Highlights
-
•
This article summarized a total of 172 published cases of immune checkpoint inhibitor (ICI)-induced diabetes mellitus (DM).
-
•
Found that glutamic acid decarboxylase antibodies positivity is related to an earlier onset of ICI-induced diabetes and a higher frequency of diabetic ketoacidosis development.
-
•
Presented a case of ICI-induced DM following obvious lipase and amylase elevation and discussed possible relationship between ICI-associated injuries to pancreatic exocrine function and endocrine function.
Keywords: Cancer immunotherapy, Immune related adverse events, Immune-induced diabetes mellitus, Diabetic ketoacidosis, Pancreatic injury
Abstract
Objective
To better understand immune checkpoint inhibitor (ICI)-induced diabetes mellitus (DM) in cancer patients.
Design and method
We present a case of ICI-induced diabetic ketoacidosis (DKA) and conduct a systematic review of the PubMed and Web of Science databases up to September 2021 to identify all published cases of ICI-induced diabetes.
Results
In addition to our case, a total of 171 published cases were identified during the literature search. Summary and statistical analyzes were conducted for all 172 cases. The median onset time from ICI initiation to DM diagnosis was 12 weeks (range: 0–122). DKA was present in 67.4% (116/172) of the cases, and low C-peptide levels were detected in 91.8% (123/134), indicating an acute onset of diabetes. Patients with positive glutamic acid decarboxylase antibodies (GADA) had an earlier onset of ICI-induced diabetes (median time 7 weeks vs. 16 weeks for GADA-negative patients, p < 0.001) and a higher frequency of DKA development (82.8 vs. 62.1%, p = 0.006). All but two patients developed insulin-dependent diabetes permanently. Immunotherapy rechallenge was reported in 53 cases after glycemia was well controlled.
Conclusion
ICI-induced DM is a serious adverse event that often presents with life-threatening ketoacidosis. GADA positivity is related to an earlier onset of ICI-induced diabetes and a higher frequency of DKA development. Close monitoring of glucose levels is needed in patients receiving ICI treatment. ICI-induced DM is usually insulin-dependent since damage to β cells is irreversible. On the premise of well-controlled glycemia, immunotherapy rechallenge is feasible.
Introduction
Immune checkpoint inhibitors (ICIs), which commonly target immune checkpoints (e.g., programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4)), unleash the power of the immune system to eradicate tumor cells. The response rate of ICIs ranges from 15 to 60% in advanced malignancies [1]. Despite the benefits for oncology treatment, activation of the immune system could induce a variety of autoimmune effects known as immune-related adverse events (irAEs).
Since a large proportion of patients do not respond to ICIs, it is necessary to identify biomarkers that predict which patients derive the most benefit. Predictive biomarker research has predominantly been focused on tumor mutational burden, PD-L1 expression, microsatellite instability-status, gut microbiota, and irAEs [2,3]. Most studies have concluded that patients with irAEs experience improved outcomes, but some studies have come to the opposite conclusion [1]. The relationship between irAEs and ICI efficacy has not been fully revealed. In particular, whether the irAE site, severity, onset time and management have an impact on ICI effectiveness deserves further exploration.
IrAEs affect a wide spectrum of organs and systems [4], commonly endocrine, pulmonary and dermatologic systems [5]. Among all these irAEs, ICI-induced diabetes mellitus (DM) is a rare but potentially life-threatening one and deserves further attention. ICI-induced DM, also known as ICI-related autoimmune diabetes, is thought to be similar to type 1 diabetes mellitus (T1DM), but the mechanism has yet to be fully elucidated. The occurrence of ICI-induced DM in both clinical trials and real-world studies ranged from 0.9 to 1.9% [6], [7], [8], [9]. Most of these cases presented as fulminant diabetes with an extremely acute onset, which could result in a life-threatening hyperglycemic hyperosmolar state or diabetic ketoacidosis (DKA) [10]. Furthermore, the pancreas exhibits not only endocrine but also exocrine functions. Patients with ICI-related pancreatic injury could present with diabetes, pancreatitis, or both. The relationship between the two types of pancreatic injuries remains unclear. It is important to study ICI-induced DM and to identify a possible link between diabetes and lipase/amylase elevation. In recent years, cases reporting ICI-induced DM have increased gradually, making it possible for us to learn more about this special disease.
Case presentation
A 43-year-old man with extensive small cell lung cancer and no history of diabetes was enrolled in the CS1003-102 trial on Jan 21, 2020. His body mass index and baseline serologic workup, including random blood glucose, serum amylase and serum lipase, were within the normal range. He was initially treated with CS1003 (anti-PD-1 monoclonal antibody, Cstone Pharmaceuticals, China) plus the combination of etoposide and carboplatin for 4 cycles followed by CS1003 monotherapy every 3 weeks for long-term maintenance. A continuous partial response was achieved after 2 cycles of treatment until the last follow-up on March 16, 2022. The patient complained of mild upper abdominal pain before cycle 3 treatment. On March 17, 2020, laboratory findings revealed elevated serum amylase and pancreatic lipase levels of 158 U/L (upper normal limit (UNL) 115 U/L) and 1179 U/L (UNL 393 U/L), respectively. Contrast-enhanced computed tomography (CT) scans of the pancreas were normal (Fig. 1). Immune-related pancreatitis was suspected, and octreotide and proton-pump inhibitors were subsequently prescribed to the patient. Both serum amylase and lipase decreased to the normal range on March 22, and his abdominal pain was relieved. Cycle 3 treatment was given as scheduled.
Fig. 1.
Contrast-enhanced computed tomography (CT) scans of the pancreas were normal.
On April 3, 2020, the patient was transferred to the emergency room with a 2-day history of polyuria, polydipsia, burning pain of the upper abdomen, fatigue, and drowsiness. Physical examination revealed impaired consciousness, dry mouth, marbled skin, cold extremities, hypotension (blood pressure: 105/45 mmHg) and tachycardia (heart rate: 108 beats per minute). Blood analysis showed marked hyperglycemia (47.19 mmol/L). Arterial blood gas analysis showing severe metabolic acidosis with respiratory compensation, together with the positive reaction of urinary ketones, supported the diagnosis of DKA. Extended biological investigations revealed glycated hemoglobin (HbA1c): 7.6%, fasting C-peptide <0.05 ng/mL, and negative T1DM-related autoantibodies. Thyroid-stimulating hormone (TSH) was slightly low, while free triiodothyronine (FT3) and free thyroxine (FT4) were in the normal range (Table 1). No HLA-typing test was performed. The patient recovered from DKA after receiving an intravenous insulin pump and intravenous fluid treatment for approximately 10 days and was prescribed subcutaneous insulin injection sequentially for long-term treatment.
Table 1.
Laboratory data on admission.
| Lab test results | Value | Reference |
| Serum | ||
| Glucose, mmol/L | 47.19 | 3.9–6.1 |
| Urea, mmol/L | 3.21 | 2.78–7.14 |
| Creatinine, μmol/L | 56 | 59–104 |
| Na, mmol/L | 134 | 135–145 |
| K, mmol/L | 3.8 | 3.5–5.5 |
| Cl, mmol/L | 98 | 96–111 |
| Ca, mmol/L | 2.0 | 2.13–2.70 |
| Albumin, U/L | 34 | 35–52 |
| Total bilirubin, μmol/L | 16 | 5.1–22.2 |
| ALT, U/L | 16 | 9–50 |
| IAA | <2 | <20 |
| ICA | 2.55 | <20 |
| GADA | <2 | <10 |
| IA2A | <2 | <10 |
| C-peptide, ng/mL | <0.05 | 0.8–4.2 |
| HbA1c,% | 7.6 | 4.5–6.3 |
| FT3, pg/mL | 1.92 | 1.8–4.1 |
| FT4, ng/dL | 1.37 | 0.81–1.89 |
| TSH, μIU/mL | 0.291 | 0.38–4.34 |
| Urine ketones, mmol/L | >=7.8 | Negative |
| Arterial blood gas | ||
| pH | 7.27 | 7.35–7.45 |
| PaCO2, mmHg | 22 | 35–45 |
| PaO2, mmHg | 118 | 83–108 |
| HCO3−, mmol/L | 9.6 | 22–27 |
ALT, glutamic-pyruvic transaminase; IAA, insulin autoantibody; ICA, islet cell autoantibody; GADA, glutamic acid decarboxylase autoantibody; IA2A, insulinoma-associated antigen-2 autoantibody; HbA1c, glycated hemoglobin; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone.
Routine tests after 4 cycles of chemoimmunotherapy showed that TSH decreased and FT3 rose to 7.46 pg/mL, without any related clinical symptoms. Thyroid peroxidase antibody (TPO-Ab) and thyroid-stimulating immunoglobulin were negative. Based on these laboratory findings, the patient was diagnosed with ICI-induced thyroiditis. However, his thyroid dysfunction returned to normal spontaneously about two months later without further intervention or suspending PD-1 antibody.
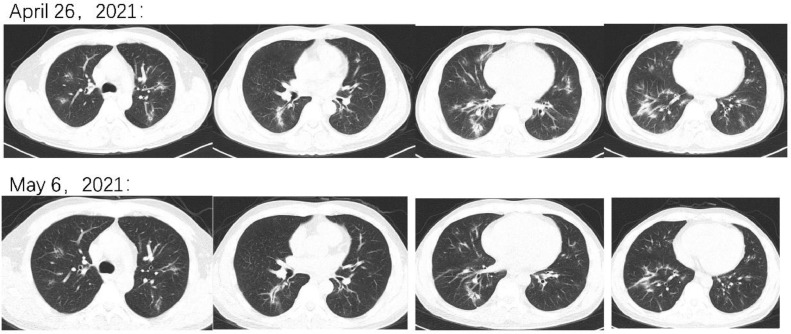
After 15 cycles of anti-PD-1 monotherapy, he developed a new cough with yellow sputum, combined with shortness of breath in April 2021. Chest CT revealed new patchy shadows in both lungs (Fig. 2). Electronic bronchoscopy suggested that the bronchial mucosa was smooth, and etiological screening of sputum and bronchoalveolar lavage fluid (BALF) was negative. BALF cytological classification showed that the number of lymphocytes was elevated significantly, which matched the diagnostic criteria of immunotherapy-associated pneumonia, and immunotherapy was consequently suspended. Prednisone (40 mg per day) was prescribed, and the dosage of subcutaneous insulin was increased accordingly. Since pulmonary infection could not be ruled out completely, moxifloxacin was also given for two weeks. Ten days later, a CT scan suggested that the patchy shadows were absorbed, and prednisone was reduced slowly.
Fig. 2.
CT on April 26, 2021 showed patchy shadows in both lungs. CT on May 6, 2021 showed that the previous patchy shadows had regressed.
Methods
The PubMed and Web of Science databases were searched up to September 2021 for case reports and case series on the subject of DM and ICIs. The title and abstract were screened for manuscript selection. Language was restricted to English. Congress reports were excluded. The search terms included ‘CTLA-4’, ‘PD-1’, ‘PD-L1’, ‘Pembrolizumab’, ‘Nivolumab’, ‘Ipilimumab’, ‘Atezolizumab’, ‘Durvalumab’, ‘immune checkpoint inhibitors’, ‘cancer immunotherapy’, ‘diabetes mellitus’, ‘autoimmune diabetes’, ‘diabetic ketoacidosis’, ‘ketoacidosis’, ‘DKA’, and ‘fulminant diabetes’. The following data were extracted from each manuscript: author, year of publication, sex and age of the patient, cancer type, checkpoint inhibitor therapy, relevant past medical history, onset time of diabetes, presence of DKA, glycemia, HbA1c, C-peptide, islet autoantibodies, serum lipase or amylase, development of other irAEs, and ICI rechallenge. Statistical analysis was carried out using the SPSS Statistics software program (version 24.0; IBM Corporation, Armonk, NY).
Results
Literature search identified a total of 151 studies, of which 102 studies were eligible. Baseline characteristics are presented in Table 2. In addition to our patients, 171 cases were identified [7,8,[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110]], with a male predominance (106/172, 61.6%) and a median age of 63 years (range 12–87). A prior diabetes history was observed in 13.4% (23/172) of the patients. Four patients had been exposed to high-dose glucocorticoids before DM diagnosis. The main tumor types were melanoma (43.6%) and lung cancer (30.2%). Pancreas metastasis was reported in two cases. Most patients were treated with anti-PD-1 or anti-PD-L1 monoclonal antibodies as monotherapy (76.2%). A total of 15.7% (n = 27) of the patients received a combination therapy of anti-PD-1/PD-L1 and CTLA-4 blockade and 6.4% (n = 11) of the patients received chemoimmunotherapy. Fifty-nine cases reported tumor regression or stable disease in response to the treatment.
Table 2.
Baseline characteristics of patients with ICI-induced diabetes mellitus.
| Characteristics | No. of Patients (%) or median (range) (N = 172) |
| Age (years) | 63 (12–87) |
| Male | 106 (61.6) |
| History of diabetes | 23 (13.4) |
| Exposure to high-dose glucocorticoids | 4 (2.3) |
| Tumor types | |
| Melanoma | 75 (43.6) |
| Lung cancer | 52 (30.2) |
| Renal cell carcinoma | 10 (5.8) |
| Breast cancer | 6 (3.5) |
| Gastrointestinal cancer | 6 (3.5) |
| Lymphoma | 5 (2.9) |
| Hepatocellular carcinoma | 2 (1.2) |
| Others | 16 (9.3) |
| Pancreas metastasis | 2 (1.2) |
| Immunotherapy | |
| Pembrolizumab | 65 (37.8) |
| Nivolumab | 59 (34.3) |
| Atezolizumab | 3 (1.7) |
| Durvalumab | 1 (0.6) |
| Other PD-1/PD-L1 monoclonal antibody | 3 (1.7) |
| Nivolumab + Ipilimumab | 21 (12.2) |
| Pembrolizumab+ Ipilimumab | 6 (3.5) |
| ICI + chemotherapy | 11 (6.4) |
| Other combination therapy* | 3 (1.7) |
| Clinical tumor response | |
| CR/PR/SD | 59 |
| PD | 2 |
| NA | 111 |
*Includes anti-PD-L1+CD137 blockade, atezolizumab+interleukin-2 and durvalumab+ Bacillus Calmette–Guérin.
PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; ICI, immune checkpoint inhibitor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NA, not available.
The median time from initiation of ICIs to diabetes diagnosis was 12 weeks (range: 0–122). One patient with previous type 2 diabetes treated with a sodium-glucose cotransporter-2 (SGLT2) inhibitor presented with DKA two days after the first dose of pembrolizumab [29], which is the shortest duration reported between immunotherapy initiation and ICI-induced DM diagnosis. The contribution of the SGLT2 inhibitor remains uncertain since published cases of DKA in the setting of combined SGLT2 and PD-1 inhibitors are rare. Six patients reported ICI-induced DM 6-14 weeks after the suspension of ICIs, including two patients [77,86] who developed diabetes during sequential treatment with ipilimumab after nivolumab. To our knowledge, no case of immune-related diabetes induced by anti-CTLA-4 antibody alone has been reported, and it is reasonable to assume that the above two cases of diabetes were mainly caused by nivolumab.
The presentation of DM related to cancer immunotherapy often follows a severe course. A total of 67.4% (116/172) of the patients presented with DKA, with a median presenting glycemia of 32.0 mmol/L (range: 10.0–109.4) and glycated hemoglobin of 8.0% (range: 6.0−13.7%). One developed a hyperosmolar hyperglycemic state. Low C-peptide levels were present in 91.8% (123/134) of the cases. Among these cases, C-peptide levels were found to be normal in 10 patients at the onset of diabetes but decreased below the reference range during follow-up. In two cases, C-peptide levels obtained post ICI-induced DM were in the normal range [34,74], indicating preserved β cell function. These two patients were finally able to discontinue insulin therapy, while all other reported cases were insulin dependent. Nine patients had normal C-peptide levels at the time of diabetes diagnosis without being retested later. Glucocorticoids were applied in 5 cases to salvage β cell function, but all failed. It should be noted that C-peptide levels of these 5 patients were below normal at the time of glucocorticoids initiation. Serum lipase or amylase levels were elevated in 46.7% (28/60) of the analyzed patients. Immunotherapy rechallenge was reported in 53 cases after glycemia was well controlled. Forty-six patients ceased ICIs definitely due to DM/DKA (7), multiple irAEs (9), progressive disease (10), financial issues (1) or unreported reasons (19) (Table 3). None of the patients died of ICI-induced DM or DKA.
Table 3.
Disease characteristics of patients with ICI-induced diabetes mellitus.
| Disease characteristics | No. of Patients (%) or median (range) (N = 172) |
| Time to diagnosis (weeks) | 12 (0–122) |
| Glycemia (mmol/l) | 32.0 (10.0–109.4) |
| HbA1c (%) | 8.0 (6.0–13.7) |
| Diabetes ketoacidosis | 116 (67.4) |
| C-peptide level | |
| Low or undetectable | 123/134 (91.8) |
| Normal* | 11/134 (8.2) |
| NA | 38 |
| Lipase/amylase | |
| Elevated | 28/60 (46.7) |
| Normal | 32/60 (53.3) |
| NA | 112 |
| Positive diabetes autoantibodies | |
| None | 81/151 (53.6) |
| At least one | 70/151 (46.4) |
| Two or more | 11/151 (7.3) |
| NA | 21 |
| Coexisting irAEs | 77 (44.8) |
| Thyroiditis/thyroid dysfunction | 53 (30.8) |
| Dermatitis/rash | 12 (7.0) |
| Hypophysitis | 9 (5.2) |
| Others | 26 (15.1) |
| Rechallenge of ICIs | |
| Yes | 53 (30.8) |
| No | 46 (26.7) |
| NA | 73 (42.4) |
* Two patients had normal C-peptide levels after ICI-induced diabetes development, the other nine patients had normal C-peptide levels at the time of diabetes diagnosis without being retested later.
HbA1c, glycated hemoglobin; NA, not available; ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse events.
A total of 44.8% of the cases reported other irAEs apart from diabetes, and thyroiditis, rash, and hypophysitis were the most reported (Table 3). At least one of the islet autoantibodies was positive in 46.4% (70/151), while two or more autoantibodies were detected in 7.3% (11/151) of the cases. The most common antibody was glutamic acid decarboxylase autoantibody (GADA), which was positive in 42.4% (64/151) of patients, followed by islet cell autoantibody (ICA) in 12.0%, insulin autoantibody (IAA) in 11.9%, insulinoma-associated antigen-2 autoantibody (IA2A) in 6.9%, and zinc transporter 8 autoantibody (ZnT8A) in 4.4%. An overview of islet autoantibodies is shown in Table 4.
Table 4.
Diabetes-related autoantibodies and ICI-induced diabetes.
| Positive | Negative | NA | Frequency,% | |
| All | 70 | 81 | 21 | 46.4 |
| GADA | 64 | 87 | 21 | 42.4 |
| ICA | 3 | 22 | 147 | 12.0 |
| IAA | 7 | 52 | 113 | 11.9 |
| IA2A | 8 | 108 | 56 | 6.9 |
| ZnT8A | 2 | 43 | 127 | 4.4 |
ICI, immune checkpoint inhibitor; GADA, glutamic acid decarboxylase autoantibody; ICA, islet cell autoantibody; IAA, insulin autoantibody; IA2A, insulinoma-associated antigen-2 autoantibody; ZnT8A, zinc transporter 8 autoantibody; NA, not available.
The onset of ICI-induced diabetes appeared earlier for patients presenting with DKA (median time 10 weeks vs. 18 weeks for patients without DKA, p = 0.013), positive islet autoantibodies (8 weeks vs. 16 weeks for autoantibody-negative patients, p < 0.001) or elevated lipase/amylase (9 weeks vs. 15 weeks for patients with normal lipase/amylase, p = 0.027). The rate of developing DKA was 82.8% among GADA-positive patients and 62.1% among GADA-negative patients (p = 0.006). The median glycemia at diagnosis was higher for patients with elevated lipase/amylase (44.0 vs. 30.0 mmol/L) or DKA (35.3 vs. 21.0 mmol/L). A summary of the results can be found in Table 5.
Table 5.
Comparison between patients with or without islet autoantibodies, elevated lipase/amylase levels and DKA.
| Islet autoantibodies | GADA | Lipase/amylase levels | DKA | |||||||||
| Positive (N = 70) | Negative (N = 81) | P value* | Positive (N = 64) | Negative (N = 87) | P value* | Elevated (N = 28) | Normal (N = 32) | P value* | Yes (N = 116) | No (N = 56) | P value* | |
| Onset time (weeks) | 8 | 16 | 0.000 | 7 | 16 | 0.000 | 9 | 15 | 0.027 | 10 | 18 | 0.013 |
| Glycemia (mmol/L) | 33.8 | 31.7 | 0.925 | 34.0 | 31.4 | 0.492 | 44.0 | 30.0 | 0.007 | 35.3 | 21.0 | 0.000 |
| HbA1c(%) | 7.9 | 7.8 | 0.656 | 7.9 | 7.8 | 0.969 | 7.8 | 7.6 | 0.232 | 8.0 | 8.2 | 0.338 |
| Lipase elevation | 10/21 | 17/37 | 0.902 | 9/18 | 18/40 | 0.724 | – | – | – | – | – | – |
| DKA | 55/70 | 52/81 | 0.053 | 53/64(82.8) | 54/87(62.1) | 0.006 | 23/28 | 19/32 | 0.055 | – | – | – |
* U test or chi-square test.
GADA, glutamic acid decarboxylase autoantibody; DKA, diabetes ketoacidosis; HbA1c, glycated hemoglobin.
Discussion
In this study, we report a patient with small cell lung cancer who received an anti-PD-1 antibody and developed rapid onset diabetes with ketoacidosis following lipase elevation. Moreover, we conduct a systematic review of 172 cases (171 published cases in addition to the case presented herein) of ICI-induced DM and summarize the clinical characteristics of these patients. Our results showed that 67.4% of the patients presented with a fulminant onset of DKA at the time of ICI-induced DM diagnosis. Positive islet autoantibodies were reported in approximately half of the tested cases (70 out of 151 or 46.4%), with GADA being the predominant antibody. This result is consistent with a previously reported rate [9,111] and differs from “classic” T1DM, where autoantibodies are present in 80−95% of patients [112,113]. In addition, patients with islet autoantibodies, regardless of the type of autoantibody, showed a significantly shorter duration between ICI initiation and ICI-induced diabetes (p < 0.001). Furthermore, GADA positivity is related to an earlier onset of ICI-induced diabetes (p < 0.001) and a higher frequency of DKA development (p = 0.006), possibly indicating a different mechanism of diabetes development between autoantibody-positive and autoantibody-negative patients. Autoantibodies have been recognized as risk factors for irAEs, for example, TPO-Ab in thyroid dysfunction and islet antibodies in ICI-induced DM [99]. This is possibly because ICIs could both facilitate preexisting autoantibody-mediated immunity and trigger the production of autoantibodies by enhancing B-cell immunity [114].
What our case distinguishes from other ICI-induced DM patients as well as the key point that bewildered physicians is that the case presents as elevation of amylase and lipase at first. It is rare for patients to develop ICI-induced pancreatitis and ICI-induced DM sequentially. ICI-induced DM is presumably caused by immune destruction of pancreatic β cells. Both preclinical evidence from mouse models and pancreatic pathological results showed that PD-1-PD-L1 signaling and CD8+ T lymphocyte infiltration play an important role in ICI-induced DM [115], [116], [117], [118]. Studies about the mechanism of how ICI-induced pancreatitis occurs are rather limited. However, increased peripancreatic CD3+ T lymphocyte and CD8+ T lymphocyte infiltration may also contribute to the development of pancreatitis, as has been reported in ICI-induced DM patients [119]. Although the mechanism underlying the collateral damage between exocrine pancreatic inflammation and β islet cells remains elusive, future research should examine the possibility of sequential damage from exocrine to endocrine pancreatic function. Upon noticing elevated levels of amylase or lipase, clinicians should not only monitor the enzymes and imaging of the pancreas but also monitor the blood glucose of patients receiving ICIs. The elevation in amylase might be a sign of the onset of fulminant DKA.
ICI-induced DM has been generally characterized by a sudden onset, loss of insulin secretion and high rate of DKA. Due to its acute disease progression, steroids are not effective when β islet cells are destroyed completely. Thus, the current measure to treat ICI-induced DM is insulin injection, similar to type 1 diabetes. The case we present rechallenged ICI after recovery from DKA. Moreover, the onset of irAEs may reflect a better prognosis in patients receiving ICIs. Our patient achieved a 2-year major partial response of small cell lung cancer, which also adds evidence to the relationship between irAE incidence and better treatment response in cancer patients.
There are several limitations of our study. The sample size was small, and the analysis included individual patient data, of whom not all parameters of interest were available. The incidence of ICI-induced DM could not be calculated by the lack of the total number of treated patients. Moreover, since data were collected from published literature, there may be publication bias resulting in a higher calculated incidence of DKA. Nevertheless, we systematically reviewed the cases reported in the literature and provided a better understanding of ICI-induced DM patients. Further studies of the mechanism of collateral damage of endocrine and exocrine function of pancreas and exploration of possible predictive biomarkers are warranted.
Conclusion
ICI-induced DM is a rare but potentially life-threatening adverse event, as DKA is often the first presentation. GADA positivity is related to an earlier onset of ICI-induced diabetes and a higher frequency of DKA development. Close monitoring of blood glucose in ICI application patients is essential. ICI-induced DM is usually insulin-dependent since the damage to β cells is irreversible, and immunotherapy rechallenge is feasible on the premise of well-controlled blood glucose. Further research on predictive biomarkers for the stratification of vulnerable patients is needed.
Authors' contributions
Minjiang Chen was the doctor in charge of the case we reported and helped with the follow-up of the patient. Jing Zhao and Wei Zhong assisted in the analysis of the results.
CRediT authorship contribution statement
Jia Liu: Formal analysis, Writing – original draft, Writing – review & editing. Yuequan Shi: Formal analysis, Writing – original draft, Writing – review & editing. Xiaoyan Liu: Formal analysis, Writing – review & editing. Dongming Zhang: Data curation, Writing – review & editing. Haoran Zhang: Data curation, Writing – review & editing. Minjiang Chen: . Yan Xu: Formal analysis, Writing – review & editing. Jing Zhao: . Wei Zhong: . Mengzhao Wang: Formal analysis, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was supported by the Youth Program of the National Natural Science Foundation of China (to Yan Xu) (Grant No. 82003309) and by the CAMS Innovation Fund for Medical Sciences (CIFMS) (to Yan Xu) (Grant No. 2021-I2M-C&T-B-014).
References
- 1.Das S., Johnson D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer. 2019;7(1):306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman A.M., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V., Stephens P.J., Daniels G.A., Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017;16(11):2598–2608. doi: 10.1158/1535-7163.Mct-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzo A., Ricci A.D. PD-L1, TMB, and other potential predictors of response to immunotherapy for hepatocellular carcinoma: how can they assist drug clinical trials? Expert Opin. Investig. Drugs. 2022;31(4):415–423. doi: 10.1080/13543784.2021.1972969. [DOI] [PubMed] [Google Scholar]
- 4.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y., Fang J., Zhou C., Liu A., Wang Y., Meng Q., Ding C., Ai B., Gu Y., Yao Y., Sun H., Guo H., Zhang C., Song X., Li J., Xu B., Han Z., Song M., Tang T., Chen P., Lu H., Shui Y., Lou G., Zhang D., Liu J., Liu X., Liu X., Gao X., Zhou Q., Chen M., Zhao J., Zhong W., Xu Y., Wang M. Immune checkpoint inhibitor-related adverse events in lung cancer: real-world incidence and management practices of 1905 patients in China. Thorac. Cancer. 2022;13(3):412–422. doi: 10.1111/1759-7714.14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., Pitot H.C., Hamid O., Bhatia S., Martins R., Eaton K., Chen S., Salay T.M., Alaparthy S., Grosso J.F., Korman A.J., Parker S.M., Agrawal S., Goldberg S.M., Pardoll D.M., Gupta A., Wigginton J.M. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang V.H.M., Mcgrath R.T., Clifton-Bligh R.J., Scolyer R.A., Jakrot V., Guminski A.D., Long G.V., Menzies A.M. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J. Clin. Endocrinol. Metab. 2019;104(11):5499–5506. doi: 10.1210/jc.2019-00423. [DOI] [PubMed] [Google Scholar]
- 8.Kotwal A., Haddox C., Block M., Kudva Y.C. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res. Care. 2019;7(1) doi: 10.1136/bmjdrc-2018-000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamatouli A.M., Quandt Z., Perdigoto A.L., Clark P.L., Kluger H., Weiss S.A., Gettinger S., Sznol M., Young A., Rushakoff R., Lee J., Bluestone J.A., Anderson M., Herold K.C. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes, 2018;67(8):1471–1480. doi: 10.2337/dbi18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akturk H.K., Michels A.W. Adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018;378(12):1163–1164. doi: 10.1056/NEJMc1801663. [DOI] [PubMed] [Google Scholar]
- 11.Abdullah H.M.A., Elnair R., Khan U.I., Omar M., Morey-Vargas O.L. Rapid onset type-1 diabetes and diabetic ketoacidosis secondary to nivolumab immunotherapy: a review of existing literature. BMJ Case Rep. 2019;12(8) doi: 10.1136/bcr-2019-229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aleksova J., Lau P.K., Soldatos G., Mcarthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-217454. bcr2016217454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhusseini M., Samantray J. Autoimmune diabetes superimposed on type 2 diabetes in a patient initiated on immunotherapy for lung cancer. Diabetes Metab. 2017;43(1):86–88. doi: 10.1016/j.diabet.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Alzenaidi A.A., Dendy J., Rejjal L. Autoimmune diabetes presented with diabetic ketoacidosis induced by immunotherapy in an adult with melanoma. J. La. State Med. Soc. 2017;169(2):49. [PubMed] [Google Scholar]
- 15.Araújo M., Ligeiro D., Costa L., Marques F., Trindade H., Correia J.M., Fonseca C. A case of fulminant type 1 diabetes following anti-PD1 immunotherapy in a genetically susceptible patient. Immunotherapy. 2017;9(7):531–535. doi: 10.2217/imt-2017-0020. [DOI] [PubMed] [Google Scholar]
- 16.Atkins P.W., Thompson D.M. Combination avelumab and utomilumab immunotherapy can induce diabetic ketoacidosis. Diabetes Metab. 2018;44(6):514–515. doi: 10.1016/j.diabet.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Bastin M., Mosbah H., Carlier A., Boudifa A., Villemain A., Hartemann A., Andreelli F. Variability in clinical presentation of diabetes mellitus during anti-PD-1 immunotherapy. Diabetes Metab. 2020;46(5):406–407. doi: 10.1016/j.diabet.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Boswell L., Casals G., Blanco J., Jiménez A., Aya F., De Hollanda A., Halperin I., Arance A.M., Mora M., Hanzu F.A. Onset of fulminant type 1 diabetes mellitus following hypophysitis after discontinuation of combined immunotherapy. A case report. J. Diabetes Investig. 2021;12(12):2263–2266. doi: 10.1111/jdi.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle V., Cundy T., Cutfield R. Rapid onset type 1 diabetes associated with the programmed cell death-1 inhibitor pembrolizumab. Intern. Med. J. 2019;49(7):930–931. doi: 10.1111/imj.14340. [DOI] [PubMed] [Google Scholar]
- 20.Capitao R., Bello C., Fonseca R., Saraiva C. New onset diabetes after nivolumab treatment. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-220999. bcr2017220999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chae Y.K., Chiec L., Mohindra N., Gentzler R., Patel J., Giles F. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol. Immunother. 2017;66(1):25–32. doi: 10.1007/s00262-016-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chokr N., Farooq H., Guadalupe E. Fulminant diabetes in a patient with advanced melanoma on nivolumab. Case Rep. Oncol. Med. 2018;2018 doi: 10.1155/2018/8981375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clontz R., Dang D.M., Hieger M.A., Becker B.A. Atezolizumab-induced autoimmune diabetes in a patient with metastatic breast cancer: a case report. Clin. Pract. Cases Emerg. Med. 2021;5(2):190–193. doi: 10.5811/cpcem.2021.2.51508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clotman K., Janssens K., Specenier P., Weets I., De Block C.E.M. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J. Clin. Endocrinol. Metab. 2018;103(9):3144–3154. doi: 10.1210/jc.2018-00728. [DOI] [PubMed] [Google Scholar]
- 25.Cuenca J.A., Laserna A., Reyes M.P., Nates J.L., Botz G.H. Critical care admission of an HIV patient with diabetic ketoacidosis secondary to pembrolizumab. Case Rep. Crit. Care. 2020;2020 doi: 10.1155/2020/8671530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Filette J.M.K., Pen J.J., Decoster L., Vissers T., Bravenboer B., Van Der Auwera B.J., Gorus F.K., Roep B.O., Aspeslagh S., Neyns B., Velkeniers B., Kharagjitsingh A.V. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur. J. Endocrinol. 2019;181(3):363–374. doi: 10.1530/eje-19-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delasos L., Bazewicz C., Sliwinska A., Lia N.L., Vredenburgh J. New onset diabetes with ketoacidosis following nivolumab immunotherapy: a case report and review of literature. J. Oncol. Pharm. Pract. 2021;27(3):716–721. doi: 10.1177/1078155220943949. [DOI] [PubMed] [Google Scholar]
- 28.Edahiro R., Ishijima M., Kurebe H., Nishida K., Uenami T., Kanazu M., Akazawa Y., Yano Y., Mori M. Continued administration of pembrolizumab for adenocarcinoma of the lung after the onset of fulminant type 1 diabetes mellitus as an immune-related adverse effect: a case report. Thorac. Cancer. 2019;10(5):1276–1279. doi: 10.1111/1759-7714.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galligan A., Xu W., Fourlanos S., Nankervis A., Chiang C., Mant A.M., Parente P., Rischin D., Krishnamurthy B., Sandhu S., Colman P.G. Diabetes associated with immune checkpoint inhibition: presentation and management challenges. Diabet. Med. 2018;35(9):1283–1290. doi: 10.1111/dme.13762. [DOI] [PubMed] [Google Scholar]
- 30.Gauci M.L., Laly P., Vidal-Trecan T., Baroudjian B., Gottlieb J., Madjlessi-Ezra N., Da Meda L., Madelaine-Chambrin I., Bagot M., Basset-Seguin N., Pages C., Mourah S., Boudou P., Lebbe C., Gautier J.F. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol. Immunother. 2017;66(11):1399–1410. doi: 10.1007/s00262-017-2033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudy C., Clévy C., Monestier S., Dubois N., Préau Y., Mallet S., Richard M.A., Grob J.J., Valéro R., Béliard S. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;38(11):e182–e183. doi: 10.2337/dc15-1331. [DOI] [PubMed] [Google Scholar]
- 32.Godwin J.L., Jaggi S., Sirisena I., Sharda P., Rao A.D., Mehra R., Veloski C. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J. Immunother. Cancer. 2017;5(1):40. doi: 10.1186/s40425-017-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakami O.A., Ioana J., Ahmad S., Tun T.K., Sreenan S., Mcdermott J.H. A case of pembrolizumab-induced severe DKA and hypothyroidism in a patient with metastatic melanoma. Endocrinol. Diabetes Metab. Case Rep. 2019;2019(1) doi: 10.1530/edm-18-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen E., Sahasrabudhe D., Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol. Immunother. 2016;65(6):765–767. doi: 10.1007/s00262-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao J.B., Renno A., Imam S., Alfonso-Jaume M., Elnagar N., Jaume J.C. Development of type 1 diabetes after cancer immunotherapy. AACE Clin. Case Rep. 2017;3(3):e242–e245. doi: 10.4158/EP161410.CR. [DOI] [Google Scholar]
- 36.Haque W., Ahmed S.R., Zilbermint M. Nivolumab-induced autoimmune diabetes mellitus and hypothyroidism in a patient with rectal neuroendocrine tumor. J. Community Hosp. Intern. Med. Perspect. 2020;10(4):338–339. doi: 10.1080/20009666.2020.1771126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harsch I.A., Konturek P.C. Acute-onset diabetes mellitus with ketoacidosis in a nivolumab-treated patient with hepatocellular carcinoma. Wiad. Lek. 2018;71(5):945–948. [PubMed] [Google Scholar]
- 38.Hatakeyama Y., Ohnishi H., Suda K., Okamura K., Shimada T., Yoshimura S. Nivolumab-induced acute-onset type 1 diabetes mellitus as an immune-related adverse event: a case report. J. Oncol. Pharm. Pract. 2019;25(8):2023–2026. doi: 10.1177/1078155218816777. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez A., Zeidan B., Desai P., Frunzi J. Diabetic ketoacidosis secondary to new onset type 1 diabetes mellitus related to pembrolizumab therapy. Cureus. 2021;13(2):e13302. doi: 10.7759/cureus.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann L., Forschner A., Loquai C., Goldinger S.M., Zimmer L., Ugurel S., Schmidgen M.I., Gutzmer R., Utikal J.S., Goppner D., Hassel J.C., Meier F., Tietze J.K., Thomas I., Weishaupt C., Leverkus M., Wahl R., Dietrich U., Garbe C., Kirchberger M.C., Eigentler T., Berking C., Gesierich A., Krackhardt A.M., Schadendorf D., Schuler G., Dummer R., Heinzerling L.M. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Huang X., Yang M., Wang L., Li L., Zhong X. Sintilimab induced diabetic ketoacidosis in a patient with small cell lung cancer: a case report and literature review. Medicine. 2021;100(19):e25795. doi: 10.1097/md.0000000000025795. (Baltimore). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes J., Vudattu N., Sznol M., Gettinger S., Kluger H., Lupsa B., Herold K.C. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55–e57. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hughes M.S., Pietropaolo M., Vasudevan M.M., Marcelli M., Nguyen H. Checking the checkpoint inhibitors: a case of autoimmune diabetes after pd-1 inhibition in a patient with HIV. J. Endocr. Soc. 2020;4(12):bvaa150. doi: 10.1210/jendso/bvaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Humayun M.A., Poole R. A case of multiple immune toxicities from Ipilimumab and pembrolizumab treatment. Hormones. 2016;15(2):303–306. doi: 10.14310/horm.2002.1656. (Athens) [DOI] [PubMed] [Google Scholar]
- 45.Ishikawa K., Shono-Saito T., Yamate T., Kai Y., Sakai T., Shimizu F., Yamada Y., Mori H., Noso S., Ikegami H., Kojima H., Tanaka H., Fujiwara S., Hatano Y. A case of fulminant type 1 diabetes mellitus, with a precipitous decrease in pancreatic volume, induced by nivolumab for malignant melanoma: analysis of HLA and CTLA-4 polymorphisms. Eur. J. Dermatol. 2017;27(2):184–185. doi: 10.1684/ejd.2016.2923. [DOI] [PubMed] [Google Scholar]
- 46.Jessel S., Austin M., Kluger H.M. Mycophenolate as primary treatment for immune checkpoint inhibitor induced acute kidney injury in a patient with concurrent immunotherapy-associated diabetes: a case report. Clin. Oncol. Case Rep. 2021;4(1):156. [PMC free article] [PubMed] [Google Scholar]
- 47.Kapke J., Shaheen Z., Kilari D., Knudson P., Wong S. Immune checkpoint inhibitor-associated type 1 diabetes mellitus: case series, review of the literature, and optimal management. Case Rep. Oncol. 2017;10(3):897–909. doi: 10.1159/000480634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kedzior S.K., Jacknin G., Hudler A., Mueller S.W., Kiser T.H. A severe case of diabetic ketoacidosis and new-onset type 1 diabetes mellitus associated with anti-glutamic acid decarboxylase antibodies following immunotherapy with pembrolizumab. Am. J. Case Rep. 2021;22 doi: 10.12659/ajcr.931702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keerty D., Das M., Hallanger-Johnson J., Haynes E. Diabetic ketoacidosis: an adverse reaction to immunotherapy. Cureus. 2020;12(9):e10632. doi: 10.7759/cureus.10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kethireddy N., Thomas S., Bindal P., Shukla P., Hegde U. Multiple autoimmune side effects of immune checkpoint inhibitors in a patient with metastatic melanoma receiving pembrolizumab. J. Oncol. Pharm. Pract. 2021;27(1):207–211. doi: 10.1177/1078155220921543. [DOI] [PubMed] [Google Scholar]
- 51.Kichloo A., Albosta M.S., Mcmahon S., Movsesian K., Wani F., Jamal S.M., Aljadah M., Singh J. Pembrolizumab-induced diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic colonic adenocarcinoma. J. Investig. Med. High Impact Case Rep. 2020;8 doi: 10.1177/2324709620951339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kikuchi F., Saheki T., Imachi H., Kobayashi T., Fukunaga K., Ibata T., Sato S., Ban N., Lyu J., Japar S., Murao K. Nivolumab-induced hypophysitis followed by acute-onset type 1 diabetes with renal cell carcinoma: a case report. J. Med. Case Rep. 2021;15(1):214. doi: 10.1186/s13256-020-02656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurihara S., Oikawa Y., Nakajima R., Satomura A., Tanaka R., Kagamu H., Shimada A. Simultaneous development of Graves' disease and type 1 diabetes during anti-programmed cell death-1 therapy: a case report. J. Diabetes Investig. 2020;11(4):1006–1009. doi: 10.1111/jdi.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kusuki K., Suzuki S., Mizuno Y. Pembrolizumab-induced fulminant type 1 diabetes with C-peptide persistence at first referral. Endocrinol. Diabetes Metab. Case Rep. 2020;2020:19–0152. doi: 10.1530/edm-19-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S., Morgan A., Shah S., Ebeling P.R. Rapid-onset diabetic ketoacidosis secondary to nivolumab therapy. Endocrinol. Diabetes Metab. Case Rep. 2018;2018:18–0021. doi: 10.1530/edm-18-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leonardi G.C., Oxnard G.R., Haas A., Lang J.P., Williams J.S., Awad M.M. Diabetic ketoacidosis as an immune-related adverse event from pembrolizumab in non-small cell lung cancer. J. Immunother. 2017;40(6):249–251. doi: 10.1097/cji.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 57.Li L., Masood A., Bari S., Yavuz S., Grosbach A.B. Autoimmune diabetes and thyroiditis complicating treatment with nivolumab. Case Rep. Oncol. 2017;10(1):230–234. doi: 10.1159/000456540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li S., Zhang Y., Sun Z., Hu J., Fang C. Anti-PD-1 pembrolizumab induced autoimmune diabetes in Chinese patient: a case report. Medicine. 2018;97(45):e12907. doi: 10.1097/md.0000000000012907. (Baltimore). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowe J.R., Perry D.J., Salama A.K., Mathews C.E., Moss L.G., Hanks B.A. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J. Immunother. Cancer. 2016;4:89. doi: 10.1186/s40425-016-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maamari J., Yeung S.J., Chaftari P.S. Diabetic ketoacidosis induced by a single dose of pembrolizumab. Am. J. Emerg. Med. 2019;37(2):376.e1–376.e2. doi: 10.1016/j.ajem.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 61.Mae S., Kuriyama A., Tachibana H. Diabetic ketoacidosis as a delayed immune-related event after discontinuation of nivolumab. J. Emerg. Med. 2021;60(3):342–344. doi: 10.1016/j.jemermed.2020.09.023. [DOI] [PubMed] [Google Scholar]
- 62.Maekawa T., Okada K., Okada H., Kado S., Kamiya K., Komine M., Murata S., Oka K., Ishibashi S., Ohtsuki M. Case of acute-onset type 1 diabetes induced by long-term immunotherapy with nivolumab in a patient with mucosal melanoma. J. Dermatol. 2019;46(12):e463–e464. doi: 10.1111/1346-8138.15061. [DOI] [PubMed] [Google Scholar]
- 63.Marchand L., Thivolet A., Dalle S., Chikh K., Reffet S., Vouillarmet J., Fabien N., Cugnet-Anceau C., Thivolet C. Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: description of pancreatic endocrine and exocrine phenotype. Acta Diabetol. 2019;56(4):441–448. doi: 10.1007/s00592-018-1234-8. [DOI] [PubMed] [Google Scholar]
- 64.Marshall S., Kizuki A., Kitaoji T., Imada H., Kato H., Hosoda M., Ishikawa M., Sakura H. Type 1 diabetes, ACTH deficiency, and hypothyroidism simultaneously induced by nivolumab therapy in a patient with gastric cancer: a case report. Case Rep. Oncol. 2020;13(3):1185–1190. doi: 10.1159/000510044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin-Liberal J., Furness A.J., Joshi K., Peggs K.S., Quezada S.A., Larkin J. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol. Immunother. 2015;64(6):765–767. doi: 10.1007/s00262-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuura N., Koh G., Konishi C., Minamino S., Takahara Y., Harada H., Kodama K., Emoto M. Fulminant onset of insulin-dependent diabetes with positive anti-GAD antibody titers during treatment with nivolumab in a patient with NSCLC. Cancer Immunol. Immunother. 2018;67(9):1417–1424. doi: 10.1007/s00262-018-2203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mellati M., Eaton K.D., Brooks-Worrell B.M., Hagopian W.A., Martins R., Palmer J.P., Hirsch I.B. Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015;38(9):e137–e138. doi: 10.2337/dc15-0889. [DOI] [PubMed] [Google Scholar]
- 68.Mengíbar J.L., Capel I., Bonfill T., Mazarico I., Espuña L.C., Caixàs A., Rigla M. Simultaneous onset of type 1 diabetes mellitus and silent thyroiditis under durvalumab treatment. Endocrinol. Diabetes Metab. Case Rep. 2019;2019(1):19–0045. doi: 10.1530/edm-19-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyauchi M., Toyoda M., Zhang J., Hamada N., Yamawaki T., Tanaka J., Harada K., Kashizaki F., Fukagawa M. Nivolumab-induced fulminant type 1 diabetes with precipitous fall in C-peptide level. J. Diabetes Investig. 2020;11(3):748–749. doi: 10.1111/jdi.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyoshi Y., Ogawa O., Oyama Y. Nivolumab, an anti-programmed cell death-1 antibody, induces fulminant type 1 diabetes. Tohoku J. Exp. Med. 2016;239(2):155–158. doi: 10.1620/tjem.239.155. [DOI] [PubMed] [Google Scholar]
- 71.Munakata W., Ohashi K., Yamauchi N., Tobinai K. Fulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical hodgkin lymphoma. Int. J. Hematol. 2017;105(3):383–386. doi: 10.1007/s12185-016-2101-4. [DOI] [PubMed] [Google Scholar]
- 72.Shah M., Maxfield L., Feroz R., Donohue K. Rapid development of type 1 diabetes mellitus after initiation of anti-PD-1 therapy. Int. J. Cancer Clin. Res. 2016;3(4):066. [Google Scholar]
- 73.Nikouline A., Brzozowski M. New DKA in a geriatric patient on immune checkpoint inhibitor therapy: a case report. CJEM. 2021;23(5):712–714. doi: 10.1007/s43678-021-00145-4. [DOI] [PubMed] [Google Scholar]
- 74.Ohara N., Kobayashi M., Ikeda Y., Hoshi T., Morita S., Kanefuji T., Yagi K., Suda T., Takada T., Hasegawa G., Sato Y., Hirano K., Kosugi S.I. Non-insulin-dependent diabetes mellitus induced by immune checkpoint inhibitor therapy in an insulinoma-associated antigen-2 autoantibody-positive patient with advanced gastric cancer. Intern. Med. 2020;59(4):551–556. doi: 10.2169/internalmedicine.3208-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okamoto M., Okamoto M., Gotoh K., Masaki T., Ozeki Y., Ando H., Anai M., Sato A., Yoshida Y., Ueda S., Kakuma T., Shibata H. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J. Diabetes Investig. 2016;7(6):915–918. doi: 10.1111/jdi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oldfield K., Jayasinghe R., Niranjan S., Chadha S. Immune checkpoint inhibitor-induced takotsubo syndrome and diabetic ketoacidosis: rare reactions. BMJ Case Rep. 2021;14(2) doi: 10.1136/bcr-2020-237217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Omodaka T., Kiniwa Y., Sato Y., Suwa M., Sato M., Yamaguchi T., Sato A., Miyake T., Okuyama R. Type 1 diabetes in a melanoma patient treated with ipilimumab after nivolumab. J. Dermatol. 2018;45(10):e289–e290. doi: 10.1111/1346-8138.14331. [DOI] [PubMed] [Google Scholar]
- 78.Patel S., Chin V., Greenfield J.R. Durvalumab-induced diabetic ketoacidosis followed by hypothyroidism. Endocrinol. Diabetes. Metab. Case Rep. 2019;2019:19–0098. doi: 10.1530/edm-19-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peyrony O., Ellouze S., Fontaine J.P., Mohamadou I., Zafrani L. Fulminant diabetes due to immune checkpoint inhibitors in the emergency department. Am. J. Emerg. Med. 2020;38(2):408.e3–408.e4. doi: 10.1016/j.ajem.2019.158495. [DOI] [PubMed] [Google Scholar]
- 80.Porntharukchareon T., Tontivuthikul B., Sintawichai N., Srichomkwun P. Pembrolizumab- and ipilimumab-induced diabetic ketoacidosis and isolated adrenocorticotropic hormone deficiency: a case report. J. Med. Case Rep. 2020;14(1):171. doi: 10.1186/s13256-020-02502-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahman W., Conley A., Silver K.D. Atezolizumab-induced type 1 diabetes mellitus in a patient with metastatic renal cell carcinoma. BMJ Case Rep. 2020;13(7) doi: 10.1136/bcr-2019-233842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakaguchi C., Ashida K., Yano S., Ohe K., Wada N., Hasuzawa N., Matsuda Y., Sakamoto S., Sakamoto R., Uchi H., Furue M., Nomura M., Ogawa Y. A case of nivolumab-induced acute-onset type 1 diabetes mellitus in melanoma. Curr. Oncol. 2019;26(1):e115–e118. doi: 10.3747/co.26.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sakurai K., Niitsuma S., Sato R., Takahashi K., Arihara Z. Painless thyroiditis and fulminant type 1 diabetes mellitus in a patient treated with an immune checkpoint inhibitor, nivolumab. Tohoku J. Exp. Med. 2018;244(1):33–40. doi: 10.1620/tjem.244.33. [DOI] [PubMed] [Google Scholar]
- 84.Samoa R.A., Lee H.S., Kil S.H., Roep B.O. Anti-PD-1 Therapy-associated type 1 diabetes in a pediatric patient with relapsed classical hodgkin lymphoma. Diabetes Care. 2020;43(9):2293–2295. doi: 10.2337/dc20-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scott E.S., Long G.V., Guminski A., Clifton-Bligh R.J., Menzies A.M., Tsang V.H. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur. J. Endocrinol. 2018;178(2):173–180. doi: 10.1530/eje-17-0810. [DOI] [PubMed] [Google Scholar]
- 86.Shiba M., Inaba H., Ariyasu H., Kawai S., Inagaki Y., Matsuno S., Iwakura H., Yamamoto Y., Nishi M., Akamizu T. Fulminant type 1 diabetes mellitus accompanied by positive conversion of anti-insulin antibody after the administration of anti-CTLA-4 antibody following the discontinuation of anti-PD-1 antibody. Intern. Med. 2018;57(14):2029–2034. doi: 10.2169/internalmedicine.9518-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skorpen P.K., Margull J. Diabetic ketoacidosis following immunotherapy for lung cancer. Tidsskr. Nor. Laegeforen. 2019;139(4) doi: 10.4045/tidsskr.18.0597. [DOI] [PubMed] [Google Scholar]
- 88.Smith-Cohn M.A., Gill D., Voorhies B.N., Agarwal N., Garrido-Laguna I. Case report: pembrolizumab-induced Type 1 diabetes in a patient with metastatic cholangiocarcinoma. Immunotherapy. 2017;9(10):797–804. doi: 10.2217/imt-2017-0042. [DOI] [PubMed] [Google Scholar]
- 89.Sothornwit J., Phunmanee A., Pongchaiyakul C. Atezolizumab-induced autoimmune diabetes in a patient with metastatic lung cancer. Front. Endocrinol. 2019;10:352. doi: 10.3389/fendo.2019.00352. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sum M., Garcia F.V. Immunotherapy-induced autoimmune diabetes and concomitant hypophysitis. Pituitary. 2018;21(5):556–557. doi: 10.1007/s11102-018-0880-8. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi A., Tsutsumida A., Namikawa K., Yamazaki N. Fulminant type 1 diabetes associated with nivolumab in a patient with metastatic melanoma. Melanoma Res. 2018;28(2):159–160. doi: 10.1097/cmr.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 92.Tang Y., Zhao Z., Wang X., Zuo W., Zhang B., Yuan T., Fu Y. A case of pembrolizumab-induced fulminant type 1 diabetes mellitus in breast cancer. Immunotherapy. 2021;13(6):483–489. doi: 10.2217/imt-2020-0222. [DOI] [PubMed] [Google Scholar]
- 93.Tassone F., Colantonio I., Gamarra E., Gianotti L., Baffoni C., Magro G., Borretta G. Nivolumab-induced fulminant type 1 diabetes (T1D): the first Italian case report with long follow-up and flash glucose monitoring. Acta Diabetol. 2019;56(4):489–490. doi: 10.1007/s00592-018-1246-4. [DOI] [PubMed] [Google Scholar]
- 94.Teramoto Y., Nakamura Y., Asami Y., Imamura T., Takahira S., Nemoto M., Sakai G., Shimada A., Noda M., Yamamoto A. Case of type 1 diabetes associated with less-dose nivolumab therapy in a melanoma patient. J. Dermatol. 2017;44(5):605–606. doi: 10.1111/1346-8138.13486. [DOI] [PubMed] [Google Scholar]
- 95.Thoreau B., Gouaillier-Vulcain F., Machet L., Mateus C., Robert C., Ferreira-Maldent N., Maillot F., Lioger B. Acute lower limb ischaemia and diabetes in a patient treated with anti-PD1 monoclonal antibody for metastatic melanoma. Acta Derm. Venereol. 2017;97(3):408–409. doi: 10.2340/00015555-2504. [DOI] [PubMed] [Google Scholar]
- 96.Tohi Y., Fujimoto K., Suzuki R., Suzuki I., Kubota M., Kawakita M. Fulminant type 1 diabetes mellitus induced by pembrolizumab in a patient with urothelial carcinoma: a case report. Urol. Case Rep. 2019;24 doi: 10.1016/j.eucr.2019.100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsang E.S., Walker E.J., Carnevale J., Fisher G.A., Ko A.H. Durable response after immune checkpoint inhibitor-related diabetes in mismatch repair deficient pancreatic cancer. Immunotherapy. 2021;13(15):1249–1254. doi: 10.2217/imt-2021-0008. [DOI] [PubMed] [Google Scholar]
- 98.Tzoulis P., Corbett R.W., Ponnampalam S., Baker E., Heaton D., Doulgeraki T., Stebbing J. Nivolumab-induced fulminant diabetic ketoacidosis followed by thyroiditis. Endocrinol. Diabetes Metab. Case Rep. 2018;2018:18–0111. doi: 10.1530/edm-18-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Usui Y., Udagawa H., Matsumoto S., Imai K., Ohashi K., Ishibashi M., Kirita K., Umemura S., Yoh K., Niho S., Osame K., Goto K. Association of serum anti-GAD antibody and HLA haplotypes with type 1 diabetes mellitus triggered by nivolumab in patients with non-small cell lung cancer. J. Thorac. Oncol. 2017;12(5):e41–e43. doi: 10.1016/j.jtho.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 100.Venetsanaki V., Boutis A., Chrisoulidou A., Papakotoulas P. Diabetes mellitus secondary to treatment with immune checkpoint inhibitors. Curr. Oncol. 2019;26(1):e111–e114. doi: 10.3747/co.26.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wen L., Zou X., Chen Y., Bai X., Liang T. Sintilimab-induced autoimmune diabetes in a patient with the anti-tumor effect of partial regression. Front. Immunol. 2020;11:2076. doi: 10.3389/fimmu.2020.02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong M., Nandi N., Sinha A. A unique case of atezolizumab-induced autoimmune diabetes. AACE Clin. Case Rep. 2020;6(1):e30–e32. doi: 10.4158/accr-2019-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu L., Li B. A case of severe diabetic ketoacidosis associated with pembrolizumab therapy in a patient with metastatic melanoma. Diabetes Metab. Syndr. Obes. 2021;14:753–757. doi: 10.2147/dmso.S297709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamaguchi H., Miyoshi Y., Uehara Y., Fujii K., Nagata S., Obata Y., Kosugi M., Hazama Y., Yasuda T. Case of slowly progressive type 1 diabetes mellitus with drastically reduced insulin secretory capacity after immune checkpoint inhibitor treatment for advanced renal cell carcinoma. Diabetol. Int. 2021;12(2):234–240. doi: 10.1007/s13340-020-00459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamoto N., Tsurutani Y., Katsuragawa S., Kubo H., Sunouchi T., Hirose R., Hoshino Y., Ichikawa M., Takiguchi T., Yukawa H., Arioka H., Saitou J., Nishikawa T. A Patient with nivolumab-related fulminant type 1 diabetes mellitus whose serum C-peptide level was preserved at the initial detection of hyperglycemia. Intern. Med. 2019;58(19):2825–2830. doi: 10.2169/internalmedicine.2780-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yilmaz M. Nivolumab-induced type 1 diabetes mellitus as an immune-related adverse event. J. Oncol. Pharm. Pract. 2020;26(1):236–239. doi: 10.1177/1078155219841116. [DOI] [PubMed] [Google Scholar]
- 107.Yun K., Daniels G., Gold K., Mccowen K., Patel S.P. Rapid onset type 1 diabetes with anti-PD-1 directed therapy. Oncotarget. 2020;11(28):2740–2746. doi: 10.18632/oncotarget.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zaied A.A., Akturk H.K., Joseph R.W., Lee A.S. New-onset insulin-dependent diabetes due to nivolumab. Endocrinol. Diabetes Metab. Case Rep. 2018;2018:17–0174. doi: 10.1530/edm-17-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zezza M., Kosinski C., Mekoguem C., Marino L., Chtioui H., Pitteloud N., Lamine F. Combined immune checkpoint inhibitor therapy with nivolumab and ipilimumab causing acute-onset type 1 diabetes mellitus following a single administration: two case reports. BMC Endocr. Disord. 2019;19(1):144. doi: 10.1186/s12902-019-0467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang A.L., Wang F., Chang L.S., Mcdonnell M.E., Min L. Coexistence of Immune Checkpoint Inhibitor-Induced Autoimmune Diabetes and Pancreatitis. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.620522. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Quandt Z., Young A., Anderson M. Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin. Exp. Immunol. 2020;200(2):131–140. doi: 10.1111/cei.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pihoker C., Gilliam L.K., Hampe C.S., Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl 2):S52–S61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 113.Verge C.F., Howard N.J., Rowley M.J., Mackay I.R., Zimmet P.Z., Egan M., Hulinska H., Hulinsky I., Silvestrini R.A., Kamath S., Al Et. Anti-glutamate decarboxylase and other antibodies at the onset of childhood IDDM: a population-based study. Diabetologia. 1994;37(11):1113–1120. doi: 10.1007/bf00418375. [DOI] [PubMed] [Google Scholar]
- 114.Liu X., Shi Y., Zhang D., Zhou Q., Liu J., Chen M., Xu Y., Zhao J., Zhong W., Wang M. Risk factors for immune-related adverse events: what have we learned and what lies ahead? Biomark. Res. 2021;9(1):79. doi: 10.1186/s40364-021-00314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Osum K.C., Burrack A.L., Martinov T., Sahli N.L., Mitchell J.S., Tucker C.G., Pauken K.E., Papas K., Appakalai B., Spanier J.A., Fife B.T. Interferon-gamma drives programmed death-ligand 1 expression on islet β cells to limit T cell function during autoimmune diabetes. Sci. Rep. 2018;8(1):8295. doi: 10.1038/s41598-018-26471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rui J., Deng S., Arazi A., Perdigoto A.L., Liu Z. Herold KC. β cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metab. 2017;25(3):727–738. doi: 10.1016/j.cmet.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ansari M.J., Salama A.D., Chitnis T., Smith R.N., Yagita H., Akiba H., Yamazaki T., Azuma M., Iwai H., Khoury S.J., Auchincloss H., Sayegh M.H. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198(1):63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pauken K.E., Jenkins M.K., Azuma M., Fife B.T. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes. 2013;62(8):2859–2869. doi: 10.2337/db12-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoneda S., Imagawa A., Hosokawa Y., Baden M.Y., Kimura T., Uno S., Fukui K., Goto K., Uemura M., Eguchi H., Iwahashi H., Kozawa J., Shimomura I. T-lymphocyte infiltration to islets in the pancreas of a patient who developed type 1 diabetes after administration of immune checkpoint inhibitors. Diabetes Care. 2019;42(7):e116–e118. doi: 10.2337/dc18-2518. [DOI] [PubMed] [Google Scholar]