Abstract
Climate change is adversely affecting the burden of infectious disease throughout the world, which is a health security threat. Climate-sensitive infectious disease includes vector-borne diseases such as malaria, whose transmission potential is expected to increase because of enhanced climatic suitability for the mosquito vector in Asia, sub-Saharan Africa, and South America. Climatic suitability for the mosquitoes that can carry dengue, Zika, and chikungunya is also likely to increase, facilitating further increases in the geographic range and longer transmission seasons, and raising concern for expansion of these diseases into temperate zones, particularly under higher greenhouse gas emission scenarios. Early spring temperatures in 2018 seem to have contributed to the early onset and extensive West Nile virus outbreak in Europe, a pathogen expected to expand further beyond its current distribution, due to a warming climate. As for tick-borne diseases, climate change is projected to continue to contribute to the spread of Lyme disease and tick-borne encephalitis, particularly in North America and Europe. Schistosomiasis is a water-borne disease and public health concern in Africa, Latin America, the Middle East, and Southeast Asia; climate change is anticipated to change its distribution, with both expansions and contractions expected. Other water-borne diseases that cause diarrheal diseases have declined significantly over the last decades owing to socioeconomic development and public health measures but changes in climate can reverse some of these positive developments. Weather and climate events, population movement, land use changes, urbanization, global trade, and other drivers can catalyze a succession of secondary events that can lead to a range of health impacts, including infectious disease outbreaks. These cascading risk pathways of causally connected events can result in large-scale outbreaks and affect society at large. We review climatic and other cascading drivers of infectious disease with projections under different climate change scenarios.
Supplementary file1 (MP4 328467 KB)
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00647-3.
Keywords: Climate change, Infectious diseases, Cascading risks, Hazard, Vulnerability, Exposure, Malaria, Dengue, Chikungunya, Lyme disease
Key Summary Points
| Why carry out this study? |
| Climate change is considered to be one of the greatest threats to human health in the twenty-first century, with significant increases in temperature extremes, heavy precipitation, and severe droughts. |
| These climate hazards can activate cascading risk pathways with a sequence of secondary, causally connected events that can disrupt critical infrastructure, vital for a functional society. |
| What was learned from the study? |
| This study examines cascading risk pathways from climate change for vector-, water-, food-, and air-borne infectious diseases in a global context. |
| Cascading effects from climate hazards include also stagnant water that serve as breeding ground for mosquitoes after a flood; contamination of drinking water after a storm surge; breakdown of vector control programs after a hurricane; cholera outbreak after a drought. |
| A narrow, siloed, and linear assessment of these risks will misinform decision- and policymakers of the magnitude and pattern of future risks, and of the opportunities to modify policies to reduce inherent vulnerabilities and enhance infectious disease control programs. |
| Elucidating cascading risk pathways from infectious diseases is a first step towards tacking infectious disease threats from climate change. |
Digital Features
This article is published with digital features, including a Talking Head video to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.19621077.
Introduction
Global surface temperature increased at an unprecedented rate over the last 50 years and will continue to do so, until at least the mid-century under all greenhouse emissions scenarios [1]. Climate futures include increases in the frequency and intensity of hot extremes, heavy precipitation, and agricultural and ecological droughts. Pathogens, vectors, hosts, and disease transmission can be sensitive to these changing conditions [2]. Specifically, pathogens develop only within a narrow temperature envelope, with development ceasing at lower or higher temperatures. Temperature influences the reproduction and extrinsic incubation period of pathogens within a vector, with higher temperatures accelerating pathogen maturation. Mosquito biting rate is also a function of temperature, which can affect disease transmission. Moreover, ambient temperature affects the spatial–temporal distribution of disease vectors that carry and transmit pathogen to humans. Disease transmission can in turn be influenced by weather patterns, albeit indirectly, by altered contact rates between human and pathogen, human and vector, or human and host.
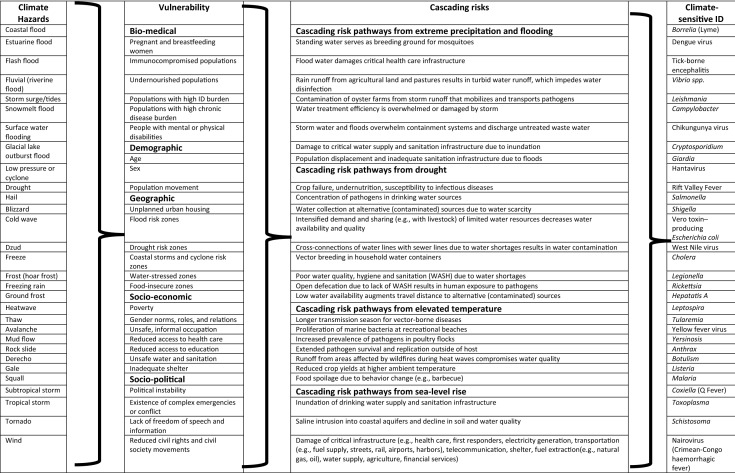
However, infectious disease transmission is also a function of underlying vulnerabilities in society (Table 1). Vulnerability is defined as the propensity or predisposition to be adversely affected by infectious disease or a susceptibility to harm and lack of capacity to cope and adapt (Fig. 1). There are multiple vulnerability factors for health impacts of climate change that can be grouped into biomedical (e.g., immunocompromised, malnourished), demographic (e.g., age, sex), geographic (e.g., land use, flood zones), socioeconomic (e.g., poverty, education), or sociopolitical (e.g., civil strife, political instability) (Table 1) [3]. From an infectious disease standpoint, vulnerability is also determined by the lack of safeguards such as door/window screens for vectors or flood barriers for storm surges [4].
Table 1.
Combination matrix of climate hazards, vulnerabilities, cascading risks, and climate-sensitive infectious disease (ID) impacts
This is not a complete list but is intended to be illustrative only
Sources: UNDRR/ISC Sendai Hazard Definition and Classification Review Technical Report; The Impacts of Climate Change on Human Health in the United States: A Scientific Assessment; Lindgren E, Andersson Y, Suk JE, Sudre B, Semenza JC. Monitoring EU Emerging Infectious Disease Risk due to Climate Change. Science 2012;336(6080):418–419. Semenza JC. Cascading risks of waterborne diseases from climate change. Nature Immunol. 2020 May;21(5):484–487
Fig. 1.
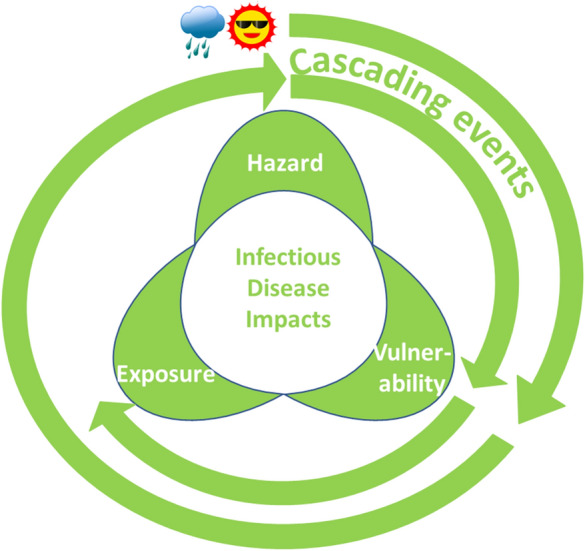
Cascading risks from infectious disease, due to a nexus of hazard, vulnerability, and exposure. Climatic hazards (e.g., extreme rain event or heat; outer spiral), amplified by societal vulnerabilities can trigger new hazards, such as floodwater contaminated with pathogens or high mosquito densities. Cascading events (inner spiral) caused by these infectious disease hazards and amplified by newly attained vulnerabilities can result in population exposure and give rise to water-borne or mosquito-borne disease outbreaks, respectively
Furthermore, infectious disease transmission depends on the exposure pattern in human populations, which is defined as the state of people, livelihoods, species, property, (eco-) systems, or other elements present in hazard zones that thereby could be adversely affected. Individuals or communities can be exposed to contaminated drinking water, vectors, or pathogens. The nexus of these three elements—hazard, vulnerability, and exposure—determines the current infectious disease impacts or future risks due to climate change (Fig. 1).
In addition to these impacts of weather and climate on pathogens, vectors, hosts, and disease transmission, a weather or climate event can activate cascading risk pathways with a sequence of secondary, causally connected events. Table 1 presents a combination matrix of how climate hazards can be combined with societal vulnerabilities that give rise to cascading risk pathways resulting in climate-sensitive infectious disease outbreaks. The dynamic interactions between climate hazard, exposure, and vulnerability set the stage for cascading risks (Fig. 1; outer spiral). For example, a hurricane can result in flooding or disrupt vector control programs as a result of infrastructure vulnerabilities. Contamination of floodwater with pathogens or high mosquito populations can cause population exposure to pathogens and then trigger cascading events (Fig. 1; inner spiral) such as water-borne or mosquito-borne outbreaks. These resulting impacts of a sequential chain reaction within natural and human systems can be significantly larger than the initial hazard and can cause additional physical, natural, social, or economic disruption (Fig. 1; inner spiral) [5]. If these secondary effects such as flooding disrupt critical infrastructure that is vital for a functional society, the consequences can be substantial. Such events can have a ripple effect across society and generate direct losses through immediate impacts or more secondary losses through consequential impacts. The combination matrix in Table 1 illustrates the predicament of climate change adaptation where virtually any climate hazard can be combined with specific vulnerabilities resulting in cascading risk pathways and infectious disease impacts. Examples of such cascading effects from climate hazards that have been described in the peer-reviewed literature include stagnant water after a flood that serves as a breeding ground for mosquitoes; contamination of drinking water after a storm; injuries from landslides or storm surges with risk of tetanus infections in populations with low vaccination coverage; or a cholera outbreak after a drought (Table 2).
Table 2.
Selected examples of climate hazards, societal vulnerabilities, and cascading events resulting in infectious disease outbreaks
| Climate hazard | Vulnerability and cascading events | Infectious disease outcome | References |
|---|---|---|---|
| Hurricane | Lack of WASH in mega-shelter after hurricane Katrina | Widespread outbreak of norovirus gastroenteritis among evacuees | Yee EL, Palacio H, Atmar RL, et al. Widespread outbreak of norovirus gastroenteritis among evacuees of Hurricane Katrina residing in a large “megashelter” in Houston, Texas: lessons learned for prevention. Clin Infect Dis. 2007;44:1032–9 |
| Typhoon | Serious flooding in Metro Manila | Outbreak of Leptospirosis | Amilasan A-shereT, Ujiie M, Suzuki M, et al. Outbreak of leptospirosis after flood, the Philippines, 2009. Emerg Infect Dis. 2012;18:91–4 |
| Cyclones: Idai and Kenneth in Mozambique | Lack of access to safe water, poor sanitation, contact with stagnant floodwater, overcrowding in the camps for displaced people | Diarrheal diseases, malaria | Mugabe VA, Gudo ES, Inlamea OF, et al. Natural disasters, population displacement and health emergencies: multiple public health threats in Mozambique. BMJ Global Health. 2021;6:e006778. doi:10.1136/bmjgh-2021-006778 |
| Heavy rainfall and elevated temperature | Contamination of surface water by compromised WASH systems | Cholera outbreaks in Yemen | Camacho A, Bouhenia M, Alyusfi R, et al. Cholera epidemic in Yemen, 2016–18: an analysis of surveillance data. Lancet Glob Health. 2018 Jun;6(6):e680–e690. https://doi.org/10.1016/S2214-109X(18)30230-4 |
| Monsoon: heavy rain | Record-breaking deluge and floods | Acute diarrhea, skin and eye infections, leptospirosis, malaria epidemic, leishmaniasis, respiratory infections, hepatitis | Baqir M, Sobani ZA, Bhamani A, et al. Infectious diseases in the aftermath of monsoon flooding in Pakistan. Asian Pac J Trop Biomed. 2012;2:76–9 |
| Floods | Health care access | Inadequate access to health care after the disaster | Jacquet GA, Kirsch T, Durrani A, Sauer L, Doocy S. Health care access and utilization after the 2010 Pakistan floods. Prehosp Disaster Med. 2016 Oct;31(5):485–91 |
| Floods, storms, droughts | Displacement | Infectious disease outbreaks including measles, cholera, cutaneous leishmaniasis, dengue | Desai AN, Ramatowski JW, Marano N, Madoff LC, Lassmann B. Infectious disease outbreaks among forcibly displaced persons: an analysis of ProMED reports 1996–2016. Confl Health. 2020 Dec;14(1):1–0 |
| Heavy rain | Overwhelmed water treatment and distribution system | Water-borne disease outbreaks, e.g., cryptosporidium | Semenza JC, Nichols G. Cryptosporidiosis surveillance and water-borne outbreaks in Europe. Euro Surveill. 2007 May;12(5):E13–14 |
| Extreme temperatures, droughts | Crop failures, undernutrition | Vulnerability to infectious diseases, especially diarrhea, pneumonia, and measles | Gwela A, Mupere E, Berkley JA, Lancioni C. Undernutrition, host immunity and vulnerability to infection among young children. Pediatr Infect Dis J. 2019 Aug 1;38(8):e175–7 |
| Droughts | Water scarcity, hygiene | Cholera outbreaks; infectious disease outbreaks |
Charnley GE, Kelman I, Green N, Hinsley W, Gaythorpe KA, Murray KA. Exploring relationships between drought and epidemic cholera in Africa using generalised linear models. BMC Infect Dis. 2021 Dec;21(1):1–2 Jofre J, Blanch AR, Lucena F. Water-borne infectious disease outbreaks associated with water scarcity and rainfall events. In: Sabater S, Barceló D, editors. Water scarcity in the Mediterranean; 2009: pp. 147–59. Berlin, Heidelberg: Springer |
Protecting and promoting health requires understanding and preparing for these causal interdependencies that permeate many aspects of society. A climate hazard can adversely affect one sector in society by surpassing a threshold that causes system failure (Fig. 1). As a result of the interconnected nature of modern society, this can have implications for other sectors [6]. The potential for cross-scale failures need to be defined in relation to the initial climate trigger. By doing so, the complexity of interactions within the public health network can be modeled mathematically. That way, cascading risk pathways can be simulated and analyzed. Understanding the weaknesses in the system can help advance adaptive capacity and intervention measures. Only then can cascading failures be anticipated and intercepted quickly with targeted measures that can prevent a public health impact. Here we critically review interlinked drivers of infectious disease transmission and elucidate the cascading risks associated with climate variability and change. We thoroughly examine cascading risk pathways from climate change for vector-, water-, food-, and air-borne infectious diseases in a global context, as opposed to a focused assessment of one infectious disease category [7] or one geographic area [4], which, to our knowledge, has not been attempted before. Such a comprehensive assessment is critical in order to elucidate cascading risk pathways from infectious diseases, in the context of the complex branching configuration of a globalized, dendritic society; indeed, it is a first step towards tackling infectious disease threats from climate change.
Methods
Peer-reviewed research articles were retrieved from PubMed using the following search terms: infectious diseases, vector-, water-, food-, air-borne diseases, climate change, climate variability, global warming, temperature, heat wave, precipitation, flooding. Disease-specific searches by pathogen name were also conducted. Keywords of the concepts and MeSH terms (when available) were used in the search strategies. A special focus was given to English language publications from the last 5 years. Of particular interest were publications that examined the specific aims of this study, climate change and cascading risks from infectious disease, and assessed the association between climate change and disease transmission. Research reports from international organizations and gray literature were also included in our analysis. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Vector-Borne Diseases
Climatic conditions indirectly affect vector-borne diseases such as mosquito- and tick-borne diseases. Alterations in environmental conditions can have secondary effects on vector populations, replication rates of pathogens, and vector–host interactions. Further, climatic events can result in cascading secondary effects that can alter the transmission pathway for vector-borne diseases discussed in this section.
Mosquito-Borne Diseases
Malaria
Malaria is caused by five species of plasmodium parasites that are transmitted by Anopheles mosquitoes. The disability-adjusted life years (DALYs) rate of malaria declined by almost 40% globally between 2007 and 2017. The largest burden of disease occurs in Africa where more than 90% of all malaria-related deaths occur [8, 9]. The RTS,S/AS01 malaria vaccine for the prevention of Plasmodium falciparum malaria in children living in regions with moderate to high transmission in combination with chemoprevention should further decrease the disease burden [10, 11]. While there have been recurrent, local outbreaks of malaria in Europe [12], the risk for widespread transmission is relatively low.
Malaria has expanded its geographic range into higher altitudes during warmer years in the highland areas of Columbia and Ethiopia [13, 14]. Thus, without interventions, it is possible that declining trends in the number of malaria DALYs will be offset by additional climate change. Cascading risk pathways from a number of hurricanes disrupted anti-malaria vector control programs and resulted in a resurgence of P. falciparum malaria in Haiti, Guatemala, and Nicaragua in the 1980s and 1990s [15, 16]. In the Amazon region, the dry season is getting longer and the rainy season that used to start at the end of October now starts at the beginning of December; this exacerbates and accelerates the burning of the rainforest. Heat stress and fires in the Amazon rainforest along with deforestation, road density, and selective logging are associated with malaria risk in the Amazon [17–19]. Smoke exposure from the Amazon fires is associated with increased respiratory illnesses such as pneumonia, acute bronchitis, and asthma in indigenous populations [20].
The Anopheles vector is projected to expand its geographic range under different climate change scenarios, in part due to an increase in temperature and an expansion of the rainy season in the tropical areas of Africa [21, 22]. Similarly, an expansion is projected in South Africa and China but a contraction in India and Southeast Asia as a result of a reduction in climatic suitability [23, 24]. In China, Plasmodium vivax and P. falciparum malaria distributions are projected to increase under higher emission scenarios, such as representative concentration pathway (RCP) 4.5 and RCP8.5 [25]. Under a multi-scenario climate change framework, these are also the areas projected to experience a lengthening of the transmission season by 1.6 additional months in the tropical highlands in the African region, the Americas, and the Eastern Mediterranean region. The improvement in climatic suitability is expected to be greater in rural areas than in urban areas, and the epidemic belt would expand towards temperate regions [26]. Similarly, a general rise in months suitable for transmission is projected to increase in India; in other areas, the transmission season is projected to contract, when the climatic conditions will be too extreme for the vector species [27].
Arboviral Diseases
Dengue
Aedes aegypti, the principal mosquito vector of many arboviral diseases, such as dengue, chikungunya, yellow fever, and Zika, has experienced a global expansion, threatening almost half of the world’s population [28, 29]. This expansion is attributed in part to the global temperature increase [30] but also globalized population movement through air traffic and urbanization, and insufficient vector control measures [31, 32]. Dengue is responsible for an estimated 10,000 deaths and 100 million symptomatic infections per year in over 125 countries [33, 34]. Dengue incidence is positively associated with temperature, precipitation, and relative humidity in several settings worldwide, including the Americas [35], India [36], and Philippines [37]. Sea surface temperature, rain, and variation in wind associated with the El Niño Southern Oscillation over the Pacific Ocean has also been used as a predictor of dengue incidence [38]. Cascading risks due to a breakdown of vector control measures in countries of Central America after hurricane Mitch in 1998 resulted in almost 40,000 cases of dengue and dengue hemorrhagic fever [16].
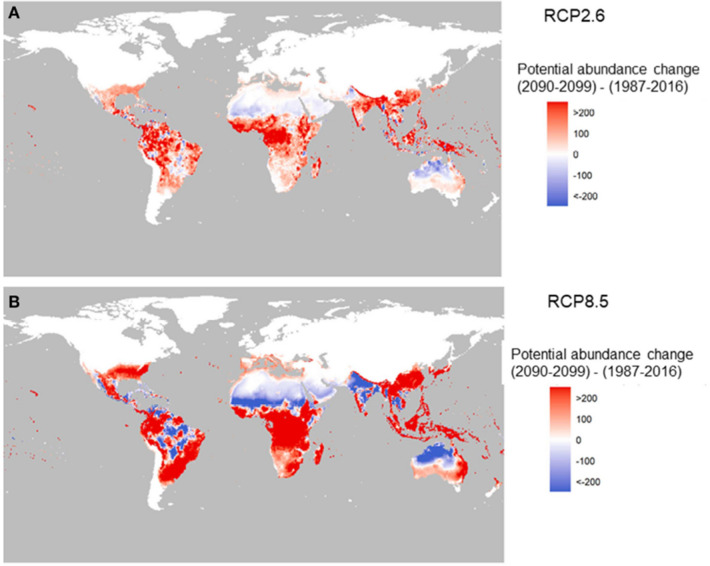
The change in potential for global abundance in the future of the dengue vector A. aegypti is shown in Fig. 2, with big increased potential in Southeast Asia, China, Japan, East Australia, and Africa [39]. More favorable temperatures and increased rainfall by 2050 from climate change could increase the suitability for dengue in southern and western Africa, southeastern USA, central Mexico, northern Argentina, and inland areas of Australia. In addition, coastal cities in eastern China and Japan are projected to become more suitable by 2050 [29]. The potential transmission season will lengthen by 4 months because of an increase in climatic suitability, particularly in lowlands in the Western Pacific region and the Eastern Mediterranean region [26].
Fig. 2.
Change in the potential abundance of A. aegypti (per larval site) over the twenty-first century (2090–2099 relative to 1987–2016). The two panels correspond to two carbon emission scenarios: RCP2.6 (a) and RCP8.5 (b).
Source: Reference [27]
Chikungunya
The chikungunya virus was first identified in Tanzania in 1952, where it caused a localized outbreak in Africa and parts of Asia, and then spread to countries around the Indian Ocean. Travel and trade have contributed to a continuous geographic spread to temperate areas [40]. The virus has also been repeatedly imported into Europe where conducive climatic conditions contributed to two large outbreaks in Italy in 2007 and 2017 [41, 42]. Projecting the chikungunya risk under RCP4.5 and RCP8.5 indicates an expansion of the transmission-suitable areas in China, sub-Saharan Africa, South America, the USA, and continental Europe, although also some contraction of the transmission risk along parts of the Adriatic coast of Europe as a result of unfavorable climatic conditions for example [43].
Zika
The Zika virus has also expanded globally, causing large outbreaks in South America in 2016 following a period of record high temperatures and severe drought conditions in 2015 [44]. Storing drinking water in open containers at home as a result of the drought might have created ideal vector breeding and exposure conditions that contributed to the outbreak. Zika could expand north with longer seasons as temperatures move towards the predicted thermal optimum (29 °C) [45].
West Nile Fever
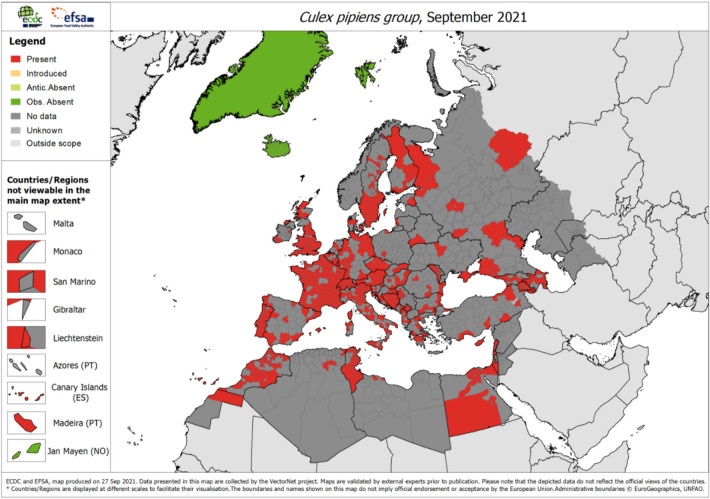
Europe experienced uncharacteristically high spring temperatures in 2018 followed by an exceptionally early and intense West Nile virus (WNV) transmission season, with 2083 cases [46, 47]. These weather anomalies could have activated the mosquito breeding season early and reduced the extrinsic incubation period, which would explain the high prevalence of WNV in mosquito vectors (Culex pipiens) (Fig. 3) and avian hosts, compared to previous years. In fact, birds infected with WNV were discovered before the virus was detected in humans, in both the Netherlands and Germany [48, 49]. In Europe, progressive expansion of WNV is projected along the edges of the current transmission areas, which can result in a cascading risk to the safety of blood banks [50]. WNV-infected, but asymptomatic donors can inadvertently contaminate the blood supply which can arrest blood transfusion services. In order to prevent cascading risk pathways affecting the blood supply, a number of steps need to be taken, such as screening, deferral and pathogen reduction strategies [51].
Fig. 3.
The vector for West Nile virus: C. pipiens mosquito distribution in Europe as of September 2021. Source: ECDC, https://www.ecdc.europa.eu/en/publications-data/culex-pipiens-group-current-known-distribution-september-2021
Tick-Borne Diseases
Lyme Disease
Vector surveillance in Canada has documented a geographic range expansion of the black-legged tick Ixodes scapularis, the main vector of Borrelia burgdorferi, the agent of Lyme disease [52]. This expansion is associated with elevated temperatures, the emergence of tick populations, increases in their range and recent geographic spread, as well as with a rapid increase in human Lyme disease cases [53–56]. In Europe, transmission by Ixodes ricinus ticks is also determined by factors besides temperature, such as host populations and habitats [57]. A higher transmission risk is projected under all RCP scenarios for I. scapularis in some areas of Canada [58]. The season of Lyme disease is projected to expand in the USA under a 2 °C warming scenario with a 20% increase of cases over the coming decades and lead to an earlier onset and longer length of the annual Lyme disease season [58, 59]. In Europe, the risk of transmission could even be reduced under the low-emission greenhouse gas scenario [60].
Tick-Borne Encephalitis
Tick-borne encephalitis (TBE) is an important zoonotic infection, with an increasing disease burden and expanding geographic range across Europe, Russia, and parts of Asia. Among a number of contributing factors, milder winters and warmer springs due to climate change have been implicated in this range expansion [61–63]. TBE is a potentially serious disease, with 3411 TBE cases reported in EU/EEA countries in 2019 of which 20 died (case fatality, 0.7%). TBE cases generally display a seasonal peak in the months of July and August. Conducive climatic conditions can result in a cascading chain of events where goats, sheep, or cows are infected with the TBE virus that can result in alimentary infection of humans after consumption of unpasteurized milk and cheese from domestic ruminants [64–66].
Rocky Mountain Spotted Fever
Rocky Mountain spotted fever is the most common fatal tick-borne disease in the USA, with 21 fatalities between 2003 and 2016, caused by Rickettsia rickettsia [67]. Warmer wetter climates have led to an expansion of tick habitat range and distribution [68, 69]. For example, the range expansion of the lone star tick is correlated with an increased incidence of spotted fever group rickettsiosis in the USA [70].
Leishmaniasis
The protozoa Leishmania infantum, the main causative agent of zoonotic visceral leishmaniasis and cutaneous leishmaniasis, are transmitted by infected Phlebotomine sandflies. Leishmaniasis is found in southern Europe and northern Africa around the Mediterranean as well as parts of Asia [71]. The primary reservoirs of human infections are domestic and stray dogs that have experienced a progressive increase of Leishmania seroprevalence rates at higher latitudes and altitudes, more so than might be expected in Mediterranean countries [72]. There has been a climate change-related expansion of sandflies into more northern latitudes and higher altitudes in Italy [73], in the Pyrenees [74], and Germany [75]. The convergence of vector dispersion and the scattering of infected dogs (e.g., through adoption services) can compound this public health issue. The climate in Central Europe is projected to become increasingly more suitable for sandflies in the future, under climate change scenarios [76].
Food-Borne Diseases
Salmonella is climate sensitive and grows in a narrow temperature envelope with a strong seasonality. An increase in ambient temperature is associated with an upsurge in Salmonella incidence in a number of settings, indicating a direct impact on replication rates [77–79]. The situation for Campylobacter is different, because the pathogen cannot replicate outside of the host. Thus, warm weather conditions may not directly affect Campylobacter replication rates but rather reflect human behavioral issues such as riskier patterns of food production/consumption or other seasonal factors [80–82]. Nevertheless, with an increase in ambient temperature and extreme weather events, the food safety risk is anticipated to increase as a result of risks from existing and emerging food-borne pathogens along the food chain [83–85].
The transmission pathway of food-borne diseases through the food chain is complex and susceptible to several climatic drivers [86]. For example, in 2017, hurricane Irma contaminated many commercial fruit and vegetable fields in Florida with pathogens and parasites, which led the US Food and Drug Administration to warn against the consumption of fresh produce that had been in contact with floodwater [87]. Hurricanes can also disrupt food processing, preparation, transport and spoil foodstuff.
Water-Borne Diseases
Water-borne diseases causing diarrheal diseases have been declining globally since the 1990s, owing to improvements in water, sanitation, and hygiene (WASH), reductions in poverty, and vaccination programs [88]. Despite this, the disease burden is considerable in low- and high-income countries [7] and is usually caused by microbial contamination of the drinking water supply [89–91]. Bacteria, protozoa, viruses, or parasites have been implicated in water-borne outbreaks due to an inability of the water treatment system to clear pathogens from the water supply [89, 91].
Water-borne outbreaks can occur because of climate variability and change followed by secondary events that are causally connected. Such cascading risk pathways can lead to a succession of system failures and damage critical infrastructure. For instance, extreme precipitation can mobilize pathogens from pastures and fields and overwhelm water treatment and distribution systems resulting in drinking water contamination [92]. In fact, empirical studies have documented cascading risks from heavy rain that give rise to water-borne outbreaks [93, 94]. Similarly, cascading risk pathways of floods can contaminate drinking water wells, treatment and distribution systems, and produce water-borne outbreaks [95, 96]. Conversely, water shortages and drought can also give rise to cascading risks and cause diarrheal diseases [97, 98], although this association has been documented inconsistently [99].
Cholera
Natural disasters can trigger a sequence of cascading events that can compromise WASH. For example, poor sanitation after a hurricane can result in cholera outbreaks [100]. Cholera, is an acute diarrheal disease, caused by the bacterium Vibrio cholerae that can result in severe morbidity and mortality; it has been associated with several climatic parameters, in situations with poor WASH and where cholera has already been seeded in the population [101–103]. For example, elevated ambient temperature is a key parameter for cholera incidence [104, 105], as well as lower and higher precipitation [106, 107]. The projected risk for non-cholera Vibrio cases, including gastroenteritis, wound infections, and septicemia, is projected to increase in the Baltic Sea region with higher sea surface temperatures [108].
Leptospirosis
Leptospira bacteria can contaminate water, soil, or food through the urine of infected animals, and cause leptospirosis, a bacterial disease that affects humans and animals. For example, recreational water use during hot weather increases the risk of exposure to water contaminated with Leptospira [109]. As part of a time series from 2006 to 2016, human leptospirosis notification was significantly associated with rainfall and land surface temperature in high-risk counties in China [110]. Floodwater or drinking water contaminated with Leptospira can cause outbreaks of leptospirosis [111, 112], vividly illustrating the nexus of climate hazard, societal vulnerability, and population exposure in creating cascading risk pathways for leptospirosis (Fig. 1).
Schistosomiasis
Infections with parasitic blood flukes of the species Schistosoma mansoni, S. japonicum, S. intercalatum, S. mekongi, and S. guineensis can cause intestinal schistosomiasis, associated with systemic inflammation. Cases have been reported in Africa, Latin America, the Middle East, and Southeast Asia [113–115]. Temperature is a determinant of the geographic distribution of flukes and a climate change assessment indicated increased transmission as well as potential shrinkages in certain areas [116]. For example, a decade-long drought between 2001 and 2009 resulted in the disappearance of significant clustering around historical transmission hot spots in coastal Kenya, due to the disappearance of the flukes from ponds along with urinary schistosomiasis transmission [117]. Conversely, extreme precipitation in June 2017 in Brazil resulted in a large schistosomiasis outbreak after a large flooding period [118].
Hotter areas in Africa are projected to experience reduced snail populations but higher populations are projected in areas with currently lower winter temperature [119, 120]. In China, currently endemic areas in Sichuan Province are projected to contract, but non-endemic areas in Sichuan and Hunan/Hubei provinces expand [116, 121].
Respiratory Infections
Respiratory infections tend to be highly seasonal, with higher incidence in the winter months, in part due to increased pathogen survival, indoor crowding, and elevated host susceptibility [122]. For example, temperature and humidity determine the incidence of influenza in temperate regions of the world. Low daily temperatures and both low and high relative humidity were associated with an increased risk of influenza incidence in Seoul, Republic of Korea [123]. Similarly, low temperature correlated with peaks of influenza virus activity in Northern Europe [124]. Conversely, warm winters tend to be followed by severe and early-onset influenza incidence the following season [125], partially due to waning population immunity to previous infections.
A respiratory disease outbreak caused by hantavirus in 1999 and 2000 in Santos, Panama was preceded by extreme precipitation [126]. Such unusual rainfall patterns may have led to increases in rodent populations and contact rates with humans [127, 128]. Infected rodents can harbor the hantavirus in their saliva, urine, or feces which can result in human exposure. Another aspect of cascading effects in a warming world is the increased risk for flooding, which can result in respiratory infections; the incidence of respiratory infections has been observed to increase after flooding in a number of settings [129–131].
Conclusion
Increasing climate variability is already leading to cascading risks from infectious disease. With projections of increases in multiple modes of climate variability with additional climate change, it is vital for health systems to prepare for more and more extreme cascading risks, taking into account multiple other drivers of outbreaks of infectious diseases. The preparation needs to consider that these changes will vary over time and space, so are inherently difficult to predict. That means health systems need to prepare for uncertainty as much as for climate change. This requires flexibility in planning modifications to vector control programs to ensure they are prepared for a range of possible futures.
Climate effects can have far-reaching implications for public health through inherent societal vulnerabilities that can magnify the impacts of cascading risk pathways (Fig. 1). A narrow, siloed, and linear assessment of these risks will misinform decision- and policymakers of the magnitude and pattern of future risks, and of the opportunities to modify policies to enhance infectious disease control programs, building from current programs. For example, current malaria control programs include treatment, bed nets, and vector control; also incorporating land use (e.g., wetland management, drainage of standing water) and socioeconomic determinants (e.g., housing, occupational exposure) can indirectly counteract the impacts of increasing climate variability on disease transmission.
A comprehensive understanding is critical of the interconnected nature of public health with social, demographic, and environmental drivers of infectious diseases. To this end, a better collaboration is warranted between public health practitioners, climate scientists, civil engineers, social scientists, network modelers, and decision-makers. Mathematical modelling of climate hazards, vulnerabilities, and exposures can improve projections of cascading events and facilitate transformative adaptation.
Failure to invest in research and health systems would jeopardize the resilience of individuals and communities to cascading impacts from infectious disease, leading to a sicker future.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
JCS had the idea for the article, performed the literature search and data analysis, and drafted the article. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Jan C. Semenza, Joacim Rocklöv, and Kris Ebi all have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Intergovernmental Panel on Climate Change (IPCC). Sixth assessment report; climate change 2021: the physical science basis. https://www.ipcc.ch/report/ar6/wg1/#SPM. Accessed 17 Apr 2022.
- 2.Wu X, Lu Y, Zhou S, Chen L, Xu B. Impact of climate change on human infectious diseases: empirical evidence and human adaptation. Environ Int. 2016;1(86):14–23. doi: 10.1016/j.envint.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 3.The impacts of climate change on human health in the United States: a scientific assessment. https://health2016.globalchange.gov/. Accessed 1 Dec 2021.
- 4.Semenza JC, Paz S. Climate change and infectious disease in Europe: impact, projection and adaptation. Lancet Reg Health Eur. 2021;1(9):100230. doi: 10.1016/j.lanepe.2021.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intergovernmental Panel on Climate Change (IPCC). https://www.ipcc.ch/report/sixth-assessment-report-working-group-ii/. Accessed 17 Apr 2022.
- 6.Lawrence J, Blackett P, Cradock-Henry NA. Cascading climate change impacts and implications. Clim Risk Manag. 2020;1(29):100234. doi: 10.1016/j.crm.2020.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza JC. Cascading risks of waterborne diseases from climate change. Nat Immunol. 2020;21(5):484–487. doi: 10.1038/s41590-020-0631-7. [DOI] [PubMed] [Google Scholar]
- 8.M’Bra RK, Kone B, Soro DP, et al. Impact of climate variability on the transmission risk of malaria in northern Côte d’Ivoire. PLoS ONE. 2018;13(6):e0182304. doi: 10.1371/journal.pone.0182304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caminade C, McIntyre KM, Jones AE. Impact of recent and future climate change on vector-borne diseases. Ann N Y Acad Sci. 2019;1436(1):157. doi: 10.1111/nyas.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS, S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359(24):2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandramohan D, Zongo I, Sagara I, et al. Seasonal malaria vaccination with or without seasonal malaria chemoprevention. N Engl J Med. 2021;385(11):1005–1017. doi: 10.1056/NEJMoa2026330. [DOI] [PubMed] [Google Scholar]
- 12.Sudre B, Rossi M, Van Bortel W, et al. Mapping environmental suitability for malaria transmission, Greece. Emerg Infect Dis. 2013;19(5):784. doi: 10.3201/eid1905.120811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siraj AS, Santos-Vega M, Bouma MJ, Yadeta D, Carrascal DR, Pascual M. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science. 2014;343(6175):1154–1158. doi: 10.1126/science.1244325. [DOI] [PubMed] [Google Scholar]
- 14.Midekisa A, Beyene B, Mihretie A, Bayabil E, Wimberly MC. Seasonal associations of climatic drivers and malaria in the highlands of Ethiopia. Parasit Vectors. 2015;8(1):1–1. doi: 10.1186/s13071-015-0954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bissell RA. Delayed-impact infectious disease after a natural disaster. J Emerg Med. 1983;1(1):59–66. doi: 10.1016/0736-4679(83)90010-0. [DOI] [PubMed] [Google Scholar]
- 16.PAHO Impact of Hurricane Mitch on Central America. Epidemiol Bull. 1998;19:1–14. [PubMed] [Google Scholar]
- 17.Vittor AY, Gilman RH, Tielsch J, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74(1):3–11. doi: 10.4269/ajtmh.2006.74.3. [DOI] [PubMed] [Google Scholar]
- 18.Olson SH, Gangnon R, Silveira GA, Patz JA. Deforestation and malaria in Mancio Lima county, Brazil. Emerg Infect Dis. 2010;16(7):1108. doi: 10.3201/eid1607.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn MB, Gangnon RE, Barcellos C, Asner GP, Patz JA. Influence of deforestation, logging, and fire on malaria in the Brazilian Amazon. PLoS ONE. 2014;9(1):e85725. doi: 10.1371/journal.pone.0085725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves L. Amazon fires coincide with increased respiratory illnesses in indigenous populations. Lancet Respir Med. 2020;8(11):e84. doi: 10.1016/S2213-2600(20)30421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akpan GE, Adepoju KA, Oladosu OR, Adelabu SA. Dominant malaria vector species in Nigeria: modelling potential distribution of Anopheles gambiae sensu lato and its siblings with MaxEnt. PLoS ONE. 2018;13(10):e0204233. doi: 10.1371/journal.pone.0204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le PV, Kumar P, Ruiz MO, Mbogo C, Muturi EJ. Predicting the direct and indirect impacts of climate change on malaria in coastal Kenya. PLoS ONE. 2019;14(2):e0211258. doi: 10.1371/journal.pone.0211258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khormi HM, Kumar L. Future malaria spatial pattern based on the potential global warming impact in South and Southeast Asia. Geospat Health. 2016;11(3):416. [DOI] [PubMed]
- 24.Laporta GZ, Linton YM, Wilkerson RC, et al. Malaria vectors in South America: current and future scenarios. Parasit Vectors. 2015;8(1):1–3. doi: 10.1186/s13071-015-1038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hundessa S, Williams G, Li S, et al. Projecting potential spatial and temporal changes in the distribution of Plasmodium vivax and Plasmodium falciparum malaria in China with climate change. Sci Total Environ. 2018;15(627):1285–1293. doi: 10.1016/j.scitotenv.2018.01.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colón-González FJ, Sewe MO, Tompkins AM, et al. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lancet Planet Health. 2021;5(7):e404–e414. doi: 10.1016/S2542-5196(21)00132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar S, Gangare V, Singh P, Dhiman RC. Shift in potential malaria transmission areas in India, using the fuzzy-based climate suitability malaria transmission (FCSMT) model under changing climatic conditions. Int J Environ Res Public Health. 2019;16(18):3474. doi: 10.3390/ijerph16183474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messina JP, Brady OJ, Golding N, et al. The current and future global distribution and population at risk of dengue. Nat Microbiol. 2019;4(9):1508–1515. doi: 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu-Helmersson J, Brannstrom A, Sewe MO, Semenza JC, Rocklöv J. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front Public Health. 2019;7:148. doi: 10.3389/fpubh.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza JC, Sudre B, Miniota J, et al. International dispersal of dengue through air travel: importation risk for Europe. PLoS Negl Trop Dis. 2014;8(12):e3278. doi: 10.1371/journal.pntd.0003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraemer MU, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(6):712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messina JP, Brady OJ, Pigott DM, Brownstein JS, Hoen AG, Hay SI. A global compendium of human dengue virus occurrence. Sci Data. 2014;1(1):1–6. doi: 10.1038/sdata.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López MS, Müller GV, Sione WF. Analysis of the spatial distribution of scientific publications regarding vector-borne diseases related to climate variability in South America. Spat Spatiotemporal Epidemiol. 2018;1(26):35–93. doi: 10.1016/j.sste.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Mutheneni SR, Morse AP, Caminade C, Upadhyayula SM. Dengue burden in India: recent trends and importance of climatic parameters. Emerg Microbes Infect. 2017;6(1):1. doi: 10.1038/emi.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvajal TM, Viacrusis KM, Hernandez LF, Ho HT, Amalin DM, Watanabe K. Machine learning methods reveal the temporal pattern of dengue incidence using meteorological factors in metropolitan Manila, Philippines. BMC Infect Dis. 2018;18(1):1–5. doi: 10.1186/s12879-018-3066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrova D, Lowe R, Stewart-Ibarra A, Ballester J, Koopman SJ, Rodó X. Sensitivity of large dengue epidemics in Ecuador to long-lead predictions of El Niño. Clim Serv. 2019;1(15):100096. doi: 10.1016/j.cliser.2019.02.003. [DOI] [Google Scholar]
- 39.Liu-Helmersson J, Brännström Å, Sewe MO, Semenza JC, Rocklöv J. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front Public Health. 2019;12(7):148. doi: 10.3389/fpubh.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yactayo S, Staples JE, Millot V, Cibrelus L, Ramon-Pardo P. Epidemiology of Chikungunya in the Americas. J Infect Dis. 2016;214(suppl_5):S441–S445. doi: 10.1093/infdis/jiw390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rezza G, Nicoletti L, Angelini R, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 42.Rocklöv J, Tozan Y, Ramadona A, et al. Using big data to monitor the introduction and spread of Chikungunya, Europe, 2017. Emerg Infect Dis. 2019;25(6):1041. doi: 10.3201/eid2506.180138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tjaden NB, Suk JE, Fischer D, Thomas SM, Beierkuhnlein C, Semenza JC. Modelling the effects of global climate change on chikungunya transmission in the 21st century. Sci Rep. 2017;7(1):1–1. doi: 10.1038/s41598-017-03566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paz S, Semenza JC. El Niño and climate change—contributing factors in the dispersal of Zika virus in the Americas? Lancet. 2016;387(10020):745. doi: 10.1016/S0140-6736(16)00256-7. [DOI] [PubMed] [Google Scholar]
- 45.Tesla B, Demakovsky LR, Mordecai EA, et al. Temperature drives Zika virus transmission: evidence from empirical and mathematical models. Proc R Soc B. 1884;2018(285):20180795. doi: 10.1098/rspb.2018.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marini G, Calzolari M, Angelini P, et al. A quantitative comparison of West Nile virus incidence from 2013 to 2018 in Emilia-Romagna, Italy. PLoS Negl Trop Dis. 2020;14(1):e0007953. doi: 10.1371/journal.pntd.0007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farooq Z, Rocklöv J, Wallin J, Abiri N, Odhiambo Sewe M, Sjödin H, Semenza JC. Artificial intelligence to predict West Nile virus outbreaks with eco-climatic drivers. Lancet Reg Health – Eur. 2022;17:100370. doi: 10.1016/j.lanepe.2022.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziegler U, Santos PD, Groschup MH, et al. West Nile virus epidemic in Germany triggered by epizootic emergence, 2019. Viruses. 2020;12(4):448. doi: 10.3390/v12040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlaskamp DR, Thijsen SF, Reimerink J, et al. First autochthonous human West Nile virus infections in the Netherlands, July to August 2020. Eurosurveillance. 2020;25(46):2001904. doi: 10.2807/1560-7917.ES.2020.25.46.2001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semenza JC, Tran A, Espinosa L, Sudre B, Domanovic D, Paz S. Climate change projections of West Nile virus infections in Europe: implications for blood safety practices. Environ Health. 2016;15(1):125–136. doi: 10.1186/s12940-016-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semenza JC, Domanović D. Blood supply under threat. Nat Clim Change. 2013;3(5):432–435. doi: 10.1038/nclimate1867. [DOI] [Google Scholar]
- 52.Clow KM, Leighton PA, Ogden NH, et al. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS ONE. 2017;12(12):e0189393. doi: 10.1371/journal.pone.0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng A, Chen D, Woodstock K, Ogden NH, Wu X, Wu J. Analyzing the potential risk of climate change on Lyme disease in eastern Ontario, Canada using time series remotely sensed temperature data and tick population modelling. Remote Sens. 2017;9(6):609. doi: 10.3390/rs9060609. [DOI] [Google Scholar]
- 54.Gasmi S, Ogden NH, Lindsay LR, et al. Emerging infections: surveillance for Lyme disease in Canada: 2009–2015. Can Commun Dis Rep. 2017;43(10):194. doi: 10.14745/ccdr.v43i10a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebi KL, Ogden NH, Semenza JC, Woodward A. Detecting and attributing health burdens to climate change. Environ Health Perspect. 2017;125(8):085004. doi: 10.1289/EHP1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burrows H, Talbot B, McKay R, et al. A multi-year assessment of blacklegged tick (Ixodes scapularis) population establishment and Lyme disease risk areas in Ottawa, Canada, 2017–2019. PLoS ONE. 2021;16(2):e0246484. doi: 10.1371/journal.pone.0246484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estrada-Peña A, de la Fuente J. Host distribution does not limit the range of the tick Ixodes ricinus but impacts the circulation of transmitted pathogens. Front Cell Infect Microbiol. 2017;11(7):405. doi: 10.3389/fcimb.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McPherson M, García-García A, Cuesta-Valero FJ, et al. Expansion of the Lyme disease vector Ixodes scapularis in Canada inferred from CMIP5 climate projections. Environ Health Perspect. 2017;125(5):057008. doi: 10.1289/EHP57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monaghan AJ, Moore SM, Sampson KM, Beard CB, Eisen RJ. Climate change influences on the annual onset of Lyme disease in the United States. Ticks Tick-Borne Dis. 2015;6(5):615–622. doi: 10.1016/j.ttbdis.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li S, Gilbert L, Vanwambeke SO, Yu J, Purse BV, Harrison PA. Lyme disease risks in Europe under multiple uncertain drivers of change. Environ Health Perspect. 2019;127(6):067010. doi: 10.1289/EHP4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindgren E, Gustafson R. Tick-borne encephalitis in Sweden and climate change. Lancet. 2001;358(9275):16–18. doi: 10.1016/S0140-6736(00)05250-8. [DOI] [PubMed] [Google Scholar]
- 62.Lukan M, Bullova E, Petko B. Climate warming and tick-borne encephalitis, Slovakia. Emerg Infect Dis. 2010;16(3):524. doi: 10.3201/eid1603.081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Semenza JC, Suk JE. Vector-borne diseases and climate change: a European perspective. FEMS Microbiol Lett. 2018;365(2):fnx244. doi: 10.1093/femsle/fnx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blomqvist G, Näslund K, Svensson L, Beck C, Valarcher JF. Mapping geographical areas at risk for tick-borne encephalitis (TBE) by analysing bulk tank milk from Swedish dairy cattle herds for the presence of TBE virus-specific antibodies. Acta Vet Scand. 2021;63(1):1–9. doi: 10.1186/s13028-021-00580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallenhammar A, Lindqvist R, Asghar N, et al. Revealing new tick-borne encephalitis virus foci by screening antibodies in sheep milk. Parasit Vectors. 2020;13(1):1–2. doi: 10.1186/s13071-020-04030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paulsen KM, Stuen S, Das Neves CG, et al. Tick-borne encephalitis virus in cows and unpasteurized cow milk from Norway. Zoonoses Public Health. 2019;66(2):216–222. doi: 10.1111/zph.12554. [DOI] [PubMed] [Google Scholar]
- 67.https://www.cdc.gov/ticks/tickbornediseases/rmsf.html. Tickborne Diseases of the United States. Accessed 17 Apr 2022.
- 68.Adem PV. Emerging and re-emerging rickettsial infections. Semin Diagn Pathol. 2019;36(3):146–51. [DOI] [PubMed]
- 69.Karbowiak G. The occurrence of the Dermacentor reticulatus tick-its expansion to new areas and possible causes. Ann Parasitol. 2014;60(1):37–47. [PubMed]
- 70.Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, Behravesh CB. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am J Trop Med Hyg. 2016;94(1):35. doi: 10.4269/ajtmh.15-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alten B, Maia C, Afonso MO, et al. Seasonal dynamics of phlebotomine sand fly species proven vectors of Mediterranean leishmaniasis caused by Leishmania infantum. PLoS Negl Trop Dis. 2016;10(2):e0004458. doi: 10.1371/journal.pntd.0004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antoniou M, Gramiccia M, Molina R, Dvorak V, Volf P. The role of indigenous phlebotomine sandflies and mammals in the spreading of leishmaniasis agents in the Mediterranean region. Eurosurveillance. 2013;18(30):20540. doi: 10.2807/1560-7917.ES2013.18.30.20540. [DOI] [PubMed] [Google Scholar]
- 73.Maroli M, Rossi L, Baldelli R, et al. The northward spread of leishmaniasis in Italy: evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop Med Int Health. 2008;13(2):256–264. doi: 10.1111/j.1365-3156.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- 74.Ballart C, Barón S, Alcover MM, Portús M, Gállego M. Distribution of phlebotomine sand flies (Diptera: Psychodidae) in Andorra: first finding of P. perniciosus and wide distribution of P. ariasi. Acta Trop. 2012;122(1):155–159. doi: 10.1016/j.actatropica.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 75.Naucke TJ, Menn B, Massberg D, Lorentz S. Sandflies and leishmaniasis in Germany. Parasitol Res. 2008;103(1):65–68. doi: 10.1007/s00436-008-1052-y. [DOI] [PubMed] [Google Scholar]
- 76.Fischer D, Moeller P, Thomas SM, Naucke TJ, Beierkuhnlein C. Combining climatic projections and dispersal ability: a method for estimating the responses of sandfly vector species to climate change. PLoS Negl Trop Dis. 2011;5(11):e1407. doi: 10.1371/journal.pntd.0001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang P, Goggins WB, Chan EY. Associations of Salmonella hospitalizations with ambient temperature, humidity and rainfall in Hong Kong. Environ Int. 2018;1(120):223–230. doi: 10.1016/j.envint.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 78.Lal A, Hales S, Kirk M, Baker MG, French NP. Spatial and temporal variation in the association between temperature and salmonellosis in NZ. Aust N Z J Public Health. 2016;40(2):165–169. doi: 10.1111/1753-6405.12413. [DOI] [PubMed] [Google Scholar]
- 79.Milazzo A, Giles LC, Zhang Y, Koehler AP, Hiller JE, Bi P. Heatwaves differentially affect risk of Salmonella serotypes. J Infect. 2016;73(3):231–240. doi: 10.1016/j.jinf.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 80.Lake IR, Colon-Gonzalez FJ, Takkinen J, et al. Exploring campylobacter seasonality across Europe using the European surveillance system (TESSy), 2008 to 2016. Eurosurveillance. 2019;24(13):1800028. doi: 10.2807/1560-7917.ES.2019.24.13.180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg A, Weinberger M, Paz S, Valinsky L, Agmon V, Peretz C. Ambient temperature and age-related notified Campylobacter infection in Israel: a 12-year time series study. Environ Res. 2018;1(164):539–545. doi: 10.1016/j.envres.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 82.Djennad A, Iacono GL, Sarran C, et al. Seasonality and the effects of weather on Campylobacter infections. BMC Infect Dis. 2019;19(1):1. doi: 10.1186/s12879-019-3840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith BA, Fazil A. Climate change and infectious diseases: the challenges: how will climate change impact microbial foodborne disease in Canada? Can Commun Dis Rep. 2019;45(4):108. doi: 10.14745/ccdr.v45i04a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akil L, Ahmad HA, Reddy RS. Effects of climate change on Salmonella infections. Foodborne Pathog Dis. 2014;11(12):974–980. doi: 10.1089/fpd.2014.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lake IR. Food-borne disease and climate change in the United Kingdom. Environ Health. 2017;16(1):53–59. doi: 10.1186/s12940-017-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semenza JC, Herbst S, Rechenburg A, et al. Climate change impact assessment of food-and waterborne diseases. Crit Rev Environ Sci Technol. 2012;42(8):857–890. doi: 10.1080/10643389.2010.534706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Food Safety News. Floodwater pathogens can’t be washed off of fresh produce. Food Safety News. 2017. https://www.foodsafetynews.com/2017/09/floodwater-pathogens-cant-be-washed-off-of-fresh-produce/. Accessed 17 Apr 2022.
- 88.Dicker D, Nguyen G, Abate D, et al. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1684–1735. doi: 10.1016/S0140-6736(18)31891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferreira DC, Graziele I, Marques RC, Gonçalves J. Investment in drinking water and sanitation infrastructure and its impact on waterborne diseases dissemination: the Brazilian case. Sci Total Environ. 2021;20(779):146279. doi: 10.1016/j.scitotenv.2021.146279. [DOI] [PubMed] [Google Scholar]
- 90.Chen L, Deng Y, Dong S, et al. The occurrence and control of waterborne viruses in drinking water treatment: a review. Chemosphere. 2021;30:130728. doi: 10.1016/j.chemosphere.2021.130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mutono N, Wright J, Mutembei H, et al. The nexus between improved water supply and water-borne diseases in urban areas in Africa: a scoping review protocol. AAS Open Res. 2020;3:12. doi: 10.12688/aasopenres.13063.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Semenza JC, Nichols G. Cryptosporidiosis surveillance and water-borne outbreaks in Europe. Euro Surveill. 2007;12(5):E13–E14. doi: 10.2807/esm.12.05.00711-en. [DOI] [PubMed] [Google Scholar]
- 93.Herrador BR, De Blasio BF, MacDonald E, et al. Analytical studies assessing the association between extreme precipitation or temperature and drinking water-related waterborne infections: a review. Environ Health. 2015;14(1):1–2. doi: 10.1186/1476-069X-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Levy K, Woster AP, Goldstein RS, Carlton EJ. Untangling the impacts of climate change on waterborne diseases: a systematic review of relationships between diarrheal diseases and temperature, rainfall, flooding, and drought. Environ Sci Technol. 2016;50(10):4905–4922. doi: 10.1021/acs.est.5b06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suk JE, Vaughan EC, Cook RG, Semenza JC. Natural disasters and infectious disease in Europe: a literature review to identify cascading risk pathways. Eur J Public Health. 2020;30(5):928–935. doi: 10.1093/eurpub/ckz111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang N, Song D, Zhang J, et al. The impact of the 2016 flood event in Anhui Province, China on infectious diarrhea disease: an interrupted time-series study. Environ Int. 2019;1(127):801–809. doi: 10.1016/j.envint.2019.03.063. [DOI] [PubMed] [Google Scholar]
- 97.Epstein A, Benmarhnia T, Weiser SD. Drought and illness among young children in Uganda, 2009–2012. Am J Trop Med Hyg. 2020;102(3):644. doi: 10.4269/ajtmh.19-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Subiros M, Brottet E, Solet JL, LeGuen A, Filleul L. Health monitoring during water scarcity in Mayotte, France, 2017. BMC Public Health. 2019;19(1):1. doi: 10.1186/s12889-019-6613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asmall T, Abrams A, Röösli M, Cissé G, Carden K, Dalvie MA. The adverse health effects associated with drought in Africa. Sci Total Environ. 2021;1(793):148500. doi: 10.1016/j.scitotenv.2021.148500. [DOI] [PubMed] [Google Scholar]
- 100.Kouadio IK, Aljunid S, Kamigaki T, Hammad K, Oshitani H. Infectious diseases following natural disasters: prevention and control measures. Expert Rev Anti Infect Ther. 2012;10(1):95–104. doi: 10.1586/eri.11.155. [DOI] [PubMed] [Google Scholar]
- 101.Jutla A, Khan R, Colwell R. Natural disasters and cholera outbreaks: current understanding and future outlook. Curr Environ Health Rep. 2017;4(1):99–107. doi: 10.1007/s40572-017-0132-5. [DOI] [PubMed] [Google Scholar]
- 102.Moore S, Dongdem AZ, Opare D, et al. Dynamics of cholera epidemics from Benin to Mauritania. PLoS Negl Trop Dis. 2018;12(4):e0006379. doi: 10.1371/journal.pntd.0006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Camacho A, Bouhenia M, Alyusfi R, et al. Cholera epidemic in Yemen, 2016–18: an analysis of surveillance data. Lancet Glob Health. 2018;6(6):e680–e690. doi: 10.1016/S2214-109X(18)30230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olago D, Marshall M, Wandiga SO, et al. Climatic, socio-economic, and health factors affecting human vulnerability to cholera in the Lake Victoria basin, East Africa. AMBIO J Hum Environ. 2007;36(4):350–358. doi: 10.1579/0044-7447(2007)36[350:CSAHFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 105.Luque Fernández MÁ, Bauernfeind A, Jiménez JD, Gil CL, Omeiri NE, Guibert DH. Influence of temperature and rainfall on the evolution of cholera epidemics in Lusaka, Zambia, 2003–2006: analysis of a time series. Trans R Soc Trop Med Hyg. 2009;103(2):137–143. doi: 10.1016/j.trstmh.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 106.Ruiz-Moreno D, Pascual M, Bouma M, Dobson A, Cash B. Cholera seasonality in Madras (1901–1940): dual role for rainfall in endemic and epidemic regions. EcoHealth. 2007;4(1):52–62. doi: 10.1007/s10393-006-0079-8. [DOI] [Google Scholar]
- 107.Hashizume M, Faruque AS, Wagatsuma Y, Hayashi T, Armstrong B. Cholera in Bangladesh: climatic components of seasonal variation. Epidemiology. 2010;21(5):706–710. doi: 10.1097/EDE.0b013e3181e5b053. [DOI] [PubMed] [Google Scholar]
- 108.Semenza JC, Trinanes J, Lohr W, et al. Environmental suitability of Vibrio infections in a warming climate: an early warning system. Environ Health Perspect. 2017;125(10):107004. doi: 10.1289/EHP2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lau CL, Smythe LD, Craig SB, Weinstein P. Climate change, flooding, urbanisation and leptospirosis: fuelling the fire? Trans R Soc Trop Med Hyg. 2010;104(10):631–638. doi: 10.1016/j.trstmh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 110.Dhewantara PW, Hu W, Zhang W, et al. Climate variability, satellite-derived physical environmental data and human leptospirosis: a retrospective ecological study in China. Environ Res. 2019;1(176):108523. doi: 10.1016/j.envres.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 111.Dechet AM, Parsons M, Rambaran M, et al. Leptospirosis outbreak following severe flooding: a rapid assessment and mass prophylaxis campaign; Guyana, January–February 2005. PLoS ONE. 2012;7(7):e39672. doi: 10.1371/journal.pone.0039672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Al-shere TA, Ujiie M, Suzuki M, et al. Outbreak of leptospirosis after flood, the Philippines, 2009. Emerg Infect Dis. 2012;18(1):91. doi: 10.3201/eid1801.101892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cissé G. Food-borne and water-borne diseases under climate change in low-and middle-income countries: further efforts needed for reducing environmental health exposure risks. Acta Trop. 2019;1(194):181–188. doi: 10.1016/j.actatropica.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mbereko A, Chimbari MJ, Manyangadze T, Mukaratirwa S. Knowledge and perceptions of schistosomiasis, a water-borne disease, in two semi-arid rural areas of South Africa (Ndumo) and Zimbabwe (Ntalale) Food Waterborne Parasitol. 2020;1(21):e00091. doi: 10.1016/j.fawpar.2020.e00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Archer J, O’Halloran L, Al-Shehri H, et al. Intestinal schistosomiasis and giardiasis co-infection in sub-Saharan Africa: can a one health approach improve control of each waterborne parasite simultaneously? Trop Med Infect Dis. 2020;5(3):137. doi: 10.3390/tropicalmed5030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang GJ, Bergquist R. Potential impact of climate change on schistosomiasis: a global assessment attempt. Trop Med Infect Dis. 2018;3(4):117. doi: 10.3390/tropicalmed3040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mutuku FM, King CH, Bustinduy AL, Mungai PL, Muchiri EM, Kitron U. Impact of drought on the spatial pattern of transmission of Schistosoma haematobium in coastal Kenya. Am J Trop Med Hyg. 2011;85(6):1065–1070. doi: 10.4269/ajtmh.2011.11-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DM Fujita, de Albuquerque Luna EJ. Natural disasters, ecotourism and adventure travel may contribute to Schistosoma mansoni in Chapada Diamantina/Bahia—Brazil—2017. Travel Med Infect Dis. 2019;31:101341. [DOI] [PubMed]
- 119.Kalinda C, Chimbari MJ, Mukaratirwa S. Effect of temperature on the Bulinus globosus—Schistosoma haematobium system. Infect Dis Poverty. 2017;6(1):1–7. doi: 10.1186/s40249-017-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCreesh N, Nikulin G, Booth M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit Vectors. 2015;8(1):1–9. doi: 10.1186/s13071-014-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu G, Fan J, Peterson AT. Schistosoma japonicum transmission risk maps at present and under climate change in mainland China. PLoS Negl Trop Dis. 2017;11(10):e0006021. doi: 10.1371/journal.pntd.0006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nichols GL, Gillingham EL, Macintyre HL, et al. Coronavirus seasonality, respiratory infections and weather. BMC Infect Dis. 2021;21(1):1–5. doi: 10.1186/s12879-021-06785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park JE, Son WS, Ryu Y, Choi SB, Kwon O, Ahn I. Effects of temperature, humidity, and diurnal temperature range on influenza incidence in a temperate region. Influenza Other Respir Viruses. 2020;14(1):11–18. doi: 10.1111/irv.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ianevski A, Zusinaite E, Shtaida N, et al. Low temperature and low UV indexes correlated with peaks of influenza virus activity in Northern Europe during 2010–2018. Viruses. 2019;11(3):207. doi: 10.3390/v11030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Towers S, Chowell G, Hameed R, et al. Climate change and influenza: the likelihood of early and severe influenza seasons following warmer than average winters. PLoS Curr. 2013;28:5. doi: 10.1371/currents.flu.3679b56a3a5313dc7c043fb944c6f138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bayard V, Kitsutani PT, Barria EO, et al. Outbreak of hantavirus pulmonary syndrome, Los Santos, Panama, 1999–2000. Emerg Infect Dis. 2004;10(9):1635. doi: 10.3201/eid1009.040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Williams RJ, Bryan RT, Mills JN, et al. An outbreak of hantavirus pulmonary syndrome in western Paraguay. Am J Trop Med Hyg. 1997;57(3):274–282. doi: 10.4269/ajtmh.1997.57.274. [DOI] [PubMed] [Google Scholar]
- 128.Engelthaler DM, Mosley DG, Cheek JE, et al. Climatic and environmental patterns associated with hantavirus pulmonary syndrome, Four Corners region, United States. Emerg Infect Dis. 1999;5(1):87. doi: 10.3201/eid0501.990110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kondo H, Seo N, Yasuda T, et al. Post-flood—infectious diseases in Mozambique. Prehosp Disaster Med. 2002;17(3):126–133. doi: 10.1017/S1049023X00000340. [DOI] [PubMed] [Google Scholar]
- 130.Biswas R, Pal D, Mukhopadhyay SP. A community-based study on health impact of flood in a vulnerable district of West Bengal. Indian J Public Health. 1999;43(2):89–90. [PubMed] [Google Scholar]
- 131.Yusof A, Siddique AK, Baqui AH, Eusof A, Zaman K. 1988 floods in Bangladesh: pattern of illness and causes of death. J Diarrhoeal Dis Res. 1991;1:310–314. [PubMed] [Google Scholar]