Abstract
Introduction
We aimed to describe patients with coexisting infective endocarditis (IE) and bacterial meningitis (BM).
Methods
We merged two large prospective cohorts, an IE cohort and a BM cohort, with only cases of definite IE and community-acquired meningitis. We compared patients who had IE and BM concurrently to patients with IE only and BM only.
Results
Among the 1030 included patients, we identified 42 patients with IE–BM (4.1%). Baseline characteristics of patients with IE–BM were mostly similar to those of patients with IE, but meningitis was the predominant presentation at admission (39/42, 92.3%). Causative pathogens were predominantly Streptococcus pneumoniae (18/42, 42.9%) and Staphylococcus aureus (14/42, 33.3%). All pneumococcal IE were associated with BM (18/18). BM due to oral and group D streptococci, Streptococcus agalactiae, and S. aureus were frequently associated with IE (14/30, 46.7%). Three-month mortality was 28.6% in patients with IE–BM, 20.5% in patients with IE, and 16.6% in patients with BM.
Conclusions
Patients with pneumococcal IE or altered mental status during IE must be investigated for BM. Patients with S. aureus, oral and group D streptococcal or enterococcal BM, or unfavorable outcome in pneumococcal meningitis would benefit from an echocardiography. Patients with the dual infection have the worst prognosis. Their identification is mandatory to initiate appropriate treatment.
Keywords: Bacterial meningitis, Infective endocarditis, Echocardiography, Staphylococcus, Streptococcus, Austrian syndrome
Key Summary Points
| The association of infective endocarditis (IE) and bacterial meningitis is rare but severe. |
| This association usually presents itself as meningitis. |
| Patients with pneumococcal IE or altered mental status must be investigated for meningitis. |
| Patients with S. aureus, oral and group D streptococcal or enterococcal meningitis must be investigated for endocarditis. |
| Unfavorable outcome in pneumococcal meningitis must be investigated for endocarditis. |
Introduction
Infective endocarditis (IE) and bacterial meningitis (BM) share low incidence, therapeutic challenges resulting from poor antibiotic diffusion to infection sites, and a mortality rate approximating 20%. Furthermore, the clinical presentation of each entity may mimic that of the other. The situation is even more complex when these two diseases develop in a single patient, given the necessity of urgent and specific therapeutic management of each condition.
This combination of IE and BM in a single patient has been described mainly through case reports. A recent Dutch nationwide cohort study on meningitis showed that 24 out of 1025 patients (2%) with BM also had IE [1]. Although the study provided original information on this rare combination, only cases initially identified with meningitis were described, without any control group reported.
To describe the natural history and clinical characteristics of this rare combination, we analyzed the cases of endocarditis–meningitis combination in a cohort of patients with IE and a cohort of patients with BM. This enabled us to compare patients with both IE and BM with patients presenting each disease alone.
Methods
Study Design
We merged the data from the AEPEI IE cohort and the COMBAT meningitis cohort. The AEPEI IE cohort is a prospective observational study that was conducted in 2008 in seven French regions by the Association pour l’Etude et la Prevention de l’Endocardite Infectieuse (AEPEI) that included all definite cases of IE. The methods used for this study—hereafter referred to as the AEPEI IE cohort—have been described elsewhere [2]. Briefly, all physicians in public and private practice who were likely to manage patients with IE were contacted and invited with frequent reminders to report every suspected case of IE. A case report form was filled out by a trained clinical research assistant and validated by an expert committee. The COMBAT meningitis cohort is a prospective observational study in which all cases of definite community-acquired bacterial meningitis identified in 69 French hospitals in 2013–2014 were reported, as described elsewhere [3]. Both cohorts received a 1-year follow-up.
Case Definition and Report Form
IE cases were classified by an expert committee using the modified Duke classification and only definite IE cases were considered [4]. Bacterial meningitis was defined by positive CSF (cerebrospinal fluid) culture and/or positive soluble antigen in CSF with or without cell reaction and/or positive polymerase chain reaction (PCR) in CSF and/or purpura fulminans (with or without positive CSF culture) with a PCR in blood and/or positive blood culture and CSF cell reaction. Patients with both IE and BM were compared to patients with IE only and to patients with BM only, first for all patients and then according to the causative microorganism (Streptococcaceae, Staphylococcus aureus).
A specific case report form was used for each cohort. Medical history, risk factors for IE, clinical presentation, laboratory and echocardiographic findings, medical and surgical treatment, and outcome were collected. Two variables were specific to the AEPEI IE cohort, namely medical or surgical procedures or situations entailing risk of bacteremia (within 3 months before hospitalization). Otherwise, most definitions from the BM cohort were similar to those of the IE cohort. Moreover, the design of the case report form, as well as the data analysis implied some common authors from the two studies [2, 3].
Furthermore, medical charts of patients with IE–BM were reviewed for the time sequence of events, the diagnosis (IE or BM) established at hospital admission, and which of the two infections pre-existed according to the medical history of patients with IE–BM in the weeks preceding hospital admission.
Statistical Methods
Continuous variables were described with median and interquartile range [IQR], and categorical variables as number of cases and percentage. Variables were compared with non-parametric tests, the Fisher’s exact test for categorical variables and the Mann–Whitney–Wilcoxon test for continuous variables. All statistical analyses were performed with SAS v9.2 (SAS Institute).
Ethical and Regulatory Issues
The AEPEI IE and COMBAT cohorts (ClinicalTrials.gov: NCT03272724 and NCT02916732, respectively) were approved by an institutional review board (CHU Besançon 12/2007 and CPP Ile de France CPP4 (IRB00003835) (2012-16NI), respectively) and the French data protection board (CNIL) (DR-2017-003 and EGY/FLR/AR128794, respectively). In both cohorts, patients were informed of the study orally and, in accordance with French law, did not have to provide written consent.
Results
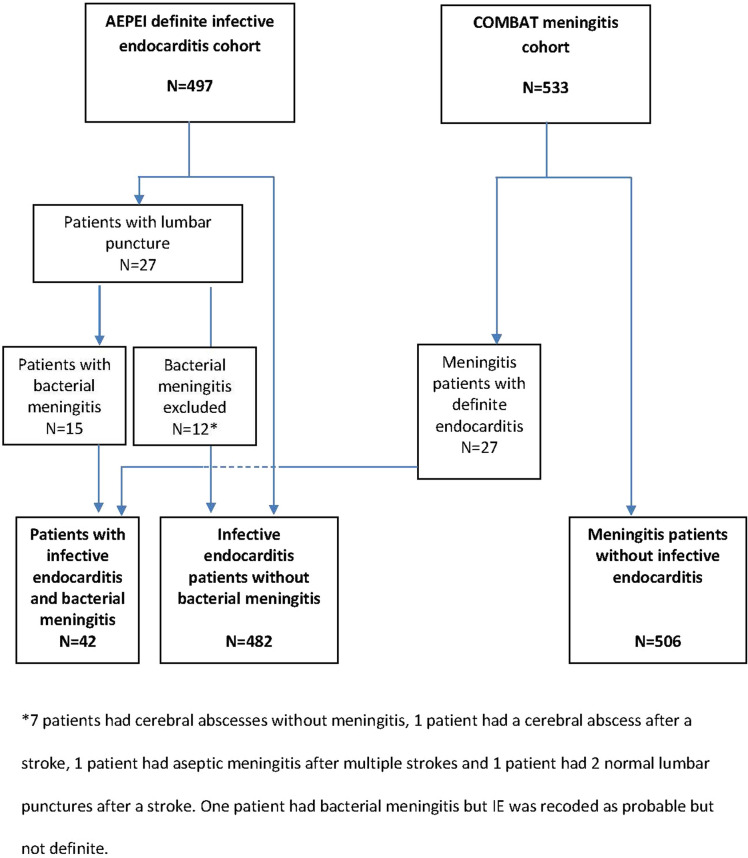
Out of the 497 patients with definite IE included in the AEPEI IE cohort, 15 patients (3%) also had meningitis. Out of the 533 patients with BM included in the COMBAT meningitis cohort, 27 (5.1%) also had definite IE. Among the 1030 patients from the merged cohorts, there were 42 patients who had both IE and meningitis (patients with IE–BM), 482 patients with IE only (patients with IE), and 506 patients with meningitis only (patients with BM) (Fig. 1).
Fig. 1.
Flowchart
Patients with IE–BM
Among the 42 patients with IE–BM, 31 (71.8%) were male, median age was 61 years [54.1–71.8], and 9 (21.4%) had a previously identified IE-predisposing cardiac condition. Streptococcaceae were the most frequent microorganisms (28 (66.6%) patients), including Streptococcus pneumoniae in 18 (42.9%) patients, and other typical IE pathogens, such as oral and group D streptococci or enterococci (Table 1). Staphylococci were represented only by S. aureus in 14 (33.3%) of the patients with IE–BM. Seven (16.7%) patients with IE–BM presented an Austrian syndrome (combination of pneumonia, meningitis, and endocarditis due to S. pneumoniae), all of them with risk factors for invasive pneumococcal disease (alcoholism (n = 5), tobacco use (n = 3), diabetes mellitus (n = 2), and AIDS (n = 1)), and a severe presentation (septic shock, heart failure, or coma), but none died. S. pneumoniae was the causative microorganism in half of the alcoholic patients with IE–BM.
Table 1.
Comparison of the characteristics of the three groups of patients according to the localization of the infections: infective endocarditis and meningitis, infective endocarditis only, and community acquired meningitis only
| IE and meningitis (n = 42) | IE only (n = 482) | Pa | Meningitis only (n = 506) | Pb | Meningitis only excluding N. meningitidis (n = 395) | Pb | |
|---|---|---|---|---|---|---|---|
| Gender: male n (%) | 31 (73.8) | 359 (74.5) | 1.000 | 275 (54.3) | 0.015 | 214 (54.2) | 0.015 |
| Age (years) median [IQR] | 61.0 [54.1–71.8] | 64.8 [52.8–75.5] (n = 481) | 0.341 | 58.2 [40.3–68.5] (n = 500) | 0.055 | 61.0 [50.5–71.8] (n = 389) | 0.609 |
| Comorbidities | |||||||
| Diabetes mellitus | 9 (21.4) | 110 (22.8) | 1.000 | 71 (14.3) (n = 495) | 0.256 | 67 (17.4) (n = 384) | 0.526 |
| Coronary diseases | 4 (9.5) | 58 (12.0) | 0.805 | 21 (4.3) (n = 494) | 0.123 | 21 (5.5) (n = 384) | 0.293 |
| Chronic cardiac failure | 4 (9.5) | 76 (15.8) | 0.373 | 28 (5.6) (n = 497) | 0.301 | 28 (7.3) (n = 386) | 0.540 |
| Chronic renal failure | 5 (11.9) | 57 (11.8) | 1.000 | 20 (4.0) (n = 496) | 0.037 | 19 (4.9) (n = 386) | 0.074 |
| Neoplasia | 1 (2.4) | 88 (18.3) | 0.005 | 54 (10.8) (n = 498) | 0.108 | 49 (12.7) (n = 387) | 0.045 |
| Liver disease | 5 (11.9) | 67 (13.9) | 1.000 | 27 (5.4) (n = 496) | 0.094 | 26 (6.8) (n = 385) | 0.213 |
| Immunodeficiency (innate or acquired, including HIV) | 6 (14.3) | 33 (6.8) | 0.114 | 55 (11.0) (n = 499) | 0.455 | 49 (12.6) (n = 388) | 0.807 |
| Alcoholism | 10 (23.8) | 58 (12.6) (n = 461) | 0.056 | 74 (14.9) (n = 495) | 0.181 | 66 (17.2) (n = 384) | 0.291 |
| IV drug use | 2 (4.8) | 28 (5.8) | 1.000 | NA | NA | ||
| Hypertension | 17 (40.5) | 227 (47.1) | 0.426 | NA | NA | ||
| Cardiac condition predisposing to endocarditis | |||||||
| Any | 9 (21.4) | ||||||
| Pre-existing valvulopathy | 9 (21.4) | 174 (37.0) (n = 470) | 0.056 | NA | NA | ||
| Endocardial lead | 1 (2.4) | 65 (13.5) | 0.048 | NA | NA | ||
| Symptoms and signs on presentation | |||||||
| NYHA III/IV | 10 (33.3) (n = 30) | 97 (77.6) (n = 125) | < 0.001 | NA | NA | ||
| Time between symptom onset and hospitalization (days) | 2.0 [0.0–4.0] (n = 39) | 3.0 [0.0–10.0] (n = 294) | 0.065 | 1.0 [0.0–2.0] (n = 487) | 0.046 | 1.0 [0.0–2.0] (n = 376) | 0.059 |
| Time between symptom onset and hospitalization n (%) | (n = 488) | 0.001 | (n = 487) | 1.000 | (n = 376) | 1.000 | |
| After hospitalization | 1 (2.4) | 31 (6.5) | 13 (2.7) | 12 (3.2) | |||
| < 1 month | 41 (97.6) | 320 (66.7) | 471 (96.7) | 362 (96.3) | |||
| 1–3 months | 0 (0.0) | 81 (16.9) | 3 (0.6) | 2 (0.5) | |||
| > 3 months | 0 (0.0) | 40 (8.3) | 0 (0.0) | 0 (0.0) | |||
| Time between hospitalization and initiation of antibiotics(days) median [IQR] | 0.0 [0.0–1.0] | 2.0 [0.0–5.0] (n = 481) | < 0.001 | 0.0 [0.0–1.0] (n = 495) | 0.011 | 0.0 [0.0–1.0] (n = 385) | 0.053 |
| Fever | 36 (92.3) (n = 39) | 406 (89.4) (n = 454) | 0.785 | 342 (69.9) (n = 495) | 0.002 | 280 (73.5) (n = 381) | 0.010 |
| Glasgow coma scale, median [IQR] | 10.0 [9.0–13.0] (n = 29) | 15.0 [15.0–15.0] (n = 416) | < 0.001 | 10.0 [7.0–12.0] (n = 331) | 0.148 | 10.0 [8.0–12.0] (n = 285) | 0.162 |
| Biological tests on presentation | |||||||
| Blood white cells, median [IQR] | 15.2 [10.6–23.7] (n = 40) | 11.6 [7.8–14.9] (n = 467) | 0.001 | 15.4 [9.9–21.0] (n = 475) | 0.542 | 15.2 [9.9–20.4] (n = 365) | 0.423 |
| CRP, median [IQR] | 258.0 [108.0–360.0] (n = 35) | 103.7 [51.0–188.0] (n = 458) | < 0.001 | 181.5 [87.0–295.0] (n = 414) | 0.061 | 177.0 [78.0–301.0] (n = 319) | 0.056 |
| Microorganisms | (n = 456) | < 0.001 | (n = 493) | < 0.001 | (n = 493) | < 0.001 | |
| Streptococcaceae | 28 (66.6) | 232 (50.9) | |||||
| S. pneumoniae | 18 (42.9) | 0 (0.0) | 265 (53.8) | 265 (69.4) | |||
| S. pyogenes, Enterococcus, other streptococci (Pneumococcus excluded) | 5 (11.9) | 87 (19.1) | 23 (4.7) | 23 (6.0) | |||
| Oral streptococci | 4 (9.5) | 83 (18.2) | 7 (1.4) | 7 (1.8) | |||
| Group D streptococci | 1 (2.4) | 62 (13.6) | 2 (0.4) | 2 (0.5) | |||
| Staphylococci | 14 (33.3) | 173 (37.9) | |||||
| S. aureus | 14 (33.3) | 125 (27.4) | 4 (0.8) | 4 (1.0) | |||
| Coagulase-negative Staphylococcus | 0 (0.0) | 48 (10.5) | 0 (0.0) | 0 (0.0) | |||
| N. meningitidis | 0 (0.0) | 0 (0.0) | 111 (22.5) | 0 (0.0) | |||
| Othersc | 0 (0.0) | 51 (11.2) | 81 (16.4) | 81 (21.2) | |||
| ≥ 2 microorganisms | 0 (0.0) | 9 (1.9) | 1.000 | 0 (0.0) | 0 (0.0) | ||
| Infection origin | (n = 471) | 0.002 | |||||
| Community acquired | 40 (95.2) | 349 (74.1) | 506 (100%) | NA | 395 (100%) | NA | |
| Hospital acquired | 1 (2.4) | 109 (23.1) | 0 (0.0) | 0 (0.0) | |||
| Healthcare related but non-hospital acquired | 1 (2.4) | 13 (2.8) | 0 (0.0) | 0 (0.0) | |||
| IE characteristics | |||||||
| Location of IE | (n = 41) | < 0.001 | |||||
| Aortic valve | 13 (31.7%) | 94 (19.5%) | NA | NA | |||
| Mitral valve | 22 (53.7%) | 128 (26.6%) | NA | NA | |||
| Aortic & mitral valve | 2 (4.9%) | 143 (29.7%) | NA | NA | |||
| Tricuspid valve | 1 (2.4%) | 34 (7.1%) | NA | NA | |||
| Tricuspid & pulmonary | 0 (0.0%) | 1 (0.2%) | NA | NA | |||
| Bilateral IE | 3 (7.3%) | 59 (12.2%) | NA | NA | |||
| Endocardial lead | 0 (0.0%) | 14 (2.9%) | NA | NA | |||
| Other | 0 (0.0%) | 2 (0.4%) | NA | NA | |||
| Undetermined | 0 (0.0%) | 7 (1.5%) | NA | NA | |||
| Vegetation | 30 (71.4%) | 420 (87.1%) | 0.010 | NA | NA | ||
| Septic shock | 20 (48.8) (n = 41) | 77 (16.0) | < 0.001 | NA | NA | ||
| Left ventricular ejection fraction < 45% | 3 (7.7) (n = 39) | 49 (11.8) (n = 416) | 0.602 | NA | NA | ||
| Cardiac abscess | 7 (16.7%) | 79 (16.4%) | 1.000 | NA | NA | ||
| Prosthesis dehiscence | 0 (0.0%) (n = 29) | 20 (19.6%) (n = 102) | 0.007 | NA | NA | ||
| Severe regurgitation | 24 (58.5%) (n = 41) | 186 (46.4%) (n = 401) | 0.144 | NA | NA | ||
| Cerebrovascular complications, (a)symptomatic | 21 (50.0) | 123 (25.5) | 0.002 | 128 (25.3) | 0.001 | 111 (28.1) | 0.005 |
| Valvular surgery | |||||||
| Rate | 18 (42.9%) | 227 (47.1%) | 0.632 | NA | NA | ||
| Time between surgery and hospitalization or diagnosis, median [IQR] | 10.5 [2.0–19.0] | 8.0 [2.0–22.0] | 0.923 | NA | NA | ||
| In-hospital outcome | |||||||
| Mortality at 30 days | 9 (21.4) | 64 (13.3) | 0.161 | 69 (13.6) | 0.169 | 64 (16.2) | 0.386 |
| Mortality at 3 months | 12 (28.6) | 99 (20.5) | 0.238 | 84 (16.6) | 0.058 | 79 (20.0) | 0.229 |
| Time between hospitalization and death, median [IQR] | 23.0 [15.0–31.0] (n = 13) | 27.0 [12.0–51.0] (n = 113) | 0.463 | 10.0 [2.0–39.0] (n = 97) | 0.134 | 11.0 [2.5–44.5] (n = 92) | 0.200 |
| Length of stay for survivors, median [IQR] | 47.0 [30.0–58.0] (n = 30) | 43.0 [27.0–66.0] (n = 481) | 0.857 | 16.0 [11.0–29.0] (n = 359) | < 0.001 | 18.0 [14.0–34.0] (n = 259) | < 0.001 |
| Length of stay, median [IQR] | 44.0 [21.0–58.0] (n = 35) | 46.0 [29.0–71.0] (n = 481) | 0.228 | 15.0 [9.0–28.0] (n = 445) | < 0.001 | 17.0 [11.0–32.0] (n = 340) | < 0.001 |
We also performed the analysis for meningitis only after withdrawal of meningitis due to Neisseria meningitis
The causative microorganisms in the patients with IE–BM from the AEPEI IE cohort were Streptococcaceae (8, out of 240, 3.3%) (3 S. pneumoniae, 1 Streptococcus dysgalactiae subsp. equisimilis, 1 Enterococcus faecalis, 1 Streptococcus oralis, 1 Streptococcus anginosus, 1 Streptococcus pyogenes), and S. aureus (7, out of 180, 3.9%) (only 1 MRSA). One patient had both a S. aureus and Streptococcus infantarius
Procedure or situations entailing risk of bacteremia were specific to the AEPEI cohort. They were actively searched among the 27 patients from the COMBAT meningitis cohort but not for the patients with BM only, therefore identified in the comparison with NA. (Procedures entailing risk were dental procedures, gastrointestinal or urogenital procedures, respiratory tract procedures, skin and soft tissue procedures, cardiac catheterization. Situations entailing risk were prolonged central venous access, active intravenous drug abuse, skin and soft tissue lesion)
NA non-applicable/non-available
aComparison of the patients with IE-BM to the IE population
bComparison of the patients with IE–BM to the meningitis population
cOthers included Listeria monocytogenes + Haemophilus influenzae + E. coli + Mycobacterium tuberculosis + Others + ≥ 2 microorganisms
Cerebrospinal fluid (CSF) showed pleocytosis (white cell count 450 [100–1200]) with predominance of polymorphonuclear leukocytes (PMNs) (90% [86–93]). Abnormally high protein levels in the CSF and hypoglycorrhachia were almost constantly reported. Direct identification of pathogens in CSF occurred in 22/42 patients and CSF culture was positive in 31/42. Among eight patients with negative CSF direct examination and culture, all had positive blood cultures, one had a CSF-positive antigen for pneumococci, and one a positive PCR. All patients with sterile CSF were already receiving antibiotics for more than 1 day.
At hospital admission, meningitis was diagnosed first in 39 (92.3%) out of the 42 patients with IE–BM (time between the two diagnoses of 4 [2–10] days); among these 39 patients, IE was subsequently searched for because of embolic complications (cerebral, ocular, cutaneous etc.) or cardiac failure revealing severe valve regurgitation. IE was diagnosed first in 3/42 patients (time between the two diagnoses of 1 [1–1.5] days), and meningitis was considered subsequently, given an unexplained altered mental status. A posteriori, review of the time sequence of events suggested that IE preceded meningitis in 18 patients, was simultaneous in 16 patients, posterior in 5 patients (all with S. pneumoniae), and undetermined in 3 patients. Endocarditis was always pre-existent or concomitant to meningitis with staphylococci.
Patients with IE–BM Compared with Patients with IE and Patients with BM
Regarding baseline characteristics, patients with IE–BM shared more similarities with patients with IE than with patients with BM, particularly for gender, age, and comorbidities.
Among all IE cases of the AEPEI cohort, 8/240 (3.3%) patients had associated Streptococcaceae BM, 7/180 (3.9%) patients had associated S. aureus BM, and 3/3 patients with S. pneumoniae IE had associated BM. Among all BM cases of the COMBAT cohort, 21/318 (6.6%) patients had associated Streptococcaceae IE (S. pneumoniae 15/280 (5.4%), oral streptococci 3/10, group D streptococci 2/4, Streptococcus agalactiae 2/5). Seven out of 11 (63.6%) patients had associated S. aureus IE. All in all, patients with BM from the COMBAT study with oral and group D streptococci, S. agalactiae, and S. aureus often had associated IE (14/30; 46.7%). No patients with BM with Neisseria meningitidis presented with IE. In absence of IE due to N. meningitidis, we also compared IE + BM with BM only after exclusion of the N. meningitidis cases.
Among patients with IE–BM, the mitral valve was the most frequently involved valve (more than 50%), followed by the aortic valve (ca. 30%), while contrary to patients with IE, other or dual localizations were exceptional. IE–BM and BM were mostly community-acquired, contrary to patients with IE, notably staphylococcus IE.
Regarding diagnosis of patients with IE–BM at hospital admission, the time interval between symptom onset and hospitalization was short, similar to meningitis or S. aureus IE, but differing from Streptococcaceae IE (involving a significantly longer time interval before hospitalization). Almost two-thirds of the patients with IE–BM (25/42) initially presented fever associated with altered mental status, and fever was almost constant in patients with IE–BM, patients with IE, and patients with S. aureus BM, but only in 73.3% of the patients with Streptococcaceae BM. Focal neurological signs appeared in the initial presentation in 8 patients out of 42, among whom four presented seizures. Glasgow coma scale was altered for patients with IE–BM and biological markers were highly elevated, similar to patients with BM, but not to patients with IE, and septic shock was significantly more frequent.
Patients with IE–BM presented more cerebrovascular events than patients with IE or BM, with no difference in cardiac surgery rate with patients with IE (42.9% vs. 47.1%, p = 0.63). Time interval before surgery tended to be shorter for patients with IE–BM with S. aureus (1.5 vs. 8.0 days) and longer for patients with Streptococcaceae IE–BM (14.5 vs. 7.0 days) (Table 2). Mortality tended to be lower among the 18 patients (42.9%) who underwent valve surgery during the initial hospital stay than among the other patients with IE–BM (2/18 (11.1%) vs. 9/24 (37.5%); p = 0.07). Three-month mortality was highest in patients with IE–BM (12; 28.6%), intermediate in patients with IE (99; 20.5%), and lowest in patients with BM (84; 16.6%). After withdrawal of meningococcal BM, 3-month mortality was similar in patients with BM and patients with IE. Length of hospital stay and survival were similar in patients with IE–BM and in patients with IE but significantly longer in both cases than in patients with BM (Table 1). Death tended to occur earlier (12 vs. 26 days) and more frequently (42.9% vs. 21.4%) among patients with IE–BM with S. aureus than in those with Streptococcaceae.
Table 2.
Comparison of the characteristics of the three groups of patients with streptococci or staphylococci infections according to the localization of the infections: infective endocarditis and meningitis, infective endocarditis only, and community acquired meningitis only
| Streptococci | Staphylococci | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IE + meningitis (n = 28) | IE only (n = 232) | Pa | Meningitis only (n = 297) | Pb | IE + meningitis (n = 14) | IE only (n = 125) | Pa | Meningitis only (n = 4) | Pb | |
| Gender: male n (%) | 21 (75.0) | 180 (77.6) | 0.812 | 159 (53.5) | 0.030 | 10 (71.4) | 89 (71.2) | 1.000 | 2 (50.0) | 0.569 |
| Age (years) median [IQR] | 61.0 [53.3–71.2] | 65.4 [53.9–76.2] | 0.214 | 60.6 [50.0–71.2] (n = 293) | 0.596 | 64.1 [54.1–71.8] | 62.9 [44.5–75.6] | 0.992 | 62.7 [40.1–72.0] | 0.873 |
| Comorbidities | ||||||||||
| Diabetes mellitus | 6 (21.4) | 44 (19.0) | 0.800 | 50 (17.3) (n = 289) | 0.604 | 3 (21.4) | 30 (24.0) | 1.000 | 1 (25.0) | 1.000 |
| Coronary diseases | 4 (14.3) | 21 (9.1) | 0.325 | 19 (6.6) (n = 289) | 0.133 | 0 (0.0) | 16 (12.8) | 0.370 | 0 (0.0) | |
| Chronic cardiac failure | 3 (10.7) | 25 (10.8) | 1.000 | 20 (6.9) (n = 291) | 0.439 | 1 (7.1) | 24 (19.2) | 0.465 | 1 (25.0) | 0.405 |
| Chronic renal failure | 3 (10.7) | 18 (7.8) | 0.482 | 13 (4.5) (n = 291) | 0.156 | 2 (14.3) | 19 (15.2) | 1.000 | 0 (0.0) | 1.000 |
| Neoplasia | 1 (3.6) | 51 (22.0) | 0.022 | 35 (12.0) (n = 292) | 0.341 | 0 (0.0) | 15 (12.0) | 0.363 | 0 (0.0) | |
| Liver disease | 4 (14.3) | 27 (11.6) | 0.756 | 16 (5.5) (n = 290) | 0.087 | 1 (7.1) | 23 (18.4) | 0.464 | 0 (0.0) | 1.000 |
| Immunodeficiency (innate or acquired, including HIV) | 4 (14.3) | 15 (6.5) | 0.133 | 39 (13.3) (n = 293) | 0.777 | 2 (14.3) | 7 (5.6) | 0.225 | 1 (25.0) | 1.000 |
| Alcoholism | 9 (32.1) | 29 (12.9) (n = 224) | 0.020 | 46 (15.9) (n = 289) | 0.038 | 1 (7.1) | 20 (17.5) (n = 114) | 0.464 | 0 (0.0) | 1.000 |
| IV drug use | 1 (3.6) | 3 (1.3) | 0.369 | 1 (7.1) | 21 (16.8) | 0.698 | ||||
| Hypertension | 12 (42.9) | 114 (49.1) | 0.555 | 5 (35.7) | 54 (43.2) | 0.777 | ||||
| Cardiac condition predisposing to endocarditis | ||||||||||
| Any | 6 (21.4) | 3 (21.4) | ||||||||
| Pre-existing valvulopathy | 6 (21.4) | 89 (39.6) (n = 225) | 0.079 | 3 (21.4) | 36 (29.3) (n = 123) | 0.757 | ||||
| Endocardial lead | 1 (3.6) | 7 (3.0) | 0.603 | 0 (0.0) | 19 (15.2) | 0.216 | ||||
| Symptoms and signs on presentation | ||||||||||
| NYHA III/IV | 7 (31.8) (n = 22) | 50 (78.1) (n = 64) | < 0.001 | 3 (37.5) (n = 8) | 22 (81.5) (n = 27) | 0.027 | ||||
| Time between symptom onset and hospitalization in days | 1.5 [0.0–3.0] (n = 26) | 6.0 [1.0–19.0] (n = 126) | 0.001 | 1.0 [0.0–2.0] (n = 284) | 0.157 | 2.0 [0.0–4.0] (n = 13) | 2.0 [0.0–6.0] (n = 97) | 0.940 | 3.0 [2.0–4.5] | 0.954 |
| Time between symptom onset and hospitalization n (%) | (n = 28) | 0.002 | (n = 284) | 1.000 | (n = 124) | 0.874 | 0.405 | |||
| After hospitalization | 0 (0.0) | 8 (3.4) | 8 (2.8) | 1 (7.1) | 11 (8.9) | 1 (25.0) | ||||
| < 1 month | 28 (100.0) | 140 (60.3) | 274 (96.5) | 13 (92.9) | 102 (82.3) | 3 (75.0) | ||||
| 1–3 months | 0 (0.0) | 59 (25.4) | 2 (0.7) | 0 (0.0) | 9 (7.3) | 0 (0.0) | ||||
| > 3 months | 0 (0.0) | 23 (9.9) | 0 (0.0) | 0 (0.0) | 2 (1.6) | 0 (0.0) | ||||
| Time between hospitalization and initiation of antibiotics (days) median [IQR] | 0.0 [0.0–1.0] | 2.0 [0.0–3.0] | < 0.001 | 0.0 [0.0–1.0] (n = 290) | 0.044 | 0.5 [0.0–1.0] | 1.0 [0.0–4.0] | 0.039 | 1.5 [0.5–3.0] | 0.151 |
| Fever | 25 (96.2) (n = 26) | 195 (91.1) (n = 214) | 0.706 | 209 (73.3) (n = 285) | 0.008 | 11 (84.6) (n = 13) | 118 (96.7) (n = 122) | 0.103 | 4 (100.0) | 1.000 |
| Glasgow coma scale, median [IQR] | 10.0 [8.0–13.0] (n = 19) | 15.0 [15.0–15.0] (n = 203) | < 0.001 | 10.0 [7.0–12.0] (n = 227) | 0.581 | 12.5 [10.0–14.0] (n = 10) | 15.0 [14.0–15.0] (n = 105) | < 0.001 | 12.5 [12.0–13.0] (n = 2) | 1.000 |
| Biological tests on presentation | ||||||||||
| Blood white cells, median [IQR] | 20.6 [10.5–26.7] (n = 27) | 10.9 [7.6–14.8] (n = 225) | 0.005 | 15.4 [10.3–21.4] (n = 278) | 0.315 | 15.0 [12.0–16.5] (n = 13) | 13.0 [8.9–16.6] (n = 121) | 0.336 | 17.0 [12.9–20.8] (n = 3) | 0.420 |
| CRP, median [IQR] | 216.5 [100.0–306.0] (n = 22) | 93.0 [54.5–142.5] (n = 224) | < 0.001 | 187.5 [91.0–318.0] (n = 238) | 0.793 | 300.0 [250.0–401.0] (n = 13) | 212.0 [121.0–293.0] (n = 113) | 0.013 | 303.0 [221.0–334.0] | 0.610 |
| Microorganisms | < 0.001 | < 0.001 | 1.000 | 1.000 | ||||||
| Streptococci | ||||||||||
| S. pneumoniaec | 18 (64.3) | 0 (0.0) | 265 (89.2) | NA | NA | NA | NA | |||
| S. pyogenes + Enterococcus + other streptococci (Pneumococcus excluded) | 5 (17.9) | 87 (37.5) | 23 (7.7) | NA | NA | NA | NA | |||
| Oral streptococci | 4 (14.3) | 83 (35.8) | 7 (2.4) | NA | NA | NA | NA | |||
| Group D streptococci | 1 (3.6) | 62 (26.7) | 2 (0.7) | NA | NA | NA | NA | |||
| Staphylococci | ||||||||||
| S. aureus | NA | NA | NA | NA | 14 (100.0) | 125 (100.0) | 4 (100.0) | |||
| ≥ 2 microorganisms | 0 (0.0) | 0 (0.0) | 1.000 | 0 (0.0) | 1.000 | 0 (0.0) | 0 (0.0) | 1.000 | 0 (0.0) | 1.000 |
| Infection origin | (n = 224) | 0.306 | (n = 124) | 0.156 | ||||||
| Community | 27 (96.4) | 202 (90.2) | 13 (92.9) | 84 (67.7) | ||||||
| Hospital acquired | 0 (0.0) | 16 (7.1) | 1 (7.1) | 37 (29.8) | ||||||
| Non hospital acquired | 1 (3.6) | 6 (2.7) | 0 (0.0) | 3 (2.4) | ||||||
| IE characteristics | ||||||||||
| Location of IE | 0.002 | (n = 13) | 0.437 | |||||||
| Aortic valve | 9 (32.1) | 47 (20.3) | 4 (30.8) | 23 (18.4) | ||||||
| Mitral valve | 15 (53.6) | 64 (27.6) | 7 (53.8) | 37 (29.6) | ||||||
| Aortic & mitral valves | 1 (3.6) | 86 (37.1) | 1 (7.7) | 28 (22.4) | ||||||
| Tricuspid valve | 1 (3.6) | 4 (1.7) | 0 (0.0) | 16 (12.8) | ||||||
| Tricuspid & pulmonary valve | 0 (0.0) | 1 (0.8) | ||||||||
| Bilateral valves | 2 (7.1) | 26 (11.2) | 1 (7.7) | 16 (12.8) | ||||||
| Endocardial lead | 0 (0.0) | 3 (2.4) | ||||||||
| Other | 0 (0.0) | 2 (0.9) | 0 (0.0) | 1 (0.8) | ||||||
| Undetermined | 0 (0.0) | 3 (1.3) | 4 (30.8) | 23 (18.4) | ||||||
| Vegetation | 19 (67.9) | 203 (87.5) | 0.010 | 11 (78.6) | 111 (88.8) | 0.380 | ||||
| Septic shock | 12 (44.4) (n = 27) | 19 (8.2) | < 0.001 | 8 (57.1) | 43 (34.4) | 0.142 | ||||
| FeVG < 45% | 2 (7.4) (n = 27) | 18 (8.7) (n = 206) | 1.000 | 1 (8.3) (n = 12) | 16 (15.5) (n = 103) | 1.000 | ||||
| Cardiac abscess | 6 (21.4) | 36 (15.5) | 0.418 | 1 (7.1) | 21 (16.8) | 0.698 | ||||
| Prosthesis dehiscence | 0 (0.0) (n = 22) | 8 (20.5) (n = 39) | 0.042 | 0 (0.0) (n = 7) | 3 (13.6) (n = 22) | 0.558 | ||||
| Severe regurgitation | 19 (67.9) | 106 (49.5) (n = 214) | 0.074 | 5 (38.5) (n = 13) | 36 (37.1) (n = 97) | 1.000 | ||||
| Cerebrovascular complications, symptomatic or not | 13 (46.4) | 59 (25.4) | 0.025 | 89 (30.0) | 0.088 | 8 (57.1) | 40 (32.0) | 0.077 | 1 (25.0) | 0.577 |
| Valvular surgery | ||||||||||
| Rate | 12 (42.9) | 117 (50.4) | 0.549 | 6 (42.9) | 43 (34.4) | 0.563 | ||||
| Time between surgery and hospitalization or diagnosis, median [IQR] | 14.5 [6.0–25.0] | 7.0 [2.0–24.0] | 0.296 | 1.5 [1.0–13.0] | 8.0 [2.0–16.0] | 0.221 | ||||
| In-hospital outcome | ||||||||||
| Mortality, 30 days | 5 (17.9) | 19 (8.2) | 0.155 | 54 (18.2) | 1.000 | 4 (28.6) | 31 (24.8) | 0.751 | 0 (0.0) | 0.524 |
| Mortality, 3 months | 6 (21.4) | 33 (14.2) | 0.397 | 64 (21.5) | 1.000 | 6 (42.9) | 45 (36.0) | 0.771 | 0 (0.0) | 0.245 |
| Delay between hospitalization and death, median [IQ R] | 26.0 [18.0–37.0] | 31.0 [12.0–60.0] | 0.891 | 9.0 [2.0–32.0] | 0.035 | 12.0 [4.0–31.0] | 23.5 [11.0–42.0] | 0.203 | – | – |
| Length of stay for survivors, median [IQR] | 44.0 [30.0–57.0] | 42.0 [27.0–59.5] | 0.887 | 17.0 [14.0–30.5] | < 0.001 | 53.0 [26.0–65.0] | 40.0 [25.0–67.0] | 0.649 | 23.0 [7.0–63.0] | 0.483 |
| Length of stay, median [IQR] | 35.0 [23.0–57.0] (n = 22) | 45.0 [30.0–68.0] (n = 232) | 0.212 | 16.0 [11.0–28.0] (n = 262) | < 0.001 | 53.0 [17.0–60.0] (n = 13) | 40.0 [25.0–72.0] | 0.838 | 23.0 [7.0–63.0] (n = 3) | 0.736 |
aComparison of the patients with IE-BM to the IE population
bComparison of the patients with IE-BM to the meningitis population
cDexamethasone use was reported only in 8/18 patients with IE–BM with S. pneumoniae
Discussion
By merging two prospective cohorts, we were able to gather and describe the largest group of patients with IE–BM, to compare these patients with those with IE only and BM only, to determine their characteristics, and to propose specific care.
Association of IE and BM is a rare (3% of IE; 5% of BM) but severe condition, with a mortality rate of 28.6%, similar to the 2% rate (24/1025 BM) and 29% (7/24) mortality reported in the literature [1]. The background characteristics of the patients with IE–BM appeared closer to those of patients with IE, but most patients with IE–BM at admission seemed more comparable to patients with BM with acute presentation, and altered mental status leading to a shorter time interval between symptom onset and hospitalization and antibiotic initiation. Fever was almost systematic for patients with IE and patients with IE–BM but not for patients with BM, in accordance with the literature [5].
Precession (and potential responsibility) of one of the two diseases over the other is difficult to ascertain. Since the characteristics of the patients with IE–BM and patients with IE are similar, it seems plausible that in most cases, endocarditis preceded meningitis onset. The marked symptomatology of meningitis, as opposed to the more silent symptomatology of endocarditis, may explain why meningitis is in the foreground even though it is a complication of endocarditis. We observed that meningitis always resulted from a bacteremia and never from contiguous infectious foci, such as otitis. This is also valid for the classic Austrian syndrome [6], favored by alcoholism and immunosuppression [7]. Besides, patients with community-acquired S. aureus meningitis almost always present with a primary infection focus such as pneumonia or endocarditis [8, 9] and with a short time to endocarditis diagnosis (3 days in [1]). Indeed, there were only four cases of S. aureus BM without IE in the BM cohort, and 77.8% of BM were associated with IE. It is noticeable that patients with IE–BM were more likely to present with cerebrovascular events, with Streptococcaceae as well as S. aureus, in line with Servy et al. [10]. The increased risk of neurological complications usually associated with S. aureus [11] may result from a comparison limited to pneumococcal meningitis [1], or a limited sample size [12, 13].
Given the urgency of initiating appropriate treatment for each of these two infections (Table 3), it is necessary to identify both infections beforehand. Considering the often silent nature of cardiac damage, the main challenge is to diagnose cardiac localization during meningitis rather than the opposite. However, the negativity of 12 out of the 27 (44.4%) lumbar punctures performed in the 497 patients of the endocarditis cohort (Fig. 1), because of neurological signs, underscores the non-specificity of the neurological symptoms in patients with IE, possibly resulting from sepsis or low cardiac flow. For typically IE-responsible microorganisms (S. aureus, group D and oral streptococci, enterococci), IE usually appeared preceding meningitis, although the flagrant meningitis symptomatology placed it in the foreground; in such meningitis cases, we suggest performing an echocardiography, regardless of the presence or not of a previously identified IE predisposing cardiac condition. In contrast, since pneumococcus IE is rare, a lack of improvement over the course of meningitis and/or a previously known IE predisposing cardiac condition and/or another focus of infection (particularly pneumonia) should prompt an echocardiography. Indeed, early diagnosis of IE concomitant with pneumococcal meningitis is difficult and even unlikely, as IE symptoms are often non-specific, and most patients with meningitis have only one set of blood cultures performed before antibiotic initiation, because of the urgency of the situation, which limits assessment of persistent bacteremia, the most common IE diagnostic criterion. For the few patients with IE diagnosed first, the challenge is to know when to perform lumbar puncture. All our cases of pneumococcal endocarditis were associated with meningitis as were 40% of the 111 cases reported by de Egea et al. [14], suggesting the need for systematic or at least a low threshold to perform lumbar puncture in patients with pneumococcal IE. Coagulase-negative staphylococci were responsible for 10.5% of the IE cohort, but they were never isolated from a combined meningeal localization, either in the Dutch study or in ours.
Table 3.
Potential consequences of misdiagnosing one disease in patients with combined infective endocarditis and bacterial meningitis
| If considering | Pneumococcus | Streptococcaceae (without Pneumococcus) | S. aureus |
|---|---|---|---|
| Meningitis only |
Treatment will be too short (2 weeks vs. 4–6 weeks) Missing surgical options |
Treatment will be too short (2 weeks vs. 4–6 weeks) For E. faecalis, an association is optimal for IE (amoxicillin + gentamicine or amoxicillin + ceftriaxone). For other Strepto, an association with Genta is often used Missing surgical options |
Treatment will be too short (2–3 weeks vs. 4–6 weeks) Missing surgical options Missing association with rifampicin and gentamicin for prosthetic valve or intracardial device |
| IE only |
Absence of dexamethasone Potentially underdosed ceftriaxone (2 g × 2 vs. 75 or 100 mg/kg/day) |
Potentially underdosed ceftriaxone (2 g × 2 vs. 75 or 100 mg/kg/day) |
Vancomycin may be underdosed (plasmatic target 40–60 mg/l for meningitis vs. 20–30 mg/l for IE) Cloxacillin concentrations likely to be insufficient in cerebrospinal fluid |
Patients with IE–BM tended towards higher mortality, possibly in relation to the increased mortality associated with S. aureus IE [2], and a higher risk of cerebrovascular complications (ca. 50%), consistently with the Dutch study [1]. The higher mortality was not due to a different rate of cardiac surgery, as compared to patients with IE, which suggests that associated BM may not be a contraindication for cardiac surgery in patients with IE. In accordance with the literature, anticoagulants did not favor cerebrovascular complications [1, 15–17].
Our study has some limitations. Some definitions varied between the two cohorts, notably with regard to cerebrovascular complications. However, even though our new definition took into account all potentially described cerebrovascular events for homogeneity, we cannot formally rule out a reporting bias between the two cohorts. Meningitis may have been underreported in the endocarditis cohort, and endocarditis in the meningitis cohort. Nonetheless, the population-based design of our study limits the risk of referral bias [18], its prospective design limits reporting bias, and the consistency between our cohorts and the literature suggests minimal biases. Another limitation could be a change in the epidemiology between the IE cohort which was formed in 2008, and the BM cohort, formed in 2013–2015. Notwithstanding this noticeable delay, it is unlikely that there is a significant change as IE guidelines were updated in 2015 without any changes recommended between 2008 and 2015, and the BM guidelines published in 2008 focused on treatment rather than on diagnosis. In the meantime, changes in vaccine recommendation concerned pathogens unrelated to IE or BM (such as pertussis, HPV, or MMR in 2014) and the extension of pneumococcal vaccine to adults at risk was published at the end of 2013, with a practical implementation posterior to the constitution of our BM cohort. Consequently, significant changes in the epidemiology of IE and BM between 2008 and 2015 or in physicians’ practices are unlikely. Finally, the lack of precise information on the timing and doses of antibiotics precluded any analyses on the impact of appropriate medical treatment.
Conclusion
The combination of IE and BM is a rare event, with a dismal prognosis. Patients with pneumococcal IE or altered mental status during IE must be investigated for bacterial meningitis. Likewise, patients with S. aureus, oral and group D streptococcal or enterococcal MB, or unfavorable outcome in pneumococcal meningitis would benefit from blood cultures and echocardiography. Early diagnosis may allow better outcomes through timely surgery when needed, and optimal antibacterial regimen.
Acknowledgements
The authors are indebted to Isabelle Petigenet for data management and statistical analysis. The authors also thank the participants of the studies.
Funding
The AEPEI IE cohort was funded by a research grant from the French Ministry of Health (PHRC 2007), grants from the Société Française de Cardiologie, the European Society of Clinical Microbiology and Infectious Diseases, and Novartis France. The sponsor was Délégation à la Recherche Clinique et au Développement, Centre Hospitalier Universitaire de Besançon. The COMBAT cohort was funded by Assistance Publique—Hôpitaux de Paris, Inserm, The French Society of Infectious Diseases (SPILF), and Pfizer Laboratory. It was also supported by the Observatoire de la Resistance du Pneumocoque (ORP) and Santé Publique France. The sponsor of the study and the funding sources had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit it for publication. The Rapid Service Fee was funded by the University Hospital of Poitiers, to which the corresponding author is affiliated.
Medical Writing
The authors thank Jeffrey Arsham for English editing, the funding for his assistance has been provided by the University Hospital of Poitiers, to which the corresponding author is affiliated.
Author Contributions
Guillaume Béraud, Sarah Tubiana, Marie-Line Erpelding, Bruno Hoen and Xavier Duval were responsible for the conception and design of the study, analysis and interpretation of data. Guillaume Béraud drafted the article and Sarah Tubiana, Vincent Le Moing, Pierre Tattevin, Bruno Hoen and Xavier Duval revised the article. All the authors participated in acquisition of data, read and approved the version to be submitted.
Compliance with Ethics Guidelines
Guillaume Béraud, Sarah Tubiana, Marie-Line Erpelding, Vincent Le Moing, Catherine Chirouze, Isabelle Gorenne, Pauline Manchon, Pierre Tattevin, Veronique Vernet, Emmanuelle Varon, Bruno Hoen and Xavier Duval all have nothing to disclose.
Disclosures
The AEPEI IE and COMBAT cohorts (ClinicalTrials.gov: NCT03272724 and NCT02916732, respectively) were approved by an institutional review board (CHU Besançon 12/2007 and CPP Ile de France CPP4 (IRB00003835)(2012-16NI), respectively) and the French data protection board (CNIL)(DR-2017-003 and EGY/FLR/AR128794, respectively). In both cohorts, patients were informed of the study orally and, in accordance with French law, did not have to provide written consent.
Data Availability
Individual-level data will not be made publicly available with this article. Requests for sharing data for scientific research can be directed to the corresponding author. All proposals will be subject to scientific review by the AEPEI and COMBAT scientific committee.
AEPEI study group on Infective Endocarditis: Principal investigators: B. Hoen, X. Duval. Other members: F. Alla, A. Bouvet, S. Briançon, E. Cambau, M. Celard, C. Chirouze, N. Danchin, T. Doco-Lecompte, F. Delahaye, X. Duval, J. Etienne, B. Iung, V. Le Moing, JF. Obadia, C. Leport, C. Poyart, M. Revest, C. Selton-Suty, C. Strady, P. Tattevin, and F. Vandenesch. Region study coordinating investigators: Y. Bernard, S. Chocron, C. Chirouze, B. Hoen, P. Plesiat, I. Abouliatim, C. De Place, P. Tattevin, M. Revest, P.Y Donnio, F. Alla, J.P Carteaux, T. Doco-Lecompte, C. Lion, N. Aissa, C. Selton-Suty, B. Baehrel, R. Jaussaud, P. Nazeyrollas, C.Strady , V. Vernet, E. Cambau, X. Duval, B. Iung, P. Nataf, C. Chidiac, M. Celard, F. Delahaye, J.F. Obadia, F. Vandenesch, H. Aumaître, J. M. Frappier, V. Le Moing, E. Oziol, A. Sotto, C. Sportouch. Centre National de Référence des Streptocoques: C. Poyart, A. Bouvet. Centre National de Référence des staphylocoques: F. Vandenesch. M. Celard, M. Bes. Investigators: P. Abassade, E. Abrial, C. Acar, JF. Alexandra, N. Amireche, D. Amrein, P. Andre, M. Appriou, MA. Arnould, A. Atoui, F. Aziza, N. Baille, N. Bajolle, P. Battistella, S. Baumard, A. Ben Ali, J. Bertrand, S. Bialek, M. Bois Grosse, M. Boixados, F. Borlot, A. Bouchachi, O. Bouche, S. Bouchemal, JL. Bourdon, A. Bouvet, L. Brasme, J.F. Bruntz, J. Cailhol, M.P. Caplan, B. Carette, JP. Carteaux, O. Cartry, C. Cazorla, M. Celard, H. Chamagne, H. Champagne, G. Chanques, B. Chevalier, C. Chirouze, F. Chometon, C. Christophe, N. Colin De Verdiere, V. Daneluzzi, L. David, N. Danchin, P. De Lentdecker, V. Delcey, P. Deleuze, X. Duval, B. Deroure, V. Descotes-Genon, K. Didier Petit, A. Dinh, V. Doat, F. Duchene, F. Duhoux, M. Dupont, S. Ederhy, O. Epaulard, M. Evest, JF. Faucher, E. Fauveau, T. Ferry, M. Fillod, T. Floch, T. Fraisse, J.M. Frapier, L. Freysz, B. Fumery, B. Gachot, S. Gallien, P. Garcon, A. Gaubert, JL. Genoud, S. Ghiglione, C. Godreuil, I. Gandjbakhch, A. Grentzinger, L. Groben, D. Gherissi, A. Hagege, N. Hammoudi, F. Heliot, P. Henry, B. Hoen, P. Houriez, L. Hustache-Mathieu, O. Huttin, S. Imbert, B. Iung, S. Jaureguiberry, M. Kaaki, A. Konate, J. M. Kuhn, S. Kural Menasche, A. Lafitte, B. Lafon, F. Lanternier, V. Le Chenault, V. Le Moing, C. Lechiche, S. Lefevre Thibaut, A. Lefort, J. Lemoine, L. Lepage, C. Leport, C. Lepousé, J. Leroy, P. Lesprit, L. Letranchant, G. Loncar, C. Lorentz, I. Magnin-Poull, A. Makinson, H. Man, M. Mansouri, O. Marçon, JP. Maroni, V. Masse, F. Maurier, F. Mechaï, O. Merceron, D. Messika-Zeitoun, Z. Metref, V. Meyssonnier, C. Mezher, S. Micheli, M. Monsigny, S. Mouly, B. Mourvillier, O. Nallet, P. Nazerollas, V. Noel, E. Oziol, B. Payet, A. Pelletier, P. Perez, J. S. Petit, F. Philippart, E. Piet, C. Plainvert, B. Popovic, J. M. Porte, P. Pradier, R. Ramadan, M. Revest, J. Richemond, M. Rodermann, M. Roncato, I. Roigt, O. Ruyer, M. Saada, J. Schwartz, C. Selton-Suty, M. Simon, B. Simorre, S. Skalli, F. Spatz, C. Strady, J. Sudrial, L. Tartiere, A. Terrier De La Chaize, MC. Thiercelin, D. Thomas, M. Thomas, L. Toko, F. Tournoux, A. Tristan, JL. Trouillet, L. Tual, F. Verdier, V. Vernet Garnier, V. Vidal, P. Weyne, M. Wolff, A. Wynckel, N. Zannad, P. Y. Zinzius. COMBAT STUDY GROUP. Principal investigator: X. Duval. Steering Committee: B. Hoen, B. Mourvillier, M.-C. Ploy, S. Tubiana, E. Varon. Scientific committee: steering committee and the following members F. Caron, P-E. Bollaert, O. Gaillot, M-K. Taha, C. Poyart, S. Bonacorsi, F. Vandenesch, E. Cambau, M. Lecuit, A. Gravet, B. Frachet, T. Debroucker, D. Levy-Bruhl, F. Raffi, M. Preau. COMBAT Clinical Centers: N. Anguel, L. Argaud, S. Arista, L. Armand-Lefevre, S. Balavoine, R. Baraduc, G. Barnaud, G. Beraud, L. Bernard, G. Bernars, D. Bertei, E. Bessede, T. Billard Pomares, C. Biron, S. Bland, J. Boileau, P. Boubeau, S. Bourdon, A. Bousquet, S. Boyer, A. Bozorg-Grayeli, L. Bret, C. Bretonniere, F. Bricaire, E. Brocas, M. Brun, J. Buret, C. Burucoa, J. Cabalion, M. Cabon, E. Cambau, G. Camuset, C. Canevet, F. Caron, A. Carricajo, B. Castan, E. Caumes, C. Cazanave, A. Chabrol, T. Challan-Belval, V. Chanteperdrix-Marillier, C. Chaplain, C. Charlier-Woerther, H. Chaussade C. Chirouze, B. Clair, J. Colot, J-M. Conil, H. Cordel, P. Cormier, J. Cousson, P. Cronier, E. Cua, A. Dao-Dubremetz, S. Dargere, N. Degand, S. Dekeyser, D. Delaune, E. Denes, P.-F. Dequin, D. Descamps, E. Descloux, J.-L. Desmaretz, J.-L. Diehl, J. Dimet, A. Dinh, X. Duval, L. Escaut, C. Fabe, F. Faibis, C. Flateau, N. Fonsale, E. Forestier, N. Fortineau, A. Gagneux-Brunon, C. Garandeau, M. Garcia, D. Garot, S. Gaudry, F. Goehringer, A. Gravet, V. Gregoire-Faucher, M. Grosset, C. Gubavu, I. Gueit, D. Guelon, T. Guimard, J. Guinard, T. Hadou, J.-P. Helene, S. Henard, B. Henry, A-C. Hochart, B. Hoen, G. Illes, S. Jaffuel, I. Jarrin, F. Jaureguy, C. Joseph, M.-E. Juvin, S. Kayal, S. Kerneis, F.Lacassin, I. Lamaury, P. Lanotte, E. Laurens, H. Laurichesse, C. Le Brun, V. Le Moing, P. Le Turnier, H. Lecuyer, S. Ledru, C. Legrix, A. Lemaignen, C. Lemble, L. Lemee, O. Lesens, M. Levast, C. Lhommet, S. Males, E. Malpote, G. Martin-Blondel, M. Marx, R. Masson, O. Matray, A. Mbadi, F. Mechai, G. Mellon, A. Merens, M.-C. Meyohas, A. Michon, J. Mootien Yoganaden, D. Morquin, S. Mouly, N. Mrozek, S. Nguyen, Y. Nguyen, M. Ogielska, E. Oziol, B. Page, S. Patrat-Delon, I. Patry, A. Pechinot, S. Picot, D. Pierrejean, L. Piroth, C. Plassart, P. Plessis, M.-C. Ploy, L. Portel, P. Poubeau, M. Poupard, C. Poyart, T. Prazuck, L. Quaesaet, F. Raffi, A. Ramanantsoa, C. Rapp, L. Raskine, J. Raymond, M. Revest, A. Riche, S. Robaday-Voisin, F. Robin, J.-P. Romaszko, F. Rousseau, A-L. Roux, C. Royer, M. Saada, D. Salmon, C. Saroufim, J.-L. Schmit, M. Sebire, C. Segonds, V. Sivadon-Tardy, N. Soismier, O. Son, S. Sunder, F. Suy, D. Tande, J. Tankovic, N. Valin, N. Van Grunderbeeck, F. Vandenesch, E. Varon, R. Verdon, M. Vergnaud, V. Vernet-Garnier, M. Vidal, V. Vitrat, D. Vittecoq, F. Vuotto. Coordination and statistical analyses (Clinical trial unit, Hôpitaux Universitaires Paris Nord Val de Seine, AP-HP, Paris): I. Gorenne, C. Laouenan, E. Marcault, F. Mentre, B. Pasquet, C. Roy, S. Tubiana. Scientific partnership: SPLIF, CMIT, SRLF, SFM, REIVAC, SFORL, APNET, SPF. Partners: ORP (M.-C. Ploy), GPIP/ACTIV (C. Levy). Sponsor: DRCI APHP. ClinicalTrial. Gov identification number: NCT01730690.
Footnotes
The members of the AEPEI and the COMBAT study groups are listed in the Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bruno Hoen and Xavier Duval contributed equally.
Contributor Information
Guillaume Béraud, Email: guillaume@beraud.pro.
the AEPEI study group:
J. F. Obadia, C. Leport, C. Poyart, M. Revest, C. Selton-Suty, C. Strady, P. Tattevin, F. Vandenesch, Y. Bernard, S. Chocron, C. Chirouze, B. Hoen, P. Plesiat, I. Abouliatim, C. De Place, P. Tattevin, M. Revest, P. Y. Donnio, F. Alla, J. P. Carteaux, T. Doco-Lecompte, C. Lion, N. Aissa, C. Selton-Suty, B. Baehrel, R. Jaussaud, P. Nazeyrollas, C. Strady, V. Vernet, E. Cambau, X. Duval, B. Iung, P. Nataf, C. Chidiac, M. Celard, F. Delahaye, J. F. Obadia, F. Vandenesch, H. Aumaître, J. M. Frappier, V. Le Moing, E. Oziol, A. Sotto, C. Sportouch, C. Poyart, A. Bouvet, F. Vandenesch, M. Celard, M. Bes, P. Abassade, E. Abrial, C. Acar, J. F. Alexandra, N. Amireche, D. Amrein, P. Andre, M. Appriou, M. A. Arnould, A. Atoui, F. Aziza, N. Baille, N. Bajolle, P. Battistella, S. Baumard, A. Ben Ali, J. Bertrand, S. Bialek, M. Bois Grosse, M. Boixados, F. Borlot, A. Bouchachi, O. Bouche, S. Bouchemal, J. L. Bourdon, A. Bouvet, L. Brasme, J. F. Bruntz, J. Cailhol, M.P. Caplan, B. Carette, J. P. Carteaux, O. Cartry, C. Cazorla, M. Celard, H. Chamagne, H. Champagne, G. Chanques, B. Chevalier, C. Chirouze, F. Chometon, C. Christophe, N. Colin De Verdiere, V. Daneluzzi, L. David, N. Danchin, P. De Lentdecker, V. Delcey, P. Deleuze, X. Duval, B. Deroure, V. Descotes-Genon, K. Didier Petit, A. Dinh, V. Doat, F. Duchene, F. Duhoux, M. Dupont, S. Ederhy, O. Epaulard, M. Evest, J. F. Faucher, E. Fauveau, T. Ferry, M. Fillod, T. Floch, T. Fraisse, J. M. Frapier, L. Freysz, B. Fumery, B. Gachot, S. Gallien, P. Garcon, A. Gaubert, J. L. Genoud, S. Ghiglione, C. Godreuil, I. Gandjbakhch, A. Grentzinger, L. Groben, D. Gherissi, A. Hagege, N. Hammoudi, F. Heliot, P. Henry, B. Hoen, P. Houriez, L. Hustache-Mathieu, O. Huttin, S. Imbert, B. Iung, S. Jaureguiberry, M. Kaaki, A. Konate, J. M. Kuhn, S. Kural Menasche, A. Lafitte, B. Lafon, F. Lanternier, V. Le Chenault, V. Le Moing, C. Lechiche, S. Lefevre Thibaut, A. Lefort, J. Lemoine, L. Lepage, C. Leport, C. Lepousé, J. Leroy, P. Lesprit, L. Letranchant, G. Loncar, C. Lorentz, I. Magnin-Poull, A. Makinson, H. Man, M. Mansouri, O. Marçon, J. P. Maroni, V. Masse, F. Maurier, F. Mechaï, O. Merceron, D. Messika-Zeitoun, Z. Metref, V. Meyssonnier, C. Mezher, S. Micheli, M. Monsigny, S. Mouly, B. Mourvillier, O. Nallet, P. Nazerollas, V. Noel, E. Oziol, B. Payet, A. Pelletier, P. Perez, J. S. Petit, F. Philippart, E. Piet, C. Plainvert, B. Popovic, J. M. Porte, P. Pradier, R. Ramadan, M. Revest, J. Richemond, M. Rodermann, M. Roncato, I. Roigt, O. Ruyer, M. Saada, J. Schwartz, C. Selton-Suty, M. Simon, B. Simorre, S. Skalli, F. Spatz, C. Strady, J. Sudrial, L. Tartiere, A. Terrier De La Chaize, M. C. Thiercelin, D. Thomas, M. Thomas, L. Toko, F. Tournoux, A. Tristan, J. L. Trouillet, L. Tual, F. Verdier, V. Vernet Garnier, V. Vidal, P. Weyne, M. Wolff, A. Wynckel, N. Zannad, and P. Y. Zinzius
the COMBAT study group:
X. Duval, B. Hoen, B. Mourvillier, M.-C. Ploy, S. Tubiana, E. Varon, F. Caron, P.-E. Bollaert, O. Gaillot, M.-K. Taha, C. Poyart, S. Bonacorsi, F. Vandenesch, E. Cambau, M. Lecuit, A. Gravet, B. Frachet, T. Debroucker, D. Levy-Bruhl, F. Raffi, M. Preau, N. Anguel, L. Argaud, S. Arista, L. Armand-Lefevre, S. Balavoine, R. Baraduc, G. Barnaud, G. Beraud, L. Bernard, G. Bernars, D. Bertei, E. Bessede, T. Billard Pomares, C. Biron, S. Bland, J. Boileau, P. Boubeau, S. Bourdon, A. Bousquet, S. Boyer, A. Bozorg-Grayeli, L. Bret, C. Bretonniere, F. Bricaire, E. Brocas, M. Brun, J. Buret, C. Burucoa, J. Cabalion, M. Cabon, E. Cambau, G. Camuset, C. Canevet, F. Caron, A. Carricajo, B. Castan, E. Caumes, C. Cazanave, A. Chabrol, T. Challan-Belval, V. Chanteperdrix-Marillier, C. Chaplain, C. Charlier-Woerther, H. Chaussade, C. Chirouze, B. Clair, J. Colot, J.-M. Conil, H. Cordel, P. Cormier, J. Cousson, P. Cronier, E. Cua, A. Dao-Dubremetz, S. Dargere, N. Degand, S. Dekeyser, D. Delaune, E. Denes, P.-F. Dequin, D. Descamps, E. Descloux, J.-L. Desmaretz, J.-L. Diehl, J. Dimet, A. Dinh, X. Duval, L. Escaut, C. Fabe, F. Faibis, C. Flateau, N. Fonsale, E. Forestier, N. Fortineau, A. Gagneux-Brunon, C. Garandeau, M. Garcia, D. Garot, S. Gaudry, F. Goehringer, A. Gravet, V. Gregoire-Faucher, M. Grosset, C. Gubavu, I. Gueit, D. Guelon, T. Guimard, J. Guinard, T. Hadou, J.-P. Helene, S. Henard, B. Henry, A.-C. Hochart, B. Hoen, G. Illes, S. Jaffuel, I. Jarrin, F. Jaureguy, C. Joseph, M.-E. Juvin, S. Kayal, S. Kerneis, F. Lacassin, I. Lamaury, P. Lanotte, E. Laurens, H. Laurichesse, C. Le Brun, V. Le Moing, P. Le Turnier, H. Lecuyer, S. Ledru, C. Legrix, A. Lemaignen, C. Lemble, L. Lemee, O. Lesens, M. Levast, C. Lhommet, S. Males, E. Malpote, G. Martin-Blondel, M. Marx, R. Masson, O. Matray, A. Mbadi, F. Mechai, G. Mellon, A. Merens, M.-C. Meyohas, A. Michon, J. Mootien Yoganaden, D. Morquin, S. Mouly, N. Mrozek, S. Nguyen, Y. Nguyen, M. Ogielska, E. Oziol, B. Page, S. Patrat-Delon, I. Patry, A. Pechinot, S. Picot, D. Pierrejean, L. Piroth, C. Plassart, P. Plessis, M.-C. Ploy, L. Portel, P. Poubeau, M. Poupard, C. Poyart, T. Prazuck, L. Quaesaet, F. Raffi, A. Ramanantsoa, C. Rapp, L. Raskine, J. Raymond, M. Revest, A. Riche, S. Robaday-Voisin, F. Robin, J.-P. Romaszko, F. Rousseau, A.-L. Roux, C. Royer, M. Saada, D. Salmon, C. Saroufim, J.-L. Schmit, M. Sebire, C. Segonds, V. Sivadon-Tardy, N. Soismier, O. Son, S. Sunder, F. Suy, D. Tande, J. Tankovic, N. Valin, N. Van Grunderbeeck, F. Vandenesch, E. Varon, R. Verdon, M. Vergnaud, V. Vernet-Garnier, M. Vidal, V. Vitrat, D. Vittecoq, F. Vuotto, I. Gorenne, C. Laouenan, E. Marcault, F. Mentre, B. Pasquet, C. Roy, and S. Tubiana
References
- 1.Lucas Marjolein J, Brouwer Matthijs C, van der Ende A, van de Beek D. Endocarditis in adults with bacterial meningitis. Circulation. 2013;127(20):2056–2062. doi: 10.1161/CIRCULATIONAHA.113.001545. [DOI] [PubMed] [Google Scholar]
- 2.Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54(9):1230–1239. doi: 10.1093/cid/cis199. [DOI] [PubMed] [Google Scholar]
- 3.Tubiana S, Varon E, Biron C, et al. Community-acquired bacterial meningitis in adults: in-hospital prognosis, long term disability and determinants of outcome in a multicentre prospective cohort. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 5.Bijlsma Merijn W, Brouwer Matthijs C, Soemirien KE, et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis. 2016;16(3):339–347. doi: 10.1016/S1473-3099(15)00430-2. [DOI] [PubMed] [Google Scholar]
- 6.Austrian R. Pneumococcal endocarditis, meningitis, and rupture of the aortic valve. AMA Arch Intern Med. 1957;99(4):539–544. doi: 10.1001/archinte.1957.00260040039004. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Juanatey C, Testa A, Mayo J, Gonzalez-Gay Miguel A. Austrian syndrome: report of two new cases and literature review. Int J Cardiol. 2006;108(2):273–275. doi: 10.1016/j.ijcard.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Brouwer Matthijs C, Keizerweerd Gabriella D, Brouwer Matthijs C, et al. Community acquired Staphylococcus aureus meningitis in adults. Scand J Infect Dis. 2009;41(5):375–377. doi: 10.1080/00365540902744766. [DOI] [PubMed] [Google Scholar]
- 9.Le Moing V, Alla F, Doco-Lecompte T, et al. Staphylococcus aureus bloodstream infection and endocarditis–a prospective cohort study. PLoS ONE. 2015;10(5):e0127385. doi: 10.1371/journal.pone.0127385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Servy A, Valeyrie-Allanore L, Alla F, et al. Prognostic value of skin manifestations of infective endocarditis. JAMA Dermatol. 2014;150(5):494–500. doi: 10.1001/jamadermatol.2013.8727. [DOI] [PubMed] [Google Scholar]
- 11.Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R, Kotilainen P. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000;160(18):2781–2787. doi: 10.1001/archinte.160.18.2781. [DOI] [PubMed] [Google Scholar]
- 12.Corral I, Martín-Dávila P, Fortún J, et al. Trends in neurological complications of endocarditis. J Neurol. 2007;254(9):1253–1259. doi: 10.1007/s00415-006-0512-5. [DOI] [PubMed] [Google Scholar]
- 13.Duval X, Iung B, Klein I, et al. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med. 2010;152(8):497–504. doi: 10.7326/0003-4819-152-8-201004200-00006. [DOI] [PubMed] [Google Scholar]
- 14.de Egea V, Patricia M, Maricela V, et al. Characteristics and outcome of Streptococcus pneumoniae endocarditis in the XXI Century: a systematic review of 111 cases (2000–2013) Medicine (Baltimore) 2015;94(39):e1562. doi: 10.1097/MD.0000000000001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schut Ewout S, Lucas Marjolein J, Brouwer Matthijs C, Vergouwen Mervyn DI, van der Ende A, van de Beek D. Cerebral infarction in adults with bacterial meningitis. Neurocrit Care. 2012;16(3):421–427. doi: 10.1007/s12028-011-9634-4. [DOI] [PubMed] [Google Scholar]
- 16.Dickerman Stuart A, Elias A, Bruno B, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS) Am Heart J. 2007;154(6):1086–1094. doi: 10.1016/j.ahj.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Snygg-Martin U, Rasmussen RV, Hassager C, Bruun NE, Andersson R, Olaison L. Warfarin therapy and incidence of cerebrovascular complications in left-sided native valve endocarditis. Eur J Clin Microbiol Infect Dis. 2011;30(2):151–157. doi: 10.1007/s10096-010-1063-3. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Hidalgo N, Almirante B, Tornos P, et al. Prognosis of left-sided infective endocarditis in patients transferred to a tertiary-care hospital–prospective analysis of referral bias and influence of inadequate antimicrobial treatment. Clin Microbiol Infect. 2011;17(5):769–775. doi: 10.1111/j.1469-0691.2010.03314.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual-level data will not be made publicly available with this article. Requests for sharing data for scientific research can be directed to the corresponding author. All proposals will be subject to scientific review by the AEPEI and COMBAT scientific committee.
AEPEI study group on Infective Endocarditis: Principal investigators: B. Hoen, X. Duval. Other members: F. Alla, A. Bouvet, S. Briançon, E. Cambau, M. Celard, C. Chirouze, N. Danchin, T. Doco-Lecompte, F. Delahaye, X. Duval, J. Etienne, B. Iung, V. Le Moing, JF. Obadia, C. Leport, C. Poyart, M. Revest, C. Selton-Suty, C. Strady, P. Tattevin, and F. Vandenesch. Region study coordinating investigators: Y. Bernard, S. Chocron, C. Chirouze, B. Hoen, P. Plesiat, I. Abouliatim, C. De Place, P. Tattevin, M. Revest, P.Y Donnio, F. Alla, J.P Carteaux, T. Doco-Lecompte, C. Lion, N. Aissa, C. Selton-Suty, B. Baehrel, R. Jaussaud, P. Nazeyrollas, C.Strady , V. Vernet, E. Cambau, X. Duval, B. Iung, P. Nataf, C. Chidiac, M. Celard, F. Delahaye, J.F. Obadia, F. Vandenesch, H. Aumaître, J. M. Frappier, V. Le Moing, E. Oziol, A. Sotto, C. Sportouch. Centre National de Référence des Streptocoques: C. Poyart, A. Bouvet. Centre National de Référence des staphylocoques: F. Vandenesch. M. Celard, M. Bes. Investigators: P. Abassade, E. Abrial, C. Acar, JF. Alexandra, N. Amireche, D. Amrein, P. Andre, M. Appriou, MA. Arnould, A. Atoui, F. Aziza, N. Baille, N. Bajolle, P. Battistella, S. Baumard, A. Ben Ali, J. Bertrand, S. Bialek, M. Bois Grosse, M. Boixados, F. Borlot, A. Bouchachi, O. Bouche, S. Bouchemal, JL. Bourdon, A. Bouvet, L. Brasme, J.F. Bruntz, J. Cailhol, M.P. Caplan, B. Carette, JP. Carteaux, O. Cartry, C. Cazorla, M. Celard, H. Chamagne, H. Champagne, G. Chanques, B. Chevalier, C. Chirouze, F. Chometon, C. Christophe, N. Colin De Verdiere, V. Daneluzzi, L. David, N. Danchin, P. De Lentdecker, V. Delcey, P. Deleuze, X. Duval, B. Deroure, V. Descotes-Genon, K. Didier Petit, A. Dinh, V. Doat, F. Duchene, F. Duhoux, M. Dupont, S. Ederhy, O. Epaulard, M. Evest, JF. Faucher, E. Fauveau, T. Ferry, M. Fillod, T. Floch, T. Fraisse, J.M. Frapier, L. Freysz, B. Fumery, B. Gachot, S. Gallien, P. Garcon, A. Gaubert, JL. Genoud, S. Ghiglione, C. Godreuil, I. Gandjbakhch, A. Grentzinger, L. Groben, D. Gherissi, A. Hagege, N. Hammoudi, F. Heliot, P. Henry, B. Hoen, P. Houriez, L. Hustache-Mathieu, O. Huttin, S. Imbert, B. Iung, S. Jaureguiberry, M. Kaaki, A. Konate, J. M. Kuhn, S. Kural Menasche, A. Lafitte, B. Lafon, F. Lanternier, V. Le Chenault, V. Le Moing, C. Lechiche, S. Lefevre Thibaut, A. Lefort, J. Lemoine, L. Lepage, C. Leport, C. Lepousé, J. Leroy, P. Lesprit, L. Letranchant, G. Loncar, C. Lorentz, I. Magnin-Poull, A. Makinson, H. Man, M. Mansouri, O. Marçon, JP. Maroni, V. Masse, F. Maurier, F. Mechaï, O. Merceron, D. Messika-Zeitoun, Z. Metref, V. Meyssonnier, C. Mezher, S. Micheli, M. Monsigny, S. Mouly, B. Mourvillier, O. Nallet, P. Nazerollas, V. Noel, E. Oziol, B. Payet, A. Pelletier, P. Perez, J. S. Petit, F. Philippart, E. Piet, C. Plainvert, B. Popovic, J. M. Porte, P. Pradier, R. Ramadan, M. Revest, J. Richemond, M. Rodermann, M. Roncato, I. Roigt, O. Ruyer, M. Saada, J. Schwartz, C. Selton-Suty, M. Simon, B. Simorre, S. Skalli, F. Spatz, C. Strady, J. Sudrial, L. Tartiere, A. Terrier De La Chaize, MC. Thiercelin, D. Thomas, M. Thomas, L. Toko, F. Tournoux, A. Tristan, JL. Trouillet, L. Tual, F. Verdier, V. Vernet Garnier, V. Vidal, P. Weyne, M. Wolff, A. Wynckel, N. Zannad, P. Y. Zinzius. COMBAT STUDY GROUP. Principal investigator: X. Duval. Steering Committee: B. Hoen, B. Mourvillier, M.-C. Ploy, S. Tubiana, E. Varon. Scientific committee: steering committee and the following members F. Caron, P-E. Bollaert, O. Gaillot, M-K. Taha, C. Poyart, S. Bonacorsi, F. Vandenesch, E. Cambau, M. Lecuit, A. Gravet, B. Frachet, T. Debroucker, D. Levy-Bruhl, F. Raffi, M. Preau. COMBAT Clinical Centers: N. Anguel, L. Argaud, S. Arista, L. Armand-Lefevre, S. Balavoine, R. Baraduc, G. Barnaud, G. Beraud, L. Bernard, G. Bernars, D. Bertei, E. Bessede, T. Billard Pomares, C. Biron, S. Bland, J. Boileau, P. Boubeau, S. Bourdon, A. Bousquet, S. Boyer, A. Bozorg-Grayeli, L. Bret, C. Bretonniere, F. Bricaire, E. Brocas, M. Brun, J. Buret, C. Burucoa, J. Cabalion, M. Cabon, E. Cambau, G. Camuset, C. Canevet, F. Caron, A. Carricajo, B. Castan, E. Caumes, C. Cazanave, A. Chabrol, T. Challan-Belval, V. Chanteperdrix-Marillier, C. Chaplain, C. Charlier-Woerther, H. Chaussade C. Chirouze, B. Clair, J. Colot, J-M. Conil, H. Cordel, P. Cormier, J. Cousson, P. Cronier, E. Cua, A. Dao-Dubremetz, S. Dargere, N. Degand, S. Dekeyser, D. Delaune, E. Denes, P.-F. Dequin, D. Descamps, E. Descloux, J.-L. Desmaretz, J.-L. Diehl, J. Dimet, A. Dinh, X. Duval, L. Escaut, C. Fabe, F. Faibis, C. Flateau, N. Fonsale, E. Forestier, N. Fortineau, A. Gagneux-Brunon, C. Garandeau, M. Garcia, D. Garot, S. Gaudry, F. Goehringer, A. Gravet, V. Gregoire-Faucher, M. Grosset, C. Gubavu, I. Gueit, D. Guelon, T. Guimard, J. Guinard, T. Hadou, J.-P. Helene, S. Henard, B. Henry, A-C. Hochart, B. Hoen, G. Illes, S. Jaffuel, I. Jarrin, F. Jaureguy, C. Joseph, M.-E. Juvin, S. Kayal, S. Kerneis, F.Lacassin, I. Lamaury, P. Lanotte, E. Laurens, H. Laurichesse, C. Le Brun, V. Le Moing, P. Le Turnier, H. Lecuyer, S. Ledru, C. Legrix, A. Lemaignen, C. Lemble, L. Lemee, O. Lesens, M. Levast, C. Lhommet, S. Males, E. Malpote, G. Martin-Blondel, M. Marx, R. Masson, O. Matray, A. Mbadi, F. Mechai, G. Mellon, A. Merens, M.-C. Meyohas, A. Michon, J. Mootien Yoganaden, D. Morquin, S. Mouly, N. Mrozek, S. Nguyen, Y. Nguyen, M. Ogielska, E. Oziol, B. Page, S. Patrat-Delon, I. Patry, A. Pechinot, S. Picot, D. Pierrejean, L. Piroth, C. Plassart, P. Plessis, M.-C. Ploy, L. Portel, P. Poubeau, M. Poupard, C. Poyart, T. Prazuck, L. Quaesaet, F. Raffi, A. Ramanantsoa, C. Rapp, L. Raskine, J. Raymond, M. Revest, A. Riche, S. Robaday-Voisin, F. Robin, J.-P. Romaszko, F. Rousseau, A-L. Roux, C. Royer, M. Saada, D. Salmon, C. Saroufim, J.-L. Schmit, M. Sebire, C. Segonds, V. Sivadon-Tardy, N. Soismier, O. Son, S. Sunder, F. Suy, D. Tande, J. Tankovic, N. Valin, N. Van Grunderbeeck, F. Vandenesch, E. Varon, R. Verdon, M. Vergnaud, V. Vernet-Garnier, M. Vidal, V. Vitrat, D. Vittecoq, F. Vuotto. Coordination and statistical analyses (Clinical trial unit, Hôpitaux Universitaires Paris Nord Val de Seine, AP-HP, Paris): I. Gorenne, C. Laouenan, E. Marcault, F. Mentre, B. Pasquet, C. Roy, S. Tubiana. Scientific partnership: SPLIF, CMIT, SRLF, SFM, REIVAC, SFORL, APNET, SPF. Partners: ORP (M.-C. Ploy), GPIP/ACTIV (C. Levy). Sponsor: DRCI APHP. ClinicalTrial. Gov identification number: NCT01730690.