Abstract
Phospholamban p.Arg14del is reported to cause hereditary cardiomyopathy with malignant ventricular tachycardia (VT) and advanced heart failure. However, the clinical courses of Japanese cardiomyopathy patients with phospholamban p.Arg14del remain uncharacterized. We identified five patients with this variant. All patients were diagnosed with dilated cardiomyopathy (DCM), developed end-stage heart failure and experienced VT requiring implantable cardioverter defibrillator discharge. Four patients survived after implantation of a left ventricular assist device (LVAD), while one patient who refused LVAD implantation died of heart failure. Based on the severe course of the disease, we propose genetic screening for phospholamban p.Arg14del in DCM patients.
Keywords: phospholamban, dilated cardiomyopathy, genetic testing, heart failure, ventricular tachycardia
Introduction
PLN, which encodes phospholamban, is a gene responsible for inherited cardiomyopathy (1). The PLN p.Arg14del (R14del) is a high-penetration pathogenic variant that exhibits phenotypes of dilated cardiomyopathy (DCM) or arrhythmogenic right ventricular cardiomyopathy (ARVC) (2). Compared to DCM patients without R14del, R14del DCM patients show a higher prevalence of appropriate implantable cardioverter-defibrillator (ICD) discharge, cardiac transplantation, and a family history of sudden cardiac death (SCD) at 50 years old (2). According to a cohort from the Netherlands, which has the largest number of R14del carriers, R14del was identified in 12% ARVC and 15% DCM cases, all of whom were heterozygous (3), indicating that this variant is autosomal dominant. Based on the bias in the presence of carriers, R14del is suggested to be a founder mutation originating in the northern part of the Netherlands (4).
Because of its poor prognosis and high prevalence, a research network including many researchers and clinicians has been established to elucidate the genotype-phenotype relationship in R14del cardiomyopathy and develop a disease-specific treatment in Europe and the United States (5). However, R14del is poorly recognized in Japan, and the clinical features of this variant in the Asian ethnicity have not been reported.
In the present study, we searched for cardiomyopathy patients with R14del in a Japanese cardiovascular genome cohort and identified five with this variant.
Case Report
Methods
To identify cardiomyopathy patients with PLN R14del variants, we analyzed the genomes of 396 patients with intrinsic cardiomyopathy at our hospital between 2010 and 2018 (including 211 DCM and 8 ARVC cases). Written informed consent was obtained from all patients before enrollment. This study was approved by the Institutional Review Board of Osaka University (accession number: 680).
Genetic analyses were performed as previously described (6). In brief, genomic DNA was extracted from the peripheral blood, followed by whole-exome sequencing. We searched the sequencing results for rare pathogenic variants in 57 cardiomyopathy-related genes that were listed as causative genes for cardiomyopathy (7,8) (Table 1). Rare pathogenic variants were identified by applying the following criteria: (i) exonic variants excluding synonymous variants or splice-site variants; (ii) variants included in the gene list; (iii) variants with a minor allele frequency <0.1% in multiple variation databases; (iv) variants listed as disease-causing mutations or likely disease-causing mutations in the Human Gene Mutation Database (2018.1), or pathogenic mutations or likely pathogenic mutations in ClinVar (clinvar_20170905).
Table 1.
List of Cardiomyopathy-related Genes.
| ABCC9 | ACTC1 | ACTN2 | ANKRD1 | BAG3 | CACNA1C | CRYAB | CSRP3 | DES | DMD | |||||||||
| DSC2 | DSG2 | DSP | EMD | EYA4 | FHL1 | FHL2 | FKTN | FLNC | GLA | |||||||||
| ILK | JPH2 | JUP | LAMA4 | LAMP2 | LDB3 | LMNA | MYBPC3 | MYH6 | MYH7 | |||||||||
| MYL2 | MYL3 | MYLK3 | MYPN | NEXN | PKP2 | PLN | PRKAG2 | PSEN1 | PSEN2 | |||||||||
| PTPN11 | RAF1 | RBM20 | RIT1 | RYR2 | SCN5A | SGCB | SGCD | TCAP | TMEM43 | |||||||||
| TNNC1 | TNNI3 | TNNT2 | TPM1 | TTN | TTR | VCL |
Results
We identified five patients with the R14del variant. The procedure of pathogenic variant filtering and the list of the rare variants identified in these patients are shown in Tables 2 and 3, respectively. Since no other known cardiomyopathy-causing variant was identified, R14del was considered the causative variant in each case.
Table 2.
The Procedure of Filtering Rare Pathogenic Variants Causing Cardiomyopathy from 5 Patients with R14del.
| Filtering criteria | Pt. 1 | Pt. 2 | Pt. 3 | Pt. 4 | Pt. 5 |
|---|---|---|---|---|---|
| (i) Exonic variants excluding synonymous ones or splice-site variants | 14,972 | 14,794 | 15,546 | 15,247 | 15,276 |
| (ii) Variants included in 57 genes in Table 1 | 127 | 123 | 127 | 104 | 116 |
| (iii) Variants with a minor allele frequency <0.1% in multiple variation databases | 3 | 2 | 2 | 4 | 4 |
| (iv) Variants listed as DM/DM? in HGMD or P/LP in ClinVar | 1 | 1 | 1 | 1 | 1 |
The number of variants remained after each filtering criteria is shown. We used the Human Genome Variation Database version 2.3 (HGVD), the Tohoku Medical Megabank Organization 3.5kJPNv2 (Tommo) and genome aggregation database v2.1.1 (gnomAD) as variation databases in filtering criteria (iii).
DM: disease causing mutation, DM?: likely disease causing mutation, P: pathogenic, LP: likely pathogenic
Table 3.
List of Rare Variants on Cardiomyopathy-related Genes.
| Patient ID | Position (hg19) |
Ref | Alt | Zygosity | Gene | Consequence | CADD phred | HGVD frequency | Tommo frequency | gnomAD frequency | HGMD class | ClinVar Clinical significance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt. 1 | 6:118880120 | AAG | - | Heterozygous | PLN | p.R14del | . | . | . | 7.1E-6 | DM | Pathogenic |
| Pt. 1 | 12:33030874 | C | T | Heterozygous | PKP2 | p.G314R | 23.8 | . | . | . | . | |
| Pt. 1 | 14:23865557 | G | A | Heterozygous | MYH6 | p.R789C | 16.42 | . | . | 7.1E-6 | . | |
| Pt. 2 | 2:179399484 | G | C | Heterozygous | TTN | p.S24888C | 16.29 | . | 0.0006 | . | . | |
| Pt. 2 | 6:118880120 | AAG | - | Heterozygous | PLN | p.R14del | . | . | . | 7.1E-6 | DM | Pathogenic |
| Pt. 3 | 2:179487494 | G | A | Heterozygous | TTN | p.P5874L | 23 | . | . | . | . | |
| Pt. 3 | 6:118880120 | AAG | - | Heterozygous | PLN | p.R14del | . | . | . | 7.1E-6 | DM | Pathogenic |
| Pt. 4 | 2:179497119 | G | T | Heterozygous | TTN | p.T5436N | 13.54 | 0.0004 | 0.0006 | 7.1E-6 | . | |
| Pt. 4 | 6:118880120 | AAG | - | Heterozygous | PLN | p.R14del | . | . | . | 7.1E-6 | DM | Pathogenic |
| Pt. 4 | 7:151273505 | C | T | Heterozygous | PRKAG2 | p.G59S | 26.3 | . | . | . | . | |
| Pt. 4 | 10:112541500 | C | T | Heterozygous | RBM20 | p.A378V | 13.97 | . | . | . | . | |
| Pt. 5 | 2:179469726 | C | T | Heterozygous | TTN | p.V8995I | 19.17 | . | . | 7.5E-5 | . | Uncertain significance |
| Pt. 5 | 2:179636100 | A | G | Heterozygous | TTN | p.W2606R | 21.6 | . | . | . | . | |
| Pt. 5 | 6:118880120 | G | C | Heterozygous | FKTN | p.E456D | 13.57 | . | . | . | . | Uncertain significance |
| Pt. 5 | 9:108397527 | AAG | - | Heterozygous | PLN | p.R14del | . | . | . | 7.1E-6 | DM | Pathogenic |
Variants remained after filtering criteria (iii) in Table 2 are shown with their annotation.
CADD phred score was obtained using CADD v1.3.
DM: disease causing mutation
Case Series
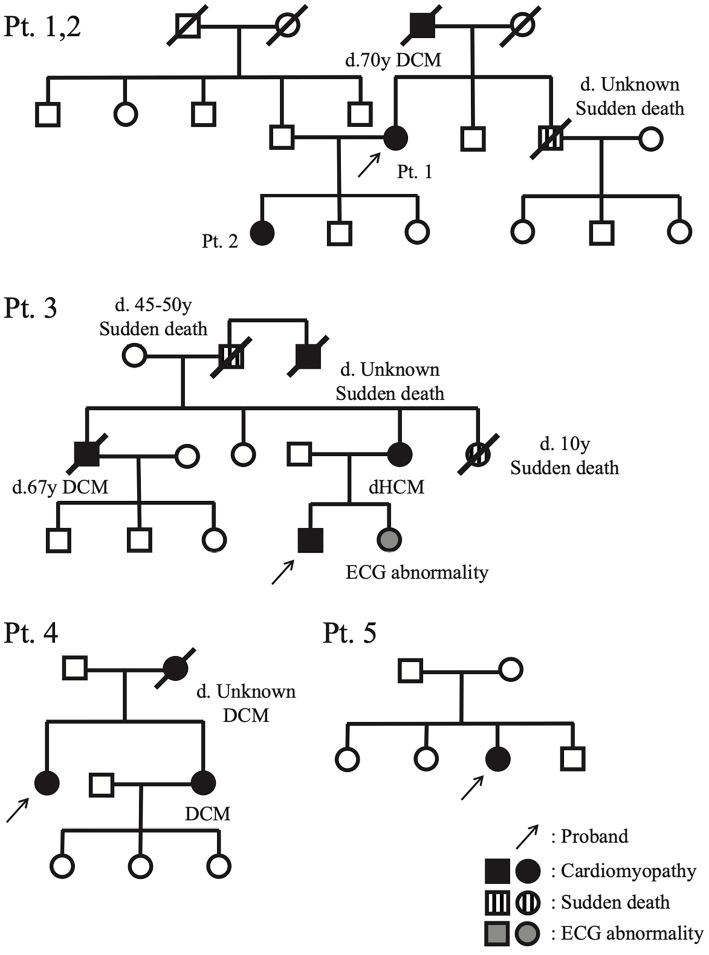
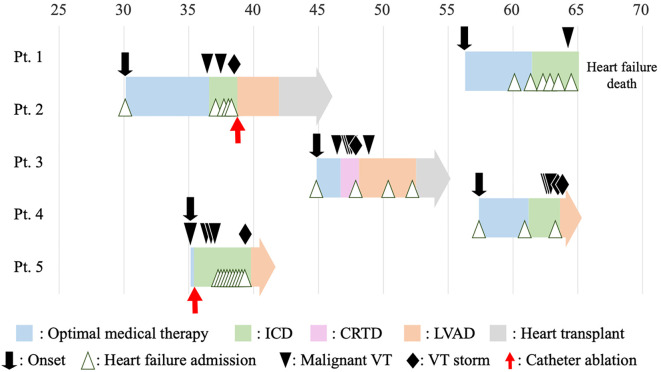
The family pedigree charts of these patients (Fig. 1), clinical courses (the median follow-up period from the onset was 9 years) (Fig. 2) and the main clinical characteristics and examination findings at the initial diagnosis (Table 4) are shown.
Figure 1.
Pedigree with proband.
Figure 2.
The clinical courses of the five patients. ‘Malignant VT’ is defined as VT that requires cardiopulmonary resuscitation or an appropriate implantable cardioverter defibrillator discharge. ‘VT storm’ is defined as multiple episodes of malignant VT in a single day. CRTD: cardiac resynchronization therapy defibrillator, ICD: implantable cardioverter defibrillator, LVAD: left ventricular assist device, VT: ventricular tachycardia
Table 4.
Baseline Characteristics.
| Characteristic | Pt.1 | Pt.2 | Pt.3 | Pt.4 | Pt.5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 56 | 29 | 44 | 57 | 35 | |||||
| Sex | F | F | M | F | F | |||||
| Disease onset | HF | HF | HF | HF | VT | |||||
| Family history | ||||||||||
| DCM | + | + | + | + | - | |||||
| SCD <45y | - | - | + | - | - | |||||
| Symptoms | ||||||||||
| Syncope | - | - | - | - | + | |||||
| NYHA | II | III | III | II | I | |||||
| ECG abnormalities | ||||||||||
| Low voltage | - | - | - | + | + | |||||
| Reduced R amplitude | - | + | + | + | + | |||||
| Repolarization abnormalities | + | - | - | - | - | |||||
| TTE | ||||||||||
| LVDd/LVDs (mm) | 53/44 | 68/59 | 74/71 | 54/48 | 46/43 | |||||
| IVS/PW (mm) | 9/6 | 9/7 | 10/10 | 8/8 | 8/6 | |||||
| LVEF (%) | 35 | 35 | 9 | 24 | 30 |
‘Low voltage’ is defined as QRS peak-to-peak amplitude in leads I, II, and III <0.5 mV, the sum of the amplitudes <1.5 mV, and amplitude in all precordial leads <1.0 mV (3). ‘Reduced R amplitude’ is defined as R waves <3 mm for at least five of six limb leads or <5 mm for five of six precordial leads (8). ‘Repolarization abnormalities’ is defined as fulfilling the major or minor criteria as described in the modified ARVC/D task force criteria (18).
ARVC/D: arrhythmogenic right ventricular cardiomyopathy/dysplasia, DCM: dilated cardiomyopathy, ECG: electrocardiogram, IVS: interventricular septum, LVDd: left ventricular end-diastolic dimension, LVDs: end-systolic dimension, LVEF: left ventricular ejection fraction, PW: posterior wall, SCD: sudden cardiac death, TTE, transthoracic echocardiography
Four patients (1, 2, 3 and 4) had family histories of DCM, and three (1, 2 and 3) had family histories of SCD (Fig. 1). The parents of Patient 5 did not show cardiac symptoms or abnormal findings in the available medical records.
At the initial visit, four out of five patients had already developed heart failure symptoms, such as dyspnea or edema, while one patient had syncope due to ventricular tachycardia (VT) (Table 4). All of them had clinical features of DCM, but none of them had developed clinical symptoms before 25 years old, which was consistent with the previous findings (3). The median age of the onset was 44 years old, with a wide range reported (20s to 50s). All patients had developed end-stage heart failure by 47 years old, within 10 years of the onset. Of note, although Patients 1 and 2 were mother and daughter, the onset was significantly earlier in Patient 2 than in Patient 1. All patients received guideline-based standard medical treatments. Patient 1 refused further invasive treatment after ICD implantation and ultimately died of heart failure at 64 years old. The other four patients underwent left ventricular assist device (LVAD) implantation after registration for heart transplantation. Finally, Patients 2 and 3 underwent heart transplantations, and the other two patients were on the waiting list and continued their outpatient visits.
Remarkably, all patients had malignant ventricular arrhythmias (defined as VT requiring cardiopulmonary resuscitation or an appropriate implantable cardioverter-defibrillator intervention) and underwent ICD or cardiac resynchronization therapy defibrillator (CRTD) implantation, as shown in Fig. 2. The median age at the first VT was 46 years old. Patients 2, 3 and 4 underwent ICD or CRTD implantation for secondary prevention because they experienced cardiopulmonary arrest due to VT outside the hospital. Appropriate ICD therapy was administered to all of the patients. Patient 1 experienced only anti-tachycardia pacing, while Patients 2, 3, 4 and 5 experienced ICD/CRTD discharge repeatedly. VT did not always occur with worsening heart failure. For Patients 3 and 5, it occurred at Ney York Heart Association class I and II, respectively. As shown in Fig. 1, acute circulatory failure caused by frequent ventricular tachycardia greatly affected the decision for LVAD implantation.
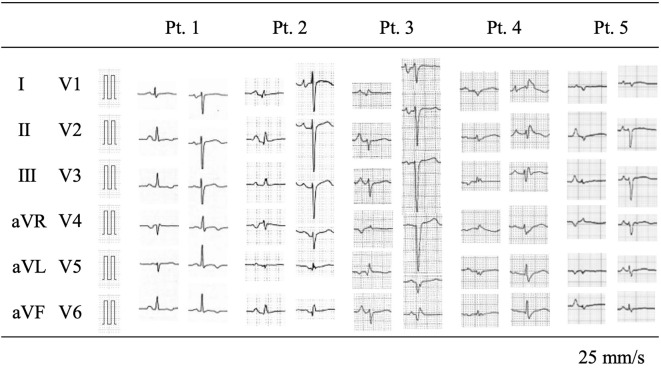
Regarding the examination findings at the diagnosis, electrocardiograms (ECGs) showed characteristic similarities consistent with previous reports (9). A low voltage was observed in 3 patients (60%), and a reduced R amplitude [defined as R waves <3 mm for at least 5 of 6 limb leads or <5 mm for 5 of 6 precordial leads (9)] was observed in 4 patients (80%), while repolarization abnormalities were found in only 1 patient (20%) (Fig. 3 and Table 4). All patients presented with a sinus rhythm and a narrow QRS duration. Echocardiography showed left ventricular dilatation and a markedly reduced left ventricular ejection fraction (LVEF) in all cases (Fig. 1). The size of the left ventricle varied from case to case. Histopathological findings showed interstitial fibrosis in all cases to varying degrees, and the diagnosis was consistent with DCM.
Figure 3.
Findings of 12-lead electrocardiograms at the initial visit (or oldest available).
Discussion
In this case series, we summarized the clinical findings of five DCM patients carrying the PLN variant R14del in Japan. All patients exhibited malignant VT that resulted in end-stage heart failure.
R14del was first identified in a Greek family in 2006 (10) and has since been reported to be a pathogenic variant causing hereditary cardiomyopathy in the United States (11), Germany (9), Canada (12) and China (13). Based on genomic allele frequencies, 6 of every 100,000 people are carriers of this variant worldwide. Carriers are particularly concentrated in the northern part of the Netherlands (4). All Dutch patients had the same haplotype, suggesting a founder effect (14). However, the origin of this variant and its spread around the world have not been fully clarified. Our five cases were not concentrated in a specific region. Although we did not perform a haplotype analysis, a further analysis of the haplotypes of the five patients may aid in identifying the origin of R14del in Japan.
We identified 5 patients with R14del variants among 211 DCM patients (2.4%). According to a meta-analysis of more than 8,000 patients with DCM (15), the entire frequency of PLN variants was 2%, and R14del was the most common among them. However, it is not appropriate to simply compare these values, as our cohort mainly consisted of patients with severe heart failure requiring advanced treatment beyond optimal medical therapy. Although R14del is reported to have high penetration, a substantial portion of R14del carriers are asymptomatic without any abnormalities on medical examinations, and the phenotypes of symptomatic carriers, such as the age of the onset, vary among individuals (3,10). Therefore, either of the parents of Patient 5 might have been an asymptomatic carrier of R14del, and rare variants other than R14del (Table 3), which may have some pathogenic effect, may influence the phenotypes of each patient. Further surveys are needed to discuss the prevalence of R14del in patients with DCM or the variety of phenotypes among carriers of R14del in Japan.
Phospholamban is a small molecule consisting of 52 amino acids. It regulates calcium transport. It is located in the cardiac sarcoplasmic reticulum (SR) and is reversibly phosphorylated by cAMP-dependent protein kinase (16). Dephosphorylated phospholamban binds to sarco/endoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) and inhibits its Ca2+ reuptake to the SR, while phosphorylated phospholamban loses its inhibitory effect against SERCA2a (10). Although R14del phospholamban itself inhibits SERCA2a more weakly than the wild type in vitro (17), heterozygous R14del phospholamban behaves as a super inhibitor of SERCA2a in cardiomyocytes (10). While the detailed mechanism remains unclear, it results in sarcoplasmic Ca2+ overload. Long-term abnormalities in Ca2+ transients may consequently lead to myocardial fibrosis and subsequently heart failure. Several PLN variants other than R14del are reported to cause DCM, including Arg9Cys (18), Arg9Leu (19), Arg9His (19), Arg25Cys (20) and Leu39stop (21). All variants lead to the development of heart failure, but only R14del and Arg25Cys are associated with ventricular arrhythmias. The complexity of the interaction with the abovementioned SERCA2a is speculated to result in the unique phenotype of R14del, even though all variants except Leu39stop lead to suppression of SERCA2a activity.
Owing to this abnormal function of mutant phospholamban, R14del cardiomyopathy exhibits a unique phenotype. First, it is characterized by a low voltage on an ECG despite left ventricular dilatation (2,8), which is consistent with our case series. Three and four of the five ECGs fulfilled the low voltage and poor R progression criteria, respectively. These ECG abnormalities are considered to reflect cardiac fibrosis in R14del cardiomyopathy, which is a frequent finding on histological examinations (10), as was noted in the present five cases. This ECG finding may be a clue suggesting the presence of R14del in patients with idiopathic DCM.
The high prevalence of ventricular arrhythmias and heart failure after adolescence is also characteristic of R14del cardiomyopathy, as was observed in our case series. The Dutch research group reported R14del clinical features in a cohort of 295 carriers (3). In the cohort, malignant ventricular arrhythmias and end-stage heart failure were observed in 36% and 24% of 125 symptomatic R14del carriers, respectively, during the 42-month observation period. Regarding the timing of ICD implantation, the cohort identified 2 risk factors of malignant VT: LVEF <45% and a history of sustained/nonsustained VT. They suggested setting these two risk factors as indications for ICD implantation (3). All of our patients were implanted with an ICD or CRTD, which worked correctly at least once, and in most cases, repeatedly. If Patients 2 and 3 had been treated according to these indications, they might not have experienced cardiopulmonary arrest due to malignant VT. In addition, the timing of LVAD implantation should be carefully considered because of the possibility of unexpected circulatory disruption due to VT. Once patients develop circulatory collapse, they may miss the chance to undergo LVAD implantation. Therefore, we propose performing the operation early rather than attempting other more conservative treatments when patients require repeated inotropic support.
In addition, family care, including genetic counseling, is also important in hereditary cardiomyopathy. Most of the patients in our study had a family history of DCM, and three had a family history of sudden death. As shown in the Dutch cohort and in our series, the disease may not be detected before patients have children because of its relatively late onset. Indeed, three of our patients had children, and in fact, the daughter had a more severe course of illness than the mother in the parent-child case. Considering the risk of sudden cardiopulmonary arrest due to ventricular arrhythmias, even in asymptomatic patients, in the Dutch cohort (3), risk stratification of asymptomatic family members is an important and valuable reason for genetic testing. The European Society of Cardiology guidelines recommend genetic testing for familial DCM with a severe course, citing the PLN variant as an example (22). We also consider genetic screening to be valuable in the management of patients with DCM and their families. Furthermore, based on our experience with five cases of PLN R14del, attention should be paid to the severe course of the disease, including SCD, when this variant is detected.
At present, there is no silver bullet for treating R14del cardiomyopathy. A homozygous R14del knock-in mouse model exhibited severe cardiac phenotype similar to that in humans and failed to respond to standard medical therapy (23). However, subcutaneous administration of PLN antisense oligonucleotides rescued their phenotype, according to the latest report (24). In the future, such gene therapy may be used as precision medicine.
In conclusion, we reported the clinical course of five patients with DCM caused by the PLN R14del variant. The onset of the disease occurs after adolescence, and its clinical course is characterized by severe heart failure with malignant ventricular arrhythmias, which are associated with a poor prognosis. We propose genetic screening, including PLN R14del, in DCM patients, especially those with marked ventricular arrhythmias.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Hershberger RE, Morales A. Dilated Cardiomyopathy Overview. 13. [Google Scholar]
- 2. van der Zwaag PA, van Rijsingen IAW, Asimaki A, et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail 14: 1199-1207, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Rijsingen IAW, van der Zwaag PA, Groeneweg JA, et al. Outcome in phospholamban R14del carriers: results of a large multicentre cohort study. Circ Cardiovasc Genet 7: 455-465, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Hof IE, van der Heijden JF, Kranias EG, et al. Prevalence and cardiac phenotype of patients with a phospholamban mutation. Neth Heart J 27: 64-69, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doevendans PA, Glijnis PC, Kranias EG. Leducq transatlantic network of excellence to cure phospholamban-induced cardiomyopathy (CURE-PLaN). Circ Res 125: 720-724, 2019. [DOI] [PubMed] [Google Scholar]
- 6. Yamada N, Asano Y, Fujita M, et al. Mutant KCNJ3 and KCNJ5 potassium channels as novel molecular targets in bradyarrhythmias and atrial fibrillation. Circulation 139: 2157-2169, 2019. [DOI] [PubMed] [Google Scholar]
- 7. Kitaoka H, Tsutsui H, Kubo T, et al. the Japanase Circulation Society Joint Working Group. JCS/JHFS 2018 guideline on the diagnosis and treatment of cardiomyopathies 85: 1590-1689, 2021. [DOI] [PubMed] [Google Scholar]
- 8. Musunuru K, Hershberger RE, Day SM, et al. ; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Genetic testing for inherited cardiovascular diseases: a scientific statement from the American Heart Association. Circ Genom Precis Med 13: 2020. [DOI] [PubMed] [Google Scholar]
- 9. Posch MG, Perrot A, Geier C, et al. Genetic deletion of arginine 14 in phospholamban causes dilated cardiomyopathy with attenuated electrocardiographic R amplitudes. Heart Rhythm 6: 480-486, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Haghighi K, Kolokathis F, Gramolini AO, et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci 103: 1388-1393, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeWitt MM, MacLeod HM, Soliven B, McNally EM. Phospholamban R14 deletion results in late-onset, mild, hereditary dilated cardiomyopathy. J Am Coll Cardiol 48: 1396-1398, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Cheung CC, Healey JS, Hamilton R, et al. Phospholamban cardiomyopathy: a Canadian perspective on a unique population. Neth Heart J 27: 208-213, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang XL, Xie J, Lan RF, et al. Genetic basis and genotype-phenotype correlations in Han Chinese patients with idiopathic dilated cardiomyopathy. Sci Rep 10: 2226, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Zwaag PA, van Rijsingen IAW, de Ruiter R, et al. Recurrent and founder mutations in the Netherlands - Phospholamban p.Arg14del mutation causes arrhythmogenic cardiomyopathy. Neth Heart J 21: 286-293, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kayvanpour E, Sedaghat-Hamedani F, Amr A, et al. Genotype-phenotype associations in dilated cardiomyopathy: meta-analysis on more than 8000 individuals. Clin Res Cardiol 106: 127-139, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Tada M, Kirchberger MA, Repke DI, Katz AM. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3’:5’-monophosphate-dependent protein kinase. J Biol Chem 249: 6174-6180, 1974. [PubMed] [Google Scholar]
- 17. Ceholski DK, Trieber CA, Young HS. Hydrophobic imbalance in the cytoplasmic domain of phospholamban is a determinant for lethal dilated cardiomyopathy. J Biol Chem 287: 16521-16529, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmitt JP. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299: 1410-1413, 2003. [DOI] [PubMed] [Google Scholar]
- 19. Medeiros A, Biagi DG, Sobreira TJP, et al. Mutations in the human phospholamban gene in patients with heart failure. Am Heart J 162: 1088-1095.e1, 2011. [DOI] [PubMed] [Google Scholar]
- 20. Liu GS, Morales A, Vafiadaki E, et al. A novel human R25C-phospholamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia. Cardiovasc Res 107: 164-174, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haghighi K, Kolokathis F, Pater L, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 111: 869-876, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC): Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129-2200, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Eijgenraam TR, Boukens BJ, Boogerd CJ, et al. The phospholamban p.(Arg14del) pathogenic variant leads to cardiomyopathy with heart failure and is unresponsive to standard heart failure therapy. Sci Rep 10: 9819, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grote Beverborg N, Später D, Knöll R, et al. Phospholamban antisense oligonucleotides improve cardiac function in murine cardiomyopathy. Nat Commun 12: 5180, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]