Highlights
-
•
A robust and selective LC-MS/MS method for quantification of 11 urinary steroids was developed and validated.
-
•
Optimized preparation of calibration standards improves method linearity and accuracy.
-
•
Pre-analytical sample stability was extensively validated.
Abbreviations: ACA, adrenocortical adenoma; ACC, adrenocortical carcinoma; DHEA, dehydroepiandrosterone; Etio, etiocholanolone; IS, internal standard; LC-MS/MS, liquid chromatography tandem mass spectrometry; LLOQ, lower limit of quantification; MRM, multiple reaction monitoring; 5-PD, 5-pregnen-3β,20α-diol; PD, 5β-pregnan-3α,20α-diol; 5-PT, 5-pregnen-3β,17,20α-triol; PT, 5β-pregnan-3α,17,20α-triol; QC, quality control; R2, coefficient of determination; SPE, solid phase extraction; ULOQ, upper limit of quantification
Keywords: Adrenal tumors, Adrenocortical carcinoma, Steroid profiling, Urinary steroids, Mass spectrometry, LC–MS/MS
Abstract
Introduction
Preoperative diagnostic workup of adrenal tumors is based on imaging and hormone analyses, but charged with uncertainties. Steroid profiling by liquid chromatography tandem mass spectrometry (LC-MS/MS) in 24-h urine has shown potential to discriminate benign and malignant adrenal tumors. Our aim was to develop and validate a specific and accurate LC-MS/MS method for the quantification of deconjugated urinary marker steroids, to evaluate their pre-analytical stability and to apply the method to clinical samples of patients with adrenal tumors.
Methods
A method for the quantification of 11 deconjugated steroids (5-pregnenetriol, dehydroepiandrosterone, cortisone, cortisol, α-cortolone, tetrahydro-11-deoxycortisol, etiocholanolone, pregnenolone, pregnanetriol, pregnanediol, and 5-pregnenediol) in human urine was developed and validated based on international guidelines. Steroids were enzymatically deconjugated and extracted by solid phase extraction before LC-MS/MS quantification in positive electrospray ionization mode.
Results
Excellent linearity with R2 > 0.99 and intra- and inter-day precisions of < 10.1 % were found. Relative matrix effects were between 96.4 % and 101.6 % and relative recovery was between 98.2 % and 115.0 %. Sufficient pre-freeze stability for all steroids in urine was found at 20–25 °C for seven days and at 4–6 °C for up to 28 days. Samples were stable during long-term storage at −20 °C and −80 °C for 6 months.
Conclusions
A sensitive and robust LC-MS/MS method for the quantification of 11 urinary steroids was developed and validated according to international guidelines. Pre-analytical matrix stability was evaluated and the suitability of the method for the analysis of clinical samples and prospective validation studies was shown.
Introduction
Adrenal tumors are among the most common neoplasms in humans. With the increasingly frequent use of cross-sectional imaging, incidental adrenal masses are observed in 3–5 % [1], [2], [3], [4]. While most of these are benign adrenocortical adenomas (ACA) that are more frequent with increased age [5], [6], [7], rare but aggressive adrenocortical carcinomas (ACC) have an annual incidence of only 0.7–2.0 cases per million population and a peak incidence between 40 and 50 years of age [8]. Early diagnosis of ACC is crucial because complete surgical removal is the only chance of cure [9]. Malignancy assessment is based on both imaging techniques and hormonal workup [9], [10], [11], which is often tedious and charged with uncertainties. Quantification of cortisol in serum and urine with or without dynamic testing helps to determine the presence of autonomous cortisol secretion and Cushing’s syndrome [12], [13]. Profiling of a broader set of steroid hormones by liquid chromatography tandem mass spectrometry (LC-MS/MS) in serum or plasma has been proposed to accelerate the diagnosis of ACC [14], [15]. The circadian rhythm underlying most steroids increases the variability of results rendering standardized sampling conditions essential. Urine collection over 24 h is a non-invasive procedure and overcomes the problem of circadian variability as net steroid output over a day can be assessed, and both diurnal fluctuations and dilution effects can be considered. Steroid quantification by LC-MS/MS has increasingly replaced immunoassays over the last decades due to its improved analytical specificity and the possibility to quantify several biomarkers in a single run by multiple reaction monitoring (MRM) [16], [17]. Bancos, Taylor et al. recently published a prospective validation study using urine steroid metabolomics in conjunction with imaging features for the differential diagnosis of adrenal incidentalomas [18], which resulted in a positive predictive value of 76.4 % for ACC detection. Our aim was to develop an LC-MS/MS method with higher clinical diagnostic value through optimal analytical accuracy suitable for clinical routine application. We selected a marker set of 11 deconjugated urinary steroids to meet this demand.
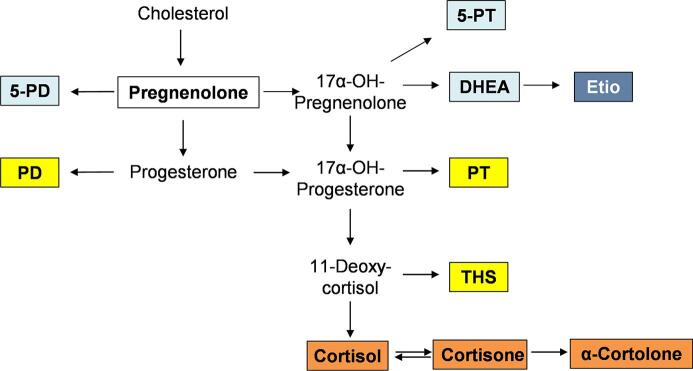
The selected marker panel included urinary steroid metabolites with the highest clinical diagnostic value for ACC diagnosis according to published reports [18], [19], [20]. Arlt et al. found nine steroid markers as most relevant for differentiation between ACC and ACA, including tetrahydro-11-deoxycortisol (THS), 5-pregnen-3β,17,20α-triol (5-PT), 5-pregnen-3β,20α-diol (5-PD), 5β-pregnan-3α,17,20α-triol (PT), etiocholanolone (Etio), and 5β-pregnan-3α,20α-diol (PD) [19]. This finding was largely confirmed by Hines et al., who found Etio, dehydroepiandrosterone (DHEA), 5-PT, 5-PD, PD, 17-hydroxypregnanolone, PT, and THS to be the strongest indicators of ACC [20]. Bancos et al. investigated a panel of 15 urinary steroids that included the markers mentioned above, as well as androsterone, 11β-hydroxyandrosterone, 11β-hydroxyetiocholanolone, cortisol, cortisone, tetrahydrocortisone, and β-cortolone [18]. By excluding strongly intercorrelated steroids, we were able to narrow down the marker set further. Pregnenolone was included additionally as a representative precursor in steroid biosynthesis, which we found useful in preliminary experiments using various biomaterials and cell culture experiments (data not shown). Fig. 1 depicts the simplified pathway of steroid biosynthesis. In Supplemental Fig. S1 the chemical structures of the 11 marker substances are shown with their molecular masses and corresponding quantifier transitions in positive MRM mode.
Fig. 1.
Simplified pathway of steroid biosynthesis and metabolism with boxed diagnostic analytes included in the quantification method. Colors indicate their role in steroid metabolism. White: early steroid hormone precursor, light blue: androgen precursor, dark blue: androgen, yellow: glucocorticoid precursor, orange: glucocorticoid.
Moreover, the focus was on validation of the pre-analytical stability of the urinary steroids, which is relevant for sample storage and handling in clinical practice. In an outpatient setting, most patients collect 24-h urine at home and send the sample to the laboratory by mail with unknown consequences on stability. Steroid degradation or intramolecular rearrangements might falsify the determined concentration and possibly lead to a misclassification of the adrenal tumor differential diagnosis. To our knowledge, for most analytical methods pre-analytical stability is usually validated for up to 24 h at room temperature and maximally 48 h at refrigerator temperature, e.g. in a stability study for urinary estrogens [21]. The necessity of pre-analytical stability assessment of urine samples in metabolomic analysis is frequently referred to, however only general recommendations for cooled or even frozen sample storage and transport are given without addressing steroid hormone metabolites specifically [22], [23], [24], [25], [26].
Herein we describe a validated LC-MS/MS method for the quantification of a panel of 11 urinary steroids. We investigated the pre-analytical stability of urinary steroid metabolites at room temperature and refrigerator temperature for up to 28 days and the method was successfully applied to 24-h urine samples collected from adrenal tumor patients.
Materials and methods
Instrumentation and materials
An Agilent 1290 HPLC (G4226A autosampler, infinityBinPump, G1316C column oven, G1330B thermostat) coupled to a QTRAP 6500 + MS-system (SCIEX, Framingham, USA) was used for LC-MS/MS measurements. LC-MS/MS data acquisition and quantification was performed with Analyst 1.6.3 (Sciex). The analytical column was an Acquity UPLC Premier HSS T3 1.8 µm 2.1x50mm (Waters GmbH, Eschborn, Germany) and offline solid phase extraction (SPE) was performed on SepPak tC18 100 mg 96-well Plates (Waters GmbH, Eschborn, Germany). Cortisol, pregnenolone, DHEA-d6, β-glucuronidase/arylsulfatase from Helix pomatia, and Sigmatrix urine diluent were purchased from Sigma-Aldrich (Taufkirchen, Germany). Etio, 5-PD, PD, PT, cortisone, α-cortolone, 5-PT, and DHEA were purchased from Steraloids (Newport, RI, USA) and THS from Cayman Chemical (Ann Arbor, MI, USA). THS-d5, Etio-d5, and PT-d5 were obtained from IsoSciences (Ambler, PA, USA) and PD-d5 and pregnenolone-d4 from Toronto Research Chemicals Inc. (Toronto, Canada). MS-grade methanol and water were purchased from VWR International GmbH (Darmstadt, Germany). Formic acid, ammonium acetate, and acetic acid were from Thermo Fisher Scientific Inc. (Schwerte, Germany).
Standard preparation
Stock solutions of all compounds were prepared in methanol at a concentration of 1.0 mg/mL (5-PT, DHEA, cortisone, cortisol, α-cortolone, Etio, pregnenolone, PT) or 0.5 mg/mL (THS, PD, 5-PD). From these, two methanolic working solutions were prepared (working solution 1: pregnenolone, PD, α-cortolone, Etio, 5-PD, cortisone, cortisol; and working solution 2: 5-PT, PT, THS, DHEA). Deuterated internal standards (IS) were dissolved in methanol (1 mg/mL), combined, and diluted with water/methanol (1:1) to an IS mix with the following concentrations: 5000 ng/mL (THS-d5), 2500 ng/mL (PD-d5), 1000 ng/mL (Etio-d5, PT-d5, DHEA-d6), and 500 ng/mL (cortisol-d4, pregnenolone-d4).
Two approaches for preparation of calibration standards and quality controls (QC) were performed and compared regarding linearity of calibration curves. First, standards were prepared by spiking the two working solutions into steroid-free urine matrix, resulting in six calibration levels. Second, working solutions were spiked into a mixture of steroid-free urine matrix and methanol (1:1). Four QC levels – lower limit of quantitation (LLOQ), low, medium, and high – were prepared analogously to the calibration standards from separate stock solutions. Table 1 lists calibration range and QC concentrations for each analyte.
Table 1.
Calibration range and QC concentrations of the steroid standards.
| Analyte | Calibration range [ng/mL] | QC LLOQ [ng/mL] | QC low [ng/mL] | QC medium [ng/mL] | QC high [ng/mL] |
|---|---|---|---|---|---|
| 5-PT | 20–5000 | 20 | 100 | 1000 | 2500 |
| DHEA | 20–5000 | 20 | 100 | 1000 | 2500 |
| Cortisone | 10–1000 | 10 | 20 | 200 | 500 |
| Cortisol | 10–1000 | 10 | 20 | 200 | 500 |
| α-cortolone | 50–5000 | 50 | 100 | 1000 | 2500 |
| THS | 20–5000 | 20 | 100 | 1000 | 2500 |
| Etio | 50–5000 | 50 | 100 | 1000 | 2500 |
| Pregnenolone | 5–500 | 5 | 10 | 100 | 250 |
| PT | 20–5000 | 20 | 100 | 1000 | 2500 |
| PD | 50–5000 | 50 | 100 | 1000 | 2500 |
| 5-PD | 50–5000 | 50 | 100 | 1000 | 2500 |
QC quality control, LLOQ lower limit of quantitation.
Sample preparation
150 µL of sample (calibration standard, QC, or urine sample) were gently mixed with 300 µL of deconjugation buffer consisting of 30 µL of β-glucuronidase/arylsulfatase (glucuronidase activity: 6.9 U/mL at 25 °C with 4-nitrophenylglucuronide, arylsulfatase activity: 19 U/mL at 25 °C with 5-nitrophenylsulfate) and 270 µL of ammonium acetate buffer (pH 4.9, 0.2 M). Next, samples underwent an incubation for 3 h at 55 °C for enzymatic deconjugation of sulfate and glucuronide moieties. Final incubation conditions were established by systematic variations of enzyme amount and incubation time. Increasing enzyme concentration or deconjugation time did not lead to an additional increase of 9 out of 11 deconjugated metabolites, indicating quantitative deconjugation. Deconjugated steroid concentrations using various amounts of arylsulfatase/glucuronidase mix are shown in Supplemental Fig. S2. 30 µL of IS mix were added to the incubated samples followed by addition of 180 µL of methanol to urine samples and 180 µL of urine matrix/methanol (1:1) to calibration standards and QC samples to ensure equal solvent composition in all samples before SPE. The last step was left out when calibration standards and QC samples were prepared in pure steroid-free urine matrix without methanol. For offline-SPE, the SepPak tC18 100 mg 96-well plate was pre-conditioned sequentially with 1 mL of methanol and 1 mL of water per well. Incubated samples were loaded, followed by two washing steps with 700 µL of water. Extracted steroids were eluted into a collection plate using 2 × 300 µL of methanol in two consecutive steps. Following complete solvent evaporation at 50 °C under a gentle flow of nitrogen, samples were reconstituted in 150 µL of methanol and diluted with 150 µL of water. 10 µL of the extracted sample were injected into the HPLC system. Samples with concentrations above the upper limit of quantification (ULOQ) were incubated again and diluted with a mixture of steroid-free urine matrix and ammonium acetate buffer, pH 4.9, 0.2 M (1:2) according to the calibration range.
LC-MS/MS conditions
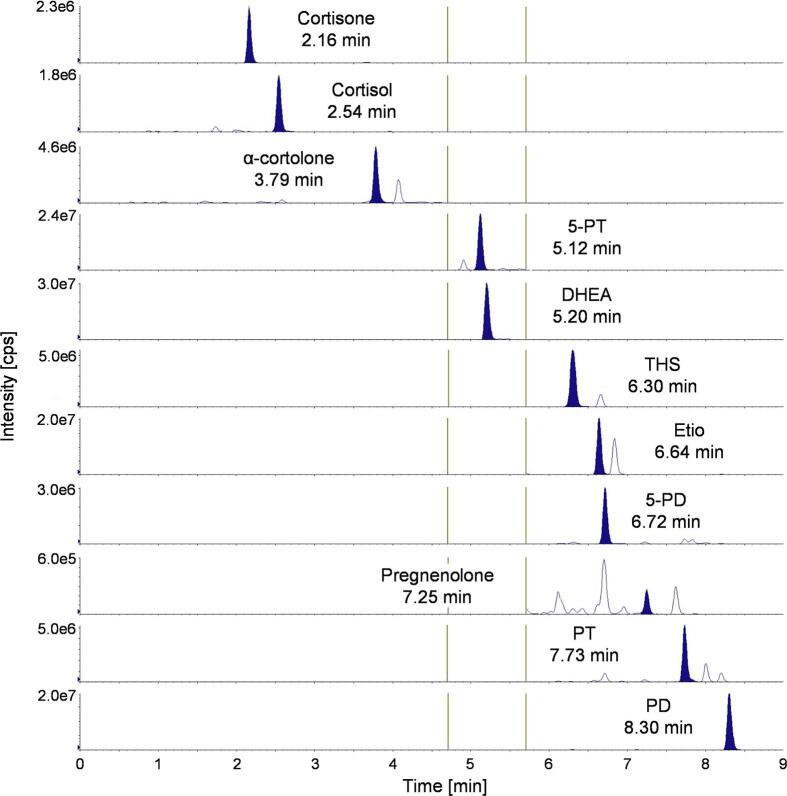
The column oven temperature was set to 45 °C. Source and gas parameters were set as follows: curtain gas: 40 psi, collision gas: medium, ion spray voltage: 4500 V, temperature: 500 °C, ion source gas 1: 50 psi, and ion source gas 2: 30 psi. Mobile phases consisted of MS-grade water with 0.1 % (V/V) formic acid (mobile phase A) and MS-grade methanol with 0.1 % (V/V) formic acid (mobile phase B). The flow rate was set to 500 µL/min with a gradient as follows: 0.0–1.0 min: 45 % B; 1.0–8.5 min: from 45 % to 80 % B; 8.5–9.0 min: from 80 % to 98 %B; 9.0–10.0 min: 98 % B; 10.0–10.5: from 98 % to 45 % B; 10.5–12.0 min: 45 % B. After 9.0 min of run time, the valve position switched to waste. To increase the number of data points per peak, MRM transitions were measured in three periods. The first period lasted from 0 to 4.7 min (detection of cortisone, cortisol, and α-cortolone), the second period from 4.7 to 5.7 min (detection of 5-PT and DHEA), and the third period from 5.7 to 9.0 min (detection of THS, Etio, 5-PD, pregnenolone, PT, and PD). One minute of automatic re-equilibration time preceded each analytical run. For every analyte, a quantifier and a qualifier MRM transition were identified and optimized to maximum intensity. Compound-specific MS-parameters for analytes and IS are listed in Supplemental Table S1. Chromatographic separation in a urine sample of an ACC patient and analyte retention times are shown in Fig. 2. Isobaric compounds are baseline separated from the analyte peaks.
Fig. 2.
Extracted ion chromatograms of the 11 quantifier MRM transitions with corresponding retention times in a urine sample of an ACC patient. Vertical lines represent borders of the three periods.
Method validation
The LC–MS/MS method was validated based on current guidelines for bioanalytical method validation by the European Medicines Agency (2011) [27] and the Food and Drug Administration (2018) [28].
Selectivity
Six different lots of human urine were evaluated to test whether endogenous compounds were interfering with the seven deuterated IS. To this end, urine samples were measured with and without IS, respectively, taking into consideration the ratio between the two as the blank IS response percentage.
Sensitivity and carry-over
The limit of detection (LOD) was defined as the concentration with a signal-to-noise ratio (S/N) > 3. Carry-over was determined by a solvent injection after injection of the highest calibrator. Acceptance criteria were fulfilled by an analyte peak area measured in the blank of less than 20 % of the analyte peak area at the LLOQ.
Accuracy, precision, and reinjection reproducibility
Precision was defined as the percent coefficient of variation (%CV) and accuracy as the ratio of calculated concentration to nominal concentration. Inter-day accuracy and precision were determined in three independent runs with four QC levels (LLOQ, low, medium, and high), each measured in four replicates. Intra-day accuracy and precision were calculated from six replicates of the four QC levels within one validation run. Reinjection reproducibility was determined by the %CV of five injections from the same processed sample.
Matrix effect and recovery
Matrix effects were evaluated by comparing the responses (analyte peak area for absolute matrix effect and the ratio of analyte peak area to IS peak area for relative matrix effect) of matrix QC samples versus QC samples prepared in water (Eq. (1)).
| (1) |
Recovery was calculated by comparing analytes’ responses (analyte peak area for absolute recovery and the ratio of analyte peak area to IS peak area for relative recovery) in processed QC samples via SPE versus post-extract spiked samples (Eq. (2)).
| (2) |
QC samples were measured in triplicate at three concentration levels (low, medium, and high) for matrix effect and recovery.
Dilution integrity
To cover the case of patient samples with steroid concentrations above the calibration range, dilution integrity was tested with QC samples prepared in a concentration of twofold the ULOQ. After enzymatic hydrolysis, samples were diluted with a mixture of steroid-free urine matrix and ammonium acetate buffer, pH 4.9, 0.2 M (1:2) in a fourfold and a tenfold dilution to concentration levels within the calibration range. Each dilution was prepared in six replicates.
Stability
Steroid stability in urine before freezing and processing was determined at room temperature (20–25 °C) and in the refrigerator (4–6 °C) for 28 days (pre-freeze stability). At each time point, a triplicate of a the patient urine pool was transferred from the evaluation temperature to −80 °C and, once all of the time points were passed, all of the samples were measured together in a single run. Stability of temperature conditions during enzymatic hydrolysis was tested by comparison of pooled urine samples after pre-heating (3 h, 55 °C) against unheated samples. To exclude an effect of the 55 °C heating phase during incubation, enzymatic hydrolysis was performed for 6 h at 30 °C.
Stock solution stability was measured after six months by comparing freshly prepared stock solutions with the original stock solutions at two concentration level (diluted to 100 ng/ml and 500 ng/ml). Long-term stability of frozen samples was determined for up to six months with QC sample storage at −20 °C and −80 °C. Three QC levels (low, medium, and high) were measured in triplicate. Autosampler stability of the processed sample was investigated over 24 h. Freeze-thaw stability was determined with three cycles of a triplicate of QC standards at three concentration levels.
Application to clinical samples
The method was applied to 24-h urine samples of 19 patients with an adrenal tumor diameter ≥ 2 cm, composed of 4 ACC patients and 15 ACA patients. The four ACC cases were all of a classical type. Three of these ACC cases were functional and one case was non-functional. 24-h Urine samples were collected consecutively between January and May 2019 as part of the European Network for the study of adrenal tumors (ENSAT) registry study, which has been approved by the local ethics committee of the University of Würzburg (#88/11). All patients provided written informed consent. Total collection volume was documented and a urine aliquot was stored at −20 °C in a urine Monovette® (Sarstedt, Nümbrecht, Germany). Diagnosis was made following standard workup (imaging, hormone measurements in serum, and histology after adrenalectomy, if available) [9], [10]. After measurement of steroid concentrations in ng/mL, the steroid excretion in µg/24 h was normalized via the individual total collection volume. The tumor diameter and Hounsfield units (HU) in unenhanced computed tomography (CT) were documented from the patients’ imaging records.
Statistics
Statistical analyses were performed using IBM SPSS version 26. Urinary steroid excretion data were found not to be normally distributed by applying the Shapiro-Wilk test. Groups were compared using the Mann Whitney U test with p-values < 0.05 considered statistically significant. Correlations between steroid excretions and tumor diameter were tested by determination of the Pearson correlation coefficient (Pearson r).
Results
Sample preparation
Preparation of calibration standards and QC samples was initially based on spiking methanolic working solutions into pure steroid-free urine matrix. However, early experiments illustrated the need for optimization for the standard and calibration preparation due to insufficient linearity of some steroids; for example PD with R2 = 0.9448 (Supplemental Fig. S3 A). Improved linearity (R2 = 0.9994 for PD) was found after spiking a mixture of methanol and steroid-free urine matrix (1:1) with methanolic working solutions (Supplemental Fig. S3 B). Method validation and measurements of patient urine samples were thus conducted with a calibration and QC samples prepared in methanol and steroid-free urine matrix (1:1). To ensure equal solvent composition and extraction properties for calibration standards and clinical urine samples during SPE, 180 µL of methanol were added to each urine sample after enzymatic hydrolysis. Accordingly, 180 µL of a 1:1 mixture of methanol and steroid-free urine matrix were added to calibration standards and QC samples.
Method validation
Calibration curves were plotted with peak area ratios (analyte/IS) against the nominal concentration of each analyte. Cortisone, cortisol, DHEA, Etio, α-cortolone, PD, and PT showed the best results with a linear curve fit with 1/x-weighting and a quadratic curve fit with 1/x2-weighting was used for 5-PT, THS, pregnenolone, and 5-PD. All calibration curves showed coefficients of determination (R2) > 0.99. R2 of calibration curves of five validation runs are listed as mean (SD) in Table 2.
Table 2.
Validation results.
| 5-PT | DHEA | Cortisone | Cortisol | α-corto-lone | THS | Etio | Preg-nenolone | PT | PD | 5-PD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute matrix effekt (%) | 97.3 | 97.9 | 99.0 | 98.3 | 97.2 | 102.0 | 96.4 | 101.1 | 98.5 | 100.1 | 96.4 |
| Relative matrix effekt (%) | 99.5 | 97.9 | 100.8 | 100.2 | 98.8 | 99.7 | 101.5 | 96.4 | 98.9 | 101.6 | 98.3 |
| Absolute recovery (%) | 116.5 | 117.1 | 121.4 | 120.1 | 120.1 | 120.7 | 116.1 | 108.9 | 111.8 | 106.0 | 113.6 |
| Relative recovery (%) | 99.2 | 99.8 | 101.3 | 101.7 | 115.0 | 98.3 | 101.7 | 101.6 | 98.2 | 101.2 | 107.4 |
| Inter-day accuracy (%) | 97.8 | 100.6 | 100.3 | 99.6 | 101.7 | 99.8 | 101.9 | 99.3 | 101.2 | 100.2 | 98.9 |
| Inter-day accuracy LLOQ (%) | 105.1 | 100.7 | 102.0 | 102.3 | 98.7 | 99.6 | 102.4 | 112.4 | 100.3 | 100.5 | 109.4 |
| Intra-day accuracy (%) | 100.3 | 99.1 | 100.0 | 99.3 | 95.1 | 97.2 | 99.4 | 102.3 | 98.9 | 100.6 | 102.8 |
| Intra-day accuracy LLOQ (%) | 109.0 | 107.1 | 100.2 | 94.6 | 91.4 | 101.1 | 107.3 | 106.7 | 98.7 | 105.2 | 111.4 |
| Inter-day precision (%CV) | 2.3 | 2.6 | 2.0 | 1.9 | 2.4 | 8.3 | 2.9 | 3.4 | 2.5 | 2.4 | 2.7 |
| Inter-day precision LLOQ (%CV) | 3.3 | 2.7 | 2.1 | 4.3 | 2.8 | 10.1 | 3.3 | 2.4 | 3.0 | 2.4 | 2.8 |
| Intra-day precision (%CV) | 3.3 | 2.8 | 2.2 | 2.1 | 3.0 | 8.8 | 3.3 | 3.6 | 2.9 | 3.2 | 3.3 |
| Intra-day precision LLOQ (%CV) | 3.8 | 2.5 | 2.1 | 1.6 | 2.0 | 7.1 | 1.4 | 2.1 | 5.6 | 2.8 | 3.9 |
| LOD (ng/mL) | 2.0 | 2.0 | 1.0 | 1.0 | 5.0 | 4.0 | 5.0 | 2.5 | 2.0 | 5.0 | 10.0 |
| Dilution Integrity (%) | 103.7 | 104.8 | 108.7 | 109.4 | 104.6 | 105.2 | 108.0 | 107.1 | 107.9 | 111.3 | 115.2 |
| Reinjection reproducibility (%CV) | 0.9 | 1.2 | 0.9 | 1.0 | 1.6 | 5.8 | 2.0 | 3.4 | 2.2 | 1.6 | 2.7 |
| Linearity, R2, n = 5 (mean, SD) | 0.9963 (0.0022) | 0.9993 (0.0005) | 0.9998 (0.0002) | 0.9995 (0.0003) | 0.9992 (0.0009) | 0.9947 (0.0067) | 0.9994 (0.0004) | 0.9963 (0.0023) | 0.9996 (0.0003) | 0.9994 (0.0002) | 0.9940 (0.0015) |
For selectivity, the blank IS response percentage was <1.0 % for cortisol-d4, Etio-d4, THS-d4, PD-d4, PT-d4, and pregnenolone-d4, and 1.3 % for DHEA-d4. All steroids were baseline separated from co-eluting isobaric substances. Steroid identification was verified by monitoring of the quantifier-to-qualifier ion ratio and comparison of quantifier and qualifier retention times with the retention time of the corresponding analytical standard.
The LOD of each analyte is listed in Table 2. No relevant carry-over was found in any analyte or IS.
Inter- and intra-day accuracy and precision was acceptable for all analytes both at the LLOQ and as the mean of QC levels low, medium, and high. Highest imprecisions were calculated for THS with an inter-day (im)precision at the LLOQ of 10.1 %. The highest %CV after five reinjections of the same processed sample was also detected for THS with 5.8 %. All results for accuracy, precision, and reinjection reproducibility are listed in Table 2.
Low matrix effects were detected for the artificial steroid-free urine matrix. Absolute matrix effects were between 96.4 % (Etio, 5-PD) and 102.0 % (THS) and relative matrix effects between 96.4 % (pregnenolone) and 101.6 % (PD). Absolute recovery was found to be between 106.0 % (PD) and 121.4 % (cortisone). However, relative recovery was closer to 100 % for all analytes, as suitable internal standards normalize the positive recovery effect detected for the analyte peak areas. Results for matrix effects and recovery are listed in Table 2.
Dilution of QC samples with concentrations above the ULOQ did not affect accuracy and precision. The mean accuracy of both dilution levels was considered as dilution integrity and is listed in Table 2 for all analytes.
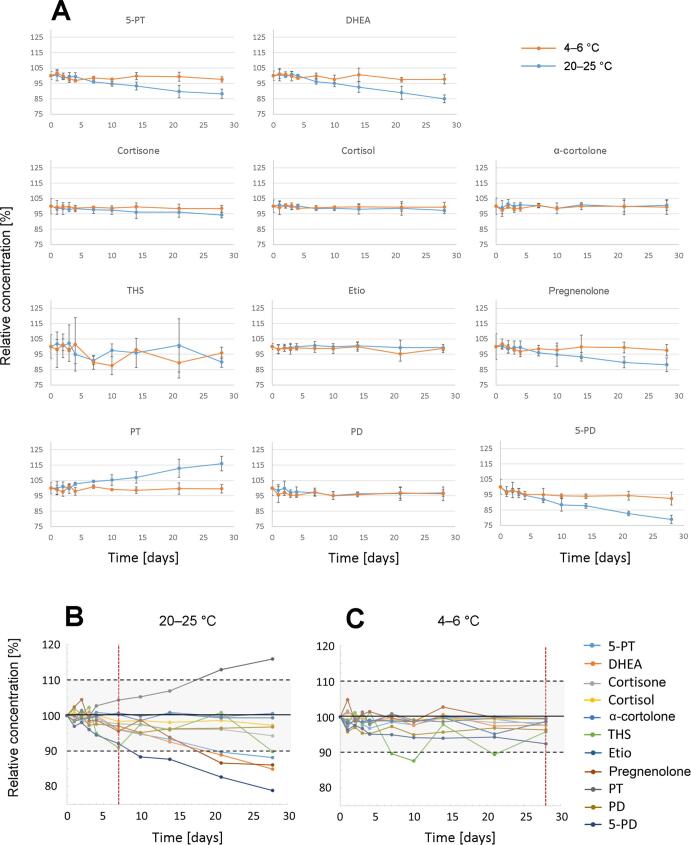
Evaluation of pre-freeze stability of the urinary steroids at 20–25 °C and at 4–6 °C showed variable stability (Fig. 3). While all 11 steroids were stable for the period of 28 days at 4–6 °C (+/- 10 % of the initial concentration at day 0), changes from baseline of less than +/- 10 % were found for seven days at 20–25 °C. Cortisone, cortisol, Etio, α-cortolone, and PD did not show any alteration over the period of 28 days for both conditions. However, significant degradation at room temperature was present for 5-PT, DHEA, pregnenolone, and 5-PD, whereas PT concentration significantly increased over time at room temperature.
Fig. 3.
(A) Pre-freeze stability of urinary steroids at 20–25 °C (blue line) and at 4–6 °C (orange line) shown as mean of three independent measurements. Steroids were stable at 20–25 °C for seven days (B) and at 4–6 °C for 28 days (C) within the prespecified limits of 90–110 % of the initial concentration.
Stock solutions were stable for up to at least six months with concentration changes below +/-15 %. Results of stock solution stability and pre-freeze stability are listed in Supplemental Table S2. Concentration changes below +/-15 % were observed in spiked QC samples for up to 6 months at −20 °C and −80 °C, as well as for 3 freeze–thaw cycles and for 24 h of the processed sample in the autosampler at 4 °C. Results of the long-term stability, autosampler stability, and freeze–thaw stability are listed in Supplemental Table S3.
Steroids showed sufficient stability for 3 h at 55 °C with change in concentration ≤ 5 % (Supplemental Table S4).
Application to clinical urine samples
Steroid concentrations were measured in 24-h urine samples of adrenal tumor patients (n = 19) and normalized to µg/24 h via total collection volume. Steroid excretions of ACC vs ACA samples were compared by Mann-Whitney U test (Table 3).
Table 3.
Comparison of steroids in ACC vs ACA urine samples in µg/24 h by Mann-Whitney U test.
| ACC (n = 4) | ACA (n = 15) | p-Value | ||
|---|---|---|---|---|
| Analytes | Mean (SD), range [µg/24 h] | Mean (SD), range [µg/24 h] | Samples below LOD [n] | |
| 5-PT | 1190 (899), 695–2534 |
81.2 (94.7), 14.3–347 |
– | 0.001 |
| DHEA | 2695 (4518), 235–9468 |
133 (435), 1.4–1702 |
2 | 0.004 |
| Cortisone | 231 (164), 56.2–383 |
124 (56.1), 38.5–241 |
– | 0.357 |
| Cortisol | 406 (472), 80.6–1087 |
109 (64.4), 26.1–224 |
– | 0.221 |
| α-cortolone | 1858 (189), 1609–2046 |
1741 (938), 371–3753 |
– | 0.221 |
| THS | 1656 (2102), 326–4788 |
198 (164), 33.5–556 |
– | 0.002 |
| Etio | 6712 (6201), 1305–15300 |
856 (665), 82.6–2192 |
– | 0.004 |
| Pregnenolone | 10.3 (8.9), 0.0–20.9 |
0.9 (2.3), 0.0–7.1 |
14 | 0.027 |
| PT | 2513 (1535), 1261–4680 |
596 (503), 80.6–2000 |
– | 0.002 |
| PD | 1154 (1369), 279–3188 |
184 (218), 32.6–928 |
– | 0.004 |
| 5-PD | 237 (88.3), 118–331 |
101 (33.2), 50.4–168 |
– | 0.004 |
| Tumor diameter [cm] | 7.0 (2.2), 4.4–9.7 |
3.6 (1.4), 2.2–7.0 |
0.006 | |
| Tumor HU in unenhanced CT, n | ||||
| ≤10 | 0 | 9 | ||
| >10 | 4 | 4 | ||
| n/a | 0 | 2 |
LOD limit of detection.
Steroid excretions of 5-PT, cortisone, cortisol, PT, and 5-PD were positively correlated with the tumor diameter, with cortisone and cortisol showing a highly significant correlation (p < 0.004), whereas 5-PT, PT, and 5-PD were slightly below the level of significance (Supplemental Table S5). The four patients with ACC had an attenuation of >10 HU in unenhanced CT. Nine of the patients with ACA had an attenuation ≤ 10 HU, while four ACA had > 10 HU and two cases had no available unenhanced CT images.
Discussion
We have developed and validated an LC-MS/MS method for the quantification of deconjugated urinary steroids and applied it to a set of 24-h urine samples of adrenal tumor patients. Steroids are excreted mainly as urinary sulfate or glucuronide conjugates and may even be measured directly as intact conjugates by LC-MS/MS [29], [30], [31], [32]. However, due to a lack of commercially available steroid conjugate standards for most diagnostically relevant steroid precursor metabolites and the large number of possible metabolites, most published quantitative methods include a deconjugation step [18], [20], [33], [34], [35], [36]. To capture the total urinary steroids including sulfates and glucuronides as well as the free steroid fraction, we performed a hydrolysis step and quantified deconjugated steroids. Measuring deconjugated urinary steroids for the hormonal workup of adrenal tumors has been performed by others, but most previously published methods were based on gas chromatography mass spectrometry (GC–MS) [19], [37], [38], [39], [40], [41]. GC–MS provides an excellent resolution, but sample pre-treatment is laborious and time consuming as derivatization steps are necessary. Two previously published works describe LC-MS/MS methods for the quantification of deconjugated steroids in urine for the application in the diagnostic workup of adrenal tumors [18], [20]. Hines et al. isolated the steroids from urine by liquid–liquid extraction [20], which has the drawbacks of a time-intensive and difficult to standardize extraction process [16]. Bancos, Taylor et al. used offline-SPE for steroid extraction [18], which was, likewise, our preferred extraction method due to its excellent recoveries and reproducibility, and lower organic solvent usage; we consider these major advantages of SPE in comparison to liquid–liquid extraction.
In contrast with other published methods, we focused on a panel of 11 urinary steroids to will facilitate clinical implementation and reduce cost. An improved analytical accuracy was obtained by the usage of seven stable isotope labelled IS, which allows more accurate measurements to be achieved over the existing methods that use fewer IS [18], [20] due to the reduction of potential matrix interferences. Moreover, we have overcome linearity issues of steroids in synthetic steroid-free urine matrix by modifying sample preparation. As linearity improved for critical analytes after addition of methanol to the urine matrix, we hypothesize that insufficient standard solubility may lead to inhomogeneous distribution within the samples during sample preparation in the absence of methanol. It is possible that precipitated steroids in real urine samples are dissolved by the addition of methanol after incubation and before SPE. This step also ensures an equal solvent composition in calibration standards, quality control samples and real urine samples.
As our method focused on deconjugated steroids, sample pre-treatment included a deconjugation step with Arylsulfatase/Glucuronidase. The combined enzymatic activity in the digestive juice of Helix pomatia is suitable for cleavage of sulfate and glucuronide esters for both (i) steroids excreted mainly as glucuronides like etiocholanolone, and (ii) mainly sulfated steroids like DHEA [32]. Enzymatic hydrolysis is complex and requires the optimization of enzyme type and concentration, incubation time, and temperature [42]. Both Hines et al. and Bancos, Taylor et al. use a mixture of glucuronidase/arylsulfatase and incubation conditions of 2 h at 50 °C and 3 h at 60 °C, respectively [18], [20]. In our sample preparation procedure, a 3 h-incubation at 55 °C with 30 µL of the liquid digestive juice of Helix pomatia resulted in most reproducible quantitative results.
We analyzed pre-freeze urinary steroid stability, which is highly relevant for clinical practice. There is a paucity of data pertaining to the acceptable storage duration and steroid stability in urine samples at room temperature, or at refrigerator temperature before long-term storage in a freezer, as most previously published methods have focused on clinical studies rather than routine clinical implementation. We were able to determine sufficient stability of 90–110 % of all steroids for at least seven days at 20–25 °C and for 28 days at 4–6 °C. This information gives confidence for the frequently performed postal dispatch of 24-h urine samples.
Finally, we showed the successful application of the method to 19 urine samples of adrenal tumor patients. Specific quantification was achieved by chromatographic baseline separation of analytes from isobaric compounds. After normalization of steroid concentrations to steroid excretion in µg/24 h, significant differences between the urine samples of ACC and benign tumor patients were found in 8 of the 11 analyzed steroids. Even with our small sample size, the method showed potential value for broad application to clinical samples. The method showed excellent sensitivity for the detected urinary steroids, as only two patients with ACA had urinary DHEA below the LOD and 14 samples contained no measurable pregnenolone, which is generally excreted in very low concentrations. The steroid excretion ranges in µg/24 h are comparable to previously published data of absolute values, even though they were measured by GC–MS/MS [19], [20], [39]. The overall increase of steroid excretion in patients with ACC compared to ACA is also in accordance with other studies. Most groups identified THS to be the most discriminative steroid to classify the tumors [19], [38], [39], whereas our results show the lowest p-values for 5-PT, followed by THS and PT.
In conclusion, a robust and specific LC-MS/MS method with optimized analytical accuracy was developed, validated, and applied to a modest set of clinical samples. Significant clinical diagnostic performance may be achieved by combining targeted metabolic profiling of urinary steroids via LC-MS/MS with bioinformatic algorithms of characteristic steroid patterns to improve the differentiation between ACC and benign tumors in clinical routine.
Ethical approval
This study was part of the European Network for the study of adrenal tumors (ENSAT) registry, which has been approved by the local ethics committee of the University of Würzburg (#88/11).
Funding
This work was supported by the Bayerische Forschungsstiftung, Forschungsverbund FORTiTher, AZ-1365-18 and by the Deutsche Forschungsgemeinschaft Project number 314061271 (CRC/Transregio 205/1 “The Adrenal: Central relay of health and disease”).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Chromsystems Instruments & Chemicals GmbH for financial support within FORTiTher and for analytical support and fruitful discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmsacl.2022.07.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Herrera M.F., Grant C.S., van Heerden J.A., Sheedy P.F., Ilstrup D.M. Incidentally discovered adrenal tumors: an institutional perspective. Surgery. 1991;110(6):1014–1021. [PubMed] [Google Scholar]
- 2.Bovio S., Cataldi A., Reimondo G., Sperone P., Novello S., Berruti A., Borasio P., Fava C., Dogliotti L., Scagliotti G.V., Angeli A., Terzolo M. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J. Endocrinol. Invest. 2006;29(4):298–302. doi: 10.1007/BF03344099. [DOI] [PubMed] [Google Scholar]
- 3.Young W.F., Jr. Clinical practice. The incidentally discovered adrenal mass. N. Engl. J. Med. 2007;356(6):601–610. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 4.Song J.H., Chaudhry F.S., Mayo-Smith W.W. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am. J. Roentgenol. 2008;190(5):1163–1168. doi: 10.2214/AJR.07.2799. [DOI] [PubMed] [Google Scholar]
- 5.Barzon L., Sonino N., Fallo F., Palu G., Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur. J. Endocrinol. 2003;149(4):273–285. doi: 10.1530/eje.0.1490273. [DOI] [PubMed] [Google Scholar]
- 6.Elhassan Y.S., Alahdab F., Prete A., Delivanis D.A., Khanna A., Prokop L., Murad M.H., O'Reilly M.W., Arlt W., Bancos I. Natural history of adrenal incidentalomas with and without mild autonomous cortisol excess: a systematic review and meta-analysis. Ann. Intern. Med. 2019;171(2):107. doi: 10.7326/M18-3630. [DOI] [PubMed] [Google Scholar]
- 7.Sherlock M., Scarsbrook A., Abbas A., Fraser S., Limumpornpetch P., Dineen R., Stewart P.M. Adrenal Incidentaloma. Endocr. Rev. 2020;41(6):775–820. doi: 10.1210/endrev/bnaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fassnacht M., Kroiss M., Allolio B. Update in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2013;98(12):4551–4564. doi: 10.1210/jc.2013-3020. [DOI] [PubMed] [Google Scholar]
- 9.Fassnacht M., Dekkers O.M., Else T., Baudin E., Berruti A., de Krijger R.R., Haak H.R., Mihai R., Assie G., Terzolo M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018;179(4):G1–G46. doi: 10.1530/EJE-18-0608. [DOI] [PubMed] [Google Scholar]
- 10.Fassnacht M., Arlt W., Bancos I., Dralle H., Newell-Price J., Sahdev A., Tabarin A., Terzolo M., Tsagarakis S., Dekkers O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016;175(2):G1–G34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 11.Fassnacht M., Assie G., Baudin E., Eisenhofer G., de la Fouchardiere C., Haak H.R., de Krijger R., Porpiglia F., Terzolo M., Berruti A. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31(11):1476–1490. doi: 10.1016/j.annonc.2020.08.2099. [DOI] [PubMed] [Google Scholar]
- 12.Nieman L.K., Biller B.M., Findling J.W., Newell-Price J., Savage M.O., Stewart P.M., et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008;93(5):1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogg N., Kurlbaum M., Deutschbein T., Grasl B., Fassnacht M., Kroiss M. Method-specific cortisol and dexamethasone thresholds increase clinical specificity of the dexamethasone suppression test for cushing syndrome. Clin. Chem. 2021;67(7):998–1007. doi: 10.1093/clinchem/hvab056. [DOI] [PubMed] [Google Scholar]
- 14.Taylor D.R., Ghataore L., Couchman L., Vincent R.P., Whitelaw B., Lewis D., Diaz-Cano S., Galata G., Schulte K.-M., Aylwin S., Taylor N.F. A 13-Steroid serum panel based on LC-MS/MS: use in detection of adrenocortical carcinoma. Clin. Chem. 2017;63(12):1836–1846. doi: 10.1373/clinchem.2017.277624. [DOI] [PubMed] [Google Scholar]
- 15.Schweitzer S., Kunz M., Kurlbaum M., Vey J., Kendl S., Deutschbein T., Hahner S., Fassnacht M., Dandekar T., Kroiss M. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur. J. Endocrinol. 2019;180(2):117–125. doi: 10.1530/EJE-18-0782. [DOI] [PubMed] [Google Scholar]
- 16.Kushnir M.M., Rockwood A.L., Roberts W.L., Yue B., Bergquist J., Meikle A.W. Liquid chromatography tandem mass spectrometry for analysis of steroids in clinical laboratories. Clin. Biochem. 2011;44(1):77–88. doi: 10.1016/j.clinbiochem.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Keevil B.G. LC-MS/MS analysis of steroids in the clinical laboratory. Clin. Biochem. 2016;49(13–14):989–997. doi: 10.1016/j.clinbiochem.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Bancos I., Taylor A.E., Chortis V., Sitch A.J., Jenkinson C., Davidge-Pitts C.J., Lang K., Tsagarakis S., Macech M., Riester A., Deutschbein T., Pupovac I.D., Kienitz T., Prete A., Papathomas T.G., Gilligan L.C., Bancos C., Reimondo G., Haissaguerre M., Marina L., Grytaas M.A., Sajwani A., Langton K., Ivison H.E., Shackleton C.H.L., Erickson D., Asia M., Palimeri S., Kondracka A., Spyroglou A., Ronchi C.L., Simunov B., Delivanis D.A., Sutcliffe R.P., Tsirou I., Bednarczuk T., Reincke M., Burger-Stritt S., Feelders R.A., Canu L., Haak H.R., Eisenhofer G., Dennedy M.C., Ueland G.A., Ivovic M., Tabarin A., Terzolo M., Quinkler M., Kastelan D., Fassnacht M., Beuschlein F., Ambroziak U., Vassiliadi D.A., O'Reilly M.W., Young W.F., Biehl M., Deeks J.J., Arlt W., Glöckner S., Sinnott R.O., Stell A., Fragoso M.C., Kastelan D., Pupovac I.D., Simunov B., Cazenave S., Haissaguerre M., Tabarin A., Bertherat J., Libé R., Kienitz T., Quinkler M., Langton K., Eisenhofer G., Beuschlein F., Brugger C., Reincke M., Riester A., Spyroglou A., Burger-Stritt S., Deutschbein T., Fassnacht M., Hahner S., Kroiss M., Ronchi C.L., Palimeri S., Tsagarakis S., Tsirou I., Vassiliadi D.A., Basile V., Ingargiola E., Reimondo G., Terzolo M., Canu L., Mannelli M., Ettaieb H., Haak H.R., Kerkhofs T.M., Biehl M., Feelders R.A., Hofland J., Hofland L.J., Grytaas M.A., Husebye E.S., Ueland G.A., Ambroziak U., Bednarczuk T., Kondracka A., Macech M., Zawierucha M., Paiva I., Dennedy M.C., Sajwani A., Sherlock M., Crowley R.K., Ivovic M., Marina L., Deeks J.J., Sitch A.J., Arlt W., Bancos I., Chortis V., Giligan L.C., Hughes B.A., Lang K., Ivison H.E., Jenkinson C., Manolopoulos K., O'Neil D.M., O'Reilly M.W., Papathomas T.G., Prete A., Shackleton C.H.L., Taylor A.E., Asia M., Sutcliffe R.P., Guest P., Skordilis K., Bancos C., Chang A., Davidge-Pitts C.J., Delivanis D.A., Erickson D., Natt N., Nippoldt T.B., Thomas M., Young W.F., Jr. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8(9):773–781. doi: 10.1016/S2213-8587(20)30218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arlt W., Biehl M., Taylor A.E., Hahner S., Libé R., Hughes B.A., Schneider P., Smith D.J., Stiekema H., Krone N., Porfiri E., Opocher G., Bertherat J., Mantero F., Allolio B., Terzolo M., Nightingale P., Shackleton C.H.L., Bertagna X., Fassnacht M., Stewart P.M. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011;96(12):3775–3784. doi: 10.1210/jc.2011-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hines J.M., Bancos I., Bancos C., Singh R.D., Avula A.V., Young W.F., Grebe S.K., Singh R.J. High-resolution, accurate-mass (HRAM) mass spectrometry urine steroid profiling in the diagnosis of adrenal disorders. Clin. Chem. 2017;63(12):1824–1835. doi: 10.1373/clinchem.2017.271106. [DOI] [PubMed] [Google Scholar]
- 21.Fuhrman B.J., Xu X., Falk R.T., Hankinson S.E., Veenstra T.D., Keefer L.K., Ziegler R.G. Stability of 15 estrogens and estrogen metabolites in urine samples under processing and storage conditions typically used in epidemiologic studies. Int. J. Biol. Markers. 2010;25(4):185–194. [PMC free article] [PubMed] [Google Scholar]
- 22.Bernini P., Bertini I., Luchinat C., Nincheri P., Staderini S., Turano P. Standard operating procedures for pre-analytical handling of blood and urine for metabolomic studies and biobanks. J. Biomol. NMR. 2011;49(3–4):231–243. doi: 10.1007/s10858-011-9489-1. [DOI] [PubMed] [Google Scholar]
- 23.Coppens A., Speeckaert M., Delanghe J. The pre-analytical challenges of routine urinalysis. Acta Clin. Belg. 2010;65(3):182–189. doi: 10.1179/acb.2010.038. [DOI] [PubMed] [Google Scholar]
- 24.Stevens V.L., Hoover E., Wang Y., Zanetti K.A. Pre-analytical factors that affect metabolite stability in human urine, plasma, and serum: a review. Metabolites. 2019;9(8) doi: 10.3390/metabo9080156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Dominguez R., Gonzalez-Dominguez A., Sayago A., Fernandez-Recamales A. Recommendations and best practices for standardizing the pre-analytical processing of blood and urine samples in metabolomics. Metabolites. 2020;10(6) doi: 10.3390/metabo10060229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi H., Guo Z., Jia X., Liu H., Ma L., Xue L. The key points in the pre-analytical procedures of blood and urine samples in metabolomics studies. Metabolomics. 2020;16(6):68. doi: 10.1007/s11306-020-01666-2. [DOI] [PubMed] [Google Scholar]
- 27.Guideline on bioanalytical method validation. Committee for Medicinal Products for Human Use (EMEA/CHMP/EWP/192217/2009): European Medicines Agency, EMA; 2011.
- 28.Guidance for Industry: Bioanalytical Method Validation. U.S. Department of Health and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research (CDER) and Center for Veterinary Medicine (CVM); 2018.
- 29.Badoud F., Grata E., Boccard J., Guillarme D., Veuthey J.-L., Rudaz S., Saugy M. Quantification of glucuronidated and sulfated steroids in human urine by ultra-high pressure liquid chromatography quadrupole time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2011;400(2):503–516. doi: 10.1007/s00216-011-4779-8. [DOI] [PubMed] [Google Scholar]
- 30.Ikegawa S., Hasegawa M., Okihara R., Shimidzu C., Chiba H., Iida T., Mitamura K. Simultaneous determination of twelve tetrahydrocorticosteroid glucuronides in human urine by liquid chromatography/electrospray ionization-linear ion trap mass spectrometry. Anal. Chem. 2009;81(24):10124–10135. doi: 10.1021/ac9018632. [DOI] [PubMed] [Google Scholar]
- 31.Fabregat A., Pozo O.J., Marcos J., Segura J., Ventura R. Use of LC-MS/MS for the open detection of steroid metabolites conjugated with glucuronic acid. Anal. Chem. 2013;85(10):5005–5014. doi: 10.1021/ac4001749. [DOI] [PubMed] [Google Scholar]
- 32.Wang R., Hartmann M.F., Wudy S.A. Targeted LC-MS/MS analysis of steroid glucuronides in human urine. J. Steroid Biochem. Mol. Biol. 2021;205 doi: 10.1016/j.jsbmb.2020.105774. [DOI] [PubMed] [Google Scholar]
- 33.Cho H.J., Kim J.D., Lee W.Y., Chung B.C., Choi M.H. Quantitative metabolic profiling of 21 endogenous corticosteroids in urine by liquid chromatography-triple quadrupole-mass spectrometry. Anal. Chim. Acta. 2009;632(1):101–108. doi: 10.1016/j.aca.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 34.Son H.H., Yun W.S., Cho S.H. Development and validation of an LC-MS/MS method for profiling 39 urinary steroids (estrogens, androgens, corticoids, and progestins) Biomed. Chromatogr. 2020;34(2) doi: 10.1002/bmc.4723. [DOI] [PubMed] [Google Scholar]
- 35.Allende F., Solari S., Campino C., Carvajal C.A., Lagos C.F., Vecchiola A., Valdivia C., Baudrand R., Owen G.I., Fardella C.E. LC-MS/MS method for the simultaneous determination of free urinary steroids. Chromatographia. 2014;77(7-8):637–642. doi: 10.1007/s10337-014-2638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y., Cai Z. Determination of hormones in human urine by ultra-high-performance liquid chromatography/triple-quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2020;34(Suppl 1) doi: 10.1002/rcm.8583. [DOI] [PubMed] [Google Scholar]
- 37.Taylor N.F. Urinary steroid profiling. Methods Mol. Biol. 2013;1065:259–276. doi: 10.1007/978-1-62703-616-0_17. [DOI] [PubMed] [Google Scholar]
- 38.Kerkhofs T.M., Kerstens M.N., Kema I.P., Willems T.P., Haak H.R. Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm Cancer. 2015;6(4):168–175. doi: 10.1007/s12672-015-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velikanova L.I., Shafigullina Z.R., Lisitsin A.A., Vorokhobina N.V., Grigoryan K., Kukhianidze E.A., Strelnikova E.G., Krivokhizhina N.S., Krasnov L.M., Fedorov E.A., Sablin I.V., Moskvin A.L., Bessonova E.A. Different types of urinary steroid profiling obtained by high-performance liquid chromatography and gas chromatography-mass spectrometry in patients with adrenocortical carcinoma. Horm Cancer. 2016;7(5-6):327–335. doi: 10.1007/s12672-016-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenders N.F., Greenfield J.R. Urinary steroid profiling in diagnostic evaluation of an unusual adrenal mass. Endocrinol Diabetes Metab Case Rep. 2019;2019 doi: 10.1530/EDM-19-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiu S.C., Chan A.O., Taylor N.F., Lee C.Y., Loung P.Y., Choi C.H., et al. Use of urinary steroid profiling for diagnosing and monitoring adrenocortical tumours. Hong Kong Med J. 2009;15(6):463–470. [PubMed] [Google Scholar]
- 42.Dwivedi P., Zhou X., Powell T.G., Calafat A.M., Ye X. Impact of enzymatic hydrolysis on the quantification of total urinary concentrations of chemical biomarkers. Chemosphere. 2018;199:256–262. doi: 10.1016/j.chemosphere.2018.01.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.