Abstract
Chylous ascites (CA) is the accumulation of fluid with a high triglyceride content in the peritoneal cavity. Only two cases in the literature have reported CA with hyperthyroidism. A 28-year-old previously healthy woman presented with gradual-onset abdominal swelling, exertional dyspnea, and diarrhea. Hyperthyroidism and heart failure were diagnosed using laboratory investigation and echocardiography. Ultrasonography revealed a large amount of ascites. The ascitic fluid was milky with elevated triglyceride levels. Treatment with anti-thyroid therapy and diuretics improved all symptoms, and the free triiodothyronine (T3) level normalized after five days. Hyperthyroidism and heart failure should be considered as reversible causes of CA.
Keywords: chylous ascites, thyroid storm, hyperthyroidism, heart failure
Introduction
Chylous ascites (CA) is an unusual type of ascites characterized by a milky ascitic fluid having a high concentration of triglycerides (>200 mg/dL). It is a clinically important disease that may lead to severe complications due to the loss of fluid, proteins, and nutrients. The reported incidence of CA is approximately 1 in 20,000 admissions at large tertiary-care referral centers (1).
It usually occurs due to trauma and rupture of the lymphatics or an increased peritoneal lymphatic pressure secondary to obstruction (2). The underlying etiologies for CA have been classified as traumatic, congenital, infectious, neoplastic, postoperative, cirrhotic, and cardiogenic (2-4). Hyperthyroidism might be associated with the development of CA, and only two such cases have been reported in the literature (5,6). Furthermore, heart failure has been reported as a reversible cause of CA (5,7-11).
In this report, we describe a rare case of CA due to hyperthyroidism and heart failure, which improved rapidly after administration of an anti-thyroid drug and diuretic therapy.
Case Report
A 28-year-old woman with a history of atopic dermatitis presented with a 1-month history of abdominal swelling, exertional dyspnea, diarrhea, and palpitations. Her family history was unremarkable. She denied any history of cigarette smoking, heavy alcohol consumption, or substance abuse. She also denied a history of abdominal trauma, surgery, or pelvic irradiation. Her vital signs were as follows: blood pressure, 133/96 mmHg; heart rate, 171 beats/min with an irregular rhythm; respiratory rate, 16 breaths/min; body temperature, 36.7°C; and oxygen saturation, 99% on 2 L/min of oxygen via nasal cannula. The patient had an enlarged thyroid gland, jugular venous distension, and severe pitting edema in both legs. Her abdomen was distended without tenderness. Shifting dullness was noted, while hepatosplenomegaly was not observed.
Laboratory investigations revealed a decreased platelet count (56×103/μL). The hemoglobin level was 11.1 g/dL, and the white blood cell count was 6,100 /μL. Liver function tests revealed that the aspartate transaminase and alanine transaminase levels were within their reference ranges, but the alkaline phosphatase (652 IU/L) and gamma-glutamyl transferase (100 IU/L) levels were elevated. Furthermore, the serum creatinine and brain natriuretic peptide levels were 0.32 mg/dL and 341 pg/mL, respectively. Thyroid function tests revealed that the thyroid-stimulating hormone (TSH), free triiodothyronine (T3), and free thyroxine (T4) levels were <0.01 mIU/L, >20.0 pg/mL, and 3.36 ng/dL, respectively (Table 1). Thyroid ultrasonography revealed a diffusely enlarged thyroid gland and a diffusely increased blood flow. The serum TSH receptor antibody concentration was 11.9 IU/L (reference range, <2.0 IU/L). The patient was diagnosed with thyroid storm secondary to Graves' disease based on the Burch-Wartofsky Point Scale (BWPS) score and the Japan Thyroid Association (JTA) criteria (12,13). Our patient had a BWPS score of 50 points: thermoregulatory dysfunction, 0 points; cardiovascular, 35 points; congestive heart failure, 5 points; gastrointestinal-hepatic dysfunction, 10 points; central nervous system disturbance, 0 points; and precipitating event; 0 points. She also had three of the symptoms listed in the JTA criteria (a fever, tachycardia, and heart failure) and met both the diagnostic criteria.
Table 1.
Laboratory Data on Admission.
| Complete blood cell count | |||
| White blood cell | 6,000 | /µL | |
| Hemoglobin | 11.1 | g/dL | |
| Platelets | 56×103 | /µL | |
| Coagulation test | |||
| PT-INR | 1.51 | ||
| aPTT | 39.1 | s | |
| Blood chemistry | |||
| Albumin | 3.6 | g/dL | |
| Aspartate aminotransferase | 41 | U/L | |
| Alanine aminotransferase | 40 | U/L | |
| Lactate dehydrogenase | 243 | U/L | |
| Alkaline phosphatase | 652 | U/L | |
| γ-glutamyl transpeptidase | 100 | U/L | |
| Total bilirubin | 1.18 | mg/dL | |
| Urea nitrogen | 9.9 | mg/dL | |
| Creatinine | 0.32 | mg/dL | |
| Creatine phosphokinase | 27 | U/L | |
| Serological tests | |||
| C-reactive protein | 0.24 | mg/dL | |
| Brain natriuretic peptide | 341 | pg/mL | |
| Endocrine tests | |||
| Thyroid stimulating hormone | <0.008 | mg/dL | |
| Free triiodothyronine | >20.0 | pg/mL | |
| Free thyroxine | 3.36 | IU/L | |
| Thyrotropin receptor antibodies* | 11.9 | IU/L |
*Reference range: <2.0 IU/L
PT-INR: prothrombin time and international normalized ratio, aPTT: activated partial thromboplastin time
Electrocardiography revealed atrial fibrillation with a rapid ventricular response. Transthoracic echocardiography (TTE) revealed diffuse left ventricular hypokinesis with an ejection fraction of 49%, moderate mitral valve regurgitation, and severe tricuspid regurgitation. The estimated pulmonary artery pressure was 38 mmHg. Abdominal ultrasonography and computed tomography revealed a large amount of ascites. The liver had a smooth surface, sharp edges, normal size, and normal parenchymal pattern. Diagnostic paracentesis revealed a milky ascitic fluid (Fig. 1) The analysis of this fluid (Table 2) revealed an elevated triglyceride level (212 mg/dL), high serum-ascites albumin gradient (≥1.1 g/dL), and high protein concentration (>2.5 mg/dL). Based on the appearance of the ascitic fluid and the presence of elevated triglyceride levels in it, the patient was diagnosed with CA. Cytology and culture results of the ascitic fluid were ultimately negative, and a urinalysis revealed no proteinuria or hematuria.
Figure 1.

Milky appearance of the ascitic fluid.
Table 2.
Biochemical Analyses of the Ascitic Fluid and Blood.
| Ascitic fluid | Blood | |||
|---|---|---|---|---|
| Total protein (g/dL) | 3.1 | 7.0 | ||
| Albumin (g/dL) | 1.7 | 3.7 | ||
| Lactate dehydrogenase (IU/L) | 97 | 243 | ||
| Triglyceride (mg/dL) | 212 | 67 | ||
| Total cholesterol (mg/dL) | 40 | 93 |
Traumatic, congenital, and postoperative etiologies for CA were ruled out based on her history. An infectious etiology was also unlikely due to the negative culture and cytology results of the ascitic fluid. A neoplastic etiology was unlikely due to the absence of findings suggestive of malignancy on abdominal imaging studies. Finally, a cirrhotic etiology was ruled out due to the absence of a risk of cirrhosis (no heavy alcohol consumption, substance abuse, and transfusion and negative results for the hep B surface antigen and hepatitis C antibody) and normal ultrasound findings of the liver and spleen.
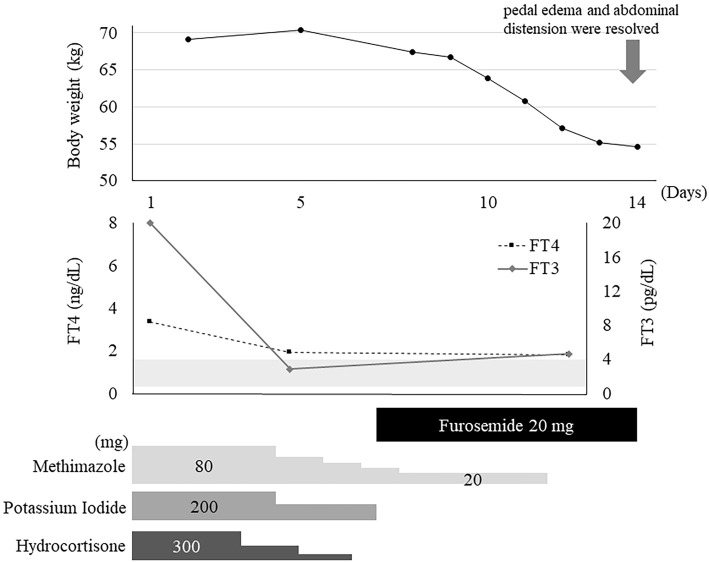
We initiated treatment with glucocorticoids, a beta-blocker, potassium iodide, and methimazole for the thyroid crisis secondary to Graves' disease. Furosemide was also administered to treat the heart failure. The patient's free T3 and free T4 levels improved and had almost normalized by day 5, but the atrial fibrillation and thrombocytopenia persisted. Symptoms of dyspnea, edema, and abdominal distension gradually improved. Corticosteroids were tapered on day 6. The patient's pedal edema and abdominal distension were resolved by day 14. Her body weight decreased by 15 kg over 2 weeks (Fig. 2). She was discharged on day 21 and continued using an antithyroid drug in an outpatient setting. On day 56, TTE revealed the complete resolution of systolic dysfunction and tricuspid regurgitation. There was no recurrence of thyrotoxicosis, heart failure, or ascites for up to 10 months after discharge.
Figure 2.
Clinical course of the patient.
Discussion
We encountered a rare case of CA associated with heart failure secondary to thyrotoxicosis. CA associated with hyperthyroidism has been described in only two case reports (5,6). Hsieh et al. reported a case of CA as a manifestation of thyrotoxic cardiomyopathy, wherein the CA rapidly disappeared with administration of anti-thyroid drugs and diuretics. Therefore, the authors suggested thyrotoxic cardiomyopathy as the main cause of CA (5). In the second case of CA with hyperthyroidism, the patient presented with intractable diarrhea and dehydration without heart failure; the CA resolved as the thyroid hormone levels normalized with methimazole treatment (6). This might suggest that hyperthyroidism itself leads to CA via an unknown mechanism.
Excluding cases with constrictive pericarditis, only six cases of CA associated with heart failure have been reported (5,7-11). Some pathophysiological hypotheses have been proposed. In heart failure, increased caval and hepatic venous pressures lead to an increase in the hepatic lymph production (14). An elevated lymphatic pressure secondary to portal hypertension can cause endothelial compromise or rupture of the dilated serosal lymphatic channels, leading to CA (7). Furthermore, in heart failure, a high pressure in the left subclavian vein restricts lymphatic drainage from the thoracic duct (8). As a result, the lymphatic venous collaterals are incapable of handling a normal lymphatic flow, and the chylous fluid leaks into the peritoneal cavity (8).
The serum albumin ascites gradient (SAAG) should be evaluated to determine whether ascites is associated with portal hypertension or other causes (15). Generally, an SAAG ≥1.1 g/dL suggests portal hypertension. In cases of ascites due to heart failure-induced portal hypertension without cirrhosis, the serum albumin level is generally within normal limits. An SAAG ≥1.1 g/dL and an ascitic fluid protein level of >2.5 mg/dL were reported to have a sensitivity and specificity of 63% and 93%, respectively, for ascites due to heart failure (16). Even among patients with CA, SAAG was reported to be effective in distinguishing ascites caused by portal hypertension from ascites due to other etiologies (2-4). In our case, an analysis of the ascitic fluid revealed an elevated SAAG (2.0 g/dL) and protein level (3.1 mg/dL), compatible with the diagnosis of portal hypertension secondary to heart failure; notably, other findings of portal hypertension, such as splenomegaly or esophageal varices, were absent.
In addition to the findings of the present case, we reviewed the analytical findings of ascites from the six previously reported cases of CA with heart failure (Table 3) (5,7-11). Among these 7 cases, SAAG was described in 5 cases, including 4 with an SAAG ≥1.1 g/dL. The ascitic fluid protein level was described in 5 cases, 4 of which had a protein level >2.5 mg/dL. Both parameters were described in four cases; three cases had an SAAG ≥1.1 g/dL and an ascitic fluid protein level >2.5 mg/dL. These observations suggest that the SAAG and ascitic fluid protein levels might be useful for detecting the cardiac cause in patients with CA. The prognosis of these cases was better than that of cases with CA due to other etiologies, such as malignancy and trauma (5,7-10). In the present case, CA improved rapidly after treatment with an antithyroid drug and diuretics. All six other cases of CA due to heart failure were reported to have a good response to diuretic therapy. Therefore, empirical diuresis should be considered for patients with CA when a cardiac cause is suspected based on an ascitic fluid analysis.
Table 3.
SAAG and the Ascitic Fluid Protein Levels among Cases of CA with Heart Failure.
| References | Year | SAAG (g/dL) |
Ascitic fluid protein (mg/dL) |
|||
|---|---|---|---|---|---|---|
| Our case | 2021 | 2.0 | 3.1 | |||
| 5 | 2010 | 1.9 | 2.3 | |||
| 7 | 2005 | 1.3 | 5.6 | |||
| 8 | 1995 | 3.9 | NA | |||
| 9 | 2011 | -1.5 | 5.4 | |||
| 10 | 2019 | NA | NA | |||
| 11 | 1996 | NA | 5.1 |
SAAG: serum-ascites albumin gradient, CA: chylous ascites, NA: not available
Although hyperthyroidism and heart failure might be possible causes of CA, CA is still very rare among patients with these conditions; this suggests the existence of other unknown factors contributing to its pathogenesis.
Conclusion
To our knowledge, this is the second case report of CA due to heart failure and hyperthyroidism in the literature. On encountering CA, heart failure and hyperthyroidism should be considered as possible and reversible causes, as antithyroid and diuretic treatment are effective with a good prognosis.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Press OW, Press NO, Kaufman SD. Evaluation and management of chylous ascites. Ann Intern Med 96: 358-364, 1982. [DOI] [PubMed] [Google Scholar]
- 2. Bhardwaj R, Vaziri H, Gautam A, Ballesteros E, Karimeddini D, Wu GY. Chylous ascites: a review of pathogenesis, diagnosis and treatment. J Clin Transl Hepatol 6: 1-9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lizaola B, Bonder A, Trivedi HD, Tapper EB, Cardenas A. Review article: the diagnostic approach and current management of chylous ascites. Aliment Pharmacol Ther 46: 816-824, 2017. [DOI] [PubMed] [Google Scholar]
- 4. Al-Busafi SA, Ghali P, Deschênes M, Wong P. Chylous ascites: evaluation and management. ISRN Hepatol 2014: 1-10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsieh M-H, Chen C-C, Wang T-Y, Chang C-T. Chylous ascites as a manifestation of thyrotoxic cardiomyopathy in a patient with untreated Graves' disease. Thyroid 20: 653-655, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Hiroi N, Sakamoto Y, Urita Y, Higa M, Kuboki K, Yoshino G. Graves' disease with intractable diarrhea, chylous ascites, and chylothorax: a case report. Thyroid 17: 1299-1303, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Ridruejo E, Mandó OG. Chylous ascites as the main manifestation of left ventricular dysfunction: a case report. BMC Gastroenterol 5: 1-4, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Villena V, De Pablo A, Martin-Escribano P. Chylothorax and chylous ascites due to heart failure. Eur Respir J 8: 1235-1236, 1995. [DOI] [PubMed] [Google Scholar]
- 9. Cakmak HA, Yenidünya G, Karadağ B, Ongen Z. Development of chylothorax and chylous ascites in a patient with congestive heart failure. Turk Kardiyol Dern Ars 39: 495-498, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Trêpa M, Baggen Santos R, Fontes Oliveira M, Silveira I, Pires J, Torres S. Val30Met familial amyloid polyneuropathy, heart failure, and chylous ascites: an unexpected combination. Rev Esp Cardiol 72: 431-433, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am 22: 263-277, 1993. [PubMed] [Google Scholar]
- 12. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid 22: 661-679, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogasawara M, Harada K, Tamura M, Ito T, Kishkurno S, Takada G. Chylous ascites associated with atrioventricular valve regurgitation after Blalock-Taussig shunt. J Pediatr Gastroenterol Nutr 23: 501-503, 1996. [DOI] [PubMed] [Google Scholar]
- 14. Starling E. The influence of mechanical factors on lymph production. J Physiol 16: 224-267, 1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 49: 2087-2107, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology 59: 1043-1051, 2014. [DOI] [PubMed] [Google Scholar]