Abstract
The degradation of the flavonol quercetin and the flavone luteolin by Eubacterium ramulus, a strict anaerobe of the human intestinal tract, was studied. Resting cells converted these flavonoids to 3,4-dihydroxyphenylacetic acid and 3-(3,4-dihydroxyphenyl)propionic acid, respectively. The conversion of quercetin was accompanied by the transient formation of two intermediates, one of which was identified as taxifolin based on its specific retention time and UV and mass spectra. The structure of the second intermediate, alphitonin, was additionally elucidated by 1H and 13C nuclear magnetic resonance analysis. In resting-cell experiments, taxifolin in turn was converted via alphitonin to 3,4-dihydroxyphenylacetic acid. Alphitonin, which was prepared by enzymatic conversion of taxifolin and subsequent purification, was also transformed to 3,4-dihydroxyphenylacetic acid. The coenzyme-independent isomerization of taxifolin to alphitonin was catalyzed by cell extract or a partially purified enzyme preparation of E. ramulus. The degradation of luteolin by resting cells of E. ramulus resulted in the formation of the intermediate eriodictyol, which was identified by high-performance liquid chromatography and mass spectrometry analysis. The observed intermediates of quercetin and luteolin conversion suggest that the degradation pathways in E. ramulus start with an analogous reduction step followed by different enzymatic reactions depending on the additional 3-hydroxyl group present in the flavonol structure.
Flavonoids are polyphenolic compounds which are present in foods and beverages of plant origin. The daily intake of flavonoids calculated on the basis of the aglycones was estimated to range from approximately 3 to 70 mg in different countries, and it may well exceed these values in regions with a very high intake of tea and vegetables (5, 10, 13). In vivo data on absorption and metabolism after oral intake are contradictory. However, a major part of ingested flavonoids are not absorbed and are largely degraded by the intestinal microflora.
It was shown in vitro that flavonoids are potent antioxidants and inhibitors of ubiquitous enzymes, and their anticarcinogenic properties were demonstrated with different cell lines (for a review, see reference 8). Due to these properties, flavonoids are reported to protect against cancer, coronary heart disease, and stroke. In order to judge the potential beneficial health effects of flavonoids in humans, studies on their fate in the gastrointestinal tract, including transformation by bacteria, are necessary. Intestinal bacteria play important roles not only in deconjugation of flavonoids but also in their further degradation. The bacterial metabolites, which possibly exert biological activities different from those of the original flavonoids, may be absorbed and further metabolized in the human body. Therefore, it is essential to study their conversion by intestinal bacteria and to identify and characterize the fermentation products formed. Although some flavonoid-degrading species, their substrates, and some of the final products are known (2, 15, 18, 22), information on the anaerobic degradation pathways, intermediates, and the enzymes involved is lacking.
Eubacterium ramulus, a strict anaerobe resident in the human intestinal tract, grows with quercetin-3-glucoside (isoquercitrin) as the sole carbon and energy source. The only intermediates detected in this degradation were quercetin and phloroglucinol, the fermentation products being 3,4-dihydroxyphenylacetic acid, butyrate, and acetate (20). Furthermore, E. ramulus was found to be able to split the ring system of several other flavonols and flavones, forming the corresponding hydroxyphenylacetic and hydroxyphenylpropionic acids, respectively. Degradation pathways of flavonols and flavones were proposed, which include reduction of the heterocyclic C-ring of the aglycon, yielding dihydroflavonols and flavanones, respectively, followed by ring fission. Cleavage of the resulting chalcones might subsequently give rise to the respective phenolic acids (19). E. ramulus was detected in fecal samples from each of 20 persons tested at cell numbers which average 0.16% of the total flora (21). Therefore, E. ramulus may be considered a common inhabitant of the human intestine and a key organism for flavonoid degradation in this habitat.
In order to test the proposed flavonoid degradation pathways, the fermentation of the flavonol quercetin and the flavone luteolin by resting cells of E. ramulus was studied. In this report we describe the detection and identification of intermediates of quercetin and luteolin degradation, respectively.
MATERIALS AND METHODS
Organism.
E. ramulus strain wK1, previously isolated from a human fecal sample (20), was used throughout the study. The organism will be made available upon request.
Chemicals.
Quercetin, luteolin, and eriodictyol were purchased from Roth (Karlsruhe, Germany), taxifolin was purchased from Sigma (Deisenhofen, Germany), and 3,4-dihydroxyphenylacetic acid and 3-(3,4-dihydroxyphenyl)propionic acid were purchased from Fluka (Deisenhofen, Germany). High-pressure liquid chromatographic (HPLC)-grade methanol (Fluka) was used throughout the experiments.
Growth media and anoxic techniques.
The anoxic techniques were essentially those of Hungate (11) and Bryant (3). A gas phase of N2-CO2 (80:20, vol/vol) was used. The anoxic workstation (MK 3; DW Scientific, Shipley, Great Britain) had a gas phase of N2-CO2-H2 (80:10:10, vol/vol/vol). E. ramulus strain wK1 (20) was grown under strictly anoxic conditions in tubes fitted with butyl-rubber stoppers and screw caps. The medium (ST medium) contained the following compounds per liter: 9 g of tryptically digested meat peptone, 1 g of proteose peptone, 3 g of meat extract, 4 g of yeast extract, 6 g of glucose, 3 g of NaCl, 2 g of Na2HPO4, 0.5 ml of Tween 80, 0.25 g of cystine, 0.25 g of l-cysteine–HCl, 0.1 g of MgSO4 · 7 H2O, 5 mg of FeSO4 · 7 H2O, and 3.4 mg of MnSO4 · 2 H2O. The pH after autoclaving at 121°C for 20 min was between 6.8 and 7.1.
Preparation of resting cell suspensions and degradation experiments.
The E. ramulus cultures grown overnight in ST medium were transferred into the anoxic workstation and were prepared for centrifugation (10,000 × g, 15 min). After centrifugation, the cells were washed once with 50 mM potassium phosphate buffer (pH 6.9) containing either 1.4 mM cysteine or 5 mM dithiothreitol, and the pellet was resuspended in the same buffer to an optical density indicated in the experiments. Aliquots of this cell suspension were each transferred into 250-ml serum bottles and used for the resting-cell experiments.
Degradation experiments were performed by adding defined amounts of flavonoids dissolved in dimethyl sulfoxide (DMSO) with a syringe. The bottles were incubated at either 37 or 19°C in a water bath equipped with a rotary shaker (120 rpm). At different times, aliquots were taken with a syringe and immediately centrifuged (12,000 × g, 5 min), and the supernatant was directly analyzed by HPLC. The pellets were lyophilized (Alpha 2-4; Christ, Osterode, Germany) and dissolved in dimethylformamide or methanol for further analysis by HPLC. For a comparison, the supernatant and the pellet together were lyophilized and dissolved in the same solvents for further analysis by HPLC.
Preparation of cell extracts and partially purified enzyme preparations.
The cell extracts were prepared in the presence or absence of oxygen at 4°C from E. ramulus cultures grown overnight in ST medium supplemented with 0.1 mM quercetin. The cells were centrifuged (10,000 × g, 15 min), washed once with 50 mM potassium phosphate buffer (pH 6.9), resuspended in the same buffer supplemented with DNase, and ruptured by twofold passage through a French pressure cell at 130 MPa (SLM Instruments, Rochester, N.Y.). Cell extracts (average, 15 mg of protein/ml) were obtained by centrifugation at 18,000 × g for 20 min. The cytoplasmic fraction was prepared by centrifugation at 100,000 × g for 45 min.
The enzyme enrichment was performed at 4°C under aerobic conditions using a fast-performance liquid chromatography system (Amersham Pharmacia Biotech, Freiburg, Germany). The cytoplasmic fraction (average, 13 mg of protein/ml) was loaded onto a DEAE-Sephacel (Amersham Pharmacia Biotech) column (6 by 2.5 cm) equilibrated with 50 mM potassium phosphate buffer (pH 7.2). Elution was done with a gradient of KCl (0 to 1 mM) in 50 mM potassium phosphate buffer (pH 7.2) at a flow rate of 2 ml/min. Fractions with taxifolin-transforming activity were used for the characterization of the taxifolin transformation and the preparation of alphitonin.
Determination of the taxifolin-transforming activity.
Taxifolin transformation was detected by HPLC analysis. The assay contained 60 μM taxifolin (added from a 1.2 mM stock solution in DMSO) in 50 mM potassium phosphate buffer (pH 6.9). The final DMSO concentration was 5%. The reaction was started by the addition of cell extract (average, 150 μg of protein), soluble enzyme fraction (average, 130 μg of protein), or partially purified enzyme preparation (average, 42 μg of protein), respectively. The assay was performed in the presence or absence of oxygen at room temperature. For HPLC analysis, samples were taken at different times and mixed with one volume of methanol-H2O-acetic acid (50:45:5, vol/vol/vol) to stop the reaction. Control reactions were devoid of enzyme or contained enzyme preparations inactivated by incubation for 1 h at 50°C.
HPLC.
Flavonoids and aromatic metabolites were measured using an HPLC system with a diode array detector (Gynkotek, Munich, Germany). The HPLC system was equipped with a pump Model 480, degasser ERC-5535, autosampler GINA 160, a column oven, a diode array detector UVD-320, and a reversed-phase C18 column (LiChroCART 250-4 LiChrospher 100 RP-18, 5 μm; 250 by 4 mm; Merck, Darmstadt, Germany). The column temperature was maintained at 37°C. Aqueous 0.1% trifluoroacetic acid (TFA) (solvent system A) and methanol (solvent system B) served as the mobile phase in a gradient mode (B from 5 to 30% in 20 min, from 30 to 50% in 5 min, from 50 to 80% in 10 min, from 80 to 100% in 4 min) with a flow rate of 1 ml/min and detection at 280 nm. TFA was replaced by aqueous 1.6% formic acid (FA) for isolation of metabolites to be analyzed by mass spectrometry. All compounds except alphitonin were identified by their retention times and UV spectra (λ = 200 to 355 nm) in comparison to reference substances. Calibration curves were used for quantification.
Sample preparation for ESI-MS.
Selected incubation supernatants from degradation experiments were used for identification of the different compounds by electrospray ionization mass spectrometry (ESI-MS). The samples (250 μl) were run on the HPLC system using the FA-methanol gradient, and the different peaks were manually collected for further analysis.
Mass spectrometry.
Coupled HPLC-ESI-MS or ESI-MS using flow injection (10 μl/min) was performed depending on the purity and concentration of the samples. Moreover, collision-induced dissociation tandem mass spectrometry was carried out to obtain a specific fragmentation.
For analysis, a triple quadrupole mass spectrometer fitted with a Z-spray API electrospray source (Quattro II; Micromass, Manchester, United Kingdom) was used. The HPLC system (2960; Waters, Milford, Mass.) was equipped with a reversed-phase C18 column (LiChroCART 250-4 LiChrospher 100 RP-18, 5 μm; 250 by 4 mm; Merck) and a 996 PDA detector. The mobile phase was a gradient of aqueous formic acid and methanol similar to those used for isolation of the metabolites (described above) with a flow rate of 0.5 ml/min and was split 6:1 prior to introduction into the mass spectrometer. MS analyses were carried out in either positive or negative ionization mode. The temperature of the ion source was maintained at 100°C. The desolvation temperature was 350°C, and the desolvation gas N2 had a flow rate of 400 liters/h. The cone and capillary voltage used for the analysis of quercetin and luteolin were 50 V and 3.7 kV, respectively, and 20 V and 3.0 kV for the analysis of the metabolites. Product ion scans of [M+H]+ were performed at low-energy collisions (15 to 30 eV) using argon as the collision gas (1.5 × 102 to 2.8 × 102 mPa). The obtained molecular ion peaks and mass spectra were compared to those of reference substances.
In parallel to NMR analyses, the alphitonin and taxifolin preparations were subjected to MS with electron impact ionization (EI-MS, 70 eV) using a Varian MAT CH6 spectrometer (Varian, Palo Alto, Calif.). EI-MS of taxifolin (m/z, percent): 304 (M+, 28), 275 (32), 153 (92), 123 (68). EI-MS of alphitonin (m/z, %): 304 (M+, 12), 126 (100), 123 (75).
Preparation of alphitonin for NMR analysis.
Taxifolin (8.8 mg) was incubated with 4 ml of the partially purified taxifolin-transforming preparation until no further alphitonin formation was observed (210 min). Samples of maximally 250 μl each were injected onto the HPLC column. Alphitonin and the nontransformed taxifolin were separated using the TFA-methanol gradient. The fractions of both substances were manually collected, pooled, and dried by vacuum centrifugation (RC 10.22.; Jouan, Saint-Nazaire, France).
NMR analysis.
1H NMR spectra (300 MHz) and 13C NMR spectra (75 MHz) were recorded on a Varian Gemini 300 in DMSO-d6. 13C NMR signals were assigned on the basis of attached proton test (APT). Nontransformed taxifolin, which was obtained from the alphitonin preparation as described above, and commercially available taxifolin gave identical 1H NMR spectra. 1H NMR of taxifolin: δ 4.51 (dd, J = 11.2, 6.1 Hz, 1H, 3-H), 5.00 (d, J = 11.2 Hz, 1H, 2-H), 5.76 (d, J = 6.1 Hz, 1H, 3-OH), 5.88, 5.93 (each d, J = 2.0 Hz, 2H, 6-H, 8-H), 6.76 (s, br, 2H, 5′-H, 6′-H), 6.89 (s, br, 1H, 2′-H), 8.97, 9.03, 10.82, 11.91 (each s, 4H, OH). 1H NMR of alphitonin: δ 2.82, 2.88 (each d, J = 14.0 Hz, 2H, CH2), 5.73, 5.79 (each d, J = 1.8 Hz, 2H, 5-H, 7-H), 6.39 (dd, J = 8.2, 1.9 Hz, 1H, 6′-H), 6.51 (d, J = 8.2 Hz, 1H, 5′-H), 6.56 (d, J = 1.9 Hz, 1H, 2′-H). 13C NMR of alphitonin: δ 41.55 (CH2), 90.39, 96.39 (C-5, C-7), 101.98 (C-2), 106.19 (C-3a), 115.65, 118.59 (C-2′, C-5′), 121.93 (C-6′), 125.66 (C-1′), 144.45, 154.08 (C-3′, C-4′), 158.64 (C-7a), 168.69, 172.56 (C-4, C-6), 193.60 (C-3).
RESULTS
In order to elucidate the pathways of flavonol and flavone transformation by E. ramulus, the fermentation of quercetin and luteolin by resting cells was investigated. Flavonoid conversion was followed by analysis of samples by HPLC. The individual samples were centrifuged, and the pellets were lyophilized and dissolved in methanol. Dimethylformamide was less suitable as the solvent. Both the resulting solution and the supernatant were examined. This method was found to be advantageous compared to the lyophilization of the whole sample because of a better recovery. The detected metabolites were identified by their specific retention times using two different HPLC gradients, and their UV spectra were recorded in the TFA-methanol system. In addition, mass spectrometry was applied for confirmation of the results.
In the first fermentation experiments using resting cells, only the final products of flavonoid degradation were observed (data not shown). However, intermediates could be observed by taking the samples immediately after adding the substrates and decreasing the cell density and the incubation temperature.
Quercetin degradation by resting cells of E. ramulus.
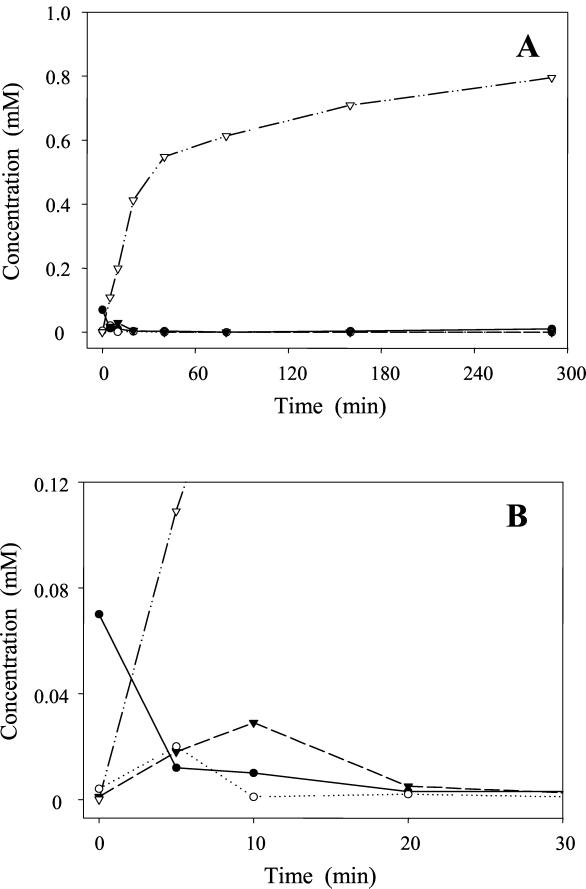
Resting-cell suspensions of E. ramulus degraded 1 mM quercetin to produce 0.8 mM 3,4-dihydroxyphenylacetic acid within 4.8 h (Fig. 1A). Retention times of quercetin were 31.5 min with the TFA-methanol gradient (UV spectrum, λmax = 260 nm) and 31.1 min with the FA-methanol gradient. Because of its low solubility in buffer, only small amounts of quercetin were recovered from the supernatant of the cell suspension. In contrast, 3,4-dihydroxyphenylacetic acid appeared exclusively in the supernatant. The identity of the latter compound was confirmed on the basis of its retention times (11.6 min, TFA-methanol gradient; 10.5 min, FA-methanol gradient) and UV spectrum (λmax = 286 nm) compared to the reference substance. MS analysis (flow injection) of the product gave the expected [M+H]+ of m/z 169 in the positive mode and [M-H]− of m/z 167 in the negative mode. The loss of 44 (CO2) resulting in the in-source fragment ion m/z 123 was observed in the negative mode and represents a typical fragmentation of phenolic acids (12). The degradation of quercetin was accompanied by the formation of two transient soluble intermediates (Fig. 1B): one of these was identified as taxifolin based on its retention times (25.4 min, TFA-methanol gradient; 23.9 min, FA-methanol gradient) and UV spectrum (λmax = 294 nm), which were identical to those of the reference substance. ESI-MS analysis showed the respective molecular ion peak of m/z 305 [M+H]+. The second intermediate with clearly different retention times of 17.8 min (TFA-methanol gradient) and 16.3 min (FA-methanol gradient) exhibited a UV spectrum (λmax = 295 nm) similar to that of taxifolin. ESI-MS analysis (LC-MS and direct injection) of this second intermediate resulted in an ion peak of m/z 305 [M+H]+ which was identical to that of taxifolin. However, in comparison to taxifolin a high rate of in-source fragmentation occurred, characterized by relatively high intensities of m/z 287 and m/z 259, indicating the loss of water and CO, respectively (data not shown). For further characterization, the compound was purified and the structure was elucidated by 1H and 13C NMR analysis (see below). It was unambiguously identified as alphitonin [2-(3,4-dihydroxbenzyl)-2,4,6-trihydroxybenzofuran-3-one], an isomeric form of taxifolin. The MS/MS spectrum of alphitonin is shown in comparison to that of taxifolin (Fig. 2). A reference substance was not available.
FIG. 1.
(A). Time course of quercetin degradation by resting cells of E. ramulus (optical density at 600 nm, 12; temperature, 37°C). The initial concentration of quercetin was 1 mM. The concentrations of quercetin (●), taxifolin (○), alphitonin (▾), and 3,4-dihydroxyphenylacetic acid (▿) in the supernatant are shown. It should be noted that the majority of the quercetin was recovered from the pellet. (B). Initial phase of the degradation to show the intermediates.
FIG. 2.
Product ion spectra (MS/MS) of the m/z 305 [M+H]+ of alphitonin (A) and taxifolin (B). The MS/MS spectrum of taxifolin shows a reverse-Diels-Alder fragment ion of m/z 153, with high intensity. This fragment was not observed in the case of alphitonin.
Degradation of taxifolin and alphitonin by resting cells of E. ramulus.
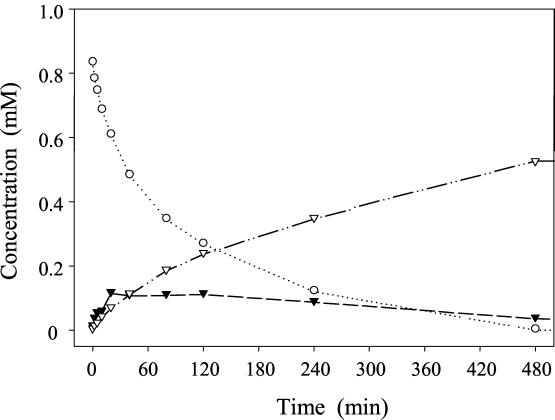
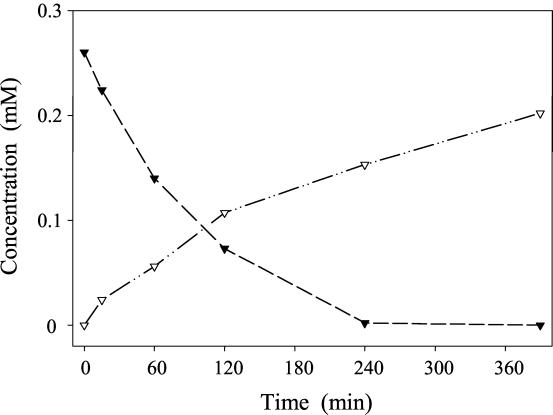
Since taxifolin was identified as an intermediate in the quercetin degradation pathway, its transformation was studied with resting cells of E. ramulus (Fig. 3). The conversion of 1 mM taxifolin resulted in the formation of 3,4-dihydroxyphenylacetic acid. Small amounts of alphitonin were formed transiently. Besides the HPLC retention times and UV spectra, LC-MS measurements were utilized to prove the identity of both substances. Incubation of 0.26 mM alphitonin with resting cells of E. ramulus led to the formation of 3,4-dihydroxyphenylacetic acid (Fig. 4). The identity of this compound was confirmed by its retention times and UV and mass spectra. Using LC-MS/MS, the same pattern of daughter ions of m/z 169 [M+H]+ (77, 123, 105) was observed for the product formed from both taxifolin and alphitonin. The same pattern was obtained for the reference substance of 3,4-dihydroxyphenylacetic acid.
FIG. 3.
Time course of taxifolin degradation by resting cells of E. ramulus (optical density at 600 nm, 2.6; temperature, 19°C). The initial concentration of taxifolin was 1 mM. Concentrations of taxifolin (○), alphitonin (▾), and 3,4-dihydroxyphenylacetic acid (▿) are given.
FIG. 4.
Time course of alphitonin degradation by resting cells of E. ramulus (optical density at 600 nm, 6; temperature, 19°C). The initial concentration of alphitonin was 0.26 mM. Concentrations of alphitonin (▾) and 3,4-dihydroxyphenylacetic acid (▿) are given.
Transformation of taxifolin by cell extracts of E. ramulus.
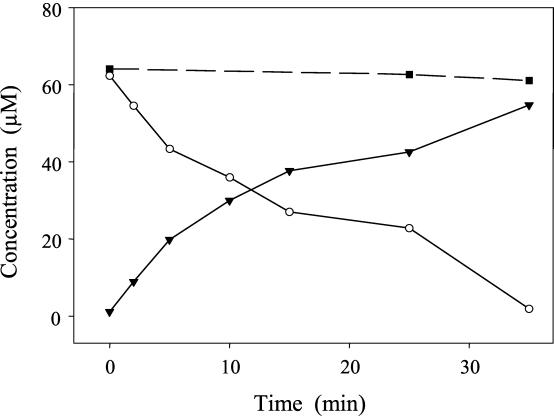
The transformation of taxifolin (60 μM) was also catalyzed by cell extracts, the soluble enzyme fraction, and a partially purified enzyme prepared from E. ramulus extracts (Fig. 5), respectively. No transformation was observed without the enzyme preparation or with an inactivated enzyme preparation. The relatively small amount of taxifolin used in these experiments was completely transformed to alphitonin as the final product in the presence or absence of oxygen without the addition of any coenzyme. The enriched enzyme preparation was also tested for transformation of the dihydroflavone eriodictyol. However, neither with nor without oxygen was a conversion observed.
FIG. 5.
Transformation of taxifolin by a partially purified enzyme preparation of E. ramulus. The initial concentration of taxifolin was 60 μM. Concentrations of taxifolin (○) and alphitonin (▾) are given. For a control, taxifolin was incubated without enzyme (▪).
Structural elucidation of alphitonin.
The alphitonin formed by taxifolin transformation and purified by HPLC was highly pure as judged by NMR analysis. Taxifolin was completely absent from this preparation. By using the APT technique, alphitonin could be distinguished from taxifolin due to the CH2 signal in the 13C NMR spectrum of alphitonin (Fig. 6). The structure of alphitonin could be unequivocally deduced from the NMR data. In particular, 13C and 1H NMR signals indicated that the ring A was being nonsymmetrically substituted. Thus, a ring-open chalcone structure could be excluded. According to the structure of alphitonin, a signal for the benzylic carbon (41.55 ppm) was assigned; the diastereotopic hydrogens gave two doublets (2.82, 2.88 ppm; J = 14.0 Hz).
FIG. 6.
13C NMR (APT) spectrum of alphitonin, obtained from taxifolin transformation by an enzyme preparation of E. ramulus followed by purification with HPLC. Using the APT technique, downward signals indicate CH3 and CH carbons, whereas upward signals indicate CH2 and C carbons.
Luteolin degradation by resting cells of E. ramulus.
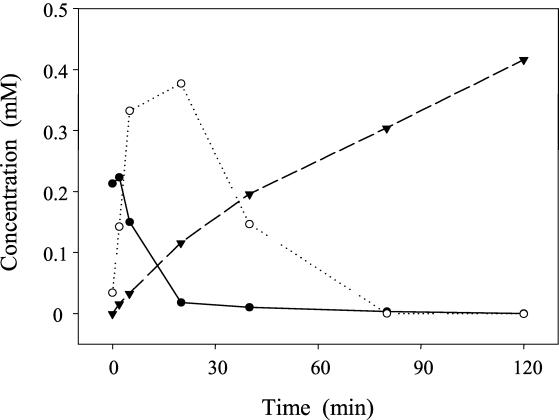
Resting cells of E. ramulus were also used to investigate the degradation of the flavone luteolin, whose structure lacks the 3-hydroxyl group present in quercetin. Luteolin (0.5 mM; TFA-methanol and FA-methanol gradients, 32.4 min, λmax = 348 nm) was transformed via the intermediate eriodictyol to 0.4 mM 3-(3,4-dihydroxyphenyl)propionic acid within 120 min (Fig. 7). Luteolin was found exclusively in the pellet of the centrifuged samples. Eriodictyol was predominantly recovered from the supernatant, whereas the pellet contained approximately 10% of this compound. The final product, 3-(3,4-dihydroxyphenyl)propionic acid, was almost exclusively found in the supernatant. The identity of eriodictyol was confirmed by its retention times (29.8 min, TFA-methanol gradient; 29.2 min, FA-methanol gradient) and UV spectrum (λmax = 294 nm). Similarly, the identity of 3-(3,4-dihydroxyphenyl)propionic acid as the product of the luteolin degradation was demonstrated on the basis of its retention times (16.3 min, TFA-methanol gradient; 14.5 min, FA-methanol gradient) and UV spectrum (λmax = 286 nm). MS analysis (flow injection) gave the expected molecular ion peaks of eriodictyol (m/z 289 [M+H]+) and 3-(3,4-dihydroxyphenyl)propionic acid (m/z 183 [M+H]+, m/z 181 [M-H]−).
FIG. 7.
Time course of luteolin degradation by resting cells of E. ramulus (optical density at 600 nm, 6; temperature, 19°C). The initial concentration of luteolin was 0.5 mM. The concentrations of luteolin (●), eriodictyol (○), and 3-(3,4-dihydroxyphenyl)propionic acid (▾) recovered from the supernatant and the pellet are shown. Immediately after starting the transformation a luteolin concentration of 0.22 mM was observed, probably as a result of very fast uptake and metabolization.
DISCUSSION
These investigations were done in order to get insight into the pathway of flavonoid degradation by a relevant bacterial species of the human intestinal tract, E. ramulus. The flavonol quercetin was chosen because quercetin glycosides are highly abundant dietary flavonoids. The ability of E. ramulus to grow on quercetin-3-glucoside was previously shown. Quercetin and phloroglucinol were detected as intermediates in the transformation of quercetin-3-glucoside. The formation of phloroglucinol indicated that E. ramulus is capable of splitting the heterocyclic C-ring of quercetin (20). The degradation of quercetin was also reported for other human intestinal bacteria and for species from the bovine rumen. Examples include Butyrivibrio sp. C3 (4), Clostridium orbiscindens (22), Pediococcus Q-05 (16), and Eubacterium oxidoreducens (17). In contrast to E. oxidoreducens, which is able to grow on the aglycon quercetin as the sole carbon and energy source in the presence of hydrogen or formate as reductants (17), the growth of E. ramulus with quercetin was strictly dependent on glucose, which could be replaced neither by hydrogen nor by formate (20). However, as described herein, resting cells of E. ramulus are able to convert quercetin and its flavone analogue, luteolin. This offered the opportunity to study these transformations quantitatively in the absence of glucose and other media components.
In accordance with previous reports using growing cultures (19, 20), quercetin was transformed by resting cells of E. ramulus to 3,4-dihydroxyphenylacetic acid (Fig. 1 and structures in Fig. 8). In the course of fermentation, the enol carbon C-3 is transformed to the carboxyl group of 3,4-dihydroxyphenylacetic acid. Thus, this bacterial degradation of quercetin does not occur via reverse reactions of its biosynthesis in plants. In the quercetin synthesis pathway of plants (for a review, see reference 6), the intermediate taxifolin is formed by hydroxylation of eriodictyol (structure in Fig. 9).
FIG. 8.
Proposed pathway of degradation of quercetin by E. ramulus.
FIG. 9.
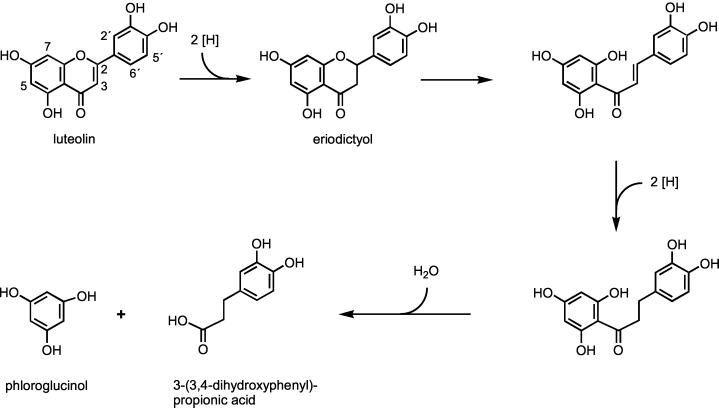
Proposed pathway of degradation of luteolin by E. ramulus.
Two intermediates of the quercetin degradation were identified, taxifolin and alphitonin (Fig. 1). Separate experiments showed that both taxifolin and alphitonin were transformed to 3,4-dihydroxyphenylacetic acid (Fig. 3 and 4). The degradation of taxifolin to 3,4-dihydroxyphenylacetic acid is in accordance with previous results using growing cells of E. ramulus (19). Alphitonin was identified as an intermediate of the conversion of taxifolin to 3,4-dihydroxyphenylacetic acid (Fig. 3). From these data, the pathway of the quercetin degradation shown in Fig. 8 could be deduced. It starts with the reduction of the double bond in the 2,3-position of quercetin, resulting in the formation of taxifolin. The following ring contraction to the identified isomeric alphitonin probably occurs by a ring opening-recyclization mechanism via a chalcone or diketone structure. However, this postulated chalcone (or tautomeric diketone) could not be observed, presumably because of the fast cyclization to alphitonin. We cannot distinguish whether this cyclization is part of an enzyme-catalyzed reaction or whether it occurs spontaneously. Neither the α-hydroxychalcone [2-hydroxy-3-(3,4-dihydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propenone] nor the diketone [3-(3,4-dihydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propane-1,2-dione] have been described in the literature so far. As a result of the ring contraction to alphitonin, a benzylic CH2 group is already formed as it finally appears in the product, 3,4-dihydroxyphenylacetic acid. An oxidative decarboxylation step is postulated for the conversion of alphitonin to phloroglucinol and 3,4-dihydroxyphenylacetic acid. Whereas 3,4-dihydroxyphenylacetic acid was identified as a final product, phloroglucinol was shown to undergo further degradation to butyrate and acetate (L. Schoefer, personal communication).
This is the first report describing alphitonin as an intermediate of bacterial metabolism. The elucidation of structure became necessary because a reference substance for this compound was not available, and the molecular masses of alphitonin and the corresponding chalcone structure are identical. The structure of alphitonin was unambiguously deduced from the NMR data, which were in agreement with the data reported by Kiehlmann and Li (14). These authors demonstrated the nonenzymatic isomerization of taxifolin to alphitonin under drastic conditions (115°C for 4 days). The structure of alphitonin, isolated from the heartwood of Alphitonia excelsa, was reported in 1960 (1). The compound was also identified in the wood of Alphitonia petriei but not in that of Alphitonia whitei (7). Remarkably, no studies on the biological activity of alphitonin have been reported so far. Our results, however, indicate that alphitonin appears as an intermediate of intestinal metabolism of the abundant flavonoid quercetin and might be absorbed in the human intestinal tract.
We also investigated the degradation of the flavone luteolin by resting cells of E. ramulus. The proposed degradation pathway is shown in Fig. 9. Initial reduction led to the formation of eriodictyol, similar to the transformation of quercetin to taxifolin. Eriodictyol was identified as an intermediate in the resting-cell fermentation (Fig. 7). By the ensuing ring cleavage, a chalcone structure could be formed which may be further reduced to a dihydrochalcone. However, neither of these compounds nor a fused five-membered structure similar to alphitonin was observed. The final product of luteolin degradation was 3-(3,4-dihydroxyphenyl)propionic acid, which was identified in fermentation experiments with resting cells (Fig. 7), comparable to the results obtained previously with growing cells of E. ramulus (19), which fermented luteolin and eriodictyol in the presence of glucose. Phloroglucinol is certainly another intermediate, as postulated for several flavonoid degradation pathways (9, 18, 22), but its further degradation occurred instantly. Similar to our results, it was reported that eriodictyol is converted to 3-(3,4-dihydroxyphenyl)propionic acid by a strain of Clostridium butyricum (18).
The comparison of the pathways (Fig. 8 and 9) reveals that degradation of both quercetin and luteolin by E. ramulus starts with the reduction of the double bond in the 2,3- position prior to the C-ring fission. The steps that follow differ due to the 3-hydroxyl group in the quercetin molecule, which is the only difference from the luteolin structure. This hydroxyl group seems to be a prerequisite for the formation of the alphitonin structure following C-ring cleavage. In contrast, the fission of the heterocyclic ring of eriodictyol, which results from luteolin, would lead directly to a chalcone structure, and for further transformation a second reduction step is assumed. These postulated intermediates could not be observed during luteolin conversion by resting cells, although such chalcones and dihydrochalcones are known to be stable (6). It was shown previously that the dihydrochalcone phloretin is also degraded by growing cells of E. ramulus in the presence of glucose (19). The final products resulting from the B-ring of quercetin and luteolin, respectively, differ in one carbon atom within the side chain, indicating an additional decarboxylation step in the case of quercetin degradation. The notion that the degradation of flavonols and flavones by E. ramulus occurs by two different pathways and involves different enzymes is supported by the finding that the taxifolin-transforming enzyme preparation did not transform eriodictyol, either in the presence or in the absence of oxygen (data not shown).
In conclusion, the fermentation by resting cells constitutes an advantageous method for the detection of intermediates of flavonoid degradation by intestinal bacteria. These bacterial metabolites should be included in investigations concerning the flavonoid effects after ingestion by humans. However, the characterization of the involved enzymes and the reaction mechanisms requires further studies with cell-free systems. This approach has already been initiated for the taxifolin isomerization as described above, and it will be continued in ongoing studies in our laboratories.
ACKNOWLEDGMENTS
This work was supported in part by the Deutsche Forschungsgemeinschaft (grant no. INK 26/A1-1, B1-1).
We thank Sabine Zimmerman for technical assistance.
REFERENCES
- 1.Birch A J, Ritchie E, Speake R N. The structure of alphitonin. J Chem Soc. 1960;1960:3593–3599. [Google Scholar]
- 2.Bokkenheuser V D, Shackleton C H, Winter J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem J. 1987;248:953–956. doi: 10.1042/bj2480953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant M P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972;25:1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- 4.Cheng K J, Jones G A, Simpson F J, Bryant M P. Isolation and identification of rumen bacteria capable of anaerobic rutin degradation. Can J Microbiol. 1969;15:1365–1371. doi: 10.1139/m69-247. [DOI] [PubMed] [Google Scholar]
- 5.de Vries J H, Janssen P L, Hollman P C, van Staveren W A, Katan M B. Consumption of quercetin and kaempferol in free-living subjects eating a variety of diets. Cancer Lett. 1997;114:141–144. doi: 10.1016/s0304-3835(97)04645-4. [DOI] [PubMed] [Google Scholar]
- 6.Dimmock J R, Elias D W, Beazely M A, Kandepu N M. Bioactivities of chalcones. Curr Med Chem. 1999;6:1125–1149. [PubMed] [Google Scholar]
- 7.Guise G B, Ritchie E, Taylor W C. Further constituents of Alphitonia species. Aust J Chem. 1962;15:314–321. [Google Scholar]
- 8.Harborne J B, Williams C A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 9.Hattori M, Shu Y Z, el-Sedawy A I, Namba T, Kobashi K, Tomimori T. Metabolism of homoorientin by human intestinal bacteria. J Nat Prod. 1988;51:874–878. doi: 10.1021/np50059a010. [DOI] [PubMed] [Google Scholar]
- 10.Hertog M G, Hollman P C, Katan M B, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer. 1993;20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- 11.Hungate R E. A roll tube method for cultivation of strict anaerobes. In: Norris J R, Ribbons D W, editors. Methods in microbiology. 3B. New York, N.Y: Academic Press; 1969. pp. 117–132. [Google Scholar]
- 12.Justesen U, Arrigoni E. Electrospray ionisation mass spectrometric study of degradation products of quercetin, quercetin-3-glucoside and quercetin-3-rhamnoglucoside, produced by in vitro fermentation with human faecal flora. Rapid Commun Mass Spectrom. 2001;15:477–483. doi: 10.1002/rcm.250. [DOI] [PubMed] [Google Scholar]
- 13.Justesen U, Knuthsen P, Leth T. Determination of plant polyphenols in Danish foodstuffs by HPLC-UV and LC-MS detection. Cancer Lett. 1997;114:165–167. doi: 10.1016/s0304-3835(97)04651-x. [DOI] [PubMed] [Google Scholar]
- 14.Kiehlmann E, Li E P M. Isomerization of dihydroquercetin. J Nat Prod. 1995;58:450–455. [Google Scholar]
- 15.Kim D H, Jung E A, Sohng I S, Han J A, Kim T H, Han M J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch Pharm Res. 1998;21:17–23. doi: 10.1007/BF03216747. [DOI] [PubMed] [Google Scholar]
- 16.Kim D-H, Han S-B, Bae E-A, Han M J. Intestinal bacterial metabolism of rutin and its relation to mutagenesis. Arch Pharm Res. 1996;19:41–45. [Google Scholar]
- 17.Krumholz L R, Crawford R L, Hemling M E, Bryant M P. A rumen bacterium degrading quercetin and trihydroxybenzenoids with concurrent use of formate or H2. Prog Clin Biol Res. 1986;213:211–214. [PubMed] [Google Scholar]
- 18.Miyake Y. Metabolism of antioxidant in lemon fruit (Citrus limon BURM. f.) by human intestinal bacteria. J Agric Food Chem. 1997;45:3738–3742. [Google Scholar]
- 19.Schneider H, Blaut M. Anaerobic degradation of flavonoids by Eubacterium ramulus. Arch Microbiol. 2000;173:71–75. doi: 10.1007/s002030050010. [DOI] [PubMed] [Google Scholar]
- 20.Schneider H, Schwiertz A, Collins M D, Blaut M. Anaerobic transformation of quercetin-3-glucoside by bacteria from the human intestinal tract. Arch Microbiol. 1999;171:81–91. doi: 10.1007/s002030050682. [DOI] [PubMed] [Google Scholar]
- 21.Simmering R, Kleessen B, Blaut M. Quantification of the flavonoid-degrading bacterium Eubacterium ramulus in human fecal samples with a species-specific oligonucleotide hybridization probe. Appl Environ Microbiol. 1999;65:3705–3709. doi: 10.1128/aem.65.8.3705-3709.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winter J, Popoff M R, Grimont P, Bokkenheuser V D. Clostridium orbiscindens sp. nov., a human intestinal bacterium capable of cleaving the flavonoid C-ring. Int J Syst Bacteriol. 1991;41:355–357. doi: 10.1099/00207713-41-3-355. [DOI] [PubMed] [Google Scholar]