these cases will resolve with a 24- or 48-h fast, and we characterize these cases as ‘bloat.’ Some fish seem to be particularly susceptible to chronic bloat and will be treated multiple times throughout their life. The other half of our cases of floating manifest similarly to bloat but often with milder loss of buoyancy and these fish do not recover after a 48-h fast. Different etiologies are likely to underlie a bloated appearance in betta fish. Those that recover may have ingested too much air during feeding, while those that do not recover may have organ damage associated with bacterial infection.18
Conclusion
While the use of Betta splendens in the laboratory is currently limited, their use presents an opportunity for researchers in a variety of scientific fields. The husbandry protocols we developed have allowed us to successfully introduce and maintain thousands of fish in our colony, and we have achieved consistent mating success, high levels of growth and survival throughout development, and widespread prevention of illness. We have accelerated the generation time of betta to 10 wk from a common standard of at least 16 wk, facilitating genetic tractability. By following the procedures outlined here, researchers interested in using betta in their laboratories will be able to maintain a thriving betta colony under standard conditions that allow them to generate and raise the large cohorts required for genetic and genomic analyses, and to perform studies of betta development, neurobiology, and behavior.
Acknowledgments
We thank Sonia Thomas, Christol Pollard, and the entire Zuckerman Institute animal husbandry and veterinary staff. We would also like to thank the many members of the betta breeding community including Salvador Alemany, Leo Buss, Gerald Griffin, Liz Hahn, Sieg Illig, Heidi Mae Burkle, Karen MacAuley, Aurelia Ogles, Holly Rutan, and Natthacha Frank Sriboribun for providing fish and sharing their fishkeeping expertise. We also thank Peter Lichtenthal for assistance in collecting early larval growth data. Animal experimentation protocols were approved by the Columbia University Institutional Animal Care and Use Committee (AC-AAAT1482). Funding: Searle Scholarship, Sloan Foundation Fellowship, and National Institutes of Health grants R34NS116734 and R35GM143051 to AB.
References
- 1.Ackerman PA, Wicks BJ, Iwama GK, Randall DJ. 2006. Low levels of environmental ammonia increase susceptibility to disease in chinook salmon smolts. Physiol Biochem Zool 79:695–707. 10.1086/504615. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad SF, Laopichienpong N, Singchat W, Suntronpong A, Pongsanarm T, Panthum T, Ariyaraphong N, Bulan J, Pansrikaew T, Jangtarwan K, Subpayakom N, Dokkaew S, Muangmai N, Duengkae P, Srikulnath K. 2020. Next-generation sequencing yields complete mitochondrial genome assembly of peaceful betta fish, Betta imbellis (Teleostei: Osphronemidae). Mitochondrial DNA B Resour 5:3856–3858. 10.1080/23802359.2020.1841582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JM, Nicoletto PF. 1997. Response of Betta splendens to computer animations of males with fins of different length. Copeia 1997:195–199. 10.2307/1447858. [DOI] [Google Scholar]
- 4.Amiri MH, Shaheen HM. 2012. Chromatophores and color revelation in the blue variant of the Siamese fighting fish (Betta splendens). Micron 43:159–169. 10.1016/j.micron.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Bekoff M. 2007. Encyclopedia of human-animal relationships: a global exploration of our connections with animals. Westport (CT): Greenwood Press. [Google Scholar]
- 6.Biokani S, Jamili S, Amini S, Sarkhosh J. 2014. The study of different foods on spawning efficiency of Siamese fighting fish (Species: Betta splendens, Family: Belontiidae). Mar Sci 4:33–37. [Google Scholar]
- 7.Boeuf G, Payan P. 2001. How should salinity influence fish growth? Comp Biochem Physiol C Toxicol Pharmacol 130:411–423. 10.1016/S1532-0456(01)00268-X. [DOI] [PubMed] [Google Scholar]
- 8.Braddock JC, Braddock ZI. 1955. Aggressive behavior among females of the Siamese fighting fish, Betta splendens. Physiol Biochem Zool 28:152–172. 10.1086/physzool.28.2.30163682. [DOI] [Google Scholar]
- 9.Braddock JC, Braddock ZI. 1959. The development of nesting behaviour in the Siamese fighting fish Betta splendens. Anim Behav 7:222–232. 10.1016/0003-3472(59)90012-0. [DOI] [Google Scholar]
- 10.Bronstein PM. 1981. Social reinforcement in Betta splendens: A reconsideration. J Comp Physiol Psychol 95:943–950. 10.1037/h0077841. [DOI] [Google Scholar]
- 11.Bronstein PM. 1981. Commitments to aggression and nest sites in male Betta splendens. J Comp Physiol Psychol 95:436–449. 10.1037/h0077780. [DOI] [PubMed] [Google Scholar]
- 12.Bronstein PM. 1982. Breeding, paternal behavior, and their interruption in Betta splendens. Anim Learn Behav 10:145–151. 10.3758/BF03212262. [DOI] [Google Scholar]
- 13.de Bruin JPC. 1980. Telencephalon and Behavior in Teleost Fish, p 175–201. In: Ebbesson SOE, editor. Comparative Neurology of the Telencephalon. Boston, MA: Springer US. [Google Scholar]
- 14.Cain NW, Anderson R, Stein L, Jessen C. 1980. Effects of prior social experience on agonistic responding by Siamese fighting fish (Betta splendens). Anim Learn Behav 8:491–496. 10.3758/BF03199639. [DOI] [Google Scholar]
- 15.Cain NW, Jessen C, Flanagan M. 1980. Social responsiveness and physical space as determinants of agonistic behavior in Betta splendens. Anim Learn Behav 8:497–501. 10.3758/BF03199640. [DOI] [Google Scholar]
- 16.Chambel J, Costa R, Gomes M, Mendes S, Baptista T, Pedrosa R. 2014. Hydrogen peroxide, iodine solution and methylene solution highly enhance the hatching rate of freshwater ornamental fish species. Aquacult Int 22:1743–1751. 10.1007/s10499-014-9779-1. [DOI] [Google Scholar]
- 17.Clotfelter ED, Curren LJ, Murphy CE. 2006. Mate choice and spawning success in the fighting fish Betta splendens: The importance of body size, display behavior and nest size. Ethology 112:1170–1178. 10.1111/j.1439-0310.2006.01281.x. [DOI] [Google Scholar]
- 18.Dong HT, Senapin S, Phiwsaiya K, Techatanakitarnan C, Dokladda K, Ruenwongsa P, Panijpan B. 2018. Histopathology and culturable bacteria associated with “big belly” and “skin nodule” syndromes in ornamental Siamese fighting fish, Betta splendens. Microb Pathog 122:46–52. 10.1016/j.micpath.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Duarte SC, Vasconcellos BdFe, Vidal Júnior MV, Ferreira AV, Mattos DdC, Branco AT. 2012. Ontogeny and embryonic description of Betta splendens, Perciformes (Regan, 1910). Rev Bras Saúde Prod Anim 13:880–893. 10.1590/S1519-99402012000300025. [DOI] [Google Scholar]
- 20.Evans CS. 1985. Display vigour and subsequent fight performance in the Siamese fighting fish, Betta splendens. Behav Processes 11:113–121. 10.1016/0376-6357(85)90053-1. [DOI] [PubMed] [Google Scholar]
- 21.Evans JJ, Pasnik DJ, Brill GC, Klesius PH. 2006. Un-ionized ammonia exposure in Nile tilapia: Toxicity, stress response, and susceptibility to Streptococcus agalactiae. N Am J Aquac 68:23–33. 10.1577/A05-032.1. [DOI] [Google Scholar]
- 22.Fan G, Chan J, Ma K, Yang B, Zhang H, Yang X, Shi C, Law H, Ren Z, Xu Q, Liu Q, Wang J, Chen W, Shao L, Gonçalves D, Ramos A, Cardoso SD, Guo M, Cai J, Xu X, Wang J, Yang H, Liu X, Wang Y. 2018. Chromosome-level reference genome of the Siamese fighting fish Betta splendens, a model species for the study of aggression. Gigascience 7:giy087. 10.1093/gigascience/giy087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox C, Merali Z, Harrison C. 2006. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav Brain Res 175:1–8. 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 24.França I de F, Heluy GM, Schultz ÉB, Vianna W de O, Pereira MM, Ramos LRV. 2021. Sex reversal in Siamese fighting fish larvae by thermal management. Bol Inst Pesca 47:e676. 10.20950/1678-2305/bip.2021.47.e676. [DOI] [Google Scholar]
- 25.Gauthier DT, Rhodes MW. 2009. Mycobacteriosis in fishes: A review. Vet J 180:33–47. 10.1016/j.tvjl.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Ghittino P. 1972. The principal aspects of bacterial fish diseases in Italy. Sym Zool S 30:25–38. [Google Scholar]
- 27.Goodrich HB, Mercer RN. 1934. Genetics and colors of the Siamese fighting fish, Betta splendens. Science 79:318–319. 10.1126/science.79.2049.318. [DOI] [PubMed] [Google Scholar]
- 28.Grabowski J, Thompson T. 1967. Bubble-nest building and visual reinforcement in Siamese fighting fish (Betta splendens). University of Minnesota. [Google Scholar]
- 29.Graham JB. 1997. Air-breathing fishes: Evolution, diversity, and adaptation. San Diego (CA): Academic Press. [Google Scholar]
- 30.Groth WO. 1970. Embryology of the Siamese fighting fish Betta splendens. Duke University. [Google Scholar]
- 31.Haller J. 1992. Group size modifies the patterns and muscle carbohydrate effects of aggression in Betta splendens. Physiol Behav 52:287–290. 10.1016/0031-9384(92)90273-5. [DOI] [PubMed] [Google Scholar]
- 32.Halperin JRP, Dunham DW, Ye S. 1992. Social isolation increases social display after priming in Betta splendens but decreases aggressive readiness. Behav Processes 28:13–31. 10.1016/0376-6357(92)90045-F. [DOI] [PubMed] [Google Scholar]
- 33.Harlioʇlu M, Mi≡e Yonar S. 2008. The importance of temperature, individual size and habitat arrangement on the bubble nest construction of Siamese fighting fish (Betta splendens Regan, 1910). Int J SciTechnol 3:53–58. [Google Scholar]
- 34.Holder E, Conmy R, Venosa A. 2015. Comparative laboratory-scale testing of dispersant effectiveness of 23 crude oils using four different testing protocols. J Environ Prot (Irvine Calif) 06:628–639. 10.4236/jep.2015.66057. [DOI] [Google Scholar]
- 35.Jaroensutasinee M, Jaroensutansinee K. 2001. Bubble nest habitat characteristics of wild Siamese fighting fish. J Fish Biol 58:1311–1319. 10.1111/j.1095-8649.2001.tb02288.x. [DOI] [Google Scholar]
- 36.Kang C-K, Lee T-H. 2010. The pharyngeal organ in the buccal cavity of the male Siamese fighting fish, Betta splendens, supplies mucus for building bubble nests. Zoolog Sci 27:861–866. 10.2108/zsj.27.861. [DOI] [PubMed] [Google Scholar]
- 37.Khoo G, Lim TM, Phang VPE. 2012. Ultrastructure of erythrophores and xanthophores of the Siamese fighting fish, Betta splendens. Isr J Aquacult-Bamid 64:64. 10.46989/001c.20620. [DOI] [Google Scholar]
- 38.Khoo G, Lim TM, Phang VPE. 2014. Cellular basis of metallic iridescence in the Siamese fighting fish, Betta splendens. Isr J Aquacult-Bamid 65:66. 10.46989/001c.20672. [DOI] [Google Scholar]
- 39.Khoo L. 2000. Fungal diseases in fish. Semin Avian Exot Pet Med 9:102–111. 10.1053/AX.2000.4623. [DOI] [Google Scholar]
- 40.Kowasupat C, Panijpan B, Ruenwongsa P, Sriwattanarothai N. 2012. Betta mahachaiensis, a new species of bubble-nesting fighting fish (Teleostei: Osphronemidae) from Samut Sakhon Province, Thailand. Zootaxa 3522:49–60. 10.11646/zootaxa.3522.1.3. [DOI] [Google Scholar]
- 41.Kwon YM, Vranken N, Hoge C, Lichak MR, Norovich AL, Francis KX, Camacho-Garcia J, Bista I, Wood J, McCarthy S, Chow W, Tan HH, Howe K, Bandara S, von Lintig J, Rüber L, Durbin R, Svardal H, Bendesky A. 2022. Genomic consequences of domestication of the Siamese fighting fish. Sci Adv 8:eabm4950. 10.1126/sciadv.abm4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas GA. 1968. A study of variation in the Siamese fighting fish, Betta splendens, with emphasis on color mutants and the problem of sex determination. Iowa State University. [Google Scholar]
- 43.Lusiastuti M, Taukhid T, Anggi I, Caruso D. 2017. Dry green leaves of Indian almond (Terminalis catappa) to prevent streptococcal infection in juveniles of the Nile tilapia. B Eur Assoc Fish Path 37:119–125. [Google Scholar]
- 44.Mandal SC, Sahu NP, Singh Kohli MP, Das P, Gupta SK, Munilkumar S. 2010. Replacement of live feed by formulated feed: Effect on the growth and spawning performance of Siamese fighting fish (Betta splendens, Regan, 1910). Aquac Res 41:1707–1716. 10.1111/j.1365-2109.2010.02564.x. [DOI] [Google Scholar]
- 45.Marino-Neto J, Sabbatini RM. 1988. A stereotaxic atlas for the telencephalon of the Siamese fighting fish (Betta splendens). Braz J Med Biol Res 21:971–986. [PubMed] [Google Scholar]
- 46.Marrone RL, Pray SL, Bridges CC. 1966. Norepinephrine elicitation of aggressive display responses in Betta splendens. Psychon Sci 5:207–208. 10.3758/BF03328355. [DOI] [Google Scholar]
- 47.Meliska CJ, Meliska JA, Peeke HVS. 1980. Threat displays and combat aggression in Betta splendens following visual exposure to conspecifics and one-way mirrors. Behav Neural Biol 28:473–486. 10.1016/S0163-1047(80)91842-7. [DOI] [PubMed] [Google Scholar]
- 48.Mifsud C, Rowland SJ. 2008. Use of salt to control ichthyophthiriosis and prevent saprolegniosis in silver perch, Bidyanus bidyanus. Aquac Res 39:1175–1180. 10.1111/j.1365-2109.2008.01981.x. [DOI] [Google Scholar]
- 49.Miron DS, Silva LVF, Golombieski JI, Baldisserotto B. 2003. Efficacy of different salt (NaCl) concentrations in the treatment of Ichthyophthirius multifiliis-infected silver catfish, Rhamdia quelen, fingerlings. J Appl Aquac 14:155–161. 10.1300/J028v14n01_12. [DOI] [Google Scholar]
- 50.Le Morvan C, Troutaud D, Deschaux P. 1998. Differential effects of temperature on specific and nonspecific immune defences in fish. J Exp Biol 201:165–168. 10.1242/jeb.201.2.165. [DOI] [PubMed] [Google Scholar]
- 51.Nagel ML, Summerfelt RC. 1977. Nitrofurazone for control of the microsporidan parasite Pleistophora Ovariae in golden shiners. Prog Fish Cult 39:18–23. 10.1577/1548-8659(1977)39[18:NFCOTM]2.0.CO;2. [DOI] [Google Scholar]
- 52.Noga E. 2010. Fish disease: diagnosis and treatment. 2nd Edition. Ames (IA): Wiley-Blackwell. 10.1002/9781118786758 [DOI] [Google Scholar]
- 53.Ogata Y, Kurokura H. 2012. Use of the freshwater rotifer Brachionus angularis as the first food for larvae of the Siamese fighting fish Betta splendens. Fish Sci 78:109–112. 10.1007/s12562-011-0420-1. [DOI] [Google Scholar]
- 54.Panijpan B, Sriwattanarothai N, Laosinchai P. 2020. Wild betta fighting fish species in Thailand and other Southeast Asian countries. Sci Asia 46:382–391. 10.2306/scienceasia1513-1874.2020.064. [DOI] [Google Scholar]
- 55.Peters HM. 1978. On the mechanism of air ventilation in Anabantoids (Pisces: Teleostei). Zoomorphologie 89:93–123. 10.1007/BF00995663. [DOI] [Google Scholar]
- 56.Prakhongcheep O, Muangmai N, Peyachoknagul S, Srikulnath K. 2018. Complete mitochondrial genome of mouthbrooding fighting fish (Betta pi) compared with bubble nesting fighting fish (B. splendens). Mitochondrial DNA B Resour 3:6–8. 10.1080/23802359.2017.1413294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prost S, Petersen M, Grethlein M, Hahn SJ, Kuschik-Maczollek N, Olesiuk ME, Reschke J-O, Schmey TE, Zimmer C, Gupta DK, Schell T, Coimbra R, De Raad J, Lammers F, Winter S, Janke A. 2020. Improving the chromosome-level genome assembly of the Siamese fighting fish (Betta splendens) in a university master’s course. G3 (Bethesda) 10:2179–2183. 10.1534/g3.120.401205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puello-Cruz AC, Velasco-Blanco G, Martínez-Rodríguez IE, Felix-Ramos E, Voltolina D. 2010. Growth and survival of Siamese fighting fish, Betta splendens, larvae at low salinity and with different diets. J World Aquac Soc 41:823–828. 10.1111/j.1749-7345.2010.00425.x. [DOI] [Google Scholar]
- 59.Rainwater FL. 1967. Courtship and reproductive behavior of the Siamese fighting fish Betta splendens Regan (Pisces, Belontiidae). Oklahoma State University. [Google Scholar]
- 60.Ramos A, Alex D, Cardoso SD, Gonçalves D. 2021. Androgens and corticosteroids increase in response to mirror images and interacting conspecifics in males of the Siamese fighting fish Betta splendens. Horm Behav 132:104991. 10.1016/j.yhbeh.2021.104991. [DOI] [PubMed] [Google Scholar]
- 61.Reed B, Jennings M. 2011. Guidance on the housing and care of zebrafish, Danio rerio. Southwater, UK: Royal Society for the Prevention of Cruelty to Animals. [Google Scholar]
- 62.Reyes-Bustamante H, Ortega-Salas AA. 2002. Initial sexual maturity and fecundity of two Anabantids under laboratory conditions. N Am J Aquac 64:224–227. . [DOI] [Google Scholar]
- 63.Rnic A. 1975. Effects of pH on the rate of aggressive display for mirror image reinforcement in Siamese fighting fish (Betta splendens). Aggress Behav 1:213–215. . [DOI] [Google Scholar]
- 64.Saekhow S, Thongprajukaew K, Phromkunthong W, Sae-khoo H. 2018. Minimal water volume for intensively producing male Siamese fighting fish (Betta splendens Regan, 1910). Fish Physiol Biochem 44:1075–1085. 10.1007/s10695-018-0495-z. [DOI] [PubMed] [Google Scholar]
- 65.Sangvara B, Ariyakulkaln P, Ukong S. 1993. Effect of temperature on the ovarian development in siamese fighting fish, Betta splendens. Silpakorn University. [Google Scholar]
- 66.Simpson MJA. 1968. The display of the Siamese fighting fish, Betta splendens, p 1–73. In: Cullen JM, Beer CG, editors. Animal Behaviour Monographs. Vol. 1. London: Balliere, Tindal and Cassell. [Google Scholar]
- 67.Singchat W, Ahmad SF, Laopichienpong N, Suntronpong A, Pongsanarm T, Panthum T, Ariyaraphong N, Subpayakom N, Dokkaew S, Muangmai N, Duengkae P, Srikulnath K. 2020. Complete mitochondrial genome of Mahachai betta, Betta mahachaiensis (Teleostei: Osphronemidae). Mitochondrial DNA B Resour 5:3059–3061. 10.1080/23802359.2020.1797578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smart G. 1976. The effect of ammonia exposure on gill structure of the rainbow trout (Salmo gairdneri). J Fish Biol 8:471–475. 10.1111/j.1095-8649.1976.tb03990.x. [DOI] [Google Scholar]
- 69.Smith HM. 1945. The fresh-water fishes of Siam, or Thailand. Bulletin of the United States National Museum. Washington D.C. 10.5479/si.03629236.188.1 [DOI] [Google Scholar]
- 70.Sullivan M, Lawrence C, Blache D. 2016. Why did the fish cross the tank? Objectively measuring the value of enrichment for captive fish. Appl Anim Behav Sci 174:181–188. 10.1016/j.applanim.2015.10.011. [DOI] [Google Scholar]
- 71.Svärdson G, Wickbom T. 1942. The chromosomes of two species of Anabantidae (Teleostei), with a new case of sex reversal. Hereditas 28:212–216. 10.1111/j.1601-5223.1942.tb03276.x. [DOI] [Google Scholar]
- 72.Thompson TI. 1963. Visual reinforcement in Siamese fighting fish. Science 141:55–57. 10.1126/science.141.3575.55. [DOI] [PubMed] [Google Scholar]
- 73.Thor DH, Weisman MH, Boshka SC. 1967. Chemical suppression of fighting in the Siamese fighting fish. Psychon Sci 9:161–162. 10.3758/BF03330809. [DOI] [Google Scholar]
- 74.Tieman DM, Goodwin AE. 2001. Treatments for Ich infestations in Channel Catfish evaluated under static and flow-through water conditions. N Am J Aquac 63:293–299. . [DOI] [Google Scholar]
- 75.Valentin FN, do Nascimento NF, da Silva RC, Fernandes JBK, Giannecchini LG, Nakaghi LSO. 2015. Early development of Betta splendens under stereomicroscopy and scanning electron microscopy. Zygote 23:247–256. 10.1017/S0967199413000488. [DOI] [PubMed] [Google Scholar]
- 76.Verbeek P, Iwamoto T, Murakami N. 2007. Differences in aggression between wild-type and domesticated fighting fish are context dependent. Anim Behav 73:75–83. 10.1016/j.anbehav.2006.03.012. [DOI] [Google Scholar]
- 77.Vu T-D, Iwasaki Y, Shigenobu S, Maruko A, Oshima K, Iioka E, Huang C-L, Abe T, Tamaki S, Lin Y-W, Chen CK, Lu MY, Hojo M, Wang HV, Tzeng SF, Huang HJ, Kanai A, Gojobori T, Chiang TY, Sun HS, Li WH, Okada N. 2020. Behavioral and brain-transcriptomic synchronization between the two opponents of a fighting pair of the fish Betta splendens. PLoS Genet 16:e1008831. 10.1371/journal.pgen.1008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wallbrunn HM. 1958. Genetics of the Siamese Fighting Fish, Betta splendens. Genetics 43:289–298. 10.1093/genetics/43.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang L, Sun F, Wan ZY, Ye B, Wen Y, Liu H, Yang Z, Pang H, Meng Z, Fan B, Alfiko Y, Shen Y, Bai B, Lee MSQ, Piferrer F, Schartl M, Meyer A, Yue GH. 2021. Genomic basis of striking fin shapes and colors in the fighting fish. Mol Biol Evol 38:3383–3396. 10.1093/molbev/msab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Sun F, Wan ZY, Yang Z, Tay YX, Lee M, Ye B, Wen Y, Meng Z, Fan B, Alfiko Y, Shen Y, Piferrer F, Meyer A, Schartl M, Yue GH. 2022. Transposon-induced epigenetic silencing in the X chromosome as a novel form of dmrt1 expression regulation during sex determination in the fighting fish. BMC Biol 20:5. 10.1186/s12915-021-01205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuanon JAS, Salaro AL, Veras GC, Tavares MM, Chaves W. 2009. Acute and chronic salinity tolerance in adult siamese fighting fish, Betta splendens. Rev Bras Zootecn 38:2106–2110. 10.1590/S1516-35982009001100005. [Article in Portuguese]. [DOI] [Google Scholar]