Abstract
A 54-year-old Japanese woman was admitted to our ward because of recurrent chest pain at rest for 2 months. She had been treated with nivolumab, an immune checkpoint inhibitor for inoperable advanced hypopharyngeal cancer for 21 months. She had no chest pain after cessation of nivolumab treatment. Cardiac catheterization confirmed the presence of vasospastic angina. Benidipine 8 mg was started, and she had no chest pain even after resuming therapy with nivolumab. Vasospastic angina is an adverse effect of nivolumab.
Keywords: chest pain, cancer, oncology, adverse event
Introduction
The development of immune checkpoint inhibitors (ICIs) targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death-1 (PD-1) has significantly improved the treatment of a variety of cancers. These new drugs aid the immune system in controlling the division of neoplastic cells. The immunologic tolerance can thus be altered, increasing the risk of reactions mediated by self-directed antigens. These reactions are termed immune-related adverse events (irAEs) (1). Cardiovascular irAEs are relatively rare and have a variety of clinical presentations. Myocarditis is well known because it is potentially life-threatening and can range from subclinical to fulminant (2).
Recently, acute coronary syndrome (ACS) or myocardial infarction has been reported from the early to late phases of starting treatment with ICIs (3,4). However, there has been only one report of vasospastic angina in the early phase of ICI initiation (5).
We herein report a case of vasospastic angina that occurred 1.5 years after the initiation of nivolumab, a PD-1 inhibitor.
Case Report
A 54-year-old Japanese woman was admitted to our ward because of chest pain at rest. She had been diagnosed with hypopharyngeal cancer (squamous cell carcinoma, cT4aN2bM0, stage IVa), and chemoradiotherapy had been started 29 months earlier by an otorhinolaryngologist in our hospital for inoperable advanced hypopharyngeal cancer. Chemotherapy was initiated with TPF [docetaxel intravenous injection (i.v.) 75 mg/m2 on day 1; cisplatin (i.v.) 75 mg/m2 on day 1; 5-fluorouracil 24-h continuous infusion by pump 750 mg/m2 on days 1-5]. However, after completion of the second cycle, chemotherapy ceased because of leukocytopenia and lack of reduction in tumor size. The therapy was then changed to PF [cisplatin (i.v.) 100 mg/m2 on day 1, 5-fluorouracil 24 h continuous infusion by pump 750 mg/m2 on days 1 to 5] with radiation therapy. PF was also ineffective and was thus changed to cetuximab, an anti-epidermal growth factor receptor (EGFR) monoclonal antibody (400 mg/m2, (i.v.) (first time) and 250 mg/m2, (i.v.) (starting the second time) for eight cycles] with radiation therapy (70 Gy in total).
However, these therapies were ineffective for her tumor, and the treatment was changed to the ICI nivolumab (240 mg/every 2 weeks) 20 months before admission. After starting nivolumab, the tumor gradually decreased, and magnetic resonance imaging (MRI) did not detect the tumor or metastasis 12 months after the initiation of therapy with nivolumab. She had been treated with amlodipine (7.5 mg/day) for hypertension. She had no allergic diseases, dyslipidemia, and diabetes mellitus. Her father had died of pulmonary emphysema at 57 years old, and her older sister had hypertension. She had been smoking since she was 20 years old, but she had stopped on being diagnosed with this cancer at 51 years old. She had experienced menopause at 50 years old.
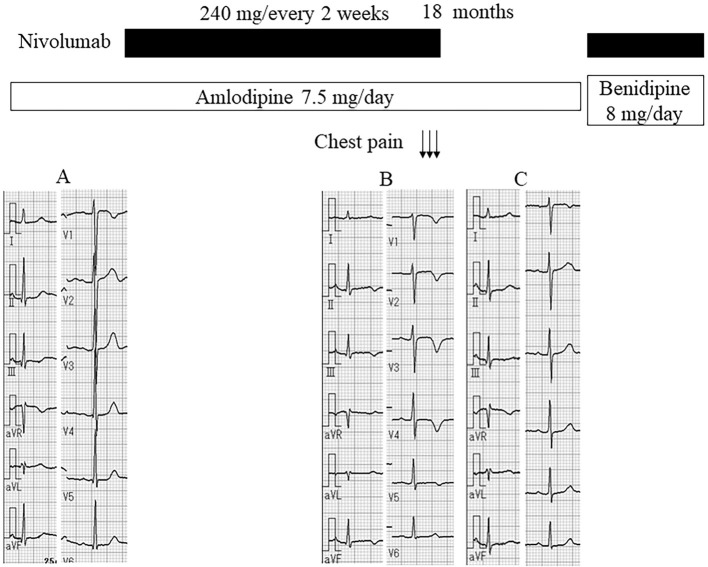
She first experienced chest pain at rest after getting up in the morning 2 months earlier with 3 repeated episodes that lasted for 5-10 minutes each. Although electrocardiography (ECG) showed normal findings before starting nivolumab treatment (Fig. 1A), a negative T wave in the V2-5 leads was noted (Fig. 1B), and she was referred to our department.
Figure 1.
Time course before and after starting nivolumab treatment. A: Electrocardiography (ECG) before starting nivolumab treatment. B: ECG after chest pain attacks. C: ECG after stopping nivolumab treatment.
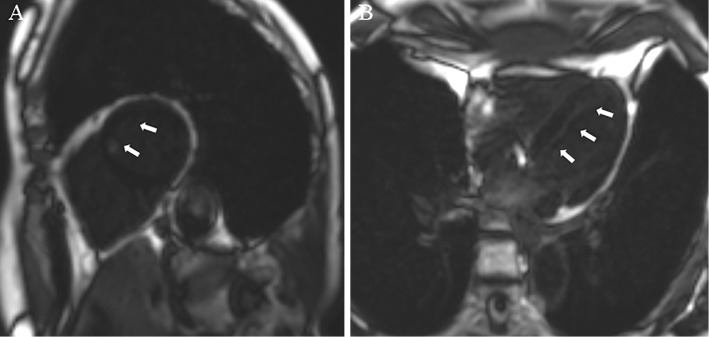
She had no chest pain after cessation of nivolumab treatment, and the ECG findings were normalized (Fig. 1C). Chest radiography was normal, and echocardiography revealed a normal left ventricular function. Gadolinium-enhanced cardiac MRI revealed late gadolinium enhancement at the endocardial region in the anteroseptal wall of the left ventricle (Fig. 2) with a normal intensity on T2-weighted imaging.
Figure 2.
Cardiac magnetic resonance imaging. A: Gadolinium-enhanced cardiac magnetic resonance imaging (arrows: late gadolinium enhancement) (sagittal section). B: Gadolinium-enhanced cardiac magnetic resonance imaging (arrows: late gadolinium enhancement) (transverse section).
The findings of a physical examination were as follows: height 154 cm, body weight 45.3 kg, blood pressure 98/64 mmHg, pulse rate 80/min and regular, and body temperature 36.7℃. Laboratory data showed normal findings, including N-terminal pro brain natriuretic peptide (36 pg/mL), high-sensitive troponin T (0.004 ng/mL), serum glucose and lipid profile (fasting plasma glucose, 92 mg/dL; hemoglobin A1c, 5.5%; low-density lipoprotein cholesterol, 125 mg/dL; high-density lipoprotein cholesterol, 47 mg/dL; triglyceride, 77 mg/dL).
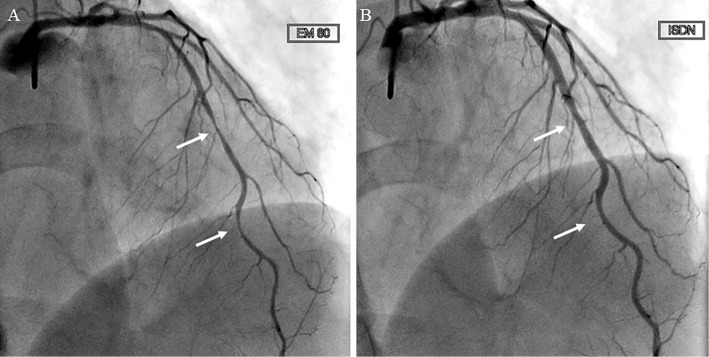
After admission, cardiac catheterization was performed, and coronary vasospasm was provoked by ergonovine (80 μg) in the anterior descending branch of the left coronary artery (Fig. 3A), which was resolved by intracoronary isosorbide dinitrate administration, although there was 25% stenosis in segments 7 and 8 of the left anterior descending artery (LAD) (Fig. 3B). Benidipine 8 mg was started, and she had no chest pain even after resuming nivolumab treatment.
Figure 3.
Coronary angiography. A: Intracoronary injection of ergonovine provoked 90% stenosis in the anterior descending branch of the left coronary artery (arrows). B: After intracoronary injection of isosorbide dinitrate, coronary angiography showed 25% stenosis in segments 7 and 8 of the anterior descending branch of the left coronary artery (arrows).
Discussion
Of the 5 patients who received nivolumab treatment (after being injected 2-16 times) for ACS confirmed by coronary angiography (CAG), 3 of them died. Therefore, ACS was deemed to be related to ICI usage, and it is sometimes difficult to differentiate from myocarditis without CAG.
There has only been one reported case of vasospastic angina related to nivolumab (5). The patient was a 57-year-old Japanese man who was admitted to the hospital with severe chest pain after undergoing 2 cycles of nivolumab for the treatment of renal cell carcinoma with lung and bone metastases (5).
In our report, our patient had chest pain at rest 18 months after the initiation of treatment with nivolumab. She had never experienced chest pain before and had quit smoking after the diagnosis of cancer. Furthermore, the chest pain disappeared after cessation of nivolumab. Therefore, we concluded that her vasospasm was related to nivolumab treatment. Our patient developed vasospastic angina later than in the previously reported case, wherein it was observed after starting nivolumab treatment. This may be related to the prescription of amlodipine, a calcium channel blocker for hypertension and/or milder organic atherosclerotic lesions in the coronary arteries, in the present patient.
Although the mechanisms of vasospasm are unknown, the PD-1/programmed cell death pathway downregulates the proatherogenic T cell response, and its inhibition increases atherosclerotic plaques associated with an increase in T cells and macrophages (6,7). Thus, these mechanisms may also be related to vasospasm of mild atherosclerosis due to nivolumab treatment.
Furthermore, before the nivolumab treatment, the present patient had received several types of chemotherapies, including 5-fluorourcil and cisplatin, which can induce endothelial injury (8). Thus, vasospasm by nivolumab may be related to such chemotherapy-induced vascular damage.
Further studies are needed to elucidate the precise mechanism as well as the incidence rate, inducing factors, and patients’ characteristics of vasospasm related to ICIs.
In conclusion, we should consider vasospastic angina and other cardiovascular irAEs in patients with chest pain after initiating therapy with ICIs. Recommended investigations include CAG, including vasospasm provocation test, as well as cardiac MRI.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158-168, 2018. [DOI] [PubMed] [Google Scholar]
- 2. Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71: 1755-1764, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cautela J, Rouby F, Salem JE, et al. Acute coronary syndrome with immune checkpoint inhibitors: a proof-of-concept case and pharmacovigilance analysis of a life-threatening adverse event. Can J Cardiol 36: 476-481, 2020. [DOI] [PubMed] [Google Scholar]
- 4. Drobni ZD, Alvi RM, Taron J, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 142: 2299-2311, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Otsu K, Tajiri K, Sakai S, Ieda M. Vasospastic angina following immune checkpoint blockade. Eur Heart J 41: 1702, 2020. [DOI] [PubMed] [Google Scholar]
- 6. Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest 117: 2974-2982, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bu DX, Tarrio M, Maganto-Garcia E, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol 31: 1100-1107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. ; ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 37: 2768-2801, 2016. [DOI] [PubMed] [Google Scholar]