Abstract
Any abnormal activation of primordial follicles and subsequent depletion can irreversibly diminish the ovarian reserve, which is one of the major chemotherapy-induced adverse effects in young patients with cancer. Herein, we investigated the effects of rapamycin on the activation and development of ovarian follicles to evaluate its fertility-sparing therapeutic value in a cyclophosphamide (CTX)-treated mouse model. Based on ovarian histomorphological changes and follicle counting in 50 SPF female C57BL/6 mice, daily administration of 5 mg/kg rapamycin for 30 days was deemed an ideal dosage and duration for administration in subsequent experiments. Compared with the control group, rapamycin treatment inhibited the activation of quiescent primordial follicles, with no obvious side effects observed. Finally, 48 mice were randomly divided into four groups: control, rapamycin-treated, cyclophosphamide-treated, and rapamycin intervention. Body weight, ovarian histomorphological changes, number of primordial follicles, DDX4/MVH expression, apoptosis of follicular cells, and expression of apoptosis protease-activating factor (APAF)-1, cleaved caspase 3, and caspase 3 were monitored. Co-administration of rapamycin reduced primordial follicle loss and the development of follicular cell apoptosis, thereby rescuing the ovarian reserve after CTX treatment. On analyzing the mTOR signaling pathway, we observed that rapamycin significantly decreased CTX-mediated overactivation of mTOR and its downstream molecules. These findings suggest that rapamycin exhibits potential as an ovarian-protective agent that could maintain the ovarian primordial follicle pool and preserve fertility in young female patients with cancer undergoing chemotherapy.
Keywords: Cyclophosphamide, Ovarian reserve, Primordial follicle, Rapamycin
The primordial follicle pool is approximately assembled at birth and represents the female reproductive capacity. Abnormal activation and depletion of primordial follicles within this fixed pool can irreversibly diminish ovarian reserve and premature ovarian insufficiency (POI) [1]. Given that survival rates are gradually increasing in patients with cancer with advances in available treatments, decreasing long-term side effects and improving the quality of life among cancer survivors have drawn increasing attention. POI is a major side effect of chemotherapy in young cancer survivors. The degree of gonadal toxicity and damage to ovarian function depends on the type, dose, drug regimen of the chemotherapeutic agent, patient age, and initial ovarian reserve prior to treatment [2]. Depletion of the residual primordial follicle pool, damage to ovarian stromal components, and compromised ovarian vascularity are considered to be pathological mechanisms of chemotherapy-induced POI [3].
Although embryo or oocyte cryopreservation has proven to be an effective method for fertility preservation, widespread application of this procedure remains limited owing to its time consumption and cost [4]. Ovarian tissue cryopreservation requires surgical intervention to remove ovarian tissue, followed by subsequent auto-transplantation; therefore, transplantation of ovarian tissue for fertility preservation is yet to be performed [5]. More importantly, it may reintroduce abnormal cells into malignant ovarian tumors. To some extent, it highlights the benefits of using gonadotropin-releasing hormone analogs (GnRHa) for suppressing follicle growth and reduction of ovarian blood flow before and during chemotherapy in patients with cancer [6]. However, most assessments have only been performed in female patients with breast cancer and lymphoma, and inconsistencies have been reported in different randomized controlled trials (RCTs) [7]. Folliculogenesis is initiated from the primordial follicle stage to the primary follicle, secondary follicle, and ultimately, the Graafian follicle, also called the preovulatory antral follicle. As primordial follicle activation is gonadotropin-independent [8], suppression of the hypothalamic–pituitary–ovarian (HPO) axis by GnRHa may only protect a fraction of follicles. The benefits of GnRHa intervention benefits remain controversial.
A growing number of studies have focused on the signaling pathways in primordial follicles during chemotherapeutic drug exposure. Cyclophosphamide (CTX), an alkylating agent, has been used as a chemotherapeutic drug for a wide range of cancers, simultaneously inducing ovarian dysfunction, including accelerated loss of ovarian reserve, dysregulated folliculogenesis, and steroid disorder [9]. CTX can cause depletion of primordial follicles, as well as apoptosis and atresia of growing follicles [9]. A study has reported that CTX could activate the growth of the quiescent primordial follicle and subsequently result in a “burnout” effect and follicle depletion by activating the AKT/mTOR pathway in oocytes and granulosa cells [10]. Elevated mTOR activity in oocytes has been shown to activate primordial follicles [11]. In addition, our previous study has shown that CTX induces primordial follicle loss and the development of follicle apoptosis via upregulation of the mTOR signaling pathway [12]. Given the detrimental effects of CTX on ovarian follicles, there is an urgent need to identify protective agents against CTX without obvious side effects. Therefore, the present study supplements our previous findings. Rapamycin is an immunosuppressive drug that is considered a specific inhibitor of the mTOR pathway. Rapamycin could be a potential ovarian-protective agent based on the critical role of the mTOR signaling pathway in primordial follicle activation and growing follicle development. Rapamycin was shown to the follicle pool reserve [13] and protect against Pten-deficiency- or CTX-induced POI [14, 15]. In the present study, we verified the effectiveness of rapamycin in protecting the follicle pool with or without CTX damage, as well as examined different doses and durations of rapamycin treatment along with potential side effects on other organs; this could promote the potential application of rapamycin for ovarian follicle preservation. We aimed to pharmacologically suppress primordial follicle activation to protect the entire follicle pool at the earliest stage, thereby expanding pharmacological fertility preservation. We hope that this mTOR inhibitor can block primordial follicle loss and be employed as an effective and safe intervention against CTX-induced POI.
Materials and Methods
Antibodies and other reagents
Rabbit monoclonal antibodies against mTOR (2983), p-mTOR (S2448) (5536), p70S6K (2708), p-p70S6K (T389) (9234), rpS6 (2217), p-rpS6 (S240/244) (5364), eIF4B (3592), p-eIF4B (S422) (3591), APAF-1 (8969), cleaved caspase 3 (9664), caspase 3 (9662), and β-tubulin (2128) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against DDX4/MVH (ab13840) and Ki-67 (ab66155) were purchased from Abcam (Cambridge, UK). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (7074) was purchased from Cell Signaling Technology. The in situ cell death detection kit was obtained from Roche (Basel, Switzerland). CTX (C7068) was purchased from Sigma-Aldrich (St. Louis, MO, USA), and rapamycin (IPA1021) was purchased from Gene Operation (Ann Arbor, MI, USA).
Animals
Five-week-old SPF C57BL/6 female mice were obtained from Shanghai Slack Laboratory Animal Co., Ltd. (Shanghai, China) and housed under standard laboratory conditions. Rapamycin was dissolved in dimethyl sulfoxide (DMSO) and diluted with normal saline. After acclimatization for 5 days, 50 mice were randomly divided into 4 groups: control group (equal volume saline containing equivalent DMSO), low-dose rapamycin (5 mg/kg), middle-dose rapamycin (10 mg/kg), and high-dose rapamycin (20 mg/kg). All mice were intraperitoneally injected once daily and sacrificed after 15 or 30 days. Detailed comparisons were conducted between the control group and the low-dose rapamycin group after 30 days. A single dose of 120 mg/kg CTX was commonly used to establish a mouse model of ovarian dysfunction[16, 17]. To determine the underlying mechanism of rapamycin against CTX-mediated ovarian dysfunction, 48 mice were randomly divided into four groups: control group (CC: equal volume saline containing equivalent DMSO), rapamycin treatment group (RC: 5 mg/kg rapamycin for 30 days), CTX-treated group (MC: a single dose of 120 mg/kg CTX at day 24), and rapamycin intervention group (RM: 5 mg/kg rapamycin for 30 days with a single dose of 120 mg/kg CTX at day 24). Retroorbital sinus punctures were performed to collect blood samples, and serum was obtained by centrifugation, which was subsequently cryopreserved at −80°C for serum marker detection. Tissues were harvested from mice and immediately placed in 4% paraformaldehyde for histological assessment or stored at −80°C for western blotting. All animal experiments were approved by the Experimental Animal Ethical Committee of Fudan University.
Histomorphology
The ovary, lung, liver, heart, kidney, spleen, and thymus were fixed in 4% paraformaldehyde for 48 h and then dehydrated and embedded in paraffin. Paraffin-embedded tissues were serially sectioned to a thickness of 4 mm. For histomorphological observation, the largest organ sections were then rehydrated and stained with hematoxylin and eosin (HE).
Quantification of ovarian follicles
The ovaries were serially sectioned, and follicles in five random sections of each ovary were counted. Data are presented as the average number of follicles in each section. Primordial and developing follicles were classified as previously described [18]. Briefly, primordial follicles contain an oocyte surrounded by a single layer of squamous granulosa cells. Follicles ranging from secondary to antral follicles are called developing follicles. Secondary follicles contain more than one layer of granulosa cells. Early antral follicles generally possess only one or two small areas of follicular fluid (antrum), whereas antral follicles exhibit a single large antral space. Preovulatory follicles have a rim of cumulus cells surrounding the oocyte.
Immunohistochemical analysis
Briefly, sections were routinely dewaxed, hydrated, incubated in 0.01 M citrate buffer (pH 6.0), and heated in a microwave for 30 min. Endogenous peroxidase activity was inhibited using 3% H2O2 for 10 min. Non-specific binding was blocked with 10% normal goat serum for 60 min. All sections were incubated with primary antibodies against DDX4/MVH (1:200) or Ki-67 (1:100) for 24 h at 4°C. The samples were then incubated for 45 min at room temperature. Antigens were visualized by diaminobenzidine (DAB) staining after incubation with HRP-conjugated secondary antibodies for 30 min. The slides were counterstained with hematoxylin, dehydrated, and mounted. Meanwhile, the negative control was treated by substituting phosphate-buffered saline (PBS) for primary antibodies.
Detection of serum markers
To evaluate the potential toxicity of rapamycin on the liver and kidney, the levels of serum alanine transaminase (ALT), aspartate transaminase (AST), and creatinine (Cr) were measured using corresponding detection kits (Biovision, USA) according to the manufacturer’s instructions.
TUNEL assay
This in situ cell death detection kit was used to detect follicle apoptosis. After deparaffinization and rehydration, the slides were incubated in a freshly prepared 0.1% Triton X-100 permeabilization solution supplemented with 0.1% citrate buffer for 8 min. Next, the slides were immersed in Tris-HCl, 0.1 M (pH 7.5), containing 3% bovine serum albumin and 20% normal bovine serum, for 30 min at room temperature. Then, slides were incubated in 50 μl of TUNEL reaction mixture for 1 h at 37°C. Finally, sections were rinsed for evaluation.
Western blotting
Total protein was extracted from mouse ovaries using RIPA lysis buffer containing the protease inhibitor phenylmethylsulfonyl fluoride (PMSF). Protein content was determined using a BCA Protein Assay Kit. Proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were subsequently incubated in 5% skimmed milk-PBST (PBS containing 0.1% Tween 20) for 1 h and incubated overnight at 4°C with specific antibodies against different proteins. HRP-conjugated goat anti-rabbit IgG was used to detect proteins using enhanced chemiluminescence. β-Tubulin expression was measured as an internal control.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). P-values were determined by performing independent samples t-test between two groups, and one-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test was used to compare parametric variables among three or more study groups. Statistical significance was set at P < 0.05.
Results
Rapamycin reduced primordial follicle activation and follicular development
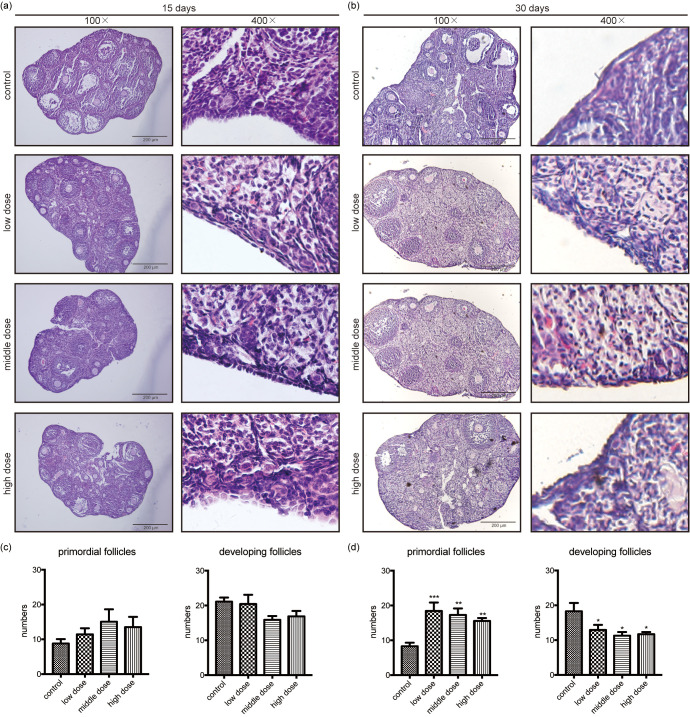
To establish the effects of rapamycin on folliculogenesis, we conducted a pilot study to examine different doses and durations of rapamycin exposure. Mice received intraperitoneal injections of vehicle or different doses of rapamycin (5, 10, or 20 mg/kg) daily for 15 or 30 days. Mice were then sacrificed after administration of the final dose. The ovaries of mice administered different rapamycin doses over 15 days exhibited numerous follicles at different stages, without obvious differences from the control group (Figs. 1a and 1c). Notably, a greater reserve of primordial follicles and a reduction in developing follicles were detected in the ovaries of mice treated with different rapamycin doses for 30 days (Figs. 1b and 1d). Nevertheless, no significant differences were observed among the different rapamycin doses administered for 30 days. Based on our initial observations, 5 mg/kg rapamycin exposure for 30 days demonstrated notable effects on folliculogenesis. Comparing the ovaries of mice treated with the vehicle and 5 mg/kg rapamycin for 30 days, we found that primordial follicles were aggregated, with less reduction noted in the rapamycin group (Fig. 2a). To determine the effect of rapamycin on developing follicles, Ki-67 expression, a marker of growth and proliferation, was assayed and showed an obvious decrease in the number of developing follicles in the rapamycin group (Fig. 2b). Accordingly, 5 mg/kg rapamycin once daily for 30 days was selected as the ideal dosage and duration in the follow-up study.
Fig. 1.
Rapamycin treatment for 30 days inhibits primordial follicle activation and follicle growth. (a) Ovaries from mice treated with different rapamycin doses (0, 5, 10 and 20 mg/kg) for 15 days show no obvious differences from the control group. (b) Ovaries from mice treated with different rapamycin doses (0, 5, 10 and 20 mg/kg) for 30 days present a substantial change in the number of primordial and developing follicles. (c) Based on follicle quantification, no obvious difference can be observed in primordial follicles and developing follicles when treated with different rapamycin doses for 15 days when compared with the control group. (d) An increased primordial follicle reserve and significantly decreased developing follicles can be observed in ovaries treated with different rapamycin doses for 30 days when compared with the control group. Data are presented as mean ± standard error of the mean (SEM). * P < 0.05, ** P < 0.01, *** P < 0.001. Scale bar = 200 μm. 100 ×, magnified 100 times; 400 ×, magnified 400 times.
Fig. 2.
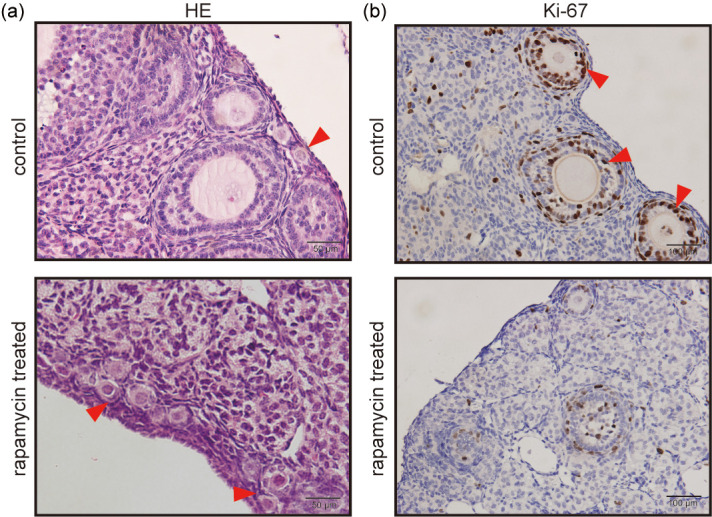
Representative images for HE staining and Ki-67 expression in mouse ovaries treated with rapamycin at 5 mg/kg for 30 days. (a) HE staining shows that primordial follicles are aggregated and reserved more in the rapamycin-treated group. Red arrows indicate primordial follicles. Scale bar = 50 μm. (b) Immunohistochemical analysis of Ki-67 shows a decrease in the number of developing follicles in the rapamycin group. Red arrows indicate Ki-67 positive cells. Scale bar = 100 μm. HE, hematoxylin and eosin.
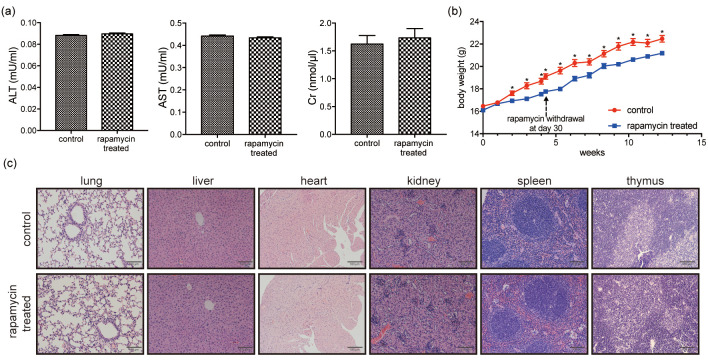
Rapamycin treatment at 5 mg/kg for 30 days induced no obvious side effects
After treatment with 5 mg/kg rapamycin for 30 days, no obvious abnormalities were observed at necropsy. Markers of toxicity were compared between control and rapamycin-treated mice, including body weights, serum levels of ALT, AST, and Cr, and pathohistological examination of primary organs. No statistical difference in serum ALT, AST, and Cr levels was detected between the rapamycin-treated and control groups (Fig. 3a). Histomorphological examination of the main organs showed no gross differences (Fig. 3c). Weight loss occurred due to rapamycin administration; however, body weight increased at similar rates to the control group after withdrawing rapamycin (Fig. 3b).
Fig. 3.
Rapamycin administration at 5 mg/kg for 30 days did not induce obvious side effects. (a) No statistically significant differences in serum ALT, AST, and Cr levels can be observed between the rapamycin-treated and control groups. (b) Body weight curve of mice in rapamycin-treated and control groups: weight loss occurs following rapamycin administration, with weight gain occurring at similar rates to the control group after rapamycin withdrawal at day 30. (c) Histomorphology of the main organs shows no gross differences between the rapamycin-treated and control groups. Scale bar = 100 μm. Data are presented as mean ± SEM. * P < 0.05. ALT, alanine transaminase; AST, aspartate transaminase; Cr, creatinine.
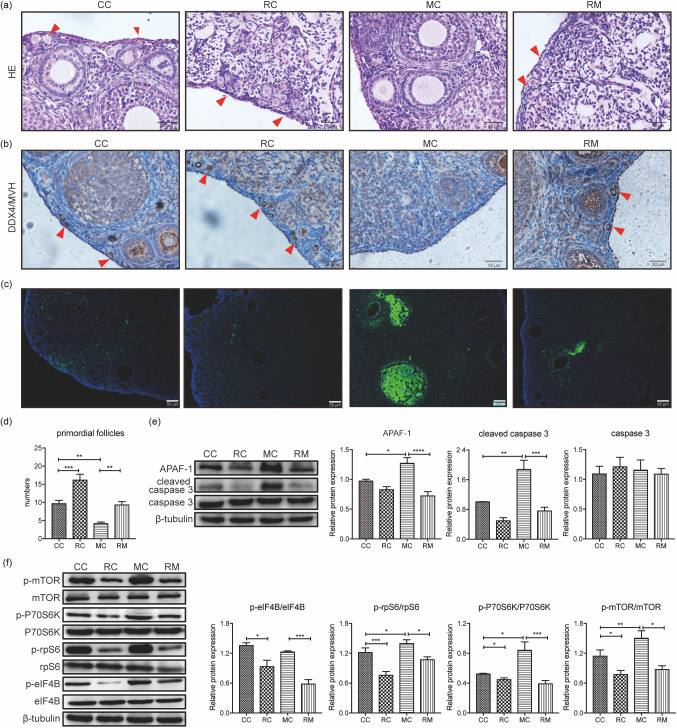
Rapamycin prevented CTX-induced primordial follicle loss and apoptosis of developing follicles
We next examined the impact of rapamycin intervention on CTX-induced primordial follicle loss using female mice pretreated with rapamycin (5 mg/kg) for 30 days. The number of primordial follicles was significantly higher in mice treated with rapamycin alone than in the control group (P < 0.05); these results were in accordance with the ovarian expression of DDX4/MVH (Figs. 4a, b, and d). Compared with the control group, the ovaries of the CTX-treated group showed marked cortical fibrosis, along with a significant reduction in the number of primordial follicles (P < 0.05). Rapamycin administration to CTX-treated mice enhanced DDX4/MVH expression and increased the number of primordial follicles when compared with the CTX-treated group (P < 0.05) (Figs. 4a, b, and d). These results indicated that rapamycin pretreatment significantly rescued primordial follicle loss induced by CTX exposure.
Fig. 4.
Rapamycin prevents CTX-induced primordial follicle loss and apoptosis of developing follicles via the mTOR signaling pathway. (a, b and d) The ovaries of the CTX-treated group show a significant reduction in the number of primordial follicles when compared with the control group. In mice treated with rapamycin alone, the number of primordial follicles is significantly increased when compared with the control group. Compared with the CTX-treated group, rapamycin treatment increases the number of primordial follicles. These results are in line with the ovarian expression of DDX4/MVH. Red arrows mark the primordial follicles. (c) TUNEL-positive cells appear widespread in large developing follicles in the CTX-treated group. Few TUNEL-positive follicle cells can be observed in sections from the control, rapamycin-treated and rapamycin intervention groups. (e) APAF-1 and cleaved caspase-3 levels are notably elevated after CTX treatment, with no change in the total caspase-3 levels. Rapamycin pretreatment could neutralize changes in APAF-1 and cleaved caspase-3. (f) CTX treatment significantly increases mTOR pathway activation, as determined by phosphorylation of mTOR, p70S6K, rpS6, and eIF4B, compared with the untreated control group. Rapamycin pretreatment attenuates the increased phosphorylation of mTOR and its downstream molecules. Data are presented as mean ± standard error of the mean (SEM). * P < 0.05, ** P < 0.01, *** P < 0.001. Scale bar = 50 μm. CC, control group; RC, rapamycin-treated group, 5 mg/kg rapamycin for 30 days; MC, CTX-treated group, a single dose of 120 mg/kg CTX at day 24; RM, rapamycin intervention group, 5 mg/kg rapamycin for 30 days with a single dose of 120 mg/kg CTX at day 24. CTX, cyclophosphamide.
As shown in Fig. 4c, TUNEL staining of ovarian sections from the rapamycin intervention group contained a reduced number of apoptotic developing follicles, especially granulosa cells, compared with ovaries from mice that received CTX alone, which showed widespread TUNEL-positive cells in large developing follicles. Few TUNEL-positive follicle cells were detected in sections from control or rapamycin-treated groups, where no gross apoptotic death was observed in pre-granulosa cells or oocytes of primordial follicles in any ovary. The same positive trend was observed considering the expression of apoptotic markers, such as APAF-1 and cleaved caspase-3 (Fig. 4e). Following CTX exposure, APAF-1 and cleaved caspase-3 levels were elevated without changes in total caspase-3 levels (Fig. 4e); however, rapamycin pretreatment significantly reversed this increase in apoptotic markers. Collectively, these results demonstrated that CTX treatment could induce hyperactivation of the developing follicle apoptosis, and rapamycin pretreatment could neutralize these changes to some extent.
Rapamycin alleviated the CTX-induced overactivation of the mTOR signaling pathway
To examine the molecular evidence of increased primordial follicle loss and apoptosis of developing follicle apoptosis after CTX and rapamycin treatment, we analyzed the effects of treatments on the mTOR signaling pathway. Protein analysis of whole ovaries was conducted to assess changes in mTOR phosphorylation and its downstream molecules. CTX treatment significantly stimulated mTOR pathway activity, as shown by the phosphorylation of mTOR, p70S6K, rpS6, and eIF4B, compared with the untreated control group. Pretreatment with rapamycin effectively attenuated the phosphorylation of mTOR and its downstream molecules (Fig. 4f). These results revealed that rapamycin could alleviate CTX-induced activation of the mTOR pathway, indicating the protective effect of rapamycin against CTX via the mTOR pathway.
Discussion
It is well-established that chemotherapy improves the survival rates of patients with malignant tumors; however, gonadal injury remains a major side effect. Several techniques, such as embryo and oocyte cryopreservation, ovarian tissue cryopreservation, and ovarian transposition, have been reported for fertility preservation. Although these techniques could help adult females and girls undergo chemotherapy, risks and limitations are known to persist [19]. The total number of primordial follicles is determined before birth, and primordial follicles remain dormant for years or decades. The activation of primordial follicles and follicle development consist of multiple stages. To develop new pharmaceutical agents for preserving the ovarian reserve, it is necessary to understand the mechanisms mediating chemotherapy-induced follicle loss. In the present study, we observed that rapamycin could reduce primordial follicle activation and growth of developing follicles without obvious side effects. Moreover, rapamycin could protect the ovarian reserve against CTX-induced primordial follicle loss and apoptosis of developing follicles. Additionally, our findings suggested that the mTOR signaling pathway participates in the regulation of primordial follicle activation. The balance of the mTOR signaling pathway is important for maintaining the primordial follicle pool, which has also been extensively studied using genetic models [20, 21]. Accordingly, we demonstrated that the mTOR inhibitor rapamycin played an important role in primordial follicle activation and apoptosis of developing follicles, and pretreatment with rapamycin could rescue CTX-induced primordial follicle loss and apoptosis of developing follicles (Fig. 5).
Fig. 5.
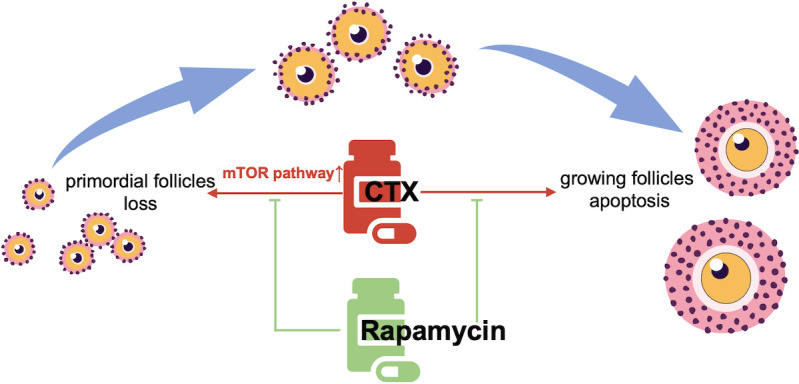
Illustrative diagram showing the protective function of rapamycin on CTX-induced ovarian reserve injury. CTX exposure causes loss of primordial follicles and apoptosis in developing follicles via activation of the mTOR signaling pathway. Rapamycin intervention alleviates the overactivation of the mTOR signaling pathway, thereby protecting the primordial follicle pool and reducing apoptosis of developing follicles. CTX, cyclophosphamide.
First, we showed that daily administration of rapamycin promoted follicular quiescence and maintained the ovarian primordial follicle pool. Mouse ovaries contain only primordial follicles at birth, and the entire process from the primordial to the large antral follicle stage, containing fully grown oocytes, requires 18–24 days [22]. Herein, the ovaries of mice treated with rapamycin for 30 days exhibited no obvious change in the number of primordial and developing follicles. However, the number of primordial follicles was significantly increased in the ovaries of rapamycin-treated mice for 30 days, while the number of developing follicles was decreased. Furthermore, Ki-67 staining results revealed that the growth of some developing follicles was arrested following rapamycin treatment. Dou et al. have reported that 2 weeks of rapamycin administration could preserve primordial follicles, increase oocyte quality, and improve the ovarian inflammatory microenvironment, expanding ovarian lifespan [23]. Accumulated data suggest that rapamycin inhibits primordial follicle activation and subsequent follicle growth, and rapamycin 5 mg/kg once daily for 30 days might confer a preventive effect against the rapid burnout of primordial follicles, thereby protecting the ovarian reserve.
However, whether CTX-induced primordial follicle loss could be attributed to a burnout effect following activation or direct rapid apoptosis remains controversial. Some studies have suggested that CTX causes apoptosis of primordial follicles directly by damaging the double-strand DNA of primordial follicles [3, 24]. Studies have also indicated that the ‘burnout’ effect could induce a reduction in primordial follicles following the activation and growth initiation of primordial follicles, as determined by the changing pattern of follicle numbers and TUNEL-negative primordial follicles even at 4 h/12 h/24 h and 3/7 days post-CTX exposure [10, 14, 25]. Despite the lack of direct evidence, numerous researchers have admitted the rationality of both theories and proposed a mechanism by which oocytes abandon their follicular quiescence to initiate DNA damage signaling pathways resulting in oocyte apoptosis [10, 25,26,27]. Consistent with previous studies [10, 14], we did not observe primordial follicle apoptosis on day 7 after CTX exposure. Nonetheless, the reduced number of TUNEL-positive cells and decreased levels of APAF-1 and cleaved caspase-3 in the rapamycin intervention group indicated that the addition of rapamycin reduced the CTX-induced apoptosis of developing follicles, which contributed to the protection of ovarian function. Since CTX has been proven to damage growing follicles, the negative regulation from growing follicles controlling dormant follicle activation could be disturbed. It is possible that the primordial follicles were no longer in a dormant state but actually in a ‘ready’ state to activate, leaving them relatively vulnerable to apoptosis. As rapamycin can preserve the follicle pool reserve by inhibiting follicle activation, future studies are warranted to determine whether it maintains the primordial follicle pool against CTX damage in this general manner.
We further elucidated the critical role of the mTOR signaling pathway in fertility preservation. In addition to its well-known role in cell growth, proliferation, mRNA translation, autophagy, and nutrient signaling, the mTOR signaling pathway is a key regulator of follicle activation and growth [28]. Follicular activation may be initiated by upregulation of the mTOR signaling pathway in pre-granulosa cells [29]. In addition, the balanced activity of mTORC1, a major molecule of the mTOR signaling pathway, in mouse oocytes, is indispensable for the physiological process of primordial follicle activation via upregulation of cyclin A expression and subsequent activation of certain cyclin-dependent kinases in oocytes [30]. Our study also found progressive activation of primordial follicles in CTX-treated ovaries, with overactivation of mTOR, p70S6K, rpS6, and eIF4B. Rapamycin, a specific mTOR inhibitor, effectively blocks the phosphorylation of mTOR and its downstream molecules. However, the mechanism through which upstream mTOR signaling governs the activation of primordial follicles remains elusive. Zhang et al. have suggested that KITL functions downstream of mTOR in somatic cells to regulate their communication with oocytes during follicle formation [31].
Rapamycin was first produced from several actinomycete species and used as an immunosuppressive compound [32]. With progressive research, rapamycin and its analogs were shown to exhibit antitumor potential [33, 34]. In addition to their fertility-protective activity, patients with cancer may benefit from their simultaneous antitumor effects. Furthermore, rapamycin administration did not induce notable side effects on other organs based on preliminary evaluations, as determined by assessing serum ALT, AST and Cr levels and pathohistological examination. However, weight loss was observed during the rapamycin administration, although body weight increased again at a rate similar to normal mice. Another study in mice has shown that 4 mg/kg of rapamycin biweekly for 93 days slightly reduces body weight, although this finding was not statistically significant [35]. The rapamycin-mediated reduction in food intake and induction of caloric restriction conditions could explain this finding [13]. Interestingly, caloric restriction can significantly enhance the follicle pool reserve by suppressing the activation of primordial follicles and the development of follicles at different stages [35, 36]. It is worth investigating whether rapamycin protects the ovarian reserve under caloric restriction conditions. In general, there is no obvious restriction on the use of rapamycin for ovarian reserve preservation, which could save time for patients with cancer when compared with techniques such as embryo and oocyte cryopreservation.
In conclusion, this study identified the role of rapamycin in terms of affording protection against CTX-induced ovarian damage by suppressing phosphorylation of the mTOR signaling pathway. Moreover, studies have found that solely freezing and thawing ovarian tissue can significantly increase the expression of the mTOR signaling pathway in primordial follicles. Conversely, rapamycin-mediated suppression of the mTORC1 pathway could preserve the primordial follicle pool [37]. This finding indicates that altered expression of activator proteins regulating the primordial follicle reserve and growth may lead to primordial follicle loss and that rapamycin treatment can protect the ovarian reserve after chemotherapy and ovarian tissue cryopreservation and transplantation. Although the underlying mechanisms require further investigation, this study provides novel insights into fertility preservation by regulating primordial follicle activation in females.
Conflicts of interests
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81771587).
References
- 1.De Vos M, Devroey P, Fauser BCJM. Primary ovarian insufficiency. Lancet 2010; 376: 911–921. [DOI] [PubMed] [Google Scholar]
- 2.Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 2010; 53: 727–739. [DOI] [PubMed] [Google Scholar]
- 3.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011; 3: 782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinolas C, Raad J, Sonigo C, Sifer C, Sermondade N, Grynberg M. Medical techniques of fertility preservation in the male and female. J Visc Surg 2018; 155(Suppl 1): S3–S9. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Lee Y, Lee S, Kim T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet Gynecol Sci 2018; 61: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonard RCF, Adamson DJA, Bertelli G, Mansi J, Yellowlees A, Dunlop J, Thomas GA, Coleman RE, Anderson RA, Anglo Celtic Collaborative Oncology Group and National Cancer Research Institute Trialists.GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol 2017; 28: 1811–1816. [DOI] [PubMed] [Google Scholar]
- 7.Blumenfeld Z. Fertility preservation and GnRHa for chemotherapy: debate. Cancer Manag Res 2014; 6: 313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction 2009; 137: 1–11. [DOI] [PubMed] [Google Scholar]
- 9.Qin X, Zhao Y, Zhang T, Yin C, Qiao J, Guo W, Lu B. TrkB agonist antibody ameliorates fertility deficits in aged and cyclophosphamide-induced premature ovarian failure model mice. Nat Commun 2022; 13: 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med 2013; 5: 185ra62. [DOI] [PubMed] [Google Scholar]
- 11.Adhikari D, Zheng W, Shen Y, Gorre N, Hämäläinen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet 2010; 19: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen XY, Xia HX, Guan HY, Li B, Zhang W. Follicle loss and apoptosis in cyclophosphamide-treated mice: what’s the matter? Int J Mol Sci 2016; 17: 836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene 2013; 523: 82–87. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Xie Y, Li S, Liang Y, Qiu Q, Lin H, Zhang Q. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res 2017; 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adhikari D, Risal S, Liu K, Shen Y. Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS One 2013; 8: e53810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai X, Yi X, Wang Y, Xia W, Tao J, Wu J, Miao D, Chen L. PQQ dietary supplementation prevents alkylating agent-induced ovarian dysfunction in mice. Front Endocrinol (Lausanne) 2022; 13: 781404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Y, Xu Y, Wang Y. Protective roles and mechanisms of rosmarinic acid in cyclophosphamide-induced premature ovarian failure. J Biochem Mol Toxicol 2020; 34: e22591. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 1968; 17: 555–557. [DOI] [PubMed] [Google Scholar]
- 19.Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2018; 36: 1994–2001. [DOI] [PubMed] [Google Scholar]
- 20.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008; 319: 611–613. [DOI] [PubMed] [Google Scholar]
- 21.Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle 2010; 9: 1673–1674. [DOI] [PubMed] [Google Scholar]
- 22.Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci USA 2002; 99: 2890–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dou X, Sun Y, Li J, Zhang J, Hao D, Liu W, Wu R, Kong F, Peng X, Li J. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell 2017; 16: 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oktem O, Oktay K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res 2007; 67: 10159–10162. [DOI] [PubMed] [Google Scholar]
- 25.Bellusci G, Mattiello L, Iannizzotto V, Ciccone S, Maiani E, Villani V, Diederich M, Gonfloni S. Kinase-independent inhibition of cyclophosphamide-induced pathways protects the ovarian reserve and prolongs fertility. Cell Death Dis 2019; 10: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Long H, Cong Y, Gao H, Lyu Q, Yu S, Kuang Y. Quercetin prevents primordial follicle loss via suppression of PI3K/Akt/Foxo3a pathway activation in cyclophosphamide-treated mice. Reprod Biol Endocrinol 2021; 19: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedoschi GM, Navarro PA, Oktay KH. Novel insights into the pathophysiology of chemotherapy-induced damage to the ovary. Panminerva Med 2019; 61: 68–75. [DOI] [PubMed] [Google Scholar]
- 28.Sulaimanov N, Klose M, Busch H, Boerries M. Understanding the mTOR signaling pathway via mathematical modeling. Wiley Interdiscip Rev Syst Biol Med 2017; 9: e1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update 2015; 21: 779–786. [DOI] [PubMed] [Google Scholar]
- 30.Tong Y, Li F, Lu Y, Cao Y, Gao J, Liu J. Rapamycin-sensitive mTORC1 signaling is involved in physiological primordial follicle activation in mouse ovary. Mol Reprod Dev 2013; 80: 1018–1034. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Liu W, Sun X, Kong F, Zhu Y, Lei Y, Su Y, Su Y, Li J. Inhibition of mTOR signaling pathway delays follicle formation in mice. J Cell Physiol 2017; 232: 585–595. [DOI] [PubMed] [Google Scholar]
- 32.Yoo YJ, Kim H, Park SR, Yoon YJ. An overview of rapamycin: from discovery to future perspectives. J Ind Microbiol Biotechnol 2017; 44: 537–553. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Zhang L, Zhang X, Xing X. Rapamycin enhanced the antitumor efficacy of oxaliplatin in cisplatin-resistant ovarian cancer cells A2780cis both in vitro and in vivo. J Chemother 2015; 27: 358–364. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Gao L, Liu X, Yuan C, Wang G. Improved antitumor effect of ionizing radiation in combination with rapamycin for treating nasopharyngeal carcinoma. Oncol Lett 2017; 14: 1105–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia DN, Saccon TD, Pradiee J, Rincón JAA, Andrade KRS, Rovani MT, Mondadori RG, Cruz LAX, Barros CC, Masternak MM, Bartke A, Mason JB, Schneider A. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience 2019; 41: 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo LL, Chen XC, Fu YC, Xu JJ, Li L, Lin XH, Xiang YF, Zhang XM. The effects of caloric restriction and a high-fat diet on ovarian lifespan and the expression of SIRT1 and SIRT6 proteins in rats. Aging Clin Exp Res 2012; 24: 125–133. [DOI] [PubMed] [Google Scholar]
- 37.Celik S, Ozkavukcu S, Celik-Ozenci C. Altered expression of activator proteins that control follicle reserve after ovarian tissue cryopreservation/transplantation and primordial follicle loss prevention by rapamycin. J Assist Reprod Genet 2020; 37: 2119–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]