Abstract
This paper presents a novel potentiometric approach for the determination of palonosetron HCl using two sensors; ionophore-free and ionophore-doped ones. The two sensors successfully determined the cited drug in the range of 1 × 10–5–1 × 10–2 M with respective Nernstian slopes of 54.9 ± 0.25 and 59.3 ± 0.16 mV/decade. Incorporating calix[8]arene as an ionophore resulted in a lower detection limit (LOD = 3.1 × 10–6 M) and enhanced selectivity when compared to the ionophore-free sensor (LOD = 7.9 × 10–6 M). This modification was also associated with faster response for the ionophore-doped sensor (response time = 20 s) compared to the ionophore-free one (response time = 30 s). The two sensors showed a stable response over a pH range of 3.0–8.0. They successfully determined palonosetron HCl in presence of its oxidative degradation products. They were also used for direct determination of the drug in commercially available parenteral solution without any interference from other dosage forms’ additives.
Subject terms: Analytical chemistry, Electrochemistry
Introduction
Palonosetron HCl (PALO), chemically designed as (S)-2-[(3S)-Quinuclidin-3-yl]-2,3,3a,4,5,6-hexahydro-1H-benzo[de]isoquinolin-1-onehydrochloride1, is a highly selective and potent second generation serotonin (5-HT3) receptor antagonist. It is characterized by strong binding affinity and long plasma elimination half-life. PALO shows efficacy in preventing nausea and vomiting resulting from highly emetogenic chemotherapy2. The drug is official only in United States Pharmacopeia (USP)3 where it is determined by a reversed-phase high performance liquid chromatographic (HPLC) method using acetonitrile: water: trifluoroacetic acid (280: 720: 0.67, v/v/v) as a mobile phase.
Literature survey revealed various analytical methods for PALO determination, including spectrophotometric4, high performance thin layer chromatographic (HPTLC)5–7 and HPLC ones7–19. According to previously conducted stability study by our research group, the drug was found to be susceptible to oxidation where two stability-indicating chromatographic methods were developed7. Although all these reported methods are suitable for PALO determination, they have two main disadvantages; (i) expensive equipment needed, and (ii) tedious sample preparation steps.
The simplicity, sensitivity and selectivity associated with potentiometric field have promoted its application in different types of samples; biomarkers, inorganic ions and pharmaceuticals analysis20–26. This potentiometric technique depends on presence of an ionizable function group in the analyte of interest leading to its selective partitioning to a plasticized lipophilic membrane in which suitable ion-exchanging salts as well as complexation agents (ionophores) are incorporated for such purpose27. These lipophilic membranes are widely utilized with different substrates to construct disposable ion-selective electrodes (ISEs)21,22,28.
Among the mostly widely used ionophores are calixarenes which are chemically consisting of phenol units linked via alkylidene groups. This provides them their unique cavity-shaped configuration that leads to formation of typical host–guest complexes with different compounds depending on some factors, such as; cavity-size, conformation and substituents. In nutshell, calixarenes are suitable for a variety of applications in ISEs fabrication22,29,30.
Here, we develop the first ISE for the potentiometric determination of PALO. We also exploit the advantages of selectivity enhancement, related to calix[8]arene incorporation in potentiometric ISE, in order to demonstrate its use as a stability-indicating one. Two ISEs are fabricated and their performance characteristics are compared; one without incorporation of ionophore (ionophore-free senor), and another one utilizing calix[8]arene as an ionophore (ionophore-doped senor).
Results and discussion
Ion-selective membranes are usually prepared from PVC, electroactive substance (ion-association complex) and a plasticizer. The role of PVC is to provide an inert solid support structure in which the rest of components are embedded. The plasticizer dissolves the ion association complex, plasticizes the membrane and affects the lipophilicity of PVC membrane. It also alters the distribution coefficient (K) of different species thus affecting the performance characteristics of electrode31. In potentiometric applications, selectivity and sensitivity are the main aim that guide optimization plan during method development. A key component in ISE fabrication that can significantly improve selectivity and sensitivity is doping the PVC polymeric membrane with an ionophore32. The response of membranes containing ionophores is largely governed by molecular recognition where the analyte functions as the guest and the ionophore plays the role of host33. ISEs containing ionophores have been shown to increase the selectivity of the sensor toward the detection of specific analytes27. Calixarenes are widely used as ionophores for various ions via dipole–dipole interactions where they can make stable host–guest inclusion complexes with different types of cation substrates. As a result, they have been largely exploited for the development of a number of ISEs22,29,34.
Stability study
As per our previously reported work, PALO was subjected to forced acidic, alkaline, oxidative, photolytic and thermal degradation conditions7. Observed degradation was noticed only under oxidative condition upon refluxing with 6% H2O2 for 6 h. Three degradation products were separated on HPTLC plate and mass analysis was then conducted by means of Advion compact mass spectrometer7. The obtained mass spectra of PALO along with its three oxidative degradation products are shown in Figure S1, supplemental information. As a result and in this work, calix[8]arene was incorporated as an ionophore and the fabricated ISE was compared to the ionophore-free one in terms of sensitivity and selectivity for PALO determination in presence of its oxidative degradation products. The pKa value of PALO is ≈ 8.81, so at pH 5.0, the studied drug has a positive charge. The use of TPB as counter ion for the cationic PALO in the two proposed ion-sensitive membrane sensors was suggested.
Response characteristics of the proposed sensors
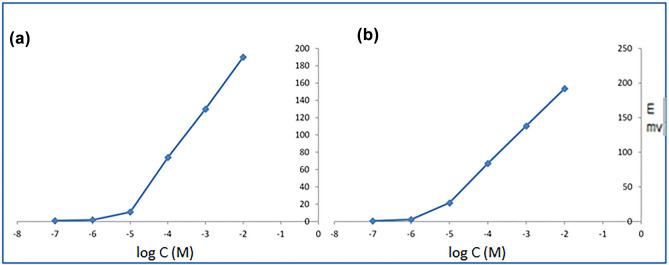
The performance characteristics of the two proposed ISEs were assessed according to the IUPAC standards27, Table 1. Two calibration curves were constructed, showing the same linearity range; 1 × 10–5 to 1 × 10–2 M as displayed in Fig. 1. The obtained slopes were 54.9 ± 0.25 and 59.3 ± 0.16 mV/decade for ionophore-free and ionophore-doped sensors, respectively, with respective detection limits of 7.9 × 10–6 and 3.1 × 10–6 M. The enhanced responses of ionophore-doped sensor are attributed to; (1) presence of rigid calix[8]arene cavity which selectively complex with PALO, (2) fast complexation-decomplexation kinetics for reversible transduction processes, and (3) high lipophilicity (especially with 8 phenolic units of calix[8]arene) preventing complexed PALO from leaching from nonpolar membrane into aqueous phase35.
Table 1.
Electrochemical response characteristics of the investigated PALO sensors.
| Parameter | Ionophore-free sensor | Ionophore-doped sensor |
|---|---|---|
| Slope (mV/decade)a | 54.9 | 59.3 |
| Intercept (mV) | 302.4 | 308.8 |
| LOD (mol/L)b | 7.9 × 10–6 | 3.1 × 10–6 |
| Regression coefficient | 0.9995 | 0.9998 |
| Response time (s) | 30 | 20 |
| Temperature | 25 °C | |
| Working pH range | 3.0–8.0 | |
| Concentration range (M) | 1.0 × 10–5–1.0 × 10–2 | |
| Stability (days) | 20 | 30 |
| Repeatabilityc | 0.68–0.82 | 0.52–0.79 |
| Intermediate precisiond | 1.06–1.85 | 0.87–1.02 |
| Accuracy (R %) | 99.69 | 100.04 |
| Emegrand vial 0.25 mg/5 mL (Mean ± RSD%)e | 101.50 ± 1.7 | 102.14 ± 0.5 |
aAverage of four determinations.
bLimit of detection calculated at the interception of extrapolated arms in potential profile.
cThe intraday (n = 3) RSD% of concentrations 10–2, 10–3 and 10–4 M PALO.
dThe interday (n = 3) RSD% of concentrations 10–2, 10–3 and 10–4 M PALO.
eThe average of five determinations.
Figure 1.
Profile of the potential in mV against log concentration of PALO at pH 5.0 for (a) ionophore-free sensor and (b) ionophore-doped sensor.
Effect of pH
The effect of pH on the response of the proposed sensor is investigated by using 1 × 10–2 M and 1 × 10–3 M PALO standard solutions at different pH values, ranging from 3.0–11.0. The obtained potentials were recorded at each pH value. It was found that the investigated electrode showed a stable response over a pH range of 3.0–8.0, Fig. 2.
Figure 2.
Effect of pH on the performance of ionophore-free sensor.
Effect of temperature
The effect of temperature on the response of the proposed ISE was studied. 1 × 10–2 M and 1 × 10–3 M PALO standard solutions were used whereas a fairly stable response is observed upon increasing temperature in the range of 25–35 °C. These results indicate reasonable thermal stability of the proposed electrode up to 35 °C, Fig. 3.
Figure 3.
Effect of temperature on the performance of ionophore-free sensor.
Selectivity of the proposed sensors
Selectivity of proposed PALO sensors in presence of interfering ions was evaluated by separate solutions method. Four inorganic ions; Na+ (as citrate), K+, Ca2+ and Mg2+ (as chlorides), oxidative degradation products as well as structurally related granisetron were selected for this study. The two proposed sensors showed non-Nernstian responses to the inorganic ions, Fig. 4. This is attributed to the hydrophobic nature of ion selective membrane which hinders the hydrophilic inorganic ions exchange. As a result, no need to determine selectivity coefficients for those ions36. On the other hand, oxidative degradation products and structurally related drugs (granisetron & ondansetron) showed near-Nernstian responses to monovalent cations (≈ 45 mV/decade) over the considered range, Fig. 4. Selectivity coefficients were consequently calculated, Table 2. As shown, about one order of magnitude enhancement was noticed for ionophore-doped sensor. This supports its exceedances over ionophore-free one as stability-indicating sensor. In nutshell, incorporation of calix[8]arene, during fabrication of ISE, improved the selectivity of the proposed ionophore-doped sensor as compared to the ionophore-free one.
Figure 4.
The responses of (a) ionophore-free sensor and (b) ionophore-doped sensor as function of log concentration for some inorganic ions, PALO oxidative degradation products and PALO structurally related drugs (granisetron & ondansetron) in selectivity measurements.
Table 2.
Potentiometric selectivity coefficient () for the investigated PALO sensors using the separate solutions method.
| Interferent | Selectivity coefficient for ionophore-free sensora | Selectivity coefficient for ionophore-doped sensora |
|---|---|---|
| Oxidative degradation products | 6.0 × 10–1 | 7.6 × 10–2 |
| Granisetron | 2.2 × 10–1 | 4.1 × 10–2 |
| Ondansetron | 2.6 × 10–1 | 4.5 × 10–2 |
aAverage of three separate determinations.
Application to dosage form
The two proposed sensors were successfully applied for the determination of PALO in Emegrand vial, Table 1. It is worth noting that this potentiometric method offers the advantage of direct PALO determination without any pretreatment steps.
Statistical analysis and sensors evaluation
The results obtained for the potentiometric analysis of PALO were statistically37 compared with those obtained by applying an official HPLC method3. The calculated values of t and F are less than their corresponding tabulated ones, which reveals that there is no significant difference between the suggested and official method with respect to accuracy and precision, Table 3. Moreover, a point-by-point comparison with the two reported stability-indicating chromatographic methods was conducted, Table 4. Besides the no need for preliminary drug preparation, our proposed sensors achieved a comparable quantification limit (LOQ) as well as ability of PALO determination in presence of other structurally related drugs.
Table 3.
Statistical comparison between the results obtained by the proposed ISE potentiometric method and the official method for determination of PALO in its pure form.
| Parameter | ISE potentiometric methoda | Official methodb | |
|---|---|---|---|
| Ionophore-free sensor | Ionophore-doped sensor | ||
| Mean | 99.69 | 100.04 | 100.33 |
| SD | 0.65 | 0.35 | 0.38 |
| SE | 0.21 | 0.12 | 0.22 |
| n | 9 | 9 | 3 |
| Variance | 0.42 | 0.12 | 0.14 |
| Student’s t-test | 2.10 (2.23)c | 1.16 (2.23)c | NA |
| F-test | 3 (19.37)c | 1.17(4.46)c | NA |
a9 determinations of 3 concentration levels by the proposed ISE method.
b3 determinations of 3 concentrations by USP 41, HPLC–UV method for PALO.
cThe values in parentheses are the corresponding tabulated values of t and F at p = 0.05.
Table 4.
An overview on the reported stability-indicating chromatographic methods compared to the proposed potentiometric method for the determination of PALO.
| Reference No | LOQ | Timea | Application | |
|---|---|---|---|---|
| 7 | HPLC | 0.1 µg mL−1 | 10 min |
Dosage form In presence of degradation products |
| HPTLC | 0.1 µg band−1 | 20 min | ||
| This work | 1.0 × 10–5 M | 20 s |
Dosage form In presence of degradation products In presence of structurally related drugs; granisetron & ondansetron |
|
aTime required for acquiring data from one sample measurement.
Conclusions
This work demonstrates the first potentiometric method for palonosetron HCl determination. Two ion-selective electrodes were fabricated; one without incorporation of ionophore (ionophore-free senor) and the second one utilizing calix[8]arene as an ionophore (ionophore-doped senor). The incorporation of calix[8]arene, as an ionophore, improved the limit of detection as well as the selectivity of the proposed sensor towards the most likely formed degradation products and the structurally related drugs. Selectivity assessment revealed that the fabricated sensors could be applied as stability-indicating ones and in direct determination of the cited drug in presence of its dosage form additives. The proposed method was found to be sensitive, rapid, easy to use, selective, simple and more economic for palonosetron HCl determination as compared to other reported ones. The method showed also good applicability for determination of the cited drug in its marketed dosage form (Emegrand vials) promoting its use in different quality control laboratories.
Methods
Materials and reagents
Palonosetron hydrochloride working standard (99.29%) was supplied from National Organization for Drug Control and Research (NODCAR, Giza, Egypt).
Commercially available Emegrand (0.25 mg/5 mL) vial for I.V., batch no.:171260, (Delta Grand Pharma, Egypt) was purchased from the Egyptian market.
Oxidative degradation products were prepared following our reported protocol7. Briefly, the intact drug was refluxed with 6% H2O2 for 6 h. The solution was then evaporated and the resulted residue was dissolved in methanol.
Sodium tetraphenyl borate (TPB) [Sigma-Aldrich, Steinheim, Germany], polyvinylchloride (PVC) [Fluka Chemie GmbH, St. Louis, USA], nitrophenyl octyl ether (NPOE) [Fluka Chemie GmbH, St.Louis, USA], tetrahydrofuran (THF) [BDH, Poole, England], calix[8]arene [Sigma-Aldrich, Steinheim, Germany] and double distilled deionized water [Otsuka, Cairo, Egypt].
The Britton-Robinson buffers38 in the pH range of 2.0–12.0 were prepared by mixing equal volumes of 0.04 M acetic acid, 0.04 M boric acid and 0.04 M phosphoric acid. The required pH values were adjusted using 0.2 M NaOH standard solution.
Instrumentation
Ag/AgCl reference electrode (Thermo Scientific Orion 90–02, MA, USA). pH meter for pH adjustments and potential measurements (Hanna 211). Magnetic stirrer (Bandelin Sonorex, Rx 5105). HPTLC aluminum plates 10 × 10 cm precoated with 0.25 mm silica gel 60 F254 (Merck, Germany) were used for stability study. The plates were developed at ambient temperature using a mixture of methanol: ammonia (10: 0.5, v/v) as the developing system in a CAMAG twin-trough chamber previously saturated with the developing system for 30 min. Advion compact mass spectrometer (CMS, USA) provided with ESI ion source was utilized for mass analysis.
Fabrication of ion-selective electrodes
The ion-selective sensors were prepared by mixing PVC (190 mg), NPOE (0.38 mL) and TPB (5 mg) for preparation of the ionophore-free sensor. PVC (190 mg), NPOE (0.38 mL), TPB (5 mg) and Calix[8]arene (10 mg) for preparation of ionophore-doped one. The membrane components were dissolved in THF (6.0 mL), and poured into Petri dishes which were then covered with filter papers and left to stand overnight at room temperature allowing the THF to evaporate. Membranes with a thickness of 0.1 mm were obtained where 8-mm diameter disks were cut using a cork borer. Disks were then affixed to a PVC tips using THF and affixed onto the end of a glass body electrode. The electrodes were then filled with an inner-filling solution (IFS) with equal volumes of 10–4 M PALO and 10–4 M KCl. A Ag/AgCl wire was placed in the IFS and served as an internal reference electrode. Each sensor was conditioned by placing them in a solution containing 10–4 M PALO for 24 h prior to use and was stored in the same solution when not in use.
Sensors calibration
Calibration of the conditioned PALO sensors is performed by immersing them separately in conjunction with double junction Ag/AgCl reference electrode in different PALO standard solutions (1 × 10–5 to 1 × 10-2 M) prepared in buffer pH 5.0. The ISEs were allowed to equilibrate while stirring and potential difference (emf) readings were the recorded (within ± 1 mV). The membrane sensors were washed between measurements with the buffer. The recorded potentials were finally plotted as a function of logarithm PALO concentrations in buffer pH 5.0 at 25 °C. A diagram for measurement process is shown in Fig. 5.
Figure 5.
A diagram for measurement process using the proposed method. Figure 5 was created using BioRender (https://biorender.com).
Selectivity studies
The potentiometric selectivity coefficients () of the proposed ISEs were evaluated according to IUPAC27 guidelines using the separate solutions method39,40. Calibration curves for some inorganic cations, oxidative degradation products as well as a structurally related organic ions, granisetron and ondansetron, were constructed using the two proposed sensors. Potentials for the same concentration (1 × 10–4 M) of PALO cation and interfering ions were measured separately, and the rearranged Nicolsky–Eisenman equation was applied36,39.
where EA and EB are potentials measured for ion of interest (with ZA charge) and interfering ion (with ZB charge), respectively, and (2.303RT/ZAF) is the slope of the calibration curve in mV/decade.
Application to pharmaceutical dosage form
Five vials of Emegrand were emptied and transferred into a 50-ml volumetric flask. The volume was completed to the mark with Robinson buffer pH 5.0 to prepare a solution of 8.4 × 10–4 M PALO. The emfs produced by immersing the prepared electrode in conjunction with the double junction Ag/AgCl reference electrode in the prepared solution at 25 °C were recorded. The concentration of PALO was calculated from the following regression equations:
where Y is the potential in mV and X is the logarithm of the concentration in M.
Supplementary Information
Author contributions
M.A.T.: methodology, software, validation, formal analysis, investigation, funding acquisition, project administration, writing—original draft, writing—review & editing. D.A.E.: methodology, software, validation, formal analysis, investigation, funding acquisition, project administration, writing—original draft, writing—review & editing. N.F.Y.: conceptualization, methodology, software, validation, visualization, supervision, project administration, funding acquisition, writing—original draft. S.M.A.: conceptualization, methodology, software, formal analysis, data curation, visualization, supervision, project administration, funding acquisition, writing—review & editing.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17349-y.
References
- 1.Brayfield A. Martindale: The complete drug reference. 39. Pharmaceutical Press; 2018. [Google Scholar]
- 2.De Leon A. Palonosetron (Aloxi): A second-generation 5-HT(3) receptor antagonist for chemotherapy-induced nausea and vomiting. Proc. (Bayl. Univ. Med. Cent.) 2006;19:413–416. doi: 10.1080/08998280.2006.11928210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Pharmacopeia,. (2018).
- 4.Parambi DGT, Mathew SM, Ganesan V. Estimation of palonosetron hydrochloride (a 5-HT3 antagonists) in pharmaceutical dosage form by UV spectrophotometric method. Int. J. Chem. Sci. 2011;4:1619–1624. [Google Scholar]
- 5.Damle MC, Agrawal AA. Development and validation of stability indicating HPTLC method for estimation of palonosetron hydrochloride. Am. J. PharmTech Res. 2015;5:275–286. [Google Scholar]
- 6.Jain PS, Chavan RS, Bari PR, Patil SS. Surana stability-indicating HPTLC method for estimation of palonosetron hydrochloride. J. Adv. Drug Delivery. 2015;2:2. [Google Scholar]
- 7.Tantawy MA, Alweshahy S, Elshabasy DA, Youssef NF. Butyl-based reversed-phase high-performance liquid chromatography and silica normal-phase high-performance thin-layer chromatography methods for the determination of palonosetron in the presence of degradation products and dosage form additives. JPC J. Planar Chromatogr. Modern TLC. 2020;33:149–160. doi: 10.1007/s00764-020-00014-3. [DOI] [Google Scholar]
- 8.Murthy V, Srinivas M, Kumar K, Mukkanti K. Development and validation of a stability-indicating LC method for determining palonosetron hydrochloride, its related compounds and degradation products using naphthalethyl stationary phase. J. Pharm. Biomed. Anal. 2011;56:429–435. doi: 10.1016/j.jpba.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Parekh D, Patel CJ, Patel MM. Stability-indicating RP-HPLC method development and validation for estimation of palonosetron hydrochloride in its parenteral dosage form. World J. Pharm. Res. 2018;7:723–736. [Google Scholar]
- 10.Pathi PJ, Raju NA. The estimation of palonosetron hydrochloride in parenterals by RP-HPLC. Asian J. Pharm. Technol. 2012;2:77–79. [Google Scholar]
- 11.Inturi S, Inturi R, Venkatesh G. A validated novel RP-HPLC method development for the estimation of palonosetron hydrochloride in bulk and softule dosage forms. Pharm. Sin. 2011;2:223–234. [Google Scholar]
- 12.Li P, et al. Liquid chromatography-electrospray quadrupole linear ion trap mass spectrometry method for the quantitation of palonosetron in human plasma and urine: Application to a pharmacokinetic study. J. Chromatogr. B. 2012;895–896:10–16. doi: 10.1016/j.jchromb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Determination of palonosetron in human urine by LC–MS/MS. Bioanalysis. 2011;3:1337–1342. doi: 10.4155/bio.11.110. [DOI] [PubMed] [Google Scholar]
- 14.Yang S, et al. Determination of palonosetron in human plasma by ultra performance liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2012;57:13–18. doi: 10.1016/j.jpba.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Ding L, Chen Y, Yang L, Wen A. Determination of palonosetron in human plasma by liquid chromatography-electrospray ionization-mass spectrometry. J. Pharm. Biomed. Anal. 2007;44:575–580. doi: 10.1016/j.jpba.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Feng F, Le W, Wang H, Zhu L. Sensitive and selective LC-MS-MS assay for the quantification of palonosetron in human plasma and its application to a pharmacokinetic study. Chromatographia. 2008;68:193–199. doi: 10.1365/s10337-008-0712-5. [DOI] [Google Scholar]
- 17.Tian K, Chen H, Tang J, Chen X, Hu Z. Enantioseparation of palonosetron hydrochloride by micellar electrokinetic chromatography with sodium cholate as chiral selector. J. Chromatogr. A. 2006;1132:333–336. doi: 10.1016/j.chroma.2006.08.090. [DOI] [PubMed] [Google Scholar]
- 18.Radhakrishnanand P, Subba Rao DV, Himabindu V. Validated chiral LC method for the enantiomeric separation of palonosetron hydrochloride. Chromatographia. 2008;69:369–373. doi: 10.1365/s10337-008-0887-9. [DOI] [Google Scholar]
- 19.Xiao-rong Y, Min S, Tai-jun H. Direct enantiomeric separation of palonosetron hydrochloride by chiral HPLC. Chin. J. New Drugs. 2008;10:2. [Google Scholar]
- 20.Bakker E, Pretsch E. Modern potentiometry. Angew. Chem. Int. Ed Engl. 2007;46:5660–5668. doi: 10.1002/anie.200605068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yehia AM, Saad AS, Tantawy MA. USB multiplex analyzer employing screen-printed silver electrodes on paper substrate; A developed design for dissolution testing. J. Pharm. Biomed. Anal. 2020;186:113272. doi: 10.1016/j.jpba.2020.113272. [DOI] [PubMed] [Google Scholar]
- 22.Yehia AM, Farag MA, Tantawy MA. A novel trimodal system on a paper-based microfluidic device for on-site detection of the date rape drug "ketamine". Anal. Chim. Acta. 2020;1104:95–104. doi: 10.1016/j.aca.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Özbek O, Isildak Ö, Isildak I. A potentiometric biosensor for the determination of valproic acid: Human blood–based study of an anti–epileptic drug. Biochem. Eng. J. 2021;176:108181. doi: 10.1016/j.bej.2021.108181. [DOI] [Google Scholar]
- 24.Isildak Ö, Özbek O. Application of potentiometric sensors in real samples. Crit. Rev. Anal. Chem. 2021;51:218–231. doi: 10.1080/10408347.2019.1711013. [DOI] [PubMed] [Google Scholar]
- 25.Özbek O, Isildak Ö. Potentiometric PVC membrane sensor for the determination of anti-epileptic drug levetiracetam in pharmaceutical formulations. ChemistrySelect. 2022;7:e202103988. doi: 10.1002/slct.202103988. [DOI] [Google Scholar]
- 26.Wadie M, Marzouk HM, Rezk MR, Abdel-Moety EM, Tantawy MA. A sensing platform of molecular imprinted polymer-based polyaniline/carbon paste electrodes for simultaneous potentiometric determination of alfuzosin and solifenacin in binary co-formulation and spiked plasma. Anal. Chim. Acta. 2022;1200:339599. doi: 10.1016/j.aca.2022.339599. [DOI] [PubMed] [Google Scholar]
- 27.Bakker E, Bühlmann P, Pretsch E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997;97:3083–3132. doi: 10.1021/cr940394a. [DOI] [PubMed] [Google Scholar]
- 28.Guinovart T, Parrilla M, Crespo GA, Rius FX, Andrade FJ. Potentiometric sensors using cotton yarns, carbon nanotubes and polymeric membranes. Analyst. 2013;138:5208–5215. doi: 10.1039/c3an00710c. [DOI] [PubMed] [Google Scholar]
- 29.El-Sayed MA. Advantages of the incorporation of 2-hydroxyl propyl beta cyclodextrin and calixarene as ionophores in potentiometric ion-selective electrodes for rivastigmine with a kinetic study of its alkaline degradation. Sens. Actuators B Chem. 2014;190:101–110. doi: 10.1016/j.snb.2013.08.065. [DOI] [Google Scholar]
- 30.Kelani K, Hegazy M, Hassan A, Tantawy M. A new comparative potentiometric method for analysis of omarigliptin using three different sensors. Electroanalysis. 2021 doi: 10.1002/elan.202100653. [DOI] [Google Scholar]
- 31.Janata J, Josowicz M, Vanýsek P, DeVaney DM. Chemical sensors. Anal. Chem. 1998;70:179–208. doi: 10.1021/a1980010w. [DOI] [PubMed] [Google Scholar]
- 32.Abd El-Rahman MK, Zaazaa HE, ElDin NB, Moustafa AA. Just-dip-it (potentiometric ion-selective electrode): An innovative way of greening analytical chemistry. ACS Sustain. Chem. Eng. 2016;4:3122–3132. doi: 10.1021/acssuschemeng.6b00138. [DOI] [Google Scholar]
- 33.Xiao KP, Bühlmann P, Nishizawa S, Amemiya S, Umezawa Y. A chloride ion-selective solvent polymeric membrane electrode based on a hydrogen bond forming ionophore. Anal. Chem. 1997;69:1038–1044. doi: 10.1021/ac961035d. [DOI] [Google Scholar]
- 34.Bakker E, Telting-Diaz M. Electrochemical sensors. Anal. Chem. 2002;74:2781–2800. doi: 10.1021/ac0202278. [DOI] [PubMed] [Google Scholar]
- 35.Diamond D, Nolan K. Peer reviewed: calixarenes: Designer ligands for chemical sensors. Anal. Chem. 2001;73:22–29. doi: 10.1021/ac012376g. [DOI] [PubMed] [Google Scholar]
- 36.Safwat N, Mahmoud AM, Abdel-Ghany MF, Ayad MF. In situ monitoring of triclosan in environmental water with subnanomolar detection limits using eco-friendly electrochemical sensors modified with cyclodextrins. Environ. Sci. Process. Impacts. 2021;23:457–466. doi: 10.1039/D0EM00387E. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel MR, Stephens L. Statistics. Schaum’s Outline Series; 1961. [Google Scholar]
- 38.Britton HTS, Robinson RA. CXCVIII.—Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931;2:1456–1462. doi: 10.1039/JR9310001456. [DOI] [Google Scholar]
- 39.Umezawa Y, Bühlmann P, Umezawa K, Tohda K, Amemiya S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations (technical report) Pure Appl. Chem. 2000;72:1851–2082. doi: 10.1351/pac200072101851. [DOI] [Google Scholar]
- 40.Bakker E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996;143:L83–L85. doi: 10.1149/1.1836608. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.