Abstract
Methods
This study was conducted among 60 rats, and groups consist of control, three separate groups for RJ, dimethylhydrazine (DMH), and vitamin E, and two separate treated groups with DMH + RJ and DMH + vitamin E. Additionally, the cytotoxicity of royal jelly was examined on HT-29 cell line. Findings. Based on the in vitro assessment using MTT assay, the LC50 of royal jelly was 1.781 mg/ml, and the highest cytotoxicity was observed at 25 mg/ml concentration after 48 hours. Meanwhile, in the in vivo study, after the 13th week, compared to the DMH group, the rats exposed to DMH + royal jelly experienced a significant less oxidative stress (P < 0.05) and a significantly greater total antioxidant capacity (TAC) level (P < 0.05). The expression of proliferating cell nuclear antigen (PCNA), platelet-derived growth factor (PDGF), and carcinoembryonic antigen (CEA) proteins significantly decreased among the animals receiving DMH + royal jelly compared to the DMH group. The pathological examinations revealed less congestion, necrosis, inflammation, and cell proliferation in the colon tissue of the RJ-treated group than that of the DMH group. Overall, the biochemical indices were better in the treatment groups in comparison with the DMH group.
Conclusion
The results represented the clinical usability of royal jelly, as a substance with anticancer properties, to prevent and treat colorectal cancer. This issue is related to its effective antioxidant potential, which even exhibits more effectiveness than the vitamin E, which is known as a strong antioxidant.
1. Introduction
Cancer is among the conventional causes of the disease-caused death in the world [1]. Regarding colorectal cancer (CRC), a rise in reactive oxygen species (ROS) value plays a critical role in the development of the disease. According to Guéraud [2], a high ROS level influences the different signaling pathways related to proliferation, tumor survival, invasion, and metastasis. The body uses various mechanisms for adjusting the ROS concentration like the antioxidant-based enzymatic system. In addition, peptic ulcers, necrosis, and inflammations are closely associated with the cancers [3]. Lipid peroxidation products have been introduced as the oxidative stress biomarkers to determine the cell injury extent. Several researchers have utilized dimethylhydrazine (DMH) and its metabolites as toxic compounds for inducing the inflammation, precancer phases, and cancer lesions, especially in colon tissue [4]. The lesions include the different levels of necrosis and inflammation up to the cancer [5–7]. The DMH mostly affects by methylating tissue, damaging mucus, and generating free radicals [8]. Dolara et al. [9] outlined that oral antioxidants can diminish the effects and lesions induced by the DMH.
Many factors are applied to detect the severity and prognosis of colorectal cancer. CEA, as one of the markers of CRC, is a glycoprotein produced during embryonic stage until birth [10]. Despite the low serum CEA concentration, its content elevates in adults when cancer is developing [11]. PCNA, an antigen for proliferating cell nucleus, operates like a DNA clamp for the DNA polymerase in eukaryotic cells and is essential for proliferation. In the proliferating cells, more PCNA are often synthesized and expressed [12] than normal cells, which indicates an upregulated cell proliferation and is a reliable index of tumor cell proliferation [13]. Another protein that plays an important role of a brake for cell divisions is the APC [14]. Some studies have suggested the expression of PDGF, another cell division and growth factor, as a sign of the enhancing uncontrollable cell growth and tumorigenesis in cancers and tumors [15, 16]. Pan et al. [17] mentioned that this protein may cause cancer metastasis.
Royal jelly is secreted from the salivary glands of worker honeybees. The presence of the low values of this substance in the diet of female honeybees converts them into the workers, while the larvae fed an adequate level of royal jelly would develop into the queen [18]. Royal jelly (RJ) is rich in free amino acids, polypeptides, sugar, fatty acids, and minerals, the constituents of which can inhibit the tumor growth and prevent malignancies from invading. Kimura [19] showed the antitumor effect of oral royal jelly on Syrian mice. This compound regulates anticancer activity by influencing N-acetylation and inhibiting 2-aminofluorene [20]. Based on the results of the in vitro and in vivo studies, royal jelly as a medicinal agent can cope with the oxidative stress through affecting metabolic pathways and reducing free radicals [21]. Due to the great antioxidant properties of royal jelly, it becomes an ideal option for being used with other treatments such as chemotherapy [22]. The other advantages of consuming this substance in animal models are its effects on cancer factors and an increase in patients' lifespan. Furthermore, royal jelly positively affects drug-caused toxicity. Along with the decreasing inflammation and oxidative stress, being easy to access and produce can be addressed as the other benefits of utilizing this compound [23].
2. Method
2.1. Royal Jelly Preparation
Fresh royal jelly was purchased from Mazandaran province in the north of Iran (52.35° E and 36.47° N). Additionally, a part of the substance was analyzed through employing the gas chromatography-mass spectroscopy (GC-MS) technique.
2.2. Analysis of Royal Jelly Based on the GC-MS Technique
Royal jelly was injected into a Shimadzu GCMS-TQ8040 NX by using a microsyringe, followed by scanning for 45 minutes. The analysis process was repeated three times. Then, the components were specified by comparing their spectra with those in Wiley and NIST/EPA/NIH34-44 spectral mass libraries. The values lower than 1% were removed from the table [24].
2.3. Cell Culture
HT-29 cells, as a human intestinal cancer cell line, were placed in the RPMI 1640 medium with 10% fetal bovine serum (FBS) within a 75 ml flask. They were laid into a plate for cultivation after reaching the cell confluence of 75%, achieving appropriate cell morphology, and being in growth logarithmic phase. Further, trypsin-EDTA was applied to detach cells from the flask floor, and cells were counted by using the Neubauer slide [25].
2.4. MTT
The cells were cultivated in 96-well plates so that 7000 cells were cultured in each well. The exposure stages of royal jelly began after 48 hours, when cell frequency reached 70% in each well. Furthermore, 200 mg of royal jelly was dissolved into 500 μl of DMSO, made to 2 ml volume with 1500 μl of medium, and serially diluted up to 10 times. Regarding each dilution, three wells were considered for treating with royal jelly. The control (three wells) was prepared by dissolving 500 μl of DMSO in 1500 μl of medium and rediluting up to 10 times serially. Serial dilution was performed through mixing 1 ml of the previous solution with 1 ml of medium in each time. Following the exposure, the plates were incubated for 48 hours. Then, 5 mg/ml MTT powder was dissolved in PBS, 50 μl of which was added into each well after suctioning its supernatant, and placed in an incubator for 3 hours. Finally, 150 μl of DMSO was poured into each well to dissolve colored crystals. It was subjected to an ELIZA reader with the main and background wavelength of 570 and 360 nm, respectively. The results were calculated by using the Excel program, viability percentage was transferred by using the Prism software, and linear regression was determined [25].
2.5. Animals under Study
In the present study, 60 male Wistar rats weighing 200-210 g with the age of 8 weeks were held in the animal house of the Pasture Institute of Iran under a 12hours light/dark cycle at 20-23°C and 60-70% relative humidity. During the study, they had access to a standard level of food and water, and the intended ethical protocols were observed (IR.IAU.BABOL.REC.1399.100), alongside with the ARRIVE guidelines 2.0 [26].
2.6. Study Design
Vitamin E and DMH were purchased from Merck (Germany). The Wistar rats were randomly categorized into six 10-member groups. The rats in the first group exposed to no treatment and received normal saline gavage as the control group. The second and third groups received royal jelly with 300 mg/kg concentration [27] and vitamin E with 180 mg/kg dose [28] by gavage once per week, respectively. Those in the fourth group were subcutaneously injected with 30 mg/kg of DMH once a week [29]. In addition, 300 mg/kg of royal jelly and 180 mg/kg of vitamin E were, respectively, administered to the DMH-treated animals in the fifth and sixth groups.
All animals survived after the 13th week, which then were completely anesthetized intraperitoneally by using 10 mg/kg ketamine (10%, Bremer Pharma GmbH) and 80 mg/kg Xylazine (2%, AlfasanDiergeneesmiddelen BV) cocktail [30]. Following the blood sampling from the rats, colon tissue samples were collected, two parts of which were kept separately in formalin and a freezer at -80°C for histological assessment and tissue homogenate preparation, respectively.
2.7. Blood and Serum Tests
The blood samples were taken in two separate tubes, one of which included EDTA for CBC (Celltac Es MEK-7300 K, Nihon Kohden). Another tube without the anticoagulant were centrifuged (EBA 20, Hettich®) and utilized to measure all serum markers by using a BIOLIS24i autoanalyzer (Tokyo BoekiMedisys Inc.).
2.8. Colon Tissue Homogenization
To prepare tissue homogenate, 250 mg of colon tissue was homogenized in 1 ml of 50 mM phosphate buffer solution and 0.1 M EDTA with pH 7.4 in each group. Then, the mixture was centrifuged at 4°C and 12000 rpm for 20 min, the supernatant of which was isolated and held at -80°C until oxidative stress marker determination. The protein content of the homogenates was obtained by using bovine serum albumin (standard) based on the Bradford assay [31].
2.9. Malondialdehyde (MDA) Concentration Measurement
MDA was assessed on a TebPazhouhan Razi Kit. The tissue supernatant and reagents were allowed to reach room temperature half an hour prior to the start of the experiment. The reagents were heated up to 50°C on a bain-marie and were vortexed when any crystal was detected. The deionized water was applied to double the volume of thiobarbituric acid, which was mixed with the reagents of HOAC (×5), alkali (×10), in a 1 : 1 : 2 ratio (HOAC : alkali : thiobarbituric acid). Further, 200 μl of sample (or standard) was poured into 800 μl of working solution, followed by closing its lid, placing in a bain-marie at 95°C for 45 min, and cooling it in ice water containers quickly. After a 15-minute centrifugation at 3000 rpm, the samples were transferred into plate wells and their absorbance was examined at 550 nm.
2.10. Superoxide Dismutase (SOD) Level Determination
This stage was performed by using a SOD Activity Assay Kit (Nasdox). To evaluate SOD concentration, 50 μl of the homogenate supernatant was added into sample wells, and the control received an equal volume of deionized water, to which both R1 and R2 were poured, respectively. Finally, the absorbance of the mixtures was read at 405 nm after room temperature incubation for 5 min in the absence of light.
2.11. Total Antioxidant Capacity (TAC)Assay
A TAC Assay Kit (Naxifer) was used for assaying a TAC level in colon tissue. Following a half-hour placement of reagents at room temperature, 2.2 ml of R2b was poured into each R2a bottle and was completely vortexed until dissolution to produce R2 solution. The R2 was mixed with an equal volume of R3 reagent and was vortexed, to which a fivefold volume of R1 was added. Regarding each well, 5 μl of sample (or standard) and 250 μl of working solution were, respectively, added, the optical absorbance of which was determined at 593 nm after 5 min.
2.12. Protein Expression by Suing Western Blot Analysis
In each group, a part of colon tissue (1 g) was frozen and lyzed with the RIPA buffer, followed by achieving tissue homogenate by using an electric homogenizer. The tissue homogenates were centrifuged at 12000 rpm for 10 min and western-blotted to obtain protein. The blots were incubated with APC, PCNA, CEA, and PDGF (primary antibodies) at 4°C for 12 hours, as well as the proper secondary antibodies related to peroxidase conjugate, respectively. This study used β-actin antibody as an internal control protein, relative to which the percentage of other antibodies were reported. The resultant membranes were read after washing with TBS after 10 minutes. Regarding each protein, the expression level were measured in all samples, the area under its diagram was computed by using the ImageJ software, and the ratio of this area to the β-actin protein was determined [25, 32].
2.13. Histology
In the pathological assessment, paraffin blocks (TE100, PouyaAbzarAzma) were prepared by washing colon tissues with sterile normal saline, lying in 10% formalin buffer, fixating (DS2080/H, Did Sabz Co.), dehydrating, and passaging. After cooling the blocks (TE100, PouyaAbzarAzma), the five-microns sections (DS4055, Did Sabz Co.) were H&E stained and examined by using an Olympus CX23 optical microscope. In the histological analysis, Kruskal-Wallis and Mann-Whitney U assays were employed for the histopathological scoring between the groups, as well as determining mitotic index to compare the significance of their differences [33, 34].
2.14. Data Analysis
The statistical analysis was carried out by analyzing the data of stress and inflammatory markers, CBC, serum tests, and the ratios of western blot proteins by using the SPSS 26 software based on the one-way ANOVA and Duncan's post hoc tests. The P value less than 0.05 was considered significant.
3. Results
3.1. Analysis of Royal Jelly through Using the GC-MS Technique
The result of GC-MS analysis indicated the existence of natural antioxidants, as well as a high value of 10-hydroxy-8-decenoic acid (10H8DA), 5-hydroxymaltol (HMT), and 5-hydroxymethylfurfural (5HM) (Table 1).
Table 1.
Results of analyzing royal jelly by using the GC-MS method. Retention time (RT): the duration between injection (initial time) and detection.
| Chemical constituents | Retention time | Peak area(%) | Molecular weight(mg/mol) | Molecular formula |
|---|---|---|---|---|
| 5-Hydroxymaltol | 8.674 | 5.98 | 142.12 | C6H6O4 |
| (+)-Verbenone | 9.505 | 4.96 | 150.21 | C10H14O |
| 5-Hydroxymethylfurfural | 10.158 | 18.97 | 126.11 | C6H6O3 |
| 10-Hydroxy-8-decenoic acid | 15.829 | 13.81 | 186.25 | C10H18O3 |
| (Z)-8-Tetradecenal | 15.927 | 1.67 | 210.36 | C14H26O |
| Alpha-linolenic acid | 20.074 | 14.65 | 278.42 | C18H30O2 |
| Ostreasterol | 29.967 | 9.04 | 398.7 | C28H46O |
3.2. MTT
The first figure displays the cytotoxicity of royal jelly with different concentrations on HT-29 cell lines. As depicted, the cytotoxicity is maximized at 25 mg/ml, and LC50 is equal to 1.781 mg/ml after 48 hours (Figures 1 and 2).
Figure 1.

Viability level of HT-29 cells, as well as LC50. The results were read after 48 hours.
Figure 2.

Comparison between the effects of royal jelly with various concentrations on cancer cells viability. Results are expressed as the mean ± standard error in mg/ml.
3.3. Hematological Parameters
The blood profile assessment reflected less RBC, hemoglobin, RDW, MCHC, MCV, MCH, and hematocrit (HCT) level in the DMH group compared to the control group. In addition, both treatment groups experienced better status in comparison with the DMH group. No significant difference was observed among other groups (Table 2).
Table 2.
Comparison between RBC parameters.
| Groups | RBC (×106/μl) | HGB (g/dl) | HCT (%) | MCV (fl) | MCH (pg) | MCHC (g/dl) | RDW (%) |
|---|---|---|---|---|---|---|---|
| Control | 8.12 ± 0.22 | 13.64 ± 0.45 | 40.7 ± 1.46 | 51.06 ± 0.78 | 17.16 ± 0.34 | 33.56 ± 0.24 | 14.35 ± 0.35 |
| Royal Jelly | 8.89 ± 0.19∗ | 14.59 ± 0.33∗ | 43.59 ± 0.92 | 49.76 ± 0.52 | 16.61 ± 0.29 | 33.38 ± 0.21 | 14.08 ± 0.5 |
| Vitamin E | 8.32 ± 0.19 | 14.15 ± 0.27 | 41.2 ± 2.04 | 50.24 ± 0.5 | 17.08 ± 0.35 | 33.6 ± 0.48 | 13.76 ± 0.32 |
| DMH | 7.54 ± 0.37 | 12.94 ± 0.47 | 39.19 ± 1.78 | 49.43 ± 1.68 | 16.99 ± 0.29 | 32.44 ± 0.33 | 13.51 ± 0.31 |
| DMH + royal jelly | 8.59 ± 0.26∗ | 14.1 ± 0.45 | 42.28 ± 1.49 | 50.08 ± 0.53 | 16.86 ± 0.18 | 33.69 ± 0.21∗ | 14.03 ± 0.37 |
| DMH + vitamin E | 8.09 ± 0.24 | 13.24 ± 0.4 | 39.88 ± 1.55 | 49.51 ± 0.87 | 17.11 ± 0.24 | 33.59 ± 0.64 | 13.61 ± 0.39 |
∗ P < 0.05: significant compared to the DMH group. All results are expressed as the mean ± standard error. n = 10.
3.4. WBC
As shown in Table 3, the WBC count significantly increased in the DMH group compared to the control group (P < 0.05), followed by a substantial decrease among the rats in the DMH + royal jelly group. Further, the DMH group exhibits a greater neutrophil percentage and a significantly lower lymphocyte percentage (P < 0.05), the status of which becomes better in the DMH + royal jelly-treated group. However, the platelet level was not significantly different between the groups (Table 3).
Table 3.
Comparison between platelet and WBC parameters.
| Groups | WBC (×103/μl) | Neutrophil (%) | Lymphocyte (%) | PLT (×103/μl) |
|---|---|---|---|---|
| Control | 7.93 ± 0.44∗ | 59.08 ± 3∗ | 45 ± 3.6∗ | 734 ± 23.84 |
| Royal Jelly | 7.43 ± 0.65∗ | 59.75 ± 3.42∗ | 42.25 ± 2.35∗ | 716.75 ± 29.32 |
| Vitamin E | 7.6 ± 0.48∗ | 62.16 ± 3.64 | 42.21 ± 2.71∗ | 791.13 ± 23.13 |
| DMH | 9.58 ± 0.75 | 72.4 ± 5.05 | 26.93 ± 2.96 | 786.63 ± 51.79 |
| DMH + royal jelly | 8.34 ± 0.33 | 64.15 ± 4.06 | 34.16 ± 4.34 | 739.25 ± 28.55 |
| DMH + vitamin E | 8.2 ± 0.48 | 70.5 ± 3.18 | 29.71 ± 2.76 | 803.63 ± 32.88 |
∗ P < 0.05: significant compared to the DMH group. All results are expressed as the mean ± standard error. n = 10.
3.5. Biochemical and General Serum Inflammatory Markers
The results related to the serum total protein (TP) concentration revealed that the TP content was higher in the DMH group than that of the control group, which significantly diminished after receiving the royal jelly (P < 0.05).
Blood albumin value was reduced by administering the DMH, but no significant difference was found between the groups (Figure 3). In addition, the results demonstrated a rise in the LDH, CRP, and CPK levels in the DMH group, in which the rise of the CRP and LDH was significant. Following the treatment with royal jelly, a decrease was seen in the CPK and CRP levels in the DMH + RJ group compared to the DMH group (Figure 3).
Figure 3.

Comparison between general serum biochemical indices. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001: significant compared to the DMH group. All results are expressed as the mean ± standard error. n = 5.
3.6. MDA Concentration in Colon Tissues
The DMH group had a significantly higher MDA level than the control group (P < 0.01), while this value was lower in the DMH + royal jelly-exposed group (Figure 4).
Figure 4.

MDA, SOD, and TAC levels in the different groups. ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001: significant compared to the DMH group. All results are expressed as the mean ± standard error. n = 5.
3.7. SOD Level in Colon Tissues
The use of DMH resulted in the diminishing SOD concentration in the colon tissue compared to the control group. In this regard, a significant enhancement was obtained among the rats treated with royal jelly in comparison with the DMH group, which was a higher number than the vitamin E-receiving group (Figure 4).
3.8. TAC in Colon Tissues
In terms of TAC, a significant increase was found in the both treatment groups compared to the DMH group (P < 0.001) (Figure 4).
3.9. Protein Expression Level (Western Blot)
As demonstrated in Table 4, the ratio of protein expression in the groups reflects a significantly more expression of the CEA in the DMH group than that of the control group (P < 0.05), which is also significantly lower than that of the rats receiving DMH + royal jelly (P < 0.05). In the DMH group, a significantly higher level of the PCNA and PDGF was detected compared to the control group (P < 0.05), while they both significantly declined after treating with the royal jelly (P < 0.05). This improvement in status was greater in the animals treated with DMH + royal jelly than those of receiving DMH + vitamin E. Finally, the APC was significantly less expressed in the DMH group in comparison with the control group, followed by an increase in the both treatment groups with insignificant (P > 0.05) difference from the DMH group (Table 4).
Table 4.
Comparison between the expression levels of proteins relative to that of β-actin in colon tissue.
| Groups | CEA | PCNA | PDGF | APC |
|---|---|---|---|---|
| Control | 0.39 ± 0.01∗ | 0.72 ± 0.03∗ | 0.76 ± 0.02∗ | 0.99 ± 0.02∗ |
| DMH | 0.82 ± 0.09 | 1.49 ± 0.07 | 1.38 ± 0.05 | 0.65 ± 0.06 |
| DMH + RJ | 0.48 ± 0.04∗ | 1.01 ± 0.1∗ | 0.94 ± 0.04∗ | 0.80 ± 0.05 |
| DMH + Vit E | 0.71 ± 0.04 | 1.02 ± 0.05∗ | 0.98 ± 0.11∗ | 0.79 ± 0.06 |
∗ P < 0.05: significant compared to the DMH group. All results are expressed as the mean ± standard error. n = 5.
3.10. Histological Observations
This figure includes scoring and comparing pathological lesions such as the mitotic index, necrosis, and inflammatory cell infiltration level between all groups (with the average number of mitoses in 10 HPF at the tumor area, [33]) (Figure 5).
Figure 5.
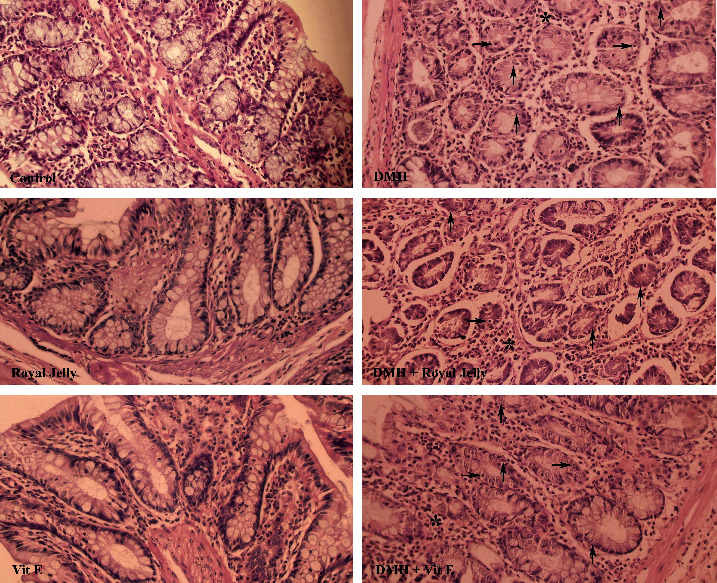
Comparison of colon tissues indices of the different groups. Normal tissue conditions in the control, royal jelly, and vitamin E groups. Mitosis, necrosis, and inflammatory cells which are illustrated with upward arrows, right arrows, and ∗, respectively, which can be seen in the DMH and treatment groups. ×40 magnification, H&E staining.
The observations revealed no significant difference between the control, royal jelly, and vitamin E groups with respect to necrosis, mitosis, and inflammatory cells, although the indices of the DMH group significantly differed from those of the control (P < 0.01). Necrosis, mitosis, and inflammatory cells significantly were reduced among the rats exposed to DMH + royal jelly in comparison with the DMH group (P < 0.01). RJ consuming led to a significant diminution in the mitotic index compared to the vitamin E-receiving group (Table 5).
Table 5.
Comparison between the types of colon tissue injuries in different inflammatory groups.
| Groups | Necrosis | Inflammatory cells in filtration | Mitosis |
|---|---|---|---|
| Control | 0.20 ± 0.13 | 0.10 ± 0.10 | 0.30 ± 0.15 |
| Royal Jelly | 0.10 ± 0.10 | 0.10 ± 0.10 | 0.10 ± 0.10 |
| Vitamin E | 0.10 ± 0.10 | 0.10 ± 0.10 | 0.20 ± 0.13 |
| DMH | 2.10 ± 0.23a, e, f | 1.90 ± 0.28a, e | 20.70 ± 1.08a, e, f |
| DMH + royal jelly | 1.00 ± 0.26b, e | 0.80 ± 0.20b, e | 8.80 ± 0.89b, e, g |
| DMH + vitamin E | 1.20 ± 0.25c, f | 1.30 ± 0.30c | 16.70 ± 1.21c, f, g |
P values less than 0.0001 were considered statistically significant. All results are expressed as the mean ± standard error. aStatistically significant differences were observed between the control and DMH groups. bStatistically significant differences were observed between the control and DMH + royal jelly-exposed groups. cStatistically significant differences were observed between the control and DMH + vitamin E-exposed groups. eStatistically significant differences were observed between the DMH and DMH + royal jelly-exposed groups. n = 10.
4. Discussion
The oral consumption of royal jelly exhibited anticancer activity during the pretumorigenesis phases of colorectal cancer [23]. This substance also resulted in reducing tissue stress and diminishing inflammation in precarcinogenesis stage. Considering the antitumor and anticancer properties of royal jelly, it significantly modulated the expression level of CEA, APC, PCNA, and PDGF proteins and regulated blood and serum factors positively. Besides, the administration of the RJ among the Wistar rats led to no adverse or toxic effect.
Some researchers have proposed the cytotoxicity of royal jelly on MRC-5 [35] and PC3 cell lines [36]. In the present study, this compound represented appropriate cytotoxicity in the HT-29 cell culture medium, as one of the most resistant and invasive cancer cell lines. In the MTT assay, RJ showed a proper anticancer ability with a low LC50.
The results suggested the ability of royal jelly to kill human intestinal precancerous and cancer cells, which is consistent with those of some earlier studies [20, 23, 37]. Based on the results of the in vitro study using MTT assay, royal jelly at 25 mg/kg concentration exhibited the highest cytotoxicity against cancer cells. The presence of the antioxidants such as 10H8DA, HMT, and 5HM in royal jelly is likely responsible for the main part of its anticancer properties [38]. HMT is a strong antioxidant and a free-radical scavenger [39], which specifically protects erythrocytes against the ROS [40] and decreases the MDA level [41]. Further, 5HM is a phenolic compound with antioxidant and anti-inflammatory effects, which reduces inflammatory cells in the sites and improves tissue stress conditions [42].
In this study, colorectal cancer was induced in rats following the use of DMH for 13 consecutive weeks. Exposing to the DMH results in the higher expression of oncogenes, disrupting the apoptosis process through the two methylation and mutation mechanisms and simultaneously enhancing the ROS value in some tissues [8]. After administering the DMH, the RBC was the first body cell which experienced a reduced stability (Table 2), since the oxidative stress is among the causes of disturbing RBC's normal level in blood [43], leading to other abnormalities and diseases such as cancer [44, 45]. Maintaining the oxidant balance in the body is considered very important, and therapeutic agents should have a high antioxidant ability to compensate for this elevation in the blood free radicals [46–49]. The promoted stress in erythrocytes disrupts the immune system [50]. Thus, the WBC count raises by revealing neoplasm in which the percentage of phagocyte increases [51, 52]. Furthermore, the lipid peroxidation causes interference in the erythrocyte function and worsens the condition through creating an oxidative stress in the cell membranes, destructing them and releasing their substances in blood [53].
Among the biomarkers that reflect the acute body status [54–56], the CPK level was enhanced in the DMH group, which may be attributed to the heart damage, myocardial microinfarctions, skeletal muscle, and/or liver problems. The significant elevation in the CRP value (P < 0.05) was another biomarker in this regard, which was significantly diminished by treating with DMH + royal jelly. In the DMH group, a significant improvement was found in the regard of the LDH level (P < 0.05), which demonstrates vast destructions at the cellular level. However, this value was reduced after consumption of the royal jelly. Similarly, the results of the previous studies suggested that the royal jelly could inhibit inflammation and upregulates cell healing [57, 58].
In the present study, the DMH led to a significant elevation in the tissue stress in colon, which is the same with that of Wei et al. [59]. In addition, the SOD, an antioxidant pathway enzyme, was declined in the DMH group compared to the control group. Also, the DMH group had a significantly higher MDA concentration than the control group (P < 0.05), which indicated an increase in the tissue stress, as well as the existence of the ROS metabolites [60]. So, the promoted lipid peroxidation in the colon tissue following the exposure to the DMH was one of the reasons for enhancing stress [61]. Further, an increase in the lipid peroxidation products exhibited an inflammatory and carcinogenesis effect and accelerated the secondary neoplasms, along with leading to the more oxidative stress [62]. A reduction in the TAC level in the DMH group decreased the body's ability to fight against the oxidative stress and intensified the peroxidation-caused lesions, which has the same consequences as the previous studies [63, 64]. After administering the royal jelly, a significant enhancement was observed in the TAC level compared to the DMH group (P < 0.05) due to its antioxidant properties. Therefore, an increase was occurred in the power of the antioxidant system in the body against the free radicals and metabolites that were generated in the lipid peroxidation cycle. This issue caused a better antioxidant balance in the body, which is consistent with the results that were obtained by Milani et al. [65] and Wielsøe et al. [66].
Furthermore, the DMH led to a various tissue lesions in the colon, which was in line with the results of Hamiza et al. [67]. Scoring and staging the lesions, based on the protocols provided in the previous studies [34, 68, 69], demonstrated that the DMH-treated rats exhibited the signs of necrosis, inflammatory cells, and a higher mitotic index, which are attributed to the methylation and increased free radicals in the tissue [70]. Compared to the DMH group, the consumption of royal jelly, similar to previous study by Nagai et al. [71], significantly diminished the mitotic index, necrosis, and inflammatory cells because of the reducing of the free radicals, which were caused by the declining lipid peroxidation in the colon tissue (P < 0.01), whereas they were increased by the DMH at the first place [72].
Finally, the western blot revealed that the CEA level was significantly more expressed in the DMH group compared to the control group (P < 0.05). This issue is related to the fact that this protein is often promoted by the cancerous cell secretions [73]. The DMH caused the occurred changes, leading to a disturbance in the tissue oxidant balance, methylation, mutation, and carcinogenesis, respectively. Accordingly, a significant rise was found in the PCNA protein expression in the groups receiving DMH in comparison to the control group (P < 0.05). However, royal jelly significantly decreased the PCNA level (P < 0.05) and prevented the cancer by inhibiting the proliferation. In fact, royal jelly represents its antitumor and anticancer activity by stopping the cancer cells from reproducing. Additionally, the declined APC protein expression caused chromosomal instability, mutation, and cancer. In the DMH group, the expression of this protein was lower than the control group (P < 0.05), which reflects the loss of chromosomal stability, an increase in unbridled cell divisions, and cell carcinogenesis. The rats exposed to DMH + royal jelly experienced an enhancement in the reexpression of the APC gene, leading to a greater concentration of the APC protein in the cells and more chromosomal stability, leading to a controlled cell reproduction cycle and decelerated tumorigenesis. The results revealed a significant improvement in the PDGF content of the DMH group in comparison with the control group (P < 0.05), followed by a significant reduction in the DMH + royal jelly-treated group because of the inhibition of this factor from being produced by consuming the royal jelly (P < 0.05). Similarly, Okda et al. [74] outlined that administering indomethacin + vitamin D combination significantly diminishes the elevation which was occurred in the tissue stress, PDGF, and CEA marker levels due to the exposure to DMH based on a similar technique.
5. Conclusion
Royal jelly can effectively reduce the oxidative stress and free radicals through controlling the pathway of lipid peroxidation and improves the power of body's antioxidant defense system significantly. This substance controls the cell division markers, which results in a decreased uncontrollable cell division, a declined rate of carcinogenesis, and a lower extent of tumorigenesis. Overall, RJ can be used as an agent for fighting the colorectal cancer due to its cytotoxicity level on cancerous cells and has a potential for raising the tissue's TAC.
Data Availability
Upon request, data supporting the conclusion of our study are accessible by corresponding author.
Ethical Approval
All experiments and procedures of this study were reviewed and approved by the Research Ethics Committees of Islamic Azad University-Babol Branch (IR.IAU.BABOL.REC.1399.100).
Conflicts of Interest
The authors have no conflicts of interest to declare. All coauthors have seen and agree with the contents of the manuscript, and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
References
- 1.Lei L., Zhang J., Decker E. A., Zhang G. Roles of lipid peroxidation-derived electrophiles in pathogenesis of colonic inflammation and colon cancer. Frontiers in Cell and Developmental Biology . 2021;9 doi: 10.3389/fcell.2021.665591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guéraud F. 4-Hydroxynonenal metabolites and adducts in pre-carcinogenic conditions and cancer. Free Radical Biology and Medicine . 2017;111:196–208. doi: 10.1016/j.freeradbiomed.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov S. I. Inflammation and colorectal cancer: colitis-associated neoplasia. Seminars in Immunopathology . 2013;35(2):229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S. H., Thulasingam S., Chellappan D. R., Chinnaswamy P., Nagarajan S. Morin and Esculetin supplementation modulates c-myc induced energy metabolism and attenuates neoplastic changes in rats challenged with the procarcinogen 1,2 - dimethylhydrazine. European Journal of Pharmacology . 2017;796:20–31. doi: 10.1016/j.ejphar.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Glauert H. P., Bennink M. R. Metabolism of 1, 2-Dimethylhydrazine by Cultured Rat Colon Epithelial Cells. Nutrition and Cancer . 1983;5(2):78–86. doi: 10.1080/01635588309513782. [DOI] [PubMed] [Google Scholar]
- 6.Perše M., Cerar A. Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. Journal of Biomedicine and Biotechnology . 2011;2011:14. doi: 10.1155/2011/473964.473964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shree A., Sultana S. Colonoprotective efficacy of gallic acid against 1, 2-dimethylhydrazine induced colon carcinogenesis in Wistar rats. Free Radical Biology and Medicine . 2019;145:S38–S39. [Google Scholar]
- 8.Eslami M., Yousefi B., Kokhaei P., et al. Importance of probiotics in the prevention and treatment of colorectal cancer. Journal of Cellular Physiology . 2019;234(10):17127–17143. doi: 10.1002/jcp.28473. [DOI] [PubMed] [Google Scholar]
- 9.Dolara P., Luceri C., Filippo C. D., et al. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis . 2005;591(1-2):237–246. doi: 10.1016/j.mrfmmm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Gan N., Jia L., Zheng L. A sandwich electrochemical immunosensor using magnetic DNA nanoprobes for carcinoembryonic antigen. International Journal of Molecular Sciences . 2011;12(11):7410–7423. doi: 10.3390/ijms12117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy M. J. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clinical Chemistry . 2001;47(4):624–630. doi: 10.1093/clinchem/47.4.624. [DOI] [PubMed] [Google Scholar]
- 12.Adan A., Kiraz Y., Baran Y. Cell proliferation and cytotoxicity assays. Current Pharmaceutical Biotechnology . 2016;17(14):1213–1221. doi: 10.2174/1389201017666160808160513. [DOI] [PubMed] [Google Scholar]
- 13.Bologna-Molina R., Mosqueda-Taylor A., Molina-Frechero N., Mori-Estevez A. D., Sánchez-Acuña G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Medicina Oral, Patologia Oral y Cirugia Bucal . 2013;18(2):e174–e179. doi: 10.4317/medoral.18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Joumaa M. M., Taleb R. I., Rizk S., Borjac J. M. Protective effect of Matricaria chamomilla extract against 1, 2-dimethylhydrazine-induced colorectal cancer in mice. Journal of Complementary and Integrative Medicine . 2020;17(3) doi: 10.1515/jcim-2019-0143. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y., Tanaka F., Yoshikawa Y., et al. PDGF-BB is a novel prognostic factor in colorectal cancer. Annals of Surgical Oncology . 2008;15(8):2129–2136. doi: 10.1245/s10434-008-9943-9. [DOI] [PubMed] [Google Scholar]
- 16.Olsen R. S., Dimberg J., Geffers R., Wågsäter D. Possible role and therapeutic target of PDGF-D signalling in colorectal cancer. Cancer Investigation . 2019;37(2):99–112. doi: 10.1080/07357907.2019.1576191. [DOI] [PubMed] [Google Scholar]
- 17.Pan H.-D., Peng Y. F., Xiao G., Gu J. High levels of serum platelet-derived growth factor-AA and human epidermal growth factor receptor-2 are predictors of colorectal cancer liver metastasis. World Journal of Gastroenterology . 2017;23(7):1233–1240. doi: 10.3748/wjg.v23.i7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roger A., Rubira N., Nogueiras C., Guspi R., Baltasar M., Cadahia A. Anaphylaxis caused by royal jelly. Allergologia et Immunopathologia . 1995;23(3):133–135. [PubMed] [Google Scholar]
- 19.Kimura Y. Antitumor and antimetastatic actions of various natural products. Studies in Natural Products Chemistry . 2008;34:35–76. doi: 10.1016/S1572-5995(08)80024-5. [DOI] [Google Scholar]
- 20.Premratanachai P., Chanchao C. Review of the anticancer activities of bee products. Asian Pacific Journal of Tropical Biomedicine . 2014;4(5):337–344. doi: 10.12980/APJTB.4.2014C1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocot J., Kiełczykowska M., Luchowska-Kocot D., Kurzepa J., Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: possible medical application. Oxidative Medicine and Cellular Longevity . 2018;2018:29. doi: 10.1155/2018/7074209.7074209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai T., Inoue R., Suzuki N., Nagashima T. Antioxidant properties of enzymatic hydrolysates from royal jelly. Journal of Medicinal Food . 2006;9(3):363–367. doi: 10.1089/jmf.2006.9.363. [DOI] [PubMed] [Google Scholar]
- 23.Miyata Y., Sakai H. Anti-cancer and protective effects of royal jelly for therapy-induced toxicities in malignancies. International Journal of Molecular Sciences . 2018;19(10):p. 3270. doi: 10.3390/ijms19103270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ubaid J. M., Kadhim M. J., Hameed I. H. Study of bioactive methanolic extract of Camponotus fellah using gas chromatography–mass spectrum. International Journal of Toxicological and Pharmacological Research . 2016;8(6):434–439. [Google Scholar]
- 25.Li Y., Liu C., Xiao D., et al. Trillium tschonoskii steroidal saponins suppress the growth of colorectal cancer cells in vitro and in vivo. Journal of Ethnopharmacology . 2015;168:136–145. doi: 10.1016/j.jep.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 26.Percie du Sert N., Hurst V., Ahluwalia A., et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Journal of Cerebral Blood Flow & Metabolism . 2020;40(9):1769–1777. doi: 10.1177/0271678X20943823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed A. A.-R., Galal A. A., Elewa Y. H. Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochemica . 2015;117(7):649–658. doi: 10.1016/j.acthis.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Guoxian Z., Zhenhong Z., Xiaoli L. The effects of vitamin E on reproduction performance in laying hens fed with different diets. China Feed . 2011;3 [Google Scholar]
- 29.Kaur J., Sanyal S. Diclofenac, a selective COX-2 inhibitor, inhibits DMH-induced colon tumorigenesis through suppression of MCP-1, MIP-1α and VEGF. Molecular Carcinogenesis . 2011;50(9):707–718. doi: 10.1002/mc.20736. [DOI] [PubMed] [Google Scholar]
- 30.Struck M. B., Andrutis K. A., Ramirez H. E., Battles A. H. Effect of a short-term fast on ketamine–xylazine anesthesia in rats. Journal of the American Association for Laboratory Animal Science . 2011;50(3):344–348. [PMC free article] [PubMed] [Google Scholar]
- 31.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry . 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Jia Y., Ma Z., Liu X., et al. Metformin prevents DMH-induced colorectal cancer in diabetic rats by reversing the Warburg effect. Cancer Medicine . 2015;4(11):1730–1741. doi: 10.1002/cam4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y.-J., Ketter R., Steudel W. I., Feiden W. Prognostic significance of the mitotic index using the mitosis marker anti–phosphohistone H3 in meningiomas. American Journal of Clinical Pathology . 2007;128(1):118–125. doi: 10.1309/HXUNAG34B3CEFDU8. [DOI] [PubMed] [Google Scholar]
- 34.Hosseini S. M., Hejazian L. B., Amani R., Siahchehreh Badeli N. Geraniol attenuates oxidative stress, bioaccumulation, serological and histopathological changes during aluminum chloride-hepatopancreatic toxicity in male Wistar rats. Environmental Science and Pollution Research . 2020;27(16):20076–20089. doi: 10.1007/s11356-020-08128-1. [DOI] [PubMed] [Google Scholar]
- 35.Musa M., Nasir N. F. M., Thirumulu K. P. Evaluation of royal jelly as an alternative to fetal bovine serum in cell culture using cell proliferation assays and live cell imaging. African Journal of Traditional, Complementary and Alternative Medicines . 2014;11(1):148–155. [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammadi Abandansari R., Parsian H., Kazerouni F., Porbagher R., Zabihi E., Rahimipour A. Effect of simultaneous treatment with royal jelly and doxorubicin on the survival of the prostate cancer cell line (pc 3): an in vitro study. International Journal of Cancer Management . 2018;11(4) doi: 10.5812/ijcm.13780. [DOI] [Google Scholar]
- 37.Nakaya M., Onda H., Sasaki K., Yukiyoshi A., Tachibana H., Yamada K. Effect of royal jelly on bisphenol A-induced proliferation of human breast cancer cells. Bioscience, Biotechnology, and Biochemistry . 2007;71(1):253–255. doi: 10.1271/bbb.60453. [DOI] [PubMed] [Google Scholar]
- 38.You M., Miao Z., Pan Y., Hu F. Trans-10-hydroxy-2-decenoic acid alleviates LPS-induced blood-brain barrier dysfunction by activating the AMPK/PI3K/AKT pathway. European Journal of Pharmacology . 2019;865, article 172736 doi: 10.1016/j.ejphar.2019.172736. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L., Chen J., Su J., et al. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. Journal of Agricultural and Food Chemistry . 2013;61(44):10604–10611. doi: 10.1021/jf403098y. [DOI] [PubMed] [Google Scholar]
- 40.Shapla U. M., Solayman M., Alam N., Khalil M. I., Gan S. H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: effects on bees and human health. Chemistry Central Journal . 2018;12(1):1–18. doi: 10.1186/s13065-018-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulbricht R. J., Northup S. J., Thomas J. A. A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Toxicological Sciences . 1984;4(5):843–853. doi: 10.1093/toxsci/4.5.843. [DOI] [PubMed] [Google Scholar]
- 42.Demirci M. A., Ipek Y., Gul F., Ozen T., Demirtas I. Extraction, isolation of heat-resistance phenolic compounds, antioxidant properties, characterization and purification of 5-hydroxymaltol from Turkish apple pulps. Food Chemistry . 2018;269:111–117. doi: 10.1016/j.foodchem.2018.06.147. [DOI] [PubMed] [Google Scholar]
- 43.Shiono H., Yagi Y., Chikayama Y., Miyazaki S., Nakamura I. Oxidative damage and phosphatidylserine expression of red blood cells in cattle experimentally infected with Theileria sergenti. Parasitology Research . 2003;89(3):228–234. doi: 10.1007/s00436-002-0742-0. [DOI] [PubMed] [Google Scholar]
- 44.Vaupel P. The role of hypoxia-induced factors in tumor progression. The Oncologist . 2004;9(S5):10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- 45.Gomollón F., Gisbert J. P. Anemia and inflammatory bowel diseases. World Journal of Gastroenterology: WJG . 2009;15(37):4659–4665. doi: 10.3748/wjg.15.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiala E. S. Investigations into the metabolism and mode of action of the colon carcinogens 1, 2-dimethylhydrazine and azoxymethane. Cancer . 1977;40(S5):2436–2445. doi: 10.1002/1097-0142(197711)40:5+<2436::AID-CNCR2820400908>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 47.Clemens M. R., Waller H. D. Lipid peroxidation in erythrocytes. Chemistry and Physics of Lipids . 1987;45(2-4):251–268. doi: 10.1016/0009-3084(87)90068-5. [DOI] [PubMed] [Google Scholar]
- 48.Gali-Muhtasib H., Ocker M., Kuester D., et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. Journal of Cellular and Molecular Medicine . 2008;12(1):330–342. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghadi F. E., Ghara A. R., Bhattacharyya S., Dhawan D. K. Selenium as a chemopreventive agent in experimentally induced colon carcinogenesis. World Journal of Gastrointestinal Oncology . 2009;1(1):74–81. doi: 10.4251/wjgo.v1.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghatreh-Samani M., Esmaeili N., Soleimani M., Asadi-Samani M., Ghatreh-Samani K., Shirzad H. Oxidative stress and age-related changes in T cells: is thalassemia a model of accelerated immune system aging? Central-European Journal of Immunology . 2016;41(1):116–124. doi: 10.5114/ceji.2015.56973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laoui D., van Overmeire E., Movahedi K., et al. Mononuclear phagocyte heterogeneity in cancer: different subsets and activation states reaching out at the tumor site. Immunobiology . 2011;216(11):1192–1202. doi: 10.1016/j.imbio.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Vieira-de-Abreu A., Campbell R. A., Weyrich A. S., Zimmerman G. A. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Seminars in Immunopathology . 2012;34(1):5–30. doi: 10.1007/s00281-011-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacob R. F., Mason R. P. Lipid peroxidation induces cholesterol domain formation in model membranes. Journal of Biological Chemistry . 2005;280(47):39380–39387. doi: 10.1074/jbc.M507587200. [DOI] [PubMed] [Google Scholar]
- 54.Southern J. T., Schiller C. M. Utilization of blood analyses to evaluate metabolic changes in control and 1,2-dimethylhydrazine-treated adult male Fischer rats. Cancer Letters . 1981;14(1):47–54. doi: 10.1016/0304-3835(81)90008-2. [DOI] [PubMed] [Google Scholar]
- 55.Arigesavan K., Sudhandiran G. Carvacrol exhibits anti-oxidant and anti-inflammatory effects against 1, 2-dimethyl hydrazine plus dextran sodium sulfate induced inflammation associated carcinogenicity in the colon of Fischer 344 rats. Biochemical and Biophysical Research Communications . 2015;461(2):314–320. doi: 10.1016/j.bbrc.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 56.Jisha N., Vysakh A., Vijeesh V., Latha M. S. Ethyl acetate fraction of Muntingia calabura L. exerts anti-colorectal cancer potential via regulating apoptotic and inflammatory pathways. Journal of Ethnopharmacology . 2020;261, article 113064 doi: 10.1016/j.jep.2020.113064. [DOI] [PubMed] [Google Scholar]
- 57.Saritas N., Yildiz K., Büyükipekci S., Coskun B. Effect of different levels of royal jelly on biochemical parameters of swimmers. African Journal of Biotechnology . 2011;10(52):10718–10723. doi: 10.5897/AJB11.1862. [DOI] [Google Scholar]
- 58.Abdel-Hafez S. M. N., Rifaai R. A., Abdelzaher W. Y. Possible protective effect of royal jelly against cyclophosphamide induced prostatic damage in male albino rats; a biochemical, histological and immuno-histo-chemical study. Biomedicine & Pharmacotherapy . 2017;90:15–23. doi: 10.1016/j.biopha.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 59.Wei W., Li R., Liu Q., et al. Amelioration of oxidative stress, inflammation and tumor promotion by tin oxide-sodium alginate-polyethylene glycol-allyl isothiocyanate nanocomposites on the 1, 2-dimethylhydrazine induced colon carcinogenesis in rats. Arabian Journal of Chemistry . 2021;14(8, article 103238) doi: 10.1016/j.arabjc.2021.103238. [DOI] [Google Scholar]
- 60.Amerizadeh F., Rezaei N., Rahmani F., et al. Crocin synergistically enhances the antiproliferative activity of 5-flurouracil through Wnt/PI3K pathway in a mouse model of colitis-associated colorectal cancer. Journal of Cellular Biochemistry . 2018;119(12):10250–10261. doi: 10.1002/jcb.27367. [DOI] [PubMed] [Google Scholar]
- 61.Lokeshkumar B., Sathishkumar V., Nandakumar N., Rengarajan T., Madankumar A., Balasubramanian M. P. Anti-oxidative effect of myrtenal in prevention and treatment of colon cancer induced by 1, 2-dimethyl hydrazine (DMH) in experimental animals. Biomolecules & Therapeutics . 2015;23(5):471–478. doi: 10.4062/biomolther.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.López-Mejía A., Ortega-Pérez L. G., Magaña-Rodríguez O. R., et al. Protective effect of Callistemon citrinus on oxidative stress in rats with 1, 2-dimethylhydrazine-induced colon cancer. Biomedicine & Pharmacotherapy . 2021;142, article 112070 doi: 10.1016/j.biopha.2021.112070. [DOI] [PubMed] [Google Scholar]
- 63.Costa N., Verediano T., Viana M., Vaz-Tostes M. Yacon (Smallanthus sonchifolius): effect on integrity of intestinal barrier, inflammatory response and oxidative stress in animal model of colon cancer (P06-052-19) Current Developments in Nutrition . 2019;3(Supplement_1) doi: 10.1093/cdn/nzz031.P06-052-19. [DOI] [Google Scholar]
- 64.Gulmez C., Atakisi O. Kumiss supplementation reduces oxidative stress and activates sirtuin deacetylases by regulating antioxidant system. Nutrition and Cancer . 2020;72(3):495–503. doi: 10.1080/01635581.2019.1635628. [DOI] [PubMed] [Google Scholar]
- 65.Milani E., Nikfar S., Khorasani R., Zamani M. J., Abdollahi M. Reduction of diabetes-induced oxidative stress by phosphodiesterase inhibitors in rats. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology . 2005;140(2):251–255. doi: 10.1016/j.cca.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Wielsøe M., Long M., Ghisari M., Bonefeld-Jørgensen E. C. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers In Vitro. Chemosphere . 2015;129:239–245. doi: 10.1016/j.chemosphere.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 67.Hamiza O. O., Rehman M. U., Tahir M., et al. Amelioration of 1, 2 dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in Wistar rats. Asian Pacific Journal of Cancer Prevention . 2012;13(9):4393–4402. doi: 10.7314/APJCP.2012.13.9.4393. [DOI] [PubMed] [Google Scholar]
- 68.Krenn V., Morawietz L., Häupl T., Neidel J., Petersen I., König A. Grading of chronic synovitis—a histopathological grading system for molecular and diagnostic pathology. Pathology-Research and Practice . 2002;198(5):317–325. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 69.Ghassami E., Varshosaz J., Minaiyan M., Nasirikenari M., Hoseini S. M. Biodistribution, safety and organ toxicity of docetaxel-loaded in HER-2 aptamer conjugated Ecoflex® nanoparticles in a mouse xenograft model of ovarian cancer. Recent Patents on Nanotechnology . 2019;13(1):49–58. doi: 10.2174/1872210513666181128162403. [DOI] [PubMed] [Google Scholar]
- 70.Abdel-Rasol M. A., El-Beih N. M., Yahya S. S., El-Sayed W. M. The antitumor activity of ginger against colorectal cancer induced by dimethylhydrazine in rats. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) . 2022;22(8):1601–1610. doi: 10.2174/1871520621666210903112813. [DOI] [PubMed] [Google Scholar]
- 71.Nagai T., Sakai M., Inoue R., Inoue H., Suzuki N. Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chemistry . 2001;75(2):237–240. doi: 10.1016/S0308-8146(01)00193-5. [DOI] [Google Scholar]
- 72.Karthikkumar V., Sivagami G., Viswanathan P., Nalini N. Rosmarinic acid inhibits DMH-induced cell proliferation in experimental rats. Journal of Basic and Clinical Physiology and Pharmacology . 2015;26(2):185–200. doi: 10.1515/jbcpp-2014-0044. [DOI] [PubMed] [Google Scholar]
- 73.Hao C., Zhang G., Zhang L. Serum CEA levels in 49 different types of cancer and noncancer diseases. Progress in Molecular Biology and Translational Science . 2019;162:213–227. doi: 10.1016/bs.pmbts.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 74.Okda T., Abd-Elghaffar S., Katary M., Abd-Alhaseeb M. Chemopreventive and anticancer activities of indomethacin and vitamin D combination on colorectal cancer induced by 1, 2-dimethylhydrazine in rats. Biomedical Reports . 2021;14(2):1–1. doi: 10.3892/br.2020.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, data supporting the conclusion of our study are accessible by corresponding author.


