Abstract
The effect of mycotoxins (MT) on broiler performance without or with the inclusion of yeast cell wall extract (YCWE, Mycosorb, Alltech, Inc., KY) was evaluated in a random-effects meta-analysis. Data was extracted from 25 research experiments with a total of 10,307 broilers. Broilers fed MT had lower (P < 0.001) body weight gain (BWG, −217 g), reduced feed intake (FI, −264 g), increased feed conversion ratio (FCR, 0.12), and greater mortality by 2.01%. Inclusion of YCWE improved (P < 0.001) BWG (59 g) and FI (65 g), lowered FCR (−0.05), and reduced mortality by 1.74%. Additionally, change in European Production Efficiency Factor (EPEF) was assessed. Feeding MT lowered (P < 0.001) EPEF while YCWE increased (P < 0.001) EPEF. Finally, the carbon footprint of production was evaluated. Control fed birds produced an estimated 1.93 kg CO2-equivalent/kg liveweight (LW), while MT fed broilers produced 2.13 kg CO2-equivalent/kg LW and YCWE inclusion lowered this to 2.03 kg CO2-equivalent/kg LW which resulted in −25 tonnes less CO2-equivalent output per 100,000 birds with YCWE. In conclusion, mycotoxins can play a role in reducing broiler performance and farm production output, as well as increase the carbon footprint. Inclusion of YCWE in feed under a mycotoxin challenge can improve broiler performance and output, as well as lower carbon footprint, which could play a role in farm efficiency, profitability, and environmental sustainability.
Key words: broiler, carbon footprint, performance, mycotoxin, yeast cell wall extract
INTRODUCTION
Mycotoxins, secondary metabolites produced by fungi, are common contaminants of agricultural crops (Devreese et al., 2013). In fact, a recently published review indicated that the frequency of detectable mycotoxins is up to 60 to 80% globally (Eskola et al., 2020). In the future, contamination by mycotoxins may further rise given increasing global temperatures, elevated carbon dioxide concentrations, drought stress, and extreme weather events (Eskola et al., 2020; Perrone et al., 2020). Agricultural practices aimed at improving environmental sustainability, such as the adoption of no-till methods or reduction of pesticide use, could conversely play a further detrimental role leading to mold development and augmented mycotoxin incidences (Jouany, 2007). Each of these factors can influence fungal growth and distribution as well as the type and concentrations of mycotoxins produced.
The agricultural industry should be aware of the presence of mycotoxins as these natural contaminants can negatively affect farm productivity and profitability. From a crop perspective, mycotoxin contamination could affect profitability and production efficiency due to reduced crop yields, feedstuff/feed rejections, or additional costs required for handling and testing of materials (Wu and Mitchell, 2016). Economic losses may also be associated within animal production due to diminished performance or health (Kipper et al., 2020; Holanda and Kim, 2021). Poultry can be susceptible to a wide array of mycotoxins through effects on growth, feed intake, feed efficiency, gut health, and immunity (Mogadam and Azizpour, 2011; Kolawole et al., 2020; Weaver et al., 2020b). Historically, these effects would be viewed as lost economic performance because animals may not reach their production optimum resulting in reduced profitability. However, it is now increasingly important to also view the contribution of mycotoxins through the lens of environmental sustainability and their potential negative impact. Although the global greenhouse gas (GHG) emissions from chickens is relatively low at 0.6 gigatonnes CO2-equivalents (CO2-eq) and represents about 8% of the livestock sector's emissions, the scale and growth rate of the poultry industry will still require emission reductions (MacLeod et al., 2013). Of the sources of emissions associated with the chicken meat supply chain, feed production contributes 78%. As such, the contribution of feed to the overall carbon footprint of the system is significant. The presence of mycotoxins in feed may further add to this carbon footprint of production by increasing feed waste and reducing feed efficiency.
Despite the widespread challenge of mycotoxins, there have been positive developments in management strategies. Methods include post-harvest cleaning or processing of contaminated grains; important breakthroughs in analytical technologies and extraction techniques enabling better mycotoxin quantification and monitoring; and the use of feed additives, supplements or feed ingredients that minimize the effects of mycotoxins on the animal (Čolović et al., 2019; Weaver et al., 2020a). Among those, the yeast cell wall extract (YCWE) rich in complex insoluble carbohydrates, has demonstrated considerable ability for the binding of several mycotoxins both in vitro, ex vivo, and in vivo (Aravind et al., 2003; Čolović et al., 2019; Kolawole et al., 2019; Jaćević et al., 2020; Weaver et al., 2020b).
Over the years, numerous papers have been published on the use of YCWE technology during mycotoxin challenges. However, it can be difficult to assess the overall impact of YCWE use when comparing different research publications. As such, we have conducted a meta-analysis using a statistical approach to integrate and quantify the overall outcome across a prior collection of experimental work (Sauvant et al., 2008). Our paper is an attempt to synthesis the current published and unpublished data in order to make a generalized statement on the effects of mycotoxins and YCWE on broiler performance. Our objectives in this study were to 1) assess the impact of mycotoxins on performance parameters and mortality of broilers; 2) to quantify the beneficial effects of YCWE (Mycosorb, Alltech Inc., Nicholasville, KY) inclusion during mycotoxin challenges; and 3) to determine the extent of YCWE use during a mycotoxin challenge to return broiler performance back to the control. Additionally, results from this meta-analysis were used to evaluate the possible roles of mycotoxins and YCWE on the economic and environmental impact of broiler production. To our knowledge, this is the first time a study has evaluated the influence of mycotoxins and mitigation strategies on the environmental sustainability of broiler production.
MATERIALS AND METHODS
Literature Search and Selection of References
Both published studies and unpublished trial reports that evaluated the effect of YCWE (Mycosorb, Alltech Inc.) inclusion during mycotoxin challenges on the impact to broiler performance were used in this meta-analysis. Available unpublished trials were included in this meta-analysis to provide a broader range of data in hopes of publication bias (Sterne et al., 2001). Materials were retrieved in April 2021 from either Alltech's internal bibliography database or through literature searches conducted through online databases (Google Scholar, Agricola, Pubmed) using keywords of “mycotoxins” and “broilers” in all searches, along with at least one additional keyword of “yeast cell wall extract,” “esterified glucomannan,” “glucomannan polymer,” “Mycosorb,” or “Alltech.” There were no date restrictions placed on the search engines. All literature, both published and unpublished, was subject to selection screening according to the following criteria: 1) all trials must be conducted with broiler chickens; 2) the experiment must contain at least one challenge treatment with mycotoxins alone (MT) as well as at least one YCWE + mycotoxin treatment that contained the specific YCWE product being investigated (Mycosorb, Alltech, Inc.); 3) the inclusion of a control treatment without detectable mycotoxins or minimal mycotoxin content was desired but not required; 4) mycotoxin type and concentrations needed to be provided, as well as the YCWE inclusion rate; 5) at least one variable of performance including body weight gain (BWG), feed intake (FI), feed conversion ratio (FCR), or mortality rate must be provided; and 6) the number of days in the experimental period and the number of animals in the trial was reported. This screening process resulted in a selection of 25 references that consisted of 18 journal publications and 7 unpublished studies that were either technical reports or presented at international conferences. Results from each trial were partitioned into treatments of either control, MT or YCWE.
Data Extraction
Broiler BWG, which was provided in most references, was used as a measure of performance in this meta-analysis. In cases where BWG was not provided, we calculated total gain by using the published average daily gain multiplied by the number of trial days. Similarly, FI was the variable used in our meta-analysis and in cases where this was not provided in a reference, FI was calculated from average daily feed intake multiplied by the number of trials days. Most references did provide FCR, but for those that did not, FCR was calculated using FI divided by BWG. We also assessed mortality percentage among birds. A total of 9 trials provided information on bird survival rates. If a reference listed livability percentage only, we calculated mortality rate by subtracting livability rate from 100%. We also assessed changes to the European Production Efficiency Factor (EPEF) between treatments. Only 2 of the trials used provided published EPEF values, however, we were able to manually calculate the EPEF for any trial used in this meta-analysis that listed measures of body weight (BW), FCR, number of days on trial, and mortality rate. The EPEF value was calculated using the following equation (Weaver et al., 2020b),
where the livability is expressed in %; BW is expressed in kg; the age in days; and the FCR is kg of feed consumed per kg of BWG.
Statistical Analysis
Mean effect size was calculated from raw mean differences of each response variable (i.e., BWG, FI, FCR, mortality) from each treatment group (control, MT, YCWE) that was reported in each study. Estimates of standard deviation (SD), and sample size for each treatment group were also collected from each study. There were instances where standard deviation was not reported in the studies, but the coefficient of variation (CV) was reported. In those instances, we estimated standard deviation by multiplying the mean by the CV. There were instances where neither the CV nor SD was reported. In those cases, we computed a SD that was an average from the other studies from available data for each treatment group.
Generally, our meta-analysis model can initially be defined as,
where i is the observed effect size in the i-th study, θ represents the unknown true effect size of the i-th study, and e is the associated sampling error with ei ∼ N(0,vi). The sampling variances (vi) were utilized directly from the studies or imputed using methods described above. A data transformation to assume normally distributed estimates was not used.
Additionally, we assumed, a priori, the existence of heterogeneity in effect sizes among studies. Differences in experimental design, methodology, and personnel can vary and may present additional variability that prevents generalizing patterns among response variables (Bornstein et al., 2010). Thus, we added an additional error term to our model effectively creating a hierarchical random-effects model (Thompson et al., 2001; Gelman and Hill, 2007). This error can be described as,
where θi represents the observed effect size in the i-th study, μ represents an average true effect size from a distribution of true effect sizes, and ζi is the error associated with the distribution of effect sizes (i.e., between study heterogeneity) with ζi ∼ N(0, τ2). Variance associated with the distribution of effect sizes (τ2) was estimated using restricted maximum likelihood (Viechtbauer, 2005). Overall, the random-effects model can be defined as,
where is the observed effect size of i independent effect size estimates. The random-effects model permitted studies of varying sample size to be equally weighted, which prevented our average true effect size estimate from being heavily influenced by studies with large sample sizes, a feature not possible with a fixed-effects model (Bornstein et al., 2010). Furthermore, the use of a random-effects model can permit more generalized conclusions regarding the effects of mycotoxins and YCWE present in the diets of broiler chickens. We also explored the potential for publication bias through the use of the Egger test (Egger et al., 1997). This test examines asymmetry in constructed funnel plots of effect size and standard error which may suggest publication bias. Finally, we evaluated between-study heterogeneity using the calculated heterogeneity statistic (I2) (Higgins et al., 2003; Von Hippel, 2015). This statistic is calculated as:
where Q is the calculated chi-squared statistic and df is the degrees of freedom associated with a comparison. Smaller values of I2 indicate lower heterogeneity, while larger values indicate higher heterogeneity (Higgins et al., 2003). We conducted our random-effects meta-analysis using R (R Development Core Team, 2021), R Studio (RStudio Team, 2021), and the package metafor (Viechtbauer, 2010). We gauged statistical significance at an alpha value of 0.05.
We also conducted a paired t test to examine the response of each treatment group on EPEF. This was not incorporated into our meta-analysis framework because measures of variance were not provided by nearly all of the studies. We gauged statistical significance at an alpha value of 0.05. This was conducted in RStudio using built in functions.
Simulation Assessment: Environmental Impact of Mycotoxins and YCWE
The results obtained from the meta-analysis were used in a simulation assessment to evaluate how mycotoxins without or with YCWE would influence the economic and environmental impact of broiler production. Environmental impact assessment was conducted by way of a life cycle assessment (LCA) by using the Alltech E-CO2 Poultry EA (Broiler) model (Alltech E-CO2, Stamford, United Kingdom), a bespoke carbon footprint tool employed commercially in the broiler industry. This model was designed following Intergovernmental Panel on Climate Change guidelines (Intergovernmental Panel on Climate Change (IPCC) 2019, Eggleston et al., 2006) and is independently accredited by the Carbon Trust according to the British Standards Institute's PAS:2050 specification for LCA standards (BSI, 2011). Embedded emissions associated with the cultivation, production, and delivery of purchased feeds, which comprise the majority of a broiler footprint, were estimated using the latest data sourced from FeedPrint software developed by Wageningen University and Blonk Milieu Advies (Vellinga et al., 2013). Feed emissions data were thus comparable with commercially available industry tools, and compatible with the LCA methodology employed in model as the process level data were also compliant with the PAS:2050 specification. The LCA boundary was defined as from ‘cradle-to-farm-gate’, that is covering emissions arising in all inputs and stages through the supply chain and on-farm processes, up to the point at which the product birds leave the farm. Therefore, this analysis did not account for emissions attributed to subsequent downstream processing, packaging, or transport of chicken meat beyond the farm gate. This ‘cradle-to-farm-gate’ approach is consistent with methodologies applied in previous poultry production system emission studies (Leinonen et al., 2012; Tallentire et al., 2017).
Calculations considering dietary formulations were based on a standard European wheat-soybean meal diet (Table 1). Broiler performance variables utilized were based on industry standards and breed performance objectives (Burns et al., 2008; Aviagen, 2019; Van Limbergen et al., 2020), with differences between treatments based on the results from this meta-analysis when feeding mycotoxins without or with YCWE. Emission intensity was estimated for each of 3 broiler production scenarios, comprising: a baseline control; broilers consuming MT; and the subsequent effect of including YCWE during mycotoxin challenges. Each scenario began with a flock of 100,000 birds placed on farm and the period of each assessment was the time to reach finishing weight. The output emission intensity was presented using 3 functional units: kg CO2-eq/bird; kg CO2-eq/kg liveweight; and kg CO2-eq/kg carcass weight leaving the farm gate.
Table 1.
Inputs used for a simulation assessment to evaluate the impact of mycotoxins without or with yeast cell wall extract (YCWE) on the economic and environmental impact of broiler production.
| Treatments2 |
|||
|---|---|---|---|
| Item1 | Control | Mycotoxins | YCWE |
| Number of broilers | 100,000 | 100,000 | 100,000 |
| Days on feed | 40 | 40 | 40 |
| Body weight gain, g | 2,697.00 | 2,479.80 | 2,545.28 |
| Average daily gain, g/d | 67.43 | 62.00 | 63.63 |
| Finishing weight, kg | 2.74 | 2.52 | 2.59 |
| FCR3 | 1.57 | 1.69 | 1.64 |
| Mortality, % | 3.52 | 5.59 | 3.85 |
| Dietary inputs | |||
| Wheat | 64 | 64 | 64 |
| Soybean Meal | 22 | 22 | 22 |
| Protein, % | 19.7 | 19.7 | 19.7 |
| Metabolizable Energy, MJ/kg | 13 | 13 | 13 |
| YCWE inclusion, kg/t | 1.3 | ||
Inputs for performance of birds fed control, mycotoxins, and yeast cell wall extract (YCWE) diets were based on industry standards and breed performance objectives [56-58], with differences between treatments based on the results from this meta-analysis when feeding mycotoxins without or with YCWE.
Treatments represent control, diets reported to be without mycotoxins or minimal mycotoxin contamination; Mycotoxin, diets with reported mycotoxin contamination; and yeast cell wall extract (YCWE; Mycosorb, Alltech, Inc.) diets reported to contain both mycotoxins and YCWE.
FCR, feed conversion ratio.
RESULTS
Research Characteristics
A total of 25 trials were used for this meta-analysis, 18 of which were published in scientific journals (Table 2). The studies were published over a 21-yr period (1999 to 2020) and were conducted in 11 countries (4 each from Brazil and Iran; 3 from Canada and India; 2 each from China, Turkey, Serbia, and United States; one from Colombia, Thailand, and Croatia). There were 10,307 broilers included: 2,149 fed control; 3,146 fed MT alone; and 4,994 fed YCWE during mycotoxin challenges. Trials were conducted over 20 to 56 d (average 35.5 d). Most trials used breeds of Ross, Cobb, or Arbor Acres with 5 trials not listing the breed type. Most studies used male birds or mixed sex, although there were some female-only trials, and 7 did not provide information on bird sex. Mycotoxin types reported by these studies in the MT rations included aflatoxins/aflatoxin B1 (21 treatments; 917 μg/kg average; 7.4 μg/kg minimum; 3,033 μg/kg maximum), ochratoxins/ochratoxin A (9 treatments; 1,118 μg/kg average; 1.4 μg/kg minimum; 2,000 μg/kg maximum), deoxynivalenol (8 treatments; 3.4 mg/kg average; 0.73 mg/kg minimum; 9.5 mg/kg maximum), 3-acetyl-deoxynivalenol (1 treatment, value of 26.1 μg/kg), 15-acetyl-deoxynivalenol (2 treatments; 171 μg/kg average; 42 μg/kg minimum; 300 μg/kg maximum), fumonisins (5 treatments; 3.9 mg/kg average; 0.7 mg/kg minimum; 7.3 mg/kg maximum), zearalenone (7 treatments; 376 μg/kg average; 54 μg/kg minimum; 680 μg/kg maximum), T-2 toxin (14 treatments; 1.4 mg/kg average; 0.03 mg/kg minimum; 3.0 mg/kg maximum), diacetoxyscirpenol (1 treatment, value of 1 mg/kg), and fusaric acid (3 treatments; 13.1 mg/kg average; 0.59 mg/kg minimum; 20.3 mg/kg maximum). Most trials had diets naturally contaminated with mycotoxins, or mycotoxins formed by culture following mold inoculation, but a few studies used pure mycotoxins. The average dose rate of YCWE was 1.3 kg/t, with 24.3% using 0.5 kg/t, 35.1% using 1.0 kg/t, 27.0% using 2.0 kg/t and 13.5% using other inclusion rates of YCWE between 0.25 and 4.0 kg/t.
Table 2.
Description of studies utilized for the random-effects meta-analysis examining: (1) the effect of control; (2) mycotoxins alone; (3) or yeast cell wall extract (YCWE) inclusion during mycotoxin challenges, on the performance of broilers.
| Trial information1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Location | Breed | Sex | Birds/trt2 | Days | Source3 | Type4 | YCWE, kg/t5 | Reference |
| USA | Cobb | Male | 200 | 42 | Natural | DON, 3ADON, 15ADON, FA |
0.25, 0.5, 1.0, 2.0, 4.0 | Weaver et al., 2020b |
| Iran | Ross-308 | Male | 30 | 35 | Natural | AF | 0.5, 1.0 | Mogadam and Azizpour, 2011 |
| India | - | Mixed | 70 | 35 | Natural | AF, OCH, ZEA, T2 | 0.5 | Aravind et al., 2003 |
| Turkey | Ross-308 | Male | 40 | 20 | Culture | AF | 0.5, 1.0 | Basmacioglu et al., 2005 |
| Brazil | Cobb | Male | 240 | 28 | Natural | AF, OCH, DON, T2, ZEA, FUM |
0.5. 1.0 | T.P. Ribeiro and A. Back (MercoLab, Brazil, personal communication) |
| Colombia | Ross-308 | Male | 30 | 28 | Pure | T2 | 2.0 | Diaz et al., 2005 |
| India | - | Mixed | 60 | 35 | Culture | AF, T2 | 1.0 | Girish and Devegowda, 2006 |
| Iran | Ross | Mixed | 180 | 41 | Natural | AF | 0.5, 1.0, 1.5 | Kamalzadeh et al., 2009 |
| Thailand | Arbor Acres | - | 45 | 42 | Natural | AF | 0.5, 1.0 | Khajarern and Khajarern, 1999 |
| China | Arbor Acres | Male | 48 | 32 | Culture | AFB1, OTA, T2 | 0.5 | Liu et al., 2011 |
| Serbia | Ross-308 | Mixed | 20 | 21 | Culture | OTA | 2.0 | Nedeljković-Trailović et al., 2015 |
| Iran | Ross-308 | - | 100 | 42 | Culture | AFB1 | 2.0 | Nemati et al., 2015 |
| Serbia | Ross | Mixed | 20 | 21 | Pure | T2 | 2.0 | Nešić et al., 2011 |
| Croatia | - | - | 120 | 42 | Pure | DAS | 1.0 | Pavicic et al., 2001 |
| India | - | Mixed | 60 | 35 | Culture | AF, OTA, T2 | 1.0 | Raju and Devedowda, 2000 |
| Brazil | Cobb | Female | 196 | 35 | Culture | AF | 1.0 | Rossi et al., 2010 |
| Iran | Ross-308 | - | 100 | 42 | Culture | AF | 2.5 | Saki et al., 2018 |
| Brazil | Cobb 500 | Male | 66 | 20 | Natural | FB1 | 2.0 | Silva et al., 2010 |
| Canada | Cobb | - | 90 | 56 | Natural | DON, T2 | 2.0 | T. Smith (University of Guelph, Canada, personal communication) |
| Canada | Ross | Male | 90 | 56 | Natural | DON, FA, ZEA | 2.0 | Swamy et al., 2002 |
| Canada | Ross | Male | 90 | 56 | Natural | DON, 15ADON, FA, ZEA | 2.0 | Swamy et al., 2004 |
| Brazil | Ross-308 | Male | 240 | 40 | Natural/Pure | AF, DON, ZEA, FUM, T2 | 1.0 | Vieira et al., 2004 |
| China | Arbor Acres | - | 175 | 41 | Natural | AF, OCH, DON, T2, FUM, ZEA | 0.5, 1.0 | Wang et al., 2006 |
| Turkey | Ross-308 | - | 30 | 21 | Culture | AF | 0.75 | Yildirim et al., 2011 |
| USA | - | - | 25 | 21 | Culture | AF | 1.0, 2.0 | Zhao et al., 2010 |
Trial information: data collected from the 25 studies utilized in this meta-analysis. Information not supplied from the study is indicated by “-”.
Birds/trt: number of birds per treatment from control, mycotoxin or mycotoxin plus yeast cell wall extract (YCWE; Mycosorb, Alltech, Inc.)
Source: source of mycotoxin contamination from naturally contaminated feedstuffs, artificial culture of feedstuff, or addition of pure mycotoxin to the ration.
Abbreviations: AFB1, aflatoxin B1; AF, aflatoxins; DAS, diacetoxyscirpenol; DON, deoxynivalenol; FB1,: fumonisin B1; FUM: fumonisins; 3ADON, 3-acetyl-deoxynivalenol; 15ADON, 15-acetyl-deoxynivalenol; FA, fusaric acid; OCH, ochratoxins; ; OTA, ochratoxin A; T2, T-2 toxin; ZEA, zearalenone.
YCWE, kg/t: inclusion rate of yeast cell wall extract (Mycosorb, Alltech, Inc.) in kg per ton utilized in study treatments.
Effects of Mycotoxins on Broiler Performance Parameters
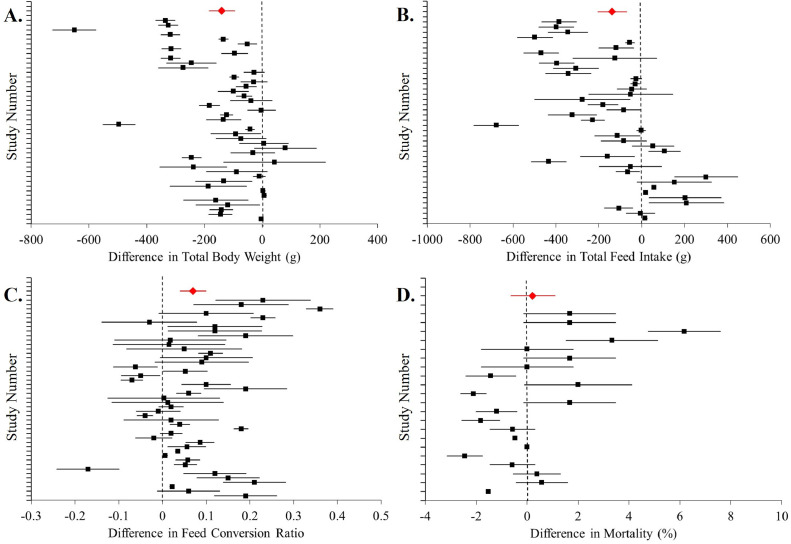
The difference between broilers fed MT diets and those fed control diets was investigated. Broilers consuming MT had significantly lower (P < 0.001) mean total BWG by 217.20 grams than birds fed control diets (Table 3). Forest plot depiction of study comparisons showed that among all trials, 30 of 33 comparisons were below zero, indicating the broilers fed MT treatments had significantly lower BWG than birds fed control (Figure 1A). No trials showed an increase in BWG for MT fed birds, and only 3 of the 33 comparisons resulted in no difference between treatments. Total FI was also significantly lower (P < 0.001) by 264.44 grams for birds fed MT versus birds fed control diets (Table 3). Many of the trials included in the meta-analysis showed significantly lower FI for MT birds with effect sizes below zero (Figure 1B).
Table 3.
Mean effect size estimates from a random-effect meta-analysis for feeding control, mycotoxins alone or yeast cell wall extract (YCWE) inclusion during mycotoxin challenges on the performance of broiler chickens.
| Heterogeneity test |
|||||||
|---|---|---|---|---|---|---|---|
| Item1 | No. Comp.2 | Mean effect size | 95% CI3 | P-value | I2 (%)4 | P-value | Eggar P-value5 |
| Body Weight Gain, g | |||||||
| Mycotoxin - Control | 33 | −217.20 | −155.70, −278.68 | <0.001 | 99.60 | <0.001 | 0.398 |
| YCWE - Mycotoxin | 46 | 65.48 | 41.35, 89.61 | <0.001 | 97.79 | <0.001 | 0.069 |
| YCWE - Control | 43 | −139.61 | −95.90, −183.31 | <0.001 | 99.34 | <0.001 | 0.914 |
| Total Feed Intake, g | |||||||
| Mycotoxin - Control | 32 | -264.44 | −174.70, −354.19 | <0.001 | 99.56 | <0.001 | 0.124 |
| YCWE - Mycotoxin | 44 | 99.39 | 50.29, 148.49 | <0.001 | 98.93 | <0.001 | 0.019 |
| YCWE - Control | 39 | −137.04 | −69.72, −204.37 | <0.001 | 99.44 | <0.001 | 0.911 |
| FCR6 | |||||||
| Mycotoxin - Control | 34 | 0.12 | 0.08, 0.16 | <0.001 | 98.93 | <0.001 | 0.844 |
| YCWE - Mycotoxin | 48 | −0.05 | −0.02, −0.08 | 0.001 | 98.93 | <0.001 | 0.309 |
| YCWE - Control | 45 | 0.07 | 0.04, 0.10 | <0.001 | 98.87 | <0.001 | 0.459 |
| Mortality Rate, % | |||||||
| Mycotoxin - Control | 15 | 2.07 | 1.35, 2.79 | <0.001 | 99.92 | <0.001 | 0.030 |
| YCWE - Mycotoxin | 24 | −1.74 | −1.24, −2.24 | <0.001 | 99.91 | <0.001 | 0.130 |
| YCWE - Control | 21 | 0.22 | 0.64, 1.08 | 0.615 | 99.98 | <0.001 | 0.001 |
Treatments represent control, diets reported to contain undetectable or minimal mycotoxin contamination; Mycotoxin, diets with reported mycotoxin contamination; and yeast cell wall extract (YCWE; Mycosorb, Alltech, Inc.) diets reported to contain both mycotoxins and YCWE. Effects of mycotoxins or YCWE were determined by the differences between treatments of mycotoxin minus control, YCWE minus mycotoxin, and YCWE minus control.
No. Comp.: number of different trial comparisons for each treatment and performance variable available from the 25 references used in this meta-analysis.
95% CI, 95% confidence interval.
I2: percentage of between-study variation.
Eggar P-value: Eggar test for asymmetry for gauging publication bias.
FCR, feed conversion ratio.
Figure 1.
Differences between broilers fed mycotoxin contaminated diets and control diets with undetectable or minimal mycotoxin content. The effect sizes with 95% confidence intervals between treatments are show (in black) for studies included in the meta-analysis for total body weight gain in grams (A), total feed intake in grams (B), feed conversion ratio (C) and percent mortality (D). The mean effect size and 95% confidence interval for response variable are depicted in red.
There was a significant impact of mycotoxins on FCR. Broilers fed the MT diets had 0.12 increase in FCR over those fed control (P < 0.001). Mortality was 2.07% greater (P < 0.001) among birds fed MT from those fed control diets (Table 3). Similar to BWG and FI, many of the studies showed significant differences in FCR and mortality rates between most control and MT treatments while relatively fewer showed no difference between treatment comparisons (Figures 1C and 1D). The FCR values were significantly higher for MT fed birds in 23 of 34 treatment comparisons, and only one of the 34 comparisons showed MT lowering FCR. Mortality rate was higher in 11 of 15 treatment comparisons, with no trials showing mortality to be lowered by MT.
Effects of YCWE During Mycotoxin Challenges on Broiler Performance Parameters
Broilers fed diets containing YCWE during the mycotoxin challenge had 65.48 grams greater BWG (P < 0.001) and 99.39 grams greater FI (P < 0.001) than those fed MT alone (Table 3). Twenty-one out of 46 comparisons had YCWE fed broilers with greater BWG than MT fed birds, while 23 comparisons showed no difference between treatments (Figure 2A). Treatment comparisons for FI showed a slightly greater number of trials with nonsignificant comparisons, although 17 of 44 trials did show YCWE to have increased FI in contrast to MT fed broilers (Figure 2B). There was a significant impact of YCWE on FCR, with these broilers having lower FCR by −0.05 than those fed MT alone (P < 0.001). The results for FCR showed that 16 of 48 comparisons between YCWE and MT indicated FCR was lowered by YCWE inclusion during the mycotoxin challenge, while 29 treatment comparisons resulted in no difference (Figure 2C). Feeding broilers YCWE resulted in 1.74% lower mortality rates (P < 0.001) compared to those fed MT (Table 3). Many of the studies included in the meta-analysis showed significant differences in mortality rates between YCWE and MT treatments as shown when the 95% confidence interval does not cross zero (Figure 2D).
Figure 2.
Differences between broilers fed diets with yeast cell wall extract (YCWE, Mycosorb, Alltech, Inc.) during mycotoxin challenges and broilers fed mycotoxin contaminated diets without YCWE. The effect sizes with 95% confidence intervals between treatments are shown (in black) for studies included in the meta-analysis for total body weight gain in grams (A), total feed intake in grams (B), feed conversion ratio (C) and percent mortality (D). The mean effect size and 95% confidence interval for response variable are depicted in red.
Comparison of Control and YCWE During Mycotoxin Challenges on Broiler Performance Parameters
Although we observed that broilers consuming YCWE had consistently improved performance over broilers consuming MT, the inclusion of YCWE did not fully return performance to that of the control for many of the measured parameters. Broilers fed YCWE diets had lower (P < 0.001) BWG (−139.61 grams) and FI (−137.04 grams) than broilers fed control (Table 3). Most comparisons between treatments for BWG and FI were below zero (Figures 3A and 3B) indicating that YCWE during the mycotoxin challenge did not restore fully initial control performances. However, some comparisons were not significant between YCWE and control. The FCR was higher by 0.07 for broilers consuming YCWE compared with control (P < 0.001). Sixteen out of 45 comparisons between YCWE and control were not significantly different, while 5 comparisons showed FCR was lowered by YCWE inclusion during the mycotoxin challenge in contrast to control (Figure 3C). Mortality rate response behaved differently to the other investigated parameters, where YCWE fed broilers had restored (P = 0.615) mortality rate to the one observed for the unchallenged control birds. Most treatment comparisons for mortality (12 of 21) showed no difference between YCWE and control, while 7 showed reduced mortality beyond that of the control with YCWE inclusion (Figure 3D).
Figure 3.
Differences between broilers fed diets with yeast cell wall extract (YCWE, Mycosorb, Alltech, Inc.) during mycotoxin challenges and broilers fed control diets without YCWE and undetectable or minimal mycotoxin contamination. The effect sizes with 95% confidence intervals between treatments are shown (in black) for studies included in the meta-analysis for total body weight gain in grams (A), total feed intake in grams (B), feed conversion ratio (C) and percent mortality (D). The mean effect size and 95% confidence interval for response variable are depicted in red.
Between-Study Heterogeneity and Publication Bias
The results for heterogeneity (I2) indicated significant differences (P < 0.001) between studies for all performance parameters tested (Table 3). Reported I2 values were above 97.79%. Eggar's test for asymmetry did not indicate publication bias for all treatment effects on BWG and FCR (P > 0.05; supplementary materials, Figures S1, S2, and S3). For FI there was indication of potential publication bias for YWCE vs. MT (P < 0.05) but not other treatment comparisons. Potential publication bias was also indicated for mortality for the comparison between MT vs. control and YCWE vs. control (P < 0.05).
Influence of Mycotoxins and YCWE on European Production Efficiency Factor
Results from the paired t test for calculation of EPEF showed that the MT fed birds had lower (P < 0.001) EPEF by a mean difference of −59.36 than broilers fed control treatments (Table 4). When comparing YCWE to the MT fed birds, the EPEF was greater (P < 0.001) by a mean difference of 16.81. However, birds fed YCWE during the mycotoxin challenge did have lower EPEF with a mean of −38.35 (P < 0.001) in contrast to the control fed birds.
Table 4.
Effect of feeding control, mycotoxin or yeast cell wall extract (YCWE) inclusion during mycotoxin challenges on the European Performance Efficiency Factor (EPEF) of broilers.
| Item1 | No. comparisons2 | Mean effect size | 95% CI3 | P-value |
|---|---|---|---|---|
| Mycotoxin - Control | 16 | −59.36 | −37.36, −81.37 | <0.001 |
| YCWE - Mycotoxin | 25 | 16.81 | 11.92, 21.71 | <0.001 |
| YCWE - Control | 22 | −38.35 | −21.84, −54.85 | <0.001 |
Treatments represent control, diets reported to contain undetectable or minimal mycotoxin contamination; Mycotoxin, diets with reported mycotoxin contamination; and yeast cell wall extract (YCWE; Mycosorb, Alltech, Inc.) diets reported to contain both mycotoxins and YCWE. Effects of mycotoxins or YCWE were determined by the differences between treatments of mycotoxin minus control, YCWE minus mycotoxin, and YCWE minus control.
No. Comparisons: number of different trial comparisons for each treatment and performance variable available from the 25 references used in this meta-analysis.
95% CI, 95% confidence interval.
Influence of Mycotoxins and YCWE on Broiler Farm Output and Carbon Footprint
Simulation of the impact of mycotoxins and YCWE on farm production performance and carbon footprint of broiler production was completed (Table 5). Calculations showed that there were 96,480 saleable control fed broilers per 100,000 birds placed, which generated 264.4 tonnes liveweight (LW). In contrast, there were 94,410 saleable MT fed birds which generated only 237.9 tonnes LW per 100,000 birds placed. Inclusion of YCWE during the mycotoxin challenge increased the number of saleable birds to 96,150 and increased total output to 249.0 tonnes LW. Results for carbon footprint assessment showed that control fed birds generated 5.29 kg CO2-eq/bird or 1.93 kg CO2-eq/kg LW. Birds fed MT generated 5.36 kg CO2-eq/bird or 2.13 kg CO2-eq/kg LW. The inclusion of YCWE resulted in lower emissions at 5.25 kg CO2-eq/bird (2.03 kg CO2-eq/kg LW).
Table 5.
Simulation for the impact on farm production performance parameters and carbon footprint (CO2-eg) per 100,000 broilers placed consuming mycotoxins without or with yeast cell wall extract (YCWE) based on variables obtained from the meta-analysis.
| Treatments1 |
||||
|---|---|---|---|---|
| Parameters | Control | Mycotoxins | YCWE | YCWE vs MT2 |
| Number of saleable birds | 96,480 | 94,410 | 96,150 | 1740 |
| Liveweight produced, tonnes | 264.4 | 237.9 | 249.0 | 11.1 |
| Emissions/bird, kg CO2-eq/bird | 5.29 | 5.36 | 5.24 | −0.12 |
| Emissions/kg liveweight (LW), kg CO2-eq/kg LW | 1.93 | 2.13 | 2.03 | −0.10 |
| Emissions/kg carcass, kg CO2-eq/kg carcass | 2.77 | 3.05 | 2.91 | −0.14 |
Treatments represent control, diets reported to contain undetectable or minimal mycotoxin contamination; Mycotoxin, diets with reported mycotoxin contamination; and yeast cell wall extract (YCWE; Mycosorb, Alltech, Inc.) diets reported to contain both mycotoxins and YCWE.
YCWE vs MT: differences between YCWE and mycotoxin treatments.
DISCUSSION
Mycotoxins represent a challenge for poultry production globally. Grains used in feed manufacturing often contain multiple mycotoxins which could play a detrimental role in the performance and health of broilers (Kolawole et al., 2020; Weaver et al., 2020b; Koletsi et al., 2021; Weaver et al., 2021). Despite the challenge that mycotoxins pose, mycotoxin management strategies have been developed to minimize the negative effects of mycotoxins on broilers. The information in this meta-analysis may help poultry producers gain a better understanding, and provide to our knowledge for the first time, better insight of how mycotoxin contamination of feed could be influencing the production, profitability, and carbon footprint of their operation. Simultaneously this meta-analysis provides evidence for an effective mycotoxin management program with the use of a proprietary YCWE product (Mycosorb).
Although individual trials provide good reference for the impact of mycotoxins or the benefits of management strategies, research results can vary due to the variety of biotic, abiotic, and regional factors even in experimental research environments, and it can be hard to assess the overall impact. In contrast, a meta-analytical study can be used to integrate, summarize and quantify results across prior research (Sauvant et al., 2008). Our meta-analysis provides a good representation of different production systems across many regions. These trials also had a wide range of mycotoxin types and concentrations, as well as a range of YCWE inclusion rates, which could better represent what is found in a real production setting. Therefore, we are able to draw conclusions regarding the impact of mycotoxins on broilers and the benefit of YCWE inclusion during mycotoxin challenges.
Our meta-analysis indicated high between-study heterogeneity (I2 > 90%; Higgins et al., 2003). A large I2 value suggests high variability between studies, which may reduce the utility of an average effect size due to the lack of commonality between studies. However, the I2 statistic can depend substantially on the sampling error, or within-study variability (Borenstein et al., 2017). Many of the studies included in the meta-analysis had very high sample sizes and thus low sampling error. The I2 statistic is a measure of the variability not associated with sampling error, and therefore, it is expected that with low sampling error the value for between-study error will be large. Furthermore, it could be expected that between-study heterogeneity may exist considering that the studies included in this meta-analysis were conducted in different countries under differing management strategies. Other publications have also found high heterogeneity when conducting meta-analysis. Upon observation of high heterogeneity, Salami et al. (2022) stratified their study based on several factors related to product usage, management, and layer age. Their results indicated that the subgroup analysis did not explain most of the heterogeneity as there are numerous factors that could influence data variation. For example, one factor that may affect outcome to mycotoxin challenge is the feeding program or nutritional status of the bird. However, an almost equal number of papers included in this meta-analysis did not report dietary composition as those that did include this information. A previous meta-analysis investigating mycotoxin effects on broilers also found a similar challenge, with only 41% of papers reporting feed composition (Kipper et al., 2020). Thus, we caution the interpretation and utility of the calculated I2 statistic in our analysis and acknowledge that further future research could investigate the many factors influencing broiler performance during mycotoxin challenge and YCWE inclusion. Overall, our research does address the goal of assessing the general trend for mycotoxin impacts on broiler performance and the use of YCWE.
Despite the higher heterogeneity between studies, most of our data comparisons showed no publication bias. Publication bias, or the tendency for research to be published based on a certain outcome rather than independent of outcome, occurs in the scientific field (Rothstein et al., 2005). This can be problematic in a meta-analysis in which an average effect size is used to generalize a phenomenon (i.e., effects of mycotoxins on feed intake). It may suggest the mean effect sizes we extracted from the literature do not accurately characterize the actual range of plausible effects. The Eggar test examines asymmetry between calculated effect sizes and associated standard errors via a funnel plot (Egger et al., 1997). Asymmetry may be more likely if the studies used in the analysis do not include a range of effect sizes and errors (Sterne et al., 2011). However, we were able to minimize risks of publication bias through a comprehensive literature search that included published and unpublished studies from many parts of the world. Therefore, we are confident our calculated mean effect sizes can be used to generally describe these phenomena.
Mycotoxin consumption by poultry can negatively impact BWG, FI, and FCR by several different mechanisms such as causing damage to, or affecting the function of, the intestinal tract, internal organs, and immune system (Raju and Devegowda, 2002; Awad et al., 2011; Manafi, 2011; Weaver et al., 2020b). The results of our meta-analysis showed that broilers challenged by a range of different mycotoxin types and concentrations had overall reduced BWG by −217.2 grams (13% reduction), lower FI by −264.4 grams (9% reduction), and increased FCR by 12 points (7% increase) when compared with birds fed control diets that contained undetectable or minimal mycotoxin contamination. Our analysis also indicated that the negative impact on growth performance appear to be consistent across publications, with 90.9% of treatment comparisons showing MT reduced broiler BWG, 81.3% reduced FI, and 68% increasing FCR. Previously published meta-analytical studies on the mycotoxin impacts on broiler performance have found similar results. Kipper et al. (2020) analyzed the results from 158 publications and showed nearly identical results with BWG reduced by 15%, FI by 9%, and FCR increased by 7% when broilers consumed a range of mycotoxin types and concentrations. Kipper et al. (2020) also reviewed the impact of individual mycotoxins on broilers, finding that most mycotoxins played a role in lowering BGW, while fumonisins had a lesser impact on FI and zearalenone played a minimal role in FCR (Kipper et al., 2020). As a result of these various meta-analysis results, it is suggested that mycotoxins have a constant negative impact on broiler BWG, FI and FCR.
The effect of mycotoxins on FCR is particularly important as FCR is a measurement of broiler feed utilization with a higher value indicating less efficiency. Feed represents a large portion of cost for the poultry industry, accounting for approximately 60 to 80% of production expenses (Kolawole et al., 2020). As such, the impact of mycotoxins on FCR could result in increased feed costs and significant impact on the overall profitability of the farm. Further negative impacts on profitability will also result from changes to the mortality rate, which we showed to increase by 2.07% when MT were consumed by broilers. This result is supported by results from a previous meta-analysis publication which indicated that feeding broilers mycotoxins significantly increased mortality, with aflatoxins and deoxynivalenol being primary drivers of this observation (Andretta et al., 2011).
As poultry are often sold on a per-weight basis, changes to BWG, FCR and of course mortality can result in reduced farm profitability. The impact of mycotoxins on these three variables can be further described by calculation of EPEF, which assesses economic efficiency of broilers (Weaver et al., 2020b). The results of our meta-analysis showed that control fed birds had an EPEF of 264, that was further reduced by 59.4 (22.5%) to 204 for broilers consuming MT. Few studies have investigated the role of mycotoxins on EPEF, but those that have do show that mycotoxins can play a role as we have observed. Broilers housed in a commercial production setting and consuming feed naturally contaminated with mycotoxins had a 14% lower EPEF than control birds (EPEF of 274 vs. 319) (Weaver et al., 2020b). Andretta et al. (2011) also showed a significant impact of mycotoxins on EPEF, with control fed birds having a similar EPEF of 261, but with mycotoxin fed birds having a greater reduction in EPEF by 40%. The benefit of EPEF is that it can be used to express farm production efficiency and profitability. Szőllősi and Szűcs (2014) modeled the impact of EPEF on broiler production in Hungary. Their model showed that a 20% reduction in EPEF could result in a 15% reduction in production cost but a decline in output by 22%. This effect results in decreased income by 1.5 thousand EUR/flock or 9.5 thousand Euro/year, and a significant 20.6 thousand Euro less annual income than can be achieved by the greater/average EPEF. As such, our observed 22.5% change in EPEF due to mycotoxins could be playing an important role in farm profitability.
Although mycotoxins have a clear impact on the performance, profitability, and sustainability of broiler production, management techniques are available to help minimize this challenge. Certain agronomic practices used during field growth of the plant may help lower mold growth and mycotoxin challenge, as can proper storage management of feedstuffs and feed ingredients (Jouany, 2007). To help minimize direct effects on the animal, feed supplements or ingredients, such as YCWE are available. YCWE contains an insoluble complex carbohydrate network formed from chains of β-(1,3)- and β-(1,6)-D-glucans, which play a role in the formation of a highly organized tridimensional structure (Yiannikouris et al., 2021). Mycotoxins have been demonstrated to interact with the β-D-glucan chains through hydrogen bonding and van der Waals interactions (Yiannikouris et al., 2006). Furthermore, the YCWE structure is shown to resist digestion, retain its binding capacity throughout the digestive tract, and be minimally impacted by pH changes (Yiannikouris et al., 2021). As a result, YCWE is shown to be efficient in sequestering mycotoxins and decreasing the adverse effects on animals (Weaver et al., 2020b). To our knowledge this is the first meta-analysis assessing the use of YCWE for broilers during mycotoxin challenges.
Despite the negative impact of mycotoxins on broiler performance, results of our meta-analysis showed that the use of YCWE during mycotoxin challenges resulted in significantly greater BWG by 65.48 g, increased FI by 99.39 g, and a 5-point reduction in FCR. These improvements in performance may be explained by the ability of the β-D-glucans in YCWE to sequester mycotoxins, thereby reducing the harmful effect of mycotoxins (Yiannikouris et al., 2006). The benefit of including YCWE when broilers are exposed to mycotoxins was shown in many of the trial comparisons used in this meta-analysis. In fact, 45.7% of the treatment comparisons showed BWG of broilers to be improved when YCWE was included, while 38.6% of comparisons showed increased FI, and 33.3% resulted in lowered FCR. Furthermore, no trials included in the meta-analysis showed a reduction in BWG or FI when including YCWE during the mycotoxin challenges. Although some treatment comparisons did indicate a lack of difference between YCWE and MT, this observation may have resulted from an improper YCWE inclusion rate or issues related to the homogeneous distribution in the diet of YCWE used in relation to the mycotoxin challenge. Furthermore, the severity of mycotoxin challenges was used in some of the research trials, for example concentrations of aflatoxins at 1 to 3 mg/kg (Basmacioglu et al., 2005; Zhao et al., 2010; Yildirim et al., 2011; Saki et al., 2018), ochratoxins at 2 mg/kg (Raju and Devegowda, 2000; Nedeljković-Trailović et al., 2015) or T-2 toxin at 3 mg/kg (Raju and Devegowda, 2000), may have resulted in negative impacts on broilers which were too great to fully overcome through product inclusion.
Beyond the beneficial implications on performance improvement during mycotoxin challenges, the inclusion of YCWE also lowered bird mortality by −38.2%. This insight on the ability of a mycotoxin mitigation strategy such as YCWE to minimize the impact of mycotoxins on bird mortality is quite unique, as few other publications have investigated this parameter. Furthermore, we saw that this statistical reduction in mortality was observed in many of the treatment comparisons (67%) used in this meta-analysis. The other 33% of the comparisons resulted in no significant difference between YCWE and MT treatments. Interestingly, these treatments that showed no difference were from the trials with higher mycotoxin levels including 3 mg/kg aflatoxins (Vieira et al., 2004), 3 mg/kg T-2 toxin, 2 mg/kg ochratoxin A, or combinations of these high mycotoxin levels (Raju and Devegowda, 2000), as well as moderate YCWE inclusion of 1 kg/tonne. Furthermore, results of this meta-analysis showed that the mortality rate of broilers fed YCWE during the mycotoxin challenge was returned to that of birds fed the control diets.
As shown in this meta-analysis, the collective effects of mycotoxins on performance and mortality can impact the efficiency of broiler production, quantifiable as EPEF, and could result in economic loss for producers. Conversely, the inclusion of YCWE significantly increased the EPEF by 16.8 (7.4%) from 226.7 to 243.5. Although the EPEF was not returned to that of the control birds, inclusion of YCWE returned about one third of the EPEF when broilers consumed mycotoxins. It is important to note that trials in this meta-analysis not only had a wide range of mycotoxin types and concentrations, but also a range of YCWE inclusion rates, which may or may not have aligned with the manufacturer recommended inclusion based on the mycotoxin contamination level. Overall, based on our results, we suggest that the use of YCWE could improve the profitability of broiler production when mycotoxin contamination of feed is a challenge. Adapting the YCWE inclusion rate to match the actual mycotoxin challenge level could maximize the EPEF.
Although the impact of mycotoxins and benefit of YCWE on broiler performance and health is certainly of interest, understanding their contribution to GHG emissions is an increasingly important consideration as governments and industries across the globe commit to reducing GHG. To our knowledge, the potential role that mycotoxins may play in the carbon footprint of broiler production has not yet been investigated. Carbon footprint refers to the total amount of GHG emissions attributed to the production of a product along a supply chain, expressed as kg or tonnes of CO2-eq, and may consider emissions from consumption, end-of-life recovery, and disposal (MacLeod et al., 2013). Furthermore, the CO2-eq emission is a standard measurement allowing for comparing emissions of different GHG types.
We believe that through the impacts on bird performance, feed efficiency and health, mycotoxins may play a role in CO2-eq and sustainability. To assess this concept, we utilized data obtained from this meta-analysis to simulate the impact of mycotoxins without or with YCWE on CO2-eq. Metrics for performance of birds being fed control, MT and YCWE diets were based on industry standards and breed performance objectives (Aviagen, 2019; Van Limbergen et al., 2020; Szőllősi et al., 2021), with differences between treatments based on the results from this meta-analysis when feeding mycotoxins without or with YCWE. Considering these inputs, it is estimated that control fed broilers could generate a total of 264.4 tonnes LW per 100,000 birds placed, whereas MT fed birds generated only 237.9 tonnes LW per 100,000 birds placed as a result of reduced growth and greater mortality. Despite the mycotoxin challenge, the inclusion of YCWE increased final bird body weight, increased the number of saleable birds by 1,740, and increased total output by 11 tonnes to 249.0 tonnes LW. As a result, it can be estimated that the use of YCWE during a mycotoxin challenge results in an increase of total protein output and profitability of a production system.
The environmental impact should also be considered. For control fed birds, CO2-eq was estimated at 5.29 kg CO2-eq/bird (1.93 kg CO2-eq/kg LW) which is in line with other estimates of broiler CO2-eq production at 5.24 kg CO2-eq per bird marketed (Burns et al., 2008). In contrast, the feeding of diets containing mycotoxins increased the CO2-eq to 5.36 kg/bird (2.13 kg CO2-eq/kg LW) which would result in an additional 46.62 tonnes of CO2-eq produced per 100,000 birds. This increase occurred due to poor feed utilization and a greater amount of deadstock. Although we are not aware of any research assessing the role that mycotoxins play in GHG production, other publications have indicated that changes to feed efficiency or dietary composition can alter the emissions of GHG (Sell-Kubiak et al., 2017; Salami et al., 2021). Although mycotoxins are certainly not the only component playing a role in GHG emissions, it is interesting to consider their presence as a potential addition to total emissions.
We have already shown in this meta-analysis a potential for YCWE to improve broiler performance, and it is also shown in this simulation that YCWE could play a role in reducing GHG emissions when mycotoxins are being simultaneously consumed by broilers. In fact, our calculations show that broilers fed YCWE had lower emissions at 5.25 kg CO2-eq/bird (2.03 kg CO2-eq/kg LW) and overall, produced −25.41 tonnes less CO2-eq per 100,000 birds than MT fed birds. This reduction in carbon footprint could equate to 30 fewer round-trip transatlantic flights from London to New York, or the annual average use of 17 cars in the U.K. It is important to note that further improvement in environmental impacts could be obtained from the reduction in days on feed to slaughter, which was not accounted for in this simulation, which would impact feed costs, energy, labor, and fuel requirements, as well as final output of GHG emissions. Future assessment of the impacts of both mycotoxins and YCWE are needed, with direct investigation being made at the farm-level. Despite some unknowns, results from this meta-analysis and simulation example are the first of its kind to indicate the impact of mycotoxins on the sustainability of broiler production and the potential environmental benefits of using a mycotoxin management strategy such as YCWE.
CONCLUSIONS
This meta-analysis provides a summary across available literature for the impacts of mycotoxins with or without YCWE on the performance of broilers. Consumption of mycotoxin contaminated feed by broilers resulted in consistent negative effects on BWG, FI, FCR and mortality. Mycotoxin consumption also resulted in poorer EPEF, reduced production outputs and potentially greater estimated GHG emissions. Although the feeding of YCWE to broilers during the mycotoxin challenge did not fully return performance to that of birds consuming the control with no mycotoxin contamination, results from this meta-analysis did show that YCWE inclusion significantly improved all performance parameters as well as reduced mortality rate. Furthermore, the inclusion of YCWE resulted in improved EPEF and lowered GHG emissions compared to feeding mycotoxins alone. As such, the inclusion of YCWE during mycotoxin challenges could result in not only improved broiler performance and health, but also enhanced profitability and environmental sustainability of broiler production.
Acknowledgments
ACKNOWLEDGMENTS
The authors thank the members of Alltech ECO2 (Alltech, Inc., Stamford, UK) for their assessment of the environmental impact of mycotoxins and YCWE on broiler production, as well as for their assistance in writing of methods relating to this carbon footprint simulation discussed in our meta-analysis.
DISCLOSURES
The authors A.C.W., N.A. and A.Y. are employees of Alltech Inc., which produces and markets Mycosorb, the commercial product evaluated in this meta-analysis. This does not influence compliance of publishing policies.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2022.102043.
Appendix. Supplementary materials
Supplementary figures S1, S2 and S3 are available online
REFERENCES
- Andretta I., Kipper M., Lehnen C.R., Hauschild L., Vale M.M., Lovatto P.A. Meta-analytical study of productive and nutritional interactions of mycotoxins in broilers. Poult. Sci. 2011;90:1934–1940. doi: 10.3382/ps.2011-01470. [DOI] [PubMed] [Google Scholar]
- Aravind K.L., Patil V.S., Devegowada G., Umakantha B., Ganpule S.P. Efficacy of esterified glucomannan to counteract mycotoxicosis in naturally contaminated feed on performance and serum biochemical and hematological parameters in broilers. Poult. Sci. 2003;82:571–576. doi: 10.1093/ps/82.4.571. [DOI] [PubMed] [Google Scholar]
- Aviagen. 2019. Ross 308/Ross 308 FF broiler performance objectives 2019. 0419-AVNR-107. Accessed Feb. 2022. http://eu.aviagen.com/tech-center/download/1339/Ross308-308FF-BroilerPO2019-EN.pdf. Accessed 02/08/2022
- Awad W.A., Hess M., Twarużek M., Grajewski J., Kosicki R., Böhm J., Zentek J. The impact of the Fusarium mycotoxin deoxynivalenol on the health and performance of broiler chickens. Int. J. Mol. Sci. 2011;12:7996–8012. doi: 10.3390/ijms12117996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basmacioglu H., Oguz H., Ergul M., Col R., Birdane Y.O. Effect of esterified glucomannan on performance, serum biochemistry and haematology in broilers exposed to aflatoxin. Czech J. Anim. Sci. 2005;50:31–39. [Google Scholar]
- Bornstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. A basic introduction to fixed-effect and random-effect models for meta-analysis. Res. Synth. Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- Borenstein M., Higgins J.P.T., Hedges L.V., Rothstein H.R. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res. Synth. Methods. 2017;8:5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- British Standards Institution (BSI) British Standards Institute; London, UK: 2011. PAS 2050:2011 Specification for the Assessment of Lifecycle Greenhouse Gas Emissions of Goods and Services. [Google Scholar]
- Burns R.T., Li H., Xin H., Gates R.S., Overhults D.G. Greenhouse gas (GHG) emissions from broiler houses in the Southeastern United States. Page 181 in Proc. Agric. Biosystems Eng. Conf; Providence, RI; 2008. [Google Scholar]
- Čolović R., Puvača N., Cheli F., Avantaggiato G., Greco D., Duragić O., Kos J., Pinotti L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins. 2019;11:617. doi: 10.3390/toxins11110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreese M., De Backer P., Croubels S. Overview of the most important mycotoxins for the pig and poultry husbandry. Vlaams Diergeneeskundig Tijdschrift. 2013;82:171–180. [Google Scholar]
- Diaz G.J., Cortés A., Roldán L. Evaluation of the efficacy of four feed additives against the adverse effects of T-2 toxins in growing broiler chickens. J. Appl. Poult. Sci. 2005;14:226–231. [Google Scholar]
- Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskola M., Kos G., Elliott C.T., Hajšlová J., Mayar S., Krska R. Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’ of 25% Crit. Rev. Food Sci. Nutr. 2020;60:2773–2789. doi: 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- Gelman A., Hill J. Cambridge Univ. Press; New York, NY: 2007. Data Analysis Using Regression and Hierarchical/Multilevel Models. [Google Scholar]
- Girish C.K., Devegowda G. Efficacy of glucomannan-containing yeast product (Mycosorb) and hydrated sodium calcium aluminosilicate in preventing the individual and combined toxicity of aflatoxin and T-2 toxin in commercial broilers. Asian-Aust. J. Anim. Sci. 2006;19:877–883. [Google Scholar]
- Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holanda D.M., Kim S.W. Mycotoxin occurrence, toxicity, and detoxifying agents in pig production with an emphasis on deoxynivalenol. Toxins. 2021;13:171. doi: 10.3390/toxins13020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) In: In volume 4: Agriculture, Forestry and Other Land Use. Eggleston H.S., Buendia L., Miwa K., Ngara T., Tanabe K., editors. IPCC; IGES, Japan: 2006. 2006 IPCC Guidelines for National Greenhouse Gas Inventories. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) In: In Volume 4: Agriculture, Forestry and Other Land Use. Calvo Buendia E., Tanabe K., Kranjc A., Baasansuren J., Fukuda M., Ngarize S., Osako A., Pyrozhenko Y., Shermanau P., Federici S., editors. IPCC; Switzerland: 2019. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. [Google Scholar]
- Jaćević V., Dumanović J., Lazarević M., Nepovimova E., Resanović R., Milovanović Z., Wu Q., Kuča K. Antidotal potency of the novel, structurally different adsorbents in rats acutely intoxicated with the T-2 toxin. Toxins. 2020;12:643. doi: 10.3390/toxins12100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouany J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007;137:342–362. [Google Scholar]
- Kamalzadeh A., Hosseini A., Moradi S. Effects of yeast glucomannan on performance of broiler chickens. Int. J. Agric. Biol. 2009;11:49–53. [Google Scholar]
- Khajarern J., Khajarern S. Protective effects on Mycosorb against aflatoxicosis in ducklings and broilers. Proc. Alltech's 15th Annual Symp. Biotechnol; Lexington, KY; Feed Industry; 1999. [Google Scholar]
- Kipper M., Andretta I., Ribeiro A.M.L., da Silva Pires P.G., Franceschina C.S., Cardinal K.M., de Oliveira Moraes P., Schroeder B. Assessing the implications of mycotoxins on productive efficiency of broilers and growing pigs. Sci. Agric. 2020;77 [Google Scholar]
- Kolawole O., Meneely J., Greer B., Chevallier O., Jones D.S., Connolly L., Elliott C. Comparative in vitro assessment of a range of commercial feed additives with multiple mycotoxin binding claims. Toxins. 2019;11:659. doi: 10.3390/toxins11110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolawole O., Graham A., Donaldson C., Owens B., Abia W.A., Meneely J., Alcorn M.J., Connolly L., Elliott C.T. Low doses of mycotoxin mixtures below EU regulatory limits can negatively affect the performance of broiler chickens: a longitudinal study. Toxins. 2020;12:433. doi: 10.3390/toxins12070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletsi P., Schrama J.W., Graat E.A.M., Wiegertjes G.F., Lyons P., Pietsch C. The occurrence of mycotoxins in raw materials and fish feeds in Europe and the potential effects of deoxynivalenol (DON) on the health and growth of farmed fish species – a review. Toxins. 2021;13:403. doi: 10.3390/toxins13060403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen I., Williams A., Wiseman J., Guy J., Kyriazakis I. Predicting the environmental impacts of chicken systems in the United Kingdom through a life cycle assessment: broiler production systems. Poult. Sci. 2012;91:8–25. doi: 10.3382/ps.2011-01634. [DOI] [PubMed] [Google Scholar]
- Liu Y.L., Meng G.Q., Wang H.R., Zhu H.L., Hou Y.Q., Wang W.J., Ding B.Y. Effect of three mycotoxin adsorbents on growth performance, nutrient retention and meat quality in broilers fed on mould-contaminated feed. Br. Poult. Sci. 2011;52:255–263. doi: 10.1080/00071668.2011.559453. [DOI] [PubMed] [Google Scholar]
- MacLeod M., Gerber P., Mottet A., Tempio G., Falcucci A., Opio C., Vellinga T., Henderson B., Steinfeld H. Food Agric. Organization United Nations (FAO); Rome, Italy: 2013. Greenhouse Gas Emissions From Pig and Chicken Supply Chains: A Global Life Cycle Assessment. [Google Scholar]
- Manafi M. Evaluation of different mycotoxin binders on broiler breeders induced with aflatoxin B1: effects on visceral organ weight and organ lesions parameters. Adv. Environ. Biol. 2011;5:3795–3799. [Google Scholar]
- Mogadam N., Azizpour A. Ameliorative effect of glucomannan-containing yeast product (Mycosorb) and sodium bentonite on performance and antibody titers against Newcastle disease in broilers during chronic mycotoxin challenge. Afr. J. Biotechnol. 2011;10:17372–17378. [Google Scholar]
- Nedeljković-Trailović J., Trailović S., Resanović R., Milićević D., Jovanovic M., Vasiljevic M. Comparative investigation of the efficacy of three different adsorbents against OTA-induced toxicity in broiler chickens. Toxins. 2015;7:1174–1191. doi: 10.3390/toxins7041174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemati Z., Karimi A., Besharati M. Effects of aflatoxin B1 and yeast cell wall supplementation on the growth performance of broilers. Page 117–120 in Proc. Int'l Conf. Innovations Chemical Agric. Eng; Kuala Lumpur, Malaysia; 2015. [Google Scholar]
- Nešić K., Resanović R., Jakić-Dimić D., Nešić V. Efficiency of various feed additives on the performance of broilers treated with T-2 toxin. Biotechnol. Anim. Husb. 2011;27:705–711. [Google Scholar]
- Pavicic P., Spring P., Fuchs N., Nemanic A. Efficacy of esterified glucomannan to reduce the toxicity of diacetoxyscirpenol in broiler chickens. Proc. 13th European Symp. Poult. Nutr; Blankenberge, Belgium; World's Poultry Science Association; 2001. [Google Scholar]
- Perrone G., Ferrara M., Medina A., Pascale M., Magan N. Toxigenic fungi and mycotoxins in a climate change scenario: ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms. 2020;8:1496. doi: 10.3390/microorganisms8101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A Language and Environment for Statistical Computing. [Google Scholar]
- RStudio Team . RStudio, PBC; Boston, MA: 2021. RStudio: Integrated Development for R.http://www.rstudio.com/ Version 1.4.1106. accessed May 2021. [Google Scholar]
- Raju M.V.L.N., Devegowda G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin) Br. Poult. Sci. 2000;41:640–650. doi: 10.1080/713654986. [DOI] [PubMed] [Google Scholar]
- Raju M.V.L.N., Devegowda G. Esterified-glucomannan in broiler chicken diet contaminated with aflatoxin, ochratoxin and T-2 toxin: evaluation of its binding ability (in vitro) and efficacy as immunomodulator. Asian-Aust. J. Anim. Sci. 2002;15:1051–1056. [Google Scholar]
- Rossi P., Rutz F., de Lima G.J.M.M., Nunes J.K., Anciuti M.A., de Moraes P.V.D., da Silva J.G.C., da Silveira M.H.D., Maier J.C. Effect of sterified glucomannan adsorbent on growth performance and visceral characterization of broiler chickens. R. Bras. Agroc. 2010;16:91–100. [Google Scholar]
- Rothstein H.R., Sutton A.J., Borenstein M. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. John Wiley & Sons; Hoboken, New Jersey: 2005. Publication bias in meta-analysis. [Google Scholar]
- Saki A., Rahmani A., Mahmoudi H., Tabatabaei M.M., Zamani P., Khosravi A.R. The ameliorative effect of Mycosorb in aflatoxin contaminated diet of broiler chickens. J. Livestock Sci. Technol. 2018;6:39–47. [Google Scholar]
- Salami S.A., Moran C.A., Warren H.E., Taylor-Pickard J. Meta-analysis and sustainability of feeding slow-release urea in dairy production. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami S.A., Ross S.A., Patsiogiannis A., Moran C.A., Taylor-Pickard J. Performance and environmental impact of egg production in response to dietary supplementation of mannan oligosaccharide in laying hens: a meta-analysis [e-pub ahead of print] Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell-Kubiak E., Wimmers K., Reyer H., Szwaczkowski T. Genetic aspects of feed efficiency and reduction of environmental footprint in broilers: a review. J. Appl. Genet. 2017;58:487–498. doi: 10.1007/s13353-017-0392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvant D., Schmidely P., Daudin J.J., St-Pierre N.R. Meta-analysis of experimental data in animal nutrition. Anim. 2008;2:1203–1214. doi: 10.1017/S1751731108002280. [DOI] [PubMed] [Google Scholar]
- Silva W.T.M., Nunes R.V., Santin E., Nunes C.G.V., Frank R., Schone R.A., Hofferber T.R. Performance and immune response of broilers fed diets containing corn naturally contaminated with fumonisin. Proc. Alltech's 26th Annual Symp. Biotechnol. Feed Industry; Lexington, KY; 2010. [Google Scholar]
- Sterne J.A.C., Egger M., Smith G.D. Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A.C., Sutton A.J., Ioannidis J.P.A., Terrin N., Jones D.R., Lau J., Carpenter J., Rucker G., Harbord R.M., Schmid C.H., Tetzlaff J., Deeks J.J., Peters J., Macaskill P., Schwarzer G., Duval S., Altman D.G., Moher D., Higgins J.P.T. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomized controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- Swamy H.V.L.N., Smith T.K., Cotter P.F, Boermans H.J., Sefton A.E. Effects of feeding blends of grains naturally contaminated with fusarium mycotoxins on production and metabolism in broilers. Poult. Sci. 2002;81:966–975. doi: 10.1093/ps/81.7.966. [DOI] [PubMed] [Google Scholar]
- Swamy H.V.L.N., Smith T.K., Karrow N.A., Boermans H.J. Effects of feeding blends of grains naturally contaminated with fusarium mycotoxins on growth and immunological parameters of broiler chickens. Poult. Sci. 2004;83:533–543. doi: 10.1093/ps/83.4.533. [DOI] [PubMed] [Google Scholar]
- Szőllősi L., Szűcs I. An economic approach to broiler production. A case study from Hungary. Polish Assoc. Agric. Econ. Agribus. 2014;16:275–281. [Google Scholar]
- Szőllősi L., Béres E., Szűcs I. Effects of modern technology on broiler chicken performance and economic indicators: a Hungarian case study. Italian J. Anim. Sci. 2021;20:188–194. [Google Scholar]
- Tallentire C.W., Mackenzie S.G., Kyriazakis I. Environmental impact trade-offs in diet formulation for broiler production systems in the UK and USA. Agric. Syst. 2017;154:145–156. [Google Scholar]
- Thompson S.G., Turner R.M., Warn D.E. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat. Methods Med. Res. 2001;10:375–392. doi: 10.1177/096228020101000602. [DOI] [PubMed] [Google Scholar]
- Van Limbergen T., Sarrazin S., Chantziaras I., Dewulf J., Ducatelle R., Kyriazakis I., McMullin P., Méndez J., Niemi J.K., Papasolomontos S., Szeleszczuk P., Van Erum J., Maes D. Risk factors for poor health and performance in European broiler production systems. BMC Vet. Res. 2020;16:287. doi: 10.1186/s12917-020-02484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga T.V., Blonk H., Marinussen M., Van Zeist W., Starmans D. Wageningen UR Livestock Research; Wageningen, The Netherlands: 2013. Methodology Used in Feedprint: A Tool Quantifying Greenhouse Gas Emissions of Feed Production and Utilization.http://webapplicaties.wur.nl/software/feedprintNL/index.asp [Google Scholar]
- Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J. Educ. Behav. Stat. 2005;30:261–293. [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48. [Google Scholar]
- Vieira S.L., Santurio J.M., Ott R.P., Viola E.S., Almeida J.G., Eichner G., Quadros V.R. Reduction of mycotoxin synergistic toxicity and histopathological evaluation of broilers after Mycosorb inclusion in the diet. Proc. Alltech's 20th Annual Symp. Biotechnol; Lexington, KY; Feed Industry; 2004. [Google Scholar]
- Von Hippel P.T. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015;15:35. doi: 10.1186/s12874-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.J., Fui S.X., Miao C.H., Feng D.Y. Effects of different mycotoxin adsorbents on performance, meat characteristics and blood profiles of avian broilers fed mold contaminated corn. Asian-Aust. J. Anim. Sci. 2006;19:72–79. [Google Scholar]
- Weaver A.C., Adams N., Yiannikouris A. Use of technology to assess and monitor multimycotoxin and emerging mycotoxin challenges in feedstuffs. Appl. Anim. Sci. 2020;36:19–25. [Google Scholar]
- Weaver A.C., King W.D., Verax M., Fox U., Kudupoje M.B., Mathis G., Lumpkins B., Yiannikouris A. Impact of chronic levels of naturally multi-contaminated feed with fusarium mycotoxins on broiler chickens and evaluation of the mitigation properties of different titers of yeast cell wall extract. Toxins. 2020;12:636. doi: 10.3390/toxins12100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A.C., Weaver D.M., Adams N., Yiannikouris A. Co-Occurrence of 35 mycotoxins: a seven-year survey of corn grain and corn silage in the United States. Toxins. 2021;13:516. doi: 10.3390/toxins13080516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Mitchell N.J. How climate change and regulations can affect the economics of mycotoxins. World Mycotoxin J. 2016;9:653–663. [Google Scholar]
- Yiannikouris A., André G., Poughon L., François J., Dussap C., Jeminet G., Bertin G., Jouany J.-P. Chemical and conformational study of the interactions involved in mycotoxin complexation with β-D-glucans. Biomacromolecules. 2006;7:1147–1155. doi: 10.1021/bm050968t. [DOI] [PubMed] [Google Scholar]
- Yiannikouris A., Apajalahti J., Kettunen H., Ojanperä S., Bell A.N.W., Keegan J.D., Moran C.A. Efficient aflatoxin B1 sequestration by yeast cell wall extract and hydrated sodium calcium aluminosilicate evaluated using a multimodal in-vitro and ex-vivo methodology. Toxins. 2021;13:24. doi: 10.3390/toxins13010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim E., Yalçinkaya İ., Kanbur M., Çinar M., Oruç E. Effects of yeast glucomannan on performance, some biochemical parameters and pathological changes in experimental aflatoxicosis in broiler chickens. Revue. Méd. Vét. 2011;162:413–420. [Google Scholar]
- Zhao J., Shirlet R.B., Dibner J.D., Uraizee F., Officer M., Kitchell M., Vazquez-Anon M., Knight C.D. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poult. Sci. 2010;89:2147–2156. doi: 10.3382/ps.2009-00608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures S1, S2 and S3 are available online