Abstract
Peritoneal lymphomatosis (PL) is a rare presentation of malignant lymphoma cases, many of which are diagnosed as diffuse large B-cell lymphoma (DLBCL) and characterized by aggressive clinical courses. We herein report a 63-year-old woman presenting with the rapid development of abdominal distention due to bulky peritoneal tumors. The pathological evaluation of a needle biopsy sample, combined with flow cytometry, yielded the diagnosis of DLBCL. Prompt chemotherapeutic intervention resulted in favorable disease control and sustained complete remission. It is necessary to diagnose cases of DLBCL presenting as PL early to ensure prompt treatment and prevent mortality.
Keywords: peritoneal lymphomatosis, diffuse large B-cell lymphoma, EPOCH-R
Introduction
Peritoneal lymphomatosis (PL) is a rare presentation of malignant lymphoma characterized by diffuse peritoneal lesions and is frequently accompanied by ascites and mesenteric lesions (1). Lymphoma cases with PL have been reported sporadically in literature, and most of them were diagnosed as aggressive B-cell lymphoma, specifically diffuse large B-cell lymphoma (DLBCL) or Burkitt's lymphoma.
It is necessary to diagnose and treat PL promptly, as delaying management can lead to death. A prompt diagnosis and chemotherapeutic intervention can result in long-term remission and saving of lives. However, the clinicopathological characteristics of PL are yet to be elucidated because of its rarity. Furthermore, appropriate management is challenging, as the clinical features of PL mimic the findings of peritoneal dissemination of carcinoma.
We herein report a case of DLBCL that presented as PL with an extremely aggressive course.
Case Report
A 63-year-old woman who had suffered from malaise, anorexia, profuse sweating, and abdominal distension for 2 weeks presented to our institution because of an intra-abdominal bulky mass suggestive of malignant lymphoma. She was in good health without any medication or remarkable medical history.
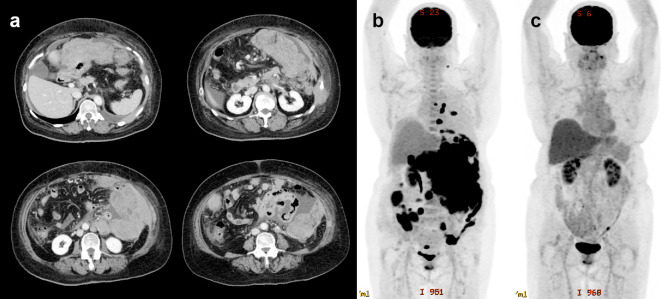
At presentation, she was afebrile but had a highly distended abdomen. A complete blood test revealed mild anemia (hemoglobin 10.8 g/dL), thrombocytosis (530×109/L), and neutrophilia (white blood cell count of 12.5×109/L with 92% neutrophils, 2% lymphocytes, and 3% monocytes). A biochemistry analysis revealed the marked elevation of lactic dehydrogenase (LDH, 3,018 U/L) and soluble interleukin-2 receptor (7,867 U/mL). In addition, the C-reactive protein level was elevated (8.0 mg/dL). No renal dysfunction or electrolyte disturbance was observed. Bone marrow aspiration and a biopsy revealed no infiltration of abnormal cells. Computed tomography (CT) and positron emission tomography (PET) revealed bulky tumors in the peritoneum that extended towards the abdominal and thoracic walls, with sporadically disseminated nodular lesions, ascites, and pleural effusion (Fig. 1a, b).
Figure 1.
Radiological findings. (a) CT showing bulky tumors in the peritoneum that extended towards the abdominal and chest walls, with ascites and pleural effusion. (b) PET/CT showing the accumulation of fluorodeoxyglucose in the peritoneal tumor and sporadically disseminated nodules. (c) PET/CT after the third course of EPOCH-R confirmed a complete metabolic response.
A needle biopsy of the chest wall lesion was promptly performed, and flow cytometry detected the B-cell population (CD19+, CD20+) with a light chain restriction (κ>>λ), highly suggestive of mature B-cell neoplasm. Therefore, we presumed the diagnosis to be aggressive B-cell lymphoma and immediately started chemotherapy with the EPOCH-R regimen (continuous administration of etoposide at 50 mg/m2/day, doxorubicin at 10 mg/m2/day, and vincristine at 0.4 mg/m2/day for 96 hours on days 1-4; cyclophosphamide at 750 mg/m2 on day 5; prednisone at 100 mg on days 1-5; rituximab at 375 mg/m2 intravenous on day 6), which resulted in the rapid improvement of symptoms and general status. Neither tumor lysis syndrome nor disseminated intravascular coagulation was observed.
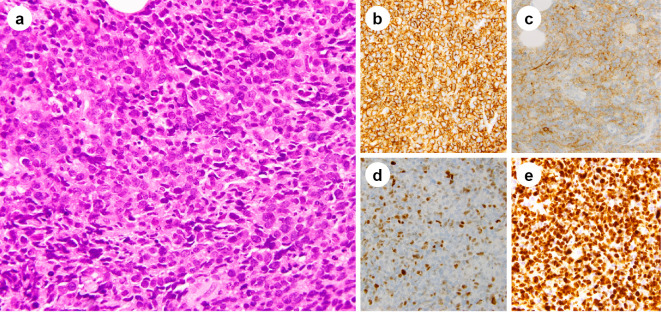
The pathological evaluation of the needle biopsy sample revealed diffuse proliferation of abnormally large cells with nuclear swelling and the concentration of nuclear chromatin, which had invaded between the muscular tissues (Fig. 2a). The aberrant cells were CD20+, CD79a+, CD3-, CD5-, bcl2+, bcl6+, MUM1+, Igκ+, and Igλ- (Fig. 2b). They were weakly positive for CD10 and c-Myc (Fig. 2c, d). Epstein-Barr virus-encoded ribonucleic acid (RNA)-positive cells were not detected by in situ hybridization. The Ki-67 labelling index was estimated to be 80-90% (Fig. 2e). Thus, a histological diagnosis of DLBCL was established.
Figure 2.
Pathological findings. (a) Hematoxylin and Eosin staining showing diffuse proliferation of abnormally large cells with nuclear swelling and concentration of nuclear chromatin. Immunohistochemistry showing that the abnormally large cells are positive for CD20 (b), and weakly positive for CD10 (c) and Myc (d). The Ki-67 labelling index is estimated to be 80-90% (e).
A cytogenetic analysis with G-band karyotyping could not be performed due to the failure to obtain cells in the mitotic phase. A fluorescence in situ hybridization analysis demonstrated no positive signals of IgH/MYC fusion or MYC amplification. The lesion was subclassified into the germinal center B-cell (GCB) subtype (2). The clinical stage was evaluated as IV-B (Lugano classification) (3), and the prognostic risk was classified as high according to both the international prognostic index (IPI) (4) and the central nervous system IPI (CNS-IPI) (5).
Two weeks after the initiation of the treatment, a marked decrease in lymphoma lesions was demonstrated on CT. A complete metabolic response was confirmed using PET/CT after the third course of EPOCH-R (Fig. 1c), and a total of six courses of EPOCH-R were completed as scheduled without any issues. Since then, the patient has not demonstrated disease relapse for more than two years without any additional therapy.
Discussion
PL is a rare presentation of lymphoma defined as the intraperitoneal spread of lymphoma lesions (1). It is radiologically characterized by diffuse peritoneal thickening and masses, and most cases are accompanied by ascites (1,6). Bowel wall thickening, retroperitoneal lymphadenopathy, and hepatosplenomegaly are also frequently observed (6). It can be difficult to diagnose PL because it can mimic other conditions involving the peritoneum, such as peritoneal carcinomatosis and tuberculous peritonitis (7). A prompt diagnosis is necessary, as PL could be curable with appropriate therapeutic intervention; however, a delay in treatment can result in death. In the present case, although the patient presented with life-threatening abdominal distention that developed abruptly within a month, prompt identification of lymphoma cells in mature B-cell lineage by flow cytometry led to the immediate initiation of effective chemotherapy, due to which the patient's life was saved.
PL can be seen in many subtypes of non-Hodgkin lymphoma, which seems to converge with DLBCL in most cases (6). Cases of DLBCL with PL presentation that have been recently reported in the literature with a detailed description of clinical course are summarized in Table (8-14). The patients were middle-aged and older, with no marked difference in proportions between the sexes. Most of them experienced general symptoms in addition to abdominal symptoms that developed within two months. Symptoms were accompanied by pleural effusion and ascites and highly elevated levels of serum LDH. The clinical stage was evaluated as IV in most cases, and the prognostic risk was classified as high based on the IPI and CNS-IPI. No specific tendencies in pathological findings were noted due to the limited descriptions. Cytogenetic abnormalities, including MYC translocation, could not be established. The present case was subclassified into the GCB subtype with MUM1 positivity; similarly, it was considered double-expressor lymphoma due to bcl2 and Myc positivity on immunohistochemistry rather than double-hit lymphoma with MYC translocation. We noted no cases with a similar phenotype among the previous studies shown in Table. Regarding the clinical outcome, although there seem to be high rates of premature mortality, prompt and appropriate therapy can result in a long-term progression-free survival in some cases. In the present case, we treated the patient with the EPOCH-R regimen, considering its superior clinical outcome over the conventional R-CHOP regimen for DLBCL cases with high IPI scores (15), which resulted in long-term disease-free remission. In addition, CNS relapses have not been documented despite the high risk according to a CNS-IPI evaluation in the previous and present cases; however, the optimal therapeutic strategy, including prophylaxis for CNS relapse, for such cases has not yet been established.
Table.
Recently Reported Cases of DLBCL That Presented as Peritoneal Lymphomatosis.
| Reference | Age/ Sex |
Symptoms | Duration of symptom | Biopsy site | Immunophenotype | Ki-67 index | Lesion sites other than peritoneum | Serum LDH at diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 74/M | Cachexy, weight loss, profuse sweating | 2 months | Peritoneum | CD20+, CD79a+, Pax5+, bcl2+, bcl6+, CD10+, MUM1+ | About 70% | Liver, ascites, pleural effusion | Elevated | Mini-CHOP | Premature death |
| 9 | 40/F | Abdominal pain, nausea, vomiting, body weight loss | 8 months | Duodenum | CD20+, CD79a+, bcl2-, bcl6-, CD10-, MUM1-, | 60-70% | duodenum | 318 U/L | Chemotherapy* | Alive without disease, 15 months after the diagnosis |
| 10 | 62/M | Appetite loss, abdominal distension | 1 month | Omentum | CD20+, bcl6+, EBV-* | Not described | Not described | 746 U/L | R-CHOP ×6 | complete metabolic response |
| 11 | 45/F | Abdominal distention | 1 month | Omentum | CD20+, CD10+, bcl2+, bcl6+, Myc+ | 80% | Pleural effusion, ascites, ovary, pleural effusion, chest wall | Not described | Not described | Not described |
| 12 | 59/M | Abdominal distention, dyspepsia, dyspnoea | Not described | Ascites | CD20+, CD10+, bcl6+, Pax5+, MUM1-, EBER- | Not described | Ascites, pleural effusion, lymph nodes | 1,223 U/L | R-CHOP ×6 | Alive without disease, >1 year after the diagnosis |
| 13 | 57/F | General weakness, malaise, abdominal distension, nausea, vomiting, oedema | 2 months | Peritoneum** | CD20+ | Not described | pleural effusion, ascites | 3,194 U/L | None | Death before treatment |
| 14 | 81/M | Abdominal pain, appetite loss, general weakness | A few weeks | Ascites | CD20+, CD79a+ | 80% | Ascites | 1,866 U/L | R-CVP | Premature death |
| This case | 63/F | Malaise, anorexia, profuse sweating, abdominal distension | 2 weeks | Chest wall | CD20+, CD79a+, bcl2+, bcl6+, MUM1+, CD10+(weak), Myc+(weak), EBER- | 80-90% | Pleural effusion, ascites, chest wall | 3,018 U/L | EPOCH-R ×6 | Alive without disease, >2 years after the diagnosis |
*Detailed information is not available
**Diagnosed postmortem by autopsy
CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisolone; EBER: Epstein-Barr virus-encoded ribonucleic acid; EBV: Epstein-Barr virus; EPOCH-R: etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone, and rituximab; LDH: lactic dehydrogenase; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone; R-CVP: rituximab, cyclophosphamide, vincristine, and prednisolone
In summary, we encountered a case of DLBCL masquerading as PL with a highly aggressive presentation. A prompt diagnosis and immediate chemotherapy resulted in long-term remission and the patient's life being saved. The further accumulation of clinical experience is necessary to clarify the clinicopathological features of DLBCL cases presenting as PL and to establish an optimal approach to treatment.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Cabral FC, Krajewski KM, Kim KW, Ramaiya NH, Jagannathan JP. Peritoneal lymphomatosis: CT and PET/CT findings and how to differentiate between carcinomatosis and sarcomatosis. Cancer Imaging 13: 162-170, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103: 275-282, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32: 3059-3068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 329: 987-994, 1993. [DOI] [PubMed] [Google Scholar]
- 5. Schmitz N, Zeynalova S, Nickelsen M, et al. CNS international prognostic index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 34: 3150-3156, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Karaosmanoglu D, Karcaaltincaba M, Oguz B, Akata D, Ozmen M, Akhan O. CT findings of lymphoma with peritoneal, omental and mesenteric involvement: peritoneal lymphomatosis. Eur J Radiol 71: 313-317, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Yoo E, Kim JH, Kim MJ, et al. Greater and lesser omenta: normal anatomy and pathologic processes. Radiographics 27: 707-720, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Chic Acevedo C, Ruiz Molina I, Contreras De Miguel E, Solis Garcia E. Peritoneal lymphomatosis. A case report. Hematol Transfus Cell Ther. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaarour M, Busack C, Munker R. Obstructing duodenal diffuse large B-cell lymphoma with peritoneal lymphomatosis with exceptional response to R-CHOP. Cureus 11: e4621, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim HB, Hong R, Na YS, Choi WY, Park SG, Lee HJ. Isolated peritoneal lymphomatosis defined as post-transplant lymphoproliferative disorder after a liver transplant: a case report. World J Clin Cases 7: 4299-4306, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Senthil R, Gangadharan VP, Nair Visakh AR, Mahadevan P, Pratap T. Peritoneal lymphomatosis mimicking peritoneal carcinomatosis from ovarian malignancy on F-18 fluorodeoxyglucose positron emission tomography/computed tomography. Indian J Nucl Med 34: 147-149, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi WY, Kim JH, Choi SJ, et al. Peritoneal lymphomatosis confused with peritoneal carcinomatosis due to the previous history of gastric cancer: a case report. Clin Imaging 40: 837-839, 2016. [DOI] [PubMed] [Google Scholar]
- 13. Curakova E, Genadieva-Dimitrova M, Misevski J, et al. NonHodgkin's lymphoma with peritoneal localization. Case Rep Gastrointest Med 2014: 723473, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim YG, Baek JY, Kim SY, et al. Peritoneal lymphomatosis confounded by prior history of colon cancer: a case report. BMC Cancer 11: 276, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: clinical outcomes of the phase III intergroup trial alliance/CALGB 50303. J Clin Oncol 37: 1790-1799, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]