Abstract
The application of genotyping to clinical isolates of Cryptosporidium has increased significantly our knowledge and understanding of the distribution and epidemiology of this parasite. However, some methods can be laborious and demand specialist technical expertise. PCR-restriction fragment length polymorphism (RFLP) techniques represent a more rapid and simple method of genotyping to support epidemiological and clinical investigations than conventional DNA analytical techniques. We describe a nested PCR-RFLP technique that identifies polymorphisms in the C. parvum thrombospondin-related adhesive protein gene locus; this method offers a sensitive and specific tool for the confirmation and investigation of disease associated with C. parvum. The potential of this enhanced method is demonstrated by its application to the confirmation and epidemiological investigation of an outbreak of cryptosporidiosis associated with a school visit to an open farm.
The protozoan parasite Cryptosporidium parvum causes self-limiting but often prolonged watery diarrhea in immunocompetent persons but severe illness and invasive infection in immunocompromised patients (3, 18). Recently, several single-nucleotide polymorphisms have been described, together with methods for their determination and analysis that have indicated the genetic relatedness of isolates of C. parvum (5, 7, 9). This information has significantly increased our knowledge and understanding of the distribution and occurrence of this protozoan parasite in both humans and animal host species. The majority of isolates belong to one of two broad genotypes: genotype 1 (or H), generally restricted to humans and a nonhuman primate (14), and genotype 2 (or C), found in both human and animal hosts (12). Other subtypes, and indeed other Cryptosporidium spp., have also been identified in humans with and without underlying immunodeficiencies by various molecular typing techniques (4). Reported methods for establishing genotype may involve technically demanding, time-consuming, or relatively expensive methodologies, such as post-PCR sequencing or the interpretation of complex gel profiles (19). Thus, while such methods may be appropriate as research tools, they are less suitable for large population-based epidemiological surveillance purposes.
We describe a simple and rapid nested PCR for the identification of type-specific polymorphisms in a C. parvum thrombospondin-related adhesive protein (TRAP-C2) gene; the method is based on simple restriction enzyme analysis (12, 16). The TRAP-C2 gene is an example of a well-characterized gene which demonstrates the polymorphic nature of this parasite genome but which has been little used as an epidemiological tool. Further, we describe the application of this method to the investigation of clinical specimens from sporadic and outbreak cases of cryptosporidiosis and explore the potential of typing systems for Cryptosporidium using PCR-restriction fragment length polymorphism (RFLP) techniques.
MATERIALS AND METHODS
Oocyst preparation.
Fresh unfixed patient stool samples containing Cryptosporidium spp. (detected by microscopy in primary testing laboratories) were stored at 4°C prior to oocyst preparation by flotation using saturated sodium chloride (NaCl) solution (13). Briefly, feces were emulsified in deionized water, and the oocysts were separated from the debris by flotation using saturated NaCl solution and centrifugation for 8 min at 1,000 × g. The floating material containing the oocysts was washed with phosphate-buffered saline, and the oocysts were resuspended in 1 ml of deionized water. Oocyst suspensions of previously characterized animal-derived C. parvum genotype 2 (Moredun and Iowa strains) and human-derived genotypes 1 and 2 were also stored at 4°C prior to DNA extraction.
DNA extraction.
A 200-μl sample of prepared oocyst suspension was incubated at 100°C for 60 min, and DNA was extracted using a QIAMP DNA mini kit (QIAGEN Ltd., Crawley, United Kingdom). Purified DNA was stored at −20°C until required.
Amplification of the TRAP-C2 gene.
A nested PCR amplification method was designed de novo based on a single PCR method described previously (12). The C. parvum-specific external primers, 5′-CAT ATT CCC TGT CCC TTG AGT TGT-3′ (CF) and 5′-TGG ACA ACC CAA ATG CAG AC-3′ (CR) (Life Technologies, Glasgow, United Kingdom), generated a 369-bp product. Each of the internal nested primers was designed to be complementary to a region within the 369-bp product generated by primers CF and CR so that they would also amplify the region containing the single-nucleotide polymorphism. The nested primers were designed so that their annealing temperatures would be significantly lower than those for CF and CR so that a multiplex (single-tube) nested PCR protocol could be developed if required. GC content and primer complementation (which can result in primer-dimer formation) were also considered. The internal primer sequences chosen, 5′-GGT AAT TGG TCA CGA-3′ (C2F) and 5′-CCA AGT TCA GGC TTA-3′ (C2R) (Life Technologies, Glasgow, United Kingdom), resulted in the generation of a 266-bp product. The final concentrations of the reaction components for both amplifications were as follows: MgCl2, 1.5 mM; each deoxynucleoside triphosphate, 0.2 mM; and 2 pmol each of primers CF and CR for the primary PCR and primers C2F and C2R for the secondary (nested) PCR. The primary PCR consisted of an initial denaturation step at 94°C for 3 min; 38 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min; and a final extension phase of 10 min at 72°C. The secondary (nested) PCR consisted of an initial denaturation step at 94°C for 3 min; 38 cycles at 94°C for 30 s, 44°C for 30 s, and 72°C for 1 min; and a final extension phase of 10 min at 72°C.
Genotype determination.
Two restriction enzymes were used, HaeIII and BstEII (Promega, Southampton, United Kingdom). The nested PCR product was subjected to digestion at 37°C for 12 to 18 h. The two enzymes used have different recognition sequences, present in only one of the two genotypes under investigation. At position 42 within the C. parvum TRAP-C2 gene, type 1 DNA has the nucleotide cytosine (in italic type), which is part of the recognition sequence for HaeIII: GG CC. At the same position, type 2 DNA has within the sequence GGTCACC the nucleotide thymine (in italic type), which is recognized and therefore digested by the restriction endonuclease BstEII.
Visualization and interpretation.
The HaeIII- and BstEII-digested PCR products were resolved using agarose (3% [wt/vol]) gel electrophoresis (Phorecus; Biogene, Cambridge, United Kingdom). Product size was confirmed by comparison with a DNA molecular-weight-standard marker (Life Technologies). The digested products were visualized using ethidium bromide (0.1 mg/100 ml) and recorded using a digital camera and KDS1D analysis software (Kodak, Rochester, N.Y.).
Evaluation of the nested TRAP-C2 protocol.
The nested PCR protocol was evaluated for specificity by excluding false-positive results using relevant non-C. parvum DNA from two other species of Cryptosporidium (one avian-derived C. baileyi isolate and three human-derived C. meleagridis isolates), DNA from the protozoan parasites Cyclospora cayetanensis and Toxoplasma gondii, and human DNA. In addition, a preparation from a known Cryptosporidium-negative human fecal sample and an amplification-negative control were also tested. In order to standardize our assay, a quality control exercise was performed in which the TRAP-C2 genotype of 122 fecal isolates from sporadic human cases of cryptosporidiosis was compared with that of the diagnostic region of the Cryptosporidium oocyst wall protein (COWP) gene (15).
Investigation of clinical cases and an outbreak of cryptosporidiosis associated with a school visit to a farm.
In addition to the application of the method to fecal specimens from sporadic cases of cryptosporidiosis, the nested PCR TRAP-C2 genotyping scheme was applied to the investigation of an outbreak of cryptosporidiosis among people who had visited an open farm in North Wales during March 1999. Thirteen individuals (12 pupils and 1 teacher from the junior class) from a group of 27 juniors and 3 teachers developed symptoms of diarrhea (9 cases), vomiting (6 cases), and abdominal pain (6 cases) 6 to 8 days after the group had visited the open farm. Specimens of acute-phase feces were submitted for nine of the symptomatic people, and Cryptosporidium spp. were detected by microscopy for eight (seven children and one adult) at Rhyl Public Health Laboratory.
Although facilities at the farm were found to be satisfactory, other risk factors at the school were excluded and animal contact remained the most plausible source of the infection. Nine days after the farm visit, two samples of lamb feces were taken from the floor of the petting area where lambs had been bottle fed by the children. Cryptosporidium spp. were detected in both samples by microscopy at the local laboratory. The eight human fecal specimens and the two lamb fecal samples were typed using the nested PCR TRAP-C2 method in our laboratory.
Other clinical samples.
The TRAP-C2 genotyping method was also used to confirm C. parvum subtypes in nonfecal clinical specimens from patients unrelated to the above outbreak and for whom a specific clinical need for genotyping had been identified, including bronchiolar lavage fluid from an immunosuppressed patient with a pulmonary infection following bone marrow and liver transplantation.
RESULTS
Modification and evaluation of the TRAP-C2 genotyping assay.
Amplification was achieved from DNA extracted from characterized isolates of C. parvum, generating a nested PCR product of the expected size (266 bp). No amplification was obtained from the C. baileyi DNA template or the three C. meleagridis isolates tested. The non-Cryptosporidium sp. DNA did not generate any amplification product, and none of the negative control samples gave a positive result.
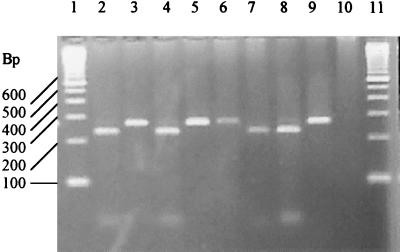
Figure 1 shows the typical results obtained after restriction enzyme digestion of the TRAP-C2 PCR product generated from characterized genotype 1 and genotype 2 isolates. Within our nested PCR, each genotype contains the recognition sequence for either BstEII or HaeIII. Since the recognition sequence for these enzymes is at the same locus on the PCR product, cleavage with either BstEII (genotype 2) or HaeIII (genotype 1) results in two fragments (227 and 39 bp).
FIG. 1.
Agarose gel electrophoresis of BstEII and HaeIII restriction endonuclease cleavage products. Lanes 1 and 11, DNA molecular-weight-standard marker. Lane 2, C. parvum (Moredun strain, genotype 2) BstEII cleavage products. Lane 3, C. parvum (Moredun strain, genotype 2) HaeIII-resistant PCR product. Lane 4, C. parvum (IOWA strain, genotype 2) BstEII cleavage products. Lane 5, C. parvum (IOWA strain, genotype 2) HaeIII-resistant product. Lane 6, C. parvum (human isolate, genotype 1) BstEII-resistant product. Lane 7, C. parvum (human isolate, genotype 1) HaeIII cleavage products. Lane 8, C. parvum (human isolate, genotype 2) BstEII cleavage products. Lane 9, C. parvum (human isolate, genotype 2) HaeIII-resistant product. Lane 10, PCR negative control.
The quality control exercise performed using a subset of isolates collected in our laboratory, in which the TRAP-C2 genotype was compared with that of the COWP gene, showed that in 100% of the 122 isolates analyzed by both methods, the genotypes were identical.
Investigation of clinical cases and an outbreak of cryptosporidiosis associated with a school visit to a farm.
The application of the nested PCR-RFLP TRAP-C2 genotyping method to the confirmed cases of cryptosporidiosis associated with the farm visit confirmed the presence of C. parvum genotype 2 in all seven of the children's feces and in the two lamb fecal samples. The identity of the genotype in the adult sample could not be established, as no PCR product could be generated.
DISCUSSION
We describe the development, evaluation, and application to outbreak investigation of a simple and rapid method that discriminates between the two most common genotypes of C. parvum occurring in human infections by identifying previously reported TRAP-C2-specific polymorphisms (12, 16). The method described here is less time-consuming and hence more readily applicable to the investigation of relatively large sample numbers, such as those associated with suspected outbreaks of unknown origin, than other methods. The information that this method can provide represents valuable microbiological evidence to support both epidemiological investigations of individual outbreaks and national surveillance.
The output generated by this method is simple, and its interpretation is unequivocal, since a simple assessment of whether the enzyme digests at a certain nucleotide position provides the user with a clear identification of the genotype. An advantage of using two restriction endonucleases is that it is also possible to detect the presence of mixed genotypes in a single specimen, since an undigested product in addition to a digested product would be present with both of the restriction enzymes HaeIII and BstEII. This strategy has also proved useful in confirming cases where PCR-RFLP typing using a single enzyme has suggested a mixed infection (unpublished observations).
The nested PCR protocol allows the analysis of samples having a low copy number of the Cryptosporidium genome, permitting the investigation of a greater variety of clinical material, such as bronchioalveolar lavage fluid, sputum, and vomit. Pulmonary infection with C. parvum genotype 1 was diagnosed in an immunosuppressed patient who had recently undergone bone marrow and liver transplantation by application of the method to DNA extracted from bronchiolar lavage fluid. In addition, the nested protocol also offers increased confidence in confirmation of the identity of the PCR product by successful annealing of the interior nested set of primers. Further, confidence in the assay is increased by use of two restriction endonucleases, since cleavage of the PCR product is only possible at specific predicted recognition sequence sites. The nested primers described here were also designed to permit the development of a multiplex (single-tube) nested PCR method, which would offer more rapid analysis without compromising sensitivity or specificity.
In common with other gene-specific polymorphism identification techniques, including those for COWP (15), TRAP-C1 (14), and polythreonine (2), our technique is rapid and simple to perform. The TRAP-C2 assay is largely specific for C. parvum. The external primers have been previously reported to be noncomplementary to and therefore will not amplify C. muris or C. serpentis (17). In addition, we have demonstrated that the protocol reported here does not result in the amplification of C. baileyi or C. meleagridis DNA. Therefore, our method represents a useful addition to the range of molecular tools currently available for the genetic characterization of isolates of C. parvum.
Consistency in genomotyping results for isolates in TRAP-C2 and COWP assays again suggests a lack of genetic recombination and supports the possibility of distinct human pathogenic species (14, 8). Thus, the single-locus approach for genotyping large numbers of samples in high-throughput laboratories is supported. In developing, describing, and evaluating a further means of investigating the genome of C. parvum, weight is added to the prospect that genotypes 1 and 2 may exist as two reproductively isolated populations of the parasite (14).
A main advantage of molecular analyses is in their application to specimens that are unsuitable for investigation by conventional methodologies. The detection of Cryptosporidium spp. in nonfecal samples is difficult and rarely reported, but PCR-RFLP methods readily detect them, have been proven sufficiently sensitive and discriminatory for application to other clinical specimens, and have been applied to bronchoalveolar lavage fluid and sputum for the diagnosis of pulmonary cryptosporidiosis in, for example, transplant recipients. In addition, due to the invasive nature of cryptosporidial infection, the detection of the organisms in gastrointestinal tract tissue is increasingly being requested. In situations such as these, a timely and accurate diagnosis of a cryptosporidial infection is of significant clinical value.
The principal purpose of using the techniques such as the one described here is for the investigation of sources of infection, thus potentially providing a scientific rationale for the epidemiological control or prevention of such outbreaks in the future (10, 11). In the outbreak described here, the farm had provided satisfactory hygiene facilities, and the school had implemented acceptable standards of cleanliness (unpublished observations); thus, descriptive epidemiology and supportive molecular analyses identified the lambs as the most likely source of the infection. However, since our technique allowed us to discriminate only between two C. parvum genotypes, we cannot in practice draw unequivocal conclusions without more discriminative data. Thus, current undertakings by the PHLS Cryptosporidium Reference Unit, in collaboration with others, include the investigation of technologies which will allow more detailed analysis of subtypes within C. parvum genotypes from both human and animal sources; such techniques include analysis of mini- and microsatellite repeats (1) and PCR-coupled single-strand conformation polymorphisms (6) as markers of genetic relatedness.
ACKNOWLEDGMENTS
We acknowledge the following individuals and groups for their contribution to this publication: Graham Williams for collecting the animal samples; Phillip Tynan, Rhyl Public Health Laboratory, for the primary diagnosis; the Outbreak Control Team; the Scottish Parasite Diagnostic Laboratory for providing C. baileyi oocysts; the PHLS Toxoplasma Reference Unit for providing T. gondii DNA; the Swansea Hospitals NHS Trust Molecular Diagnostic Unit for providing human DNA; and Dulwich Public Health Laboratory and Department of Medical Microbiology for submitting the bronchiolar lavage fluid sample for analysis.
REFERENCES
- 1.Caccio S, Homan W, Camilli R, Traldi G, Kortbeek T, Pozio E. A microsatellite marker reveals population heterogeneity within human and animal genotypes of Cryptosporidium parvum. Parasitology. 1999;120:237–244. doi: 10.1017/s0031182099005508. [DOI] [PubMed] [Google Scholar]
- 2.Carraway M, Tzipori S, Widmer G. New RFLP marker in Cryptosporidium parvum identifies mixed parasite populations and genotypic instability in response to host change. Infect Immun. 1997;65:3958–3960. doi: 10.1128/iai.65.9.3958-3960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casemore D P. Human cryptosporidiosis: clinical aspects, epidemiology and control. Proc R Coll Physicians (Edinburgh) 2000;30:287–293. [Google Scholar]
- 4.Chalmers R M, Elwin K. Implication and importance of genotyping Cryptosporidium. Communicable Dis Public Health. 2000;3:155–157. [PubMed] [Google Scholar]
- 5.Clark D P. New insights into human cryptosporidiosis. Clin Microbiol Rev. 1999;12:554–563. doi: 10.1128/cmr.12.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasser R B, Zhu X Q, Caccio S, Chalmers R, Widmer G, Morgan U, Thompson R C A, Pozio E, Browning G F. Genotyping Cryptosporidium parvum by single strand conformation polymorphism analysis of ribosomal and heat shock gene regions. Electrophoresis. 2001;22:433–437. doi: 10.1002/1522-2683(200102)22:3<433::AID-ELPS433>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Gasser R B, O'Donoghue P. Isolation, propagation and characterisation of Cryptosporidium. Int J Parasitol. 1999;29:1379–1413. doi: 10.1016/s0020-7519(99)00113-7. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons C L, Gazzard B G, Ibrahim M A A, Morris-Jones S, Ong C S L, Awad-el-Kariem F M. Correlation between markers of strain variation in Cryptosporidium parvum: evidence of clonality. Parasitol Int. 1988;47:139–147. [Google Scholar]
- 9.Morgan U M, Xiao L, Fayer R, Lal A A, Thompson R C A. Variation in Cryptosporidium: towards a taxonomic revision of the genus. Int J Parasitol. 1999;29:1733–1751. doi: 10.1016/s0020-7519(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 10.Ong C, Eisler D L, Goh S H, Tomblin J, Awad-El-Kariem F M, Beard C B, Xiao L, Sulaiman I, Lal A, Fyfe M, King A, Bowie W R, Isaac-Renton J L. Molecular epidemiology of cryptosporidiosis outbreaks and transmission in British Columbia, Canada. Am J Trop Med Hyg. 1999;61:63–69. doi: 10.4269/ajtmh.1999.61.63. [DOI] [PubMed] [Google Scholar]
- 11.Patel S, Pedraza-Diaz S, McLaughlin J, Casemore D P Outbreak Control Team South and West Devon. Incident management Team and Further Epidemiological and Microbiological Studies Subgroup North Thames 1997. 1998. Molecular characterisation of Cryptosporidium parvum from two large suspected waterborne outbreaks. Communicable Dis Public Health. 1995;1:231–233. [PubMed] [Google Scholar]
- 12.Peng M M, Xiao L, Freeman A R, Arrowood M J, Escalante A A, Weltman A C, Ong C S L, Mackenzie W R, Lal A A, Beard C B. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg Infect Dis. 1997;3:567–573. doi: 10.3201/eid0304.970423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryley J F, Meade R, Hazelhurst J, Robinson T E. Methods in coccidiosis research: separation of oocysts from faeces. Parasitology. 1976;73:311–326. doi: 10.1017/s0031182000046990. [DOI] [PubMed] [Google Scholar]
- 14.Spano F, Putignani L, Crisanti A, Sallicandro P, Morgan U M, Le Blancq S M, Tchack L, Tzipori S, Widmer G. Multilocus genotypic analysis of Cryptosporidium parvum isolates from different hosts and geographical origins. J Clin Microbiol. 1998;36:3255–3259. doi: 10.1128/jcm.36.11.3255-3259.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spano F, Putignani L, McLauchlin J, Casemore D P, Crisanti A. PCR-RFLP analysis of the Cryptosporidium wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150:207–217. doi: 10.1016/s0378-1097(97)00115-8. [DOI] [PubMed] [Google Scholar]
- 16.Sulaiman I M, Xiao L, Yang C, Escalante L, Moore A, Beard C B, Arrowood M J, Lal A A. Differentiating human from animal isolates of Cryptosporidium parvum. Emerg Infect Dis. 1998;4:681–685. doi: 10.3201/eid0404.980424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulaiman I M, Xiao L, Lal A A. Evaluation of Cryptosporidium parvum genotyping techniques. Appl Environ Microbiol. 1999;65:4431–4435. doi: 10.1128/aem.65.10.4431-4435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungar B. Cryptosporidiosis in humans (Homo sapiens) In: Dubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 59–82. [Google Scholar]
- 19.Widmer G. Genetic heterogeneity and PCR detection of Cryptosporidium parvum, p 223–239. In: Baker J R, Muller R, Rollinson D, Tzipori S, editors. Opportunistic protozoa in humans. San Diego, Calif: Academic Press, Inc.; 1998. [DOI] [PubMed] [Google Scholar]