Abstract
Objective
To investigate the serum total antibody (immunoglobulin M and immunoglobulin G) titre against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein receptor-binding domain following BNT162b2 messenger ribonucleic acid (mRNA) coronavirus disease 2019 (COVID-19) vaccination in Japanese rheumatic disease patients undergoing immunosuppressive therapy.
Methods
The serum antibody titre against SARS-CoV-2 spike protein was analysed in 123 outpatients with rheumatic diseases at Kagawa University Hospital and 43 healthy volunteers who had received 2 doses of the BNT162b2 mRNA vaccine with at least 14 days elapsing since the second dose.
Results
The antibody titre in rheumatic disease patients was significantly lower than that in healthy subjects (p<0.0001). The antibody titres of the 41 patients who received biologics or Janus kinase inhibitors and the 47 patients who received conventional immunosuppressive agents were significantly lower than those of the 35 patients who did not receive immunosuppressive agents (p<0.0001 and p<0.0001, respectively). In addition, the mean antibody titre of the 43 patients on methotrexate was significantly lower than that of the 80 patients not on methotrexate (p=0.0017).
Conclusion
Immunogenicity to the BNT162b2 mRNA COVID-19 vaccine in rheumatic disease patients was found to be reduced under immunosuppressive treatment. In particular, methotrexate seems to be associated with a decreased antibody response.
Keywords: antibody response, COVID-19, immunosuppression, mRNA vaccine, rheumatic disease
Introduction
At time of writing, the coronavirus disease 2019 (COVID-19) pandemic continues to threaten the health of people all over the world. Although the safety and efficacy of the BNT162b2 messenger ribonucleic acid (mRNA) COVID-19 vaccine has been proven in the general population (1), patients receiving immunosuppressive therapy were excluded from the trial. Currently, many patients with rheumatic diseases are receiving immunosuppressive therapy, such as methotrexate and rituximab, which have been reported to reduce immune responses to influenza and pneumococcal vaccines (2). Regarding mRNA vaccination against COVID-19, immunosuppressive medications, such as rituximab, methotrexate, glucocorticoids, abatacept and mycophenolate, have been reported to reduce the immune response to BNT162b2 vaccination (3,4). However, data on immune responses against mRNA COVID-19 vaccines in Japanese rheumatic disease patients receiving immunosuppressive therapy are limited (5).
Therefore, we investigated the immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in Japanese rheumatic disease patients undergoing immunosuppressive therapy compared with healthy control subjects.
Materials and Methods
Participants
One hundred and twenty-three adult outpatients (≥20 years old) with rheumatic diseases at Kagawa University Hospital who had received 2 doses of the BNT162b2 mRNA vaccine were recruited for the study. All patients included in the study had a rheumatologist-confirmed definite diagnosis of their respective rheumatic disease. Clinical information, such as the age, sex, type of rheumatic disease, and treatment, was obtained from medical records. Forty-three healthy volunteers vaccinated with two doses of BNT162b2 served as a control group. No patient or healthy volunteer had a history of COVID-19.
Serum was collected when at least 14 days had passed since the administration of the second dose of the vaccine, having received the second dose between March 13 and August 6, 2021. The date of vaccination was confirmed and recorded with the vaccination certificate at the outpatient visit. Immunosuppressive therapy was continued before or after COVID-19 vaccination without temporary suspension.
This study was approved by the ethics committee of Kagawa University. Informed consent was obtained from each participant. This study was carried out according to the Declaration of Helsinki.
Endpoints of the study
The primary end point was immunogenicity against the BNT162b2 mRNA COVID-19 vaccine in rheumatic disease patients compared with controls measured at least two weeks after the second vaccine dose. The secondary endpoint was the effect of immunosuppressive treatments on the vaccine's immunogenicity.
Measurement of antibody response
The serum total antibody (IgM and IgG) titre to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) S protein receptor-binding domain was assessed by an enzyme-linked immunosorbent assay (ELISA) using Elecsys Anti-SARS-CoV-2 S RUO (Roche, Basel, Switzerland) according to the manufacturer's instructions.
Statistical analyses
All values reported are medians with interquartile ranges (IQR) unless otherwise noted. Antibody levels were compared using the Mann-Whitney U, Kruskal-Wallis and Steel-Dwass tests. All p values were 2-sided, and a p value <0.05 was considered significant. Data were analysed using the JMPⓇ Pro14 software program (SAS Institute, Cary, USA).
Results
Study population
The study enrolled 123 patients with rheumatic diseases and 43 healthy controls who had received 2 doses of the BNT162b2 mRNA vaccine. The clinical features of healthy subjects and rheumatic disease patients are shown in Table. The average age±standard deviation (SD) was 65.6±15.0 years for the rheumatic disease patients and 50.4±12.6 years for the healthy subjects, with the former being significantly older than the latter (p<0.0001). The median (IQR) duration from the second immunization to blood sampling was significantly shorter in the rheumatic disease patients than in the healthy subjects [29 (19-48) vs. 57 (55-58), P<0.0001]. Among the 123 patients with rheumatic disease, rheumatoid arthritis was the most common disease (n=54), followed by Sjögren syndrome (n=24), spondyloarthritis (n=11) and antineutrophil cytoplasmic antibody-associated vasculitis (n=10), with various other diseases comprising the remaining 24 cases (Table).
Table.
Characteristics of Study Participants.
| Healthy control | All RMD patients | With IS | Bio or JAKi | Conv. IS only | Without IS | |
|---|---|---|---|---|---|---|
| N | 43 | 123 | 88 | 41 | 47 | 35 |
| Age, y | 50.4±12.6 | 65.6±15.0 | 66.7±14.5 | 66.2±15.0 | 67.2±14.1 | 62.9±16.1 |
| Sex | ||||||
| Male, n (%) | 14 (32.6) | 24 (19.5) | 18 (20.5) | 10 (24.4) | 8 (17.0) | 6 (17.1) |
| Female, n (%) | 29 (67.4) | 99 (80.5) | 70 (79.5) | 31 (75.6) | 39 (83.0) | 29 (82.9) |
| Median time after second immunization for blood sample, d (interquartile range) | 57.0 (55.0, 58.0) |
29.0 (19.0, 48.0) |
28.0 (20, 46.3) |
27.0 (18.0, 40.5) |
31.0 (22.0, 52.0) |
36.0 (17.0, 56.0) |
| Rheumatoid disease diagnosis, n (%) | ||||||
| Rheumatoid arthritis | 54 (43.9) | 47 (53.4) | 26 (63.4) | 21 (44.7) | 7 (20.0) | |
| Systemic lupus erythematosus | 8 (6.5) | 6 (6.8) | 0 (0) | 6 (12.8) | 2 (5.7) | |
| Antiphospholipid syndrome | 5 (4.1) | 2 (2.3) | 0 (0) | 2 (4.3) | 3 (8.6) | |
| Sjögren syndrome | 24 (19.5) | 11 (12.5) | 2 (4.9) | 9 (19.1) | 13 (37.1) | |
| Systemic sclerosis | 4 (3.3) | 1 (1.1) | 1 (2.4) | 0 (0) | 3 (8.6) | |
| Polymyositis/dermatomyositis | 9 (7.3) | 7 (8.0) | 1 (2.4) | 6 (12.8) | 2 (5.7) | |
| Anti-neutrophil cytoplasmic antibody associated vasculitis | 10 (8.1) | 10 (11.4) | 7 (17.1) | 3 (6.4) | 0 (0) | |
| IgG4-related disease | 5 (4.1) | 3 (3.4) | 0 (0) | 3 (6.4) | 2 (5.7) | |
| Spondyloarthritis | 11 (8.9) | 11 (12.5) | 7 (17.1) | 4 (8.5) | 0 (0) | |
| Othersa | 14 (11.4) | 7 (8.0) | 2 (4.9) | 5 (10.6) | 7 (20.0) | |
| Medical exposure, n (%) | ||||||
| IS | ||||||
| Conv IS | 67 (54.5) | 67 (76.1) | 20 (48.8) | 47 (100) | ||
| Methotrexate (8.7±3.1 mg/week) | 43 (35.0) | 43 (48.9) | 15 (36.6) | 28 (59.6) | ||
| Azathioprine | 9 (7.3) | 9 (10.2) | 3 (7.3) | 6 (12.8) | ||
| Mycophenolate mofetil | 2 (1.6) | 2 (2.27) | 0 (0) | 2 (4.3) | ||
| Mizoribine | 2 (1.6) | 2 (2.27) | 0 (0) | 2 (4.3) | ||
| Tacrolimus | 16 (13.0) | 16 (18.2) | 1 (2.4) | 15 (31.9) | ||
| Cyclosporine | 7 (5.7) | 7 (8.0) | 1 (2.4) | 6 (12.8) | ||
| Biologics | 35 (28.5) | 35 (39.8) | 35 (85.4) | |||
| Tumour necrosis factor inhibitors | 10 (8.1) | 10 (11.4) | 10 (24.4) | |||
| IL-6 inhibitors | 5 (4.1) | 5 (5.7) | 5 (12.2) | |||
| Rituximab | 6 (4.9) | 6 (6.8) | 6 (14.6) | |||
| Abatacept | 7 (5.7) | 7 (8.0) | 7 (17.1) | |||
| IL-17 or IL-23 inhibitors | 6 (4.9) | 6 (6.8) | 6 (14.6) | |||
| Mepolizumab | 1 (0.8) | 1 (1.1) | 1 (2.4) | |||
| JAKi | 6 (4.9) | 6 (6.8) | 6 (14.6) | |||
| Baricitinib | 6 (4.9) | 6 (6.8) | 6 (14.6) | |||
| Glucocorticoids (PSL 3.4±2.7 mg/d) | 62 (50.4) | 47 (53.4) | 19 (46.3) | 28 (59.6) | 15 (42.9) |
RMD: rheumatic disease, IS: immunosuppressant, Bio: biologics, JAKi: janus kinase inhibitor, Conv: conventional, IL: interleukin, PSL: prednisolone
aPolymyalgia rheumatica (n=1), Behçet’s disease (n=2), Takayasu’s arteritis (n=2), IgA vasculitis (n=1), relapsing polychondritis (n=1), undifferentiated connective tissue disease (n=1), fibromyalgia syndrome (n=2), adult-onset Still’s disease (n=1), TAFRO syndrome (n=1), macrophage activating syndrome (n=1), hypergammaglobulinemia (n=1)
A total of 71.5% (n=88) patients with rheumatic diseases received immunosuppressive treatment. Among the therapeutic agents, we defined biologics, Janus kinase (JAK) inhibitors, methotrexate, azathioprine, mycophenolate mofetil, mizoribine, tacrolimus and cyclosporine as immunosuppressive agents. Concomitant use of glucocorticoids was not included under immunosuppressive agents because the mean dose was low (prednisolone 3.4±2.4 mg/day). Conventional immunosuppressive medication monotherapy (methotrexate, azathioprine, mycophenolate mofetil, mizoribine, tacrolimus or cyclosporine) was used in 38.2% (n=47). Biologics and JAK inhibitors were used as monotherapy or in combination with conventional immunosuppressive drugs in 33.3% (n=41). Glucocorticoids were used in 50.4% (n=62).
Immunogenicity against the BNT162b2 mRNA vaccine in rheumatic disease patients
The antibody titre in rheumatic disease patients was significantly lower than that in healthy subjects [241.0 (69.4-703.7) U/mL vs. 741.6 (509.2-1,103.0) U/mL, p<0.0001] (Fig. 1).
Figure 1.
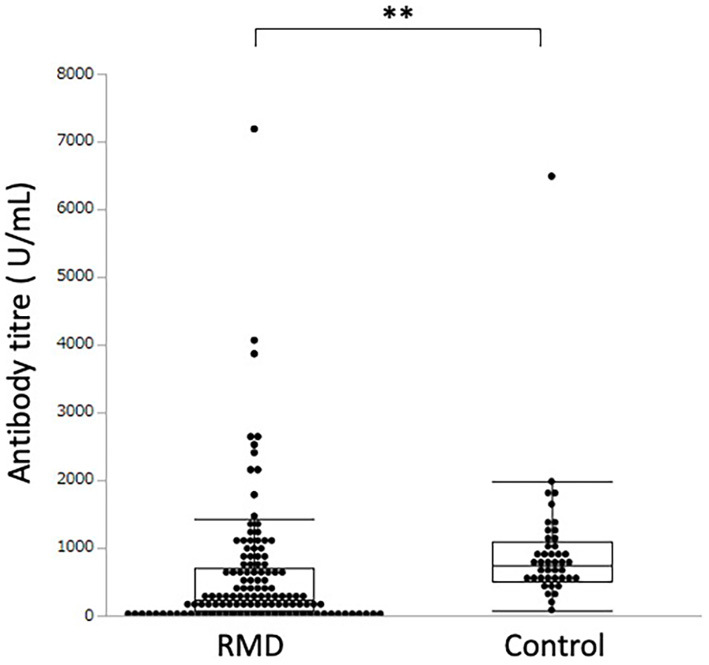
Serum antibody titre against the SARS-CoV-2 spike protein in patients with rheumatic diseases (RMD) and healthy controls. ** indicates a p value less than 0.01.
Effect of immunosuppressive treatments on the immunogenicity of the BNT162b2 mRNA vaccine
The antibody titres of 41 patients treated with biologics or JAK inhibitors and 47 patients with conventional immunosuppressive medication monotherapy were 108.2 (20.6-304.8) U/mL and 226.0 (46.7-584.1) U/mL, respectively, which were significantly lower than the value in the 35 patients who did not receive immunosuppressive medication [927.0 (314.8-1392.0) U/mL, p<0.0001 and p<0.0001, respectively] (Fig. 2). The antibody titre of the 43 patients (35.0%) treated with methotrexate was significantly lower than that of the 80 patients (65.0%) who did not receive methotrexate [median (IQR) antibody titre: 168.2 (43.5-307.9) U/mL vs. 391.4 (83.3-1,026.7) U/mL, p=0.0017] (Fig. 3).
Figure 2.
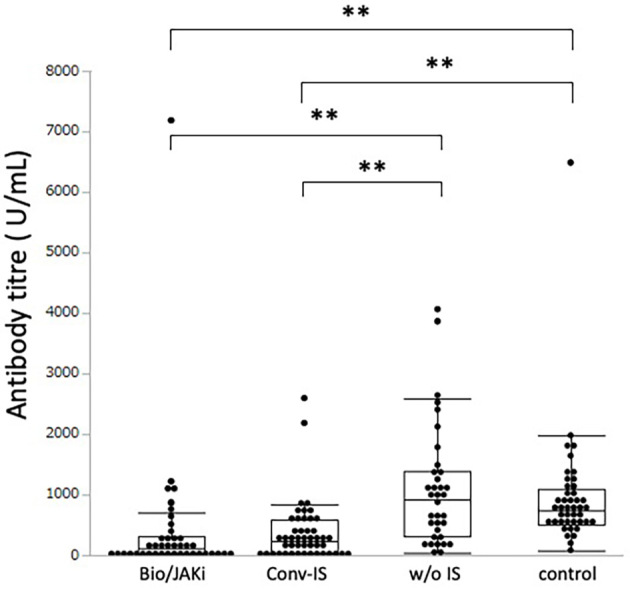
Serum antibody titre against the SARS-CoV-2 spike protein in patients with rheumatic diseases treated with biologics/Janus kinase inhibitors, conventional immunosuppressants, or no immunosuppressants and healthy controls. ** indicates a p value less than 0.01. Bio/JAKi: biologics or Janus kinase inhibitors, Conv-IS: conventional immunosuppressant, w/o IS: without immunosuppressant, control: healthy control
Figure 3.
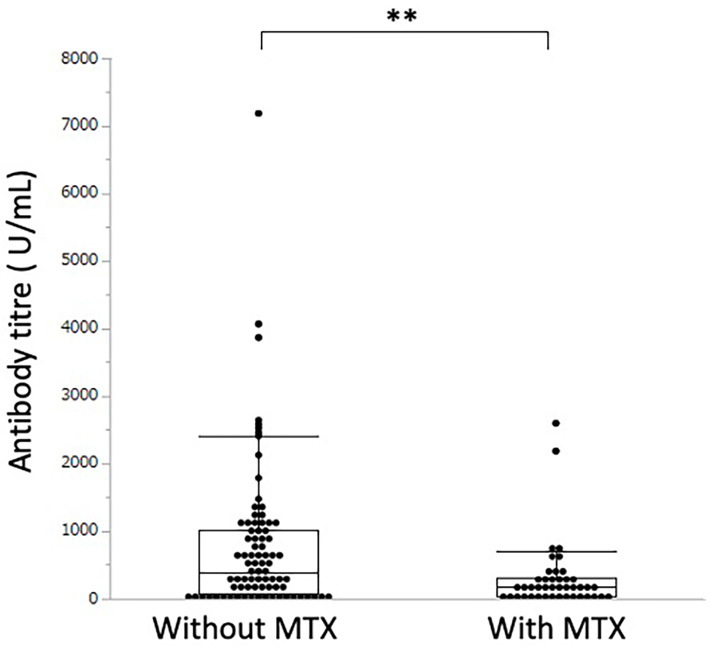
Serum antibody titre against the SARS-CoV-2 spike protein in patients with rheumatic diseases treated with or without methotrexate (MTX). ** indicates a p value less than 0.01.
Methotrexate was used without or with biologics/JAK inhibitors in 22.8% (n=28) and 12.2% (n=15) of rheumatic patients, respectively. The antibody titre of patients using methotrexate without biologics or JAK inhibitors was significantly lower than that of the patients using neither biologics, JAK inhibitors nor methotrexate [median (IQR) antibody titre: 205.0 (27.4-344.7) U/mL vs. 593.6 (212.3-1,089.3) U/mL, p=0.0029]. There was no significant difference in the antibody titres between the groups using methotrexate in combination with biologics or JAK inhibitors and the patients not using methotrexate with biologics or JAK inhibitors [median (IQR) antibody titre: 130.3 (54.9-194.6) U/mL vs. 77.1 (9.5-527.6) U/mL, p=0.9981].
There were also no significant differences in antibody titres between the groups using methotrexate with biologics or JAK inhibitors and those using methotrexate without biologics or JAK inhibitors [median (IQR) antibody titre: 130.3 (54.9-194.6) U/mL vs. 205.0 (27.4-344.7) U/mL, p=0.8024] (Fig. 4).
Figure 4.
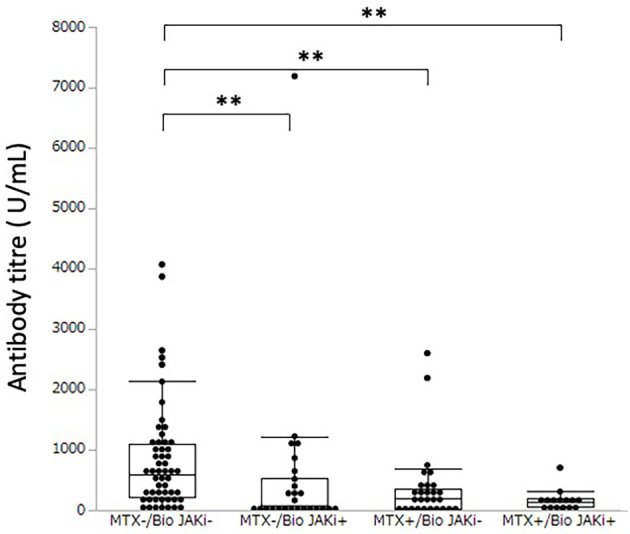
Serum antibody titre against the SARS-CoV-2 spike protein in rheumatic disease patients with or without methotrexate and with or without biologics/Janus kinase inhibitors. ** indicates a p value less than 0.01. MTX-/Bio JAKi-: without methotrexate and also without biologics nor Janus kinase inhibitors; MTX-/Bio JAKi+: without methotrexate, but with either biologics or Janus kinase inhibitors; MTX+/Bio JAKi-: with methotrexate but without biologics nor Janus kinase inhibitors; MTX+/Bio JAKi+: with methotrexate and also with either biologics or Janus kinase inhibitors.
The patients treated with glucocorticoids showed significantly lower antibody titres than the patients without glucocorticoids [median (IQR) antibody titre: 129.9 (16.4-418.3) U/mL vs. 443.3 (204.4-1,020.0) U/mL, p<0.0001].
Discussion
There are several reports on the age-dependent immune response to BNT162b2 vaccination (6-8). The rheumatic disease patients in this study were significantly older than the healthy subjects. While there was no significant association between the age and antibody titre in healthy subjects with a correlation coefficient of -0.06 [95% confidence interval (CI) -0.35 to +0.25], a very weak negative correlation between them was observed in rheumatic disease patients with the correlation coefficient of -0.19 (95% CI -0.36 to -0.01). Although the difference in age might have affected the results, we consider it possible to compare the antibody titres of rheumatic disease patients with those of healthy subjects because the expected variation by age is small.
The antibody titre is believed to decrease with time after BNT162b2 vaccination (9,10). In the present study, although the median time after second immunization for blood samples from rheumatic disease patients was significantly shorter than that in healthy subjects, there was no significant association between the median time after the second immunization until blood sampling and the antibody titre in healthy subjects with a correlation coefficient of +0.03 (95% CI -0.27 to +0.33) or in rheumatic disease patients with a correlation coefficient of -0.05 (95% CI -0.22 to +0.13).
In a German single-centre cohort, anti-SARS-CoV-2 antibody titres to mRNA vaccines were significantly lower in patients with chronic inflammatory diseases as compared with healthy controls (11). In a multicentre study in Israel, IgG antibody levels against SARS-CoV-2 spike S1/S2 proteins were significantly lower among patients with autoimmune inflammatory rheumatic diseases, 95.2% of whom were treated with immunomodulatory medications, than in the general population (3). Japanese patients with rheumatic disease in this study also showed significantly lower antibody titres to SARS-CoV-2 S protein receptor-binding domain than did healthy controls.
In another German study, although patients with immune-mediated inflammatory disease showed lower antibody responses to the BNT162b2 mRNA COVID-19 vaccine than did healthy controls, there were no marked differences by treatment regimen [no treatment, conventional synthetic disease modifying anti-rheumatic drugs (DMARDs), biologic DMARDs/targeted synthetic DMARDs], so they concluded that the disease itself rather than the treatment affected the immune response (12). However, the antibody titres of both patients treated with biologics or JAK inhibitors and those receiving conventional immunosuppressive monotherapy were significantly lower than those in patients without immunosuppressive medication in our study. In other words, immune responses to the BNT162b2 mRNA COVID-19 vaccine in Japanese rheumatic patients were reduced under immunosuppressive treatment. Differences in patient backgrounds, such as the type of disease and medication, might have led to the differences in results between our study and previous reports.
It is well known that methotrexate impairs antibody responses to influenza and pneumococcal vaccines (13,14). Methotrexate is one of the most frequently used immunosuppressive drugs in rheumatic diseases globally, and its effects on immune responses raised by COVID-19 vaccines have caused some concern. Haberman et al. reported that methotrexate hampers immune responses to BNT162b2 mRNA COVID-19 vaccination in immune-mediated inflammatory disease, and the mean antibody titre in patients on methotrexate was lower than that of patients not receiving methotrexate in their study (4). Bugatti et al. reported that methotrexate impaired the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. The antibody titre in patients treated with methotrexate was significantly lower than that in patients not on methotrexate (15). Mahil et al. reported that the humoral immune response to a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with psoriasis receiving methotrexate monotherapy was impaired compared with that in healthy controls and patients on targeted biological therapy (16). In our study, patients with rheumatic disease treated with methotrexate showed significantly lower antibody titres than those not on methotrexate. In addition, this trend was observed among patients without any biologics or JAK inhibitors. Methotrexate is often used in combination with biologics and JAK inhibitors, so it is important to consider these concomitant medications in order to understand the effect of methotrexate itself on the immunogenicity of COVID-19 vaccination. Our results, together with the above-mentioned report, suggest that methotrexate itself weakens the humoral immune response to mRNA COVID-19 vaccination.
Several data suggesting that glucocorticoids impair SARS-CoV-2 vaccine responses have been reported (3,15,17). In our study, patients with rheumatic disease who were treated with glucocorticoids showed significantly lower antibody titres than those not on glucocorticoids, despite the relatively low dose of glucocorticoids administered (prednisolone, 3.4±2.7 mg/day). The dose-dependent effect of glucocorticoids on the vaccine response was not analysed in our study because the mean dose was low. The mean glucocorticoid doses of previous studies were 6.2 and 3.8 mg/day (3,15). These results suggest that even low doses of glucocorticoids might affect the response to vaccination.
There are several limitations associated with our study. First, as mentioned above, there was a considerable difference in the median duration from the second vaccination to serum collection between the rheumatic disease patients and the control group. Although there was no clear association between the time since the second vaccination until serum collection and the antibody titre in rheumatic disease patients, it may still have affected the results of this study. Second, the antibody titre might not reflect the total vaccine efficacy. Both humoral and cellular immune responses are important in developing immunity to a virus, so the antibody titre might reflect only part of the overall response. Furthermore, the level of the antibody needed for virus immunity remains unclear. Further studies are needed to clarify the relationship between the antibody titre and vaccine efficacy. Third, this study includes a variety of rheumatic diseases and various immunosuppressants. Each immunosuppressant and rheumatic disease shows a different response to BNT162b2 vaccination (18). Finally, although no participants in this study reported any history of COVID-19, the serum antibody level was not measured before vaccination. It is therefore difficult to conclusively say whether or not participants had been exposed to SARS-CoV-2.
In conclusion, immune responses to the BNT162b2 mRNA COVID-19 vaccine in Japanese rheumatic disease patients were found to be reduced under immunosuppressive treatment. In particular, methotrexate treatment seems to be associated with a decreased antibody response. At present, how high the antibody titre needs to be to prevent infection or severe disease is unclear. Although BNT162b2 mRNA COVID-19 vaccination in rheumatic disease patients results in a lower antibody titre than in healthy subjects, the effectiveness of the vaccination has never been ruled out in this population. Therefore, rheumatic disease patients should be advised to take prophylactic measures against COVID-19 in addition to recommendations to receive BNT162b2 vaccination. Further studies with larger numbers of participants are expected to clarify the effects of individual immunosuppressants and diseases on the efficacy of mRNA COVID-19 vaccination.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Gillian Campbell, PhD, for editing a draft of this manuscript.
References
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383: 2603-2615, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 66: 1016-1026, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 80: 1330-1338, 2021. [DOI] [PubMed] [Google Scholar]
- 4. Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 80: 1339-1344, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kageyama T, Ikeda K, Tanaka S, et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect 27: 1861.e1-1861.e5, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis 73: 2065-2072, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 26: 2100096, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richards NE, Keshavarz B, Workman LJ, Nelson MR, Platts-Mills TAE, Wilson JM. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open 4: e2124331, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim N, Shin S, Minn D, et al. SARS-CoV-2 infectivity and antibody titer reduction for 6 months after second dose of BNT162b2 mRNA vaccine in healthcare workers: a prospective cohort study. J Infect Dis. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Israel A, Shenhar Y, Green I, et al. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines (Basel) 10: 64, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 80: 1306-1311, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon D, Tascilar K, Fagni F, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis 80: 1312-1316, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ribeiro ACM, Guedes LKN, Moraes JCB, et al. Reduced seroprotection after pandemic H1N1 influenza adjuvant-free vaccination in patients with rheumatoid arthritis: implications for clinical practice. Ann Rheum Dis 70: 2144-2147, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Kapetanovic MC, Roseman C, Jönsson G, Truedsson L, Saxne T, Geborek P. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum 63: 3723-3732, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Bugatti S, De Stefano L, Balduzzi S, et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis 80: 1635-1638, 2021. [DOI] [PubMed] [Google Scholar]
- 16. Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol 3: e627-e637, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deepak P, Kim W, Paley MA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv. Forthcoming. [Google Scholar]
- 18. Friedman MA, Curtis JR, Winthrop KL. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 80: 1255-1265, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


