Abstract
Background
The efficacy and safety of tralokinumab, a fully human monoclonal antibody that specifically neutralizes interleukin-13, plus topical corticosteroids (TCS) as needed were evaluated over 32 weeks in the phase III ECZTRA 3 trial. Significantly more tralokinumab- versus placebo-treated patients achieved the primary endpoints of Investigator’s Global Assessment (IGA) score of 0/1 and 75% improvement in Eczema Area and Severity Index (EASI-75) and all confirmatory endpoints at Week 16.
Objective
This post hoc analysis investigated the impact of tralokinumab plus TCS on atopic dermatitis (AD) severity, symptoms, and health-related quality of life (QoL) over the entire 32-week treatment period of ECZTRA 3, including all patients initiated on tralokinumab irrespective of the response achieved at Week 16.
Methods
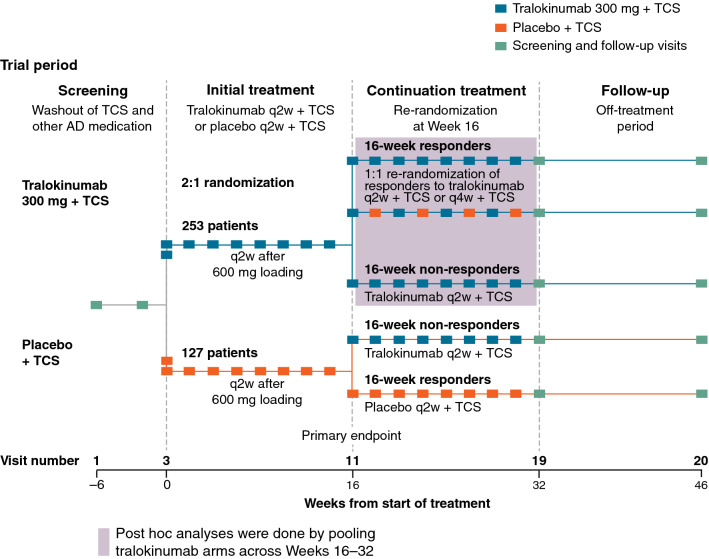
Patients were randomized 2:1 to receive subcutaneous tralokinumab 300 mg or placebo every 2 weeks (q2w) with TCS as needed for an initial 16 weeks. At Week 16, patients who achieved the clinical response criteria (IGA 0/1 and/or EASI-75) with tralokinumab were re-randomized 1:1 to tralokinumab q2w or every 4 weeks (q4w), with TCS as needed, for another 16 weeks. Patients not achieving the clinical response criteria with tralokinumab received tralokinumab q2w plus TCS from Week 16. All patients randomized to tralokinumab in the initial treatment period were pooled for this analysis, irrespective of response at Week 16 or dosing regimen beyond Week 16.
Results
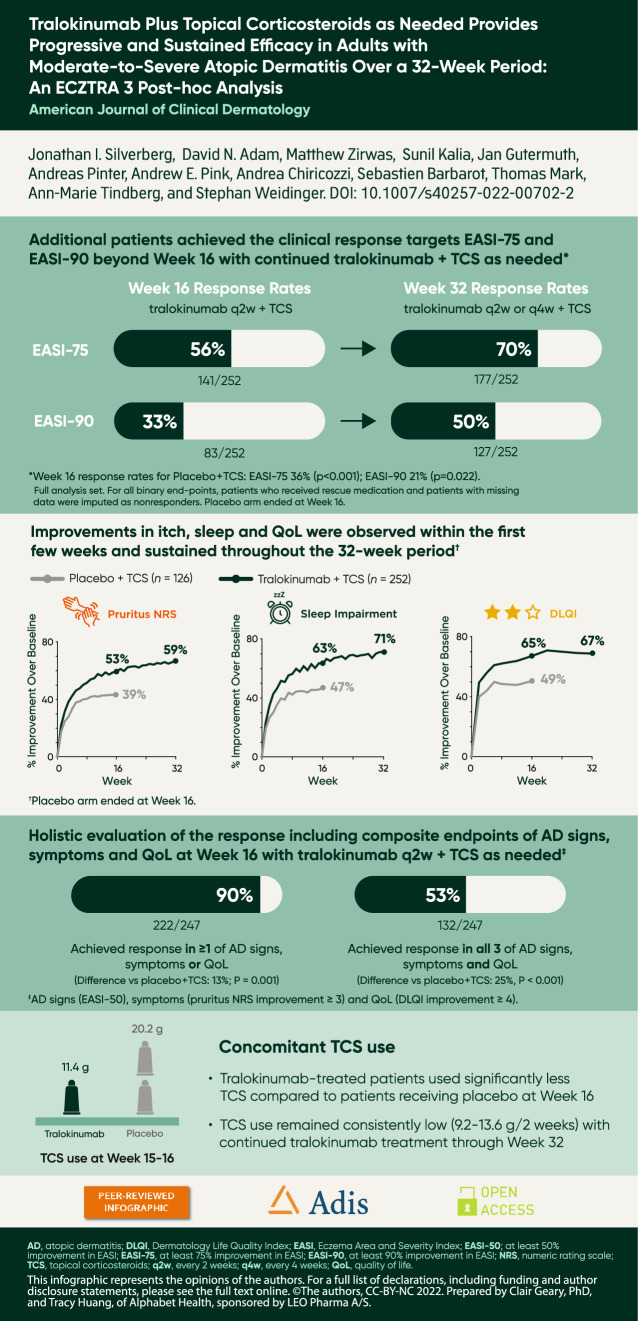
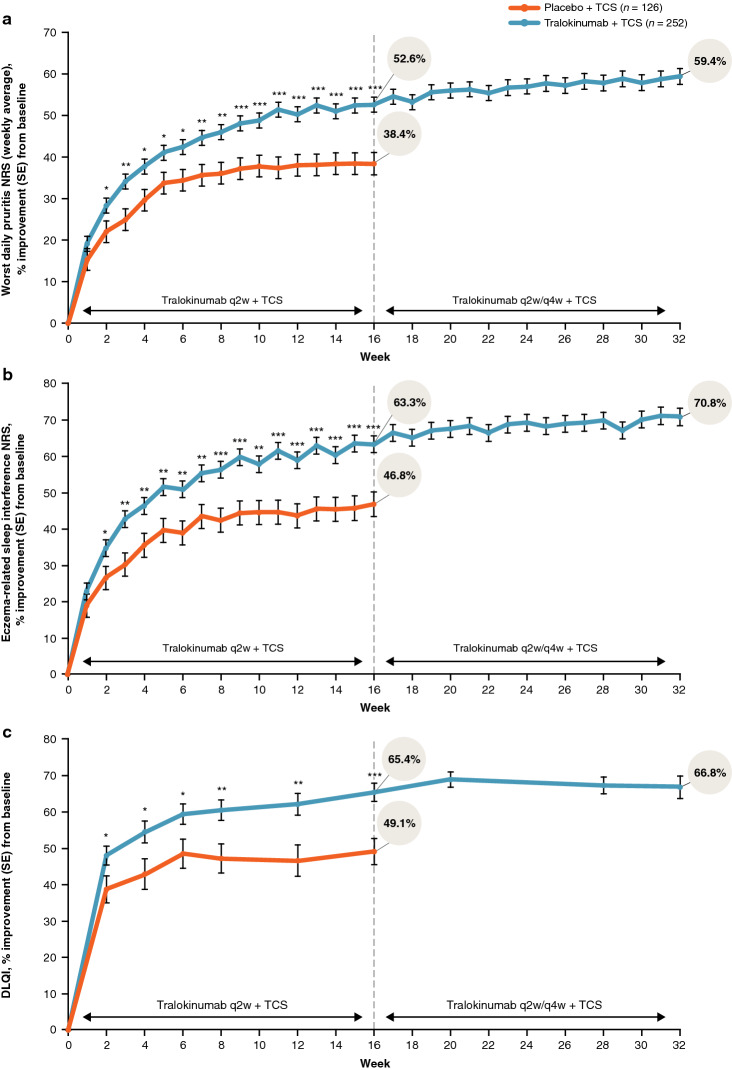
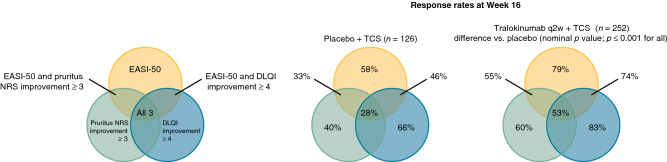
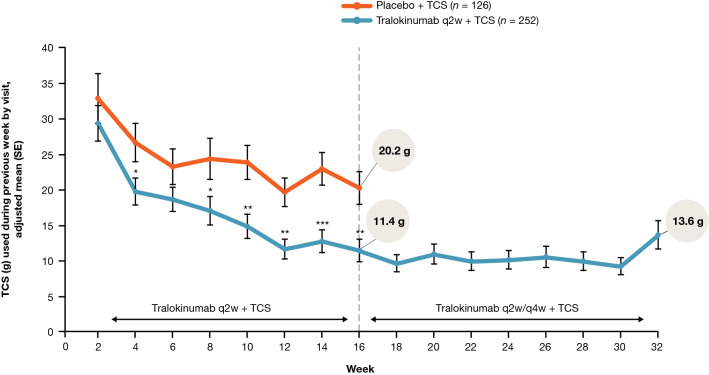
Continued tralokinumab (q2w, N = 164; q4w, N = 69) plus TCS treatment provided progressive improvements from Week 16 onwards in AD signs, with 70.2% (177/252) of patients achieving EASI-75 and 50.4% (127/252) achieving EASI-90 at Week 32. Improvements in patient-reported outcomes were observed within the first few weeks of tralokinumab q2w plus TCS treatment and were sustained throughout the 32-week period. At Week 32, patients initiated on tralokinumab q2w plus TCS achieved a relative improvement versus baseline of 70.8% (standard error (SE), 2.4) in eczema-related sleep interference numeric rating scale (NRS) and 66.8% (SE, 3.1) in Dermatology Life Quality Index (DLQI). Mean TCS use during Weeks 16–32 ranged from 9.2 to 13.6 g (SE, 1.2–2.0) q2w. Most patients (89.9% (222/247)) initiated on tralokinumab q2w plus TCS achieved a meaningful improvement in at least one of the three disease domains, including AD signs (EASI-50), symptoms (pruritus NRS improvement ≥ 3), and QoL (DLQI improvement ≥ 4) at Week 16. Of patients initiated on tralokinumab q2w plus TCS, 53.4% (132/247) achieved a clinically meaningful improvement in all three domains at Week 16 (vs. placebo, 28.5% (35/123); p < 0.001).
Conclusions
Continued tralokinumab treatment plus TCS as needed provides progressive and sustained improvements in AD signs, symptoms, and health-related QoL over 32 weeks.
Clinical trial registration
NCT03363854; study start date: 22 February 2018; primary completion date: 8 March 2019; study completion date: 26 September 2019.
Infographic
Video abstract: What is the impact of tralokinumab plus topical corticosteroids in adults with moderate-to-severe atopic dermatitis over 32 weeks? (MP4 216,988 KB)
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-022-00702-2.
Plain Language Summary: Treatment with tralokinumab plus topical corticosteroids provided continued improvements over a 32-week study period
Atopic dermatitis (AD) is a chronic inflammatory disease that causes excessively dry and itchy skin that can negatively impact sleep and overall quality of life for patients. Topical corticosteroids (TCS) are the most common medication used for AD, but they are not able to control the most severe cases. Tralokinumab is a treatment injected under the skin that targets an immune messenger protein called interleukin 13, which plays a key role in driving the signs and symptoms of AD. The ECZTRA 3 clinical trial, funded by LEO Pharma, compared the use of TCS as needed with either tralokinumab or placebo in over 350 adult patients with moderate-to-severe AD over a 32-week period. After 16 weeks, more patients taking tralokinumab plus TCS had clear or almost clear skin compared with patients taking placebo plus TCS. Patients taking tralokinumab also used less TCS than patients taking placebo. In new analyses presented here, we found that the proportion of patients with clear or almost clear skin continued to increase with on-going treatment from Week 16 to Week 32. Tralokinumab plus TCS treatment also led to clinically meaningful improvements in outcomes important to patients, including itch, sleep, and quality of life. Improvements occurred early, within the first few weeks of therapy, and lasted through Week 32. Our assessment of multiple outcomes over time clearly demonstrates the positive impact of tralokinumab on different aspects of AD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-022-00702-2.
| Digital Features Digital Features for this article can be found at https://doi.org/10.6084/m9.figshare.20154776. |
Key Points
| Atopic dermatitis (AD) has a multitude of symptoms that affect patients beyond the efficacy endpoints used by regulatory authorities when evaluating treatment success. To fully understand the ability of treatments to control disease, other endpoints and timepoints need to be considered. |
| To provide a better understanding of the impact of tralokinumab over time on multiple disease domains, we assessed the timing, magnitude, and sustainability of the effect over the entire 32-week treatment period of the ECZTRA 3 trial, including all patients initiated on tralokinumab irrespective of the response achieved at Week 16. Tralokinumab plus topical corticosteroids provided progressive and sustained improvements in both clinician- and patient-reported outcomes, including AD signs, symptoms, and health-related quality of life over 32 weeks. |
| These results provide a more complete overview of the benefits of tralokinumab over time, which may help inform clinical decisions. |
Introduction
There remains a need for improved symptom control and reduced burden in patients with moderate-to-severe atopic dermatitis (AD). A large cross-sectional survey of US physicians and their patients with AD found that 42% of patients were identified as having inadequately controlled disease [1]. In recent years, advances in our understanding of the underlying pathophysiology have led to the discovery and regulatory approval of multiple new systemic treatments for AD [2].
AD is a chronic disease, requiring long-term management. The use of Investigator’s Global Assessment (IGA) 0/1 (clear/almost clear) and 75% improvement in Eczema Area and Severity Index (EASI-75) after 16 weeks of treatment as primary outcomes in clinical studies is largely driven by guidance from regulatory authorities such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) [3]. However, these measures and timepoints do not sufficiently capture disease control, and patients with AD report a wide variety of therapeutic needs beyond lesion control, including itch and sleep disturbances [4]. A more comprehensive assessment of the impact on multiple disease domains and at timepoints beyond Week 16 is needed to inform clinical decisions to initiate, continue, discontinue, or modify treatment [5].
Tralokinumab is a fully human immunoglobulin G4 monoclonal antibody that specifically binds to the interleukin (IL)-13 cytokine with high affinity, preventing interaction with the IL-13 receptor and subsequent downstream IL-13 signaling [6–8]. Tralokinumab met all primary and secondary endpoints at Week 16 in the pivotal phase III trials (ECZTRA 1, 2, and 3), which included 1,976 adult patients with moderate-to-severe AD [9, 10]. Tralokinumab was recently approved for the treatment of moderate-to-severe AD in adult patients who are candidates for systemic therapy in the European Union, Canada, and the USA [11–13].
The ECZTRA 3 trial (NCT03363854) assessed the efficacy and safety of tralokinumab in combination with a topical corticosteroid (TCS) used on active lesions. Significantly more patients treated with tralokinumab every 2 weeks (q2w) plus TCS versus patients treated with placebo plus TCS achieved the primary endpoints of IGA 0/1 (38.9% vs. 26.2%; p = 0.015) and EASI-75 (56.0% vs. 35.7%; p < 0.001), as well as EASI-50 (79.4% vs. 57.9%; p < 0.001) and EASI-90 (32.9% vs. 21.4%; p = 0.022) at Week 16, as reported previously [10]. All confirmatory endpoints in the test hierarchy were also met. Most patients who achieved the primary endpoint maintained their response up to Week 32 on tralokinumab q2w plus TCS (89.6% maintained IGA 0/1 and 92.5% maintained EASI-75) and on tralokinumab every 4 weeks (q4w) plus TCS (77.6% maintained IGA 0/1 and 90.8% maintained EASI-75). To obtain further insight into the impact of tralokinumab on multiple disease domains and over time, here we assessed the timing, magnitude, and sustainability of the effect on signs, symptoms, and health-related quality of life (QoL) over the entire 32-week treatment period. To reflect clinical practice, we pooled all patients who started on tralokinumab in the initial treatment period irrespective of the response achieved at Week 16. Safety outcomes for ECZTRA 3 were previously reported for the different treatment arms [10].
Methods
Study Design and Patients
The design and methodology of ECZTRA 3 (NCT03363854) have been published previously [10]. Briefly, ECZTRA 3 was a double-blind, randomized, placebo-controlled, 32-week, phase III trial conducted across 68 sites in Europe and North America. Eligible patients were ≥ 18 years of age, with a diagnosis of AD for ≥ 1 year, an EASI score of ≥ 16, an IGA score of 3 or 4, and worst daily pruritus numeric rating scale (NRS) average score of ≥ 4.
Following a 2- to 6-week screening period, including washout of prior AD medications (2 weeks for topical treatments; 4 weeks for systemic treatments), patients were randomized 2:1 to subcutaneous tralokinumab 300 mg q2w with TCS as needed or placebo q2w with TCS as needed for 16 weeks, and received tralokinumab 600 mg (loading dose) or placebo on Day 0 (Fig. 1). At Week 16, patients who achieved the clinical response criteria with tralokinumab (IGA 0/1 and/or EASI-75) were re-randomized 1:1 to tralokinumab q2w or q4w plus TCS as needed for a further 16 weeks; the objective of the continuation period at Weeks 16–32 was to evaluate the ability to maintain the Week 16 response (IGA 0/1 and/or EASI-75) with two different dosing interval options: q2w and q4w. Patients who achieved clinical response with placebo continued to receive placebo (q2w) to maintain blinding of the study, and patients not achieving the clinical response criteria at Week 16 (from tralokinumab or placebo) received tralokinumab q2w plus TCS as needed from Week 16 onwards (Fig. 1).
Fig. 1.
Trial design. AD atopic dermatitis, q2w every 2 weeks, q4w every 4 weeks, TCS topical corticosteroid
Patients were provided TCS (mometasone furoate 0.1% cream; Europe class 3 (potent); US class 4 (mid-strength)) in kit sizes of 180–200 g q2w, free of charge, and were instructed to apply a thin layer of supplied TCS once daily to areas with active lesions as needed. The quantity of TCS used was measured based on used and unused tubes returned at bi-weekly visits. Patients were instructed to apply an emollient twice daily to lesional skin only when TCS was not applied. Rescue treatment (topical and systemic medications) was permitted to control intolerable AD symptoms at investigator discretion.
Endpoints
Endpoints evaluating AD extent and severity up to Week 32 included the proportion of patients achieving IGA 0/1; the proportion of patients achieving 50%, 75%, and 90% improvement in EASI (EASI-50, EASI-75, and EASI-90); and percentage improvement in EASI versus baseline. Endpoints evaluating patient-reported outcomes up to Week 32 included relative improvement from baseline in weekly average of worst daily pruritus NRS, eczema-related sleep interference NRS, and Dermatology Life Quality Index (DLQI). The proportion of patients achieving a response equivalent to established levels of clinically meaningful improvements at Week 16 in three disease-specific domains—AD signs (EASI-50) [14], symptoms (≥ 3-point pruritus NRS improvement) [15], and QoL (≥ 4-point DLQI improvement) [16]—was also assessed. Other endpoints included quantity of concomitant TCS used up to Week 32 (assuming no TCS was used from non-returned tubes).
Statistical Analysis
Statistical analyses followed pre-specifications [10]. Post hoc analyses were based on the full analysis set and were conducted by pooling all patients treated with tralokinumab in the initial treatment period (n = 252) irrespective of the response achieved at Week 16 and the dosing regimen (q2w or q4w) received beyond Week 16 (Fig. S1, see Electronic Supplementary Material (ESM)). Additionally, pre-specified analysis by Week 16 response is provided. For binary endpoints, the differences in response rates between treatment groups were analyzed using the Cochran-Mantel-Haenszel test stratified by region and baseline disease severity. Patients with missing data or who received rescue medication prior to the visit were assumed to be non-responders. For continuous endpoints, changes from baseline were analyzed using a repeated-measurements model with an unstructured (compound symmetric when needed for convergence) covariance matrix among visits within patients. Data collected after initiation of rescue medication or permanent discontinuation of investigational medicinal product were excluded from the analysis, but each patient contributed information to all timepoints via the covariance matrix. For waterfall plots illustrating percentage improvement in EASI at the individual patient level, last observation carried forward was applied for patients using rescue treatment, for patients discontinuing study medication, and for patients with missing data for other reasons up to Week 32.
Results
Patients
Overall, 380 patients were randomized in ECZTRA 3 to receive tralokinumab q2w plus TCS (n = 253) or placebo q2w plus TCS (n = 127) in the initial treatment period, with 233 patients continuing to receive tralokinumab (q2w (n = 164) or q4w (n = 69)) during the continuation period (Weeks 16–32) (Fig. S1, see ESM). One patient from each treatment group did not receive a treatment dose and was excluded from the analysis. Baseline demographics and disease characteristics were similar across treatment groups (Table 1). Overall, 46% of patients had severe disease (IGA score of 4) at baseline with a mean EASI score of 29.4, a mean DLQI score of 17.5, and a mean weekly average of worst daily pruritus NRS score of 7.7.
Table 1.
Patient baseline demographics and disease characteristics
| All randomized (N = 380) | Placebo q2w + TCS (N = 127) | Tralokinumab q2w + TCS (N = 253) | |
|---|---|---|---|
| Mean age, years | 39.1 | 37.7 | 39.8 |
| Male, n (%) | 209 (55.0) | 84 (66.1) | 125 (49.4) |
| Race, n (%) | |||
| White | 288 (75.8) | 85 (66.9) | 203 (80.2) |
| Black or African American | 35 (9.2) | 12 (9.4) | 23 (9.1) |
| Asian | 41 (10.8) | 24 (18.9) | 17 (6.7) |
| Native Hawaiian or other Pacific Islander | 2 (0.5) | 1 (0.8) | 1 (0.4) |
| Other | 14 (3.7) | 5 (3.9) | 9 (3.6) |
| Mean duration of atopic dermatitis, years (SD) | n = 379; 28.2 (16.0) | n = 126; 28.7 (15.0) | n = 253; 28.0 (16.5) |
| Mean BSA involvement, % (SD) | 48.1 (24.2) | 49.0 (25.9) | 47.6 (23.3) |
| IGA, n (%) | |||
| Moderate (IGA 3) | 202 (53.2) | 66 (52.0) | 136 (53.8) |
| Severe (IGA 4) | 176 (46.3) | 60 (47.2) | 116 (45.8) |
| Missinga | 2 (0.5) | 1 (0.8) | 1 (0.4) |
| Mean EASI score (SD) | n = 378; 29.4 (12.3) | n = 126; 30.4 (12.8) | n = 252; 28.8 (12.0) |
| Mean DLQI score (SD) | n = 375; 17.5 (7.1) | n = 125; 17.2 (7.2) | n = 250; 17.6 (7.1) |
| Mean weekly average of worst daily pruritus NRS score (SD) | n = 377; 7.7 (1.5) | n = 126; 7.9 (1.5) | n = 251; 7.7 (1.5) |
| Mean weekly average of eczema-related sleep NRS (SD) | n = 377; 7.0 (2.1) | n = 126; 7.1 (2.2) | n = 251; 6.9 (2.1) |
BSA body surface area involvement, DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, NRS numeric rating scale, q2w every 2 weeks, SD standard deviation, TCS topical corticosteroids
aTwo patients (one in each arm) did not receive a treatment dose and were not included in the full analysis
Clinician- and Patient-Reported Outcomes up to Week 32
Lesion Extent and Severity
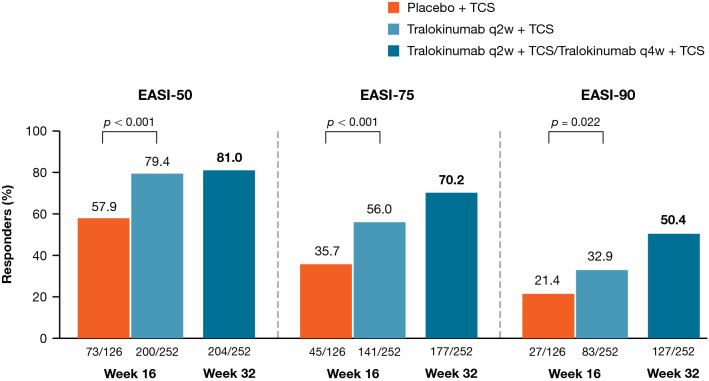
Analyzing the efficacy of tralokinumab treatment over the entire 32-week treatment period, irrespective of the response achieved at Week 16 and the dosing regimen (q2w or q4w) received in the continuation treatment period (n = 252), EASI-50 response rates were sustained from Week 16 (79.4%) to Week 32 (81.0%) in the pooled tralokinumab group, while EASI-75 and EASI-90 response rates continued to improve beyond Week 16 to 70.2% and 50.4%, respectively, at Week 32 (Fig. 2 and Fig. S2, see ESM).
Fig. 2.
EASI response rates at Weeks 16 and 32. Composite estimand (primary analysis): Patients who received rescue medication were considered non-responders. Patients with missing data were imputed as non-responders. EASI Eczema Area and Severity Index, EASI-50 at least 50% improvement in EASI, EASI-75 at least 75% improvement in EASI, EASI-90 at least 90% improvement in EASI, q2w every 2 weeks, q4w every 4 weeks, TCS topical corticosteroid
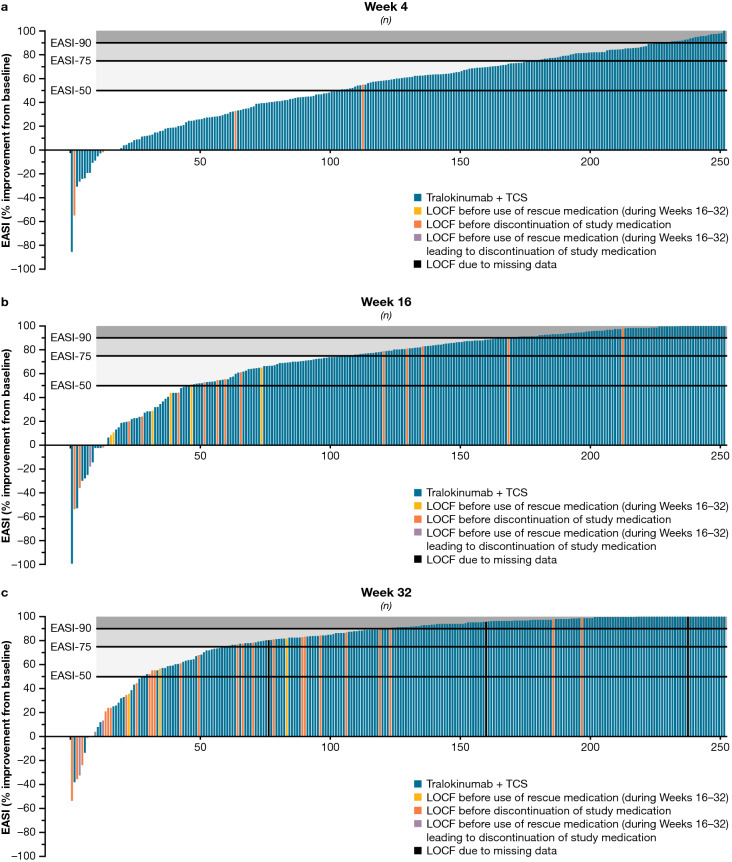
At Week 16, the least squares mean percentage improvement in EASI from baseline was 71.2% with tralokinumab (p < 0.001 vs. placebo); this increased to 84.2% at Week 32 with continued tralokinumab (Table 2 and Fig. S3, see ESM). Figure 3 provides further granularity into EASI responses over time, including the outcome for each of the 252 patients initiated on tralokinumab plus TCS at Weeks 4, 16, and 32.
Table 2.
Efficacy endpoints at Weeks 16 and 32
| Outcome | Week 16 | Week 32 | |
|---|---|---|---|
| Tralokinumab q2w plus TCS | Placebo q2w plus TCS | Tralokinumab q2w/q4w plus TCS | |
| IGA 0/1 responders, n (%) | 98/252 (38.9) | 33/126 (26.2) | 123/252 (48.8) |
| Difference, %, vs. placebo (95% CI); p value | 12.4 (2.9–21.9); p = 0.015 | ||
| EASI-75 responders, n (%) | 141/252 (56.0) | 45/126 (35.7) | 177/252 (70.2) |
| Difference, %, vs. placebo (95% CI); p value | 20.2 (9.8–30.6); p < 0.001 | ||
| EASI-50 responders, n (%) | 200/252 (79.4) | 73/126 (57.9) | 204/252 (81.0) |
| Difference, %, vs. placebo (95% CI); p value | 21.3 (11.3–31.3); p < 0.001 | ||
| EASI-90 responders, n (%) | 83/252 (32.9) | 27/126 (21.4) | 127/252 (50.4) |
| Difference, %, vs. placebo (95% CI); p value | 11.4 (2.1–20.7); p = 0.022 | ||
| Adjusted mean percentage improvement in EASI ± SE | 71.2 ± 2.2 | 55.3 ± 3.2 | 84.2 ± 1.4 |
| Difference, %, vs. placebo (95% CI); p value | 15.9 (8.2–23.6); p < 0.001 | ||
| Adjusted mean percentage improvement in worst daily pruritus NRS (weekly average) ± SE | 52.6 ± 1.8 | 38.4 ± 2.7 | 59.4 ± 1.9 |
| Difference, %, vs. placebo (95% CI); p value | 14.2 (7.9–20.5); p < 0.001 | ||
| Adjusted mean percentage improvement in eczema-related sleep NRS (weekly average) ± SE | 63.3 ± 2.3 | 46.8 ± 3.4 | 70.8 ± 2.4 |
| Difference, %, vs. placebo (95% CI); p value | 16.5 (8.5–24.5); p < 0.001 | ||
| Adjusted mean percentage improvement in DLQI total scores ± SE | 65.4 ± 2.5 | 49.1 ± 3.6 | 66.8 ± 3.1 |
| Difference, %, vs. placebo (95% CI); p value | 16.4 (7.6–25.1); p < 0.001 | ||
CI confidence interval, DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, EASI-50 at least 50% improvement in EASI, EASI-75 at least 75% improvement in EASI, EASI-90 at least 90% improvement in EASI, IGA Investigator’s Global Assessment, NRS numeric rating scale, q2w every 2 weeks, q4w every 4 weeks, SE standard error, TCS topical corticosteroids
Fig. 3.
Distribution of percentage improvement in EASI from baseline in all patients initiated on tralokinumab q2w at a Week 4, b Week 16, and c Week 32. a, b Tralokinumab q2w + TCS (n = 252), full analysis set. LOCF applied for patients using rescue medication/discontinuing study medication during the initial period (last observation available prior to using rescue medication/discontinuing study medication during the initial period). c Tralokinumab q2w/q4w + TCS (n = 252), full analysis set. LOCF applied for patients using rescue medication (during Weeks 16–32) or discontinuing study medication (last observation available prior to using rescue medication (during Weeks 16–32) or discontinuing study medication) and for patients with missing data for other reasons at Week 32 (last observation available). EASI Eczema Area and Severity Index, EASI-50 at least 50% improvement in EASI, EASI-75 at least 75% improvement in EASI, EASI-90 at least 90% improvement in EASI, LOCF last observation carried forward, q2w every 2 weeks, q4w every 4 weeks, TCS topical corticosteroids
Video 1 Percentage improvement in EASI from baseline to Week 32 in all patients initiated on tralokinumab q2w. (MP4 5305 KB)
In the subgroup of patients who achieved the pre-defined response criteria (IGA 0/1 and/or EASI-75) at Week 16 and were subsequently re-randomized to tralokinumab q2w (n = 69) or q4w (n = 69) plus TCS, the proportion of patients achieving EASI-90 continued to increase to 72.5% and 63.8%, respectively, at Week 32 (Fig. S4a, see ESM). In the subgroup of patients who did not achieve the pre-defined response criteria at Week 16 and continued receiving tralokinumab q2w plus TCS (n = 95), 34.7% achieved EASI-90 at Week 32 (Fig. S4a, see ESM).
Patient-Reported Outcomes
Patient-reported outcomes, including pruritus NRS, sleep NRS, and DLQI, improved from baseline to Week 16 and were sustained through Week 32 in the pooled tralokinumab-treated patient group (n = 252; Table 2 and Fig. 4). Weekly average of worst daily pruritus NRS and eczema-related sleep interference NRS improved throughout the initial 16-week treatment period, with a mean improvement of 52.6% with tralokinumab plus TCS versus 38.4% with placebo plus TCS at Week 16 for itch, and an improvement of 63.3% with tralokinumab plus TCS versus 46.8% with placebo plus TCS for sleep (both p < 0.001). Sustained improvements in pruritus NRS and sleep NRS of 59.4% and 70.8%, respectively, were observed through Week 32 in the pooled tralokinumab-treated patient group (Fig. 4a, b).
Fig. 4.
Least squares mean percentage improvement by visit in a weekly average of worst daily pruritus NRS, b weekly average in eczema-related sleep NRS, and c DLQI. Hypothetical estimand: treatments were reassigned at Week 16 and the placebo arm was only followed up to Week 16. The tralokinumab arm was followed beyond Week 16 and the different dosing (q2w or q4w) was ignored. Rescue medication was reset at Week 16. Data collected after permanent discontinuation of study medication or initiation of rescue medication were not included. In case of no post-baseline assessments before initiation of rescue medication, the Week 2 change was imputed as 0. Repeated-measurements model: Endpoint = Treatment*Week + Baseline*Week + Region + Baseline IGA. Compound symmetry was assumed for the covariance matrix. *p < 0.05 vs. placebo + TCS; **p < 0.01 vs. placebo + TCS; ***p < 0.001 vs. placebo + TCS. DLQI Dermatology Life Quality Index, IGA Investigator’s Global Assessment, NRS numeric rating scale, q2w every 2 weeks, q4w every 4 weeks, SE standard error, TCS topical corticosteroids
DLQI total scores improved with tralokinumab plus TCS versus placebo plus TCS, with a mean improvement of 65.4% with tralokinumab plus TCS compared to 49.1% with placebo plus TCS at Week 16 (p < 0.001). In the pooled tralokinumab-treated group, improvements in DLQI were sustained with continued tralokinumab plus TCS treatment through Week 32 (Fig. 4). In addition, the improvement in DLQI from study baseline was sustained through Week 32 in Week 16 responder subgroups who were re-randomized to tralokinumab q2w (83%) and q4w (77%) during the continuation period (Fig. S4b, see ESM).
Evaluation of Clinically Meaningful Response at Week 16
More patients receiving tralokinumab plus TCS achieved a clinically meaningful response in each of the three disease-specific domains measured—AD signs (EASI-50), symptoms (pruritus NRS ≥ 3 improvement), and/or QoL (DLQI ≥ 4 improvement)—compared with placebo plus TCS at Week 16 (Table 3). Most patients (89.9%) who were initiated on tralokinumab q2w plus TCS achieved a clinically meaningful response in at least one of the three domains at Week 16, and 75.3% achieved a response in AD signs in combination with symptoms or QoL (p ≤ 0.001 vs. placebo for both) (Table 3 and Fig. 5). Of the patients who were initiated on tralokinumab q2w plus TCS, 53.4% (132/247) achieved a clinically meaningful improvement in all three domains at Week 16 (difference vs. placebo, 25.0%; 95% confidence interval 14.9–35.2; p < 0.001).
Table 3.
Composite efficacy endpoints at Week 16
| Outcome | Week 16 | |
|---|---|---|
| Tralokinumab q2w plus TCS | Placebo q2w plus TCS | |
| EASI-50 or itch improvement of NRS ≥ 3 points or DLQI improvement of ≥ 4 points responders, n (%) | 222/247 (89.9) | 95/123 (77.2) |
| Difference, %, vs. placebo (95% CI); p value | 12.5 (4.2–20.8); p = 0.001 | |
| EASI-50 and itch improvement NRS ≥ 3 points responders, n (%) | 137/251 (54.6) | 42/126 (33.3) |
| Difference, %, vs. placebo (95% CI); p value | 21.3 (11.0–31.6); p < 0.001 | |
| EASI-50 and itch improvement NRS ≥ 3 points and DLQI improvement ≥ 4 points | 132/247 (53.4) | 35/123 (28.5) |
| Difference, %, vs. placebo (95% CI); p value | 25.0 (14.9–35.2); p < 0.001 | |
| EASI-50 and itch improvement NRS ≥ 3 points or DLQI improvement ≥ 4 points | 186/247 (75.3) | 64/123 (52.0) |
| Difference, %, vs. placebo (95% CI); p value | 23.2 (12.8–33.5); p < 0.001 | |
Patients with pruritus NRS < 3 and DLQI < 4 at baseline were excluded from the analysis where pruritus and DLQI is implicated
CI confidence interval, DLQI Dermatology Life Quality Index, EASI-50 at least 50% improvement in Eczema Area and Severity Index, NRS numeric rating scale, q2w every 2 weeks, TCS topical corticosteroids
Fig. 5.
Composite endpoint response rates at Week 16. DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, EASI-50 at least 50% improvement in EASI, NRS numeric rating scale, q2w every 2 weeks, TCS topical corticosteroids
Topical Corticosteroid Use up to Week 32
Tralokinumab plus TCS-treated patients used less of the supplied TCS compared with placebo plus TCS-treated patients at Weeks 15–16 (11.4 g (SE, 1.6) vs. 20.2 g (SE, 2.3); p = 0.002) and TCS use remained consistently low with continued tralokinumab treatment, with mean TCS use during Weeks 16–32 ranging from 9.2 to 13.6 g (SE, 1.2–2.0) every 2 weeks (Fig. 6).
Fig. 6.
TCS use from baseline to Week 32 by visit. Assuming no TCS was used from non-returned tubes. Hypothetical estimand: treatments were reassigned at Week 16 and the placebo arm was only followed up to Week 16. The tralokinumab arm was followed beyond Week 16 and the different dosing (q2w or q4w) was ignored. Rescue medication was reset at Week 16. Data collected after permanent discontinuation of study medication or initiation of rescue medication were not included. In case of no post-baseline assessments before initiation of rescue medication, the Week 2 change was imputed as 0. Repeated-measurements model: Endpoint = Treatment*Week + Baseline*Week + Region + Baseline IGA. Compound symmetry was assumed for the covariance matrix. *p < 0.05 vs. placebo + TCS; **p < 0.01 vs. placebo + TCS; ***p < 0.001 vs. placebo + TCS. IGA Investigator’s Global Assessment, q2w every 2 weeks, q4w every 4 weeks, SE standard error, TCS topical corticosteroids
Discussion
The pivotal ECZTRA 3 trial was designed to evaluate the efficacy of tralokinumab plus TCS versus TCS alone at Week 16 according to pre-defined thresholds established by guidance from regulatory authorities. All primary and secondary objectives were met, confirming the efficacy of tralokinumab q2w plus TCS for EASI-75, IGA 0/1, pruritus NRS, SCORing Atopic Dermatitis, and DLQI observed at Week 16 [10]. Tralokinumab plus TCS was well tolerated, with an overall frequency and severity of adverse events comparable with TCS alone over 32 weeks [10].
Since AD is a chronic condition requiring long-term treatment, in this post hoc analysis we evaluated the efficacy of tralokinumab plus TCS as needed over 32 weeks. Data were pooled from all patients initiated on tralokinumab q2w, no matter the response achieved at Week 16 or the dosing regimen (q2w or q4w) used between Weeks 16 and 32. This provides a better understanding of the clinical response achieved over time in all patients initiated on tralokinumab treatment at study start, reflecting clinical practice not bound by the design limitations of a pivotal phase III trial. In addition, because no single outcome captures the full burden of a multidimensional disease such as AD, a more holistic evaluation of the Week 16 response was performed.
Importantly, this analysis demonstrates that additional patients achieved the clinical response targets preferred by regulatory authorities (IGA 0/1 or EASI-75) beyond Week 16, indicating that response rates progressively improve over time with continued tralokinumab therapy. The proportion of patients achieving EASI-75 increased from 56% at Week 16 to 70% at Week 32, and the proportion achieving EASI-90 progressively improved to 50% at Week 32 with no indication of reaching a plateau (Fig. S2c, see ESM). This is clinically relevant, as Week 16 appears to be too early for evaluating the full benefit of tralokinumab on lesions in some patients. However, this analysis also identified a group of “super-responders” achieving EASI-75, or even EASI-90 response, after only 4 weeks of treatment with tralokinumab q2w plus TCS (Fig. 3a; Video 1, online version only).
Improvements observed with tralokinumab plus TCS in disease domains important to patients with AD, such as itch, sleep, and DLQI, occurred early, within the first few weeks of therapy, and were then sustained through Week 32.
Examining the Week 16 response from a holistic perspective, the vast majority (89.9%) of patients treated with tralokinumab q2w plus TCS achieved a clinical response equivalent to established levels of clinically meaningful improvements in AD signs (EASI-50), symptoms (pruritus NRS improvement ≥ 3), or QoL (DLQI improvement ≥ 4), and 75.3% achieved a meaningful reduction in lesion extent and severity in combination with an improvement in either pruritus or QoL.
The range of outcomes reported in this study shows progressive and sustained improvements using tralokinumab in both clinician- and patient-reported outcomes. When evaluating treatment success in clinical practice for a chronic disease such as AD, it is important to look beyond the primary endpoints and timepoints recommended by regulatory authorities. Considering the chronicity of AD, the impact of an AD treatment must be considered against the short- and long-term needs of each patient and the relevant risks and benefits of existing or alternative treatment options [5, 17]. Including patients in shared decision-making maximizes patient adherence and treatment success [18].
Limitations of this analysis include post hoc pooling of patients after re-randomization to two different dosing regimens (q2w and q4w) at Week 16. As reported previously, both tralokinumab q2w and q4w treatment regimens were able to maintain an EASI-75 response at Week 32 in nine out of ten patients [10]. Furthermore, the improvements in EASI-90 response rates and DLQI were similar between the q2w and q4w treatment arms during Weeks 16–32 (Fig. S4, see ESM); this supports pooling of the two treatment arms in the current analysis and expected real-world clinical practice.
The high response rates at Week 16 in the group treated with TCS alone should also be acknowledged. This may reflect the provision of TCS (mometasone furoate 0.1% cream) free of charge at bi-weekly visits in ECZTRA 3. Importantly, all patients treated with tralokinumab used approximately 50% less TCS at Week 16 and the mean use remained low (around 5–7 g/week) in the continuation period (Fig. 6), further demonstrating the effectiveness of tralokinumab.
Another limitation is the lack of a placebo comparator arm beyond Week 16. When designing the ECZTRA 3 study, comparison between tralokinumab plus TCS versus TCS alone was limited to Week 16. In order to ensure that all patients with uncontrolled moderate-to-severe AD at Week 16 had access to active systemic therapy, patients initially randomized to placebo who did not achieve the primary outcomes at Week 16 were assigned to receive tralokinumab q2w in addition to TCS during Weeks 16–32. This prevented the inclusion of the majority of patients initiated on placebo in any comparative analyses beyond Week 16.
Despite these limitations, the current analyses provide a more complete picture of the benefits of tralokinumab over time, which may help guide clinicians in their decision-making. It is difficult to compare the results of tralokinumab and other systemic treatment options, mainly because no comparative study has been carried out and between-trial comparisons should be made with caution. Based on recent trials comparing Janus kinase (JAK) inhibitors and dupilumab, JAK inhibitors appear to have a faster onset of action than dupilumab [19–21]; however, the response rates seem to even out over time. The proportion of patients achieving EASI-75 at Week 32 with tralokinumab plus TCS reported in our analysis (70%) is similar to the proportion of patients achieving EASI-75 over time reported in other systemic-plus-TCS studies in adults with moderate-to-severe AD [22, 23].
Conclusions
In summary, tralokinumab plus TCS as needed provided progressive and sustained improvements over 32 weeks in the extent and severity of AD and in patient-reported outcomes in patients with moderate-to-severe AD. TCS use, as needed, remained low in patients treated with tralokinumab compared with placebo over 32 weeks, demonstrating the TCS-sparing effects of tralokinumab.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing and editorial assistance were provided by Amy Graham, PhD, and Lauren Smith, BA, from Complete HealthVizion, funded by LEO Pharma A/S.
Declarations
Funding
The tralokinumab ECZTRA 3 trial was sponsored by LEO Pharma A/S (Ballerup, Denmark).
Conflicts of interest/Competing interests
JIS reports personal fees from AbbVie, Afyx, AnaptysBio, Aobiome, Arena, Asana, Aslan, BioMX, Bluefin, Bodewell, Boehringer Ingelheim, Celgene, Connect Biopharma, Dermavant, Dermira, DS Biopharma, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, Kymab, LEO Pharma, Luna, Menlo, Novartis, Pfizer, RAPT, Realm, Regeneron, and Sanofi Genzyme, grants and/or personal fees from Galderma, GlaxoSmithKline and Pfizer, and stock/stock options from AbbVie, Arcutis, and Eli Lilly outside the submitted work. DNA has been an investigator, consultant, advisory board member, speaker for and/or received honoraria from AbbVie, Actelion, Amgen, Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Coherus, Dermira, Dermavant, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Stiefel, Sun Pharma, UCB, and Valeant. MZ is a consultant, investigator, and/or speaker for AbbVie, All Free Care/Sun, Anaptys Bio, Arcutis, Bausch Health, Cara, Concert, Dermavant, Edessa Biotech, Eli Lilly and Company, EPI Health, Fit Bit, Galderma, Genentech, Incyte, LEO Pharma, Level-Ex, L’Oréal, LUUM, Menlo, Novartis, Oculus, Ortho Derm, Peloton, Pfizer, Regeneron, Sanofi, Trevi, and UCB, is a part owner of AsepticMD, and has worked with LEO Pharma and other companies with atopic dermatitis drugs that have received FDA approval or are expected to receive FDA approval in the next year. SK reports consulting for AbbVie, Amgen Inc, Aralez Pharmaceuticals Canada Inc, Bausch Health, Celgene Corporation, Eli Lilly and Company, Galderma SA, Johnson & Johnson, La Roche-Posay, Novartis International AG, Pfizer Inc, Sanofi Genzyme, and UCB; conducting clinical trials that have received funding from AbbVie, Amgen Inc, Bausch Health, Corbus Pharmaceuticals Holdings Inc, Eli Lilly and Company, Janssen Pharmaceuticals, LEO Pharma, Merck & Co, Novartis, Pfizer Inc, and UCB; receiving grant funding from LEO Pharma A/S and Novartis International AG; and serving as co-chair of the Canadian PSOLAR steering committee; and has received salary support from the Michael Smith Foundation for Health Research. JG is a consultant and/or speaker for and/or receives honoraria from AbbVie, Almirall, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi Genzyme, and has provided expert testimony to AbbVie and LEO Pharma. AP reports acting as an investigator, speaker, or advisor for AbbVie, Almirall-Hermal, Amgen, Biogen Idec, BioNTec, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Hexal, Janssen, LEO Pharma, MC2, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Regeneron, Roche, Sandoz Biopharmaceuticals, Sanofi Genzyme, Schering-Plough, Tigercat Pharma, and UCB Pharma. AEP has acted as an advisor/speaker/investigator for or received educational support from AbbVie, Almirall, Amgen, Anaptys Bio, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Galderma, Janssen, La Roche-Posay, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi, and UCB. AC has served as a scientific adviser and/or clinical study investigator for AbbVie, Almirall, Fresenius Kabi, Janssen, LEO Pharma, Lilly, Novartis, Sanofi Genzyme, and UCB Pharma, and as paid speaker for AbbVie, Almirall, Janssen, LEO Pharma, Lilly, Novartis, and Sanofi Genzyme. SB is an investigator or speaker for AbbVie, Almirall, Chiesi, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi-Genzyme, UCB Pharma. TM and AMT are employees of and hold stock in LEO Pharma. SW is co-principal investigator of the German atopic eczema registry TREATgermany; has received institutional research grants from La Roche-Posay, LEO Pharma, and Sanofi Deutschland GmbH; has performed consultancies for AbbVie, Almirall, Eli Lilly, GlaxoSmithKline, Kymab, LEO Pharma, Novartis, Pfizer, Regeneron, and Sanofi Genzyme; has lectured at educational events sponsored by AbbVie, Almirall, Eli Lilly, Galderma, LEO Pharma, Pfizer, Novartis, Regeneron, and Sanofi Genzyme; has received work-related travel support from AbbVie, LEO Pharma, and Sanofi Genzyme; and is involved in performing clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of psoriasis and atopic eczema.
Ethics approval
This randomized study was conducted in accordance with the ethical principles derived from international guidelines including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines and in compliance with International Council for Harmonisation guidelines for Good Clinical Practice. The clinical trial was approved by institutional review boards or ethics committees at each study site. This trial followed the Consolidated Standards of Reporting Trials reporting guideline.
Consent to participate
All patients provided written informed consent.
Consent for publication
NA.
Availability of data and material
Data will be made available, upon request to the study sponsor, following review by the external Patient and Scientific Review Board.
Code availability
NA.
Author contributions
Conceptualization: JIS (equal), DNA (equal), MZ (equal), SK (equal), JG (equal), AP (equal), AEP (equal), AC (equal), SB (equal), TM (equal), AMT (equal), SW (equal); Investigation: JIS (equal), DNA (equal), MZ (equal), SK (equal), JG (equal), AP (equal), AEP (equal), AC (equal), SB (equal), TM (equal), AMT (equal), SW (equal); Data curation: TM (lead); Formal analysis: TM (lead), AMT (supporting); Methodology: TM (equal), AMT (equal); Visualization: AMT (lead); Writing-original draft: JIS (equal), DNA (equal), MZ (equal), SK (equal), JG (equal), AP (equal), AEP (equal), AC (equal), SB (equal), TM (equal), AMT (equal), SW (equal); Writing-review & editing: JIS (equal), DNA (equal), MZ (equal), SK (equal), JG (equal), AP (equal), AEP (equal), AC (equal), SB (equal), TM (equal), AMT (equal), SW (equal).
References
- 1.Vilsbøll AW, Anderson P, Piercy J, Milligan G, Kragh N. Extent and impact of inadequate disease control in US adults with a history of moderate to severe atopic dermatitis following introduction of new treatments. Dermatol Ther (Heidelb). 2021;11(2):475–486. doi: 10.1007/s13555-021-00488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2021;21(1):21–40. doi: 10.1038/s41573-021-00266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei EX, Kirsner RS, Eaglstein WH. End points in dermatologic clinical trials: A review for clinicians. J Am Acad Dermatol. 2016;75(1):203–209. doi: 10.1016/j.jaad.2016.01.052. [DOI] [PubMed] [Google Scholar]
- 4.Augustin M, Langenbruch A, Blome C, Gutknecht M, Werfel T, Ständer S, et al. Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol. 2020;34(1):142–152. doi: 10.1111/jdv.15919. [DOI] [PubMed] [Google Scholar]
- 5.Thyssen JP, Vestergaard C, Deleuran M, de Bruin-Weller MS, Bieber T, Taieb A, et al. European Task Force on Atopic Dermatitis (ETFAD): treatment targets and treatable traits in atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34(12):e839–e842. doi: 10.1111/jdv.16716. [DOI] [PubMed] [Google Scholar]
- 6.Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75(1):54–62. doi: 10.1111/all.13954. [DOI] [PubMed] [Google Scholar]
- 7.Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, et al. Atopic dermatitis is an IL-13 dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 2019;139:1480–1489. doi: 10.1016/j.jid.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popovic B, Breed J, Rees DG, Gardener MJ, Vinall LMK, Kemp B, et al. Structural characterisation reveals mechanism of IL-13-neutralising monoclonal antibody tralokinumab as inhibition of binding to IL-13Ra1 and IL-13Ra2. J Mol Biol. 2017;429(2):208–219. doi: 10.1016/j.jmb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour JP, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2) Br J Dermatol. 2021;184(3):437–449. doi: 10.1111/bjd.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverberg JI, Toth D, Bieber T, Alexis AF, Elewski BE, Pink AE, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450–463. doi: 10.1111/bjd.19573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761180. Accessed 7 Feb 2022.
- 12.European Medicines Agency. Adtralza (tralokinumab) European Public Assessment Report. 19 Aug 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/adtralza#product-information-section. Accessed 7 Sep 2021.
- 13.Health Canada. Adtralza product information. 3 Nov 2021. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=101050. Accessed 9 Nov 2021.
- 14.Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. What are the best endpoints for Eczema Area and Severity Index and Scoring Atopic Dermatitis in clinical practice? A prospective observational study. Br J Dermatol. 2021;184(5):888–895. doi: 10.1111/bjd.19457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbé A, Nelson L, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–769. doi: 10.1111/bjd.17744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basra MKA, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27–33. doi: 10.1159/000365390. [DOI] [PubMed] [Google Scholar]
- 17.Boguniewicz M, Alexis AF, Beck LA, Block J, Eichenfield LF, Fonacier L, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract. 2017;5(6):1519–1531. doi: 10.1016/j.jaip.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Heratizadeh A, Werfel T, Wollenberg A, Abraham S, Plank-Habibi S, Schnopp C, et al. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140(3):845–53.E3. doi: 10.1016/j.jaci.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs. dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055. doi: 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–1112. doi: 10.1056/NEJMoa2019380. [DOI] [PubMed] [Google Scholar]
- 21.Reich K, Thyssen JP, Blauvelt A, Eyerich K, Soong W, Rice ZP, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis who received background topical therapy in a 26-week, randomized, head-to-head trial. Abstract presented at the EADV 30th Congress; 29 Sep–2 Oct 2021.
- 22.Silverberg JI, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, Costanzo A, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: Week 52 AD Up study results. J Allergy Clin Immunol. 2022;149(3):977–87.e14. [DOI] [PubMed]
- 23.Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.