Abstract
Efficient transport of solutes in the cerebrospinal fluid (CSF) plays a critical role in their clearance from the brain. Convective bulk flow of solutes in the CSF in the perivascular space (PVS) is considered one of the important mechanisms behind solute movement in the brain, before their ultimate drainage to the systemic lymphatic system. Acoustic pressure waves can impose radiation force on a medium in its path, inducing localized and directional fluidic flow, known as acoustic streaming. We transcranially applied low-intensity focused ultrasound (FUS) to rats that received an intracisternal injection of fluorescent CSF tracers (dextran and ovalbumin, having two different molecular weights–Mw). The sonication pulsing parameter was determined on the set that propelled the aqueous solution of toluidine blue O dye into a porous media (melamine foam) at the highest level of infiltration. Fluorescence imaging of the brain showed that application of FUS increased the uptake of ovalbumin at the sonicated plane, particularly around the ventricles, whereas the uptake of high-Mw dextran was unaffected. Numerical simulation showed that the effects of sonication were non-thermal. Sonication did not alter the animals’ behavior or disrupt the blood-brain barrier (BBB) while yielding normal brain histology. The results suggest that FUS may serve as a new non-invasive means to promote interstitial CSF solute transport in a region-specific manner without disrupting the BBB, providing potential for enhanced clearance of waste products from the brain.
Subject terms: Biotechnology, Neuroscience
Introduction
The brain bears high sensitivity/vulnerability toward changes in the extracellular environment, and demands efficient waste clearance due to its higher metabolic rate compared to the other organs1,2. Aberrant waste transport and clearance from the brain has been implicated with aging3,4, traumatic brain injury5,6, stroke7–9, idiopathic normal pressure hydrocephalus10,11, and various neurodegenerative conditions, especially with an emerging link to dementia12 and Alzheimer’s disease (AD)13. Although the exact mechanism of waste transport from the brain is not clearly understood, convective bulk flow, diffusive, or aquaporin-4 channel-mediated (known as ‘glymphatic’) transports, and their combinations mediated by the extracellular fluid in the brain have been identified to play important roles1–3,14,15.
The extracellular fluid in the brain consists of cerebrospinal fluid (CSF) and interstitial fluid (ISF) filling the interstitial space2,15. The CSF and ISF, along with metabolites and waste products from the brain, are believed to be continuously exchanged/transported through various routes before entering the lymphatic system1–3,14,15. The CSF occupies the sub-arachnoid space (SAS) between the arachnoid mater and pia mater, and the pial arteries/veins supply the blood to the brain parenchyma through penetrating vasculature. The blood–brain barrier (BBB), mainly formed by the tight junctions of cerebral vascular endothelial cells and the glia limitans that surround the brain parenchyma, separates the blood circulating in the vasculature from the brain parenchyma while allowing for passive diffusion of water and lipid/water-soluble molecules as well as for active transport of key nutrients16,17.
The CSF, separated from blood flow through the blood-cerebrospinal fluid barrier (BCSFB), moves through the perivascular space (PVS) around cerebral arterioles, venules and capillaries, and partially mediates crucial bulk movement of solutes across the brain. Arterial cardiac pulsation was identified as one of the important driving forces behind CSF flow through the PVS18, while the numerous microscopic fenestrations on the surface of the pia and leptomeningeal vessels (called ‘stomata’, ~ 3 μm in diameter in humans)19 provide potential conduits for CSF and macromolecules/macrophages to reach the PVS19,20. The large pial surface and widespread existence of solute transport routes on the cerebral meninges, combined with the mechanical pressure gradient created by arterial pulsation, allow non-diffusional, convective bulk flow of CSF to reach the PVS, which is considered one of the major mechanisms for solute transport and clearance in the brain18,21,22.
Insonification of acoustic pressure waves can impose radiation force on a medium in its path, thus inducing localized directional fluidic flow, known as acoustic streaming23–25. The acoustic streaming effect has been shown to create convective bulk flow that moves dye molecules through aqueous media in porous, soft tissue-mimicking materials26, and is used in applications such as propelling/mixing fluids in microfluidic devices27–29, or to detect the presence of fluids in the breast or ovarian cysts30,31. Advances in focused ultrasound (FUS) techniques have allowed for non-invasive and targeted transcranial delivery of acoustic pressure waves to a small region of the brain, with flexibility in controlling the depth/location of the acoustic focus32–37. Non-thermal mechanical energy conferred by the ultrasound has already been used in various applications such as brain stimulation38–40 and drug delivery36,41.
We were motivated to examine if acoustic streaming created by the application of transcranial FUS (tFUS) can regionally enhance convective flow of CSF, and thus promotes CSF solute movement in a non-thermal and non-destructive fashion. We first applied FUS in both pulsed and continuous modes to induce fluidic flow of soluble dyes (toluidine blue O, TbO, 270-Da molecular weight–Mw) in porous media (i.e., melamine foam). The pulsing parameter that resulted in the highest dye infiltration was subsequently used in rodent in vivo experiments whereby we applied tFUS to a localized brain area and examined the spatial distribution of intracisternally injected fluorescent CSF tracers (dextran and ovalbumin, having two different Mw). We hypothesized that regional administration of acoustic pressure waves to the rat brain would facilitate CSF bulk flow into the brain parenchyma, accompanied by the movement of intracisternally injected CSF tracers.
Results
FUS transducer and sonication parameters
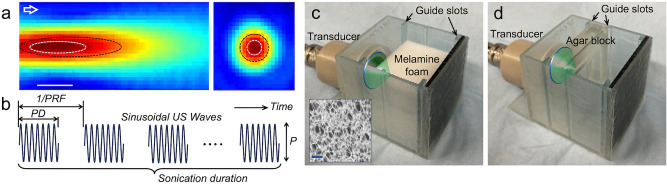
A FUS transducer, operating at a 200 kHz fundamental frequency (FF), generated an acoustic focus 11 mm away from the exit plane of the transducer. The acoustic focus, 5 mm in diameter and a 15 mm long, was defined by the profile bound at full-width at 90% maximum pressure (FW90%M; Fig. 1a in white dotted profile), or a 7 mm diameter and a 27 mm length defined at full-width-at-half-maximum (FWHM; black dotted profile in Fig. 1a). The 200 kHz FF was selected considering future transcranial delivery of ultrasound in humans. The schematics and nomenclature of the FUS pulsing parameters are described in Fig. 1b. Bursts of sinusoidal ultrasound waves with defined peak-to-peak pressure amplitudes (P), each of a specific pulse-duration (PD), were administered in a repeated fashion at a pulse repetition frequency (PRF). The PD and PRF together determine the resulting sonication duty cycle (DC) (in %, indicating the fraction of active sonication time per second). The overall duration of pulsed sonication is termed as sonication duration. Acoustic intensity, i.e., acoustic power per given area (W/cm2), is expressed in spatial-peak pulse-average intensity (ISPPA) while the spatial-peak temporal-average intensity (ISPTA) is used to represent its time-averaged value.
Figure 1.
Schematics of the experimental setup and dye infiltration test. (a) Pressure profile along the longitudinal and transverse plane of the FUS focus (bar = 10 mm). (b) Illustration of the temporal features of sonication parameters used in the experiment. (c) Experimental setup for TbO dye infiltration into the melamine block (shown without the TbO solution, inset: microscopic view of the foam, bar = 100 μm). (d) A setup for sonicating the agar block shown without the dye solution. The chamber contained guide slots to position the acoustic focus at 1 mm in front of the surface of the block. The green cones illustrate the FUS sonication to the target.
Dye infiltration test
We first examined the feasibility of using acoustic streaming to facilitate fluidic bulk flow into porous melamine foams (pore diameter range of ~ 10–100 μm), which roughly approximated the porosity of cerebral perivascular stomata19,20. TbO dye (T3260, Millipore Sigma, Burlington, MA) was dissolved at 25 µg/mL concentration in phosphate buffered saline (PBS, Thermo Fisher, Waltham, MA) for visualization. The foam was hydrated in clear PBS and transferred to a chamber with rubber pad backing (for sound absorption) that contained the dye solution (Fig. 1c, shown without the dye solution).
Two sets of experiments were taken in sequence. First, FUS was applied every second (i.e., PRF 1 Hz) to the front of the foam block, with a 1 mm gap between the acoustic focus and the surface of the block, for 10 min using different PDs (100, 250, 500, 750 ms) including a continuous wave (CW) condition. ISPPA was adjusted to 5, 2, 1, 0.67, and 0.5 W/cm2, respectively, to maintain an ISPTA of 0.5 W/cm2. The corresponding DCs were 10, 25, 50, 75, and 100%. Higher ISPPA values and longer pulse/sonication durations would generate higher acoustic streaming effects42, but accompanies a higher susceptibility for heat generation, thus ISPTA was fixed at 0.5 W/cm2. The solution was maintained at room temperature (24.2–24.6 °C) during sonication. Following sonication, foams blocks (n = 10 in each group) were cut through the midpoint along the sonication direction, then imaged using a flatbed photo scanner. The images were segmented by a threshold intensity of 150 to delineate the area of dye infiltration. Sonication administered at an ISPTA of 0.5 W/cm2 yielded visible infiltration on the foam block (example shown in Fig. 2a,b). Image analysis revealed that the use of 100 ms PD, given at a 10% DC at an ISPPA of 5 W/cm2 yielded the greatest infiltration diameter over the foam surface (16.5 ± 1.6 mm, average ± standard deviation, n = 10) and depth (6.1 ± 1.0 mm) among different PD conditions, including continuous wave (Fig. 2e,f, post-hoc Tukey HSD, P < 0.001 following ANOVA; F(4,45) = 13.3 from diameter measurement and F(4,45) = 11.6 from depth measurement).
Figure 2.
Results from the dye infiltration test. (a) Exemplary front view of a melamine foam showing dye infiltration and (b) the middle section (dashed line from the front view) showing the dye infiltration depth. The green triangle depicts the direction of sonication. (c) Front view and (d) the section view of an exemplar agar block that does not show sonication-related dye infiltration. The effects of different pulse durations (PDs in ms; CW = continuous wave) on (e) infiltration inlet diameter and (f) depth. The effect of FUS given at 100 ms PD with a DC of 10% on the (g) inlet diameter and (h) depth of TbO infiltration. ISPTA was maintained at 0.5 W/cm2 throughout the conditions. Error bar: standard error. *post-hoc Tukey HSD, P < 0.001 following ANOVA.
Another dye infiltration experiment was conducted using a fixed PD of 100 ms (which generated the highest infiltration in the first set) with different DCs (10, 20, 30, 40, and 50%) by adjusting the PRF to 1, 2, 3, 4, and 5 Hz respectively. To maintain the ISPTA at 0.5 W/cm2 across different DCs, ISPPA was adjusted to 5, 2.5, 1.67, 1.25, and 1 W/cm2, respectively. We found that the use of a 10% DC given at ISPPA of 5 W/cm2 generated the greatest diameter (17.6 ± 0.9 mm) on the foam surface (Fig. 2g with post-hoc Tukey HSD, P < 0.001 following ANOVA; F(4,45) = 20.5). In terms of infiltration depth, both 10% and 20% DCs (given at 5 and 2.5 W/cm2 ISPPA, respectively) resulted in a greater level of infiltration compared to those from using other DCs (Fig. 2h; post-hoc Tukey HSD, P < 0.001 following ANOVA: F(4,45) = 20.5). The use of a 10% DC yielded the deepest infiltration into the melamine block (6.4 ± 1.1 mm).
We also examined if FUS can facilitate solute movement in a material much denser than melamine foam. The agar hydrogel blocks (n = 10), having similar porosity as that of brain parenchyma (40–80 nm)43,44, were inserted to a cassette and immersed in the TbO-PBS solution (Fig. 1d shown without the solution). Then, FUS was delivered to the front face of the agar hydrogel block using the sonication parameter that resulted in the highest infiltration in the melamine foam (i.e., 100-ms PD at a 10% DC). The sonication did not have an impact on dye infiltration (0.57 ± 0.10 mm) compared to the unsonicated area (0.54 ± 0.11 mm; paired t-test, P > 0.1). The diameter could not be measured in the absence of visible dye marking on the agar surface (Fig. 2c,d).
Enhanced CSF tracer movement by tFUS
Rats (n = 6 in each group, total 3 groups) were anesthetized, then placed on a stereotactic frame to deliver CSF tracers intracisternally based on an established surgical protocol45. The CSF tracers were medium MW (45 kDa; similar MW to the neuroactive Aβ oligomers in AD46, ~ 8–70 kDa) Texas Red Ovalbumin (OA) and high MW (2000 kDa) fluorescein isothiocyanate (FITC)-dextran (FITC-d), being constituted at a 0.5 wt% concentration in artificial CSF (aCSF).
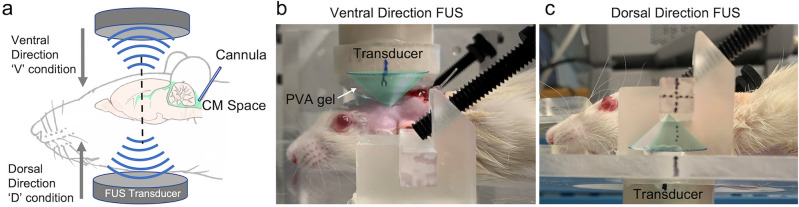
Upon administering the CSF tracers, the animal was positioned on a mechanical sonication setup that stereotactically delivered FUS sonication. With 7 min time gap between end of CSF tracer injection, FUS was transcranially delivered 2 mm caudal to the bregma in ~ 1 mm depth from the top of the head (ventral direction to the brain, noted as ‘V’ condition) as well as from the neck area (dorsal direction to the brain, ‘D’ condition, Fig. 3). A polyvinyl alcohol (PVA) hydrogel was used as a compressible acoustic coupler, and ultrasound gel was applied to all interfaces. When applying FUS through the neck, the transducer was positioned in a manner that avoided the mandibular osseous structures in its path. The sonication was given at a 100 ms PD every second (10% DC) at an ISPTA of 0.5 W/cm2 (ISPPA = 5 W/cm2; peak rarefactional pressure (Pr) = 386 kPa) for 30 min. We performed the same experiment without sonication (i.e., the control condition, noted as ‘C’ condition) while maintaining the same time sequence as the sonication experiment.
Figure 3.
Schematics of the sonication setup for enhancing CSF solute movement in rat brain. (a) Illustration of the tFUS setup and the site of the CSF tracer injection. The transducer was mounted to a 2-axis mechanical stage and the animal was positioned over the stereotactic frame that moves vertically. (b) Sonication was delivered in the ventral direction through coupling PVA hydrogel and (c) to the dorsal direction below the neck. The green cones depict the acoustic profile.
The animals’ weights were indifferent across the groups (C:V:D = 282.3 ± 17. 0 g: 278.5 ± 14.3 g: 281.7 ± 10.1 g; ANOVA; F(2,15) = 0.126; P = 0.88). The starting heart/respiratory rates were not different across the groups (C:V:D = 218.2 ± 16.4: 217.7 ± 19.8: 220.7 ± 17.8 beats-per-min [bpm], ANOVA; F(2,15) = 0.048; P = 0.95 for the heart rate; C:V:D = 60.7 ± 3.0: 61.3 ± 3.3: 58.7 ± 6.5/min; ANOVA; F(2,15) = 0.556; P = 0.59 for the respiratory rate). The ending heart/respiratory rates were not different across the groups (C:V:D = 216.2 ± 18.4: 217.0 ± 22.7: 217.3 ± 19.7 bpm; ANOVA; F(2,15) = 0.005; P = 0.99 for the heart rate; C:V:D = 59.3 ± 3.0: 57.3 ± 7.3: 58.3 ± 5.3/min; ANOVA; F(2,15) = 0.205; P = 0.82 for the respiratory rate). Heart/respiratory rates did not change before and after the sonication within each condition (paired t-test, two-tail, all P > 0.2).
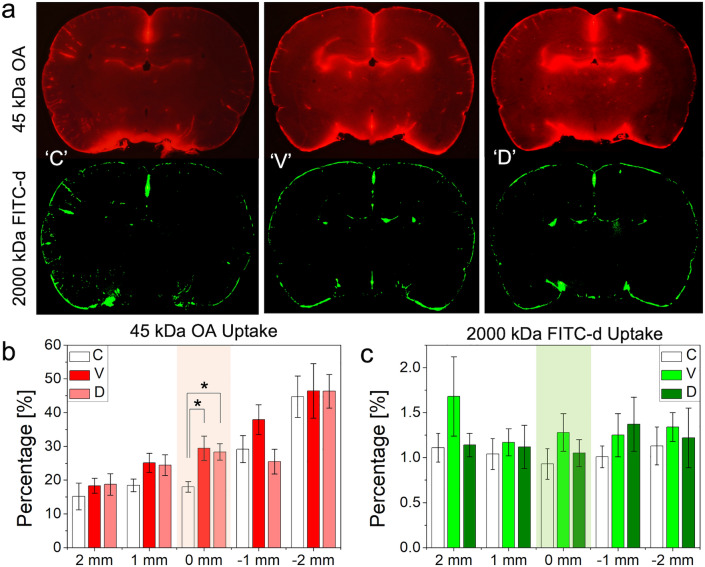
Fluorescence imaging showed that FUS increased the uptake of OA at the sonicated plane, especially around the ventricles (an example shown in Fig. 4a). Quantitative image analysis across the rostral-caudal sections with respect to the sonicated plane showed that the OA distributed much more extensively in the sonicated plane from FUS conditions (both the ‘V’ and ‘D’ conditions) compared to no sonication (post-hoc Tukey HSD, P < 0.05 following ANOVA; F(2,15) = 5.42, Fig. 4b). The direction of the sonication did not affect the degrees of enhancement (post-hoc Tukey HSD following ANOVA, P = 0.96). The spatial distribution of high MW FITC-d in the brain, on the other hand, was more restricted than that of OA (only ~ 1% uptake) and remained unaffected by the sonication (Fig. 4c; ANOVA, across all sections, F(2,15) < 1.3 and P > 0.32).
Figure 4.
Image analysis of CSF tracer distribution across the experimental condition. (a) An exemplar image of OA (in red) and FITC-d (in green) at the coronal planes of sonication across no FUS (‘C’) and FUS conditions (‘V’ and ‘D’). (b) % uptake of OA and (c) FITC-d (n = 6 in each group) across the rostral ( +) and caudal (− ) planes surrounding the sonication (shown in mm;—rostral, + caudal). Colored sections indicate the location of the acoustic focus. *: P < 0.05; error bars: standard error.
Numerical simulation of thermal analysis
Although low-intensity (ISPTA of 0.5 W/cm2) ultrasound was used, we conducted a numerical simulation to model the propagation of acoustic waves in the cranial cavity to evaluate the presence of tissue heating. The simulation was performed using a two-step process: (1) simulation of acoustic propagation and (2) subsequent thermal estimation at the brain tissue as well as at the skull tissue interfaces.
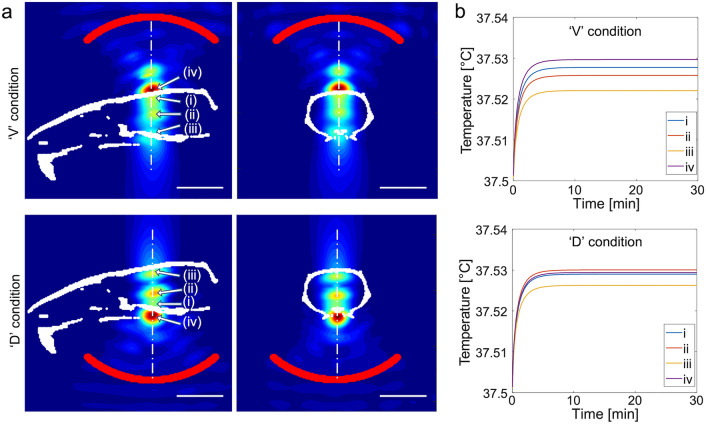
The simulated spatial distributions of the acoustic intensity obtained from a rodent skull are shown at the mid-sagittal/coronal sections along the sonication path for ventral and dorsal FUS conditions (Fig. 5a). In addition to the geometric focal areas (indicated by (i)), the clear presence of reverberation inside the cranial cavity was detected near the midpoint between the skull base and cap (i.e., nearby the ventricles, indicated by (ii)). Another local maximum was seen at the brain tissue interfacing the bone opposite to sonication (iii). The highest intensity occurred at the skull surface facing the incident FUS waves (noted as ‘iv’). This pattern was reproduced in other skull samples, and the averaged ISPPA and peak-to-peak pressure amplitude were tabulated from different locations (‘i’–‘iv’, Table 1). The acoustic reverberation yielded an in situ ISPPA greater than 5 W/cm2 at some of these local maxima (e.g., nearby the ventricles and the skull surface facing the incident acoustic waves). Despite reverberations, thermal modeling indicated that the temperature at these areas exhibited only a slight elevation of < 0.03 °C, reaching a steady-state maximum within 10 min of sonication (Fig. 5b).
Figure 5.
Numerical simulation of the acoustic propagation and thermal rise from different tissue locations. (a) Pseudo-colored relative acoustic intensity normalized with respect to the maximum value obtained from the numerical simulation of acoustic intensity along the plane of acoustic focus (obtained from one rat skull, outlined in a white cranial profile) from the ‘V’ and ‘D’ conditions. The profile of the transducer is marked by the red line. Bar = 10 mm. (b) Numerical estimation of tissue temperature at (i) the geometric acoustic focus, (ii) local maximum near the center of the brain, (iii) brain-skull interface opposite to the sonication path, as well as (iv) the skull surface facing the incident sonication.
Table 1.
Percent intensity transmission (with respect to the free-water maximum of 5 W/cm2 ISPPA), in situ ISPPA (in W/cm2), and peak-to-peak pressure (P, in kPa), numerically estimated at four different locations—(i)–(iv) from Fig. 5. Average ± standard deviation (n = 3).
| Location | ‘V’ condition | ‘D’ condition | ||
|---|---|---|---|---|
| ISPPA (W/cm2) | P (kPa) | ISPPA (W/cm2) | P (kPa) | |
| i | 4.5 ± 0.4 | 726.7 ± 29.0 | 4.4 ± 0.1 | 718.3 ± 8.0 |
| ii | 5.1 ± 0.6 | 775.3 ± 45.8 | 6.5 ± 0.4 | 873.7 ± 27.1 |
| iii | 3.9 ± 0.7 | 674.7 ± 62.6 | 5.4 ± 0.3 | 801.7 ± 24.6 |
| iv | 7.1 ± 0.5 | 913.3 ± 32.3 | 7.6 ± 1.6 | 947.0 ± 103.2 |
Histological analysis and evaluation of BBB disruption
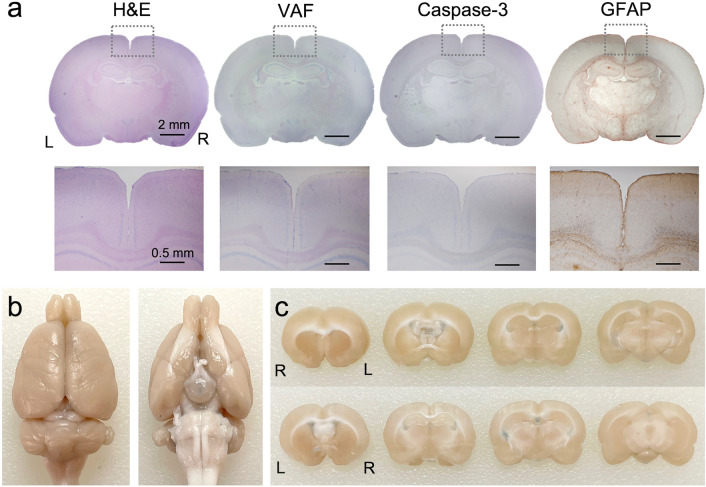
Six male SD rats (weighing 275.5 ± 8.2 g) that did not receive CSF tracer injections were divided into three groups. Two in each group underwent sonication in either ventral or dorsal direction and were subjected to different post-FUS survival duration before sacrifice (immediate, n = 2; 2 weeks, n = 2; 4 weeks, n = 2). The animals’ behavior was monitored during the survival period (30 min observation, 3 days/week). All animals showed normal behavior after the sonication sessions throughout their survival period. The histological analysis of the sonicated brain regions did not reveal any signs of tissue damage in any animal (exemplary data shown in Fig. 6a) in terms of global tissue integrity/hemorrhaging (from H&E), ischemic (VAF-toluidine blue) and apoptotic damage (caspase-3), including glial infiltrations (GFAP). Visual inspection of gross brain anatomy (ventral and caudal view) and coronal brain sections from rats that received an intravenous injection of trypan blue immediately after sonication (n = 2) revealed no visible signs of dye extravasation, suggesting the absence of BBB disruption (Fig. 6b).
Figure 6.
Exemplary histology analysis results of brain tissues and evaluation of blood–brain barrier disruption. (a) Top row: H&E, VAF-toluidine blue, caspase-3 and GFAP (from left to right column) stained microscopic images of sonicated brain slices. bar = 2 mm, Bottom row: magnified images from the defined region-of-interest (dotted rectangles). bar = 0.5 mm (b) Tail vein injection of trypan blue immediately after FUS sonication revealed no signs of blood–brain barrier disruption in a gross brain specimen and (c) 2 mm-thick coronal brain sections shown in rostral and caudal sides. L = left; R = right.
Discussion
Dye infiltration test
Previous studies have shown the utility of the acoustic streaming effect in enhancing drug delivery (e.g., anti-tumor agents) to the brain (referred to as convection-enhanced delivery)47,48. Recently, Aryal and colleagues demonstrated that application of diagnostic-intensity FUS operating at 650 kHz in a scanning mode (i.e., continuous sonication along the rectangular trajectory that loops inside of the brain in every 24 s) enhanced the brain parenchymal delivery of intrathecally-injected optical tracers (1 kDa) and panitumumab (150 kDa) in rats49. While these studies have provided promising perspectives of using acoustic pressure waves to facilitate CSF solute movement, the acoustic streaming effect involves complicated non-linear fluid dynamics for the transport of small molecules/particles through porous media24, especially in the pulsed mode of operation25,50. Thus, the effects of pulsing parameters on enhancing TbO dyes to the non-homogenous porous media (melamine foam) were first evaluated, then used to guide the rodent experiment.
Image analysis revealed that the use of 100 ms PD and a 10% DC given at ISPPA of 5 W/cm2 yielded the greatest dye infiltration diameter and depth across the tested parameters (Fig. 2e,f). The infiltration was reduced when a PD longer than 100 ms was used, including the continuous wave condition. Lower ISPPA values (≤ 2 W/cm2) used under these conditions (to maintain the ISPTA at 0.5 W/cm2) suggests that the degree of dye movement was enhanced at a higher ISPPA among the tested parameters. When a fixed PD of 100 ms was applied in different DCs (10–50% with 10% increment by adjusting the PRF), the use of low DCs (10% and 20% given at 5 and 2.5 W/cm2 ISPPA, respectively) resulted in a greater level of dye infiltration compared to the use of higher DCs (Fig. 2g,h). These findings suggest that the increasing DC alone, given at a constant ISPTA, would not enhance acoustic streaming while the use of a higher ISPPA would favor induction of acoustic streaming. The observation also agrees with previous work by Hoyos and colleagues, which estimated the velocity of fluorescent particles (800 nm in diameter) within a microfluidic channel under exposure to pulsed ultrasound waves25. However, excessively high ISPPA values would eventually create inertial cavitation, and should be avoided for biological applications.
TbO dye infiltration into the melamine foam became less evident when sonication was delivered to the agar gel blocks (Fig. 2c,d). This suggests that acoustic streaming is less likely to affect the dye movement in materials with sub-micron porosity whereby diffusion becomes the dominant mode of transport over convective bulk flow. Since the agar gel blocks, having a similar porosity to the brain parenchyma (40–80 nm43,44), were sonicated to estimate the effects of acoustic streaming, we conjecture that FUS will not likely to influence solute movement within the dense brain neuropil, in which convective bulk flow is not likely to be a dominant source43,51,52.
Enhanced CSF/interstitial tracer movement by tFUS
We acknowledge that the porosity and complexity of the melamine foams cannot replicate the complicated brain cytoarchitecture. Furthermore, the biodistribution of synthetic TbO dyes in the CSF is poorly understood. These limitations necessitated the in vivo testing in rodents using CSF tracers such as OA and FITC-d. Fluorescent images showing the uptake of OA after a 30 min of tracer injection resembled the OA distribution from previous investigations14,21,51. Sonication specifically enhanced the OA uptake at the sonicated section, regardless of the sonication direction (ventral or dorsal), showing almost doubled uptake (~ 30% occupancy, Fig. 4b) compared to the no-FUS condition (which showed only ~ 18%, Fig. 4b). Based on the numerical simulation of acoustic propagation (Fig. 5), we found that an additional acoustic focus was formed in the middle of the sonication path in the brain (nearby the ventricles), with an acoustic intensity (thus pressure level) even slightly higher than the intended ISPPA. It is likely to stem from constructive interference of incident pressure waves and reflected from the opposite end of the skull; i.e., reverberation. The formation of these local pressure maxima might have enhanced acoustic streaming near the ventricle CSF space, further propelling CSF solutes to the surrounding area, yielding the observed OA distribution pattern.
Although the acoustic focus (5 mm in diameter and 15 mm in length, defined at FW90%M) was large enough to sonicate nearly the entire brain section in its path, the slices, ≥ 1 mm rostral or caudal to the sonication, did not show differential effects from the sonication. The numerical simulation also revealed that the acoustic focus formed in a restricted area in the rostral-caudal direction, which also support the observed selective OA uptake distribution pattern in the coronal section. Heart rate, which is known to be associated with the degree of solute transport in the brain18,21,53, was indifferent across the conditions, thus its contribution to differential OA transport was unlikely. Taken together, these results suggest that FUS enhanced the movement of the OA in the CSF to the brain that received sonication, with high sensitivity to the spatial intensity profile.
We note that the fixation method employed (paraformaldehyde (PFA) perfusion) is known to greatly reduce the volume of PVS18, thus confounding the interpretation of fluorescence imaging. The PFA perfusion fixation, uniformly adopted across the experimental conditions, would not have impacted the interpretation of the results, but may have reduced the observed fluorescence level in the brain tissue. Use of imaging techniques such as in vivo two-photon microscopy18 or infrared confocal fluorescence imaging54 would be helpful to monitor CSF tracer movement in live animals as well as to probe the contribution from potential expansion of the extracellular and perivascular space that may accompany pulsed application of ultrasound55.
The use of low frequency (200 kHz) ultrasound waves in the present study, albeit advantageous for transcranial application, was prone to generating intracranial reverberations due to the longer wavelength compared to the rodent cranial dimension. The use of an animal model having larger cranium, such as sheep or non-human primates would be beneficial to reduce/eliminate reverberation. In fact, the 200 ~ 300 kHz frequency range has been safely used in studies of FUS-mediated functional brain neuromodulation among sheep40 and humans56 without the effects of reverberation. Alternatively, the use of higher frequency ultrasound can be sought after, especially applicable to assessment among small animals, with added potential merits in creating acoustic streaming effects at higher efficiency25. The use of higher frequency (650 kHz) ultrasound, operating at a lower intensity, DC (7.7%), and a shorter sonication duration (10 min) than the present study, has shown to enhance the CSF solute uptake far greater than the one observed herein49. Since acoustic streaming is also known to be governed by the wavelength and amplitude shape of acoustic pressure waves57, the effects of pulsing scheme, including the effects of amplitude-modulation of acoustic waveforms (other than standard sinusoidal waves), on FUS-mediated CSF solute transport warrant further investigation.
The distribution of high Mw (2000 kDa) FITC-d occupied much smaller areas than the uptake of OA (~ 1% uptake per given section of the brain area), which suggests that CSF solute movement is size-dependent. The results matched well with previous investigations showing inferior efficiency in transporting large exogenous macromolecules14,51,58 which shared clinical relevance to the accumulation of large aβ plaques and fibrils in AD59,60. Unlike 45 kDa OA with its movement enhanced by the sonication, FUS did not affect the movement of high Mw (2000 kDa) FITC-d in the present study (Fig. 4c), which suggests the presence of size limit of solutes that can be transported by the FUS. 150 kDa-sized intrathecally-injected panitumumab, much bigger than 45KDa OA, has shown to be efficiently transported to the brain parenchyma through the application of FUS49, and calls for further investigation of gauging the effects of FUS on moving various types and size of CSF solutes.
To estimate the solute movement patterns enhanced by the sonication, a numerical approach to model the convective flow associated with the acoustic streaming effects can be considered. However, acoustic streaming, especially in non-homogenous media such as the brain, would involve highly complicated nonlinear fluid dynamics that depend on many unknown acoustic properties in brain tissue24,25,50. In addition, complex and inhomogeneous brain cytoarchitecture are largely unknown, including anisotropic porosities and spatial orientations of specific neuropil as well as structures of perivascular CSF space43. Furthermore, contribution of the arterial pulsation on the CSF flow patterns18 is another important factor to be considered in numerical estimation. Thus, development of numerical simulations describing the effects of acoustic streaming on CSF solute transport within the entire brain volume would meet great challenges and constitutes a subject for future investigation.
Thermal analysis and safety considerations
Thermal simulations showed that low-intensity sonication used in the present work is unlikely to cause temperature elevation in the brain tissue, despite the formation of intracranial acoustic foci having a slightly higher acoustic intensity. To ensure the absence of thermal confounders, magnetic resonance imaging (MRI)-based thermometry61,62 can be adopted to monitor temperature changes caused by sonication in vivo. Nevertheless, comprehensive histological analyses obtained from the animals that received sonication (Fig. 6a), along with the observed normal animal behavior, attest to the safety of the transcranial application of pulsed FUS to the rat brain.
Regarding the mechanical impact on biological tissue, especially associated with undesirable cavitation, the use of a 386 kPa peak rarefactional pressure (Pr) in the present study did not yield signs of brain tissue damage. Although the cavitation threshold for the brain tissue was not measured from the present study, approximately 580 kPa Pr produced cavitation in an agar phantom63 (3 s continuous waves at 1 MHz) while ~ 800 kPa Pr (continuous 1 MHz sonication) was estimated to generate cavitation in water, as predicted by theoretical models64. These Pr values are much higher than the one used in the present study, which support our observation that cavitation was unlikely. As the application of longer sonication pulses tends to induce cavitation at a lower Pr than a single pulse64, further study on the systematic evaluation of cavitation threshold in the brain tissue across the different PDs and frequency range would be helpful in gauging the safety of long pulse ultrasound.
Potential application to promote waste removal from the brain
We found that non-thermal tFUS sonication facilitated regional CSF movement of 45 kDa-Mw OA in the rodent brain without disrupting the BBB (Fig. 6b). This led us to believe that the technique can be used to facilitate waste clearance from the brain, including similar-sized Aβ monomers and oligomers (precursors for plaque formation46) that are associated with Alzheimer’s disease (AD). Regional enhancement of CSF solute clearance, especially on Aβ, has been demonstrated in a murine model of early dementia65 by actively disrupting the BBB using intravenous ultrasound microbubble with concomitant application of FUS. However, the technique is vulnerable to potential risks for cavitation-related tissue damage (e.g., hemorrhaging66,67) as well as modification of local hemodynamic properties68–70. If shown to facilitate the solute removal from the brain without disrupting the BBB, the present method may provide elegant and unprecedented means to enhance clearance of waste products from the brain, including Aβ/tau proteins. Further investigation is needed to examine the detailed effects of sonication parameters on promoting the clearance of interstitial tracers, e.g., intracortically injected radio/fluorescent-labeled Aβ oligomers.
As a completely non-invasive technique, we envision that transcranial FUS can be applied to a wide spectrum of neurological conditions associated with aberrant brain lymphatic function and suboptimal clearance of the metabolic waste. For example, compromised local fluid-exchange inside the infarct core due to stroke lead to the accumulation of proinflammatory cytokines, causing brain inflammation and neurodegeneration71–73. Furthermore, as the white matter tracts in the brain consist of myelinated nerve bundles that allow for anisotropic fluid movement74,75, enhancement of convective CSF motion along the tracts may also be conceived as a future extension of the technique for possible amelioration of the impaired lymphatic clearance implicated with multiple sclerosis76,77. It is also plausible to conjecture that facilitated lymphatic function of the brain may compensate for suboptimal clearance of metabolites burden (e.g., lactate and Aβ), associated with sleep deprivation78–80.
New findings in the present study are summarized herein. First, a specific pulsing scheme (i.e., 100 ms PD operating at 10% DC) provided superior transport efficiency than continuous waves based on the dye infiltration test. Secondly, the technique can regionally transport CSF solutes (OA) having a much higher MW than most CNS drugs (typically < 1 kDa), which have been transported by existing CED techniques. On the other hand, there was a limitation on the molecule size for our technique to enhance the transport (2000 kDa dextran). The FUS-mediated regional transport of OA, bearing size similarity to the neuroactive Aβ oligomers in AD (sized ~ 8–70 kDa), would become particularly important, for example, for enhancing the transport of Aβ proteins that preferentially accumulate in a region-specific fashion81.
Conclusions
We have demonstrated that localized application of pulsed tFUS safely promoted the movement of intracisternally injected CSF tracers into the rat brain parenchyma. Although the transport of solutes in the CSF plays an instrumental role in their clearance from the brain, further investigation of the effects of tFUS on directly promoting solute clearance from the brain is urgently needed, including examination of the effects of sonication parameters beyond those tested, to position tFUS as new neurotherapeutic platform.
Methods
FUS transducer
A FUS transducer (GS200-D25-P38, Ultran Group, State College, PA) was actuated by a sinusoidal electrical waveform amplified by a linear power amplifier (240L, Electronics and Innovations, Rochester, NY). A function generator (33500B, Keysight, Santa Rosa, CA) was used to produce the initial waveform. The spatial distribution of the acoustic intensity profile was characterized in degassed water tank using a method described elsewhere in detail62,82. The FUS transducer was immersed in degassed water (< 2 ppm oxygen level; measured by colorimetric K-7512 ChemMets kit, Chemetrics, Midland, VA), and the longitudinal pressure profile along the sonication axis was mapped using a needle hydrophone (HNC-200, Onda Corp, Sunnyvale, CA) mounted to a robotic, 3-axis linear stage (Bi-Slides, Velmex Inc., Bloomfield, NY) with a 1 mm step covering a 5 cm × 3 cm rectangular area (5 mm away from the exit plane of the transducer). The focus was defined at the location having the maximum pressure, and the pressure profile perpendicular to the acoustic focus was also measured with a 1 mm step covering 3 cm × 3 cm. The pressure at the focus was calibrated with respect the input voltage using a calibrated hydrophone (HNR-500, Onda Corp, Sunnyvale, CA).
Melamine foam and agar hydrogel blocks for dye infiltration tests
Melamine foams (IEXL21, Sponge outlet, Kenmore, NY), having pore diameter range of ~ 10–100 μm, were cut 50 mm × 45 mm × 35 mm (width × height × thickness). Agarose power (A4018, low gelling temperature, Millipore Sigma) was dissolved in PBS at concentration of 2.5% w/w in 50 °C. The solution was poured into a 30 × 30 × 10 mm (width × height × thickness) mold and gelled83. The melamine foam and the agar block were inserted into a chamber that was 3D-printed (Form3, Formlabs, Somerville, MA). The chamber contained guide slots to position the acoustic focus at 1 mm in front of the surface of the block (by introducing a 12 mm gap between transducer exit plane and the surface; Fig. 1c,d).
Rodent FUS platform
The animal was positioned over an in-house sonication setup that consisted of a vertical stage that moved the stereotactic platform and a two-axis stage which controlled the transducer position in horizontal directions (each stage had 15 µm spatial precision, NLS 4, Newmark Systems, CA). The bregma location for referencing the sonication location was estimated 9 mm rostral to the interaural line.
Animal procedures and intracisternal injection of CSF tracers
The present study was carried out and reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org/). All animal procedures were conducted in compliance with the Institutional Animal Care and Use Committee (IACUC) regulations and standards, set forth by the Brigham and Women’s Hospital. Sprague–Dawley (SD) rats (all male) were socially housed (two rats/cage) under a 12 h/12 h light/dark cycle (lights on at 7 AM, off at 7 PM). The rats were allowed to access food and water ad libitum.
As the CSF tracers, 45 kDa-MW Texas Red Ovalbumin (OA, 023,021, Thermo Fisher) and 2000 kDa-MW fluorescein isothiocyanate (FITC)-dextran (FITC-d, FD2000S, Millipore Sigma) were constituted at a 0.5 wt% concentration in artificial CSF (aCSF; Tocris Bioscience, Bristol, UK). The prepared CSF tracer solution were intracisternally injected based on an established surgical protocol45. Rats (n = 6 in each group, total 3 groups) were anesthetized with an intraperitoneal injection of a mixture of 40–80 mg/kg ketamine and 10 mg/kg xylazine, then placed on a stereotactic frame (ASI Instruments, Warren, MI). Respiratory rate was measured manually and additional doses (~ half dose each) of anesthesia were administered as needed. After adequate anesthesia was achieved, the animal’s head was shaved using a clipper and depilation lotion. A temperature-controlled water-circulating pad (T-pump, Gaymar, Orchard Park, NY) was placed under the animal to maintain the animal’s body temperature.
Saline and/or cotton swabs were used to clean and dry the dura covering the cisterna magna (CM). A 30G cannula, filled with aCSF, was inserted into the center of the subarachnoid CM space at a depth of 1–2 mm, perpendicular to the covering dura. The needle insertion site was sealed with a few drops of cyanoacrylate glue (with application of accelerant). The CSF tracers were injected at a rate of 2 µL/min for 10 min using a syringe pump (Legato 100, KD Scientific, Holliston, MA), whereby the injection rate is known not to elevate the intracranial pressure21.
During the time to allow CSF tracers to distribute across the brain (with and without application of FUS), Respiratory and heart rates were measured (measured by Surgivet Hand-held pulse oximeter V1030, Smiths Medical, Dublin OH) right before and after the FUS, as heart rate is a critical factor known to affect CSF transport.
Fluorescent image analysis
Immediately after a FUS sonication session, the animal’s brain was harvested upon transcardial perfusion of 4% paraformaldehyde (PFA) in PBS (BM-155, Boston Bioproducts, Ashland, MA) and additional PFA submerge fixation (~ 24 h). The extracted brain underwent vibratome sectioning in 200 µm-thick slices (PELCO easiSlicer, Ted Pella, Redding, CA) along the rostral-caudal direction encompassing the acoustic focus. The sectioned slices were imaged with a widefield fluorescent microscope (TS100, Nikon, Japan) using an ultrawide field (23.4 × 15.6 mm) CMOS Sensor (4592 × 3056 pixel resolution, NEX-5, Sony, Japan). The sectional images from 2 mm rostral to 2 mm caudal directions from the sonication location with 1 mm gap in-between (thus, a total of 5 images) were normalized to the intensity scale ranging from 0 to 255 (8-bit image) and were processed with ImageJ software (version 1.53i, https://imagej.nih.gov/ij/)84. The brain area was first segmented, guided by the background fluorescence and corresponding bright-field image. The areas showing tracer uptake were segmented above 170% of the minimum background fluorescence value. Then, the percent area of tracer uptake with respect to the brain was derived from each animal for OA and FITC-d images and underwent one-way ANOVA followed by Tukey HSD post-hoc test for multiple comparisons.
Numerical simulation acoustic propagation and thermal analysis
For the acoustic simulation, the three-dimensional (3D) skull geometry was obtained from ex vivo rat skulls (n = 3; without the mandibular bones) using computed tomography (CT, SkyScan micro-CT, Source 60 kV, Bruker, Billerica, MA, USA) in isotropic voxels of 17 × 17 × 17 μm3. Hounsfield unit (HU) values in the images were calibrated with respect to a water tube located within the same image volume. The acquired images were subsampled to make isotropic voxels of 0.25 μm on each side, yielding a ratio of 30 pixels per wavelength in water (λ = ~ 7.5 mm at 200 kHz) to attain sufficient spatial resolution for the simulation85. The acoustic source was modeled based on our previous method82, factoring in the transducer geometry (transducer diameter of 28 mm and curvature radius of 22 mm), and positioned in the location used in the experiment. A simulation volume encompassing both the transducer and skull (183 × 275 × 266 voxels, 45.75 × 68.75 × 66.5 mm3) was used in the finite-difference time domain (FDTD)-based analysis with a time resolution of 0.05 μs. The acoustic properties of the skull, i.e., speed of sound, density, and attenuation, were used as described in our previous work86 based on the HU values of CT images87.
The resulting 3D distribution of acoustic intensity was used to estimate the temperature change at the skull and brain tissue by solving the bio-heat transfer equation55,8788 based on an FDTD scheme with discretized spatial resolution of 0.25 mm and a time resolution of 0.5 ms. The sonication parameters from the in vivo study (fundamental frequency of 200 kHz, ISPPA of 5 W/cm2, 100 ms PD, 1 Hz PRF [i.e., 10% DC] and 30 min sonication duration) were used in the estimation assuming homogenous intracranial brain tissue and an initial tissue temperature of 37.5 °C. Thermal properties of the brain (specific heat of 3600 J/kg/K, thermal conductivity of 0.528 W/K/m), skull (specific heat of 1300 J/kg/K, thermal conductivity of 0.4 W/K/m) and blood perfusion (perfusion rate of 8.24 kg/m3/s, density of 1030 kg/m3, specific heat of 3620 J/kg/K) were assigned to the simulation89. Contributions from the pulsation of CSF and thermoregulatory responses in heat transfer/removal90 were not modeled to conservatively overestimate the temperature rise in the brain tissue.
Histological analysis and evaluation of BBB disruption
We transcardially perfused the rats that were not injected with the CSF tracer (4% formaldehyde) and extracted brain after additional 24 h immerse fixation. The sonicated brain area was sectioned and stained with hematoxylin and eosin (H&E) to detect necrosis or hemorrhaging, vanadium acid fuchsin (VAF)-toluidine blue to detect the presence of ischemic neurons, glial fibrillary acidic protein (GFAP) immunohistochemistry (IHC) to detect glial infiltration, and caspase-3 for visualization of any apoptotic activity91. The integrity of the BBB was separately assessed (from 2 rats) using intravenous (via tail-vein injection) trypan blue (in saline, 0.1 g/kg, 872.9 Da) perfusion85 for 30 min right after tFUS (n = 2, each receiving sonication from dorsal or ventral direction). Transcardial perfusion fixation was performed using the same fixation protocol, and the brain was subsequently harvested and imaged using a dissection microscope.
Acknowledgements
We thank Dr. Wonhye Lee for participating in acquisition of computed tomography of rodent skulls. We thank work was partially supported by National Aeronautics and Space Administration (NASA) grant NNX16AO69A-T0415 (to SSY). The funder had no role in study design, data collection, data analysis, and decision to publish or preparation of the manuscript.
Author contributions
S.S.Y., H.C.K., J.K., and K.Y. participated in study design, equipment preparations, data acquisition, and analysis. E.K., K.K., J.V.R., and K.Y. participated in numerical simulation and data analysis. All participated in manuscript writing.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. The role of brain barriers in fluid movement in the CNS: Is there a 'glymphatic' system? Acta Neuropathol. 2018;135:387–407. doi: 10.1007/s00401-018-1812-4. [DOI] [PubMed] [Google Scholar]
- 2.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: A beginner's guide. Neurochem. Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benveniste H, et al. The glymphatic system and waste clearance with brain aging: A review. Gerontology. 2019;65:106–119. doi: 10.1159/000490349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kress BT, et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iliff JJ, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piantino J, Lim MM, Newgard CD, Iliff J. Linking traumatic brain injury, sleep disruption and post-traumatic headache: A potential role for glymphatic pathway dysfunction. Curr. Pain Headache Rep. 2019;23:62. doi: 10.1007/s11916-019-0799-4. [DOI] [PubMed] [Google Scholar]
- 7.Lv T, Zhao B, Hu Q, Zhang X. The glymphatic system: A novel therapeutic target for stroke treatment. Front. Aging. Neurosci. 2021;13:689098. doi: 10.3389/fnagi.2021.689098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji C, et al. The role of glymphatic system in the cerebral edema formation after ischemic stroke. Exp. Neurol. 2021;340:113685. doi: 10.1016/j.expneurol.2021.113685. [DOI] [PubMed] [Google Scholar]
- 9.Lin L, et al. Impaired glymphatic system in secondary degeneration areas after ischemic stroke in rats. J. Stroke Cerebrovasc. Dis. 2020;29:104828. doi: 10.1016/j.jstrokecerebrovasdis.2020.104828. [DOI] [PubMed] [Google Scholar]
- 10.Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017;140:2691–2705. doi: 10.1093/brain/awx191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringstad G, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight. 2018;3:e121537. doi: 10.1172/jci.insight.121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Mesquita S, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560:185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semyachkina-Glushkovskaya O, Postnov D, Kurths J. Blood(-)brain barrier, lymphatic clearance, and recovery: Ariadne's thread in labyrinths of hypotheses. Int. J. Mol. Sci. 2018;19:3818. doi: 10.3390/ijms19123818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today. 1996;2:106–113. doi: 10.1016/1357-4310(96)88720-X. [DOI] [PubMed] [Google Scholar]
- 17.Cammalleri A, Croce P, Lee W, Yoon K, Yoo SS. Therapeutic potentials of localized blood-brain barrier disruption by noninvasive transcranial focused ultrasound: A technical review. J. Clin. Neurophysiol. 2020;37:104–117. doi: 10.1097/WNP.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mestre H, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018;9:4878. doi: 10.1038/s41467-018-07318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Zhou J, Shi Y. Scanning electron microscopy of human cerebral meningeal stomata. Ann. Anat. 1996;178:259–261. doi: 10.1016/S0940-9602(96)80059-8. [DOI] [PubMed] [Google Scholar]
- 20.Pizzo ME, et al. Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol. 2018;596:445–475. doi: 10.1113/JP275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliff JJ, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muoio V, Persson PB, Sendeski MM. The neurovascular unit-concept review. Acta Physiol. (Oxf) 2014;210:790–798. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Du G. Acoustic streaming generated by a focused Gaussian beam and finite amplitude tonebursts. Ultrasound Med. Biol. 1993;19:167–176. doi: 10.1016/0301-5629(93)90008-C. [DOI] [PubMed] [Google Scholar]
- 24.Raghavan R. Theory for acoustic streaming in soft porous matter and its applications to ultrasound-enhanced convective delivery. J. Ther. Ultrasound. 2018;6:6. doi: 10.1186/s40349-018-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyos M, Castro A. Controlling the acoustic streaming by pulsed ultrasounds. Ultrasonics. 2013;53:70–76. doi: 10.1016/j.ultras.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 26.El Ghamrawy A, et al. Acoustic Streaming in a Soft Tissue Microenvironment. Ultrasound Med. Biol. 2019;45:208–217. doi: 10.1016/j.ultrasmedbio.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Cheung YN, Nguyen NT, Wong TN. Droplet manipulation in a microfluidic chamber with acoustic radiation pressure and acoustic streaming. Soft Matter. 2014;10:8122–8132. doi: 10.1039/C4SM01453G. [DOI] [PubMed] [Google Scholar]
- 28.Doinikov AA, Thibault P, Marmottant P. Acoustic streaming in a microfluidic channel with a reflector: Case of a standing wave generated by two counterpropagating leaky surface waves. Phys. Rev. E. 2017;96:013101. doi: 10.1103/PhysRevE.96.013101. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed D, Mao X, Shi J, Juluri BK, Huang TJ. A millisecond micromixer via single-bubble-based acoustic streaming. Lab Chip. 2009;9:2738–2741. doi: 10.1039/b903687c. [DOI] [PubMed] [Google Scholar]
- 30.Nightingale KR, Kornguth PJ, Trahey GE. The use of acoustic streaming in breast lesion diagnosis: A clinical study. Ultrasound Med. Biol. 1999;25:75–87. doi: 10.1016/S0301-5629(98)00152-5. [DOI] [PubMed] [Google Scholar]
- 31.Clarke L, Edwards A, Pollard K. Acoustic streaming in ovarian cysts. J. Ultrasound Med. 2005;24:617–621. doi: 10.7863/jum.2005.24.5.617. [DOI] [PubMed] [Google Scholar]
- 32.Hertzberg Y, Naor O, Volovick A, Shoham S. Towards multifocal ultrasonic neural stimulation: Pattern generation algorithms. J. Neural. Eng. 2010;7:056002. doi: 10.1088/1741-2560/7/5/056002. [DOI] [PubMed] [Google Scholar]
- 33.Jagannathan J, et al. High-intensity focused ultrasound surgery of the brain: Part 1–A historical perspective with modern applications. Neurosurgery. 2009;64:210–211. doi: 10.1227/01.NEU.0000336766.18197.8E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jolesz FA, Hynynen K, McDannold N, Tempany C. MR imaging-controlled focused ultrasound ablation: A noninvasive image-guided surgery. Magn. Reson. Imaging Clin. N. Am. 2005;13:545–560. doi: 10.1016/j.mric.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Schlesinger D, et al. MR-guided focused ultrasound surgery, present and future. Med. Phys. 2013;40:080901. doi: 10.1118/1.4811136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poon C, McMahon D, Hynynen K. Noninvasive and targeted delivery of therapeutics to the brain using focused ultrasound. Neuropharmacology. 2017;120:20–37. doi: 10.1016/j.neuropharm.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munoz F, Aurup C, Konofagou EE, Ferrera VP. Modulation of brain function and behavior by focused ultrasound. Curr. Behav. Neurosci. Rep. 2018;5:153–164. doi: 10.1007/s40473-018-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bystritsky A, et al. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011;4:125–136. doi: 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Bowary P, Greenberg BD. Noninvasive focused ultrasound for neuromodulation: A review. Psychiatr. Clin. North Am. 2018;41:505–514. doi: 10.1016/j.psc.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Kim HC, et al. Transcranial focused ultrasound modulates cortical and thalamic motor activity in awake sheep. Sci. Rep. 2021;11:19274. doi: 10.1038/s41598-021-98920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunevicius A, McDannold NJ, Golby AJ. Focused ultrasound strategies for brain tumor therapy. Oper Neurosurg. (Hagerstown) 2020;19:9–18. doi: 10.1093/ons/opz374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frenkel V, Gurka R, Liberzon A, Shavit U, Kimmel E. Preliminary investigations of ultrasound induced acoustic streaming using particle image velocimetry. Ultrasonics. 2001;39:153–156. doi: 10.1016/S0041-624X(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 43.Holter KE, et al. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc. Natl. Acad. Sci. U S A. 2017;114:9894–9899. doi: 10.1073/pnas.1706942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinney JP, et al. Extracellular sheets and tunnels modulate glutamate diffusion in hippocampal neuropil. J. Comp. Neurol. 2013;521:448–464. doi: 10.1002/cne.23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xavier, A. L. R. et al. Cannula Implantation into the Cisterna Magna of Rodents. J. Vis. Exp. (2018). [DOI] [PMC free article] [PubMed]
- 46.Yang T, Li S, Xu H, Walsh DM, Selkoe DJ. Large soluble oligomers of amyloid beta-protein from alzheimer brain are far less neuroactive than the smaller oligomers to which they dissociate. J. Neurosci. 2017;37:152–163. doi: 10.1523/JNEUROSCI.1698-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olbricht W, Sistla M, Ghandi G, Lewis G, Jr, Sarvazyan A. Time-reversal acoustics and ultrasound-assisted convection-enhanced drug delivery to the brain. J. Acoust. Soc. Am. 2013;134:1569–1575. doi: 10.1121/1.4812879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mano Y, et al. Intraparenchymal ultrasound application and improved distribution of infusate with convection-enhanced delivery in rodent and nonhuman primate brain. J. Neurosurg. 2016;124:1490–1500. doi: 10.3171/2015.3.JNS142152. [DOI] [PubMed] [Google Scholar]
- 49.Aryal, M., Quan, Z., Rosenthal, E. L. & Airan, R. Noninvasive ultrasonic glymphatic induction enhances intrathecal drug delivery. Cell Rep.10.2139/ssrn.3698892 (2021).
- 50.Wu J. Acoustic streaming and its applications. Fluids. 2018;3:18. doi: 10.3390/fluids3010018. [DOI] [Google Scholar]
- 51.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the 'glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife. 2017;6:e27679. doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hablitz LM, et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019;5:eaav5447. doi: 10.1126/sciadv.aav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai Z, et al. NIR-II fluorescence microscopic imaging of cortical vasculature in non-human primates. Theranostics. 2020;10:4265–4276. doi: 10.7150/thno.43533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hersh DS, et al. Pulsed ultrasound expands the extracellular and perivascular spaces of the brain. Brain Res. 2016;1646:543–550. doi: 10.1016/j.brainres.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee W, et al. Non-invasive transmission of sensorimotor information in humans using an EEG/focused ultrasound brain-to-brain interface. PLoS ONE. 2017;12:e0178476. doi: 10.1371/journal.pone.0178476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Q, Hu J. Diversity of acoustic streaming in a rectangular acoustofluidic field. Ultrasonics. 2015;58:27–34. doi: 10.1016/j.ultras.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Lee H, et al. The effect of body posture on brain glymphatic transport. J. Neurosci. 2015;35:11034–11044. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bloom GS. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 60.Peng W, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiol. Dis. 2016;93:215–225. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishihara Y, et al. A precise and fast temperature mapping using water proton chemical shift. Magn. Reson. Med. 1995;34:814–823. doi: 10.1002/mrm.1910340606. [DOI] [PubMed] [Google Scholar]
- 62.Yoo SS, et al. Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56:1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haller J, Wilkens V. Determination of acoustic cavitation probabilities and thresholds using a single focusing transducer to induce and detect acoustic cavitation events: ii. Systematic investigation in an agar material. Ultrasound Med. Biol. 2018;44:397–415. doi: 10.1016/j.ultrasmedbio.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Church CC. Frequency, pulse length, and the mechanical index. Acoust. Res. Lett. Online. 2005;6:162–168. doi: 10.1121/1.1901757. [DOI] [Google Scholar]
- 65.Lee Y, et al. Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer's disease model. Sci. Rep. 2020;10:16144. doi: 10.1038/s41598-020-73151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu HL, et al. Hemorrhage detection during focused-ultrasound induced blood-brain-barrier opening by using susceptibility-weighted magnetic resonance imaging. Ultrasound Med. Biol. 2008;34:598–606. doi: 10.1016/j.ultrasmedbio.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Yoon K, et al. Localized blood-brain barrier opening in ovine model using image-guided transcranial focused ultrasound. Ultrasound Med. Biol. 2019;45:2391–2404. doi: 10.1016/j.ultrasmedbio.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin WH, Fan CH, Ting CY, Liu HL, Yeh CK. Dynamic perfusion assessment by contrast-enhanced ultrasound in blood-brain barrier disruption. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013;2013:1152–1155. doi: 10.1109/EMBC.2013.6609710. [DOI] [PubMed] [Google Scholar]
- 69.Fan CH, et al. Detection of intracerebral hemorrhage and transient blood-supply shortage in focused-ultrasound-induced blood-brain barrier disruption by ultrasound imaging. Ultrasound Med. Biol. 2012;38:1372–1382. doi: 10.1016/j.ultrasmedbio.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Todd N, Zhang Y, Livingstone M, Borsook D, McDannold N. The neurovascular response is attenuated by focused ultrasound-mediated disruption of the blood-brain barrier. Neuroimage. 2019;201:116010. doi: 10.1016/j.neuroimage.2019.116010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaberel T, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: A new target for fibrinolysis? Stroke. 2014;45:3092–3096. doi: 10.1161/STROKEAHA.114.006617. [DOI] [PubMed] [Google Scholar]
- 72.Kylkilahti TM, et al. Achieving brain clearance and preventing neurodegenerative diseases-A glymphatic perspective. J. Cereb. Blood Flow Metab. 2021;41:2137–2149. doi: 10.1177/0271678X20982388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sundaram S, et al. Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson's disease. Neurosci. Biobehav. Rev. 2019;103:305–315. doi: 10.1016/j.neubiorev.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alexander AL, Hasan K, Kindlmann G, Parker DL, Tsuruda JS. A geometric analysis of diffusion tensor measurements of the human brain. Magn. Reson. Med. 2000;44:283–291. doi: 10.1002/1522-2594(200008)44:2<283::AID-MRM16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 75.Sepehrband F, et al. Perivascular space fluid contributes to diffusion tensor imaging changes in white matter. Neuroimage. 2019;197:243–254. doi: 10.1016/j.neuroimage.2019.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saade C, et al. Gadolinium and multiple sclerosis: Vessels, barriers of the brain, and glymphatics. AJNR Am. J. Neuroradiol. 2018;39:2168–2176. doi: 10.3174/ajnr.A5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schubert JJ, et al. Dynamic (11)C-PiB PET shows cerebrospinal fluid flow alterations in Alzheimer disease and multiple sclerosis. J. Nucl. Med. 2019;60:1452–1460. doi: 10.2967/jnumed.118.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naylor E, et al. Lactate as a biomarker for sleep. Sleep. 2012;35:1209–1222. doi: 10.5665/sleep.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lundgaard I, et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J. Cereb. Blood Flow Metab. 2017;37:2112–2124. doi: 10.1177/0271678X16661202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shokri-Kojori E, et al. Beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. U S A. 2018;115:4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palmqvist S, et al. Earliest accumulation of beta-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 2017;8:1214. doi: 10.1038/s41467-017-01150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon K, Lee W, Croce P, Cammalleri A, Yoo SS. Multi-resolution simulation of focused ultrasound propagation through ovine skull from a single-element transducer. Phys. Med. Biol. 2018;63:105001. doi: 10.1088/1361-6560/aabe37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Narayanan J, Xiong J-Y, Liu X-Y. Determination of agarose gel pore size: Absorbance measurements vis a vis other techniques. J. Phys. Conf. Ser. 2006;28:83–86. doi: 10.1088/1742-6596/28/1/017. [DOI] [Google Scholar]
- 84.Younan Y, et al. Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med. Phys. 2013;40:082902. doi: 10.1118/1.4812423. [DOI] [PubMed] [Google Scholar]
- 85.Xu L, et al. Localized disruption of blood albumin-phenytoin binding using transcranial focused ultrasound. Ultrasound Med. Biol. 2020;46:1986–1997. doi: 10.1016/j.ultrasmedbio.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 86.Pichardo S, Sin VW, Hynynen K. Multi-frequency characterization of the speed of sound and attenuation coefficient for longitudinal transmission of freshly excised human skulls. Phys. Med. Biol. 2011;56:219–250. doi: 10.1088/0031-9155/56/1/014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm(1948) J. Appl. Physiol.(1985) 1998;85:5–34. doi: 10.1152/jappl.1998.85.1.5. [DOI] [PubMed] [Google Scholar]
- 88.Constans C, Mateo P, Tanter M, Aubry JF. Potential impact of thermal effects during ultrasonic neurostimulation: Retrospective numerical estimation of temperature elevation in seven rodent setups. Phys. Med. Biol. 2018;63:025003. doi: 10.1088/1361-6560/aaa15c. [DOI] [PubMed] [Google Scholar]
- 89.Sukstanskii AL, Yablonskiy DA. Theoretical model of temperature regulation in the brain during changes in functional activity. Proc. Natl. Acad. Sci. U S A. 2006;103:12144–12149. doi: 10.1073/pnas.0604376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoon K, et al. Effects of sonication parameters on transcranial focused ultrasound brain stimulation in an ovine model. PLoS ONE. 2019;14:e0224311. doi: 10.1371/journal.pone.0224311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.