Abstract
Dissimilatory arsenate-reducing bacteria have been implicated in the mobilization of arsenic from arsenic-enriched sediments. An As(V)-reducing bacterium, designated strain GBFH, was isolated from arsenic-contaminated sediments of Lake Coeur d'Alene, Idaho. Strain GBFH couples the oxidation of formate to the reduction of As(V) when formate is supplied as the sole carbon source and electron donor. Additionally, strain GBFH is capable of reducing As(V), Fe(III), Se(VI), Mn(IV) and a variety of oxidized sulfur species. 16S ribosomal DNA sequence comparisons reveal that strain GBFH is closely related to Desulfitobacterium hafniense DCB-2T and Desulfitobacterium frappieri PCP-1T. Comparative physiology demonstrates that D. hafniense and D. frappieri, known for reductively dechlorinating chlorophenols, are also capable of toxic metal or metalloid respiration. DNA-DNA hybridization and comparative physiological studies suggest that D. hafniense, D. frappieri, and strain GBFH should be united into one species. The isolation of an Fe(III)- and As(V)-reducing bacterium from Lake Coeur d'Alene suggests a mechanism for arsenic mobilization in these contaminated sediments while the discovery of metal or metalloid respiration in the genus Desulfitobacterium has implications for environments cocontaminated with arsenious and chlorophenolic compounds.
Arsenic is the 20th most abundant element in the Earth's crust (56) and is widely distributed throughout nature as a result of weathering, dissolution, fire, volcanic activity, and anthropogenic input (13). The last includes the use of arsenic in pesticides, herbicides, wood preservatives, and dye stuffs as well as production of arsenic-containing wastes during smelting and mining operations (56). In arsenic-enriched environments, a major concern is the potential for mobilization and transport of this toxic element to groundwater and drinking water supplies. In Bangladesh, an estimated 57 million people have been exposed to arsenic through contaminated wells (9). This incident serves as an unfortunate reminder of the toxic consequences of arsenic mobilization and underscores the need to understand the factors controlling the mobility and solubility of arsenic in aquatic systems (60).
Coeur d'Alene Lake (CDAL) is the second largest lake in Idaho. As a result of a century of mining along the Coeur d'Alene River, one of two rivers feeding CDAL, lake sediments are highly enriched in trace elements including Ag, As, Cd, Pb, Sb, and Zn (31). Sediment pore waters are also trace element enriched with mean total arsenic and lead concentrations exceeding 160 and 250 μg/liter (28), respectively. Nevertheless, CDAL surface waters comply with current federal drinking water standards (28) (50 and 15 μg/liter for As and Pb [75, 76], respectively). Because residents of Northern Idaho use these waters for recreation and fishing and as a source of drinking water (82), concern remains over the possibility that contaminants could be mobilized from the sediment to the water column.
Iron is the dominant metal in the Coeur d'Alene system, constituting approximately 10% of the sediments by dry weight (14, 27). Because iron is exceptionally abundant in CDAL sediments, its transformations are likely to influence the bioavailability and mobility of trace elements such as arsenic (16). Sorption of As onto the surface of insoluble iron oxyhydroxides is well documented, as is the observation that the oxidation state of arsenic influences its propensity to sorb onto iron mineral surfaces (5, 18, 22, 50–52, 62, 63, 65). In arsenic-enriched soils and sediments, an increase in soluble As(III) is commonly observed upon establishment of reducing conditions (1, 8, 52, 53, 69). This observation has been attributed to poor sorption of As(III) onto iron oxyhydroxides (18, 62) and is consistent with the idea that As(III) is more mobile than As(V) in aquatic environments (39). Recent studies, however, suggest that under certain conditions at circumneutral pH, As(III) can sorb to iron oxyhydroxides at least as strongly as As(V) (50, 63, 74). In view of this, alternative mechanisms should be considered to explain why soluble As(III) commonly increases when soils and sediments become anoxic.
The occurrence of soluble As in reduced aquatic environments has been attributed to the reductive dissolution of solid-phase iron oxyhydroxides followed by the release of sorbed arsenic (1, 4, 8, 18, 53, 60). In such environments, the reductive dissolution of iron oxyhydroxides is largely mediated by the activity of dissimilatory iron-reducing bacteria (DIRB) (43). Our laboratory has demonstrated that the DIRB Shewanella alga BrY, an organism that cannot respire As(V), mobilizes As(V) from the solid-phase ferric arsenate mineral scorodite by reducing Fe(III) to Fe(II) (14). As(V) reduction is therefore not a prerequisite to arsenic solubilization from FeO(OH)x, even though an increase in soluble As(III) is commonly observed upon the onset of anoxia (1, 8, 52, 53). In anaerobic environments, DIRB-mediated reductive dissolution of iron minerals would likely result in an increase in As(V) concentrations, provided DIRB were not also capable of reducing As(V) (14). However, if dissimilatory arsenate-reducing organisms were also present and active, solubilized As(V) could be readily converted into As(III).
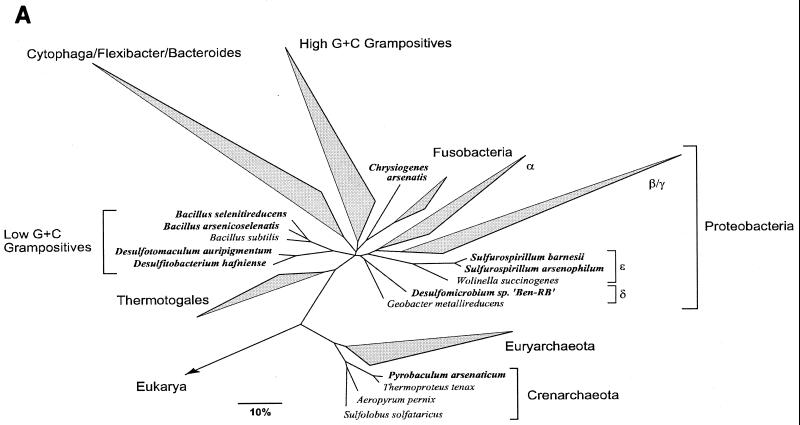
Our laboratory has demonstrated that CDAL sediments are highly reduced (16) and support biotic reduction of As(V) (28). We have hypothesized that this results from dissimilatory arsenate reduction. Dissimilatory arsenic-reducing bacteria (DAsRB) couple the reduction of As(V) to the oxidation of an organic compound or H2 and thereby conserve energy for growth (33, 46, 72). Most probable number estimates suggest that the number of cultivable DAsRB in this environment ranges from 103 to 105 cells/g (wet weight) of sediment (28). Several studies of dissimilatory arsenate reduction suggest that this transformation is likely to play a role in mobilizing arsenic from ferric oxyhydroxide minerals (3, 19, 28, 83). Incubation of either sterile As-contaminated sediments or scorodite with a pure culture of the DAsRB Sulfurospirillum arsenophilum MIT-13T resulted in elevated aqueous-phase As(III) concentration (3). When the DAsRB Sulfurospirillum barnesii strain SES-3T was incubated with As(V) that was either coprecipitated with or adsorbed to poorly crystalline iron oxyhydroxide minerals, approximately half of the resulting arsenite was retained in the solid phase and the other half was released into solution (83). The few known DAsRB are remarkable for their phylogenetic diversity and metabolic versatility (see Fig. 5A) (Table 1). This suggests that the capacity to couple growth to As(V) reduction may be widespread. If further investigation confirms that this trait is broadly distributed among the Bacteria and Archaea and that such organisms are abundant and active, DAsRB may play essential roles in mediating the reductive portion of the arsenic cycle.
FIG. 5.
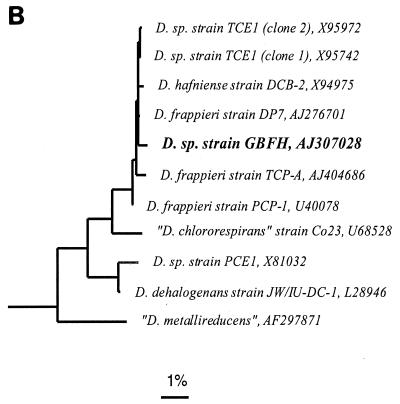
(A) Unrooted phylogenetic tree showing the positions of currently recognized As(V)-reducing microorganisms (in bold) among the major lines of prokaryotes. The tree is based on an optimized global tree reconstructed from almost full-length SSU rRNA gene sequences belonging to all three domains using various treeing programs included in the ARB package. The bar indicates 10% estimated sequence divergence. (B) Phylogenetic tree based on partial 16S rRNA gene sequences (nucleotides 98 to 1542, E. coli numbering) showing the placement of strain GBFH (in bold) in the low-GC gram-positive clade among members of the genus Desulfitobacterium. A maximum-likelihood method was used for tree reconstruction. Reference sequences (not shown) belonging to major lineages of the Bacillus-Clostridium group of bacteria were used as the out-group. The bar indicates 1% estimated sequence divergence.
TABLE 1.
Summary of relevant physiological characteristics of previously published DAsRBa
| Physiological parameter | Previously characterized DAsRB
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MIT-13T | SES-3T | BAL-1T | OREX-4T | E1HT | MLS10T | BEN-RBb | PZ6T | |
| Electron donors | ||||||||
| Lactate | + | + | + | + | + | + | + | |
| Pyruvate | + | + | + | + | − | + | ||
| H2 plus acetate | + | + | + | + | − | − | ||
| Fumarate | + | + | + | − | ||||
| Malate | + | + | + | − | ||||
| Succinate | + | + | − | − | − | |||
| Citrate | + | − | + | − | ||||
| Butyrate | +c | + | ||||||
| H2 | − | − | − | + | + | |||
| Formate | +c | +c | − | − | − | − | ||
| Acetate | − | − | + | − | − | − | − | |
| Glucose | − | − | − | + | ||||
| Glycerol | + | |||||||
| Ethanol | + | − | − | |||||
| Galactose | − | +d | ||||||
| Methanol | − | − | − | − | ||||
| Electron acceptors | ||||||||
| As(V) | + | + | + | + | + | + | + | + |
| Nitrate | + | + | + | − | + | + | − | |
| Nitrite | + | + | − | + | ||||
| Se(VI) | + | − | + | − | + | |||
| Se(IV) | − | − | − | − | + | − | ||
| Sulfate | − | − | − | + | − | − | + | |
| Thiosulfate | + | − | + | − | − | + | ||
| Sulfite | − | + | − | − | ||||
| S0 | − | + | + | |||||
| Fe(III) | + | − | − | + | − | |||
| Mn(IV) | − | + | − | |||||
| Fumarate | + | + | + | + | + | |||
| Oxygen | − | −e | − | − | − | +e | − | |
| Optima | ||||||||
| Temperature (°C) | 20 | 33 | 27 | 25–30 | 95 | |||
| pH | 7.5 | 7.5 | 6.4–7.0 | 8.5 | 9.5 | |||
| Doubling time (h) | 14 | 5 | 4 | 9 | 1.3 | |||
Blank cells indicate that no data are available with regard to the specific variable. Data for S. arsenophilum MIT-13T are from references 2, 57, and 71. Data for S. barnesii SES-3T are from references 41, 57, and 72. Data for Chrysiogenes arsenatis BAL-1T are from reference 47. Data for Desulfotomaculum auripigmentum OREX-4T are from reference 58. Data for Bacillus arseniciselenatis E1HT and Bacillus selenitireducens MLS10T are from reference 6. Data for Desulfomicrobium sp. strain Ben-RB are from reference 46. Data for Pyrobaculum arsenaticum PZ6T are from reference 33. +, growth was supported; −, growth was not supported.
Physiological characterization is minimal with no formal survey of electron donors or acceptors.
Growth occurs only in the presence of acetate as a carbon source.
Growth is fermentative rather than respiratory.
Growth is microaerophilic.
We hypothesized that the biotic generation of As(III) observed in CDAL sediment microcosms (28) arose from the activity of dissimilatory arsenate-reducing bacteria, and we sought to enrich such organisms from CDAL sediments. From thermodynamic considerations, we hypothesized that formate could serve as both the electron donor and carbon source for As(V) reduction and that energy from this reduction could be conserved for growth. Insofar as no characterized DAsRB have been enriched from CDAL and no characterized DAsRB are capable of growth on formate alone, we proposed that a formate-oxidizing DAsRB recovered from CDAL sediments would be phylogenetically distinct from previously characterized arsenate-reducers. Lastly, we hypothesized that As(V)-reducing organisms enriched from these Fe-rich sediments would also be capable of obtaining energy for growth by dissimilatory Fe(III) reduction. Such an organism could be expected to mediate diagenesis of both arsenic and iron.
MATERIALS AND METHODS
Enrichment and isolation.
Strain GBFH was isolated from an enrichment culture inoculated with sediment obtained from the Coeur d'Alene River delta, Coeur d'Alene Lake, Idaho. Sediments were collected using a gravity coring device (55) and processed as previously described (15, 16). Thirty-centimeter cores were split into two 15-cm sections and homogenized using sterile, anaerobic lake water. The resulting slurry served as an inoculum for the enrichment of As(V)-reducing bacteria. One milliliter of sediment slurry was added to 9 ml of medium, and the enrichments were incubated at 25°C in the dark. All manipulations were carried out in a LabConco anaerobic chamber containing an atmosphere of N2:CO2:H2 (75:15:10).
Strict anaerobic technique (54) was used in the preparation of the medium and the manipulation of the cultures. The medium used for enrichment of strain GBFH contained the following (per liter): NaHCO3 (2.5 g), NH4Cl (1.5 g), KH2PO4 (0.6 g), KCl (0.1 g), Wolfe's vitamin solution (10 ml), and modified Wolfe's mineral solution (10 ml). Components of the vitamin and mineral solutions can be found in ATCC medium no. 1957. Salts, trace elements, and vitamins were combined, heated, cooled under O2-free N2:CO2:H2 (75:15:10), dispensed into degassed 20-ml Balch tubes or 120-ml serum vials, sealed with butyl rubber stoppers, and autoclaved. Cysteine-HCl (1 mM), formate (10 mM), and As(V) (10 mM) were added separately from sterile, anaerobic stocks. The pH of the complete medium was 7.0.
Enrichments were analyzed for accumulation of As(III) and observed microscopically. The enrichment culture chosen for continued investigation rapidly accumulated As(III). This enrichment culture, designated strain GBFH, was passaged seven times through a terminal dilution series in liquid media and then passaged three times through agar shake tube dilutions (61). The medium for the agar shake tubes contained the same components as the enrichment medium, with the addition of 1% Bacto-agar (Difco) and 0.1 g of yeast extract (Difco)/liter. Culture purity was determined by microscopy and denaturing gradient gel electrophoresis (DGGE). Strain GBFH has been deposited in the American Type Culture Collection.
Media, cultivation, and strains.
The medium used for the growth of strain GBFH during subsequent experiments differed in two ways from the enrichment medium. Yeast extract (0.1 g/liter) was added to the medium, and the headspace was changed to N2:CO2 (4:1), as hydrogen was found to be mildly inhibitory (see Results). Unless otherwise specified, this slightly modified medium was used for the maintenance of GBFH and for all growth experiments following isolation.
Desulfitobacterium dehalogenans JW/IU-DC1T (DSMZ 9161) (77), Desulfitobacterium hafniense DCB-2T (DSMZ 10664) (12), Desulfitobacterium frappieri PCP-1T (DSMZ 12420) (7), and Desulfitobacterium chlororespirans Co23T (DSMZ 11544) (67) were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ) (Braunschweig, Germany). All organisms were initially cultured on the recommended DSMZ medium. Since all four previously characterized Desulfitobacterium species were easily cultured on the medium described in the previous paragraph, this medium was used for all comparative growth experiments. Unless otherwise stated, all growth experiments were conducted at 37°C in the dark.
Electron microscopy.
Samples for scanning electron microscopy were pipetted onto a polylysine-coated Nuclepore filter and/or coverslip, fixed with 2.5% glutaraldehyde, washed twice with 0.1 M sodium cacodylate buffer, and postfixed with 1% osmium tetroxide buffered in 1× phosphate-buffered saline, pH 7.2 (66). Samples were dehydrated in a graded ethanol series, dried with hexamethyldisilizane, mounted on aluminum stubs, and viewed on a Hitachi S-4000 scanning electron microscope. Digital images were captured with SEMages software (Advanced Database Systems). Samples for transmission electron microscopy were fixed and dehydrated as described above and then infiltrated in a graded epoxy resin and polymerized at 60°C for 2 days. Seventy-nanometer sections were collected and poststained with 2% uranyl acetate followed by lead citrate (64) and viewed using a Hitachi H-7000 transmission electron microscope. Digital images were captured with Digital Micrograph 3.3.1 (Gatan, Inc.). Samples for negative staining received no pretreatment and were stained for 10 min using either 2% uranyl acetate or 2% phosphotungstic acid.
Oxidation of formate coupled to the reduction of As(V).
Mid-log-phase formate (10 mM)- and As(V) (10 mM)-grown cells of strain GBFH were inoculated into fresh medium (10% inoculum) containing 10 mM formate and 10 mM As(V) and adjusted to pH 7.5. Triplicate cultures were inoculated for each of five experimental conditions: (i) live cells in complete medium, (ii) live cells in medium containing no formate, (iii) live cells in medium containing no As(V), (iv) heat-killed cells added to complete medium, and (v) uninoculated complete medium. Identical experiments were performed using D. frappieri and D. hafniense.
Determination of optimal temperature and pH.
For temperature and pH determinations, strain GBFH was grown in medium containing 10 mM formate and 10 mM As(V). Optimum temperature determinations were made at pH 7.0 and under headspaces of N2:CO2 (4:1) and N2:CO2:H2 (75:15:10). To determine the optimum pH, bicarbonate was eliminated and replaced with a buffer system consisting of 25 mM (each) HEPES, PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], MES (morpholineethanesulfonic acid), and Tris base. The initial pH was set using sterile, anoxic 2 N HCl or 2 N NaOH. Because As(V) respiration with formate as the carbon and electron source is proton consuming, pH increased during growth but not by more than 0.3 pH units. Growth rates were calculated using the linear portion of the semi-logarithmic plot of optical density at 420 nm (OD420) versus time.
Heat-resistant spore formation.
To test for the ability of strain GBFH to form heat-resistant spores, triplicate cultures of GBFH were grown to stationary phase on 10 mM formate and 10 mM As(V) and the presence of spores was confirmed by microscopy. Three spore-containing cultures were incubated at 85°C for 30 min, and then each culture was transferred to fresh medium and incubated at 37°C. Growth was monitored spectrophotometrically. As(III) was quantified after inoculation of cultures into fresh medium and after 8 days of incubation.
Carbon source and electron donor survey.
Strain GBFH, D. hafniense, and D. frappieri were grown to mid-log phase on 10 mM lactate and 10 mM As(V). Each organism was inoculated onto each carbon source in triplicate using a 10% inoculum. Carbon sources were added from sterile, anaerobic stock solutions to yield the concentrations given in Table 2. Carbon sources were tested using As(V) as the sole terminal electron acceptor (TEA), except fermentative cultures, which were given no As(V). Hydrogen was tested as a potential electron donor by the injection of 10 ml of H2 to the headspace of culture tubes using a sterile syringe and shaking the tubes at 125 rpm. Growth was monitored spectrophotometrically, and if growth occurred, the culture was passaged two additional times. Organisms were considered positive for carbon source utilization if 2.5 to 3 doublings occurred during the third passage and if more than 80% of the supplied As(V) was respired to As(III) after the third passage. Negative controls contained no carbon source.
TABLE 2.
Carbon source and electron donor utilization profiles for strain GBFH, D. hafniense, and D. frappieri using 10 mM As(V) as the TEA
| Carbon source | Final carbon source concn (mM) | Organisma
|
||
|---|---|---|---|---|
| GBFH | D. hafniense | D. frappieri | ||
| Pyruvate | 10 | + | + | + |
| Pyruvate | 10b | + | + | + |
| Lactate | 10 | + | + | + |
| Formate | 10 | + | + | + |
| Fumarate | 10 | + | + | + |
| Butyrate | 10 | − | + | + |
| Succinate | 10 | − | + | + |
| Malate | 10 | − | + | + |
| Ethanol | 5 | − | + | + |
| Acetate | 10 | − | − | − |
| Glycerol | 5 | − | − | − |
| Methanol | 5 | − | − | − |
| Citrate | 10 | − | − | − |
| Benzoate | 5 | − | − | − |
| H2 | 10 | − | − | − |
| H2 plus acetate | 50 | − | − | − |
| Propionate | 10 | − | − | − |
| Glucose | 10 | − | − | − |
| Galactose | 10 | − | − | − |
| Lactate | 10b | − | − | − |
| Butyrate | 5b | − | − | − |
| None | − | − | − | |
+, growth was supported; −, growth was not supported.
Cultures were grown under fermentative conditions and were not provided with As(V).
TEA survey.
To investigate the potential for strain GBFH, D. hafniense, and D. frappieri to utilize various TEAs, cells were pregrown on either 10 mM lactate and 10 mM As(V) or fermentatively grown on 11.3 mM pyruvate. A 10% inoculum was used to inoculate triplicate cultures for each electron acceptor tested. Electron acceptors were tested with 10 mM lactate serving as the electron donor and carbon source. If growth occurred, the culture was passaged two additional times. Organisms were considered positive for TEA utilization if 2.5 to 3 doublings occurred during the third passage and if reduction of the TEA occurred (see below) after the third passage. Negative controls contained no TEA. D. dehalogenans and D. chlororespirans were tested for the ability to utilize MnO2, As(V), Se(VI), and Fe(III) using the same experimental design.
Sulfate, sulfite, thiosulfate, nitrate, nitrite, fumarate, selenate, selenite, arsenate, arsenite, ferric pyrophosphate, and 3-chloro-4-hydroxyphenylacetate (3-Cl-4-OHPA) were added to the media from sterile anaerobic stock solutions to give the final concentrations shown in Table 3. Elemental sulfur (Fisher Chemicals) and MnO2 (synthesized by the method of Burdige [10]) were added as powder to Balch tubes prior to autoclaving. Fe(III) gel was prepared according to the method of Lovley and Phillips (44). To test O2 (100% air) as the TEA, cultures were grown aerobically, and 5% air was tested as a potential TEA by preparing media anaerobically and adding sterile air to 5% of the headspace volume in each Balch tube. No reductant was added during the testing of 5% air as the TEA, and all culture tubes containing air were incubated with shaking at 120 rpm.
TABLE 3.
Terminal electron accepting utilization profiles for strain GBFH, D. hafniense, and D. frappieri using 10 mM lactate as the carbon source and electron donor
| TEA | Final TEA concna | Organismb
|
||
|---|---|---|---|---|
| GBFH | D. hafniense | D. frappieri | ||
| Elemental S | 10 | + | + | + |
| Sulfite | 5 | + | + | + |
| Thiosulfate | 10 | + | + | + |
| Fumarate | 10 | + | + | + |
| MnO2 | 10 | + | + | + |
| As(V) | 10 | + | + | + |
| Fe(III) gel | 50 | + | + | + |
| Fe pyrophosphate | 3 g/liter | + | + | + |
| Se(VI) | 10 | + | + | + |
| Nitrate | 10 | − | + | + |
| Nitrite | 10 | − | − | − |
| Sulfate | 10 | − | − | − |
| O2 | 100% air | − | − | − |
| O2 | 5% air | − | − | − |
| Se(IV) | 10 | − | − | − |
| As(III) | 10 | − | − | − |
| 3-Cl-4-OHPA | 10 | − | − | − |
| None | − | − | − | |
Concentrations are in millimoles unless otherwise noted in the column.
+, growth was supported (see Materials and Methods); −, growth was not supported.
Reduction of sulfate, sulfite, thiosulfate, and elemental sulfur was tested by the addition of 0.1% FeSO4 to the culture tubes. Formation of black FeS indicated the presence of sulfide resulting from the reduction of the oxidized sulfur compounds. The reduction of MnO2 was assessed by a color change from black MnO2 to white MnCO3. Precipitation of red elemental selenium was used as an indicator of Se(VI) and Se(IV) reduction. The reduction of Fe(III) pyrophosphate was inferred by the color change from yellow to clear with siderite (FeCO3) formation, whereas the reduction of Fe(III) gel was assessed by monitoring the color change from rust to black. Direct cell counts were used to confirm growth on selenate, selenite, sulfate, and manganese. Otherwise, cell density was measured spectrophotometrically.
Oxygen sensitivity.
Strain GBFH was grown to mid-log phase on 10 mM lactate and 10 mM As(V), and a 10% inoculum was used to inoculate experimental tubes in triplicate. Sterile air was added to final concentrations of 0, 1, 2, 5, and 10% air by volume in the Balch tube headspace; reductant was not added to the experimental tubes. To test whether strain GBFH could resume growth after being exposed to 10% air, cells from the 10% air treatment were subsampled after 24 h of incubation and reinoculated into 0% air tubes. Cultures were shaken at 120 rpm at 37°C. Growth was monitored spectrophotometrically, and the accumulation of As(III) was quantified once growth was evident. Controls consisted of autoclaved cells.
D. dehalogenans inhibition by As(V).
To determine if the growth of D. dehalogenans was inhibited by the presence of As(V), D. dehalogenans cultures were pregrown on pyruvate (11.3 mM) and then inoculated in duplicate into the following eight treatments: (i) 11.3 mM pyruvate, (ii) 11.3 mM pyruvate plus 10 mM As(V), (iii) 10 mM lactate plus 10 mM 3-Cl-4-OHPA, (iv) 10 mM lactate plus 10 mM 3-Cl-4-OHPA plus 10 mM As(V), (v) 10 mM lactate plus 10 mM nitrate, (vi) 10 mM lactate plus 10 mM nitrate plus 10 mM As(V), (vii) 10 mM lactate, and (viii) 10 mM lactate plus 10 mM As(V). The same experiment was carried out with D. hafniense in order to compare the response of D. dehalogenans to that of an organism capable of reducing As(V). To determine if D. dehalogenans could survive exposure to As(V), cultures of D. dehalogenans (treatments ii, iv, and vi) were harvested after 1 week by filtration through a sterile 0.22-μm-pore-size syringe filter in the anaerobic chamber. These cells were used to inoculate medium lacking As(V) and containing the electron donor-TEA pair present in the original treatment.
Analytical techniques.
Where cell densities are reported as optical density, Balch tubes were inserted into a Spectronic 20 D+ spectrophotometer and the absorbance at 420 nm (70) was recorded. Uninoculated medium served as the blank. Where cell densities are reported as cells per milliliter, cells were stained with 4,6-diamidino-2-phenylindole (DAPI) and counted directly under epifluorescence (29) using a Zeiss Axioskop microscope.
As(III) (arsenite) was quantified spectrophotometrically as previously described (14) using a modification of the method of Johnson and Pilson (38). Because phosphate was present in the growth medium, a reduction step was necessary to quantify As(V). Briefly, unoxidized samples were prepared by acidifying a 300-μl sample with 100 μl of 25 mM HCl, and oxidized samples were prepared by oxidizing a 300-μl sample with 100 μl of KIO3 solution (5 mM KIO3 in 50 mM HCl). Reduced samples were prepared by reducing a 300-μl sample with 100 μl of cold reducing solution (1 part 1.4% sodium thiosulfate, 2 parts 14% sodium metabisulfite, and 2 parts 3.5 N sulfuric acid) (37). All treatments were incubated at 25°C for 10 min. Molybdenum-containing reaction mixture (600 μl) (73) was then added to each treatment. Samples were immediately incubated at 78°C for 10 min, followed by a 5-min incubation on ice. Absorbance was measured at 865 nm. Arsenite concentrations were determined by subtracting the oxidized sample absorbance from the unoxidized sample absorbance. Arsenate concentrations were determined by subtracting the unoxidized sample absorbance from that of the reduced samples.
Formate was quantified enzymatically using a modified spectrophotometric assay for formate dehydrogenase (30). Briefly, 800 μl of phosphate buffer, 130 μl of double-distilled H2O, 30 μl of sample, 20 μl of NAD solution, and 20 μl of formate dehydrogenase were added to a 1-ml cuvette, mixed, and incubated in the dark for 1 h. Absorbance was read at 340 nm.
Analysis of DNA and phylogeny.
To assess GBFH culture purity, an ∼600-bp fragment of the small-subunit ribosomal gene was PCR amplified and subjected to DGGE. Each 50-μl PCR mixture contained the following (stock concentrations are in parentheses): 5 μl of 10× PCR buffer (GibcoBRL), 1.25 μl of the deoxynucleoside triphosphates (10 mM [each]) (GibcoBRL), 1 μl of bovine serum albumin (20 mg/ml) (Boehringer-Mannheim), 3 μl of MgCl2 (50 mM) (GibcoBRL), 2 μl of forward primer (12.5 μM) (GibcoBRL), 2 μl of reverse primer (12.5 μM) (GibcoBRL), 0.25 μl of Taq DNA polymerase (5 U/μl) (GibcoBRL), 1 μl of DNA template (as cells), and 33.5 μl of water (high-performance liquid chromatography grade; Aldrich). The forward primer, EUB338F, contained a GC-rich clamp for DGGE (5′-CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCGACTCCTACGGGAGGCAGC-3′). The reverse primer was EUB907R and contained no GC-clamp (5′-CCGTCAATTCMTTTRAGTTT-3′). Acrylamide gels (37.5:1 acrylamide-to-N,N′-methyl-bis-acrylamide ratio, 7.5% [wt/vol]) were run for 15 h at 60°C and 65 V over a 40 to 60% denaturant gradient using the Bio-Rad D-Gene system. One hundred percent denaturant was defined as 7 M urea and 40% formamide. Gels were visualized using UV and photographed digitally using Molecular Analyst software (Bio-Rad, Munich, Germany).
For phylogenetic analyses, almost full-length bacterial 16S rRNA gene fragments were directly PCR amplified from whole GBFH cells (80). The resulting products were purified using the Prep-A-Gene DNA purification kit (Bio-Rad) and sequenced using a LICOR automated sequencer (MWG Biotech, Ebersberg, Germany). Cycle sequencing protocols based upon the chain termination technique were applied using the Thermo Sequenase fluorescent labeled primer cycle sequencing kit (Amersham, Braunschweig, Germany). The obtained sequence was added to an alignment of more than 10,000 small-subunit (SSU) rRNA sequences belonging to all three domains using the ARB sequence database and the alignment tool of the ARB program package (W. Ludwig and O. Strunk; http://www.biol.chemie.tu-muenchen.de/pub/ARB/documentation). Phylogenetic analyses were performed by applying the maximum parsimony (ARB, PHYLIP), distance matrix (ARB, PHYLIP) (21), and maximum likelihood (fastDNAml) (49) methods on different data sets.
For comparative analyses of genomic DNA, DNA was extracted by the method of Cashion et al. (11). DNA-DNA hybridization was carried out using the thermal renaturation method (17) with modifications according to the methods of Huss et al. (35) and Escara and Hutton (20) with a Gilford System 2600 spectrophotometer equipped with a Gilford 2527-R thermoprogrammer and plotter. Renaturation rates were computed by the TRANSFER.BAS program (36).
Nucleotide sequence accession numbers.
The newly determined 16S rRNA gene sequence of strain GBFH has been deposited in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession no. AJ3047028.
RESULTS
Morphology of strain GBFH.
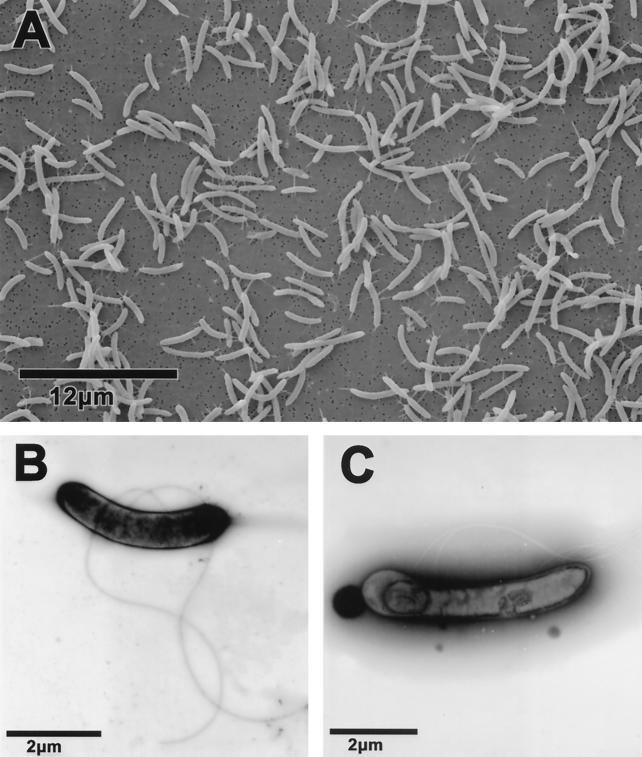
Cells of strain GBFH were curved rods measuring 2 to 4 μm in length and 0.3 to 0.5 μm in width (Fig. 1A). Strain GBFH possessed two to five laterally attached flagella (Fig. 1B) and formed endospores after entering the stationary phase (Fig. 1C). When grown on plates or shake tubes containing 1% agar, colonies of strain GBFH were white with rounded edges and approximately 1 to 2 mm in diameter.
FIG. 1.
(A) Scanning electron micrograph illustrating the cells of strain GBFH are curved rods measuring 2 to 4 μm in length and 0.3 to 0.5 μm in width. (B) Transmission electron micrograph of strain GBFH illustrating cell morphology and two laterally attached flagella. The sample was negatively stained with 2% uranyl acetate. (C) Transmission electron micrograph of endospore formation by strain GBFH. Note the five lateral flagella. The sample was negatively stained with 2% phosphotungstic acid.
Oxidation of formate coupled to the reduction of As(V).
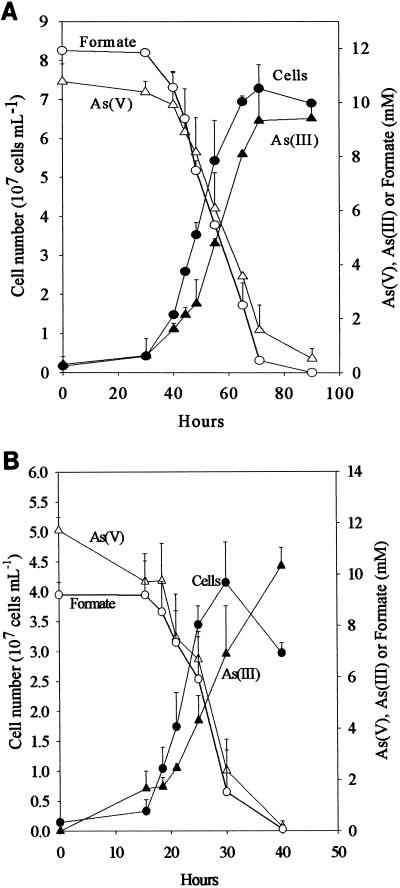
Strain GBFH coupled formate oxidation to As(V) reduction, conserving energy for growth (Fig. 2A). Figure 2A shows the concomitant increase in cell number, consumption of formate, depletion of As(V), and accumulation of As(III). D. frappieri also coupled As(V) reduction to formate oxidation and conserved energy in the process (Fig. 2B). During the growth of both D. frappieri and strain GBFH, As(V) and formate consumption were tightly coupled and As(III) accumulated concomitantly with cell growth (Fig. 2). Both organisms utilized As(V) and formate in an approximately 1:1 ratio. Controls included medium lacking formate, medium lacking As(V), heat-killed cells, and uninoculated medium. No increase in cell number, depletion of formate, or reduction of As(V) to As(III) was detected in any controls (data not shown).
FIG. 2.
Growth curve of strain GBFH (A) and D. frappieri (B) at pH 7.5 illustrating an increase in cell number (●) and As(III) (▴) and a decrease in As(V) (▵) and formate (○). Experiments were performed in triplicate, and error bars represent standard deviations.
Optimal temperature and pH.
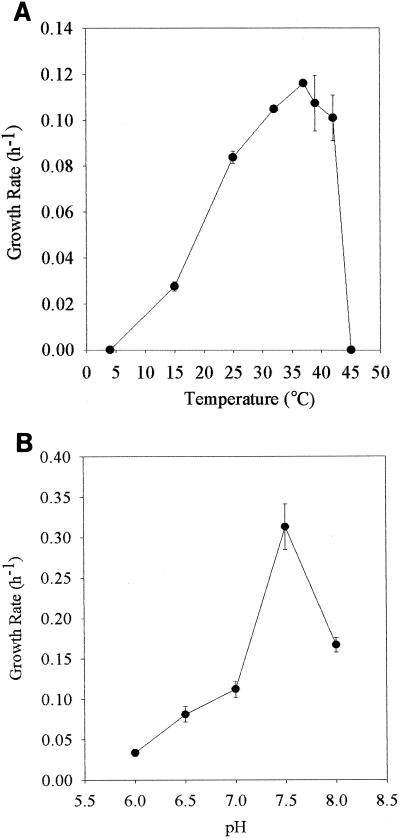
Strain GBFH grew optimally at 37°C, with a maximum growth rate of 0.12 h−1 (Fig. 3A) at pH 7.0. Strain GBFH was capable of growth and As(V) respiration at temperatures ranging from 15 to 42°C. Although growth was not observed at 4 or 45°C, small amounts of As(V) respiration (2 mM) occurred at both temperatures. Inclusion of 10% hydrogen in the headspace of culture tubes completely inhibited the growth of GBFH at 42°C. H2 reduced growth rates at all other temperatures (data not shown). The optimal pH for the growth of strain GBFH was 7.5, with a maximum growth rate of 0.313 h−1 (Fig. 3B). The growth rate at pH 7.5 (0.313 h−1) was nearly threefold higher than at pH 7.0 (0.12 h−1) and twofold higher than at pH 8 (0.167 h−1). GBFH was capable of growth and As(V) respiration from pH 6.0 to 8.0.
FIG. 3.
(A) Effect of temperature on specific growth rate (h−1) of strain GBFH grown on 10 mM formate and 10 mM As(V) at pH 7.0. (B) Effect of pH on specific growth rate of strain GBFH grown on 10 mM formate and 10 mM As(V) at 37°C. Experiments were performed in triplicate; each error bar represents one standard deviation.
Heat-resistant spore formation.
After 8 days of incubation at 37°C in fresh culture medium, all three heat-treated cultures of strain GBFH exhibited a log increase in cell number and respired all 10 mM As(V) provided (data not shown).
Carbon source and electron donor survey.
Carbon source and electron donor utilization profiles are summarized in Table 2. D. hafniense and D. frappieri exhibited identical carbon source utilization patterns. D. hafniense, D. frappieri, and strain GBFH all utilized pyruvate, lactate, formate, and fumarate. When cultured on formate and As(V) or fumarate and As(V), the yield for all three organisms was lower (OD420 ≈ 0.08 to 0.1) than when cultured on lactate and As(V) or pyruvate and As(V) (OD420 ≈ 0.15 to 2.0). Only D. hafniense and D. frappieri were able to utilize butyrate, succinate, malate, and ethanol. D. hafniense and D. frappieri grew slowly on malate and succinate. Cultures required 1 to 2 weeks to reach densities equivalent to those reached in 2 to 3 days when grown on lactate. Neither As(V) respiration nor an increase in cell density was observed when D. hafniense, D. frappieri, or strain GBFH was supplied with glycerol, methanol, citrate, benzoate, H2, H2 and acetate, propionate, glucose, or galactose. After three passages, D. hafniense, D. frappieri, and strain GBFH showed increases in cell number when acetate was provided, but in no case did cultures achieve sufficient densities to be considered positive for acetate utilization by our criteria (see Materials and Methods). Despite poor yields (OD420 ≈ 0.03), all three organisms reduced 8 to 10 mM As(V) in the presence of acetate, even after three consecutive transfers. Pyruvate supported fermentative growth of D. hafniense, D. frappieri, and strain GBFH, whereas lactate and butyrate did not support fermentative growth. Controls lacking a carbon source showed no increase in cell number and no reduction of As(V).
TEA survey.
Results of the TEA survey are summarized in Table 3. As in the carbon source utilization profile, D. hafniense and D. frappieri exhibited identical TEA utilization patterns. The inability of strain GBFH to utilize nitrate represents the singular difference between strain GBFH and D. hafniense and D. frappieri with respect to the terminal electron accepting processes tested. Cultures of all three organisms underwent a lag phase of 2 weeks when lactate- and As(V)-grown cells were inoculated in medium containing either fumarate, Se(VI), Fe(III) gel, elemental sulfur, MnO2, thiosulfate, or sulfite. No lag phase was observed when ferric pyrophosphate, As(V), or nitrate was provided as the TEA. Reduction of MnO2 resulted in a black-to-white color change, presumably from the formation of rhodochrosite (MnCO3) (45); the reduction of Se(VI) resulted in the precipitation of red elemental selenium. Cell growth on both Se(VI) and MnO2 was confirmed by direct cell count. Cells grown on elemental sulfur, thiosulfate, and sulfite produced sulfide, as inferred from the precipitation of FeS upon addition of FeSO4. Cultures of strain GBFH, D. hafniense, and D. frappieri grown on 10 mM As(V) and transferred to medium containing either sulfur, thiosulfate, or sulfite accumulated a bright yellow precipitate presumed to be orpiment (As2S3) (59) or realgar (As2S2) (33). Cultures grown on sulfate never produced detectable amounts of sulfide, and direct cell counts confirmed that no growth occurred when sulfate was provided as the sole TEA. In cultures where no TEA was provided, no growth occurred, confirming that an exogenous TEA must be supplied for growth on lactate by strain GBFH, D. hafniense, and D. frappieri.
D. dehalogenans and D. chlororespirans grew by reducing Fe(III) pyrophosphate, Se(VI), and MnO2, forming siderite, elemental selenium, and MnCO3, respectively. Cell growth on Se(VI) and MnO2 was verified by direct cell counts. Neither growth nor As(V) reduction was observed when As(V) was provided as the sole TEA. The foregoing evidence suggests that D. dehalogenans and D. chlororespirans are not capable of dissimilatory As(V) reduction, unlike D. hafniense, D. frappieri, and strain GBFH.
Oxygen sensitivity.
Strain GBFH was capable of growth and As(V) respiration when 0, 1, or 2% air was present in the headspace of the culture tubes. However, no cell growth occurred in cultures containing 5 or 10% air (data not shown). After 24 h of exposure to 10% air, strain GBFH resumed growth and As(V) reduction when reinoculated into tubes containing no air (data not shown).
D. dehalogenans inhibition by As(V).
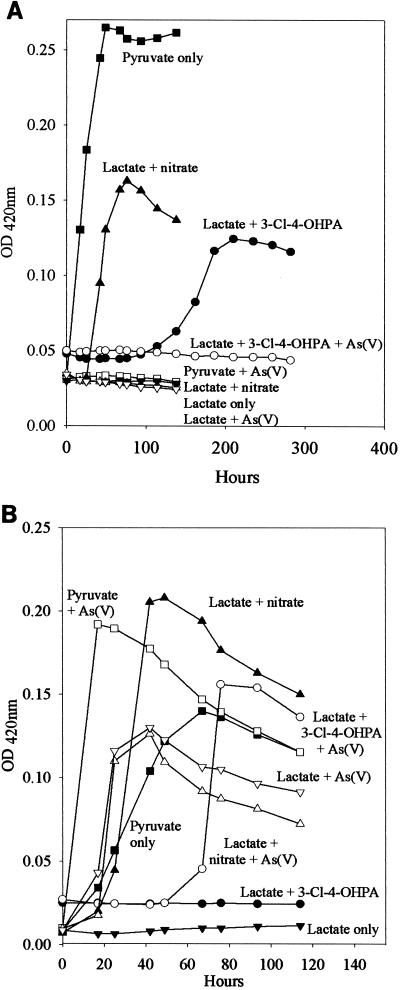
D. dehalogenans neither grew (Fig. 4A) nor respired As(V) (data not shown) when lactate was supplied as the electron donor. In contrast, these conditions supported growth (Fig. 4B) and As(V) reduction (data not shown) by D. hafniense. Neither D. dehalogenans nor D. hafniense was capable of fermentative growth on lactate (Fig. 4). However, when pyruvate was supplied, both D. dehalogenans and D. hafniense grew fermentatively. When As(V) was added as a potential respiratory TEA to medium containing pyruvate, the growth of D. dehalogenans was completely inhibited (Fig. 4A). However, D. hafniense grew more quickly and to higher densities when As(V) was added to pyruvate than when pyruvate was simply fermented (Fig. 4B). Both D. dehalogenans and D. hafniense were capable of growth on lactate when nitrate was supplied as a respiratory TEA. The addition of As(V) to medium containing lactate and nitrate completely inhibited the growth of D. dehalogenans but not that of D. hafniense. Even in the presence of nitrate, D. hafniense reduced 9.5 mM As(V) to As(III) (data not shown). When lactate and 3-Cl-4-OHPA were provided, D. dehalogenans increased in cell number (Fig. 4A) but D. hafniense did not (Fig. 4B). As noted with other treatments, when As(V) was added to medium containing lactate and 3-Cl-4-OHPA, the growth of D. dehalogenans was completely inhibited (Fig. 4). D. hafniense, however, was capable of growth on medium containing lactate, 3-Cl-4-OHPA, and As(V) (Fig. 4B). In this case, all As(V) provided was reduced to As(III) (data not shown).
FIG. 4.
Growth of D. dehalogenans (A) and D. hafniense (B) in the presence of As(V) and other electron acceptors. Cells grown fermentatively on pyruvate were inoculated into one of the following eight treatments: ▪, pyruvate only; □, pyruvate plus As(V); ●, lactate plus 3-Cl-4-OHPA; ○, lactate plus 3-Cl-4-OHPA plus As(V); ▴, lactate plus nitrate; ▵, lactate plus nitrate plus As(V); ▾, lactate alone; ▿, lactate plus As(V). Experiments were performed in duplicate; symbols represent average values.
Cultures of D. dehalogenans differed in their responses to prolonged exposure to As(V). When cultures containing As(V) and pyruvate were washed and reinoculated into pyruvate-only media, growth resumed after 1 to 2 weeks. Cultures attained densities that were only slightly lower (OD420 = 0.19) than those consisting of cells that were grown on pyruvate with no prior exposure to As(V) (OD420 = 0.26) (Fig. 4). Neither cultures originally containing As(V), lactate, and nitrate nor cultures originally containing As(V), lactate, and 3-Cl-4-OHPA resumed growth after more than 1 month of incubation in As(V)-free media (data not shown).
DGGE, phylogeny, and comparative genomic analyses.
DGGE analysis of the partial 16S ribosomal DNA sequence from strain GBFH revealed three distinct bands (data not shown), suggesting the presence of multiple rRNA operons. This likely accounts for the observation that a 97-bp region at the 5′-terminal end of the entire 16S rRNA gene of strain GBFH was not readable by direct sequencing of the PCR products. The remaining readable part of the 16S rRNA sequence was affiliated with the genus Desulfitobacterium within the Bacillus-Clostridium group of the Firmicutes. Strain GBFH clusters most closely with D. hafniense and D. frappieri (Fig. 5B). Similarity values of the partial 16S rRNA sequence of GBFH (nucleotide positions 98 to 1542, Escherichia coli numbering) with the sequences of strains of D. hafniense and D. frappieri were above 99%. Similarity values with the type strains of D. chlororespirans and D. dehalogenans were 98.2% and 97.4%, respectively. DNA-DNA hybridization experiments reveal that strain GBFH exhibits 81.2% DNA-DNA reassociation with D. frappieri and 79.7% DNA-DNA hybridization with D. hafniense. D. frappieri and D. hafniense had 88.7% DNA-DNA reassociation. DNA-DNA reassociation between these three strains and D. chlororespirans was substantially lower, ranging between 64 and 68%, whereas the reassociation between these three strains and D. dehalogenans was even lower, ranging between 41 and 46% (Table 4).
TABLE 4.
DNA-DNA reassociation values of type strains of Desulfitobacterium species and strain GBFH
| Organism | % DNA-DNA reassociation with:
|
|||
|---|---|---|---|---|
| D. frappieri PCP-1T | D. hafniense DCB-2T | Strain GBFH | D. chlororespirans Co23T | |
| D. hafniense DCB-2T | 88.7 | |||
| Strain GBFH | 81.2 | 79.7 | ||
| D. chlororespirans Co23T | 67.7 | 64.3 | 66.9 | |
| D. dehalogenans JW/IU-DC1T | 45.9 | 43.9 | 40.6 | 44.9 |
DISCUSSION
To date, eight DAsRB have been isolated in pure culture (2, 6, 33, 41, 46, 47, 58). These organisms have been isolated from diverse environments, including freshwater marshes (41, 46, 47), acidic hot springs (33), and alkaline, hypersaline sediments (6). Table 1 summarizes the known physiological properties of currently described DAsRB. Considered as a group, As(V)-reducing bacteria can obtain energy by coupling the oxidation of a wide variety of carbon compounds, and in some instances H2, to an impressive array of organic and inorganic TEAs. In view of their metabolic diversity and niche breadth, it is perhaps not surprising that DAsRB are phylogenetically diverse and include members of both the Bacteria and Archaea (Fig. 5A). These observations suggest that the capacity to couple growth to As(V) reduction may be widespread. Should further investigation reveal such organisms to be abundant and active, DAsRB are likely to play key roles in mediating the reductive portion of the arsenic cycle and in mobilizing arsenic in aquatic environments.
In this communication we describe the isolation of the Desulfitobacterium strain GBFH. GBFH is the first As(V)-reducing organism capable of conserving energy for growth by coupling the reduction of As(V) to the oxidation of formate when formate is supplied as the sole electron donor and carbon source (Fig. 2A). Controls demonstrate that formate alone cannot support growth in the absence of an exogenous TEA such as As(V). Yeast extract (0.1 g/liter) did not serve as a carbon and energy source as no growth or As(V) reduction occurred in the absence of formate. Comparative studies reveal that D. frappieri (Fig. 2B) and D. hafniense (Table 2) are also capable of coupling As(V) reduction to formate oxidation, conserving energy for growth in the process. Calculations using published thermodynamic values (26, 79) reveal this to be an energy-yielding, proton-consuming reaction, as seen in the following equations:
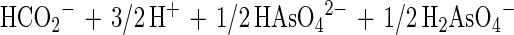 |
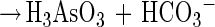 |
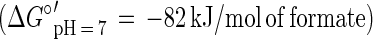 |
and
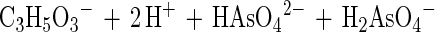 |
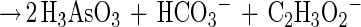 |
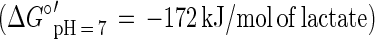 |
The oxidation of formate yields approximately half of the energy produced by the oxidation of lactate per mole of electron donor. This is consistent with the higher cell densities observed when Desulfitobacterium spp. were grown on lactate and As(V) rather than on formate and As(V). Theoretical considerations predict a 1:1 ratio of formate consumed to As(V) reduced. Analysis of growth by strain GBFH and D. frappieri shows that formate and As(V) are consumed in a ratio of approximately 1:1 (Fig. 2). Our experimental data are therefore in relatively good agreement with theoretical predictions. Because the oxidation of formate does not support ATP generation through substrate level phosphorylation, ATP generation is likely to occur via electron transport phosphorylation. Because formate is a characteristic end product of mixed acid fermentation (68) and may be an important end product in other anaerobic fermentations (23, 34), As(V) reducers capable of utilizing formate as an electron donor and carbon source for growth may have a competitive advantage over organisms unable to use this substrate.
Comparative physiology reveals that strain GBFH is phenotypically similar to its two closest relatives, D. frappieri and D. hafniense. The optimal temperature (37 to 38°C) and pH (7.5) for all three organisms are nearly identical (Fig. 3) (12), and all three organisms are capable of heat-resistant spore formation (7). Both strain GBFH and D. hafniense grow in the presence of low levels of oxygen and can resume growth following exposure to higher oxygen levels (12). Carbon source utilization profiles are identical for D. hafniense and D. frappieri with respect to the compounds tested (Table 2) and are in good agreement with previous studies (7, 12, 24). The ability of D. hafniense and D. frappieri to use butyrate, succinate, malate, and ethanol distinguishes them from strain GBFH. Although the growth of all three organisms on acetate did not produce enough cell mass to be considered positive for growth, respiration of 8 to 10 mM As(V) after three passages suggests that acetate can be used as an electron donor but not as a source of carbon for assimilation into cell mass. As with carbon utilization, the terminal electron accepting capabilities of D. hafniense and D. frappieri are identical with respect to the TEAs tested (Table 3).
The singular difference between the TEA utilization profiles of D. hafniense, D. frappieri, and strain GBFH is the inability of strain GBFH to utilize nitrate. The differences in carbon source and TEA utilization may be attributable to the natural habitat of these organisms. Strain GBFH was isolated from an oligotrophic mesotrophic (82) lake whose sediments are low in carbon (average total organic carbon = 2.1% [32]). D. hafniense and D. frappieri were both recovered from dechlorinating consortia inoculated with carbon- and nitrogen-rich sewage sludge (48) and amended with glucose (7). We may reasonably infer that D. hafniense and D. frappieri were exposed to stronger selective pressure for the utilization of glucose fermentation end products and NO3 both in their native habitat and during the enrichment process.
The ability of members of the genus Desulfitobacterium to conserve energy for growth by reducing toxic metalloids such as As(V) and Se(VI) is documented here for the first time. Desulfitobacteria have previously been isolated on the basis of their ability to reductively dechlorinate chlorinated ethenes as well as a wide variety of chlorinated phenols (7, 12, 24, 25, 48, 77). In fact, only one other member of the genus Desulfitobacterium has been enriched for an ability other than reductive dechlorination of hydrocarbons (78). D. hafniense, D. frappieri, and strain GBFH grow by coupling the reduction of both As(V) and Se(VI) to the oxidation of lactate (Table 3). Additionally, these organisms mediate the precipitation of arsenic sulfide minerals concomitant with the reduction of As(V) and oxidized sulfur species. These metabolic properties suggest that desulfitobacteria could be useful for remediating cocontaminated environments. For example, it is conceivable that such microbes could precipitate As(V) and Se(VI) as arsenic sulfides and elemental selenium while reductively dehalogenating chlorinated hydrocarbons. The ability of desulfitobacteria to reductively transform organic and metalloid contaminants simultaneously is presently under investigation.
Although all of the desulfitobacteria tested in this study grow by dissimilatory Se(VI) reduction, not all desulfitobacteria can respire As(V). D. dehalogenans cannot grow by coupling the oxidation of pyruvate or lactate to the reduction of As(V) (Fig. 4A). Moreover, the presence of As(V) completely inhibits fermentative growth on pyruvate and respiratory growth on nitrate and 3-Cl-4-OHPA (Fig. 4A). D. dehalogenans is therefore not a good choice for remediating environments cocontaminated with arsenious and chlorophenolic wastes because the presence of As(V) inhibits both respiratory and fermentative growth. Identical experiments with D. hafniense (Fig. 4B) suggest that the growth of Desulfitobacterium spp. capable of reducing As(V) is not inhibited by As(V).
Exposure to As(V) has different effects on the fermentative and respiratory metabolism of D. dehalogenans. When D. dehalogenans is grown with pyruvate and As(V), washed, and reinoculated into pyruvate medium lacking As(V), growth resumes within 1 to 2 weeks. Thus, prolonged exposure to As(V) does not appear to affect the ability of D. dehalogenans to resume fermentative growth once As(V) is eliminated. However, cultures of D. dehalogenans that are grown with lactate, nitrate, and As(V) or lactate, 3-Cl-4-OHPA, and As(V) do not resume growth in the same medium lacking As(V). This implies that prolonged exposure to As(V) permanently inhibits respiratory function, a reasonable conclusion in light of the fact that As(V) uncouples oxidative phosphorylation (56). Our results suggest that, in D. dehalogenans, ATPases responsible for oxidative phosphorylation are permanently impaired by As(V) exposure, whereas enzymes responsible for substrate-level phosphorylation are not.
Phylogenetic analysis shows that strain GBFH is related to the low-GC gram-positive bacteria (Fig. 5A) and clusters most closely with D. hafniense and D. frappieri (Fig. 5B). Determination of genomic similarities by DNA-DNA reassociation revealed that D. hafniense DCB-2T and D. frappieri PCP-1T share 89% similarity (Table 4). Interpreting reassociation values of higher than 70% as indicative of species affiliation (81), and considering their similar physiologies, the two species should be united. Strain GBFH should also be considered a member of this species because DNA-DNA reassociation values obtained for strain GBFH and D. hafniense DCB-2T and D. frappieri PCP-1T range between 78 and 81% (Table 4) and strain GBFH is phenotypically similar to D. hafniense and D. frappieri (Tables 2 and 3). Differences in DNA sequence may be sufficient to account for strain-specific differences in morphology and physiology, such as motility, the ability to utilize various electron acceptors and donors, and the ability to dechlorinate various chlorophenols. The degree of DNA similarity among these three strains, D. chlororespirans, and D. dehalogenans is below the 70% criteria for species affiliation (Table 4). In addition, phenotypic differences illustrated here and demonstrated in other publications (42, 67, 77) suggest that D. dehalogenans and D. chlororespirans are sufficiently different from each other and from other members of the genus to warrant separate species designations. The International Code of Nomenclature of Bacteria (40) describes in rule 62 that when species are united, the oldest legitimate epithet, in this case D. hafniense, is retained.
The isolation of As(V)-respiring strain GBFH from CDAL sediments is significant because it provides several mechanisms for arsenic mobilization under the reducing conditions that prevail in this environment. It has previously been shown that CDAL sediments biologically reduce As(V) to As(III) (28). The isolation of strain GBFH confirms that this habitat supports microbial populations capable of this transformation. Additionally, physiological characterization of strain GBFH reveals that this organism respires Fe(III). It has also been demonstrated that microbial Fe(III) reduction can mobilize As(V) from CDAL sediments (14). Strain GBFH could therefore contribute to the mobilization of arsenic as As(V) by mediating the reductive dissolution of Fe(III) oxyhydroxides. Finally, strain GBFH could also contribute to the mobilization of arsenic as As(III) by direct reduction of solid-phase As(V) to aqueous As(III) or by reduction of aqueous As(V) to As(III). DAsRB strain SES-3T, which also reduces Fe(III), has been shown to mediate As(III) solubilization using synthetic iron minerals with As(V) sorbed or coprecipitated on the mineral surface (83). Although specific experiments designed to test the contribution of strain GBFH to arsenic mobilization in CDAL sediments have not yet been performed, the capacity of strain GBFH to reduce Fe(III) and As(V) suggests that it could contribute to both arsenic and iron diagenesis.
Herein we report the isolation and characterization of the first organism capable of coupling growth to As(V) reduction while utilizing formate as the sole carbon source and electron donor. We also provide the first evidence that members of the genus Desulfitobacterium can utilize toxic metals or metalloids as TEAs. The ability of Desulfitobacterium spp. to reductively dechlorinate phenols, reduce metals or metalloids, and mediate precipitation of arsenic sulfides makes certain members of this genus excellent candidates for bioremediation of cocontaminated environments. Physiological and genomic comparisons suggest the species D. hafniense be emended by unifying strains GBFH, PCP-1T (formerly D. frappieri), and DCB-2T (D. hafniense) under the name D. hafniense.
Emended description of D. hafniense.
This emendation is based on the description of D. hafniense DSMZ 10664T (12), D. frappieri DSMZ 12420T (7), the present report detailing the description of strain GBFH, and comparative physiological and genomic studies conducted in this study. Cells are rod shaped, gram negative, and occur singly, in pairs, and in short chains. Dimensions vary from 2.0 to 4.0 μm in length and 0.6 to 0.7 μm in width to 3.3 to 6.0 μm in length and 0.3 to 0.5 μm in width. The bacteria are motile by one or two terminal flagella (DSMZ 10664) or two to five laterally attached flagella (strain GBFH) or are nonmotile (DSMZ 12420). Heat-resistant spores are formed. Spores are terminal. The organism is an obligate anaerobe with slight oxygen tolerance. Optimum pH is 7.5, and optimum growth temperature is 37 to 38°C. Pyruvate, lactate, formate, and fumarate (10 mM each) support growth. The growth of strains DSMZ 10664 and DSMZ 12420 was also supported by butyrate, succinate, malate (10 mM each), and ethanol (5 mM). Tryptophan and yeast extract may support growth in the presence of inorganic electron acceptors. The organism reduces nitrate to ammonia and nitrite. Elemental sulfur, thiosulfate, sulfite, Fe(III), Fe(III) gel, MnO2, fumarate, As(V), Se(VI), and Fe pyrophosphate are reduced in the presence of lactate. The following compounds may be dechlorinated by individual strains: pentachlorophenol; 2,4,5-trichlorophenol (2,4,5-TCP); 2,4,6-TCP; 2,4-dichlorophenol (2,4-DCP); 3,5-DCP; 3-Cl-4-OHPA; 2,3,4,5-tetrachlorophenol; 2,3,5,6-tetrachlorophenol; 2,3,4-TCP; 2,3,5-TCP; 2,3,6-TCP; 3,4,5-TCP; and 2,6-DCP. The organism contains cytochrome c but not desulfoviridin. The organism is indole positive and catalase negative. The organism does not liquefy gelatin. The DNA G+C content is between 46 and 47 mol%. The type strain has been isolated from municipal sludge. The type strain is D. hafniense DSMZ 10664 (strain DCB-2T).
ACKNOWLEDGMENTS
We thank Matt Morra, Don Crawford, Anthony March, and Scott Fendorf for useful discussions. We also thank the Electron Microscopy Core Lab (ICBR) at the University of Florida and the teaching staff of the 1999 Marine Biological Laboratory Microbial Diversity Course.
This work was supported in part by grants to R.F.R. from the U.S. Geological Survey (99HQGR0218) and the U.S. Department of Energy (DE-FG02-00ER63036) and by an Alumni Fellowship to A.N. from the University of Florida.
REFERENCES
- 1.Aggett J, Kriegman M R. The extent of formation of arsenic(III) in sediment interstitial waters and its release to hypolimnetic waters in Lake Ohakuri. Water Res. 1988;22:407–411. [Google Scholar]
- 2.Ahmann D, Roberts A L, Krumholz L R, Morel F M M. Microbe grows by reducing arsenic. Nature. 1994;371:750. doi: 10.1038/371750a0. [DOI] [PubMed] [Google Scholar]
- 3.Ahmann D, Krumholz L R, Hemond H F, Lovley D R, Morel-Francois M M. Microbial mobilization of arsenic from sediments of the Aberjona watershed. Environ Sci Technol. 1997;31:2923–2930. [Google Scholar]
- 4.Belzile N. The fate of arsenic in sediments of the Laurentian Trough. Geochim Cosmochim Acta. 1988;52:2293–2302. [Google Scholar]
- 5.Belzile N, Tessier A. Interactions between arsenic and iron oxyhydroxides in lacustrine sediments. Geochim Cosmochim Acta. 1990;54:103–109. [Google Scholar]
- 6.Blum J S, Bindi A B, Buzzelli J, Stolz J F, Oremland R S. Bacillus arsenicoselenatis, sp. nov., and Bacillus selenitireducens, sp. nov.: two haloalkaliphiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Arch Microbiol. 1998;171:19–30. doi: 10.1007/s002030050673. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard B, Beaudet R, Villemur R, McSween G, Lepine F, Bisaillon J G. Isolation and characterization of Desulfitobacterium frappieri sp. nov., an anaerobic bacterium which reductively dechlorinates pentachlorophenol to 3-chlorophenol. Int J Syst Bacteriol. 1996;46:1010–1015. doi: 10.1099/00207713-46-4-1010. [DOI] [PubMed] [Google Scholar]
- 8.Brannon J M, Patrick W H. Fixation, transformation, and mobilization of arsenic in sediments. Environ Sci Technol. 1987;21:450–459. doi: 10.1021/es00159a005. [DOI] [PubMed] [Google Scholar]
- 9.British Geological Survey. Phase 2 groundwater studies of arsenic contamination in Bangladesh. Nottingham, United Kingdom: British Geological Survey; 2001. [Google Scholar]
- 10.Burdige D J. Ph.D. thesis. University of California, San Diego; 1983. [Google Scholar]
- 11.Cashion P, Holder-Franklin M A, McCully J, Franklin M. A rapid method for the base ratio determination of bacterial DNA. Anal Biochem. 1997;81:461–466. doi: 10.1016/0003-2697(77)90720-5. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen N, Ahring B K. Desulfitobacterium hafniense sp. nov., an anaerobic, reductively dechlorinating bacterium. Int J Syst Bacteriol. 1996;46:442–448. [Google Scholar]
- 13.Cullen W R, Reimer K J. Arsenic speciation in the environment. Chem Rev. 1989;89:713–764. [Google Scholar]
- 14.Cummings D E, Caccavo F, Fendorf S, Rosenzweig R F. Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ Sci Technol. 1999;33:723–729. [Google Scholar]
- 15.Cummings D E, Caccavo F, Jr, Spring S, Rosenzweig R F. Ferribacterium limneticum, gen. nov., sp. nov., an Fe(III)-reducing microorganism isolated from mining-impacted freshwater lake sediments. Arch Microbiol. 1999;171:183–188. [Google Scholar]
- 16.Cummings D E, March A W, Bostick B, Spring S, Caccavo F, Jr, Fendorf S, Rosenzweig R F. Evidence for microbial Fe(III) reduction in anoxic, mining-impacted lake sediments (Lake Coeur d'Alene, Idaho) Appl Environ Microbiol. 2000;66:154–162. doi: 10.1128/aem.66.1.154-162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLay J, Cattoir H, Reynaerts A. The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem. 1970;12:133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 18.DeVitre R, Belzile N, Tessier A. Speciation and adsorption of arsenic on diagenetic iron oxyhydrides. Limnol Oceanogr. 1991;36:1480–1485. [Google Scholar]
- 19.Dowdle P R, Laverman A M, Oremland R S. Bacterial dissimilatory reduction of arsenic(V) to arsenic(III) in anoxic sediments. Appl Environ Microbiol. 1996;62:1664–1669. doi: 10.1128/aem.62.5.1664-1669.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escara J F, Hutton J R. Thermal stability and renaturation of DNA in dimethyl sulfoxide solutions; acceleration of the renaturation rate. Biopolymers. 1980;19:1315–1327. doi: 10.1002/bip.1980.360190708. [DOI] [PubMed] [Google Scholar]
- 21.Felsenstein J. Numerical methods for inferring phylogenetic trees. Q Rev Biol. 1982;57:379–404. [Google Scholar]
- 22.Fendorf S, Eick M J, Grossl P, Sparks D L. Arsenate and chromate retention mechanisms on goethite. 1. Surface structure. Environ Sci Technol. 1997;31:315–320. [Google Scholar]
- 23.Ferry J G, Wolfe R S. Anaerobic degradation of benzoate to methane by a microbial consortium. Arch Microbiol. 1976;107:33–40. doi: 10.1007/BF00427864. [DOI] [PubMed] [Google Scholar]
- 24.Gerritse J, Drzyzga O, Kloetstra G, Keijmel M, Wiersum L P, Hutson R, Collins M D, Gottschal J C. Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl Environ Microbiol. 1999;65:5212–5221. doi: 10.1128/aem.65.12.5212-5221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerritse J, Renard V, Pedro Gomes T M, Lawson P A, Collins M D, Gottschal J C. Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch Microbiol. 1996;165:132–140. doi: 10.1007/s002030050308. [DOI] [PubMed] [Google Scholar]
- 26.Hanselmann K W. Microbial energetics applied to waste repositories. Experientia. 1991;47:645–687. [Google Scholar]
- 27.Harrington J M, Laforce M J, Rember W C, Fendorf S E, Rosenzweig R F. Phase associations and mobilization of iron and trace elements in Coeur d'Alene Lake, Idaho. Environ Sci Technol. 1998;32:650–656. [Google Scholar]
- 28.Harrington J M, Fendorf S E, Rosenzweig R F. Biotic generation of arsenic(III) in metal(loid)-contaminated freshwater lake sediments. Environ Sci Technol. 1998;32:2425–2430. [Google Scholar]
- 29.Hobbie J E, Daley R J, Jasper S. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Höpner T, Knappe J. Determination with formate dehydrogenase. In: Bergmeyer U H, editor. Methods of enzymatic analysis. Deerfield Beach, Fla: Weinheim; 1974. pp. 1551–1555. [Google Scholar]
- 31.Horowitz A J, Elrick K A, Cook R B. Effect of mining and related activities on the sediment trace element geochemistry of Lake Coeur d'Alene, Idaho, USA. Part 1: surface sediments. Hydrol Process. 1993;7:403–423. [Google Scholar]
- 32.Horowitz A J, Elrick K A, Robbins J A, Cook R B. A summary of the effects of mining and related activities on the sediment-trace element geochemistry of Lake Coeur d'Alene, Idaho, USA. J Geochem Expl. 1995;52:135–144. [Google Scholar]
- 33.Huber R, Sacher M, Vollmann A, Huber H, Rose D. Respiration of arsenate and selenate by hyperthermophilic archaea. Syst Appl Microbiol. 2000;23:305–314. doi: 10.1016/S0723-2020(00)80058-2. [DOI] [PubMed] [Google Scholar]
- 34.Hungate R E, Smith W, Bauchop T, Yu I, Rabinowitz J C. Formate as an intermediate in the bovine rumen fermentation. J Bacteriol. 1970;102:389–397. doi: 10.1128/jb.102.2.389-397.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huss V A R, Festl H, Schleifer K H. Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol. 1983;4:184–192. doi: 10.1016/S0723-2020(83)80048-4. [DOI] [PubMed] [Google Scholar]
- 36.Jahnke K D. Basic computer-program for evaluation of spectroscopic DNA renaturation data from Gilford-system-2600 spectrophotometer on a PC/XT/AT type personal-computer. J Microbiol Methods. 1992;15:61–73. [Google Scholar]
- 37.Johnson D L. Simultaneous determination of arsenate and phosphate in natural waters. Environ Sci Technol. 1971;5:411–414. [Google Scholar]
- 38.Johnson D L, Pilson M E Q. Spectrophotometric determination of arsenite, arsenate, and phosphate in natural waters. Anal Chim Acta. 1972;58:289–299. [Google Scholar]
- 39.Korte N E, Fernando Q. A review of arsenic(III) in groundwater. Crit Rev Environ Control. 1991;21:1–39. [Google Scholar]
- 40.Lapage S P, Sneath P H A, Lessel E F, Skerman V B D, Seeliger H P R, Clark W A, editors. International code of nomenclature of bacteria. Washington, D.C.: American Society for Microbiology; 1992. [PubMed] [Google Scholar]
- 41.Laverman A M, Blum J S, Schaefer J K, Phillips E J P, Lovley D R, Oremland R S. Growth of strain SES-3 with arsenate and other diverse electron acceptors. Appl Environ Microbiol. 1995;61:3556–3561. doi: 10.1128/aem.61.10.3556-3561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lie T J, Godchaux W, Leadbetter E R. Sulfonates as terminal electron acceptors for growth of sulfite-reducing bacteria (Desulfitobacterium spp.) and sulfate-reducing bacteria: effects of inhibitors of sulfidogenesis. Appl Environ Microbiol. 1999;65:4611–4617. doi: 10.1128/aem.65.10.4611-4617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 44.Lovley D R, Phillips E J P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macy J M, Santini J M, Pauling B V, O'Neill A H, Sly L I. Two new arsenate/sulfate-reducing bacteria: mechanisms of arsenate reduction. Arch Microbiol. 2000;173:49–57. doi: 10.1007/s002030050007. [DOI] [PubMed] [Google Scholar]
- 47.Macy J M, Nunan K, Hagen K D, Dixon D R, Harbour P J, Cahill M, Sly L I. Chrysiogenes arsenatis gen. nov., sp. nov., a new arsenate-respiring bacterium isolated from gold mine wastewater. Int J Syst Bacteriol. 1996;46:1153–1157. doi: 10.1099/00207713-46-4-1153. [DOI] [PubMed] [Google Scholar]
- 48.Madsen T, Licht D. Isolation and characterization of an anaerobic chlorophenol-transforming bacterium. Appl Environ Microbiol. 1992;58:2874–2878. doi: 10.1128/aem.58.9.2874-2878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maidak B L, Olsen G J, Larsen N, Overbeck R, McCaughey M J, Woese C R. The ribosomal database project. Nucleic Acids Res. 1996;24:82–85. doi: 10.1093/nar/24.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manning B A, Fendorf S E, Goldberg S. Surface structures and stability of arsenic(III) on goethite: spectroscopic evidence for inner-sphere complexes. Environ Sci Technol. 1998;32:2383–2388. [Google Scholar]
- 51.Manning B A, Goldberg S. Adsorption and stability of arsenic(III) at the clay mineral-water interface. Environ Sci Technol. 1997;31:2005–2011. [Google Scholar]
- 52.Masscheleyn P H, Delaune R D, Patrick W H. Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ Sci Technol. 1991;25:1414–1419. [Google Scholar]
- 53.McGeehan S L, Naylor D V. Sorption and redox transformation of arsenite and arsenate in two flooded soils. Soil Sci Soc Am J. 1994;58:337–342. [Google Scholar]
- 54.Miller T L, Wolin M J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974;27:985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mudroch A, MacKnight S. Handbook of techniques for aquatic sediments sampling. Boca Raton, Fla: Lewis Publishers; 1994. [Google Scholar]
- 56.National Research Council. Arsenic. Washington, D.C.: National Academy of Sciences; 1977. [Google Scholar]
- 57.Newman D K, Ahmann D, Morel F M M. A brief review of microbial arsenate respiration. Geomicrobiol J. 1998;15:255–268. [Google Scholar]
- 58.Newman D K, Kennedy E K, Coates J D, Ahmann D, Ellis D J, Lovley D R, Morel F M. Dissimilatory arsenate and sulfate reduction in Desulfotomaculum auripigmentum sp. nov. Arch Microbiol. 1997;168:380–388. doi: 10.1007/s002030050512. [DOI] [PubMed] [Google Scholar]
- 59.Newman D K, Beveridge T J, Morel F M M. Precipitation of arsenic trisulfide by Desulfotomaculum auripigmentum. Appl Environ Microbiol. 1997;63:2022–2028. doi: 10.1128/aem.63.5.2022-2028.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nickson R T, McArthur J M, Ravenscroft P, Burgess W G, Ahmed K M. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem. 2000;15:403–413. [Google Scholar]
- 61.Pfennig N, Widdel F, Trüper H G. The dissimilatory sulfate-reducing bacteria. In: Starr M P, Stolp H, Trüper H G, Balows A, Schlegel H G, editors. The prokaryotes. Vol. 1. Berlin, Germany: Springer-Verlag KG; 1981. pp. 926–947. [Google Scholar]
- 62.Pierce M L, Moore C B. Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Res. 1982;16:1247–1253. [Google Scholar]
- 63.Raven K P, Jain A, Loeppert R H. Arsenite and arsenate adsorption on ferrihydrite: kinetics, equilibrium, and adsorption envelopes. Environ Sci Technol. 1998;32:344–349. [Google Scholar]
- 64.Reynolds E S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rochette E A, Li G C, Fendorf S E. Stability of arsenate minerals in soil under biotically generated reducing conditions. Soil Sci Soc Am J. 1998;62:1530–1537. [Google Scholar]
- 66.Sabatini D D, Bensch K, Barrnett R J. Cytochemistry and electron microscopy: the preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanford R A, Cole J R, Loffler F E, Tiedje J M. Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl Environ Microbiol. 1996;62:3800–3808. doi: 10.1128/aem.62.10.3800-3808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlegel H G. General microbiology. Cambridge, England: Cambridge University Press; 1992. [Google Scholar]
- 69.Seyler P, Martin J. Biogeochemical processes affecting arsenic species distribution in a permanently stratified lake. Environ Sci Technol. 1989;23:1258–1263. [Google Scholar]
- 70.Stanier R Y. The microbial world. Englewood Cliffs, N.J: Prentice-Hall; 1986. [Google Scholar]
- 71.Stolz J F, Ellis D J, Blum J S, Ahmann D, Lovley D R, Oremland R S. Sulfurospirillum barnesii sp. nov. and Sulfurospirillum arsenophilum sp. nov., new members of the Sulfurospirillum clade of the epsilon Proteobacteria. Int J Syst Bacteriol. 1999;49:1177–1180. doi: 10.1099/00207713-49-3-1177. [DOI] [PubMed] [Google Scholar]
- 72.Stolz J F, Oremland R S. Bacterial respiration of arsenic and selenium. FEMS Microbiol Rev. 1999;23:615–627. doi: 10.1111/j.1574-6976.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 73.Strickland J, Parsons T. A practical handbook of seawater analysis. Fish Res Board Can Bull. 1968;167:49–52. [Google Scholar]
- 74.Sun X H, Doner H E. An investigation of arsenate and arsenite bonding structures on goethite by FTIR. Soil Sci. 1996;161:865–872. [Google Scholar]
- 75.U.S. Environmental Protection Agency. National primary drinking water regulations for lead and copper. Fed Regist. 2000;65:1950–2015. [Google Scholar]
- 76.U.S. Environmental Protection Agency. National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Fed Regist. 2001;66:20580–20584. [Google Scholar]
- 77.Utkin I, Woese C, Wiegel J. Isolation and characterization of Desulfitobacterium dehalogenans gen. nov., sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Syst Bacteriol. 1994;44:612–619. doi: 10.1099/00207713-44-4-612. [DOI] [PubMed] [Google Scholar]
- 78.van de Pas B A, Harmsen H J, Raangs G C, de Vos W M, Schraa G, Stams A J. A Desulfitobacterium strain isolated from human feces that does not dechlorinate chloroethenes or chlorophenols. Arch Microbiol. 2001;175:389–394. doi: 10.1007/s002030100276. . [Online.] [DOI] [PubMed] [Google Scholar]
- 79.Vink B W. Stability relations of antimony and arsenic compounds in the light of revised and extended Eh-pH diagrams. Chem Geol. 1996;130:21–30. [Google Scholar]
- 80.Wallner G, Fuchs S, Spring S, Beisker W, Amann R. Flow sorting of microorganisms for molecular analysis. Appl Environ Microbiol. 1997;63:4223–4231. doi: 10.1128/aem.63.11.4223-4231.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wayne L G, Brenner D J, Colwell R R, Grimont P A D, Kandler O, Krichevsky M I, Moore L H, Moore W E C, Murray R G E, Stackebrandt E, Starr M P, Trüper H G. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol. 1987;37:463–464. [Google Scholar]
- 82.Woods P, Beckwith M. Nutrient and trace-element enrichment of Coeur d'Alene Lake, Idaho. U.S. Geological Survey Open-File Report 95–740. U.S. Washington, D.C.: Geological Survey; 1996. [Google Scholar]
- 83.Zobrist J, Dowdle P R, Davis J A, Oremland R S. Mobilization of arsenite by dissimilatory reduction of adsorbed arsenate. Environ Sci Technol. 2000;34:4747–4753. [Google Scholar]