Abstract
Context
Pheochromocytomas and paragangliomas (PPGLs) are known to be rare. However, there is scant literature reporting their epidemiology, particularly whether the diagnosis of PPGL has increased with advances in medical imaging and biochemical and genetic testing.
Objective
The primary objective of this systematic review was to determine the annual incidence of PPGLs and change over time.
Design
A systematic review was performed. Medline, Embase, PubMed, and Web of Science Core Collection databases were searched to identify studies reporting PPGL incidence. Studies were eligible for inclusion from the database’s inception until August 30, 2021.
Results
A total of 6109 manuscripts were identified; 2282 duplicates were excluded, and a further 3815 papers were excluded after abstract and/or full text review. Twelve studies were included in the final review. The incidence of PPGL ranged from 0.04 to 0.95 cases per 100 000 per year. Incidence increased over time, from approximately 0.2/100,000 individuals in studies performed before 2000, to approximately 0.6/100,000 in studies undertaken after 2010. The mode of diagnosis changed over the same time period, with more patients diagnosed from incidental imaging findings, and fewer at autopsy or from symptoms.
Conclusion
The annual incidence of PPGL has increased over time. Much of this increase is likely from incidental identification of tumors on imaging. However, the epidemiology of PPGL remains understudied, in particular, in associations with altitude, ethnicity, and genetics. To improve early detection and management guidelines, these gaps should be addressed.
Keywords: pheochromocytoma, incidence, paraganglioma, epidemiology
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors originating from the chromaffin cells of the autonomic nervous system [1]. Pheochromocytomas (PCCs) originate from the adrenal medulla, whereas paragangliomas (PGLs) arise from extra-adrenal paraganglia [2]. Paragangliomas are divided into 2 subtypes: sympathetic paragangliomas, occurring along the sympathetic trunk, and head and neck paragangliomas (HNPGL); the latter named because the majority of parasympathetic paragangliomas arise in the head and neck region [2].
Detailed epidemiological studies on PPGLs are scarce, with most conducted before the era of modern imaging and improvements in biochemical analysis. One of the oldest and most frequently cited studies, by Beard et al of patients from Rochester, Minnesota, with PPGL identified from 1950 to 1979, reported an annual PPGL incidence of 0.95/100,000 [3]. In comparison, incidence rates reported in other studies from a similar time period were far lower, between 0.04 and 0.21/100,000 [4-7], suggesting that either Rochester had an unusually high incidence of PPGL or that PPGLs may be underdiagnosed in other centers.
Over the past 5 decades, there have been significant advances in clinical medicine. For example, there is now widespread availability of high-resolution anatomical imaging and a variety of functional imaging options including longstanding techniques such as I-123 metaiodobenzylguanidine scintigraphy, and more recent positron emission tomography scanning options including 18F-fluoro-2 deoxy-D-glucose, 18-F-flurodihydroxyphenylalanine, and gallium-68 Dotatate [8, 9]. The sensitivity of biochemical detection has also improved with the development of assays for the detection of catecholamine metabolites [10]. With these advances, improved detection of PPGL is expected, and therefore higher incidence rates compared with those reported in the past century [11].
Particularly over the past 2 decades there have been marked advances in our knowledge of the genetics of PPGL [12, 13]. An increased number of germline susceptibility genes have been identified, genetic testing is more widely available, and there is an increased awareness of the importance of screening those with a known PPGL susceptibility gene. Nearly one-half of patients with PPGL harbor a germline mutation in 1 of the PPGL susceptibility genes [13, 14]. Patients with a genetic predisposition often present younger and, particularly if identified from surveillance because of a known familial germline mutation, are more likely to be asymptomatic compared with those with sporadic tumors [9]. However, geographical differences in availability and uptake of screening and the presence of founder mutations in certain regions, such as the “Black Forest” VHL mutation [15], the SDHx Dutch founder mutations [16], and the Trentino SDHD mutation [17], may result in different epidemiological patterns.
A clear understanding of the epidemiology of PPGLs is needed, especially with the recent advances in screening and investigation methods. Additionally, studying the epidemiology of the disease can help determine whether changes to the current guidelines are needed. The primary objective of this systematic review was to determine the annual incidence of PPGLs and determine any change in incidence over time.
Methods
This systematic review was performed following the PRISMA guideline [18]. Medline, Embase, PubMed, and Web of Science Core Collection databases were searched to identify manuscripts and conference abstracts using the following terms: pheochromocytoma, phaeochromocytoma, chromaffin cell, adrenal medulla, paraganglioma, chemodectoma, carotid body tumor, carotid body tumour, glomus cell tumor, glomus cell tumour, epidemiology, and incidence (see Table 1 for details). There was no specific timeframe set; studies were eligible for inclusion from the database’s inception until the August 30, 2021. Descriptive, observational studies published in English reporting original data on the annual incidence of PPGL within a defined population were eligible for inclusion. Studies that did not report the incidence rates of PPGL were excluded. Studies were excluded if they only reported malignant/metastatic PPGLs or only patients identified at autopsy. Single-center surgical series without a defined catchment area were excluded.
Table 1.
Search terms used for systematic review
| Database | Search term | Filters | Date of search | Results |
|---|---|---|---|---|
| Web of Science | ((ALL = (Pheochromocytoma OR phaeochromocytoma OR chromaffin cell OR adrenal medulla)) OR((ALL = (paraganglioma OR chemodectoma OR carotid body tumour OR carotid body tumor OR glomus cell tumour OR glomus cell tumor)) AND ALL = (epidemiology OR incidence OR prevalence) | English, human only | August 30, 2021 | 223 |
| PubMed | (((Pheochromocytoma OR phaeochromocytoma OR adrenal medulla* OR chromaffin cell) OR (Paraganglioma OR chemodectoma OR Carotid body tumo* OR glomus cell tumo*))) AND (Epidemiology OR incidence OR prevalence) | Human, English | August 30, 2021 | 1831 |
| Ovid (Medline and Embase) | 1. (pheochromocytoma or phaeochromocytoma or Adrenal medulla* or chromaffin cell).mp. [mp = ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui,sy] 2. (paraganglioma or chemodectoma or Glomus cell tumour or Glomus cell tumor or carotid body tumo*).mp. [mp = ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui,sy] 3. (Epidemiology or incidence or prevalence).mp. [mp = ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui,sy] 4. 1 or 2 5. 3 and 4 6. Remove duplicates from 5 7. Limit 6 to English language 8. Limit 7 to human |
August 30, 2021 | 76 710 22 996 5 955 315 92 794 4770 3625 3202 2794 |
Three authors (A.A., V.B., and M.S.E.) independently assessed the eligibility of all papers at each step. Following independent review, consensus was reached through discussion. Titles and abstracts were screened first followed by assessment of the complete text, and relevant articles included. References of included articles were searched for relevant papers, and those identified were evaluated for eligibility based on the same criteria. A.A. extracted data, using Microsoft Excel for Mac (version 16.56) on the following categories: study design, timeline, data source, country, postmortem cases, primary tumor, disease stage, number of cases, demographics of study participants (age and sex), tumor size, and annual incidence rates. M.S.E. and V.B. reviewed the accuracy of the extracted data. To assess the risk of bias in the included studies, we used a tool developed to appraise prevalence and incidence studies [19].
Results
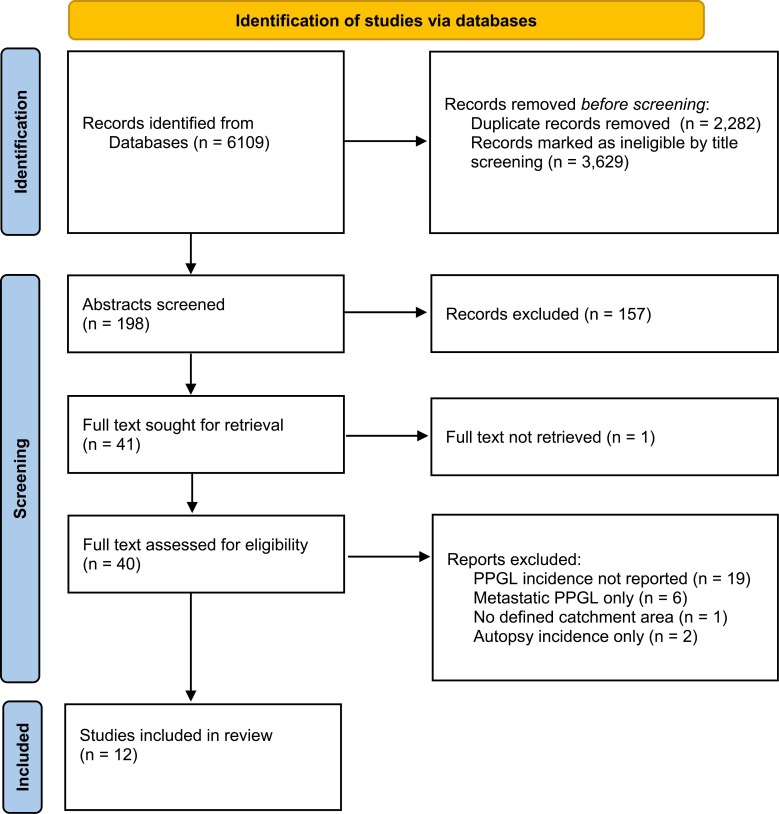
The literature review yielded 6109 titles; 2282 duplicates were removed. Upon systematic screening of the remaining titles, abstracts, and full texts, an additional 3815 papers were excluded (Fig. 1). The full text of 1 article was unable to be located despite extensive efforts, including contacting the study authors. The remaining 12 studies were included in this review [3-7, 11, 20-25].
Figure 1.
PRISMA flow chart.
The data collection period ranged from 1949 to 2019. Eight studies collected information from 1 or more registry-based systems [3, 5, 6, 11, 21-24] and 1 study did not state how patients were identified [20]. In 5 of the 8 registry-based systems, patients were identified from national registries. One group used 3 registries (Danish National Patient Registry, Danish National Pathology Registry, and Danish National Registry of Causes of Death) [21], whereas the remaining studies used a single registry [5, 6, 11, 24]. Of these, 1 was a national cancer registry [5], 1 was a pathology registry [11], 1 a national registry of hospital patients [6], and 1 a national insurance registry [24]. Of the registry-based studies, 2 sent additional questionnaires to departments to obtain additional cases and information and confirm the diagnosis [5, 6]. The remaining 3 registry-based studies were single center and used either hospital medical records system [3, 22] or a mixed approach of surgical pathology and surgical adrenalectomy lists and diagnostic codes [23]. Two studies were questionnaire-based [4, 7]. De Graeff and Horak sent a questionnaire to 28 pathology institutes, of which 23 responded [4]. Similarly, Hartley and Perry-Keene sent letters to Endocrine Society colleagues and pathology centers throughout Queensland, Australia [7]. Leung et al collected information using a combination of administrative health data, chart reviews, diagnostic imaging, anatomical pathology, and autopsy records [25]. Four studies were conducted in single centers (Rochester, Minnesota, USA [3]; South Galicia, Spain [22]; Newfoundland, Canada [23]; and Northern Ireland [20]). Overall, using the bias assessment tool, studies were deemed likely to have a low risk of bias with the exception being that of Kim et al [24], who obtained anonymous data; therefore, accuracy of the data was unable to be confirmed.
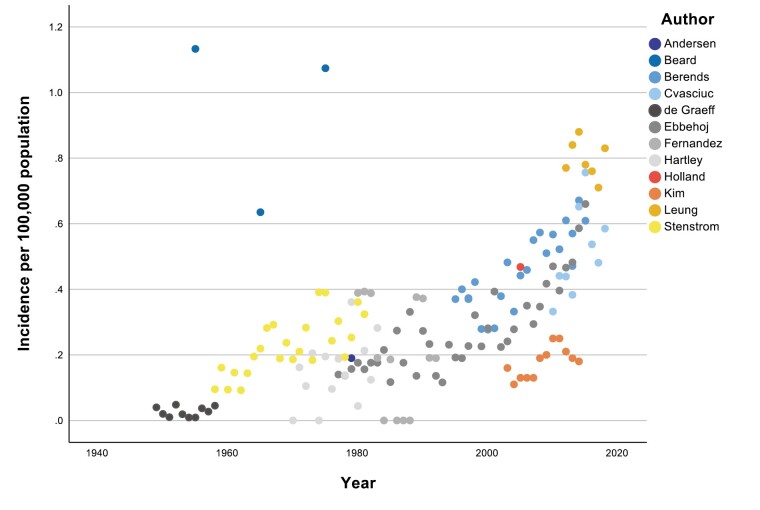
Included studies were divided into 2 groups based on the last year of data collection (Table 2). Six studies completed data collection between 1959 and 1992 [3-7, 22], whereas a further 6 studies completed data collection between 2010 and 2019 [11, 20, 21, 23-25]. Annual incidence rates (per 100,000 individuals per annum) were available for 9 studies [4, 5, 7, 11, 20-22, 24, 25]. For the other 3 studies, 5-year data were available for 1 [6] and 10-year data periods for 2 studies [3, 23]. For these 3 studies, a mean incidence was calculated for the midpoint of the study period. PPGL incidence rates over time for the 12 studies are shown in Fig. 2.
Table 2.
Characteristics of 12 included studies reporting the incidence of phaeochromocytoma and paraganglioma
| First author | Study design | Period of data collection | Country (place) | Number of cases | Demographics of study participants | PPGL incidence per 100,000 per annum |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCC | PGL total | HNPGL | Total | Male (n) | Female (n) | Age range | Median age | Mean age | |||||
| De Graeff [4] | Retrospective (nationwide) | 1949-1959 | Netherlands | 35 | 5 | 0 | 44 | 20 | 24 | 8-74 | 44 | 42.48 | 0.042 |
| Ebbehoja [21] | Retrospective (nationwide) | 1977-2006 | Denmark | NS | NS | NS | 339b | 153 | 186 | <25-75+ | 56.2 | 0.14c | |
| Beard [3] | Retrospective (single center) |
1950-1979 | Rochester, Minnesota, USA | NS | NS | NS | 13 | 7 | 6 | 21-77 | 54 | 53.1 | 0.95 |
| Stenstrom [5] | Retrospective (nationwide) | 1958-1981 | Sweden | 344 | 95 | NSd | 439 | 184 | 255 | 20-90+ | 55.8 | 0.21 | |
| Andersen [6] | Retrospective (nationwide) | 1977-1981 | Denmark | 45 | 2 | NS | 47 | 27 | 20 | 15-81 | 45 F 50 M |
0.19 | |
| Hartley [7] | Retrospective (multicenter) | 1970-1983 | Queensland, Australia | 41 | 5 | 0 | 46 | 25 | 21 | 9-80+ | 44.95 | 0.155 | |
| Fernandez- Calvet [22] | Prospective (single center) |
1980-1992 | South Galicia, Spain | 13 | 1 | NS | 14 | 6 | 8 | 21-65 | 43 | 0.206 | |
| Berendse [11] | Retrospective (nationwide) | 1995-1999 | Netherlands | 1210 | 274 | 0 | 1493f | 821 | 672 | 0-88 | 51 | 0.37c | |
| Holland [23] | Retrospective single center |
2001-2010 | Canada | 23 | 1 | NS | 24 | 13 | 11 | 13-76 | 55.6 33.6g |
0.47 | |
| Kim [24] | Retrospective (nationwide) | 2003-2014 | Korea | NS | NS | NS | 1048 | 517 | 528 | 0-80 | 47.6 | 0.18c | |
| Berendse [11] | Retrospective (nationwide) | 2011-2015 | Netherlands | 1210 | 274 | 0 | 1493f | 821 | 672 | 0-88 | 51 | 0.57c | |
| Ebbehoja [21] | Retrospective (nationwide) | 2007-2015 | Denmark | NS | NS | NS | 228 | 99 | 129 | <25-75+ | 56.2 | 0.66c | |
| Cvasciuc [20] | Retrospective (single center) | 2010-2019 | Northern Ireland | 73 | 13 | NS | 86 | 41 | 45 | 28-83 | 54.5 | 0.53 | |
| Leung [25] | Retrospective (multicentre) | 2012-2019 | Alberta, Canada | 118 | 121 | 93 | 239 | 97 | 142 | 0-80+ | 55 | 0.66 |
Abbreviations: F, female; M, male; NS, not stated; PCC, pheochromocytoma; PGL, paraganglioma; PPGL, pheochromocytoma and paraganglioma.
aSame study but incidence reported for 1977 and 2015.
bOnly 192 of 567 had clinical data available, so separation into PCC and PGL (including head and neck PGL) not available.
cAge-specific incidence rate.
dBreakdown by site: 61 abdominal, 22 peripheral nerve, 3 intrathoracic, 3 urinary bladder, and 6 miscellaneous.
eSame study but incidence reported over 2 time periods.
f9 patients had both.
gMean age for familial cases.
Figure 2.
Annual incidence of PPGL 1949 to 2018.
From the 12 studies, a total of 4060 cases were included. Of these cases, 2047 (50.4%) were female. Age was variably reported with the mean or median age ranging from 43 to 56.2 years. Berends et al reported a linear increase in age at presentation with PCC from approximately age 45 years in 1995 to almost age 55 years by 2015 [11]. Ebbehoj et al reported a younger median age for those diagnosed because of genetic causes at 34.3 years compared with 63 years for those diagnosed from imaging for cancer [21], with similar findings reported by other groups when familial cases were compared to either symptomatic apparently sporadic or incidentally identified tumors [20, 23].
Of the studies that clearly detailed the numbers of PCC and PGL, the majority of tumors arose in the adrenals (2105 vs 553 extra-adrenal). In most studies, the incidence of PCCs were higher than that of PGLs. For example, in 8 studies, PCCs made up 78.4% to 95.8% of the total PPGLs [4-7, 11, 20, 22, 23]. In only 1 study was the proportion of PGLs higher than that of PCCs at 50.6% [25]. For most studies, the number of HNPGLs was not clearly documented (Table 2).
Patients diagnosed at autopsy were included in 9 of the 12 studies [3-7, 11, 21, 22, 25]. Autopsy rates were highly variable, ranging from > 50% of deaths in the Rochester study [3] to less than 10% of deaths in a recent Canadian study [25]. Table 3 reports the proportion of PPGL diagnosed at autopsy (Table 3), with the proportion of cases diagnosed by autopsy ranging from 42% in earlier studies [5] to < 1% in more recent times [21, 25]. For all studies apart from that of Ebbehof et al [21], it was unclear whether the mode of discovery for any of the PPGLs was due to familial screening. Because of the time periods included, genetic testing was variably reported, but some studies in the pregenetic testing era had high rates of apparent clinically familial disease [22]. Ebbehoj et al reported that almost one-half of all PPGL patients (46.8%) had not undergone genetic testing, although this improved over the course of the period studied [21]. Of the 86 patients identified by Cvasciuc et al, 46 underwent genetic testing, with 52% of these being identified as having an underlying germline mutation [20].
Table 3.
Studies including autopsy-identified phaeochromocytoma and paraganglioma
| First author | Period of data collection | Total cases | Autopsy-diagnosed cases N (% of total cases) |
Symptomatic N (% of total cases) |
Incidental N (% of total cases) |
Familial screening |
|---|---|---|---|---|---|---|
| De Graeff [4] | 1949-1959 | 44 | 16 (36.4%) | 27 (61%) | 1 (2.3%)a | -b |
| Beard [3] | 1950-1979 | 13 | 5 (38.5%) | 2 (15%) | 4 (31%)a | 2c |
| Stenstrom [5] | 1958-1981 | 439 | 184 (41.9%) | NS | NS | NS |
| Andersen [6] | 1977-1981 | 47 | 8 (17.0%) | 36 (77%) | 3 (6.4%)a | 5c |
| Hartley [7] | 1970-1983 | 46 | 7 (15.2%) | NS | NS | NS |
| Ebbehoj [21]h | 1977-1986 | 42* | 10 (24.4%) | 21 (50%)d | 5 (11.9%) | 3 |
| Fernandez-Calvet [22] | 1980-1992 | 14 | 1 (7.1%) | 11 (78.6%) | 2 (14%)a | 5c |
| Ebbehoj [21]h | 1987-1996 1997-2006 |
27* 44* |
7 (25.9%) 7 (15.9%) |
13 (48.1%)e 20 (45.5%) |
2 (7.4%) 12 (27.3%)e |
1 2 |
| Berends [11] | 1995-2015 | 1493 | 72 (4.8%) | NS | NS | NS |
| Ebbehoj [21]h | 2007-2015 | 79f | 0 (0%) | 19 (24.1%) | 44 (55.7%)g | 10 |
| Leung [25] | 2012-2019 | 239 | 1 (0.4%) | NS | NS | NS |
Abbreviation: NS, not stated.
aIncidental diagnosis during surgery for another reason.
bNo apparent family screening but 7 cases appear to be likely familial including 3 with neurofibromatosis.
cFamilial cases reported but method of diagnosis unclear.
dIncluded cases with paroxysmal symptoms and hypertension, an additional 2 patients were reported to have “other” as mode of discovery.
eIncluded cases with paroxysmal symptoms and hypertension, an additional 3 patients were reported to have “other” as mode of discovery.
fOnly 192 of the entire 567 case cohort had clinical information available (42/87 for 1977-1986, 27/104 for 1987-1996, 44/148 for 1997-2006, and 79/228 for 2007-2015 time periods, respectively).
gIncluded cases with paroxysmal symptoms and hypertension, an additional 6 patients were reported to have “other” as mode of discovery.
hSame study but different timepoints.
Tumor size was only clearly documented in 4 studies [11, 21, 23, 25]. Berends et al reported a median PCC diameter of 4 cm and demonstrated a linear decrease of median PCC diameter between 1995 and 2015 [11]. Leung et al reported a median diameter of PPGLs as 3.2 cm [25]. Holland et al reported a mean tumor size of 4.5 cm for sporadic tumors compared with 3. 2cm for familial tumors with a wide overlap between both groups [23]. Ebbehof et al reported a median tumor size of 4.5 cm for the cohort with clinical data available and an increased proportion of patients over time being diagnosed with smaller tumors (ie, <4 cm) [21].
Six of the 12 studies [4, 6, 7, 22-24] reported the number of metastatic PPGL cases with the proportion of patients with metastatic disease ranging from 4.2% (Holland et al) to 17.7% (Kim et al) of total cases.
Three additional studies had a robust design and reported useful information on the incidence of PPGLs but did not meet the inclusion criteria (Table 4). Dua et al reported an increase of 37% in PGL incidence from 1998-2000 to 2009-2011 [26]. This study was excluded because only carotid body PGLs were included. Ebbehoj et al comprehensively reported the incidence of adrenal tumors, including PCC, in Olmsted county, Minnesota; however, it was excluded because PGLs were not included [27]. Of note, this study was performed in the same region as that of Beard et al, but showed a lower incidence (0.4/100,000 compared with 0.95/100,000, respectively) [3, 27]. Takayanagi et al reported the epidemiology of adrenal disorders in Japan but undertook a survey-based method with the results extrapolated to estimate the total number of patients with PCC but did not give an estimate of the incidence [28].
Table 4.
Well-conducted studies that did not meet the inclusion criteria
| First author | Reason for exclusion | Study design | Period of data collection | Country (Place) | Number of cases | Demographics of study participants | PPGL incidence per 100,000/ annum | PGL incidence per 100,000/ annum | PCC incidence per 100,000/ annum | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCC | PGL | Total | M (n) | F (n) | Age range | Median age | Mean age | ||||||||
| Dua [26] | Carotid body PGLs only | Retrospective (nationwide) | 1998-2011 | US | 684 | 684 | 271 | 413 | 53.8 | 1998 0.07 2011 0.1 |
- | ||||
| Ebbehoj [27] | PCCs only | Retrospective (multicentre) | 1995-2017 | Olmsted County, MN, USA | 14 | - | 14 | 5 | 9 | 26-70 | 46 | - | 0.4 | ||
| Takayanagi [28] | Survey | Nationwide | 1997 | Japan | 1030a | NR | 1030a | - | - | - |
Abbreviations: M, male; F, female; PCC, pheochromocytoma; PGL, paraganglioma; PPGL, pheochromocytoma and paraganglioma.
aEstimated number.
Discussion
The purpose of this systematic review was to compare studies of PPGL incidence and identify trends over time. The main finding from this systematic review is the increase in PPGL incidence over time, with the mean incidence increasing from 0.19/100,000 per annum in studies after excluding 1 outlier [3] performed before 2000 [4-6] to 0.58/100,000 per annum after excluding 1 outlier [24] in the studies after 2010 [11, 20, 21, 23, 25]. Outlying studies are discussed in the following section.
There are several potential reasons for this increase. First, there has been a substantial rise in adrenal incidentaloma detection over the years because of increasing utilization of noninvasive imaging modalities in clinical practice. For example, in the United States, computed tomography scans increased from approximately 14.4 scans per 1000 population in 1980-1982 to approximately 225 per 1000 population in 2006 [29]. Similarly, magnetic resonance imaging scanning in the United States increased from 34.3 scans per 1000 population in 1995 to 128 per 1000 population in 2019 [30]. As such, incidental lesions (adrenal or extra-adrenal) may contribute to the increase in incidence of PPGL, despite incidentally identified adrenal lesions often are not assessed according to recommended guidelines [31]. This is supported by the finding of Ebbehoj et al, in which more than one-half of the PPGLs between 2007 and 2015 were identified from imaging (adrenal incidentalomas or cancer imaging) with only 15.2% being diagnosed from paroxysmal symptoms and none at autopsy [21]. In comparison, those diagnosed before 1986 were diagnosed because of symptoms in approximately 50% of cases (including as a consequence of investigating possible secondary hypertension) and one-quarter of patients were diagnosed at autopsy [21]. Also of note, Ebbehoj et al reported that the standardized incidence rate of adrenal tumors (all subtypes combined) in Olmsted County, Minnesota, USA, increased from 4.4/100.000 person-years in 1995 to 47.8/100,000 person-years in 2017, predominantly from the increase in incidentally detected adrenal lesions [27]. Although the rise in PPGL incidence over time shown here is less marked than the 10-fold increase in adrenal tumour demonstrated by Ebbehoj et al [27], this is likely influenced by the lower autopsy rates in more recent times offsetting the increase in PPGL identified by imaging. This is important because, historically, many patients with PPGL were only diagnosed postmortem. For example, Stenstrom et al reported that 42% of patients were diagnosed at autopsy [5]. The very high rates of autopsy (>50%) also likely contributes to the very high PPGL incidence of 0.95/100,000 in the study published in 1983 by Beard et al, where 5/11 cases were diagnosed at autopsy [3]. There has been a marked reduction in autopsy rates from > 70% in some centers before 1970 to < 1% in some centers in 2013 [32]. The reasons for this are likely to be multifactorial and complex including social, cultural, legal, and financial.
In addition to advances in medical imaging, there have also been improvements in biochemical testing. Plasma-free metanephrine measurements are now widely available. They have a very high sensitivity for the diagnosis of functioning PPGL [33], making biochemical diagnosis of these tumors less challenging than previously because of the stability of these metabolites compared with plasma catecholamines. A further potential explanation for the increase in diagnosis of PPGL is the advances in knowledge of the high rates of underlying germline mutations in patients with PPGL, an increase of genetic testing, and surveillance of at-risk family members, particularly over the past decade [34]. However, it has been observed that screening and surveillance guidelines have not been widely implemented, and in some centers/countries may be limited by cost [34]. Even though PPGL genetics is being increasingly studied, most of the studies included in this review provide limited information on hereditary PPGLs.
There remain difficulties in the classification of malignant PPGL. In the absence of metastatic disease, there are no histological features that can confidently differentiate malignant from benign disease. As such, the term “malignant” was replaced with “metastatic” in 2017 [2]. Only 6 studies in this review reported the rates of metastatic disease [4, 6, 7, 22-24].
Data on tumor size was limited; therefore, it is difficult to interpret the data based on 4 studies with findings from Berends et al suggesting a possible decrease in size over time [11]. This could be again contributed to by advances in imaging, biochemical testing, and surveillance of patients known to harbor an underlying germline mutation. The possible increase in age at presentation reported by Berends et al [11] may also be explained in the context of increasing adrenal incidentaloma detection.
The results showed PCC preponderance in all but 1 of the studies [25]. This is likely because of Leung et al also including patients with HNPGLs, which make up to 20% of all PGLs [2], whereas the other 3 recent studies excluded this group of patients. This is, however, difficult to apply to the earlier 6 studies because those were published before the formal differentiation between PGLs and PCCs in 2004 [35], and it is unclear from those papers whether any patients with HNPGLs were included.
A recent meta-regression analysis reported the incidence of PPGLs but in the context of altitude and showed that increase in altitude is associated with PPGLs rather than time [36]. However, the findings of that study have been queried because of various limitations, including noninclusion of several seemingly relevant papers [37]. A limitation of our current review is the lack of information on not only altitude at the time of diagnosis but also altitude over the lifespan of the individual, the latter potentially being more relevant to tumorigenesis.
Race was rarely reported. There are very limited data on the association of race with PPGL incidence. Hartley et al reported a far higher incidence in Europeans compared with Aborigines and Pacific Islanders in Queensland [7], but in that era, access to health care was likely to be vastly different between these groups, likely leading to underdiagnosis in the latter 2 groups. Additionally, all but 1 study [24] was done in countries where the majority of the population are of European ancestry. To gain a better understanding of the role of race and PPGL, we need more studies from non-European countries and careful documentation of either patient race or ethnicity.
Two studies from this review appear to be outliers. The first is the study by Kim et al, which showed a lower rate of PPGL than expected but a higher rate of malignant disease [24]. This was the only study of patients predominantly not of European ancestry, and so may reflect a real difference in this population. However, the methodology was different from other studies using health insurance database rather than the more commonly used hospital discharges or surgical/pathology databases. Further work in this and other ethnically diverse regions would be helpful to clarify if there are marked differences according to geography or race, as potentially suggested by the findings from this group.
The other outlier is that of Beard et al, demonstrating a much higher incidence of PPGL than others in the same era, but this is the smallest study included with only 13 cases (including 2 familial cases) over 30 years [3]. It is possible that there is a true higher rate of PPGL in this population but, if so, this is not confirmed by Ebbehoj et al in the recent study of adrenal tumors, although this study only included adrenal tumors rather than both PCC and PGL [27]. Alternatively, there may be a higher detection rate as suggested by a possible association between an increased incidence in areas with university hospitals [5]. This may be due to an increase in awareness of, or investigation for PPGL, in these centers. Another possible explanation is the population used by Beard et al for the denominator was that of Rochester City [3]. The authors did not appear to account for change in population over time and it is also possible that the catchment should have been that of Olmsted County, which would decrease the incidence from 0.95/100,000 to 0.6/100,000 over the same time period [38]. Although still higher than historically comparable studies, this seems potentially more in keeping with the expected incidence from such an academic unit with a high rate of autopsy examinations.
Limitations of this review include restriction of search criteria to English language only, which raises the possibility of bias. We excluded 1 conference abstract because we were not able to obtain it, despite contacting local libraries and the abstract authors. There are missing data points; for instance, we had limited information on tumor size and race or ethnicity from most studies. In addition, because of the nature of this review, we did not have information from all studies as to how patients presented, specifically, whether from symptoms, incidental findings, or family screening because of a known hereditary mutation. Although the incidence appeared to have increased over time, it is difficult to ascertain from the information included in the literature whether this pertains to both PCC and PGL or predominantly the former. Changes in detection from family screening is expected to be seen with increased genetic testing but limited information was available on this from most of the included studies. Four single-center studies were included in the review [3, 20, 22, 23], threatening the external validity of the results and applicability to the general population. De Graeff et al and Hartley et al used questionnaires and letters to identify patients [4, 7], raising the possibility of response and recall biases. In addition, most studies identified patients from surgical or pathology databases potentially missing those patients who did not undergo surgery.
In conclusion, the incidence of PPGL appears to have increased over time, despite a marked decrease in autopsy rates, most likely from an increase in incidental PCC detection through increased rates of imaging and improved imaging technology. The epidemiology of PPGL remains understudied, and there are gaps in our knowledge, in particular, changes in detection of PGLs, including HNPGLs, the association with altitude, tumour size, geographical and racial differences and the background genetics of the populations being studied.
Glossary
Abbreviations
- HNPGL
head and neck paraganglioma
- PCC
pheochromocytoma
- PGL
paraganglioma
- PPGL
pheochromocytomas and paragangliomas
Contributor Information
Abdul Rahman Al Subhi, Waikato Clinical Campus, University of Auckland, Hamilton 3240, New Zealand.
Veronica Boyle, Waikato Clinical Campus, University of Auckland, Hamilton 3240, New Zealand.
Marianne S Elston, Email: m.elston@auckland.ac.nz, Waikato Clinical Campus, University of Auckland, Hamilton 3240, New Zealand.
Disclosures
The authors have nothing to disclose.
Data Availability
Datasets generated during and/or analyzed during the current study are available from Mendeley Data [39].
References
- 1. Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab (Seoul). 2017;32(2):152-161. Doi: 10.3803/EnM.2017.32.2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lam AK. Update on adrenal tumours. In 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28(3):213-227. Doi: 10.1007/s12022-017-9484-5 [DOI] [PubMed] [Google Scholar]
- 3. Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1983;58(12):802-804. [PubMed] [Google Scholar]
- 4. De Graeff J, Horak BJ. The incidence of phaeochromocytoma in the Netherlands. Acta Med Scand. 1964;176:583-593. [DOI] [PubMed] [Google Scholar]
- 5. Stenstrom G, Svardsudd K. Pheochromocytoma in Sweden 1958-1981. An analysis of the National Cancer Registry Data. Acta Med Scand. 1986;220(3):225-232. [PubMed] [Google Scholar]
- 6. Andersen GS, Lund JO, Toftdahl D, Strandgaard S, Nielsen PE. Pheochromocytoma and Conn’s syndrome in Denmark 1977-1981. Acta Med Scand Suppl. 1986;714:11-14. Doi: 10.1111/j.0954-6820.1986.tb08961.x [DOI] [PubMed] [Google Scholar]
- 7. Hartley L, Perry-Keene D. Phaeochromocytoma in Queensland--1970-83. Aust N Z J Surg. 1985;55(5):471-475. [PubMed] [Google Scholar]
- 8. Guerrero MA, Schreinemakers JM, Vriens MR, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg. 2009;209(6):727-732. Doi: 10.1016/j.jamcollsurg.2009.09.022 [DOI] [PubMed] [Google Scholar]
- 9. Neumann HPH, Young WF Jr., Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. Doi: 10.1056/NEJMra1806651 [DOI] [PubMed] [Google Scholar]
- 10. Darr R, Kuhn M, Bode C, Bornstein SR, et al. Accuracy of recommended sampling and assay methods for the determination of plasma-free and urinary fractionated metanephrines in the diagnosis of pheochromocytoma and paraganglioma: a systematic review. Endocrine. 2017;56(3):495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berends AMA, Buitenwerf E, de Krijger RR, et al. Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: a nationwide study and systematic review. Eur J Intern Med. 2018;51:68-73. [DOI] [PubMed] [Google Scholar]
- 12. Neumann HP, Bausch B, McWhinney SR, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346(19):1459-1466. [DOI] [PubMed] [Google Scholar]
- 13. Jhawar S, Arakawa Y, Kumar S, et al. New insights on the genetics of pheochromocytoma and paraganglioma and its clinical implications. Cancers (Basel). 2022;14(3):594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ben Aim L, Pigny P, Castro-Vega LJ, et al. Targeted next-generation sequencing detects rare genetic events in pheochromocytoma and paraganglioma. J Med Genet. 2019;56(8):513-520. [DOI] [PubMed] [Google Scholar]
- 15. Brauch H, Kishida T, Glavac D, et al. Von Hippel-Lindau (VHL) disease with pheochromocytoma in the Black Forest region of Germany: evidence for a founder effect. Hum Genet. 1995;95(5):551-556. [DOI] [PubMed] [Google Scholar]
- 16. Hensen EF, van Duinen N, Jansen JC, et al. High prevalence of founder mutations of the succinate dehydrogenase genes in the Netherlands. Clin Genet. 2012;81(3):284-288. [DOI] [PubMed] [Google Scholar]
- 17. Schiavi F, Dematte S, Cecchini ME, et al. The endemic paraganglioma syndrome type 1: origin, spread, and clinical expression. J Clin Endocrinol Metab. 2012;97(4):E637-E641. [DOI] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3): 123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cvasciuc IT, Gull S, Oprean R, Lim KH, Eatock F. Changing pattern of pheochromocytoma and paraganglioma in a stable UK population. Acta Endocrinol (Buchar). 2020;16(1):78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ebbehoj A, Stochholm K, Jacobsen SF, et al. Incidence and clinical presentation of pheochromocytoma and sympathetic paraganglioma: a population-based Study. J Clin Endocrinol Metab. 2021;106(5):e2251-e2261. [DOI] [PubMed] [Google Scholar]
- 22. Fernandez-Calvet L, Garcia-Mayor RV. Incidence of pheochromocytoma in South Galicia, Spain. J Intern Med. 1994;236(6):675-677. [DOI] [PubMed] [Google Scholar]
- 23. Holland J, Chandurkar V. A retrospective study of surgically excised phaeochromocytomas in Newfoundland, Canada. Indian J Endocrinol Metab. 2014;18(4):542-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim JH, Moon H, Noh J, Lee J, Kim SG. Epidemiology and prognosis of pheochromocytoma/paraganglioma in Korea: a nationwide study based on the National Health Insurance Service. Endocrinol Metab (Seoul). 2020;35(1):157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leung AA, Pasieka JL, Hyrcza MD, et al. Epidemiology of pheochromocytoma and paraganglioma: population-based cohort study. Eur J Endocrinol. 2021;184(1):19-28. [DOI] [PubMed] [Google Scholar]
- 26. Dua A, Spees TC, Hernandez FC, Igbadumhe AA, Algodi M, Desai SS. Trends in the incidence of carotid body tumors in the United States from 1998 to 2011. Vasc Dis Manag. 2014;11(12):E298-E302. [Google Scholar]
- 27. Ebbehoj A, Li D, Kaur RJ, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(11):894-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takayanagi R, Miura K, Nakagawa H, Nawata H. Epidemiologic study of adrenal gland disorders in Japan. Biomed Pharmacother. 2000;54(Suppl 1):164s-168s. [DOI] [PubMed] [Google Scholar]
- 29. Mettler FA Jr., Bhargavan M, Faulkner K, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950-2007. Radiology. 2009;253(2):520-531. [DOI] [PubMed] [Google Scholar]
- 30. OECD. Magnetic resonance imaging (MRI) exams (indicator). 2022. Doi: 10.1787/1d89353f-en. Accessed 3 March 2022. [DOI]
- 31. Wickramarachchi BN, Meyer-Rochow GY, McAnulty K, Conaglen JV, Elston MS. Adherence to adrenal incidentaloma guidelines is influenced by radiology report recommendations. ANZ J Surg. 2016;86(6):483-486. [DOI] [PubMed] [Google Scholar]
- 32. Shojania KG, Burton EC. The vanishing nonforensic autopsy. N Engl J Med. 2008;358(9):873-875. [DOI] [PubMed] [Google Scholar]
- 33. Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287(11):1427-1434. [DOI] [PubMed] [Google Scholar]
- 34. Alrezk R, Suarez A, Tena I, Pacak K. Update of pheochromocytoma syndromes: genetics, biochemical evaluation, and imaging. Front Endocrinol. 2018;9:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeLellis R, Lloyd R, Heitz P, Eng C.. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 36. Leung AA, Hyrcza MD, Pasieka JL, Kline GA. Incidence of pheochromocytoma and paraganglioma varies according to altitude: meta-regression analysis. Eur J Endocrinol. 2021;184(5):L21-L23. [DOI] [PubMed] [Google Scholar]
- 37. Ebbehoj A, Poulsen PL, Sondergaard E. Incidence of PPGL according to altitude - calendar time is of the essence. Eur J Endocrinol. 2021;186(1):L1-L2. [DOI] [PubMed] [Google Scholar]
- 38. 1860-2010 Rochester and Olmsted County Population. https://www.olmstedcounty.gov/sites/default/files/2020-10/1860-2010%20Rochester%20and%20Olmsted%20County%20Population.pdf. Accessed 3 March 2022.
- 39. Boyle, V. Data from: PPGL epidemiology SR search terms and dates. Mendeley Data 2022. Deposited 30 May 2022. https://data.mendeley.com/datasets/szp6hyfj7m/2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during and/or analyzed during the current study are available from Mendeley Data [39].