Abstract
Background
Influence of iguratimod on bone mineral density (BMD) and biomarkers of bone metabolism in patients with rheumatoid arthritis (RA) remains not determined. Accordingly, a meta-analysis was performed for systematical evaluation.
Methods
Relevant randomized controlled trials (RCTs) were retrieved by searching of PubMed, Embase, Cochrane's Library, China National Knowledge Infrastructure (CNKI), and Wanfang databases. A random-effect model was used to pool the results.
Results
In total, 24 RCTs including 2439 patients with RA contributed to the meta-analysis. Pooled results showed that compared to methotrexate alone, additional use of iguratimod 25 mg Bid for 12∼24 weeks significantly improved lumbar-spine BMD (mean difference [MD]: 0.12, 95% confidence interval [CI]: 0.04 to 0.20, p=0.002, I2 = 39%) in patients with RA. Moreover, treatment with iguratimod was associated with increased serum osteoprotegerin (MD: 180.36 pg/ml, 95% CI: 122.52 to 238.20, p < 0.001, I2 = 48%), and decreased serum receptor activator for nuclear factor kappa-B ligand (MD: −10.65 pmol/l, 95% CI: −15.59 to −5.72, p < 0.001, I2 = 53%). In addition, iguratimod was associated with increased bone formation markers such as the serum N-terminal middle molecular fragment of osteocalcin (MD: 4.23 ng/ml, 95% CI: 3.74 to 4.71, p < 0.001, I2 = 35%) and total procollagen type I amino-terminal propeptide (MD: 9.10 ng/ml, 95% CI: 7.39 to 10.80, p < 0.001, I2 = 86%), but decreased the bone resorption marker such as serum β-C terminal cross-linking telopeptide of type 1 collagen (MD: −0.18 pg/ml, 95% CI: −0.21 to −0.14, p < 0.001, I2 = 70%).
Conclusions
Iguratimod could prevent the bone loss and improve the bone metabolism in patients with RA.
1. Introduction
Rheumatoid arthritis (RA) is a common chronic inflammatory disease characterized by polyarthritis [1, 2]. The primary symptom of RA at early stage is synovitis-related joint swelling [3]. With the progression of the disease, damage of articular bone and cartilage occurs, which further impairs the functional ability of the patients [4]. Despite of local bone erosion, which is considered as a central feature or RA and a key determinant of disease severity and poor functional outcome [5], patients with RA are also vulnerable to systemic bone loss, osteoporosis, and fractures [6, 7]. Indeed, a previous meta-analysis showed a pooled incidence of overall and fragility fractures were 33.0 and 15.3 per 1000 person-years, respectively [8]. Therefore, impairment of bone metabolism has been recognized as one of the important pathophysiological features of RA, which may adversely affect the functional ability and survival of these patients [9].
The mechanisms underlying the pathogenesis of bone loss in patients with RA are complicated [4]. Some studies have shown that proinflammatory responses presented as increased receptor activator for nuclear factor kappa-B ligand (RANKL) and decreased osteoprotegerin (OPG) in peripheral blood could contribute to the bone loss and osteoporosis patients with RA [10–12]. Moreover, patients with RA were also shown to have a reduced level of markers for bone formation, such as the N-terminal middle molecular fragment of osteocalcin (N-MID) and the total procollagen type I amino-terminal propeptide (T-P1NP), but an increased level of markers for bone absorption, such as the β-C terminal cross-linking telopeptide of type 1 collagen (β-CTX) [13], which collectively reflect the bone loss in RA. Iguratimod (IGU) is a novel small-molecule antirheumatic drug for patients with active RA [14]. Compared with conventional treatment, IGU combined with methotrexate (MTX) has been approved to have better efficacy and safety for RA patients [15]. Moreover, monotherapy with IGU has also been proposed as a potential alternative to MTX for the treatment of RA [16]. However, influence of IGU on bone metabolism in patients with RA remains largely unknown. Therefore, we performed a meta-analysis to systematically evaluate the influence of IGU on bone mineral density (BMD) and biomarkers of bone metabolism in patients with RA.
2. Materials and Methods
This systematic review and meta-analysis was performed in accordance to the PRISMA [17,18] (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement and the Cochrane Handbook [19] guidelines.
2.1. Search Strategy
PubMed, Embase, and the Cochrane Library (Cochrane Center Register of Controlled Trials), China National Knowledge Infrastructure (CNKI), and Wanfang databases were systematically searched for relevant RCTs, using the combination of the following three groups of terms: (1) “Iguratimod” OR “alamode” OR “T-614”; (2) “bone” OR “osteoporosis” OR “rarefaction” OR “bone rarefaction” OR “bone mineral density” OR “BMD” OR “bone mass” OR “bone mineral content” OR “bone turnover” OR “bone resorption” OR “bone formation”; and (3) “random” OR “randomly”OR “randomized” OR “randomised.” The search was limited to studies in humans. We also analyzed reference lists of the original and review articles using a manual approach. Studies published from the inception of the databases until March 5, 2022, were retrieved.
2.2. Study Selection
Studies were included if they met the following criteria: (1) full-length articles published in peer-reviewed journals; (2) reported as RCTs with parallel design; (3) included adult patients with confirmed diagnosis of RA who were treated with background medications such MTX; (4) patients were randomly assigned to a treatment group of IGU, and a control group with placebo or no treatment; and (5) reported at least one of the following outcomes during follow-up, such as changes of BMD, OPG, RANKL, N-MID, T-P1NP, and β-CTX. Any parallel-group RCTs fulfilling the previously mentioned inclusion criteria were eligible for the meta-analysis, not limited to double-blind or placebo-controlled trials only. Reviews, observational studies, crossover studies, studies including patients without the diagnosis of RA, or studies did not report the outcomes of interest were excluded from the meta-analysis.
2.3. Data Extraction and Quality Assessment
Two authors independently performed the literature search, data extraction, and quality assessment according to inclusion criteria. If discrepancies occurred, they were resolved by discussion with the corresponding author. The following data was collected, such as the design characteristics, baseline characteristics of the included patients (age, gender, duration of RA, and background treatments), regimens of IGU and controls, follow-up duration, and outcomes reported. We used the seven-domain Cochrane Risk of Bias Tool [19] to evaluate the quality of the included studies, which include criteria concerning sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other potential threats to validity.
2.4. Statistical Analysis
Continuous variables were analyzed using mean difference (MD) and 95% confidence interval (CI). Changes of BMD, serum levels of OPG, RANKL, N-MID, T-P1NP, and β-CTX between baseline and endpoint in response to treatment of IGU as compared to controls were pooled separately in the meta-analyses. Cochrane's Q test was applied to evaluate the heterogeneity among the included studies. The I2 statistic was also determined, which indicates the percentage of total variation across studies that are due to the heterogeneity rather than chance [19, 20]. An I2 > 50% indicates significant heterogeneity among the trials. A random-effect model was used to pool the results since this model was considered to incorporate the potential between-study heterogeneity and could therefore minimize the influence of possible heterogeneity on the result [19]. Predefined subgroup analyses [19] were used to evaluate whether the difference of follow-up durations may affect the results. The median of the follow-up durations across the included studies was used as cutoff for defining subgroups. Potential publication bias was assessed with Egger's regression asymmetry test, or visual inspection of funnel plots if limited RCTs are included [21]. p values were two-tailed and statistical significance was set at 0.05. We used RevMan (Version 5.1; Cochrane, Oxford, UK) and Stata 12.0 software for the meta-analysis and statistical study.
3. Results
3.1. Search Results
A total of 572 articles were identified through database search, and 474 were retrieved after excluding the duplications. Subsequently, 432 were further excluded by screening of the titles and abstracts mainly because these studies were not relevant to the aim of the meta-analysis. Of the 42 potentially relevant articles for full-text review, eighteen studies were further excluded based on the reasons listed in Figure 1. Finally, the remaining 24 studies [22–45] met the inclusion criteria of the meta-analysis and were finally included for subsequent analyses.
Figure 1.
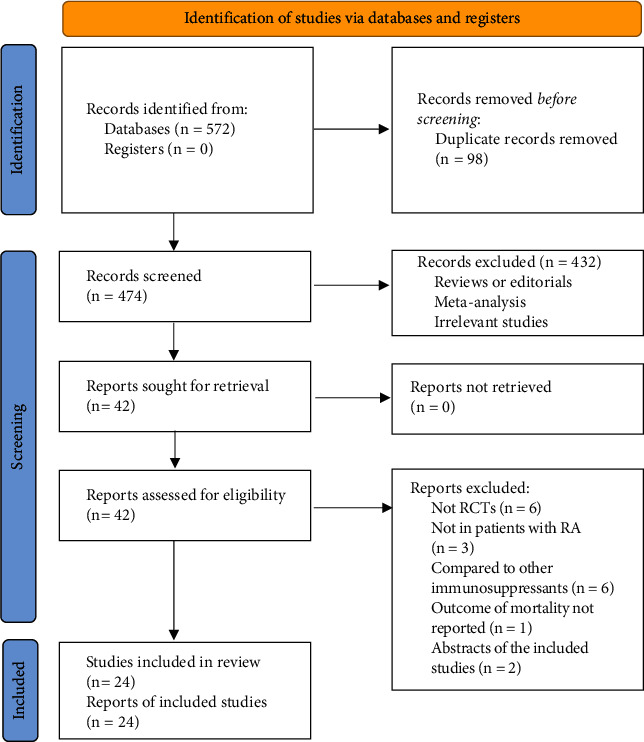
Flowchart of database search and literature identification.
3.2. Study Characteristics
Overall, 24 studies [22–45] including 2439 patients with RA contributed to the meta-analysis. The characteristics of the included studies are shown in Table 1. All of the included studies were open-label and parallel-group RCTs performed in China. All of the RCTs included patients with confirmed diagnosis of RA who were primarily treated with background therapy of MTX, hydroxychloroquine, or etanercept. Corticosteroids were not used in the patients of the included RCTs. For the three studies including patients with RA and osteoporosis, patients were additionally treated with oral calcium and vitamin D3 supplementation in two studies [31, 39], while no additional treatment for osteoporosis was applied for another study [38]. The sample sizes of the studies varied between 60 and 138. The mean ages of the patients varied between 45 and 73 years. The dosages of IGU were maintained as 25 mg bid in the intervention group, while no additional treatment was administered in the control group. The follow-up durations varied from 12 to 53 weeks.
Table 1.
Characteristics of the included RCTs, BMD, OPG, RANKL, N-MID, T-P1NP, and β-CTX.
| Author, year | Country | Study design | Diagnosis | Number of patients | Male | Age | Duration of RA | Background treatment | Intervention | Control | Follow-up duration | Outcomes reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | years | years | Weeks | |||||||||
| Tian, 2017 | China | R, OL | RA | 116 | 45.7 | 51.1 | 9.1 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
| Yan, 2018 | China | R, OL | RA | 70 | 32.9 | 56 | 11.4 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
| Du, 2018 | China | R, OL | RA | 98 | 47.9 | 48.1 | 4 | MTX | IGU 25 mg bid | No treatment | 16 | 456 |
| Cao, 2018 | China | R, OL | RA | 60 | 65 | 68 | NR | MTX | IGU 25 mg bid | No treatment | 24 | 23 |
| Chen, 2018 | China | R, OL | RA | 120 | 35 | 45.8 | 7.1 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
| Wang, 2019 | China | R, OL | RA | 100 | 41 | 61.4 | 9.3 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
| Gao, 2019 | China | R, OL | RA | 108 | 42.6 | 47.9 | 3.1 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
| Ding, 2019 | China | R, OL | RA | 76 | 42.1 | 72.7 | 8.7 | MTX | IGU 25 mg bid | No treatment | 16 | 456 |
| Zeng, 2019 | China | R, OL | RA | 80 | 61.3 | 46.6 | 7.6 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
| Xu, 2019 | China | R, OL | RA and osteoporosis | 115 | NR | NR | NR | MTX | IGU 25 mg bid | No treatment | 12 | 1 |
| Huan, 2019 | China | R, OL | RA | 128 | 32.8 | 45.6 | 2.3 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
| Yang, 2020 | China | R, OL | RA and osteoporosis | 81 | 21.1 | 45.9 | 5.5 | MTX and HCQ | IGU 25 mg bid | No treatment | 24 | 12356 |
| Chen, 2020 | China | R, OL | RA | 60 | 55 | 70.3 | NR | MTX | IGU 25 mg Bid | No treatment | 12 | 23 |
| Liang, 2020 | China | R, OL | RA | 114 | 43.9 | 53.9 | 7.3 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
| Li, 2020a | China | R, OL | RA | 120 | 55 | 46.2 | 4.1 | MTX | IGU 25 mg bid | No treatment | 53 | 12356 |
| Lun, 2020 | China | R, OL | RA and osteopenia | 60 | 11.7 | 57.5 | 5.7 | MTX | IGU 25 mg bid | No treatment | 12 | 2356 |
| Li, 2020b | China | R, OL | RA | 138 | 41.3 | 65.5 | 5 | MTX | IGU 25 mg bid | No treatment | 12 | 456 |
| Li, 2021a | China | R, OL | RA | 115 | 53.9 | 46.3 | 5.5 | MTX | IGU 25 mg bid | No treatment | 24 | 12356 |
| Fang, 2021 | China | R, OL | RA and osteoporosis | 100 | NR | 61.8 | NR | MTX | IGU 25 mg bid | No treatment | 12 | 1 |
| Li, 2021b | China | R, OL | RA | 120 | 65 | 52.9 | 5.3 | MTX | IGU 25 mg bid | No treatment | 24 | 45 |
| Jiang, 2021 | China | R, OL | RA | 120 | 51.7 | 45.5 | 5.8 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
| Hu, 2021 | China | R, OL | RA | 134 | 71.6 | 54.9 | 8.6 | MTX or etanercept | IGU 25 mg bid | No treatment | 12 | 16 |
| Zhang, 2021 | China | R, OL | RA and osteopenia | 120 | 40 | 45.7 | 0.5 | MTX | IGU 25 mg bid | No treatment | 24 | 56 |
| Sun, 2022 | China | R, OL | RA | 86 | 58.1 | 49 | 5.9 | MTX | IGU 25 mg bid | No treatment | 24 | 456 |
BMD: bone mineral density; OPG: osteoprotegerin; RANKL: receptor activator for nuclear factor kappa-B ligand; N-MID: the N-terminal middle molecular fragment of osteocalcin; T-P1NP: total procollagen type I amino-terminal propeptide; β-CTX: β-C terminal cross-linking telopeptide of type 1 collagen; RCT: randomized controlled trials; RA: rheumatoid arthritis; R: randomized; OL: open label; NR: not reported; MTX: methotrexate; HCQ: hydroxychloroquine; IGU: iguratimod; bid: twice daily.
3.3. Data Quality
The details of risks of biases of the included studies according to the Cochrane assessment tool are listed in Table 2. The details of random sequence generation were reported in five studies [22–25,32–34,37,38,40–42], and the details allocation concealment were not reported in any of the included studies. The details of withdrawals and dropouts were reported in all studies.
Table 2.
Quality evaluation via the Cochrane's Risk of Bias Tool.
| Random sequence generation | Allocation concealment | Blinding in performance | Blinding in outcome detection | Incomplete outcome data | Reporting bias | Other bias | Total | |
|---|---|---|---|---|---|---|---|---|
| Tian, 2017 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Yan, 2018 | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Du, 2018 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Cao, 2018 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Chen, 2018 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Wang, 2019 | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Gao, 2019 | High risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Ding, 2019 | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Zeng, 2019 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Xu, 2019 | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Huan, 2019 | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Yang, 2020 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Chen, 2020 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Liang, 2020 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Li, 2020a | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Lun, 2020 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Li, 2020b | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Li, 2021a | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Fang, 2021 | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Li, 2021b | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Jiang, 2021 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Hu, 2021 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 4 |
|
| ||||||||
| Zhang, 2021 | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
|
| ||||||||
| Sun, 2022 | Unclear | Unclear | High risk | High risk | Low risk | Low risk | Low risk | 3 |
3.4. Influence of IGU on BMD in Patients with RA
Five of the included studies [31,36,38–40] reported the outcome of BMD, which were all measured as the lumbar-spine BMD. Pooled results of five studies [31, 36, 38–40] including 550 patients with RA showed that compared with control, treatment with IGU significantly improved lumbar-spine BMD (MD: 0.12, 95% CI: 0.04 to 0.20, p=0.002, I2 = 39%; Figure 2(a)). Subgroup analysis showed consistent results in studies with follow-up durations of 12 weeks (MD: 0.13, 95% CI: 0.01 to 0.26, p=0.04, I2 = 68%) and 24 weeks (MD: 0.13, 95% CI: 0.01 to 0.26, p=0.04, I2 = 0%; p for subgroup difference = 1.00; Figure 2(a)).
Figure 2.
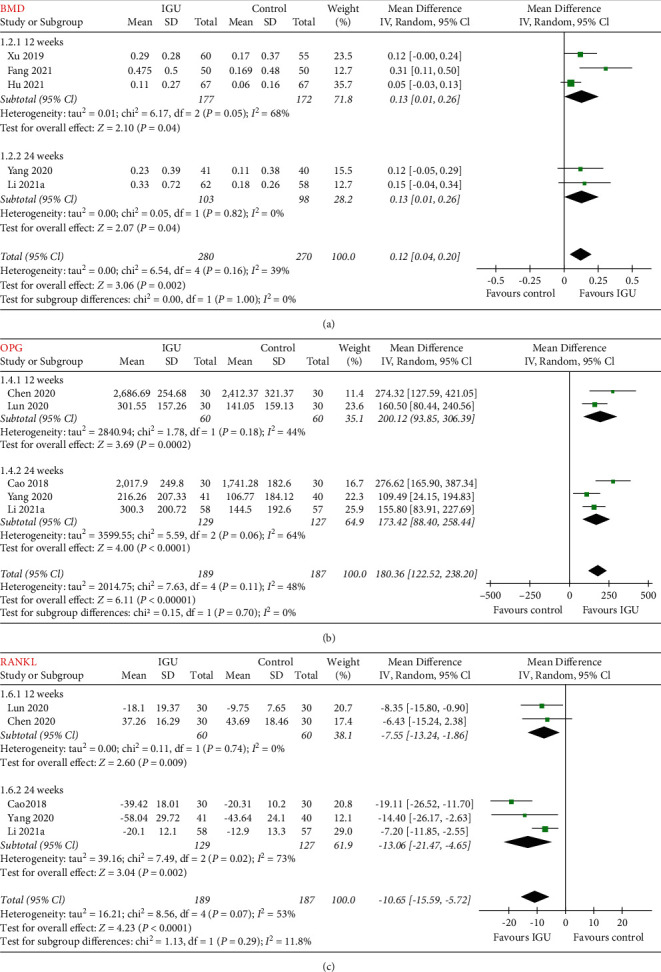
Forest plots for the meta-analyses evaluating the influences of IGU on BMD, serum OPG, and RANKL in patients with RA; (a) meta-analysis for BMD stratified by follow-up durations; (b) meta-analysis for serum OPG stratified by follow-up durations; (c) meta-analysis for serum RANKL stratified by follow-up durations.
3.5. Influence of IGU on Biomarkers of Bone Metabolism in Patients with RA
Pooled results of five studies [23,33,37,38,42] including 376 patients with RA showed that IGU was associated with increased serum OPG (MD: 180.36 pg/ml, 95% CI: 122.52 to 238.20, p < 0.001, I2 = 48%; Figure 2(b)) and decreased serum RANKL (MD: −10.65 pmol/l, 95% CI: −15.59 to −5.72, p < 0.001, I2 = 53%; Figure 2(c)). Subgroup analysis showed consistent results in studies with follow-up durations of 12 weeks and 24 weeks (p for subgroup difference = 0.70 and 0.29, resp.). In addition, pooled results also showed that IGU was associated with increased serum N-MID (14 studies [22,24–30,32,34,35,41,43,45], MD: 4.23 ng/ml, 95% CI: 3.74 to 4.71, p < 0.001, I2 = 35%; Figure 3(a)) and T-P1NP (19 studies [22,24–30,32,34–38,41–45], MD: 9.10 ng/ml, 95% CI: 7.39 to 10.80, p < 0.001, I2 = 86%; Figure 3(b)), but decreased serum β-CTX (19 studies [22,24–30,32,34–38,40–42,44,45], MD: −0.18 pg/ml, 95% CI: −0.21 to −0.14, p < 0.001, I2 = 70%; Figure 3(c)). Subgroup analyses showed consistent results in studies followed <24 weeks, or in those with 24 weeks or longer (p for subgroup difference = 0.73, 0.95, and 0.21, resp.).
Figure 3.
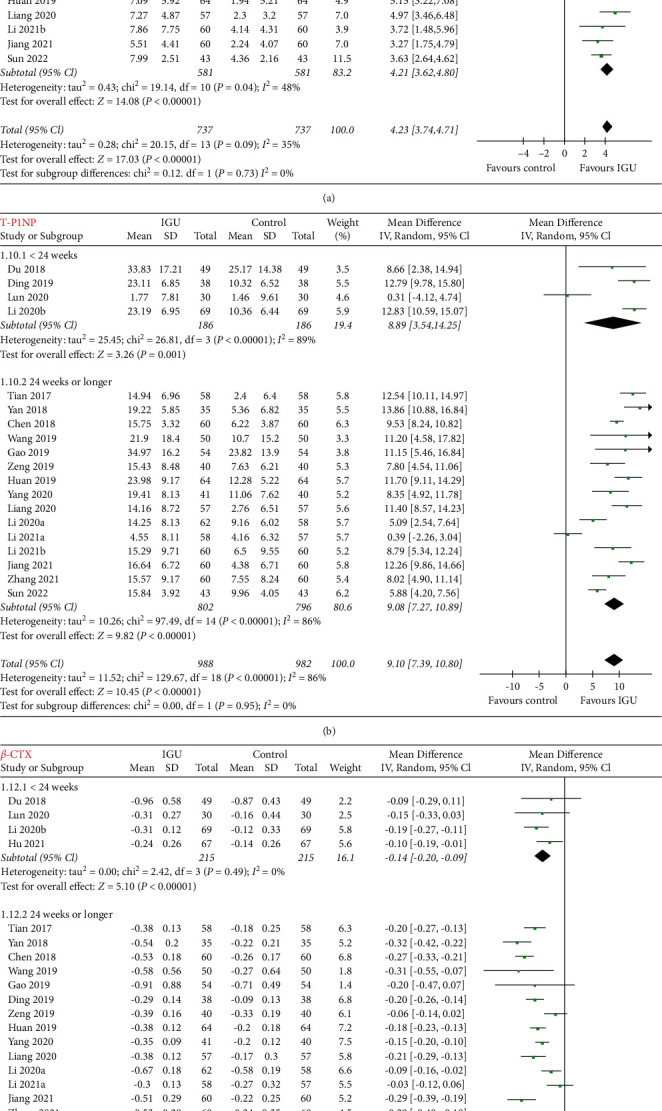
Forest plots for the meta-analyses evaluating the influences of IGU on serum N-MID, T-P1NP, and β-CTX in patients with RA; (a) meta-analysis for serum N-MID stratified by follow-up durations; (b) meta-analysis for serum T-P1NP stratified by follow-up durations; (c) meta-analysis for serumβ-CTX stratified by follow-up durations.
3.6. Publication Bias
Forest plots for the meta-analyses for the influences of IGU on BMD, OPG, RANKL, N-MID, T-P1NP, and β-CTX were shown in Figures 4(a)–4(f). The plots were symmetrical on visual inspection, suggesting low risks of publication biases. The results of Egger's regression tests also suggested low risks of publication biases (p for Egger's regression tests all >0.05).
Figure 4.
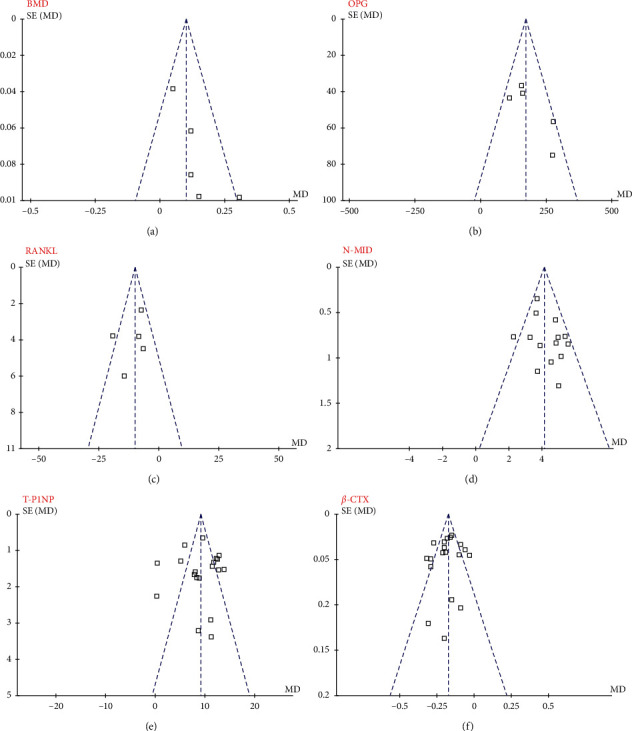
Funnel plot for the evaluation of publication biases of the meta-analyses; (a) changes of BMD; (b) changes of serum OPG; (c) changes of serum RANKL; (d) changes of serum N-MID; (e) changes of serum T-P1NP; (f) changes of serum β-CTX.
4. Discussion
In this study, by pooling the results of 24 RCTs, we found that, for patients with RA, additional treatment with IGU could significantly improve the lumbar-spine BMD as compared to controls with no additional treatment. Moreover, treatment with IGU in patients with RA was associated with significantly increased OPG and decreased RANKL in the peripheral blood. Additionally, IGU could also significantly increase serum markers of bone formation (N-MID and T-P1NP) and decrease the serum marker of bone resorption (β-CTX). Taken together, the results of the meta-analysis suggested that IGU could prevent the bone loss and improve the bone metabolism in patients with RA.
To the best of our knowledge, this may be the first meta-analysis which evaluated the influence of IGU on BMD and biomarkers of bone metabolism in patients with RA. Accumulating evidence suggests that besides synovitis-related joint damage and erosion of local bone, systemic bone loss and osteoporosis are also common in patients with RA [46]. More importantly, comorbidities related to the impairment of bone metabolism may increase the risk of fracture in these patients, which may further adversely affect the functional capacity and prognosis of patients with RA [47]. Therefore, effective prophylactic strategies are needed to prevent the bone loss and improve the bone metabolism in patients with RA [48]. The possible mechanisms for the systemic bone loss in patients with RA are complicated, including treatments related adverse influences, such as the use of corticosteroids, and inflammatory mediated mechanisms [48]. In our meta-analysis, we found that treatment with IGU significantly improved BMD as compared to control in patients with RA. These findings show that besides the validated therapeutic efficacy of IGU for relieving the symptoms of active RA, IGU may also exert additional benefit on bone metabolism in these patients. These findings are consistent with the results of several observational studies, which also suggested additional benefits of IGU on bone metabolism. A pilot nonrandomized study including 93 patients with RA showed that RANKL levels and the RANKL/OPG ratio significantly decreased in both serum and interleukin 1 beta-induced RA fibroblast-like synoviocytes after treatment with IGU [49]. Besides, another observational study also showed that IGU could stimulate bone formation in patients with RA, probably via regulating the RANKL/RANK/OPG system [50].
Subsequent meta-analyses further showed that the mechanisms underlying the potential preventative role of IGU on bone loss in patients with RA may involve the stimulating OPG, inhibiting RANKL, enhancing bone formation, and attenuating bone resorption. Subgroup analyses according to the follow-up durations of the studies showed consistent results. These findings are consistent with the findings of some preclinical studies. In a study of rat model of ovariectomy-induced osteoporosis, IGU was shown to inhibit RANKL-induced osteoclastogenesis and bone resorption in primary bone marrow mononuclear cells [51]. Moreover, a subsequent study suggested that IGU not only suppressed osteoclastogenesis by interfering with RANKL but also inhibited the bone resorption of mature osteoclasts in cultured bone marrow monocytes of the mice [52]. A recent in vitro study also showed that IGU significantly suppressed the dexamethasone-induced increase in osteoclasts, differentiation, and bone resorption activity, which also involved the regulation of the OPG/RANKL pathway [53]. Collectively, these findings indicated that iguratimod could preserve bone loss and improve bone metabolism in patients with RA. Besides, some recent studies also have highlighted the potential benefits of IGU on bone metabolism in other clinical circumstances other than RA. For example, in vitro and in vivo studies have consistently showed that IGU could effectively protect against cancer-induced bone pain and bone destruction, probably via downregulating interleukin-6 production in a nuclear factor-κB-dependent manner [54, 55]. Moreover, an early study has also suggested a directly inhibitory role of IGU on osteoclast formation and function, which may also be a candidate mechanism underlying the preventative efficacy of IGU against bone destruction [56]. However, studies investigating the molecular mechanisms underlying the potential bone protective role of IGU remain rare, and future studies are warranted.
Our study also has limitations. First, all the included studies were from China. The potential benefits of IGU on bone metabolism should be validated in patients of other ethnicities. Second, the follow-up durations were from 12 to 53 weeks. The long-term influence of IGU on BMD and bone metabolism in patients with RA should be investigated in the future. Moreover, all of the included studies were open-label studies. The findings should be validated in large-scale placebo-controlled RCTs. In addition, in two of the included studies, patients of RA and osteoporosis were also additionally treated with oral supplementation of calcium and vitamin D3. Although patients of the IGU and control group both received the previously mentioned treatments, the possible imbalance of calcium and vitamin D3 status of the body may affect the results of BMD and markers of bone metabolism [57, 58]. Finally, lumbar-spine BMD was reported in all of the included studies. Influences of IGU on BMD of other sites should be evaluated.
To sum up, the results of the meta-analysis suggest that treatment with IGU in patients with RA is associated with improved lumbar-spine BMD, increased serum OPG, decreased RANKL, increased serum markers of bone formation (N-MID and T-P1NP), and decreased serum marker of bone resorption (β-CTX). These findings indicate that IGU may attenuate systemic bone loss and improve bone metabolism in patients with RA.
Acknowledgments
This study was supported by the Hubei Provincial Health Commission Joint Fund Project (General Project, WJ2019H461).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Garcia-Gonzalez C. M., Baker J. Treatment of early rheumatoid arthritis: methotrexate and beyond. Current Opinion in Pharmacology . 2022;64 doi: 10.1016/j.coph.2022.102227.102227 [DOI] [PubMed] [Google Scholar]
- 2.Shah P., Siddique A., Thakkar A., et al. An update on novel therapeutic intervention in Rheumatoid arthritis. International Immunopharmacology . 2022;109 doi: 10.1016/j.intimp.2022.108794.108794 [DOI] [PubMed] [Google Scholar]
- 3.Figus F. A., Piga M., Azzolin I., McConnell R., Iagnocco A. Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmunity Reviews . 2021;20(4) doi: 10.1016/j.autrev.2021.102776.102776 [DOI] [PubMed] [Google Scholar]
- 4.Maeda K., Yoshida K., Nishizawa T., et al. Inflammation and bone metabolism in rheumatoid arthritis: molecular mechanisms of joint destruction and pharmacological treatments. International Journal of Molecular Sciences . 2022;23(5):p. 2871. doi: 10.3390/ijms23052871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schett G., Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nature Reviews Rheumatology . 2012;8(11):656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wysham K. D., Baker J. F., Shoback D. M. Osteoporosis and fractures in rheumatoid arthritis. Current Opinion in Rheumatology . 2021;33(3):270–276. doi: 10.1097/bor.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 7.Shim J. H., Stavre Z., Gravallese E. M. Bone loss in rheumatoid arthritis: basic mechanisms and clinical implications. Calcified Tissue International . 2018;102(5):533–546. doi: 10.1007/s00223-017-0373-1. [DOI] [PubMed] [Google Scholar]
- 8.Jin S., Hsieh E., Peng L., et al. Incidence of fractures among patients with rheumatoid arthritis: a systematic review and meta-analysis. Osteoporosis International . 2018;29(6):1263–1275. doi: 10.1007/s00198-018-4473-1. [DOI] [PubMed] [Google Scholar]
- 9.Bejarano V., Hensor E., Green M., et al. Relationship between early bone mineral density changes and long-term function and radiographic progression in rheumatoid arthritis. Arthritis Care & Research . 2012;64(1):66–70. doi: 10.1002/acr.20553. [DOI] [PubMed] [Google Scholar]
- 10.Xu S., Wang Y., Lu J., Xu J. Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arthritis-induced osteoporosis. Rheumatology International . 2012;32(11):3397–3403. doi: 10.1007/s00296-011-2175-5. [DOI] [PubMed] [Google Scholar]
- 11.Llorente I., Garcia-Castaneda N., Valero C., Gonzalez-Alvaro I., Castaneda S. Osteoporosis in rheumatoid arthritis: dangerous liaisons. Frontiers of Medicine . 2020;7 doi: 10.3389/fmed.2020.601618.601618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jura-Poltorak A., Szeremeta A., Olczyk K., Zon-Giebel A., Komosinska-Vassev K. Bone metabolism and RANKL/OPG ratio in rheumatoid arthritis women treated with TNF-alpha inhibitors. Journal of Clinical Medicine . 2021;10(13):p. 2905. doi: 10.3390/jcm10132905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fardellone P., Sejourne A., Paccou J., Goeb V. Bone remodelling markers in rheumatoid arthritis. Mediators of Inflammation . 2014;2014:5. doi: 10.1155/2014/484280.48428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H., Gao H., Wang Q., Wang M., Wu B. Molecular mechanisms and clinical application of Iguratimod: a review. Biomedicine & Pharmacotherapy . 2020;122 doi: 10.1016/j.biopha.2019.109704.109704 [DOI] [PubMed] [Google Scholar]
- 15.Zeng L., Yu G., Yang K., Hao W., Chen H. The effect and safety of iguratimod combined with methotrexate on rheumatoid arthritis: a systematic review and meta-analysis based on a randomized controlled trial. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.780154.780154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha S., Zhao J., Yang C., Zhang J. Relative efficacy and safety of iguratimod monotherapy for the treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Clinical Rheumatology . 2020;39(7):2139–2150. doi: 10.1007/s10067-020-04986-9. [DOI] [PubMed] [Google Scholar]
- 17.Page M. J., McKenzie J. E., Bossuyt P. M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ . 2021;372:p. n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page M. J., Moher D., Bossuyt P. M., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ . 2021;372:p. n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J., Thomas J., Chandler J. Cochrane Handbook for Systematic Reviews of Interventions . Hoboken, NJ, USA: John Wiley & Son; 2021. [Google Scholar]
- 20.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. BMJ . 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ . 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian J. W., Tao P. F. Effects of iguratimod combined with methotrexate on serum M-CSF, IL-6, IL-8 and bone metabolism in patients with rheumatoid arthritis. Hainan Medical Journal . 2017;2(28):391–394. [Google Scholar]
- 23.Cao L. N., Yin H. Q., Li C. H., Wang H. X., Yin S. L. Therapeutic efficacy of iguratimod for old patients with rheumatoid arthritis and its influence on bone metabolism. Chinese Journal of Gerontology . 2018;11(38):5500–5502. [Google Scholar]
- 24.Chen J., Ding Z. H., Liu J. Influence of iguratimod combined with methotrexate on serum inflammatory factors and markers of bone metabolism in patients with rheumatoid arthritis. Chinese Journal of Integrated Traditional and Western Medicine . 2018;28(7):552–555. [Google Scholar]
- 25.Du J. Influence of iguratimod combined with methotrexate on serum M-CSF, IL-8, and markers of bone metabolism in patients with rheumatoid arthritis. Mol Diagn Treat . 2018;29(1):27–29. [Google Scholar]
- 26.Yan X. Z., Wang F. L. Effect of iguratimod in combined with methotrexate on the serum related cytokines and bone metabolism in patients with rheumatoid arthritis. Changchun University of Traditional Chinese Medicine . 2018;34(2):369–372. [Google Scholar]
- 27.Ding L., He S. Z., Wang M., Wang M. X., Zou C. J., Pan M. F. Effects of iguratimod for patients with rheumatoid arthritis: influences on biomarkers of bone metabolism. Jilin Medical Journal . 2019;40(6):1269–1270. [Google Scholar]
- 28.Gao S. Efficacy of iguratimod combined with methotrexatefor for old patients with rheumatoid arthritis and its influence on inflammatory response and bone metabolism. Journal of Contemporary Medicine . 2019;25(5):133–135. [Google Scholar]
- 29.Huan W. Clinical efficacy of guratimod for patients with rheumatoid arthritis and the influence on bone metabolism. Strait Pharmaceutical Journal . 2019;31(5):149–150. [Google Scholar]
- 30.Wang Y. E. Effects of iguratimod combined with methotrexate on inflammatory indexes and bone metabolism in rheumatoid arthritis. Chin Foreign Med Treat . 2019;34(1):100–103. [Google Scholar]
- 31.Xu X. Z., Zhu X. C., Xiao C., et al. Iguratimod for the treatment of patients with rheumatoid arthritis and osteoporosis:a clinical study. Mediterranean Journal of Mathematics . 2019;32(8):1189–1191. [Google Scholar]
- 32.Zeng Q. H., Laing Y. M., Xiao C. J. Efficacy of iguratimod combined with methotrexate for rheumatoid arthritis and its effects on inflammatory reaction, bone metabolism and immunity. Latin American Journal of Pharmacy . 2019;38(12):2415–2422. [Google Scholar]
- 33.Chen Q. H., Yang Y. H. Clinical efficacy of iguratimod for rheumatoid arthritis and its impact on bone metabolism. J Gannan Med Univ . 2020;40(5):482–484. [Google Scholar]
- 34.Liang Y. L., Tsai M. C., Lin Y. C., Strong C., Lin C. Y. Poverty and the prediction of health status in adolescents from low-income families in Taiwan. Journal of Public Health . 2020;42(1):44–52. doi: 10.1093/pubmed/fdy220. [DOI] [PubMed] [Google Scholar]
- 35.Li H. Y., Zhu G. J., Zhang Y. Z., et al. A qualitative study of zoonotic risk factors among rural communities in southern China. International health . 2020b;12(2):77–85. doi: 10.1093/inthealth/ihaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Qi W. F., Li W. Protective efficacy of iguratimod on bones in patients with rheumatoid arthritis. Med Equipment . 2020;33(19):60–62. [Google Scholar]
- 37.Lun X. M., Shen Y. J., Xing Q., Xu X. N., Zhai H. L. Clinical efficacy of iguratimod in treatment of rheumatoid arthritis and its effect on serological indicators. Medieval Review . 2020;26(14):2886–2890. [Google Scholar]
- 38.Yang E. L., Li X. H., Li J., Liu S. M., Gao W. The therapeutic effect and mechanism of iguratimod on osteoporosis secondary to rheumatoid arthritis. Guangdong Medical Journal . 2020;41(22):2293–2297. [Google Scholar]
- 39.Fang L. W., Lin G. F., Zeng B. F., Yin H. F., Lin Z. W. Iguratimod as adjunvant treatment for patients with rheumatoid arthritis and osteoporosis. Chinese Journal of Clinical Rational Drug Use . 2021;14(7B):110–114. [Google Scholar]
- 40.Hu G. H., Xu Q. F., Zheng Q. P., Lin C. Z., Xu X. H. Analysis of the mechanism of the effect of iguratimod on bone mineral density and serum B-alp and CTX-I in patients with refractory rheumatoid arthritis. Progress in Modern Biomedicine . 2021;21(16):3097–3100. [Google Scholar]
- 41.Jiang X. X. Iguratimod combined with methotrexate for the treatment of patients with rheumatoid arthritis: implications on inflammation and bone metabolism. Internal Medicine . 2021;16(1):73–75. [Google Scholar]
- 42.Li W., Jiang W., Zhou M., Wu Z. Effect of combined application of iguratimod in the treatment of active rheumatoid arthritis on bone metabolism, Th17 cells and Treg cells. American Journal of Translational Research . 2021;13(3):1676–1684. [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y. F. Iguratimod combined with methotrexate on serum M-CSF, IL-6 and bone metabolism in patients with rheumatoid arthritis. Mod Diagn Treat . 2021;32(9):1383–1385. [Google Scholar]
- 44.Zhang X. H., Wang L., Liu D. Clinical efficacy of iguratimod for the treatment of patients with rheumatoid arthritis and osteopenia: influences on biomarkers of bone metabolism and peripheral immune parameters. Shaanxi Medical Journal . 2021;50(4):482–484. [Google Scholar]
- 45.Sun P. C., Li R. L. Clinical efficacy of iguratimod combined with methotrexate in the treatment of rheumatoid arthritis and the influence on patients’ bone metabolism. Clin Med Engineer . 2022;29(1):41–42. [Google Scholar]
- 46.Orsolini G., Fassio A., Rossini M., et al. Effects of biological and targeted synthetic DMARDs on bone loss in rheumatoid arthritis. Pharmacological Research . 2019;147 doi: 10.1016/j.phrs.2019.104354.104354 [DOI] [PubMed] [Google Scholar]
- 47.Vis M., Guler-Yuksel M., Lems W. F. Can bone loss in rheumatoid arthritis be prevented? Osteoporosis International . 2013;24(10):2541–2553. doi: 10.1007/s00198-013-2334-5. [DOI] [PubMed] [Google Scholar]
- 48.Yu X. H., Yang Y. Q., Cao R. R., et al. Rheumatoid arthritis and osteoporosis: shared genetic effect, pleiotropy and causality. Human Molecular Genetics . 2021;30(21):1932–1940. doi: 10.1093/hmg/ddab158. [DOI] [PubMed] [Google Scholar]
- 49.Wang X. T., Li P., Xu T. S., Ding R., Zhang X., Bi L. Q. Effect of iguratimod and methotrexate on RANKL and OPG expression in serum and IL-1β-induced fibroblast-like synoviocytes from patients with rheumatoid arthritis. Cellular and Molecular Biology . 2016;62(12):44–50. doi: 10.14715/cmb/2016.62.12.8. [DOI] [PubMed] [Google Scholar]
- 50.Wang X., Ma C., Li P., Zhao F., Bi L. Effects of iguratimod on the levels of circulating regulators of bone remodeling and bone remodeling markers in patients with rheumatoid arthritis. Clinical Rheumatology . 2017;36(6):1369–1377. doi: 10.1007/s10067-017-3668-8. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y. X., Sun Y., Ye Y. P., et al. Iguratimod prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis via inhibition of peroxisome proliferator-activated receptor-γ. Molecular Medicine Reports . 2017;16(6):8200–8208. doi: 10.3892/mmr.2017.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C. H., Ma Z. Z., Jian L. L., et al. Iguratimod inhibits osteoclastogenesis by modulating the RANKL and TNF-alpha signaling pathways. International Immunopharmacology . 2021;90 doi: 10.1016/j.intimp.2020.107219.107219 [DOI] [PubMed] [Google Scholar]
- 53.Miyama A., Ebina K., Hirao M., et al. Effects of iguratimod on glucocorticoid-induced disorder of bone metabolism in vitro. Journal of Bone and Mineral Metabolism . 2021;39(4):639–648. doi: 10.1007/s00774-021-01206-5. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y., Ye D. W., Zhang P., et al. Anti-rheumatic drug iguratimod (T-614) alleviates cancer-induced bone destruction via down-regulating interleukin-6 production in a nuclear factor-κB-dependent manner. Journal of Huazhong University of Science and Technology—Medical sciences . 2016;36(5):691–699. doi: 10.1007/s11596-016-1646-z. [DOI] [PubMed] [Google Scholar]
- 55.Sun Y., Wu Y. X., Zhang P., Peng G., Yu S. Y. Anti-rheumatic drug iguratimod protects against cancer-induced bone pain and bone destruction in a rat model. Oncology Letters . 2017;13(6):4849–4856. doi: 10.3892/ol.2017.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gan K., Yang L., Xu L., et al. Iguratimod (T-614) suppresses RANKL-induced osteoclast differentiation and migration in RAW264.7 cells via NF-κB and MAPK pathways. International Immunopharmacology . 2016;35:294–300. doi: 10.1016/j.intimp.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 57.Hagino H. Vitamin D3 analogs for the treatment of osteoporosis. Canadian Journal of Physiology and Pharmacology . 2015;93(5):327–332. doi: 10.1139/cjpp-2014-0419. [DOI] [PubMed] [Google Scholar]
- 58.Song L. Calcium and bone metabolism indices. Advances in Clinical Chemistry . 2017;82:1–46. doi: 10.1016/bs.acc.2017.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


