Abstract
Introduction
Human immunodeficiency virus (HIV) infection can be considered a chronic disease thanks to the extended use of antiretroviral treatment (ART). In this context, low-grade chronic inflammation related to gut microbiota (GM) dysbiosis and bacterial translocation (BT) among other factors has been observed despite the use of ART. In addition, different ART regimens have demonstrated differential impacts on GM. However, the role of novel integrase strand transfer inhibitors (INSTIs) has not been investigated yet. The aim of this study was to analyse the effects of INSTIs in first-line of treatment on markers of BT, inflammation, cardiovascular risk, gut permeability and GM composition and derived short-chain fatty acids.
Methods
Twenty-six non-HIV-infected volunteers and 30 HIV-infected patients (15 naïve and 15 under INSTIs regimen) were recruited. Blood samples were extracted to analyse biochemical parameters and markers of BT, inflammation, cardiovascular risk, gut permeability and bacterial metabolism. GM composition was analysed using 16S rRNA gene sequencing.
Results
Our results showed that HIV infection increased BT, inflammation, cardiovascular risk and gut permeability, whereas INSTIs counteracted these effects. Regarding GM, the reduction in bacterial richness induced by HIV infection was restored by INSTIs. Beta diversity revealed that HIV-infected people were separated from the control group independently of treatment.
Conclusions
Current antiretroviral regimens based on INSTIs are able to reverse the impact of HIV infection on BT, systemic inflammation, gut permeability and bacterial diversity/richness, reaching similar levels to those observed in an uninfected/control population. These results suggest a protective role of INSTIs in disease progression, subsequent immune activation and in the development of future age-related complications such as cardiovascular events.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00654-4.
Keywords: HIV infection, Integrase strand transfer inhibitors, Bacteriome, Bacterial translocation, Inflammation, Cardiovascular risk
Key Summary Points
| A successful treatment of Human immunodeficiency virus (HIV) infection accompanied by a complete restoration of gut dysbiosis is of great interest in order to avoid activation of the mucosal immune system, persistent inflammation and the development of long-term complications. |
| Integrase strand transfer inhibitors (INSTIs) are the preferred choice as part of the first-line regimen in treatment-naïve individuals. |
| The role of INSTIs in gut microbiota in the context of HIV infection has not yet deeply investigated. |
| INSTIs-based treatments (dolutegravir and bictegravir) were able to restore the impact of HIV infection on bacterial translocation, systemic inflammation, gut permeability and bacterial richness/diversity. |
| These results suggest a protective role of current INSTIs regimens in disease progression, subsequent immune activation and in the development of future dysbiosis-related complications. |
Introduction
Human immunodeficiency virus (HIV) infection is considered a chronic disease and life expectancy of HIV-infected patients has significantly increased thanks to the improvement in clinical management and, specifically, by the extended use of antiretroviral treatment (ART) [1, 2]. However, this infection is accompanied by both structural and functionality alterations on gut epithelial barrier, along with immunological changes and shifts in the functionality and composition of the gut microbiota (GM) despite the use of ART [3, 4]. In a normal physiological situation, microorganisms are located in the intestinal lumen interacting with the intestinal cells in a state of symbiosis, but when HIV infection occurs, a depletion of CD4+ T lymphocytes in the gut-associated lymphoid tissue (GALT) is observed. This fact is followed by a disruption of the epithelial barrier and subsequent modifications in the intestinal lumen and in the composition of GM (at least at bacteria level) [5]. This GM dysbiosis is suggested to be one of the factors related to different immune responses and treatment outcomes after ART [6]. Moreover, these facts favour the passage of microorganisms and their components to the lamina propria and, hence, to the circulation (bacterial translocation, BT), which undergoes subsequent intestinal and systemic inflammation [7]. Thus, HIV infection is accompanied by chronic inflammation which is associated with the development and progression of age-related comorbidities such as cardiovascular diseases (CVDs) that have a clear negative impact on patient’s quality of life [5]. These negative outcomes take place even in the presence of ART. Although the impact of HIV infection on GM composition, intestinal permeability and systemic inflammation has been widely described, the long-term effects of different combinations of ARTs in HIV-infected patients with undetectable viral load have been scarcely investigated. Several studies suggest that not all ART-based regimens exert similar effects on gut [8]. In this context, a recent study from Japan observed that nucleoside reverse transcriptase inhibitor (NRTI)-sparing ART (including or not integrase strand transfer inhibitors, INSTIs) alleviate the burden of intestinal dysbiosis in HIV-1-infected patients under long-term ART [9]. In line with this, a previous work from our group [8] demonstrated that ART based on INSTIs was associated with levels of systemic inflammation, soluble CD14 (sCD14) serum levels and microbial diversity similar to those observed in uninfected controls, suggesting a healthier gut and potentially fewer HIV-related complications. In this study, most of the patients had a long history of treatment and several switches and it was not possible to elucidate if these effects were specific to raltegravir (the only integrase inhibitor approved for clinical practice and the only one available when patients were recruited for that study) or could be extrapolated to other INSTIs that are recommended by international guidelines as preferred choice as part of the first-line regimen in treatment-naïve individuals.
The objective of the present work was to elucidate if previous detected alleviating effects of raltegravir on inflammation, BT, cardiovascular risk and GM are class specific. To achieve this goal, we analysed the impact of current INSTIs-containing regimens on markers of BT, inflammation, cardiovascular risk, gut permeability, and GM composition and derived metabolites in HIV-infected patients with undetectable viral load.
Methods
This study was performed following the Helsinki Declaration and was approved by the Committee for Ethics in Drug Research in La Rioja (CEImLAR) (28 February 2019, reference number 349). All participants provided their written informed consent.
Patient Recruitment
HIV-infected patients [naïve (n = 15) and under ART (n = 15)] were recruited from the Infectious Diseases Department at Hospital Universitario San Pedro (HUSP) (Logroño, Spain) from March 2019 to February 2021. Six of the naïve patients correspond to six of the ART-treated patients recruited both before treatment and after 1 year of treatment. The group of HIV-infected ART-treated patients included HIV-infected patients in first line of treatment with INSTIs (dolutegravir or bictegravir) (Supplementary Table 1) to avoid confounding effects due to previous treatments for at least 1 year and with viral load of less than 20 copies/ml in the last 6 months. In all ART-treated patients the backbone of the treatment was one NRTI or two (Supplementary Table 1). All HIV-infected patients were immune responders. The presence of acquired immunodeficiency syndrome (AIDS), via of transmission and coinfection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) was also registered. In case of coinfection, the degree of liver fibrosis was evaluated by the FibroScan® (Echosens, Paris, France) method. Patients were classified according to the METAVIR scoring system (F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and few septa; F3, numerous septa without cirrhosis; F4, cirrhosis) [10]. CD4+ and CD8+ T cell counts and viral load were measured using flow cytometry (NAVIOS EX, Beckman Coulter) and COBAS 6800 Analyzer (Roche Molecular Systems Inc., Branchburg, New Jersey, USA), respectively, as a clinical procedure in the HUSP. Healthy patients (non-HIV-infected patients) were also recruited as a control group (n = 26). For both HIV-infected patients and controls, the following exclusion criteria were applied: age less than 18 years, patients who do not sign the informed consent, pregnant women, individuals with inflammatory disease in the last 2 months, patients treated with antibiotics, anti-inflammatory drugs, immunosuppressive drugs, statins or probiotics in the last 2 months, individuals with renal insufficiency, patients with neoplasms, individuals with history of intestinal surgery (except for appendectomy or cholecystectomy), inflammatory bowel diseases (IBD), celiac disease, chronic pancreatitis or any other syndrome related to intestinal malabsorption [8]. Patients treated with statins were excluded because it was demonstrated that this therapy can cause gut dysbiosis [11, 12]. Finally, weight, height, waist circumference, systolic and diastolic pressure, alcohol consumption, and smoking habits were also registered from all participants.
Biochemical Parameters, Markers of BT, Inflammation, Cardiovascular Risk, Gut Permeability and Microbiota-Derived Short-Chain Fatty Acids
Blood samples from HIV-infected patients were collected at HUSP and blood samples from the control population were collected at the Center for Biomedical Research of La Rioja (CIBIR). In both cases, samples were collected after 12 h of fasting and were centrifugated and stored at − 80 °C for further analysis. Serum levels of glucose, triglycerides, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), aspartate aminotransferase (GOT/AST) and alanine aminotransferase (GPT/ALT) were measured at HUSP using an AutoAnalyzer (Cobas C702, Roche, Madrid, Spain). Besides, HOMA-IR (homeostatic model assessment-insulin resistance) was calculated as previously described [13]. Enzyme-linked immunosorbent assays (ELISAs) from Merck Millipore (Darmstadt, Germany) and Luminex Screening Assays from R&D (Minneapolis, USA) were used to analyse markers of BT, inflammation and cardiovascular risk in serum samples from HIV-infected patients and healthy participants. All these analyses were performed in duplicate with commercially available kits and according to the manufacturer’s instructions. Specifically, ELISA was performed to determine levels of insulin and Luminex was performed to determine the levels of markers of BT: sCD14 and lipopolysaccharide-binding protein (LBP); inflammation markers: interleukin-6, (IL-6) and tumour necrosis factor alpha, (TNFα); and cardiovascular risk markers: intracellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and plasminogen activator protein (PAI-1). Besides, trimethylamine N-oxide (TMAO), a marker of cardiovascular risk, was quantified by ultra-performance liquid chromatography (UPLC, Nexera Shimadzu) coupled to a triple quadrupole (QQQ) mass spectrometer (MS) detector (SCIEX 3200QTRAP). Moreover, faecal calprotectin levels, a marker of gut permeability, was measured using ELISA from BÜHLMANN (Amherst, USA) according to the manufacturer’s instructions. Finally, several short-chain fatty acids (SCFAs), markers of gut bacterial metabolism, were measured by 7890C Series gas chromatography (GC) coupled to a 7000C Series Triple Quad GC/MS triple quadrupole mass spectrometer (Agilent Technologies Inc., Wilmington, DE, USA) with a multipurpose sampler (MPS) automatized liquid sample injection system (Gerstel GmbH & Co. KG, Mülheim an der Ruhr, Germany).
DNA Extraction from Stool Samples and 16S rRNA Gene Sequencing
Fresh stool samples were received at CIBIR, aliquoted in tubes (150–250 mg) and stored at − 80 °C for further analysis. Then, stool samples were unfrozen, and faecal DNA was extracted using the Real Microbiome Fecal DNA Kit (Durviz Valencia, Spain) following the manufacturer’s instructions. Then, purity, concentration and quality were determined by a Nanodrop spectrophotometer 1000 (Thermo Scientific, USA), a Qubit 3.0 fluorometer (Thermo Fisher Scientific, MA, USA) and a Fragment Analyzer (Agilent, USA). Samples were amplified for the 16S rDNA hypervariable regions V3–V4 and sequencing was performed using an Illumina sequencer (MiSeq, 2 × 300 pb, paired end) at the Genomics & Bioinformatics Core Facility at CIBIR.
The first step in computational analysis was to check the quality of reads using the quality control tool FastQC program. Then, the Qiime2 pipeline [14] was used during the bioinformatic analysis. Firstly, the raw sequences already demultiplexed (mapping the barcodes to the samples they belong to) by the Illumina sequencer were denoised using the DADA2 software. Specifically, the trimming of sequencing adapters and primer regions, the filtering of noisy reads, the dereplication of our sequences to reduce repetition, the joint of paired reads, the identification of amplicon sequence variants (ASVs) at 99% of sequencing similarity and the elimination of chimeras were performed. Secondly, we used the SILVA database [15] trained with the V3–V4 amplification primers using during the wet-lab process to do the taxonomic assignation at 70% confidence. The alpha and beta diversity were analysed: α-diversity is a measure of sample-level species richness, whereas β-diversity describes inter-subject similarity of microbial composition and facilitates the identification of broad differences between samples. The measure of α-diversity was analysed using Observed Features, Chao1 index, Fisher’s alpha, Pielou’s evenness, Shannon index and Simpson index. Observed Features, Chao1 index and Fisher’s alpha are based on richness, Pielou’s evenness is based on evenness and Shannon index and Simpson index are based on diversity (richness + evenness). The measure of β-diversity was analysed using Bray–Curtis and visualized using principal coordinate analysis (PCoA) by R software (version 4.0.5) and R Studio (version 1.4.1105). Finally, the analysis of the differential composition of bacteria was carried out with the analysis of composition of microbiomes (ANCOM) methodology at phylum, order and genus taxonomic levels. This methodology accounts for the underlying structure in the data and is widely used for comparing the composition of microbiomes in two or more populations, with no assumptions of population distribution [16].
Statistical Analysis
Results are presented as mean ± standard error of the man (SEM). Categorical variables were analysed using the Chi-square or Fisher’s exact test. Normal distribution of quantitative variables was checked using the Shapiro–Wilk test. Comparisons between two groups were performed using unpaired t test or U Mann–Whitney depending on the normality of the data. Besides, in the case of longitudinal studies, the comparisons between two groups were performed by paired t test or Wilcoxon regarding the normality of the data. Comparisons between three or more groups were analysed using analysis of variance (ANOVA) followed by Tukey post hoc regardless of the normality of the data. P values less than 0.05 and false discovery rates (FDRs) less than 0.05 were considered as statistically significant. Statistical analysis was performed using GraphPad Prism 8 (GraphPad Prism®, La Jolla, California, USA), R software (version 4.0.5) and R Studio (version 1.4.1105).
Results
Clinical and Demographical Characteristics of Participants
Table 1 shows the main characteristics of the population recruited. ART-treated patients showed nadir CD4 counts of 526.53 ± 56.30 cells/µl. The average time under treatment was of 33.27 ± 5.04 months. Viral load of naïve patients was 622,252.9 ± 341,039.3 copies/ml, whereas ART-treated patients showed indetectable viral load (below 20 copies/ml), as expected. Statistically significant differences were observed between the naïve group and ART-treated patients in CD4 levels (p < 0.01) and CD4/CD8 ratio, both higher in the ART-treated group. Statistically significant differences were observed between the control and the naïve group in terms of gender (p < 0.01), age (p < 0.05), systolic blood pressure (p < 0.05), diastolic blood pressure (p < 0.05) and smoking habits (p < 0.05). In fact, men were less abundant in the control group (34.62%) in contrast to HIV-infected patients (80.00% and 86.67% in naïve and ART-treated patients, respectively). Mean age of the control group was higher than that of the naïve group. However, no difference was observed among the controls and ART-treated patients and also among the naïve and ART-treated patients. Both systolic and diastolic blood pressure were higher in the naïve group compared to the control group. No difference was observed when the controls were compared to ART-treated patients. Of note, none of the naïve HIV-infected patients suffered from hypertension. Thus, these differences could be explained as “white coat hypertension” generated in those patients that have just received the news of being HIV-positive. Smoking habits were also higher in the naïve group and ART-treated group compared to the control group (11.54% vs. 46.67% and 66.67%, respectively). Moreover, no differences were observed either in the mode of transmission or in AIDS events (only one naïve patient suffered from it), nor in the coinfection with HCV or HBV (only two ART-treated patients presented coinfection with HCV and a grade of fibrosis of F0/F1).
Table 1.
Characteristics of healthy uninfected controls and HIV-infected patients (naïve and under ART)
| Control | Naïve | ART-treated | p value | |
|---|---|---|---|---|
| Number of patients | 26 | 15 | 15 | – |
| Gender (men) | 9/26 (34.62%) | 13/15 (86.67%) ** | 12/15 (80.00%) ** | 0.002 |
| Age (years) | 43.58 ± 2.31 | 33.87 ± 2.85 * | 43.67 ± 3.39 | 0.033 |
| BMI (kg/m2) | 24.30 ± 0.69 | 23.23 ± 1.05 | 23.51 ± 0.85 | 0.616 |
| Waist circumference (cm) | 85.35 ± 2.55 | 83.83 ± 2.86 | 85.13 ± 2.15 | 0.916 |
| Systolic blood pressure (mmHg) | 120.58 ± 2.76 | 135.67 ± 5.67 * | 129.73 ± 6.02 | 0.050 |
| Diastolic blood pressure (mmHg) | 72.19 ± 1.94 | 81.87 ± 3.31 * | 77.80 ± 3.24 | 0.035 |
| Alcohol active | 3/26 (11.54%) | 0/15 (0.00%) | 1/15 (6.67%) | 0.578 |
| Smoking active | 3/26 (11.54%) | 7/15 (46.67%) * | 10/15 (66.67%) *** | 0.001 |
| CD4 (cells/µl) | – | 464.07 ± 76.46 | 850.53 ± 101.68 | 0.006 |
| CD4/CD8 ratio | – | 0.53 ± 0.13 | 0.84 ± 0.10 | 0.027 |
| Mode of transmission | – | HS: 6/15 (40.00%) | HS: 7/15 (46.67%) | 0.716 |
| – | MSM: 9/15 (60.00%) | MSM: 7/15 (46.67%) | ||
| – | Parenteral: 0/15 (0.00%) | Parenteral: 1/15 (6.66%) | ||
| AIDS | – | 1/15 (6.67%) | 0/15 (0.00%) | 1 |
| Coinfection with HCV | – | 0/15 (0.00%) | 2/15 (13.33%) | 0.483 |
| Coinfection with HBV | – | 0/15 (0.00%) | 0/15 (0.00%) | 1 |
Qualitative variables are represented in percentage while quantitative variables are represented as mean ± standard error mean. P value refers to the comparison between two (naïve vs. ART) or three (control vs. naïve vs. ART) groups, as appropriate. Statistically significant p values are in italics. Asterisks indicate statistically significant differences with respect to control group (*p < 0.05, **p < 0.01 and ***p < 0.001)
AIDS acquired immunodeficiency syndrome, ART antiretroviral treatment, BMI body mass index, HS heterosexual, MSM men who have sex with men
Table 2 shows the biochemical characterization of the population recruited for this study. Glucose levels and the insulin resistance index, the HOMA-IR, were significantly higher in the naïve group compared to the control (p < 0.05). However, differences were not observed between the controls and ART-treated group, suggesting that ART ameliorates this metabolic disturbance. Besides, triglyceride levels were higher in HIV-infected patients, being more potent in those naïve participants. Although no statistically significant differences were observed in cholesterol levels, a significant reduction on HDL was only observed in naïve patients (p < 0.001 vs. control) but disappeared in ART-treated HIV-infected patients.
Table 2.
Biochemical parameter of healthy, uninfected controls and HIV-infected patients
| Control | Naïve | ART-treated | p value | |
|---|---|---|---|---|
| Glucose (mg/dl) | 87.64 ± 1.77 | 94.73 ± 2.42 * | 93.00 ± 2.20 | 0.039 |
| Insulin (µU/ml) | 9.25 ± 1.54 | 13.16 ± 1.78 | 9.90 ± 0.57 | 0.190 |
| HOMA-IR | 2.03 ± 0.33 | 3.40 ± 0.52 * | 2.19 ± 0.16 | 0.030 |
| Triglycerides (mg/dl) | 73.28 ± 5.21 | 124.47 ± 14.95 ** | 117.87 ± 17.73 * | 0.004 |
| Cholesterol (mg/d) | 184.40 ± 5.66 | 160.27 ± 8.70 | 179.87 ± 8.76 | 0.065 |
| HDL (mg/dl) | 62.42 ± 3.83 | 39.53 ± 2.77 *** | 51.47 ± 3.76 | 0.001 |
| LDL (mg/dl) | 110.26 ± 4.26 | 95.93 ± 7.57 | 99.00 ± 6.47 | 0.167 |
| GOT/AST (U/L) | 20.88 ± 1.62 | 21.29 ± 1.74 | 18.86 ± 1.25 | 0.607 |
| GPT/ALT (U/L) | 18.68 ± 1.60 | 23.00 ± 2.29 | 18.43 ± 1.72 | 0.211 |
Variables are represented as mean ± standard error mean. P value refers to the comparison between three groups (control vs. naïve vs. ART). Statistically significant p values are in italics. Asterisks indicate statistically significant differences with respect to control group (*p < 0.05, **p < 0.01 and ***p < 0.001)
ART antiretroviral treatment, GOT/AST glutamic oxaloacetic transaminase or aspartate aminotransferase, GPT/ALT pyruvic glutamic transaminase or alanine aminotransferase, HDL high-density lipoprotein, HOMA-IR homeostatic model assessment-insulin resistance, LDL low-density lipoprotein
BT, Inflammation, Cardiovascular Risk and Gut Permeability Markers
Figure 1 shows the results obtained from the analysis of BT, inflammation and cardiovascular risk markers in the studied population. HIV infection was accompanied by a significant increase in BT (p < 0.001 for sCD14 and LBP, naïve vs. control), although this increase was completely abolished after 1 year of INSTIs-based treatment (p < 0.01 and p < 0.001 for LBP and sCD14 respectively, ART vs. naïve) (Fig. 1a). Concerning markers of inflammation, no difference was observed in IL-6. However, a significant increase was observed in naïve patients in TNFα levels compared to controls (p < 0.05) that was completely reversed in those patients under INSTIs regimen (Fig. 1b). Regarding the analysis of cardiovascular risk markers, the naïve group presented higher levels of VCAM-1 and PAI-1 compared to controls (p < 0.001 in both cases) while no difference was observed on ICAM-1 and TMAO. The increases observed in VCAM-1 and PAI-1 were clearly reversed in the ART-treated group (p < 0.001 regarding VCAM-1 and p < 0.01 regarding PAI-1, ART vs. naïve) and, consequently, no difference was observed when the ART-treated patients were compared to uninfected volunteers (Fig. 1c). In line with these results, we carried out a small pilot study in which six naïve patients were recruited and samples were also collected after 1 year under treatment. Thus, a longitudinal approach was carried out and, although the sample size was quite small (n = 6), this analysis revealed that systemic levels of VCAM-1 and PAI-1 were significantly reduced after the treatment (p < 0.05 in both cases vs. naïve), corroborating the potent effect of INSTIs on these cardiovascular markers (Supplementary Fig. 1). HIV infection was also accompanied by a statistically significant increase in faecal calprotectin levels, a marker of gut permeability, (p < 0.05 vs. control)—an increase that was not present in ART-treated patients (p = 0.291) (Fig. 2).
Fig. 1.
Levels of bacterial translocation (a), inflammation (b) and cardiovascular risk markers (c) in the studied population compared with the control group, naïve group and ART-treated group. *p < 0.05 and ***p < 0.001 vs. control, ##p < 0.01 and ###p < 0.001 vs. ART. ART antiretroviral treatment, ICAM-1 intracellular adhesion molecule 1, IL-6 interleukin-6, LBP lipopolysaccharide binding protein, PAI-1 plasminogen activator protein 1, sCD14 soluble cluster of differentiation 14, TMAO trimethylamine N-oxide, TNF-alpha tumour necrosis factor alpha, VCAM-1 vascular cell adhesion molecule 1
Fig. 2.
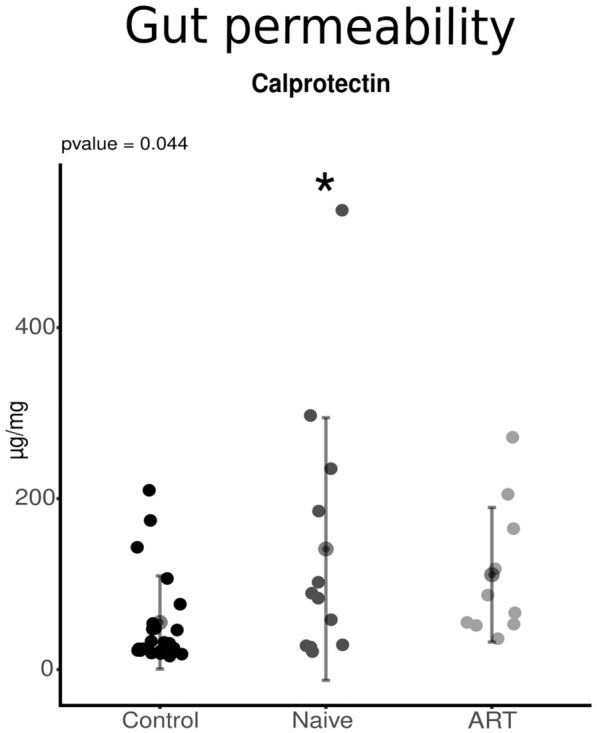
Levels of calprotectin in the studied population compared with the control group, naïve group and ART-treated group. *p < 0.05 vs. control. ART antiretroviral treatment
Gut-Derived Short-Chain Fatty Acids
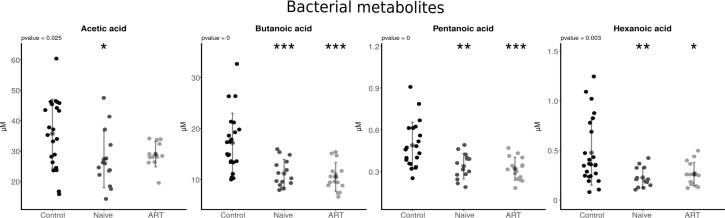
A statistically significant reduction in serum concentration of acetic acid, butanoic acid, pentanoic acid and hexanoic acid was observed in HIV-infected population (both naïve and ART-treated) compared to controls (p < 0.05 to p < 0.001). This reduction was independent of ART as can be observed in Fig. 3, except for acetic acid, where ART seems to reverse such an increase as no significant differences were observed when compared with the controls.
Fig. 3.
Levels of bacterial metabolites in the studied population compared with the control group, naïve group and ART-treated group. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control. ART antiretroviral treatment
Gut Bacteriome Diversity and Composition
Alpha Diversity
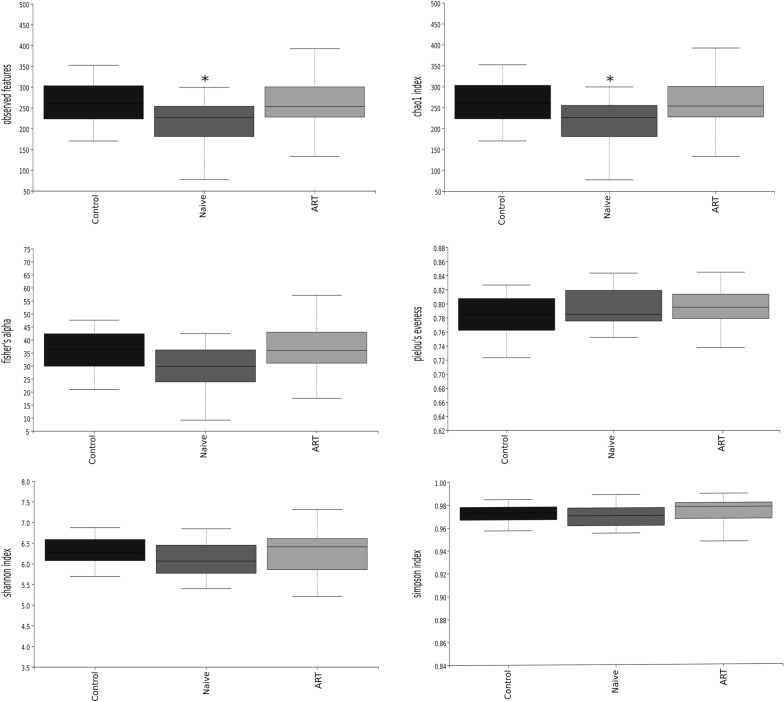
A significant decrease in Observed Features and Chao1 indexes was observed in the naïve group compared to controls (p < 0.05 in both cases). Such a decrease was not present in the ART-treated group when compared to controls, suggesting some kind of reversion due to the treatment (Fig. 4). No statistically significant differences were observed either in Fisher’s alpha or in Pielou’s evenness, nor in Shannon index or in Simpson index.
Fig. 4.
Different indexes of α-diversity of faecal samples of the studied population. *p < 0.05 vs. control. ART antiretroviral treatment
Beta Diversity
Figure 5 shows the PCoA from the studied population. The control group is clearly different from the naïve group (p < 0.01) and ART-treated group (p < 0.01). However, statically significant differences were not observed between the naïve group and ART-treated patients in terms of β-diversity as can be observed in Fig. 5.
Fig. 5.
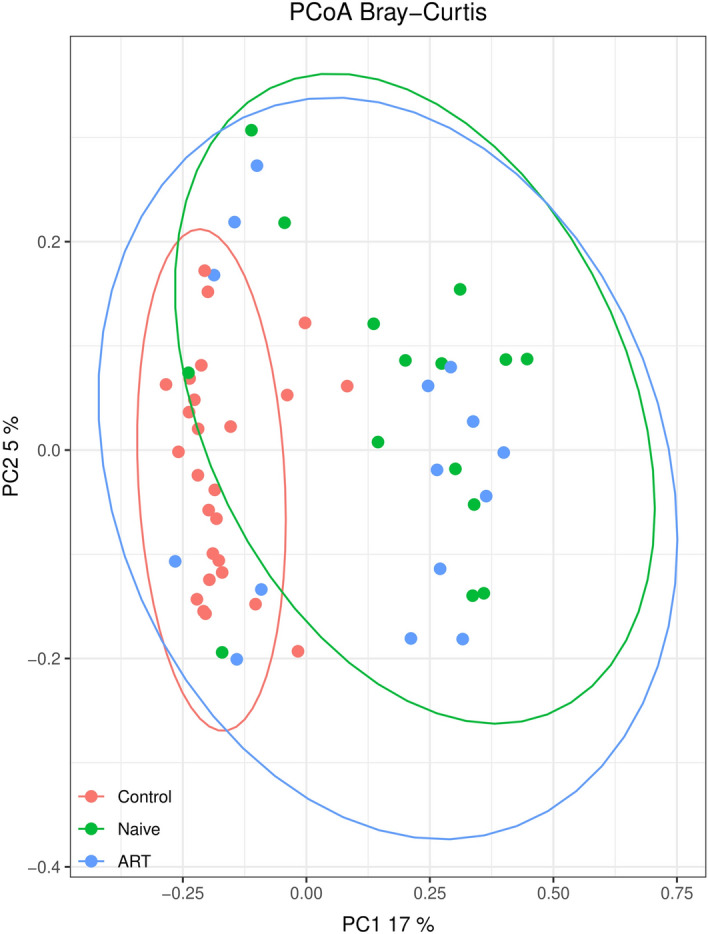
PCoAs of faecal samples from the studied population (accounting for 22% of the total variation [component 1 = 17% and component 2 = 5%]). Results are plotted according to the first two principal components. Each circle represents a sample: red circles represent the uninfected volunteers, green circles represent the naïve group and blue circles represent the ART-treated group. The clustering of samples is represented by their respective 95% confidence interval ellipse. p < 0.05 naïve vs. control and p < 0.05 ART vs. control. ART antiretroviral treatment
Differential Abundance
Concerning GM composition, a total of 16 phyla and 42 orders were detected. The two most abundant phyla in the gut were Bacteroidetes (20.27–64.21%) and Firmicutes (13.57–77.01%). At the order level, Bacteroidales (20.27–64.21%) and Clostridiales (11.41–64.47%) were the most abundant ones.
When comparing controls to naïve patients, increases in phylum level were not revealed. An increase in the order Aeromonadales (phylum Proteobacteria) and in the genus Succinivibrio (order Aeromonadales, phylum Proteobacteria) and Prevotella 2 (order Bacteroidales, phylum Bacteroidetes) were observed in the naïve group compared to controls (Table 3). In contrast, HIV infection was accompanied by a decrease in the phylum Verrucomicrobia and in the genera Erysipelotrichaceae UCG-003 (order Erysipelotrichales, phylum Firmicutes) and Catenibacterium (order Erysipelotrichales, phylum Firmicutes) in the naïve group compared to controls (Table 3). When controls were compared against ART-treated HIV-infected patients, an increase in the phyla Spirochaetes and Cyanobacteria, in the order Aeromonadales (phylum Proteobacteria) and in the genera Succinivibrio (order Aeromonadales, phylum Proteobacteria) and Catenibacterium (order Erysipelotrichales, phylum Firmicutes) were observed in the treated group (Table 3). A decrease in the phyla Bacteroidetes and Actinobacteria in the ART-treated group compared to the control group was also detected, although no specific orders or genera were decreased in this group of patients compared to healthy volunteers (Table 3).
Table 3.
Bacterial taxonomical orders that present a differential abundance in the studied population
| Control vs. | |||||||
|---|---|---|---|---|---|---|---|
| Naïve | ART | ||||||
| Category | Taxonomic group | W | Category | Taxonomic group | W | ||
| Phylum | Spirochaetes | ↑ | 9 | ||||
| Phylum | Cyanobacteria | ↑ | 6 | ||||
| Order | Aeromonadales | ↑ | 41 | Order | Aeromonadales | ↑ | 42 |
| Genus | Succinivibrio | ↑ | 285 | Genus | Succinivibrio | ↑ | 307 |
| Genus | Prevotella 2 | ↑ | 285 | Genus | Catenibacterium | ↑ | 286 |
| Phylum | Verrucomicrobia | ↓ | 9 | Phylum | Bacteroidetes | ↓ | 4 |
| Phylum | Actinobacteria | ↓ | 4 | ||||
| Genus | Erysipelotrichaceae UCG-003 | ↓ | 303 | ||||
| Genus | Catenibacterium | ↓ | 292 | ||||
ART antiretroviral treatment
Discussion
Integrase inhibitors are recommended by international guidelines [17–19] as a key component of ART in the treatment of HIV-infected patients. In particular, their efficacy, tolerability and low drug–drug interaction profile have made them the preferred choice as part of the first-line regimen in treatment-naïve individuals [20]. Here we report that current antiretroviral regimens based on INSTIs are able to reverse the impact of HIV infection on BT, systemic inflammation and bacterial diversity/richness, reaching similar levels to those observed in an uninfected/control population. These results suggest potent beneficial actions of this class of antiretrovirals on the gut and in potential age-related complications such as cardiovascular events. In addition, our study confirms that the results previously observed with raltegravir [8] can be extrapolated to other INSTIs used for treatment of HIV-infected patients.
The BT triggered by HIV infection is associated with subsequent intestinal and systemic inflammation [7] and with the predisposition of HIV-infected patients to comorbidities such as CVD [21]. Several studies have reported elevated serum levels of sCD14 and have demonstrated that this marker independently predict mortality and thrombotic risk [22, 23]. Our study clearly showed that the ART based on INSTIs reduced the levels of sCD14 and LBP, reaching similar serum levels to those observed in control population. An increase in the inflammatory cytokine TNFα in the naïve group, which was not present in the ART-treated group, was also observed in our study. This fact suggests a mild recuperation of the inflammatory state in HIV-infected people under INSTIs therapy. These results are similar to those obtained in an observational study analysing the effects of different INSTIs on markers of inflammation [24]. Thus, this could potentially reduce future HIV-related complications. In fact, we have observed that INSTIs-based treatments are able to reduce cardiovascular risk markers VCAM-1 and PAI-1 which could suggest a reduced risk of CVD, as stated in a previous study [25]. Regarding faecal calprotectin, several studies have shown that its concentrations are highly correlated with histopathological and endoscopic findings in IBD [26] and, in fact, it is used as a measurement of gut permeability and inflammation in IBD [26], although this marker has not been applied to HIV-infected patients yet. In our study, we revealed an increase of faecal calprotectin due to HIV infection that was reversed after INSTIs-based treatment. These results are in contrast with those from Eckard et al. [27] and Ancona et al. [3], who did not detect a recovery of faecal calprotectin levels after ART treatment. These differences could be due to the different treatment used, which could suggest a strong protective role of INSTIs-based treatments in terms of gut permeability.
With respect to gut bacterial composition, our results showed that HIV infection reduces bacterial richness/diversity, as previously demonstrated [28], whereas ART based on INSTIs was able to counteract this process. These results mimic those previously observed by our group with raltegravir [8] and, therefore, point towards a similar trend in all INSTIs-based regimens, confirming a class effect irrespective of the drug used. However, long-term suppressive INSTIs-based ART was not able to completely restore the compositional changes induced by HIV infection in the gut and only partial improvements were observed. Thus, HIV infection was associated with an increase in Aeromonadales order (phylum Proteobacteria) mainly triggered through the increase in genus Succinivibrio (Aeromonadales order constitutes 0.23–26.78% of the bacterial reads and Succinivibrio genus up to 26.78%). This increase was not reversed by the INSTIs and should be thoroughly studied in the future given the fact that previous studies suggested that Succinivibrio could be associated with defects in gastrointestinal functions such as diarrhea and abdominal pain [29–32], although none of the patients included in our study reported such problems. Our results also showed an increase of Prevotella 2 (phylum Bacteroidetes) associated with HIV infection, which was not present in ART-treated patients, suggesting a partial recovery after treatment. This increase observed in Prevotella could be associated with the elevated inflammation observed in naïve patients since increased Prevotella abundance is associated with augmented T helper type 17 (Th17)-mediated mucosal inflammation, which is in line with the marked capacity of Prevotella in driving Th17 immune responses in vitro. Furthermore, Prevotella stimulates epithelial cells to produce interleukin-8 (IL-8), IL-6 and C–C motif chemokine ligand 20 (CCL20), which can promote mucosal Th17 immune responses and neutrophil recruitment [33]. Thus, these results could suggest that HIV increases inflammation (confirmed by the increase in TNFα levels) and INSTIs-based treatments are able to improve this pro-inflammatory effect through the modulation of this bacterial genus, at least in part. More studies are needed in this regard. On the other hand, Verrucomicrobia phylum and Erysipelotrichaceae UCG-003 genus (phylum Firmicutes) were decreased in the naive group. In this context, Akkermansia muciniphila belonging to phylum Verrucomicrobia has been reported to be a sentinel for gut permeability having benefits in HIV-infected people [34]. Moreover, an increase in Spirochaetes and Cyanobacteria phyla and a decrease in Bacteroidetes and Actinobacteria phyla were observed in INSTIs-treated patients when compared to uninfected individuals. These effects were not observed in naïve patients, at least at phylum level. There are few studies analysing the relation of these phyla and HIV infection, but recent studies have shown that spirulina and other compounds extracted from Cyanobacteria can alleviate oxidative stress and inflammation in aspirin-induced gastric ulcer in mice [35] and could even exert anti-HIV activities [36]. Thus, the increase observed after ART treatment could be of great interest from a clinical point of view although more studies are needed. Besides, phylum Bacteroidetes has been reported to include some significant clinical pathogens [37] and has been also found to decrease in INSTI-treated patients in other studies [8, 38], which corroborates our results. Regarding Actinobacteria, this phylum includes some genera with beneficial properties to human health such as Collinsela, related to butyrate production [39], and Bifidobacterium, which has numerous positive health benefits [40]. Thus, the slight decrease observed in ART-treated patients compared to controls should be further addressed in depth. Our study showed a decrease in the concentration of acetic acid, butanoic acid, pentanoic acid (valproic acid) and hexanoic acid (caproic acid) in HIV-infected subjects, including both naïve and ART-treated patients. These SCFAs are generated by the fermentation of dietary fibre by GM and evidence indicates that they are key players in regulating the beneficial effect of dietary fibre and GM on our health [41]. Specifically, a study revealed that acetic acid suppresses colonic inflammation in germ-free (GF) mice [42] and butanoic acid has also been demonstrated to exert important actions related to cellular homeostasis such as anti-inflammatory, antioxidant and anti-carcinogenic functions [43]. On the other hand, hexanoic acid has revealed anti-inflammatory effects and a role in maintaining the integrity of the gastrointestinal epithelial barrier through regulation of mucus production and tight junction expression [44], and was previously shown to protect against dysbiosis and expansion of pathogenic bacteria in animals [45]. The fact that the levels of these SCFAs are not restored after ART treatment is interesting and should be studied further, to evaluate if a longer treatment is able to restore them.
Finally, this study has some limitations. There are some differences between the control group and the HIV-infected groups, such as gender, age and smoking habits, all which factors that could impact on GM. However, both HIV-infected groups were balanced for these factors, so the differences observed between them, which is the main aim of this study, will be independent from the aforementioned factors and could be attributed to INSTIs. Finally, we were not able to reveal if the effects observed on BT, inflammation, cardiovascular risk markers and GM are similar between dolutegravir and bictegravir regimens because of the small number of patients taking bictegravir.
Conclusions
Our study demonstrates that INSTIs, as part of the first-line regimen in the treatment of naïve individuals, partially restore BT, inflammation and gut permeability. However, the effects of these treatments on gut compositional changes are milder, probably because of the strong effects induced by HIV infection. Thus, these INSTIs-based ARTs reverse the actions of HIV infection on BT and subsequent systemic immune activation and probably on disease progression and future age-related complications such as CVD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all participants of this study and physicians involved in patient recruitment.
Funding
This work was supported by Fundación Rioja Salud and Pablo Villoslada-Blanco was granted a predoctoral grant from Consejería de Desarrollo Económico e Innovación (Government of La Rioja). The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Patricia Pérez-Matute and José A. Oteo conceived the study concept. Pilar Blanco-Navarrete, Luis Metola, Valvanera Ibarra and Jorge Alba recruited the patients. Pablo Villoslada-Blanco, María Íñiguez and Emma Recio-Fernández conducted the prosecution of the samples, the measurement of the different markers (BT, inflammation, cardiovascular risk, gut permeability and SCFAs) and the DNA extraction from stools. Pablo Villoslada-Blanco and MdT carried out the 16S rRNA gene sequencing and the bioinformatic analysis. Pablo Villoslada-Blanco and Patricia Pérez-Matute did the statistical analysis. Pablo Villoslada-Blanco wrote the manuscript. All authors read an approved the final manuscript.
Disclosures
Pablo Villoslada-Blanco, Patricia Pérez-Matute, María Íñiguez, Emma Recio-Fernández, Pilar Blanco-Navarrete, Luis Metola, Valvanera Ibarra, Jorge Alba, María de Toro and José A. Oteo all have nothing to disclose.
Compliance with Ethics Guidelines
This study was performed following the Helsinki Declaration and was approved by the Committee for Ethics in Drug Research in La Rioja (CEImLAR) (28 February 2019, reference number 349). All participants provided their written informed consent.
Data Availability
The datasets generated during and/or analysed during the current study are available in the NCBI SRA repository, http://www.ncbi.nlm.nih.gov/bioproject/819232.
Footnotes
Publisher's Note
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on ART across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11:492. [DOI] [PMC free article] [PubMed]
- 2.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1523–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancona G, Merlini E, Tincati C, et al. Long-term suppressive cART is not sufficient to restore intestinal permeability and gut microbiota compositional changes. Front Immunol Front. 2021;12:459. [DOI] [PMC free article] [PubMed]
- 4.Zaidan SM, Leyre L, Bunet R, et al. Upregulation of IL-32 isoforms in virologically-suppressed HIV-infected individuals: potential role in persistent inflammation and transcription from stable HIV-1 reservoirs. J Acquir Immune Defic Syndr. 2019;82:503. doi: 10.1097/QAI.0000000000002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villoslada-Blanco P, Pérez-Matute P, Oteo JA. Lights and shadows of microbiota modulation and cardiovascular risk in HIV patients. Int J Environ Res Public Health. 2021;18:6837. doi: 10.3390/ijerph18136837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Sun J, Wei L, et al. Altered gut microbiota correlate with different immune responses to HAART in HIV-infected individuals. BMC Microbiol. 2021;21:1–12. doi: 10.1186/s12866-020-02074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016;11:182–190. doi: 10.1097/COH.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villanueva-Millán MJ, Pérez-Matute P, Recio-Fernández E, Rosales JML, Oteo JA. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J Int AIDS Soc. 2017;20:21526. doi: 10.7448/IAS.20.1.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imahashi M, Ode H, Kobayashi A, et al. Impact of long-term antiretroviral therapy on gut and oral microbiotas in HIV-1-infected patients. Sci Rep. 2021;11:1–10. doi: 10.1038/s41598-020-80247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-Matute P, Íñiguez M, Villanueva-Millán MJ, et al. Short-term effects of direct-acting antiviral agents on inflammation and gut microbiota in hepatitis C-infected patients. Eur J Intern Med. 2019;67:47–58. doi: 10.1016/j.ejim.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Caparrós-Martín JA, Lareu RR, Ramsay JP, et al. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome. 2017;5:95. doi: 10.1186/s40168-017-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan JA, Skuse PH, Govindarajan K, et al. The influence of rosuvastatin upon the gastrointestinal microbiota and host gene expression profiles. Am J Physiol Circ Physiol. 2017;312:G488–G497. doi: 10.1152/ajpgi.00149.2016. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Qiime2. Available from: https://qiime2.org/. Accessed 17 Oct 2021.
- 15.SILVA. Available from: https://www.arb-silva.de/. Accessed 17 Oct 2021.
- 16.Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis. 2015;26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EEUU HIV guidelines. Available from: https://clinicalinfo.hiv.gov/en/guidelines. Accessed 22 Apr 2022.
- 18.EACS guidelines. Available from: https://eacs.sanfordguide.com/. Accessed 22 Apr 2022.
- 19.GESIDA guidelines. Available from: https://gesida-seimc.org/category/guias-clinicas/. Accessed 22 Apr 2022.
- 20.Richter E, Bornemann L, Korencak M, et al. Reduction of CD8 T cell functionality but not inhibitory capacity by integrase inhibitors. J Virol Am Soc Microbiol. 2022;JVI-01730. [DOI] [PMC free article] [PubMed]
- 21.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quiros-Roldan E, Castelli F, Bonito A, et al. The impact of integrase inhibitor-based regimens on markers of inflammation among HIV naïve patients. Cytokine. 2020;126:154884. doi: 10.1016/j.cyto.2019.154884. [DOI] [PubMed] [Google Scholar]
- 25.O’Halloran JA, Sahrmann J, Butler AM, Olsen MA, Powderly WG. Brief report: integrase strand transfer inhibitors are associated with lower risk of incident cardiovascular disease in people living with HIV. J Acquir Immune Defic Syndr. 2020;84:396–399. doi: 10.1097/QAI.0000000000002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricciuto A, Griffiths AM. Clinical value of fecal calprotectin. Crit Rev Clin Lab Sci. 2019;56:307–320. doi: 10.1080/10408363.2019.1619159. [DOI] [PubMed] [Google Scholar]
- 27.Eckard AR, Hughes HY, Hagood NL, et al. Fecal calprotectin is elevated in HIV and related to systemic inflammation. J Acquir Immune Defic Syndr. 2021;86:231–239. doi: 10.1097/QAI.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuddenham SA, Koay WLA, Zhao N, White JR, Ghanem KG, Sears CL. The impact of human immunodeficiency virus infection on gut microbiota α-diversity: an individual-level meta-analysis. Clin Infect Dis. 2020;70:615–627. doi: 10.1093/cid/ciz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clay PG, Crutchley RD. Noninfectious diarrhea in HIV seropositive individuals: a review of prevalence rates, etiology, and management in the era of combination antiretroviral therapy. Infect Dis Ther. 2014;3:103–122. doi: 10.1007/s40121-014-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Archin NM, Margolis DM. Emerging strategies to deplete the HIV reservoir. Curr Opin Infect Dis. 2014;27:29. doi: 10.1097/QCO.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill A, Balkin A. Risk factors for gastrointestinal adverse events in HIV treated and untreated patients. AIDS Rev. 2009;11:30–38. [PubMed] [Google Scholar]
- 32.Johnson MO, Neilands TB. Coping with HIV treatment side effects: conceptualization, measurement, and linkages. AIDS Behav. 2007;11:575–585. doi: 10.1007/s10461-007-9229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang J, Lin J, Isnard S, et al. The bacterium Akkermansia muciniphila: a sentinel for gut permeability and its relevance to HIV-related inflammation. Front Immunol. 2020;11:645. doi: 10.3389/fimmu.2020.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmoud YI, Abd El-Ghffar EA. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed Pharmacother. 2019;109:314–321. doi: 10.1016/j.biopha.2018.10.118. [DOI] [PubMed] [Google Scholar]
- 36.Schaeffer DJ, Krylov VS. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Saf. 2000;45:208–227. doi: 10.1006/eesa.1999.1862. [DOI] [PubMed] [Google Scholar]
- 37.Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubourg G, Surenaud M, Lévy Y, Hüe S, Raoult D. Microbiome of HIV-infected people. Microb Pathog. 2017;106:85–93. doi: 10.1016/j.micpath.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Qin P, Zou Y, Dai Y, Luo G, Zhang X, Xiao L. Characterization a novel butyric acid-producing bacterium Collinsella aerofaciens Subsp. Shenzhenensis Subsp. Nov. Microorganisms. 2019;7:78. doi: 10.3390/microorganisms7030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol. 2016;7:925. doi: 10.3389/fmicb.2016.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther. 2016;164:144–151. doi: 10.1016/j.pharmthera.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke JM, Young GP, Topping DL, et al. Butyrate delivered by butyrylated starch increases distal colonic epithelial apoptosis in carcinogen-treated rats. Carcinogenesis. 2012;33:197–202. doi: 10.1093/carcin/bgr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saresella M, Marventano I, Barone M, et al. Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Front Immunol. 2020;11:1390. doi: 10.3389/fimmu.2020.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Immerseel F, De Buck J, Boyen F, et al. Medium-chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar Enteritidis. Appl Environ Microbiol. 2004;70:3582–3587. doi: 10.1128/AEM.70.6.3582-3587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the NCBI SRA repository, http://www.ncbi.nlm.nih.gov/bioproject/819232.