Abstract
Introduction
‘Real-world’ data for mold-active triazoles (MATs) in the treatment of invasive fungal infections (IFIs) are lacking. This study evaluated usage of MATs in a disease registry for the management of IFIs.
Methods
Data were collected for this multicenter, observational, prospective study from 55 US centers, between March 2017 and April 2020. Eligible patients received isavuconazole, posaconazole, or voriconazole as MAT monotherapy (one MAT) or multiple/sequenced MAT therapy (more than one MAT) for prophylaxis or treatment. Patients were enrolled within 60 days of MAT initiation. The primary objective was to characterize patients receiving a MAT and their patterns of therapy. The full analysis set (FAS) included eligible patients for the relevant enrollment protocol, and the safety analysis set (SAF) included patients who received ≥ 1 MAT dose.
Results
Overall, 2009 patients were enrolled in the SAF. The FAS comprised 1993 patients (510 isavuconazole; 540 posaconazole; 491 voriconazole; 452 multiple/sequenced MAT therapies); 816 and 1177 received treatment and prophylaxis at study index/enrollment, respectively. Around half (57.8%) of patients were male, and median age was 59 years. Among patients with IFIs during the study, the most common pathogens were Aspergillus fumigatus in the isavuconazole (18.2% [10/55]) and voriconazole (25.5% [12/47]) groups and Candida glabrata in the posaconazole group (20.9% [9/43]); the lungs were the most common infection site (58.2% [166/285]). Most patients were maintained on MAT monotherapy (77.3% [1541/1993]), and 79.4% (1520/1915) completed their MAT therapies. A complete/partial clinical response was reported in 59.1% (591/1001) of patients with a clinical response assessment. Breakthrough IFIs were reported in 7.1% (73/1030) of prophylaxis patients. Adverse drug reactions (ADRs) were reported in 14.7% (296/2009) of patients (3.9% [20/514] isavuconazole; 11.3% [62/547] posaconazole; 14.2% [70/494] voriconazole).
Conclusions
In this ‘real-world’ study, most patients remained on their initial therapy and completed their MAT therapy. Over half of patients receiving MATs for IFIs had a successful response, and most receiving prophylaxis did not develop breakthrough IFIs. ADRs were uncommon.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00661-5.
Keywords: Antifungal treatment, Disease registry, Invasive fungal infections, Isavuconazole, Mold infection, Posaconazole, Prospective observational study, Real-world, Triazole, Voriconazole
Key Summary Points
| Why carry out this study? |
| There is a paucity of real-world outcomes data on the utility of mold-active triazoles (MATs) in the management of invasive fungal infections (IFIs). |
| The purpose of this registry-based observational study was to examine patient characteristics and therapy patterns of a ‘real-world’ patient population receiving a MAT for IFIs to better understand the use of these agents in current clinical practice. |
| What was learned from the study? |
| This study characterized and described the demographic characteristics of the at-risk population of patients on MAT therapy for the management of IFIs, thereby adding to the evolving data for this significant health problem. |
| MATs were associated with favorable clinical, mycologic, and radiologic responses in approximately half of patients who were assessed, and breakthrough IFIs were rare in those receiving MAT prophylaxis. However, the choice of MAT and use of therapeutic drug monitoring are physician- and center-dependent and may not be well standardized. |
| The findings from this study support targeted changes in therapy to align with guideline recommendations, which may result in positive outcomes when managing patients with, and at risk for, IFIs. |
Introduction
Invasive fungal infections (IFIs) are a major cause of morbidity and mortality, particularly in immunocompromised patients [1]. Many factors, such as the increasing use of immunosuppressive therapies, invasive medical care, and immune-modifying drugs, have contributed to the emergence of IFIs [2]. Opportunistic pathogens, including Candida and Aspergillus, are recognized causes of IFIs and are responsible for a large proportion of all reported deaths due to fungal disease [3].
As the patient population at risk for IFIs continues to expand [2], the appropriate choice of an IFI therapy is critical for successful outcomes and to manage the risk of mortality, therapy-associated toxicity, and development of resistance [4]. Currently available antifungal agents for the management of IFIs in the USA include the polyenes (amphotericin B deoxycholate and lipid formulations of amphotericin B), echinocandins, and triazoles [5, 6]. The use of amphotericin B for certain serious IFIs has declined in recent years because of the availability of alternative agents with high rates of effectiveness and more favorable adverse event profiles, such as the triazoles [7, 8]. Echinocandins have a relatively narrow antifungal spectrum, mostly restricted to Candida (for which they are the treatment of choice) and Aspergillus species [5, 6, 9]; micafungin has an FDA-approved indication for treatment as well as prophylaxis against Candida in specific populations [10]. The triazole agents include fluconazole and the newer mold-active triazoles (MATs) posaconazole, voriconazole, and isavuconazole [1]. MATs are currently the recommended agents for the treatment and prophylaxis of invasive aspergillosis according to the Infectious Diseases Society of America (IDSA) guidelines [6]. Posaconazole is recommended for prophylaxis in high-risk patients (stem cell recipients with graft-versus-host disease and neutropenic patients with hematologic malignancy) [6]. The IDSA guidelines for the management of candidiasis recommend an echinocandin as initial therapy and fluconazole as an appropriate alternative in select patients, with azole susceptibility testing recommended for all bloodstream and other clinically relevant Candida isolates [5]. The MATs differ in their profiles, such as labeled indications, pharmacokinetic profiles, spectrum of mold coverage, and potential side effects (with restrictions for use in certain at-risk populations) [1, 11–13].
Despite improvements in therapy for IFIs, their changing epidemiology means that the management of IFIs must continue to evolve [2]. There is also a paucity of real-world outcomes data on the utility of MATs in the management of IFIs for isavuconazole, posaconazole, and voriconazole. Patient registries of fungal infections provide the opportunity to examine current data relevant to real-world patients, such as clinical risk factors, management, and outcomes, thereby supporting routine clinical practice.
The purpose of this registry-based observational study was to examine patient characteristics and therapy patterns of a ‘real-world’ patient population receiving a MAT for IFIs to better understand the use of these agents in current clinical practice.
Methods
Study Design and Population
In this multicenter, observational, prospective registry study, patients receiving a MAT for the treatment or prophylaxis of IFIs were enrolled from 55 centers in the US. Data were collected between March 2017 and April 2020. The study was approved by an Institutional Review Board (IRB) at each participating study site (see Table S1 for details of the sites) and was conducted in accordance with the ethical principles based on the Declaration of Helsinki 1964 and its later amendments, the International Conference on Harmonisation, and any applicable laws and regulations. Informed consent was obtained for patients included in the study (those aged < 18 years had consent of a parent or legal guardian and, where appropriate, consent of the patient) when required by IRBs according to local guidance.
Patients were eligible for inclusion in the study if they received isavuconazole, posaconazole, or voriconazole as MAT monotherapy (one MAT) or as multiple/sequenced MAT therapies (more than one MAT), as treatment (pre-emptive, empiric, targeted, or salvage therapies) or prophylaxis (primary or secondary). Patients were included in the study under two versions of the protocol and were either enrolled within 60 days of MAT initiation (version 1.1; termed ‘index’), or receiving MATs at the time of enrollment (version 2.1; termed ‘enrollment’). The multiple/sequenced MAT therapies group received ≥ 1 MAT therapy on or after the MAT start date at index/enrollment. Patients were excluded from the study if they were currently enrolled in any clinical trial with an investigational antifungal agent, had previously participated in this registry, or had died before entering the study. Approximately 2000 patients were planned to be enrolled in the study.
Data Collection
A study protocol and an electronic case report form (eCRF) were developed. Investigators collected the required data from the electronic medical records at regular intervals; however, there were no protocol mandated visits or procedures associated with the study. As this was a prospective registry study, some data may have been retrospective, but most data were collected prospectively. Enrolled patients were followed and clinical outcomes assessed according to institutional standards of care. The completion of MAT therapy during the study period was recorded via a check box of “Completed” in the eCRF. End-of-therapy data were collected when discontinuation of therapy was confirmed (i.e., via a patient report or medical chart review) or up to 1 year after last patient enrollment. End of follow-up occurred within 90 days after the completion of therapy or up to 1 year from the date of last patient enrollment, whichever occurred first. Patients could be contacted directly by the center to confirm their therapy status or long-term outcomes. Data collection procedures were in place to limit missing and out-of-range data at the point of data entry.
Study Assessments
The primary objective of the study was to characterize patients receiving a MAT for treatment or prophylaxis of IFIs and their patterns of therapy. This included baseline demographics, the sequence of IFI therapy, disease indication, associated pathogens, diagnostic methods, use of therapeutic drug monitoring (TDM), response to therapy, treatment timing and duration, and safety assessments. Safety assessments included evaluation of the incidence of adverse drug reactions (ADRs) suspected to be causally related to the use of a MAT. Adverse events (AEs) not determined to be causally related to MAT use were not captured. ADRs were reported using MedDRA v20.0 preferred terms. Discontinuation due to AEs and the occurrence of drug-drug interactions (DDIs; identified by the investigator as an interaction resulting in an ADR or MAT dose/concomitant medication adjustment) were also recorded. During the study period, infection characteristics were documented, including the anatomic site of fungal infection and the pathogen treated. All antifungal therapies received by patients (including MATs) ≤ 90 days prior to study index/enrollment were recorded. Concomitant antifungal use was documented on or after study index/enrollment. Changes to MAT therapy (switching or discontinuation) after study enrollment/index were captured, and treatment sequences up to third-line MAT therapy were included in this study. The secondary objective was to describe and quantify the healthcare resource utilization (HCRU) associated with a MAT, including inpatient and outpatient utilization data. HCRU data included length of hospital stay, admission to the intensive care unit (ICU), and ventilator use at index/enrollment. The exploratory objectives of this study were to examine the differences in characteristics and patterns of therapy by clinically relevant subgroups of patients receiving MATs (subgroups included underlying disease state, pathogen, clinical risk factors, site of infection, and type of non-fungal infection) and describe the real-world clinical outcomes of MAT use associated with clinically relevant subgroups, such as patient response to MAT and mortality following therapy. Response to MAT therapy was assessed by an investigator at the end of therapy and included clinical, mycologic, and radiologic response assessments. A prophylactic response assessment (presence/absence of breakthrough IFI) was undertaken in patients receiving prophylactic therapy at study index/enrollment. It should be noted that patients categorized into the prophylaxis subgroup at index/enrollment could transition to treatment during the study. Further details of the investigator’s assessment of response can be found in Table S2. All-cause and fungal-specific mortalities were analyzed at the end of therapy using univariate logistic regression.
Data Analysis
In this study, the full analysis set (FAS) included patients meeting study entry criteria for the relevant enrollment protocol. The safety analysis set (SAF) included patients who received ≥ 1 MAT dose (including prior to or after study enrollment). Continuous variables were summarized descriptively, with N, mean, standard deviation (SD), median, and minimum and maximum. Categorical variables were summarized with frequencies and percentages of patients. Percentages were based on patients with no missing data. Effectiveness and safety endpoints were not compared directly across treatment groups.
All data analyses were performed using SAS® 9.4 or a higher version.
Results
Baseline Patient Demographics and Clinical Characteristics
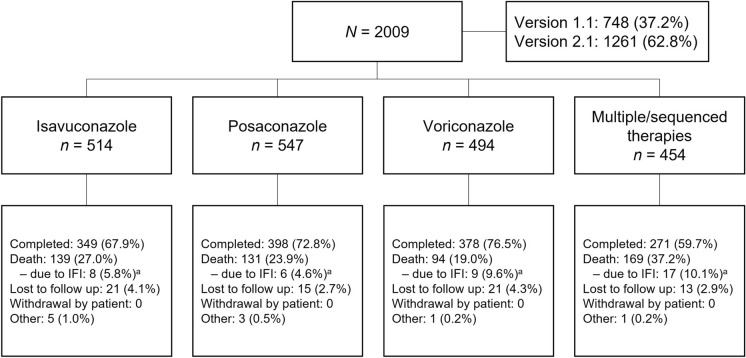
Overall, 2009 patients were enrolled and included in the SAF for this study (Fig. 1). In the SAF, 37.2% (748/2009) and 62.8% (1261/2009) of patients were included under versions 1.1 (index) and 2.1 (enrollment) of the protocol, respectively. The FAS comprised 1993 patients (510 isavuconazole, 540 posaconazole, 491 voriconazole, and 452 multiple/sequenced MAT therapies), of which 816 and 1177 patients received treatment and prophylaxis, respectively, at study index/enrollment. Patients had a mean (SD) age of 55.0 (16.9) years, and median age was 59.0 (range < 1 to 97) years (Table 1). Most patients were ≥ 18 years (97.0%), and of the patients < 18 years of age, the most common MAT monotherapy received was voriconazole (61.0% [36/59]), followed by posaconazole (18.6% [11/59]) and isavuconazole (1.7% [1/59]). Over half (57.8%) of patients were male, and the majority were white (79.4%). Hematologic malignancy (65.0%) and neutropenia (53.9%) were the most frequent underlying conditions in the FAS at study index/enrollment, and 60.6% of patients were receiving corticosteroids. Minor variations between the posaconazole group and the other MATs were observed in the FAS, such as higher proportions of patients with hematologic malignancy (79.1% versus 53.3% isavuconazole and 55.0% voriconazole) and neutropenia (67.6% vs 46.7% isavuconazole and 41.5% voriconazole). In the SAF, the majority (68.8% [1383/2009]) of patients received antifungal therapy ≤ 90 days prior to start of MAT at index/enrollment. More than half (51.1%) of all patients were receiving a non-MAT antifungal ≤ 90 days prior to start of MAT at index/enrollment (Table 1).
Fig. 1.
Patient disposition (SAF). aPercentages for cause of death by IFIs were based on the number of patients who died by the end of the study, not all enrolled patients. IFI invasive fungal infection, SAF safety analysis set
Table 1.
Baseline characteristics and demographics (FAS)
| Isavuconazole (n = 510) | Posaconazole (n = 540) | Voriconazole (n = 491) | Multiple/sequenced MAT therapies (n = 452) | Total (N = 1993) | |
|---|---|---|---|---|---|
| Male, n (%) | 290 (56.9) | 306 (56.7) | 288 (58.7) | 267 (59.1) | 1151 (57.8) |
| Age in years | |||||
| Mean ± SD | 56.7 (15.08) | 55.5 (16.33) | 52.0 (19.60) | 55.6 (16.00) | 55.0 (16.91) |
| Min | 17 | 1 | < 1 | 2 | < 1 |
| Median | 60.0 | 59.0 | 58.0 | 60.0 | 59.0 |
| Max | 92 | 97 | 86 | 84 | 97 |
| Age ≥ 18 years, n (%) | 509 (99.8) | 529 (98.0) | 455 (92.7) | 441 (97.6) | 1934 (97.0) |
| White race, n (%) | 397 (77.8) | 445 (82.4) | 382 (77.8) | 358 (79.2) | 1582 (79.4) |
| Underlying disease, n (%) | |||||
| Hematologic malignancy | 272 (53.3) | 427 (79.1) | 270 (55.0) | 326 (72.1) | 1295 (65.0) |
| Neutropenia | 238 (46.7) | 365 (67.6) | 204 (41.5) | 267 (59.1) | 1074 (53.9) |
| HSCT | 140 (27.5) | 162 (30.0) | 93 (18.9) | 154 (34.1) | 549 (27.5) |
| Solid organ transplant | 138 (27.1) | 36 (6.7) | 111 (22.6) | 67 (14.8) | 352 (17.7) |
| Solid tumor | 47 (9.2) | 40 (7.4) | 39 (7.9) | 27 (6.0) | 153 (7.7) |
| Inherited immunodeficiency disorder | 10 (2.0) | 4 (0.7) | 8 (1.6) | 4 (0.9) | 26 (1.3) |
| HIV/AIDS | 0 | 3 (0.6) | 5 (1.0) | 3 (0.7) | 11 (0.6) |
| Receiving corticosteroids | 354 (69.4) | 294 (54.4) | 277 (56.4) | 282 (62.4) | 1207 (60.6) |
| Antifungal therapy 90 days prior to MAT initiation, n (%)a | |||||
| ≥ 1 previous MATb | 263 (51.2) | 260 (47.5) | 149 (30.2) | 187 (41.2) | 859 (42.8) |
| ≥ 1 previous non-MAT antifungal therapy, n (%) | 267 (51.9) | 267 (48.8) | 237 (48.0) | 256 (56.4) | 1027 (51.1) |
The ‘total’ study population includes patients who received prophylaxis and/or treatment at index/enrollment. ‘Prophylaxis’ represents all primary and secondary prophylaxis use of MAT, and ‘treatment’ represents all pre-emptive, empiric, targeted, and salvage use of MAT
AIDS acquired immune deficiency syndrome, FAS full analysis set, HIV human immunodeficiency virus, HSCT hematopoietic stem cell transplant, MAT mold-active triazole, SD standard deviation
aSAF population
bIsavuconazole, posaconazole, or voriconazole
Infection Characteristics
During the study, IFIs were diagnosed in 14.4% (286/1993) of patients in the FAS and were reported more frequently for patients receiving multiple/sequenced MAT therapies (29.4% [133/452] than those receiving isavuconazole (12.0% [61/510]), posaconazole (8.0% [43/540]), and voriconazole (10.0% [49/491]). Proven IFI was the highest level of diagnosis in 49.2% of patients with IFIs (Table 2). Probable and possible categories accounted for most of IFI diagnoses and were reported in 30.5% and 20.2% of patients with IFIs, respectively. During the study, the most commonly treated pathogen among patients with an IFI was Aspergillus fumigatus in the isavuconazole (18.2%), voriconazole (25.5%), and multiple/sequenced MAT therapies (11.4%) groups. Candida glabrata was the most frequently recorded pathogen in the posaconazole group (20.9%) among patients with an IFI. In the SAF, 35% (7/20) of patients in the posaconazole group who were diagnosed with Candida infection received concomitant treatment with fluconazole.
Table 2.
Infection characteristics in patients with IFIs during the study (FAS)
| Isavuconazole (n = 510) | Posaconazole (n = 540) | Voriconazole (n = 491) | Multiple/sequenced MAT therapies (n = 452) | Total (N = 1993) | |
|---|---|---|---|---|---|
| Patients with IFIsa | 61 (12.0) | 43 (8.0) | 49 (10.0) | 133 (29.4) | 286 (14.4) |
| Highest level of IFI diagnosis, na | 58 | 40 | 41 | 123 | 262 |
| Proven | 26 (44.8) | 23 (57.5) | 15 (36.6) | 65 (52.8) | 129 (49.2) |
| Probable | 21 (36.2) | 12 (30.0) | 18 (43.9) | 29 (23.6) | 80 (30.5) |
| Possible | 11 (19.0) | 5 (12.5) | 8 (19.5) | 29 (23.6) | 53 (20.2) |
| Pathogen treated, nab | 55 | 43 | 47 | 123 | 268 |
| Genus species | |||||
| Aspergillus | 24 (43.6) | 9 (20.9) | 23 (48.9) | 53 (43.1) | 109 (40.7) |
| fumigatus | 10 (18.2) | 6 (14.0) | 12 (25.5) | 14 (11.4) | 42 (15.7) |
| niger | 1 (1.8) | 1 (2.3) | 2 (4.3) | 9 (7.3) | 13 (4.9) |
| flavus | 1 (1.8) | 0 | 1 (2.1) | 2 (1.6) | 4 (1.5) |
| versicolor | 0 | 0 | 3 (6.4) | 0 | 3 (1.1) |
| Not specified | 9 (16.4) | 2 (4.7) | 5 (10.6) | 23 (18.7) | 39 (14.6) |
| Candida | 17 (30.9) | 14 (32.6) | 12 (25.5) | 22 (17.9) | 65 (24.3) |
| glabrata | 8 (14.5) | 9 (20.9) | 4 (8.5) | 6 (4.9) | 27 (10.1) |
| krusei | 4 (7.3) | 1 (2.3) | 0 | 2 (1.6) | 7 (2.6) |
| parapsilosis | 3 (5.5) | 2 (4.7) | 0 | 1 (0.8) | 6 (2.2) |
| albicans | 2 (3.6) | 5 (11.6) | 7 (14.9) | 8 (6.5) | 22 (8.2) |
| dubliniensis | 1 (1.8) | 0 | 1 (2.1) | 3 (2.4) | 5 (1.9) |
| tropicalis | 1 (1.8) | 0 | 1 (2.1) | 2 (1.6) | 4 (1.5) |
| Not specified | 1 (1.8) | 1 (2.3) | 0 | 4 (3.3) | 6 (2.2) |
| Rhizopus | 3 (5.5) | 1 (2.3) | 0 | 7 (5.7) | 11 (4.1) |
| Mucor | 2 (3.6) | 0 | 1 (2.1) | 6 (4.9) | 9 (3.4) |
| Penicillium | 1 (1.8) | 1 (2.3) | 1 (2.1) | 3 (2.4) | 6 (2.2) |
| Coccidioides | 1 (1.8) | 9 (20.9) | 0 | 3 (2.4) | 13 (4.9) |
| Histoplasma | 1 (1.8) | 0 | 4 (8.5) | 5 (4.1) | 10 (3.7) |
| capsulatum | 1 (1.8) | 0 | 4 (8.5) | 3 (2.4) | 8 (3.0) |
| Scedosporium | 1 (1.8) | 0 | 0 | 3 (2.4) | 4 (1.5) |
| Fusarium | 0 | 2 (4.7) | 1 (2.1) | 7 (5.7) | 10 (3.7) |
| Other | 7 (12.7) | 7 (16.3) | 6 (12.8) | 19 (15.4) | 39 (14.6) |
Data are n (%), unless otherwise indicated
FAS full analysis set, IFI invasive fungal infection
aThe number of patients (n) with at least one IFI during the study and non-missing data for that parameter
bOnly incidences of ≥ 1% are shown
The lungs were the most common site of infection among patients with IFIs, reported in more than half (58.2%) of patients with an IFI (Table 3). Overall, 60% (1194/1993) of patients in the FAS were diagnosed with at least one non-fungal infection; bacterial (83.3% [1001/1194]) and viral (35.8% [427/1194]) infections accounted for the majority of these (Table S3). Most patients with bacterial infection were treated with one type of antibiotic (60.5% [603/996]).
Table 3.
Infection site in patients with IFIs during the study (FAS)
| Isavuconazole (n = 510) | Posaconazole (n = 540) | Voriconazole (n = 491) | Multiple/sequenced MAT therapies (n = 452) | Total (N = 1993) | |
|---|---|---|---|---|---|
| Infection site, nab | 61 | 43 | 48 | 133 | 285 |
| Lung | 36 (59.0) | 21(48.8) | 27 (56.3) | 82 (61.7) | 166 (58.2) |
| Chest | 3 (4.9) | 0 | 4 (8.3) | 10 (7.5) | 17 (6.0) |
| Oropharynx | 3 (4.9) | 4 (9.3) | 0 | 1 (0.8) | 8 (2.8) |
| Skin | 2 (3.3) | 2 (4.7) | 3 (6.3) | 7 (5.3) | 14 (4.9) |
| Maxillary sinus | 2 (3.3) | 2 (4.7) | 1 (2.1) | 7 (5.3) | 12 (4.2) |
| Abdominal cavity | 2 (3.3) | 0 | 0 | 2 (1.5) | 4 (1.4) |
| Other | 16 (26.2) | 15 (34.9) | 12 (25.0) | 37 (27.8) | 80 (28.1) |
Data are n (%), unless otherwise indicated
FAS full analysis set, IFI invasive fungal infection
aOnly incidences of ≥ 1% are shown
bThe number of patients (n) with at least one IFI during the study and non-missing infection site data
Antifungal Therapy
The majority (77.3%) of patients in the FAS had been maintained on MAT monotherapy since index/enrollment, and rates were similar among individual MATs (isavuconazole 25.6%, posaconazole 27.1%, and voriconazole 24.6%; Table 4). MAT therapy switching was observed in 22.7% of patients, and susceptibility testing was reported in 24.1% (69/286) of patients with at least one IFI during the study: isavuconazole (24.6% [15/61]), posaconazole 23.3% [10/43]), voriconazole (10.2% [5/49]), and multiple/sequenced MAT therapies (29.3% [39/133]). TDM was documented in 41.0% (816/1992) of patients in the FAS population (2366 events) and was reported less frequently for patients receiving isavuconazole (11.8% [60/510]) than those receiving posaconazole (45.3% [244/539]), voriconazole (54.0% [265/491]), and multiple/sequenced MAT therapies (54.6% [247/452]). Median TDM levels (mg/ml) were 3.2 (range 0.4–20.8) for isavuconazole, 2.3 (range 0–17.8) for voriconazole, and 1.5 (range 0–6.5) for posaconazole.
Table 4.
Treatment sequences of mold-active triazole (MAT) therapies during the study (FAS)
| Total (N = 1993) | |
|---|---|
| MAT monotherapy, n (%) | 1541 (77.3) |
| Isavuconazole | 510 (25.6) |
| Posaconazole | 540 (27.1) |
| Voriconazole | 491 (24.6) |
| Multiple/sequenced MAT therapies, n (%) | 452 (22.7) |
| Voriconazole-posaconazole | 66 (3.3) |
| Voriconazole-isavuconazole | 54 (2.7) |
| Posaconazole-voriconazole | 52 (2.6) |
| Isavuconazole-posaconazole | 46 (2.3) |
| Isavuconazole-voriconazole | 46 (2.3) |
| Posaconazole-isavuconazole | 44 (2.2) |
| Posaconazole-voriconazole-posaconazole | 26 (1.3) |
| Posaconazole-isavuconazole-posaconazole | 21 (1.1) |
| Isavuconazole-posaconazole-isavuconazole | 18 (0.9) |
| Voriconazole-posaconazole-isavuconazole | 15 (0.8) |
| Isavuconazole-voriconazole-isavuconazole | 13 (0.7) |
| Voriconazole-posaconazole-voriconazole | 10 (0.5) |
| Isavuconazole-voriconazole-posaconazole | 9 (0.5) |
| Posaconazole-voriconazole-isavuconazole | 9 (0.5) |
| Voriconazole-isavuconazole-voriconazole | 9 (0.5) |
| Voriconazole-isavuconazole- posaconazole | 7 (0.4) |
| Isavuconazole-posaconazole-voriconazole | 5 (0.3) |
| Posaconazole-isavuconazole-voriconazole | 2 (0.1) |
The ‘total’ study population includes patients who received prophylaxis and/or treatment at index/enrollment. ‘Prophylaxis’ represents all primary and secondary prophylactic use of MAT, and ‘treatment’ represents all pre-emptive, empiric, targeted, and salvage use of MAT. Monotherapy was assigned to patients receiving one therapy throughout the study. Treatment pattern is reported up to third-line therapy; patients receiving more than three therapies are counted under the sequence corresponding to their first three therapies
FAS full analysis set
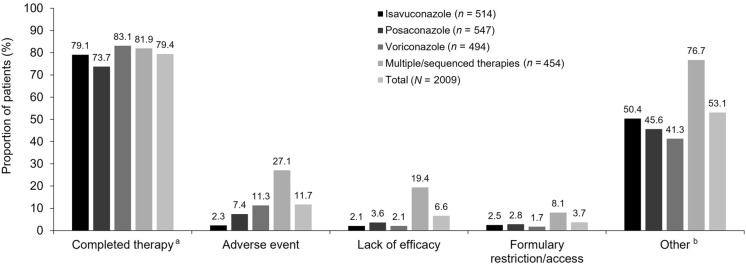
In the SAF, median duration (days) of exposure to MATs after enrollment was relatively shorter for patients receiving voriconazole (46 [range 0–810]) compared with posaconazole (61 [range 0–903]), isavuconazole (86 [range 0–1006]), and multiple/sequenced MAT therapies (108 [range 0–1061]). The majority (95.2% [1912/2009]) of patients received oral MAT therapy (isavuconazole: 90.5% [465/514], posaconazole: 98.4% [538/547], voriconazole: 92.7% [458/494], and multiple/sequenced MAT therapies: 99.3% [451/454]). In contrast, 25.1% (505/2009) of patients received intravenous MAT therapy (isavuconazole: 28.4% [146/514], posaconazole: 6.8% [37/547], voriconazole: 23.5% [116/494], and multiple/sequenced MAT therapies: 45.4% [206/454]). A small number of patients (2.4% [48/2009]) received MAT therapy via topical, nasogastric, or other administration routes. Most (79.4%) patients in the SAF were reported to have completed their MAT therapies. A numerically higher proportion of patients receiving voriconazole (83.1%) completed their MAT therapy compared with isavuconazole (79.1%) and posaconazole (73.7%) (Fig. 2). Reasons for discontinuation included AEs (11.7%), lack of efficacy (6.6%), and formulary restriction/access (3.7%). A large proportion of patients discontinued MAT therapy for ‘other’ reasons (53.1%), of which hospital admissions and hospital discharge were the most commonly reported reasons. Additional reasons for discontinuation of MAT therapy in the ‘other’ category included, but were not limited to, a lack of susceptibility of the fungal pathogen to the current MAT; decreased efficacy of the MAT; a dose increase of the MAT due to subtherapeutic MAT levels; a change in the dosage form of the study MAT (e.g., intravenous to oral) or switching the study MAT; switching the patient to another antifungal medication; a potential for DDIs relating to the MAT and another drug(s); the patient was intubated (could no longer take the prescribed oral dose), had oral dysphagia, elevated liver function tests, or was lost to follow-up; and the resolution of neutropenia or mucositis.
Fig. 2.
Reasons for discontinuation of mold-active triazoles (SAF). Patients were counted in multiple categories, but only once per category. Monotherapy was assigned to patients receiving one therapy throughout the study since index/enrollment. Percentages are based on number of patients with non-missing data for each category; isavuconazole n = 484, posaconazole n = 498, voriconazole n = 479, multiple/sequenced MAT therapies n = 454, and total N = 1915. aMultiple/sequenced MAT therapies described patients receiving more than one mold-active triazole therapy throughout the study since index/enrollment. b ‘Other’ reasons for discontinuation of mold-active triazoles included, but were not limited to, hospital visits, including hospital admission, discharge, and inpatient and outpatient switching. SAF safety analysis set
Overall, 42.7% (858/2009) of patients in the SAF received concomitant non-MAT antifungal therapy that was started on or after index/enrollment, the most common of which were micafungin (17.9% [360/2009]) and fluconazole (15.9% [319/2009]).
Safety Assessments
ADRs were reported in 14.7% of patients in the SAF and were proportionally less common in the isavuconazole group (3.9%) than in the posaconazole (11.3%) and voriconazole (14.2%) groups (Table 5). Patients in the multiple/sequenced MAT therapies group had a numerically higher proportion of ADRs (31.7%) than each of the MAT monotherapy groups. According to system organ class, elevated liver function tests were the most common ADR overall (7.3%), and the rate was numerically lower in the isavuconazole (1.2%) group than in the posaconazole (5.9%), voriconazole (8.1%), and multiple/sequenced MAT therapies (15.2%) groups. The proportions of patients with other individual ADRs were generally similar between the MAT monotherapy groups.
Table 5.
Adverse drug reactions occurring in ≥ 1% of patients in any treatment group and drug-drug interactions (SAF)
| Isavuconazole (n = 514) | Posaconazole (n = 547) | Voriconazole (n = 494) | Multiple/sequenced MAT therapiesa (n = 454) | Total (N = 2009) | |
|---|---|---|---|---|---|
| ADRs overall, n (%) | 20 (3.9) | 62 (11.3) | 70 (14.2) | 144 (31.7) | 296 (14.7) |
| Liver function test increasedb | 6 (1.2) | 32 (5.9) | 40 (8.1) | 69 (15.2) | 147 (7.3) |
| Nausea | 3 (0.6) | 11 (2.0) | 4 (0.8) | 14 (3.1) | 32 (1.6) |
| Hallucination | 0 | 0 | 3 (0.6) | 26 (5.7) | 29 (1.4) |
| QT prolonged | 1 (0.2) | 5 (0.9) | 2 (0.4) | 15 (3.3) | 23 (1.1) |
| Vomiting | 2 (0.4) | 1 (0.2) | 1 (0.2) | 5 (1.1) | 9 (0.4) |
| Photosensitivity reaction | 1 (0.2) | 0 | 5 (1.0) | 3 (0.7) | 9 (0.4) |
| Hallucination, visual | 0 | 0 | 1 (0.2) | 7 (1.5) | 8 (0.4) |
| Rash | 1 (0.2) | 0 | 1 (0.2) | 5 (1.1) | 7 (0.3) |
| Drug–drug interactions, n (%) | 13 (2.5) | 15 (2.7) | 22 (4.5) | 30 (6.6) | 80 (4.0) |
ADRs reported by preferred term using MedDRA version 20.0
ADR adverse drug reaction, MedDRA Medical Dictionary for Regulatory Activities, SAF safety analysis set
aMultiple/sequenced MAT therapies described patients receiving more than one mold-active triazole therapy throughout the study since index/enrollment
b‘Liver function test increased’ included the following preferred terms: ‘liver function test increased,’ ‘transaminases increased,’ ‘alkaline phosphatase increased,’ ‘hyperbilirubinemia,’ ‘alanine transaminase increased,’ ‘aspartate transaminase increased,’ and ‘hepatic enzyme increased’
DDIs were reported in 4.0% (80/2009) of patients overall. Of these, the MAT was withdrawn in 1.0% (21/2009) of patients. Relatively fewer DDIs were documented in patients receiving isavuconazole (2.5% [13/514]) and posaconazole (2.7% [15/547]) than in those receiving voriconazole (4.5% [22/494]) and multiple/sequenced MAT therapies (6.6% [30/484]).
Overall, 2.0% (40/2009) of patients died by the end of the study because of the IFI, and IFI-specific mortality was reported in the isavuconazole (1.6% [8/514]), posaconazole (1.1% [6/547]), voriconazole (1.8% [9/494]), and multiple/sequenced MAT therapies (3.7% [17/454]) groups. Univariate analysis in patients receiving hematopoietic stem cell transplantation revealed odds ratios (95% confidence interval [CI]) of 1.37 (1.00–1.88; p = 0.05) for all-cause mortality and 1.35 (0.52–3.50; p = 0.54) for fungal-specific mortality.
Secondary Endpoint: Healthcare Resource Utilization
Almost all patients in the FAS were treated in an inpatient setting at the time of MAT index/enrollment; only two patients in the voriconazole group were treated in an outpatient setting.
The median length of the initial hospital stay was 26 days (95% CI 25–27), ranging from 23 days (95% CI 21–26) in the isavuconazole group to 28 days (95% CI 26–30) in the posaconazole group (Table 6). Overall, around 27% of patients who were hospitalized at index/enrollment were in the ICU, ranging from 13.6% of patients in the posaconazole group to 36.6% of patients in the isavuconazole group. Most patients (85.2%) who were hospitalized did not require ventilator use. The proportion of patients using a ventilator ranged from 5.6% in the posaconazole group to 22.3% in the isavuconazole group. Patients who received voriconazole spent the longest mean duration on a ventilator (27 days versus 15–18 days in the other groups).
Table 6.
Healthcare resource utilization at index/enrollment (FAS)
| Isavuconazole (n = 510) | Posaconazole (n = 540) | Voriconazole (n = 491) | Multiple/sequenced MAT therapiesa (n = 452) | Total (n = 1993) | |
|---|---|---|---|---|---|
| n | 358 | 411 | 398 | 381 | 1548 |
| Length of initial hospital stay (days)a | |||||
| Median (95% CI) | 23.0 (21.0, 26.0) | 28.0 (26.0, 30.0) | 25.0 (22.0, 27.0) | 27.0 (25.0, 30.0) | 26.0 (25.0, 27.0) |
| In ICU at time of MAT initiation, n (%) | |||||
| Patient in ICU | 131 (36.6) | 56 (13.6) | 128 (32.2) | 99 (26.0) | 414 (26.7) |
| Ventilated at time of MAT initiation, n (%) | |||||
| Patient used ventilator | 80 (22.3) | 23 (5.6) | 68 (17.1) | 58 (15.2) | 229 (14.8) |
| Total time on ventilator (days)b | |||||
| n | 79 | 23 | 68 | 58 | 228 |
| Mean (SD) | 17.5 (39.03) | 15.1 (22.23) | 26.9 (50.73) | 16.5 (23.36) | 19.8 (38.60) |
Almost all patients were treated in an inpatient setting at the time of MAT index/enrollment; only two patients in the voriconazole group were treated in an outpatient setting and are not included in this table. The table contains healthcare resource utilization data corresponding to index MAT (protocol v1.1) or MAT at enrollment (protocol v2.1). Percentages were based on the number of patients in the FAS or n for each parameter. The n for each parameter was the number of patients with inpatient setting of care at index/enrollment with non-missing data for that parameter. Patients were counted only once for the ‘ICU at time of MAT initiation’ and ‘Ventilated at time of MAT initiation’ categories
CI confidence interval, FAS full analysis set, ICU intensive care unit, MAT mold-active triazole, SD standard deviation
aLength of stay was calculated as discharge date − admission date + 1 and was censored at the date of death or study discontinuation
bTotal time on ventilator (days) was calculated as the sum of (ventilator end date − ventilator start date + 1) for all ventilator occurrences
Exploratory Endpoint: Response Assessments
A total of 1001 of 1993 patients who received a MAT had a clinical response assessment. More than half of patients (59.1% [591/1001]) who had a clinical response assessment achieved success by resolution of some or all attributable signs of IFIs (Table 7). See Table S2 for definition of clinical response success. Response rates for those who achieved clinical success by resolution of some or all attributable signs of an IFI were similar across the MAT monotherapy groups (isavuconazole: 59.7% [184/308], posaconazole: 52.8% [95/180], and voriconazole: 59.0% [151/256]). Among the patients receiving multiple/sequenced MAT therapies, 62.6% (161/257) achieved clinical success. Overall, there was no resolution of clinical signs or symptoms in 17.3% (173/1001) of patients; this rate was numerically lower in patients receiving isavuconazole (14.6% [45/510]) and posaconazole (13.9% [25/540]) than in those receiving voriconazole (19.1% [49/491]) and multiple/sequenced MAT therapies (21.0% [54/452]). Response rates by pathogen can be found in Table S4.
Table 7.
Investigator’s assessment of patient responses to mold-active triazole therapies at study end (FAS)
| Isavuconazole (n = 510) | Posaconazole (n = 540) | Voriconazole (n = 491) | Multiple/sequenced MAT therapies (n = 452) | Total (N = 1993) | |
|---|---|---|---|---|---|
| Clinical response assessment, n | 308 | 180 | 256 | 257 | 1001 |
| Resolution of all attributable signs/symptoms | 114 (37.0) | 68 (37.8) | 82 (32.0) | 92 (35.8) | 356 (35.6) |
| Resolution of some attributable signs/symptoms | 70 (22.7) | 27 (15.0) | 69 (27.0) | 69 (26.8) | 235 (23.5) |
| No resolution of any attributable signs/symptoms | 45 (14.6) | 25 (13.9) | 49 (19.1) | 54 (21.0) | 173 (17.3) |
| No attributable signs/symptoms | 65 (21.1) | 57 (31.7) | 43 (16.8) | 33 (12.8) | 198 (19.8) |
| Results not available/patient unevaluable | 14 (4.5) | 3 (1.7) | 13 (5.1) | 9 (3.5) | 39 (3.9) |
| Mycologic response assessment, n | 227 | 116 | 194 | 199 | 736 |
| Eradication | 48 (21.1) | 20 (17.2) | 28 (14.4) | 34 (17.1) | 130 (17.7) |
| Presumed eradication | 62 (27.3) | 29 (25.0) | 66 (34.0) | 49 (24.6) | 206 (28.0) |
| Persistence | 12 (5.3) | 9 (7.8) | 17 (8.8) | 25 (12.6) | 63 (8.6) |
| Presumed persistence | 18 (7.9) | 18 (15.5) | 23 (11.9) | 26 (13.1) | 85 (11.5) |
| Indeterminate | 7 (3.1) | 3 (2.6) | 5 (2.6) | 3 (1.5) | 18 (2.4) |
| Results not available/patient unevaluable | 80 (35.2) | 37 (31.9) | 55 (28.4) | 62 (31.2) | 234 (31.8) |
| Radiologic response assessment, n | 248 | 129 | 193 | 224 | 794 |
| ≥ 90% improvement | 60 (24.2) | 36 (27.9) | 40 (20.7) | 64 (28.6) | 200 (25.2) |
| ≥ 50 to < 90% improvement | 37 (14.9) | 21 (16.3) | 40 (20.7) | 34 (15.2) | 132 (16.6) |
| ≥ 25 to < 50% improvement | 26 (10.5) | 5 (3.9) | 14 (7.3) | 11 (4.9) | 56 (7.1) |
| < 25% improvement | 39 (15.7) | 28 (21.7) | 43 (22.3) | 57 (25.4) | 167 (21.0) |
| No signs on radiologic images | 33 (13.3) | 14 (10.9) | 30 (15.5) | 25 (11.2) | 102 (12.8) |
| Results not available/patient unevaluable | 53 (21.4) | 25 (19.4) | 26 (13.5) | 33 (14.7) | 137 (17.3) |
Data shown are n (%), unless otherwise indicated. Table shows actual number of patients with an assessment (n). Since treatment groups were not randomized and assessment results were not adjusted, any perceived differences between treatment groups could be due to other confounders and not treatment effects. Note that patients who received prophylaxis may have transitioned to treatment during the course of the study; for context, 816 and 1177 patients received treatment and prophylaxis, respectively, at study index/enrollment
FAS full analysis set
There were 736 patients with a mycologic response assessment who received a MAT, of which eradication or presumed eradication was reported in nearly half (45.7% [336/736]) and persistence or presumed persistence in approximately one-fifth (20.1% [148/736]) of patients (Table 7). These rates were generally similar across all therapy groups, although the rates of eradication or presumed eradication were numerically higher in the isavuconazole (48.5% [110/227]) and voriconazole (48.5% [94/194]) groups than for posaconazole (42.2% [49/116]) and multiple/sequenced MAT therapies (41.7% [83/199]). Persistence or presumed persistence rates were numerically lower in the isavuconazole group (13.2% [30/227]) than for the other therapies (posaconazole: 23.3% [27/116], voriconazole: 20.6% [40/194]), and multiple/sequenced MAT therapies: 25.6% [51/199]).
Overall, 794 patients who received a MAT had a radiologic response assessment. Approximately half of these patients (48.9% [388/794]) achieved ≥ 25% improvement from baseline, and the response was similar across all therapy groups (isavuconazole: 49.6% [123/248], posaconazole: 48.1% [62/129], voriconazole: 48.7% [94/193]), and multiple/sequenced MAT therapies (48.7% [109/224]; Table 7).
A total of 1030 patients who received a MAT for prophylaxis at index/enrollment had a prophylactic outcome assessment. Breakthrough IFIs were reported in 7.1% (73/1030) of these patients.
Discussion
In this multicenter, prospective, observational study, we captured the demographics, clinical characteristics, therapy patterns, and outcomes of more than 2000 patients receiving a MAT for the treatment or prophylaxis of IFIs in a real-world setting.
Most patients in the study population were maintained on MAT monotherapy; however, more patients receiving voriconazole than the other MATs completed their MAT therapy. The majority (95.2%) of patients in the study received oral MAT therapy. Hematologic malignancy, use of corticosteroids, and neutropenia predominated as underlying risk factors for this population of patients, which is consistent with other reports [14]. We also found that the patients in this study with IFIs had long hospital stays, which often required ICU admission. However, it is not possible to draw any conclusions from these data without a matched control group of patients with the same underlying conditions, but without an IFI. Differences among the MATs in terms of length of hospital stay and time in the ICU were likely due to the circumstances of the individual patients. Respiratory infections were the most frequent IFIs across all therapy groups, perhaps linked to Aspergillus species being the most common pathogens in this study [15]. Candida species were also common. However, it should be noted that data reported for the most commonly treated pathogen and site of infection could be mutually exclusive in this study. For instance, although Candida was the most commonly treated species for patients in the posaconazole group, and the lungs were the most frequent site of infection, it cannot be assumed that Candida was the predominant species in the lungs for these patients. Also, some C. glabrata isolates from respiratory secretions may not have been the causative isolate. In addition, the protocol did not specify how to report potential colonization versus an invasive pathogen, and so these data were not captured.
Invasive infections due to Aspergillus species are a widely recognized and life-threatening condition in immunocompromised patients [6]. In 2016, the IDSA published guidelines strongly recommended triazoles as the preferred agents for the treatment and prevention of invasive aspergillosis, with voriconazole as primary treatment, isavuconazole as an alternative primary therapy, and posaconazole for refractory or progressive aspergillosis [6]. In the present study, a numerically higher proportion of patients received isavuconazole and voriconazole than posaconazole as MAT therapy for the most common pathogen, A. fumigatus, reflecting the IDSA guidelines. Of note, since the conclusion of this study, the Centers for Disease Control and Prevention in the US have offered screening for azole-resistant A. fumigatus [16], which could inform future studies. In terms of Candida species, > 90% of invasive disease is caused by C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei [5]. Candida glabrata was the most frequently recorded Candida species in our study, which supports the evolving epidemiology of Candida infections from the once prevalent C. albicans [17]. Indeed, it has been reported that > 30% of cases of candidemia in the US are now caused by C. glabrata [17]. However, it is important to note a possible selection bias in our study, as isavuconazole, voriconazole, and posaconazole could have been chosen by the clinician as an alternative oral option to fluconazole, given that C. glabrata is more likely than any other Candida species to be resistant to fluconazole [18]. In the present study, a numerically higher proportion of patients received posaconazole and isavuconazole than voriconazole for the treatment of known Candida species IFIs. Voriconazole is generally recommended over the other MATs for the treatment of C. glabrata that are susceptible to voriconazole, and posaconazole is recommended for the prophylaxis of invasive Candida infections and treatment of oropharyngeal candidiasis [5]. However, as this was a real-world study, the patient cohorts were representative of those who received MAT treatment or prophylaxis for IFIs in clinical practice, regardless of the guideline recommendations for first- and second-line therapies for IFIs. For example, in clinical practice an immunocompromised patient with candidemia who required prophylaxis for mold infection might be prescribed a single agent for both purposes. The choice of MAT might also be impacted by those available in an institution’s formulary or a physician’s preference based on the available evidence, such as pharmacokinetic data. In addition to these real-world study considerations, while C. glabrata was the most frequently recorded pathogen in the posaconazole group among patients with an IFI (20.9% [9/43]), the sample size of patients receiving posaconazole for C. glabrata among all patients in the posaconazole group was relatively small (1.7% [9/540]). Furthermore, 35% of patients who were diagnosed with Candida infection and received posaconazole also received concomitant fluconazole. Fluconazole is recommended in the IDSA guidelines for the treatment of infection due to C. glabrata in patients with fluconazole-susceptible isolates [5].
Our study showed that more than half (59.1%) of the patients receiving a MAT and with a clinical response assessment had a favorable clinical response, and the response rate was relatively consistent across MAT monotherapy groups (52.8–59.7%). Similar observations were reported in the SECURE study, in which 60–62% of patients with invasive mold disease who received isavuconazole or voriconazole achieved treatment success according to their clinical response assessments [12]. In an open-label, multicenter study, patients with invasive aspergillosis (n = 107) received posaconazole as salvage therapy and were compared to an external control group with IFIs (n = 86) [19]. Although this study cannot be directly compared with our study, it is interesting to note that complete or partial success was achieved in 42% of patients receiving posaconazole and 26% of the control subjects [19]. In the present study, eradication or presumed eradication was reported in nearly half (45.7%) of patients who received a MAT, and persistence or presumed persistence was reported in approximately one-fifth (20.1%) of patients with a mycologic response assessment. Again, these data are not dissimilar to those reported in the SECURE study, in which 38% and 41% of respective patients who received isavuconazole and voriconazole achieved eradication or presumed eradication [12]. Approximately half (48.9%) of patients with a radiologic response assessment achieved ≥ 25% improvement from baseline in our study. Although these rates are higher than reported in the SECURE study for isavuconazole (29%) and voriconazole (33%), achievement criteria were more stringent in the SECURE study (a combination of ≥ 25% and ≥ 50% improvement from baseline) [12]. In our study, breakthrough IFIs were reported in 7.1% of patients receiving MAT prophylaxis. Similar rates of breakthrough IFIs have been reported in other studies of isavuconazole (8.3–15.4%) [20, 21], posaconazole (3.3–13.5%) [22, 23], and voriconazole (12.5–14.1%) [24, 25].
Over the years, there has been increased interest in the utility of TDM to optimize the safety and efficacy of MATs to improve patient outcomes [26]. TDM is recommended by the IDSA for patients receiving triazole-based therapies for invasive aspergillosis, prolonged azole prophylaxis, or other therapies for which drug interactions with azoles are anticipated [6]. In particular, voriconazole shows high pharmacokinetic variability, influenced by factors such as age, genetic polymorphism of the enzyme CYP2C19, and concomitant medications [1, 27]. High variability has also been observed with the posaconazole oral suspension [28]. However, despite the IDSA guideline recommendations, TDM was only reported in around half of the patients in our study who received voriconazole and posaconazole [6]. Data generated throughout the development of isavuconazole, in addition to real-world data, have suggested that TDM is not required for this agent [26]. Therefore, it was not surprising that a relatively small proportion (12%) of patients who received isavuconazole underwent TDM in our study. Of note in the present study, median TDM levels were higher for patients who received isavuconazole compared to the other MATs, which is likely due to higher target concentrations for isavuconazole than the other agents.
In the present study, isavuconazole appeared better tolerated than other MATs, which may have resulted in the less frequent use of alternative MATs. A numerically higher proportion of ADRs was reported in the multiple/sequenced MAT therapies group than in each of the MAT monotherapy groups. The numerically lower rate of ADRs with isavuconazole compared with voriconazole is consistent with the results of a previous phase 3, double-blind, international, comparative-group study (SECURE) in which drug-related AEs were significantly less frequent in the isavuconazole group compared to the voriconazole group (42% versus 60%, respectively; p < 0.001) [12]. A lower frequency of elevated liver function tests in patients receiving isavuconazole (1.2%) than those receiving posaconazole (5.9%), voriconazole (8.1%) and multiple/sequenced MAT therapies (15.2%) was another finding of the present study and supports a lower frequency of drug-related liver toxicity reported with isavuconazole compared to voriconazole (2% versus 10%, respectively) in the SECURE study [12]. In a multicenter, phase 3, randomized, controlled trial for the primary treatment of invasive aspergillosis, a higher proportion of treatment-related elevated liver function tests occurred in the posaconazole (15% [43/288]) than in the voriconazole group (12% [35/287]; treatment difference 2.7% [95% CI − 2.9 to 8.4]) [29]. A single-center, retrospective study in 100 patients with IFIs who received isavuconazole, voriconazole, or posaconazole found no significant difference in the incidence of elevated liver function tests between agents [30]. However, a reduced incidence of QTc prolongation was found in the isavuconazole group compared to the two other groups (p = 0.037) [30]. Cardiovascular AEs, particularly QTc prolongation, and gastrointestinal effects are common concerns for patients receiving a MAT and can be a differentiating feature between the treatment options [7]. In the present study, rates of prolonged QT interval and nausea and vomiting were generally similar across the MAT monotherapies. Low rates of IFI-specific mortality were reported across the MAT monotherapy groups.
This study has some limitations. Enrolling patients under two protocols may be considered a limitation of this study; however, as both protocols involved chart review and used the same definitions of IFI and outcomes, we do not expect that the choice of protocol impacted the study results. The main difference between the two protocols was the removal of the enrollment window from the inclusion criteria and the ‘index’ terminology. This change was based on feedback from the study sites and clinical experts. For the majority of patients enrolled under protocol version 1.1, the index MAT spanned enrollment. The remaining patients under this protocol had either a different MAT or no MAT at enrollment; however, using the start date of the index MAT as the “baseline” was consistent with the study design and data collection for protocol version 1.1.
The pooling of data for three MATs with differing FDA label recommendations, safety profiles, and scopes as fungal prophylaxis and treatment could be a limitation of this study. However, the aim of this study was to provide clarity around the real-world use of these MATs in a large patient cohort, and while label and scope may differ between the MATs in this study, label use may not be representative of real-world use. There is a possibility of selection bias in relation to the patients enrolled or hospitals selected. Sites were selected based on MAT use, with favoring of higher use sites and those with a history of good antifungal clinical trial performance (sites with a higher level of subject enrollment, good data entry and follow-up parameters). To the extent possible, the distribution of sites was broadly representative of site settings for the treatment of IFIs in the US (e.g., geographically, by specialty, and community versus academic institutions). However, our study findings may not be generalizable to patients hospitalized in other countries or types of centers because of the changing epidemiology of IFIs, variations between hospitals, antifungal agent guidelines and restrictions, and other regional differences. To reflect real-world practice, there were no mandatory follow-up visits for this study. Instead, duration of follow-up was determined by the routine clinical practice for the patient and therefore varied between patients.
To limit site-specific effects, all sites underwent standardized training and used standardized documentation to complete eCRFs at enrollment and for each follow-up assessment. However, there were many missing data elements, likely from inconsistent interpretation of the eCRFs and operational methodologies by each site. Also, there were several ‘other’ categories for which a pre-specified category was not created within the eCRF, making analysis and interpretation challenging. Furthermore, patients categorized as receiving prophylaxis at index/enrollment may have transitioned to treatment at some time point during the study, but this information was not captured.
Finally, underlying patient conditions were reported but not analyzed with respect to outcomes or ADRs, which could be a limitation of the study. Outcomes would be expected to differ between patients with and without hematologic malignancies due to the high heterogeneity of underlying diseases in these patient populations (and emerging at-risk groups, such as those with chronic obstructive pulmonary disease, postoperative patients, and patients receiving newer immunosuppressive therapies [31]) and the potential for interactions between MATs and various concomitant medications [32].
Despite these limitations, the strengths of this study are its prospective, observational design, the large number of enrolled patients, and the comprehensive analysis of high-quality data.
Conclusions
These results of ‘real-world’ experience of MATs for the treatment or prophylaxis of IFIs add to the evolving data for this significant health problem. MATs were associated with favorable clinical, mycologic, and radiologic responses in approximately half of patients who were assessed, and the majority of patients who received MATs for prophylaxis had no breakthrough IFIs. However, while data showed that the underlying risk factors for IFIs remain unchanged from published studies, the choice of MAT did not always follow the recommendations of published guidelines, even when the causative pathogen was known. Furthermore, despite guideline recommendations, TDM was only reported in around half of patients who received voriconazole and posaconazole. The findings for this study support therapeutic strategies for the effective management of patients with IFIs with isavuconazole, posaconazole, and voriconazole.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was initiated and supported by Astellas Pharma Global Development, Inc., Northbrook, IL, USA. The journal’s Rapid Service Fee was funded by Astellas Pharma Inc.
Medical Writing Assistance
Medical writing support was provided by Anne-Marie Edwards, MChem, of Cello Health MedErgy, funded by Astellas Pharma Global Development, Inc. The authors would like to thank the study investigators and all patients who took part in the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conception: Luis Ostrosky-Zeichner. Study design and conduct: Luis Ostrosky-Zeichner. Data acquisition: Luis Ostrosky-Zeichner, M. Hong Nguyen, Joseph Bubalo, Barbara D. Alexander, Marisa H. Miceli, Peter G. Pappas, George R. Thompson. Analysis and interpretation: Luis Ostrosky-Zeichner, M. Hong Nguyen, Joseph Bubalo, Barbara D. Alexander, Jeanette Jiang, Yi Song, George R. Thompson. Writing: Luis Ostrosky-Zeichner, M. Hong Nguyen, Joseph Bubalo, Barbara D. Alexander, Marisa H. Miceli, Peter G. Pappas, Jeanette Jiang, George R. Thompson
Prior Presentation
These data were presented in part as an oral presentation at the 31st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), July 9–12, 2021, online, and 10th Trends in Medical Mycology (TIMM) bi-annual meeting of the European Confederation of Medical Mycology (ECMM), October 8–11, 2021, Aberdeen, Scotland, UK.
Disclosures
Luis Ostrosky-Zeichner reports grants Gilead, Pfizer, Scynexis, Cidara and Pulmocide; payment or honoraria from Pfizer and F2G; and participation on boards from Cidara, F2G, Pfizer, Eurofins and Appili. M. Hong Nguyen reports grants from Astellas Pharma Global Development, Inc. Joseph Bubalo reports payment or honoraria from medSynergy; and participation on advisory board from Pfizer. Peter G. Pappas reports grants from Scynexis, Mayne Pharma and Cidara; and consulting fees from F2G and Cidara. Barbara D. Alexander reports grants from Leadiant and Scynexis; royalties from UpToDate; participation on advisory board from HealthTrackRx; leadership or fiduciary roles in other boards, for IDSA and Mycoses Study Group; and acting as principal investigator for clinical trial sites for F2G, Scynexis, Cidara, and Shire/Takeda. Marisa H. Miceli reports grants and consulting fees from Astellas Pharma Global Development, Inc.; grants from Mayne and F2G; and consulting fees from Scynexis and PSI. Jeanette Jiang and Yi Song are employees of Astellas Pharma Global Development, Inc. George R. Thompson has nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by an Institutional Review Board (IRB) at each participating study site (see Table S1 for details of the ethics committees). The investigator(s) and all parties involved in this study conducted the study in accordance with the ethical principles based on the Declaration of Helsinki 1964 and its later amendments. Informed consent was obtained for patients included in the study (those aged < 18 years had consent of a parent or legal guardian and, where appropriate, consent of the patient) when required by IRBs according to local guidance.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available. Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jenks JD, Mehta SR, Hoenigl M. Broad spectrum triazoles for invasive mould infections in adults: which drug and when? Med Mycol. 2019;57:S168–S178. doi: 10.1093/mmy/myy052. [DOI] [PubMed] [Google Scholar]
- 2.Enoch DA, Yang H, Allyu SH, Micallef C (2017) Human fungal pathogen identification. Lion T, editor. Springer New York, New York
- 3.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:1–10. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 4.Formanek PE, Dilling DF. Advances in the diagnosis and management of invasive fungal disease. Chest. 2019;156:834–842. doi: 10.1016/j.chest.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson DT, Dimondi VP, Johnson SW, Jones TM, Drew RH. Role of isavuconazole in the treatment of invasive fungal infections. Ther Clin Risk Manag. 2016;12:1197–1206. doi: 10.2147/TCRM.S90335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledoux MP, Toussaint E, Denis J, Herbrecht R. New pharmacological opportunities for the treatment of invasive mould diseases. J Antimicrob Chemother. 2017;72:i48–58. doi: 10.1093/jac/dkx033. [DOI] [PubMed] [Google Scholar]
- 9.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 10.Vehreschild JJ, Cornely OA. Micafungin sodium, the second of the echinocandin class of antifungals: theory and practice. Future Microbiol. 2006;1:161–170. doi: 10.2217/17460913.1.2.161. [DOI] [PubMed] [Google Scholar]
- 11.Pound MW, Townsend ML, Dimondi V, Wilson D, Drew RH. Overview of treatment options for invasive fungal infections. Med Mycol. 2011;49:561–580. doi: 10.3109/13693786.2011.560197. [DOI] [PubMed] [Google Scholar]
- 12.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 13.Allen D, Wilson D, Drew R, Perfect J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev Anti Infect Ther. 2015;13:787–798. doi: 10.1586/14787210.2015.1032939. [DOI] [PubMed] [Google Scholar]
- 14.Rüping MJGT, Vehreschild JJ, Cornely OA. Patients at high risk of invasive fungal infections: when and how to treat. Drugs. 2008;68:1941–1962. doi: 10.2165/00003495-200868140-00002. [DOI] [PubMed] [Google Scholar]
- 15.Webb BJ, Ferraro JP, Rea S, Kaufusi S, Goodman BE, Spalding J. Epidemiology and clinical features of invasive fungal infection in a US health care network. Open Forum Infect Dis. 2018;5:2–9. doi: 10.1093/ofid/ofy187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. Screening for triazole-resistant Aspergillus fumigatus using agar plates. https://www.cdc.gov/fungal/lab-professionals/azole-resistant-aspergillus.html: Accessed: 22 Mar 2022.
- 17.Friedman DZP, Schwartz IS. Emerging fungal infections: new patients, new patterns, and new pathogens. J Fungi. 2019;5:67. doi: 10.3390/jof5030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berkow E, Lockhart S. Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist. 2017;10:237–245. doi: 10.2147/IDR.S118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 20.Fontana L, Perlin DS, Zhao Y, Noble BN, Lewis JS, Strasfeld L, et al. Isavuconazole prophylaxis in patients with hematologic malignancies and hematopoietic cell transplant recipients. Clin Infect Dis. 2020;70:723–730. doi: 10.1093/cid/ciz282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bose P, McCue D, Wurster S, Wiederhold NP, Konopleva M, Kadia TM, et al. Isavuconazole as primary antifungal prophylaxis in patients with acute myeloid leukemia or myelodysplastic syndrome: an open-label, prospective, phase 2 study. Clin Infect Dis. 2021;72:1755–1763. doi: 10.1093/cid/ciaa358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerolle N, Raffoux E, Socie G, Touratier S, Sauvageon H, Porcher R, et al. Breakthrough invasive fungal disease in patients receiving posaconazole primary prophylaxis: a 4-year study. Clin Microbiol Infect. 2014;20:O952–O959. doi: 10.1111/1469-0691.12688. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Xia F, Zhou H, Qiu H, Wu D, Ma X et al. Efficacy of posaconazole prophylaxis for fungal disease in hematology patients treated with chemotherapy and transplantation: an open-label, prospective, observational study. Front Microbiol 2020; 11. [DOI] [PMC free article] [PubMed]
- 24.Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trifilio S, Singhal S, Williams S, Frankfurt O, Gordon L, Evens A, et al. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant. 2007;40:451–456. doi: 10.1038/sj.bmt.1705754. [DOI] [PubMed] [Google Scholar]
- 26.Desai AV, Kovanda LL, Hope WW, Andes D, Mouton JW, Kowalski DL, et al. Exposure-response relationships for isavuconazole in patients with invasive aspergillosis and other filamentous fungi. Antimicrob Agents Chemother. 2017;61:1–9. doi: 10.1128/AAC.01034-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stott KE, Hope WW. Therapeutic drug monitoring for invasive mould infections and disease: pharmacokinetic and pharmacodynamic considerations. J Antimicrob Chemother. 2017;72:i12–i18. doi: 10.1093/jac/dkx029. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Krekels EHJ, Verweij PE, Buil JB, Knibbe CAJ, Brüggemann RJM. Pharmacokinetics and pharmacodynamics of posaconazole. Drugs. 2020;80:671–695. doi: 10.1007/s40265-020-01306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maertens JA, Rahav G, Lee D-G, Ponce-de-León A, Ramírez Sánchez IC, Klimko N, et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non-inferiority trial. Lancet. 2021;397:499–509. doi: 10.1016/S0140-6736(21)00219-1. [DOI] [PubMed] [Google Scholar]
- 30.Van Matre ET, Evans SL, Mueller SW, MacLaren R, Fish DN, Kiser TH. Comparative evaluation of isavuconazonium sulfate, voriconazole, and posaconazole for the management of invasive fungal infections in an academic medical center. Ann Clin Microbiol Antimicrob 2019;18. [DOI] [PMC free article] [PubMed]
- 31.Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol. 2011;49:S7–12. doi: 10.3109/13693786.2010.505204. [DOI] [PubMed] [Google Scholar]
- 32.Andes D, Azie N, Yang H, Harrington R, Kelley C, Tan R-D, et al. Drug–drug interaction associated with mold-active triazoles among hospitalized patients. Antimicrob Agents Chemother. 2016;60:3398–3406. doi: 10.1128/AAC.00054-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available. Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.