Abstract
A 50-year-old Japanese woman with anti-melanoma differentiation-associated gene 5 antibody (anti-MDA5 antibody)-positive dermatomyositis presenting with rapidly progressive interstitial pneumonia was treated with corticosteroids and cyclosporine. She developed nephrotic syndrome during the treatment regimen with corticosteroids and cyclosporine. A kidney biopsy revealed a thrombotic microangiopathy (TMA) glomerular lesion.
Anti-MDA5 antibody-positive dermatomyositis is prone to severe interstitial lung disease (ILD) and is often exacerbated and refractory to treatment. Renal symptoms might be due to TMA of the kidney, and this may be a sign that more intensive treatment is needed. Patients sometimes develop acute kidney injury, which may be due to the TMA.
Keywords: anti-MDA5 antibody-positive dermatomyositis, rapidly progressive interstitial pneumonia, nephrotic syndrome, thrombotic microangiopathy, cyclosporine
Introduction
Corticosteroids and cyclosporin are effective and widely used for treating nephrotic syndrome. Among connective tissue diseases, dermatomyositis (DM) and polymyositis (PM) are considered to be less likely to present with renal involvement. In recent years, several autoantibodies for DM and PM have been identified. Among them, the anti-melanoma differentiation-associated gene 5 antibody (anti-MDA5 antibody) has been found to be associated with the complication of rapidly progressive interstitial pneumonia, which has a bad prognosis. This form of interstitial pneumonia is also treated with corticosteroids and cyclosporin. We herein present a case of nephrotic syndrome during the treatment of rapidly progressive interstitial pneumonia with corticosteroids and cyclosporine.
Case Report
A 50-year-old Japanese woman presented with eczema in her hands for 9 months and then on her face. She had been admitted at a local doctor's place with pneumonia seven months prior to this admission. She had presented with edema, muscle weakness, and difficulty in walking 4 months prior. She visited another local doctor and was found to be positive for anti-MDA5 antibodies. She was referred to our hospital for review by respiratory medicine and clinical immunology specialists three months prior to the suspicion of DM. She had trouble walking to the hospital on foot and was eventually brought by an ambulance. She had no remarkable medical or family history of neuromuscular diseases. Her father had died of stomach cancer.
On her first admission, she was 160 cm tall, weighed 48 kg, and had a blood pressure of 125/93 mmHg, pulse of 111 beats/min, and temperature of 36.7ºC. She had no dry cough or shortness of breath.
She was also noted to have a heliotrope rash, Gottron's papules, V sign, shawl sign, Holster sign, and mechanic's hands. She did not have Raynaud's phenomenon.
She had difficulty lifting her head, standing from sitting, and climbing stairs. She could lift and hold her arms, and she had no dysphagia or speech disturbances. She experienced quadriceps grasp pain and she had proximal muscle weakness.
Her laboratory findings are shown in Table. Her peripheral blood cell count and biochemistry revealed the following: white blood cell count, 5,100 /μL; hemoglobin, 14.2 g/dL; platelet count, 158,000 /μL; C-reactive protein, 1.15 mg/dL; lactate dehydrogenase, 1,064 IU/L; aspartate aminotransferase, 489 IU/L; alanine aminotransferase, 118 IU/L; alkaline phosphatase, 1,409 IU/L; γ-glutamyl transpeptidase, 626 IU/L; creatine kinase (CK), 277 IU/L; blood urea nitrogen, 19 mg/dL; serum creatinine, 0.58 mg/dL; and Krebs von den Lungen (KL)-6, 946 U/mL. A connective tissue workup showed elevated anti-MDA5 antibody titres (7,000 index).
Table.
Laboratory Findings.
| <Peripheral blood> | <Blood chemistry> | <Immunological findings> | |||||||||||
| WBC | 5,100 | /μL | AST | 489 | IU/L | CRP | 1.15 | mg/dL | |||||
| RBC | 454 | ×104/μL | ALT | 118 | IU/L | HBsAg | (-) | ||||||
| Hgb | 14.2 | g/dL | ALP | 1,409 | IU/L | HCV Ab | (-) | ||||||
| Hct | 44.2 | % | γ-GTP | 626 | IU/L | TPHA | (-) | ||||||
| Plt | 15.8 | ×104/μL | LDH | 1,064 | IU/L | IgG | 2,101 | mg/dL | |||||
| <Urine> | CK | 277 | U/L | IgA | 394 | mg/dL | |||||||
| pH | 6.0 | TP | 6.2 | g/dL | IgM | 136 | mg/dL | ||||||
| SG | 1.027 | Alb | 2.2 | g/dL | IgG4 | 13.9 | mg/dL | ||||||
| Protein | (3+) | LDL-C | 66 | mg/dL | C3 | 78 | mg/dL | ||||||
| 1.88 | g/gCr | HDL-C | 22 | mg/dL | C4 | 28 | mg/dL | ||||||
| 0.96 | g/day | Na | 138 | mEq/L | CH50 | 41 | IU/mL | ||||||
| Occult blood | (+) | K | 4.3 | mEq/L | P-ANCA | <10 | U/mL | ||||||
| Glucose | (-) | Cl | 102 | mEq/L | C-ANCA | <10 | U/mL | ||||||
| Keton | (±) | Ca | 8.1 | mg/dL | Centromere Ab | <5.0 | |||||||
| β2-MG | 15,961 | ng/mL | BUN | 19 | mg/dL | ANA | (-) | ||||||
| <Urine sediment> | Cre | 0.58 | mg/dL | Anti-dsDNA | <1.0 | ||||||||
| RBC | 9 | /HPF | UA | 5.7 | mg/dL | Anti-CCP | <0.6 | ||||||
| WBC | 12 | /HPF | eGFR | 85.1 | mL/1.73 m2/m | Anti-ARSAb | <5 | ||||||
| HbA1c | 6.0 | % | Anti-Scl-70 | (-) | |||||||||
| KL-6 | 946 | U/mL | Anti-MDA5Ab | 7,000 | |||||||||
ANCA: anti-neutrophil cytoplasmic antibody, CCP: cyclic citrullinated peptide, ARS: Aminoacyl-tRNA Synthetase, Anti Scl-70: Anti Scleroderma-70
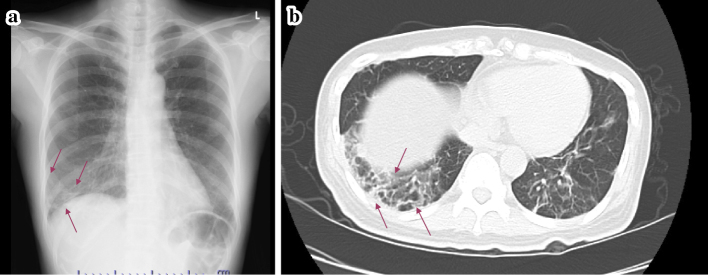
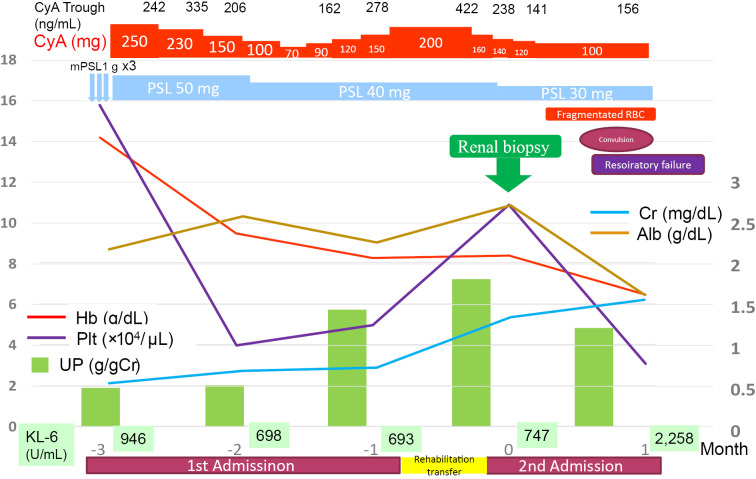
The patient's chest X-ray and computed tomography (CT) demonstrated interstitial pneumonia, which was a concern since MDA5 antibody(+) interstitial pneumonia is thought to rapidly progress (Fig. 1). The clinical course was shown in Fig. 2. She was treated with 50 mg/day of oral prednisolone (PSL) followed by pulsed methylprednisolone (1,000 mg/day for 3 consecutive days) and 250 mg/day of oral cyclosporine (CyA). The interstitial pneumonia improved, but urinary protein remained at (3+) and hypoalbuminemia (2.2 g/dL) persisted, so we were requested for a renal consultation. A renal biopsy was considered because of the persistent abnormal urinary findings despite the administration of steroids and CyA. However, a biopsy was not performed due to difficulty in moving as the patient had muscle weakness at that time. Subsequently, the dose of PSL was tapered and that of CyA adjusted to monitor its trough concentration. After one month, the dose of PSL was 40 mg/day and that of CyA 200 mg/day. Although urinary protein persisted during PSL and CyA treatment, the patient's respiratory condition remained stable and her renal function remained normal (Cr 0.77 mg/dL). She was then transferred to another hospital for further rehabilitation for muscle weakness. One month after being transferred to another hospital, she was found to have anemia, thrombocytopenia, and deteriorated renal function (Cr, 1.4 mg/dL). She was therefore re-transferred to our hospital.
Figure 1.
Chest X-ray (a) and Pulmonary CT (b) findings at admission showing interstitial pneumonia in the right lower lung (arrows).
Figure 2.
The clinical course after the 1st admission (3 months before renal biopsy: -3M) up to death (1 month after renal biopsy: 1M).
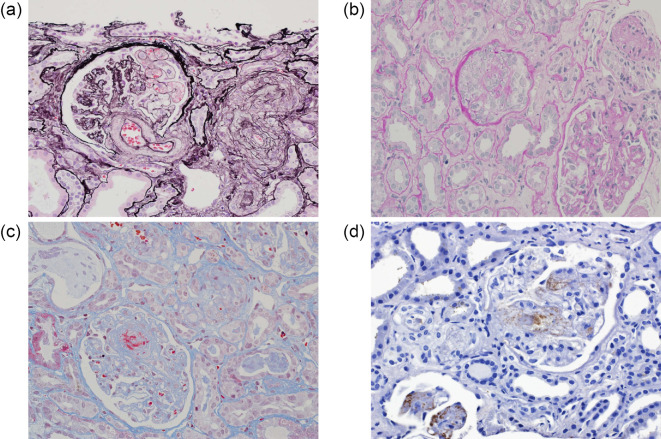
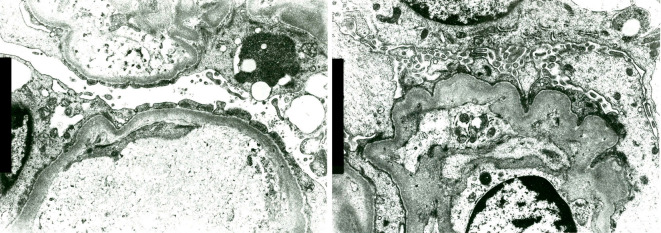
She had persistent proteinuria and hematuria that were refractory to PSL and CyA and progressed to the nephrotic range, with hypoalbuminemia and elevation of Cr. The CyA trough level was 422 ng/mL, and the dose of CyA was immediately reduced to 150 mg/day. After adjusting the dose of CyA, the patient was able to move a little with rehabilitation, and a renal biopsy was performed. The renal biopsy findings are shown in Fig. 3. Four of 17 glomeruli (24%) were crescentic, and some of them had mesangiolysis, and one of 17 glomeruli (6%) was sclerotic. Tubulointerstitial injury and arteriosclerosis were mild, and arteriolar fibrinoid necrosis was observed. Marked intimal thickening of the afferent and efferent arteries, glomerular basement membrane (GBM) duplication, endothelial detachment and shedding, enlargement of the endothelium, and partial mesangiolysis with formation of crescents, were observed. Endothelial swelling, glomerular congestion, fibrin formation, and fragmented red blood cell (RBC) at the glomerular hilum were demonstrated in Fig. 3c. Thrombi containing CD61 positive platelets in dilated glomerular capillaries are shown in Fig. 3d. All these findings were definite histological features of thrombotic microangiopathy (TMA). Immunofluorescence staining was negative for immunoglobulins and complement. Electron microscopy revealed wrinkling of the GBM, foot process effacement, degeneration and detachment of endothelial cells, and disappearance of fenestrations (Fig. 4). The pathological diagnosis was a crescent TMA glomerular lesion.
Figure 3.
Marked intimal thickening of the afferent and efferent arteries (a). GBM duplication, endothelial detachment and shedding, enlargement of the endothelium, and partial mesangiolysis with crescents (a, b) on renal biopsy. Endothelial swelling, glomerular congestion, fibrin formation, and fragmented RBC at the glomerular hilum (c). Thrombi containing CD61 positive platelets in dilated glomerular capillaries (d).
Figure 4.
Wrinkling of the GBM, foot process effacement, degeneration and detachment of endothelial cells, and disappearance of the fenestrations seen on electron microscopy.
At this point, she did not have the typical TMA symptoms, such as hemolytic anemia with crashed RBCs, progressive thrombocytopenia, and acute kidney injury (AKI). Her mild renal dysfunction (Cr 1.38 mg/dL) was suspected to be due to CyA toxicity because the trough concentration of CyA sometimes exceeded the therapeutic range (100-150 ng/mL).
Hemolytic anemia with crushed RBCs and progressive thrombocytopenia appeared 2 weeks after the renal biopsy. After that, the patient developed convulsions and symptoms of TMA. Although plasma exchange was considered, her interstitial pneumonia rapidly worsened, and she died of respiratory failure one month after undergoing the renal biopsy.
ADAMTS13 activity and its inhibitor profile were not investigated.
Discussion
Cases of renal disease as a complication of DM and PM are extremely rare. Of the 20,523 Japanese renal biopsy cases enrolled in the Japan Renal Biopsy Registry (J-RBR) between July 2007 and December 2013, 1,059 (5.2%) were due to collagen diseases and 948 due to systemic lupus erythematosus (SLE). When the remaining 110 cases were analyzed, PM/DM was observed in only one case. The only DM case showed endocapillary proliferative glomerulonephritis with immunoglobulin A (IgA) deposition (1).
Of the 65 DM or PM patients in Taiwan between 1992 and 2002, 14 (21.5%) had renal involvement. Nine (4 DM and 5 PM) patients showed acute tubular necrosis due to myoglobulinemia and myoglobinuria. Two patients with DM presented with overt proteinuria and underwent renal biopsy, which showed IgA nephropathy and membranous glomerulopathy (2).
In 150 French patients with DM and PM, renal involvement occurred in 35 (23.3%) patients, AKI in 16 (10.7%), and chronic kidney desease (CKD) in 31 (20.7%). The main cause of the AKI was drug- or myoglobinuria-induced acute tubular necrosis. Fourteen patients underwent kidney biopsy, and renal pathology revealed a wide range of renal disorders, mainly immune-complex glomerulonephritis. Five patients had a peculiar pattern of severe acute renal vascular damage consisting mainly of edematous thickening of the intima of the arterioles. A peculiar pattern of acute vascular damage is part of the spectrum of the renal diseases associated with PM/DM (3).
However, there were no cases of renal involvement of biopsy-proven TMA in PM/DM.
Regarding TMA, acquired TMA could be largely grouped into three categories: thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, and secondary TMAs. Secondary TMAs were observed in heterogeneous patient groups and were associated with drugs (calcinulin inhibitors, vascular endothelial growth factor inhibitors, etc.), connective tissue diseases, malignancies, transplantation, pregnancy, infection, and malignant hypertension. Fujimura et al. reported that 24% of TMA cases were associated with connective tissue diseases among 919 patients across Japan (Database of Nara Medical University during 1998-2008). Of the associated connective tissue diseases, SLE accounted for 42% of the cases, systemic scleroderma 23%, and PM/DM 6.4% (4).
Yamada et al. reported three patients with DM and TMA in a literature review of 13 previously reported cases. TMA with PM/DM often had a poor treatment response rate (37.5%), and even if treatment was effective, the mortality rate associated with the subsequent complications was high, and the survival rate was very low (18.8%) (5). Our patient died of rapidly progressive interstitial pneumonia.
Our patient showed mild respiratory symptoms and severe muscle weakness at admission, without any TMA symptoms. However, anti-MDA5 antibody positivity was associated with rapidly progressive respiratory dysfunction due to interstitial pneumonia, so, strong immunosuppressive therapy with corticosteroids and CyA was administered from an early stage. Fine adjustment of the dose of CyA was done while checking its trough level, which was often found to be high. The patient showed abnormal urinary findings at first admission before CyA administration, and she developed nephrotic range proteinuria 3 months after treatment with corticosteroids and CyA. The possibility of a drug (calcinulin inhibitor)-associated TMA cannot be ruled out, but it is considered to be low. Thrombocytopenia (platelet count <40,000 /μL) without fever, fragmented RBC, or psychiatric symptoms was observed one month after treatment with corticosteroids and CyA, and it was considered a side effect of the CyA. Therefore, fine adjustment of the CyA dose while checking the trough levels was performed at that time. At the first admission, renal biopsy could not be performed because of difficulty moving the patient due to muscle weakness, we could not confirm the endothelial injury, which could explain urinary findings before treatment with corticosteroids and CyA. Considering the urinary findings at first admission and the renal pathological findings (difinite TMA) at the second admission, mild endothelial injury (probably due to DM) might have already existed at first admission. At the time of renal biopsy, the thrombocytopenia had improved (platelet count: 109,000 /μL), and the symptoms of TMA, such as hemolytic anemia, fever, and psychiatric symptoms, were no longer apparent, although the renal biopsy findings showed definite TMA and the patient had a stable respiratory condition. Hemolytic anemia and convulsions became apparent two weeks after the renal biopsy, and there was an exacerbation of her respiratory status. The reduction of dose of CyA during the second admission may thus have induced the disease activity of DM and TMA.
Anti-MDA5 antibody is also known as an anti-clinically amyopathic DM (CADM) 140 antibody. The presence of anti-MDA5 antibodies in patients with CADM, especially in Asia, is significantly associated with rapidly progressive interstitial lung disease (ILD), which frequently has a poor response to treatment (6-8). Anti-MDA5 antibodies have been reported to be detected in 10-20% of DM patients, and 60-100% of anti-MDA5 antibody-positive CADM patients have been reported to develop ILD (9).
Recently, intravenous cyclophosphamide (ivCY) was administered along with corticosteroids and CyA for the treatment of severe cases of anti-MDA5 antibody-positive DM. It is regrettable that this case should have been enforced.
With regard to TMA in DM, in recent years, at least four cases of anti-MDA5 antibody-positive DM, two cases of anti-PL-7 antibody-positive DM, and one case of anti-PL-12 antibody-positive DM were reported to have TMA (10). However, the causal relationship between them is unknown.
Regrettably, the ADAMTS13 activity and its inhibitor profile were not investigated in this case, and plasma exchange should also have been a treatment option for TMA from a retrospective perspective.
Although therapeutic plasma exchange is not an established therapy for aggressive ILD, there have been some reports of interstitial pneumonia related to autoimmune diseases, including scleroderma and anti-synthetase syndrome, in which therapy-resistant interstitial pneumonia was successfully treated with plasma exchange (11-13). It is regrettable that such plasma exchange could not be performed in this case.
In conclusion, TMA can be a manifestation of renal involvement in DM/PM, especially in anti-MDA5 antibody-positive DM patients who develop severe ILD. This is the first case in which TMA has been proven by renal biopsy in severe anti-MDA5 antibody-positive DM, and this case is therefore considered to be valuable.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Ichikawa K, Konta T, Sato H, Ueda Y, Yokoyama H. The clinical and pathological characteristics of nephropathies in connective tissue diseases in the Japan Renal Biopsy Registry (J-RBR). Clin Exp Nephrol 21: 1024-1029, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Yen TH, Lai PC, Chen CC, Hsueh S, Huang JY. Renal involvement in patients with polymyositis and dermatomyositis. Int J Clin Pract 59: 188-193, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Couvrat-Desvergnes G, Masseau A, Benveniste O, et al. The spectrum of renal involvement in patients with inflammatory myopathies. Medicine (Baltimore) 93: 33-41, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: Database of Nara Medical University during 1998-2008. Intern Med 49: 7-15, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Yamada S, Yamashita H, Nakano M, et al. Thrombotic microangiopathy with polymyositis/dermatomyositis: three case reports and a literature review. Intern Med 57: 2259-2265, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 52: 1571-1576, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Sato S, Kuwana M. Clinically amyopathic dermatomyositis. Curr Opin Rheumatol 22: 639-643, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Chen Z, Cao M, Plana MN, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken) 65: 1316-1324, 2013. [DOI] [PubMed] [Google Scholar]
- 9. Chaisson NF, Paik J, Orbai AM, et al. A novel dermato-pulmonary syndrome associated with MDA-5 antibodies: report of 2 cases and review of the literature. Medicine (Baltimore) 91: 220-228, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamamoto S, Nagashima T, Akiyama Y, Nagatani K, Iwamoto M, Minota S. Fatal thrombotic microangiopathy and posterior reversible encephalopathy syndrome in a patient with anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis. Intern Med 60: 3329-3333, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omotoso BA, Ogden MI, Balogun RA. Therapeutic plasma exchange in antisynthetase syndrome with severe interstitial lung disease. J Clin Apher 30: 375-379, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Tamura K, Akiyama J, Oono K, Kadowaki S, Shimada T. A successful therapy with plasma exchange for interstitial pneumonia of progressive systemic sclerosis. Intern Med 31: 649-654, 1992. [DOI] [PubMed] [Google Scholar]
- 13. Shirakashi M, Nakashima R, Tsuji H, et al. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology (Oxford) 59: 3284-3292, 2020. [DOI] [PubMed] [Google Scholar]