Abstract
Nowadays, effective cancer therapy is a global concern, and recent advances in nanomedicine are crucial. Cancer is one of the major fatal diseases and a leading cause of death globally. Nanotechnology provides rapidly evolving delivery systems in science for treating diseases in a site-specific manner using natural bioactive compounds, which are gaining widespread attention. Nanotechnology combined with bioactives is a very appealing and relatively new area in cancer treatment. Natural bioactive compounds have the potential to be employed as a chemotherapeutic agent in the treatment of cancer, in addition to their nutritional benefits. Alginate, pullulan, cellulose, polylactic acid, chitosan, and other biopolymers have been effectively used in the delivery of therapeutics to a specific site. Because of their biodegradability, biopolymeric nanoparticles (BNPs) have received a lot of attention in the development of new anticancer drug delivery systems. Biopolymer-based nanoparticle systems can be made in a variety of ways. These systems have developed as a cost-effective and environmentally friendly solution to boost treatment efficacy. Effective drug delivery systems with improved availability, increased selectivity, and lower toxicity are needed. Recent research findings and current knowledge on the use of BNPs in the administration of bioactive chemicals in cancer therapy are summarized in this review.
Keywords: biopolymeric nanoparticles, cancer treatment, drug delivery, nanomedicines, natural bioactives
Introduction
Non–communicable diseases, such as cancer, are one of the leading causes of death worldwide. The most common type of cancer is lung cancer, which is followed by breast, prostate, and colon cancer (1). Within the next two decades, the number of new cases is expected to increase by almost 70% (2). Present treatment options for cancer include surgery, chemotherapy, and/or radiation therapy. But there are some challenges associated with the above treatment strategies like radiation therapy or chemotherapeutic drugs not only damage cancerous cells, but also harm healthy cells (3). The degree of side effects may vary from person to person depending on the type of cancer, and other variables. Chemotherapy hinders the DNA synthesis in fast–dividing cancer cells, leading to their death. It leads to various side effects as it affects healthy cells of the body (4). Additionally, high drug doses used during chemotherapy lead to increased toxicity to normal cells, with the risk of development of drug resistance (4). Thus, a major task is to strengthen the selectivity of anticancer drugs against cancerous cells while sparing normal cells and tissues. In order to treat cancer, efficient targeted delivery methods of chemotherapeutic drugs or anticancer bioactive molecules are also required. In view of the above facts, a potential approach was used to target tumor cells through nano–medicine–based preparations (5). These preparations consist of ultra–micrometer–sized carriers having the bioactive compound(s) that are capable of selectively treating tumors, thus, improving the therapeutic value of the anticancer drugs that are being used.
For the past decade, nanoparticles (NPs) have become tremendously appealing for use in biomedicine. Through enhanced permeability and retention (EPR) effects in tumor tissues, nanoparticle-based drug delivery techniques or nanocarriers can improve therapeutic efficacy and selectivity (6). Nanocarriers also have superior cellular uptake as compared to standard chemotherapeutic molecules. Among the nanocarriers, polymeric NPs, micelles, and liposomes have received significant attention (7).
The polymers utilized to prepare polymeric NPs such as poly (methyl methacrylate), polystyrene, polyacrylates, and polyacrylamide were not eco–friendly (8). The nanosystems prepared by these materials displayed chronic toxicity and inflammatory reactions. The use of new nanosystems might have huge interest to accomplish additional applications for the current large–scale characterized bioactive compounds. Nanoencapsulation, in actuality, is a low-cost technology that could allow for regulated bioactives release, which could be useful in cancer treatment. There are synthetic and natural biodegradable polymers. Polymeric nanoparticles have been created using naturally occurring biodegradable polymers, such as alginate, gelatin, chitosan, and others (9). Some researchers studied about targeted delivery systems of therapeutics through chitosan and other biopolymer based NPs for cancer therapy (10, 11). Therefore, this review focuses on recent progresses in the development of BNPs for the effective delivery of bioactive chemicals for targeted therapy of cancer.
Bioactive compounds
Bioactive substances are a natural occurrence as well as a part of our diet. Plants produce phytochemicals, which are natural bioactives capable of regulating metabolic processes and promoting good health (12). Amino acids, vitamins, enzymes, fibers, minerals, essential oils, and polyphenols are among the plant-based bioactive compounds (13). Natural biopolymers also consist of plant–derived or animal polysaccharides and proteins as well as polymers of microbial (bacteria, fungi, and yeast) sources. Several bioactive substances have the potential to be used as cancer chemotherapeutic drugs in past few years. Phytochemicals like vinca alkaloids, cephalotaxus, berberine, combretastatins, colchicine, ellipticine, and others are known for anticancerous activities against many cancer types (14). A flavonoid, chicoric acid isolated from Echinacea purpurea act as an immune stimulant by increasing the number of natural killer cells (15). A sulfur–containing compound called Ajoene isolated from Allium sativum suppresses the rate of cancer development. In a study, Shang et al. (16) reported the role of ajoene in the suppression of the growth of human breast cancer cells. Likewise, curcumin, an active chemical derived from the Curcuma longa plant, has anticancer properties. It caused the transcription factors AP1, NFkB, and STAT3 to be inhibited, halting the cell cycle in the G2/M phase (17). Similarly, another anticancer agent like Lappaol F has been obtained from Arctium lappa L. and has arrested the G2 phase of the cell cycle by promoting the G1 phase in which the p21 gene plays an important role for the same Lappaol F (18).
Citral is a bioactive ingredient found in citrus–based plant Cymbopogon citratus. Nanostructured lipid carrier–citral showed effective results against MDA–MB−231 breast cancer cells (19). Several researchers reported promising bioactive components from Cannabis sativa like delta 9-tetrahydrocannabinol (THC) and cannabinoids (CBD) which have also been investigated for their potential to treat cancer in both in vivo and in vitro studies (20–22).
A compound wedelolactone, isolated from Eclipta alba involve in the inhibition of T47D, MCF−7, and MDAMB−231 cells by exciting ER signaling (23). Curcumin is another important bioactive obtained from Curcuma longa which contains curcuminoids compounds (24). Curcumin is known to have low bioavailability because of its poor absorption and fast removal from the body. Basniwal et al. (25) have used NPs mediated delivery of curcumin to increase its bioavailability. Curcumin has been reported as a potent anticancer agent on various types of human cancers such as lung, pancreatic, prostate, breast, ovarian and others (26). Another bioactive myricetin is a bioflavonoid commonly present in food sources such as berries, red wine, vegetables, tea, and medicinal plants which is a promising anti–carcinogen with therapeutic potential reported in the breast (27), colon (28), liver (29), ovarian (30), and skin cancers (31). Geraniin is a dehydroellagitannin that is found in a variety of medicinal plants and is thought to be the most active natural chemical. Geraniin was discovered from the fruit of the Phyllanthus emblica L. plant, and found to have potent antioxidant, antimicrobial, and anticancer properties (32, 33). On MCF7 human breast cancer cells, geraniin was found to have an anticancer impact (34).
The two major classes of fat soluble antioxidants in Vitamin E are tocopherols and tocotrienols (T3) (35) that showed anticancerous properties against skin (36), stomach (37), liver (38), breast (39), colon (40), lung (41), and prostate cancer (42). Anticancer activity has been documented for a number of biologically active substances (Table 1).
Table 1.
Biologically active molecules showing anticancerous activity.
| S. No. | Bioactive compounds/ molecules | Sources | Active molecules | Effects | References |
|---|---|---|---|---|---|
| 1. | Polyphenolic compounds | Red wine, chocolate, flaxseed oil, various plants | Corilagin | Carcinogen detoxification, inhibits tumor initiation/promotion, antimutagen | (43) |
| 2. | Cyclopeptide | Nostoc | Cryptophycin | Anticancerous | (44) |
| 3. | Ajoene | Allium sativm | Ajoene | Anticancerous | (45) |
| 4. | Natural metabolites | Marine Cyanobacteria | Apratoxin A | Anticanceros | (46) |
| 5. | Gallic acid | Toona sinensis leaf extract | – | ROS-mediated anticancerous activity against prostate cancer cells | (47) |
| 6. | Polyphenol and flavonoid | Chlorella vulgaris | – | Inhibit lung cancer metastasis | (48) |
| 7. | Microcystins | Cyanobacteria | Cryptophycins | Anticancer | (49) |
| 8. | Vinorelbine, (Navelbine) | Catharanthus roseus | Vincristine | Anticancer activities lung cancer |
(50) |
| 9. | Geraniin | – | – | Anticancer activities | (32) |
| 10. | Epigallocatechin-3-gallate | – | – | Prostate cancer prevention | (51) |
| 11. | Flavonoids | Artemisia annua | Casticin | Anticancer | (52) |
| 12. | Apigenin | – | – | Anticancerous | (53) |
| 13. | Flavonoid | – | Luteolin | Anticancerous | (54) |
| 14. | Terpene | – | Curcumin | Anticancerous | (55) |
| 15. | Polyphenols | Derived from grapes, berries, red wine, peanuts | Resveratrol | Anticancerous | (56) |
Development of biopolymeric nanomaterials
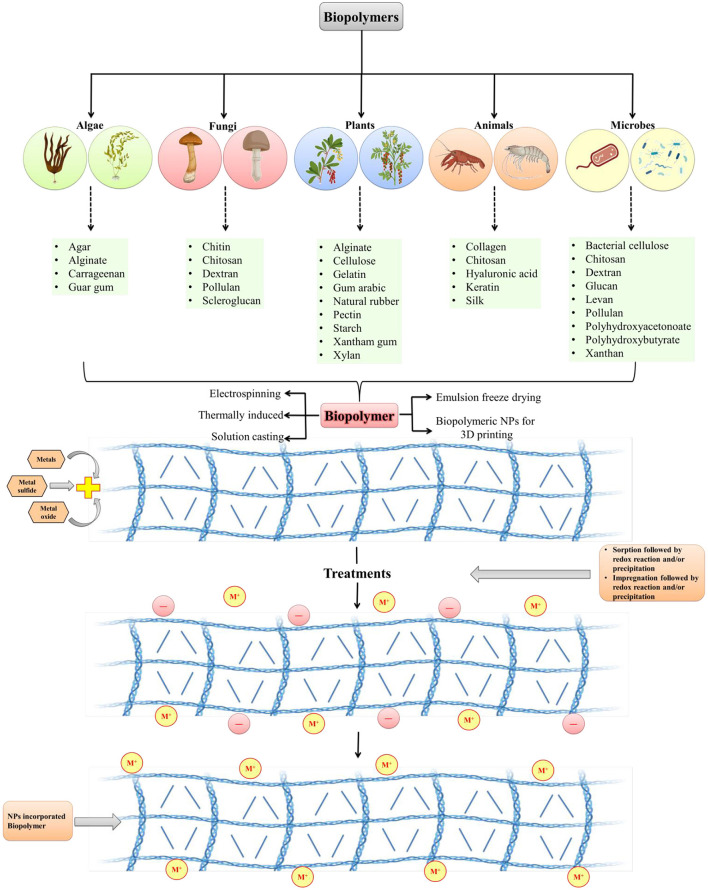
Biopolymer composites integrate the necessary qualities of two or more acceptable materials to improve the physiological and mechanical needs of biomedical regions. Because of their long-term stability, chemical substances utilized in standard processes to produce polymeric nanomaterials may cause environmental impact. Biopolymers, on the other hand, are frequently created from non-toxic monomers and are carbon-neutral. It is easier to make nanomaterials from biopolymers since they are deposited at the nanoscale. Biopolymers have been found in plants, algae, fungi, bacteria, and animals. Macromolecules include starch, alginate, chitosan, dextran, and chitin (Figure 1). There are several compounds like pectin, cellulose, lignin, and hyaluronic acid, that are used to create nanomaterials composites. In the medical field, such as in the treatment of cancer, nano biocomposites have shown encouraging outcomes in recent years such as biodegradable substances, and chitosan nanoparticles are employed to deliver anticancer drugs (58). Biopolymers are made up of diverse components and have varied physiological properties. Biopolymeric nanomaterials can be made by attaching metals to biopolymers. To construct molecular capsules, biopolymers, in particular, utilize intramolecular hydrogen bonding. Starch, for example, can be used to create polymeric nanocomposites by combining metals or metal oxides. Chitosan is also used in nanotechnology applications because of its extensive compatibility (59). To make nanomaterials, silver (Ag) can be supramolecularly incorporated into starch (60). Nanomaterials can be incorporated into biopolymers by impregnation or absorption. Physical features of NPs can differ from macro-sized bulk materials due to their greater surface area and reactivity. Nanocapsules or nanospheres can be manufactured depending on the manufacturing approach (61). Spectroscopy, microscopy, and others are among the techniques used for NPs characterization.
Figure 1.
Development of biopolymeric nanoparticles from various sources (modified and adapted from Sarkar et al. (57).
Biopolymers and their derivatives are widely utilized, and studied in the creation of hydrogels. Chemical and physical methods of producing biomaterial-based hydrogels can be distinguished. The chemical process of incorporating substituent groups into biopolymer structures, strengthening and developing new functional bioactivities as a result. Covalent bonds between the utilized polymers are created via free radical polymerization, graft copolymerization, and other polymerization techniques (6, 62, 63). The chemical reaction within the hydrogel creates everlasting bonds in the polymer system. However, reversible physical interactions such as van der Waals attraction, hydrogen bonding, polymer chain entanglements, ionic interactions, and crystallite associations hold the hydrogels together during the physical process (64). There are several nanomaterials based biopolymers employed for effective delivery of bioactives for cancer therapy (Table 2).
Table 2.
Nanoparticles based numerous biopolymeric compounds and their action in various cancer treatments.
| S. No. | Biopolymeric nanoparticles | Size (nm) | Polymer component | Actions | Bioactive molecules | Effects | Applications | References |
| 1. | Andrographolide analog chitosan based NPs | 112–240 | Octyl-grafted succinyl chitosan, naphthyl-grafted succinyl chitosan, and benzyl-grafted succinyl chitosan | Delivering anticancer medications to the sites of colon cancer | Andrographolide analog | Induces apoptosis | Colon cancer | (65) |
| 2. | DOX-verapamil/MPEG-PLA NPs | 25 | MPEG-PLA | Co-delivery system –efficiently chemotherapeutic agents and coencapsulate verapamil | Doxorubicin, Verapamil | Tumor suppression | Ovarian cancer | (66) |
| 3. | Linoleic acid conjugated SN38-loaded NPs | 109 | PEO-PBO diblock copolymer | – | Linoleic acid conjugated SN38 | EBNPs inhibit growth and promote uptake in cancer cells | Colorectal cancer | (67) |
| 4. | Curcumin- loaded Polymeric poly NPs | 200 | PLGA | Increased serum stability over free curcumin | Curcumin | Irradiating tumor cells at a low dose produces cytotoxic effects that inhibit tumor growth | Ovarian cancer | (68) |
| 5. | Epidermal growth factor receptor-targeted lipid polymeric NPs | 141.6 | EGF-PEG-DSPE | Targeting the drug delivery | Cisplatin and Doxorubicin | Enhanced cytotoxicity with anticancer activity | Lung cancer | (69) |
| 6. | Chondroitin sulfate functionalized campththecin-loaded polymeric NPs | 289 | Chitosan | Targeted drug delivery | Campththecin | Promote apoptosis | Colon cancer | (70) |
| 7. | Albendazole-loaded polyurethane NPs | 128.1 | Polyurethane | Better medication delivery | Albendazole | Increase the anticancer efficacy via inducing apoptosis | Breast cancer | (71) |
| 8. | Bortezomib loaded polymeric NPs | 199.7 | HPLA-BT | Higher drug load and its delivery | Bortezomib | Significant anticancer activity and shows higher cytotoxic effects | Breast cancer | (72) |
| 9. | Gemcitabine NPs conjugated with linoleic acid. | ~ 150 | Linoleic acid | High drug-load, improved intracellular uptake and controlled release | Gemcitabine | Induce apoptosis and improve cytotoxic activity | Thyroid cancer | (73) |
| 11. | Platinum–curcumin complexes loaded into pH and redox dual-responsive NPs | ~ 100 | mPEG-SS-PBAE-PLGA | Synergistic anticancer effects and control intracellular release | Platinum–curcumin | Improved anti-metastatic activity and synergistic anticancer implications | Lung cancer | (74) |
| 12. | Uncariatomentosa extract -PLGA & UTPCL | 300 | PCL and PLGA | Better drug delivery | Uncariatomentosa extract | – | Prostate cancer | (75) |
DOX, Doxorubicin; PBA, Phenylboronic acid; PLA, Polylactic Acid; PLGA, Polylactide-co-glycolide; HPLA-BT, N-(2- hydroxypropyl) methacrylamide (HPMA)-PLA-Biotin; MPEG-PLA, Methoxy poly(ethylene glycol)- poly(lactide) copolymer; PEO-PBO, poly (ethylene oxide)-poly (butylene oxide) DSPE-PEG, 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine- polyethylene glycol; PBAE, Poly-beta-amino ester.
Targeted delivery of bioactives via biopolymeric nanoparticles for cancer therapy
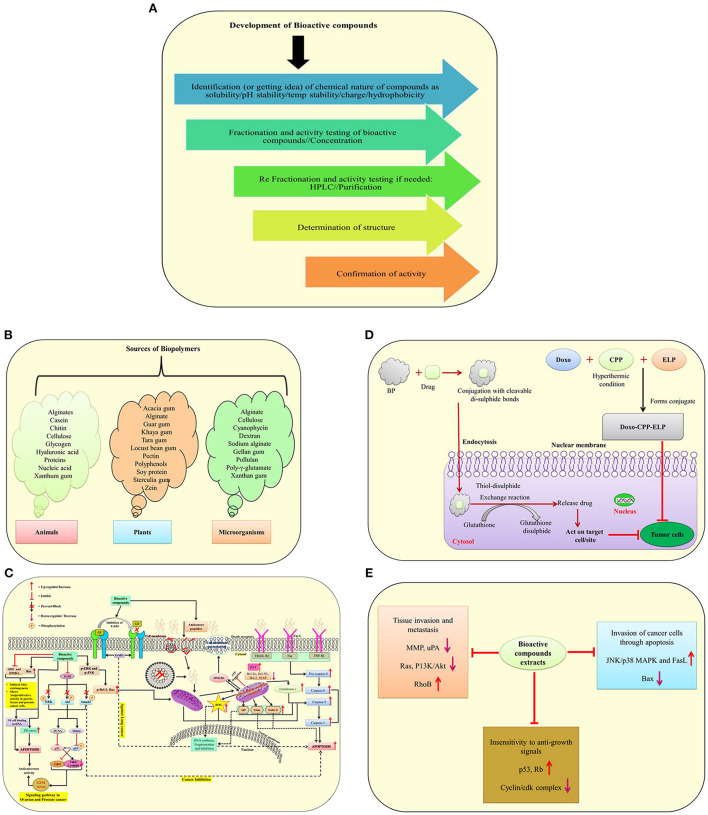
According to the World Health Organization, cancer is one of the leading causes of mortality, with more than twenty million people expected to die by the year 2025 (1). Chemotherapeutic medicines work by interfering with DNA synthesis in rapidly dividing cancer cells, slowed DNA replication, reducing therapeutic efficacy and generating a significant mortality rate in cancer patients due to their large doses (4). Bioactive molecule sources and development, as well as their involvement in drug delivery systems and molecular mechanisms against different cancer cells, are illustrated in (Figures 2A–E). Natural bioactive chemicals have an impact on cellular metabolism and signaling pathways, and they can be used to cure cancer (Figure 2C). Natural bioactive chemicals have a favorable effect on cancer treatment, according to results from both in vivo and in vitro trials (10). A study reported in cancer reports showed that resveratrol NPs has enhanced anticancer activity both in vitro and in vivo (77). Biopolymers as NPs are increasingly being employed as a different way to deliver bioactive compounds/drugs or biological macromolecules to specific body regions. Polymer nanoparticles are a novel way of medication delivery. As a result, the primary goal of biopolymer-based nanomedicine is to deliver therapeutics just to cancer cells, increasing efficacy while reducing toxicity. Nanoparticles containing natural bioactives for targeted medication delivery in various tissues enhanced bioavailability, reduced side effects, improved in vivo stability, and improved target– specific activity of bioactive chemicals in cancer treatment. In the year 2020, Mousa et al. (78) reported that nanoformulation of 3, 3′-diindolylmethane (DIM) and ellagic acid showed more effective suppression of cell viability in pancreatic cancer, tumor weight, and tumor angiogenesis as compared to with these bioactive compounds alone. New formulation strategies on lipid–based delivery systems as nanocarriers also showed improved bio–efficacy under in vivo conditions (79).
Figure 2.
A schematic illustration of bioactive molecule sources and development, as well as their involvement in drug delivery systems and molecular mechanisms against different cancer cells. (A) Development of bioactive compounds; (B) Sources of biopolymers from plants, animals and microorganisms; (C) Mode of action of anticancerous molecules via TGF–β signaling pathway, death receptor, mitochondrial receptor–induced pathways against cancer cells; (D) A mechanism of suppressing tumor cells using biopolymers in conjunction with drugs; (E) The effect of bioactive molecules from plant extracts on the underlying mechanisms of cancer development [modified and adapted from Lin et al. (76)]. ODC, Ornithine decarboxylase; DMBA, 7,12-Dimethylbenz(α)anthracene; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; NF-Kb, Transcription factor; p-ERK, Protein kinase RNA-like ER kinase; ROS, Reactive oxygen species; ENK, c-Jun-N terminal kinase; AIF, Apoptosis-inducing factor; FasL, Fas Ligand; ErbB3, Receptor tyrosine kinase; TGF-β, Transforming growth factor beta; ERK, Extracellular-signal-regulated kinase; AKT1, Serine/threonine-protein kinase; SMAD2, SMAD family member 2; Mdm2, Mouse double minute 2 homolog; PCNA, Proliferating cell nuclear antigen; p53 and p21, Tumor protein; WAF1/CIP1, Wildtype activating factor-1/cyclin-dependent kinase inhibitory protein-1; cdc2, Cell division cycle 2; HMGB1, High mobility group box 1; Smac, Second mitochondria-derived activator of caspase; EndoG, Endonuclease G; ER, Endoplasmic reticulum; TRAIL, Tumor necrosis factor (TNF)-related apoptosis-inducing ligand; TNF RI, Tumor necrosis factor receptor 1; Rb, Retinoblastoma protein; PI3K, Phosphatidylinositol-3-kinase; uPA, Urokinase plasminogen activator; BP, Biopolymers.
Biopolymer-based nanocarriers, liposomes, dendrimers, lipid-based NPs, polymeric micelles, and other new drug delivery technologies have been discovered in the last two decades that allow bioactive chemicals to be delivered into the site of action (80). Proteins (albumin, gelatin, and milk proteins), polysaccharides (cellulose, starch, pectin, chitosan, dextran and sodium alginate), and nucleic acids are examples of biopolymers (81). Biopolymer NPs were found to be effective in delivering bioactive chemicals to malignant cells in both in vivo and in vitro investigations. Biopolymeric nanoparticles containing bioactive substances have several advantages over other current delivery technologies, including non water–soluble drug delivery, stability protection, and targeted medication delivery to specific tumor cells (82). Nanoparticles deliver the therapeutic chemical, which could be natural bioactive or synthetic, directly into the target tissue/organ. Nanoparticles have a lot of potential as molecular transporters since they can deliver treatments to malignant cells without harming healthy cells (83). Polymeric pharmaceuticals, polymeric micelles, polyplexes, polymer–drug conjugates, and polymer–protein conjugates are all examples of biopolymers utilized in therapy (84).
The ways NPs are administered, as well as their physicochemical properties, have a big impact on therapeutic efficacy and biological consequences (Figure 2C). Every mode of administration has advantages and disadvantages, but the oral route of delivery is the safest and most convenient way to treat cancer. The oral mode of administration, on the other hand, has drawbacks such as gastrointestinal degradation, insolubility, and reduced absorption. As a result, current anticancer compositions aren't suited for use as oral chemotherapy. The use of biopolymers for oral delivery platforms has the potential to overcome some of the problems associated with the delivery of chemotherapy drugs. Biopolymeric formulations prevent NPs from pH degradation, enhance water solubility and stability, and target particular binding sites for selective absorption and drug release (Figure 2D) (85). Future formulations from diverse plants with anticancer properties could significantly boost the potency of anticancer medications for cancer therapy (9). The potency of recognized naturally occurring bioactive chemicals has been increased through the use of nanomaterials. In the future, herbal compounds might play a bigger role in the delivery mechanism for nano biocomposites mediated therapeutic molecules. Further research is required in this area.
Challenges and future perspectives
Every day, there are large number of patients who have advance tumors and most common concern regarding the therapy is the effectiveness and the expense of cancer medication. The effectiveness and cost of pharmaceuticals have not altered dramatically as a result of the development of new technology and drugs. Not only is the efficacy of new treatments a source of concern in India; but wealthier countries such as the United States and Europe are also experiencing issues with cancer drug delivery systems. Nanotechnology is one of the most exciting fields in the area of drug delivery systems to treat non–curable cancer disorders, especially in such a crucial situation. Nanoparticles are utilized to target and deliver drugs to cancer/tumor cells while preserving the physiology of non-tumorous cells. Researchers are working to make more consistent NPs so that the right amount of harmless therapeutics can be delivered to the right place. Metal–based (gold and silver) NPs production has progressed significantly for their applications in diagnostic and therapeutic purposes. The understanding of treatment mechanism at the molecular level will expand bioactive compounds applications for cancer therapy. Because of the regulated release of specific medications at targeted places, assessment of events during drug interaction at the tissue/cellular level, and prediction has not yet been found completely, and its application in cancer therapy/diagnosis is still limited. Animal research will provide important information on the possible use of nanoformulations as medicinal therapeutics. Acute and chronic studies are required to assess the possible harm of any type of anticancerous therapeutic molecule to humans and the environment, and their affordability is another area of research that requires further input.
Conclusions
Drugs have numerous obstacles, thus researchers are focusing on herbal medicine to reduce drug side effects and improve their effectiveness through the use of appropriate effective delivery systems. Preclinical and in vitro investigations have revealed that NPs are particularly effective at delivering chemotherapeutic medicines, molecules as well as natural components to specific tumor sites. Nanoparticles have significant advantages over alternative drug delivery systems in terms of high specificity, high stability, controlled drug release, and high drug–carrying capacity, and the ability to transport both hydrophobic and hydrophilic drug molecules. Because of their controlled release, subcellular size, and biocompatibility with cells, polymeric NPs are now widely used as drug/anticancer bioactives delivery systems in the pharmaceutical and medical areas for cancer therapy.
Author contributions
NP: conceptualization. PS, PKS, and AG: writing—original draft, reviewing and editing. SS, RS, AG, RM, and VM: reviewing, editing, and drawing figures and tables. MT and NP: reviewing, editing and finalize the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the Biotechnology Program, Department of Biochemistry, Dr. Rammanohar Lohia Avadh University, Ayodhya, India, and Department of Bioscience, Integral University, Lucknow, India for providing us with the research environment.
References
- 1.Bray F, Ferlay J, Soerjomataram L, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBACAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics. CA Cancer J Clin. (2017) 67:177–93. 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 3.Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, Kitui SK, Manyazewal T. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med. (2021) 9:20503121211034366. 10.1177/20503121211034366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Souza CA, Antony S, Thomas B, Murthy SG. Coping strategies used by cancer patients to deal with physical and psychological problems of chemotherapy. Int J Innov Res Dev. (2016) 5:36–41.7834466 [Google Scholar]
- 5.Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res (N Y). (2010) 27:2569–89. 10.1007/s11095-010-0233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Sun Q, Zhang X, Baeyens J, Su H. Self-assembled selenium nanoparticles and their application in the rapid diagnostic detection of small cell lung cancer biomarkers. Soft Matter. (2018) 14:481–9. 10.1039/C7SM01687E [DOI] [PubMed] [Google Scholar]
- 7.Conte C, Mastrotto F, Taresco V, Tchoryk A, Quaglia F, Stolnik S, et al. Enhanced uptake in 2D- and 3D- lung cancer cell models of redox responsive PEGylated nanoparticles with sensitivity to reducing extra and intracellular environments. J Control Release. (2018) 277:126–41. 10.1016/j.jconrel.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 8.Gagliardi A, Giuliano E, Venkateswararao E, Fresta M, Bulotta S, Awasthi V, et al. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front Pharm. (2021) 12:601626. 10.3389/fphar.2021.601626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagliardi A, Paolino D, Iannone M, Palma E, Fresta M, Cosco D. Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system. Int J Nanomed. (2018) 13:601–14. 10.2147/IJN.S156930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavda VP, Patel AB, Mistry KJ, Suthar SF, Wu ZX, Chen ZS, et al. Nano-drug delivery systems entrapping natural bioactive compounds for cancer: recent progress and future challenges. Front Oncol. (2022) 12:867655. 10.3389/fonc.2022.867655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharifi-Rad J, Quispe C, Butnariu M, Rotariu LS, Sytar O, Sestito S, et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. (2021) 21:318. 10.1186/s12935-021-02025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins R, Wu L, Zhang C, Davis RM, Xu B. Natural product-based nanomedicine: recent advances and issues. Int J Nanomedicine. (2015) 10:6055–74. 10.2147/IJN.S92162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, et al. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phyther Res. (2019) 13:2221–3. 10.1002/ptr.6419 [DOI] [PubMed] [Google Scholar]
- 14.Bai LY, Chiu CF, Chu PC, Lin WY, Chiu SJ, Weng JR. A triterpenoid from wild bitter gourd inhibits breast cancer cells. Sci Rep. (2016) 6:22419. 10.1038/srep22419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kooti W, Servatyari K, Behzadifar M, Samani MA, Sadeghi F, Nouri B, et al. Effective medicinal plant in cancer treatment, Part 2: review study. J Evid-Based Complementary Altern Med. (2017) 22:982–95. 10.1177/2156587217696927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang A, Cao SY, Xu XY, Gan RY, Tang GY, Corke H, et al. Bioactive compounds nad biological functions of Garlic (Allium sativum L). Foods. (2019) 8:246. 10.3390/foods8070246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lou C, Zhu Z, Zhao Y, Zhu R, Zhao H. Arctigenin, a liganan from Arctium lappa L, inhibits metastasis of human breast cancer cells through the downregulation of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol Rep. (2016) 37:179–84. 10.3892/or.2016.5269 [DOI] [PubMed] [Google Scholar]
- 18.Sun Q, Liu K, Shen X, Jin W, Jiang L, Sheikh SM, et al. A novel anticancer agent isolated from plant Arctium Lappa L. Mol Cancer Ther. (2014) 13:49–59. 10.1158/1535-7163.MCT-13-0552 [DOI] [PubMed] [Google Scholar]
- 19.Nordin N, Yeap SK, Rahman HS, Zamberi NR, Abu N, Mohamad NE, et al. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cell. Sci Rep. (2019) 9:1614. 10.1038/s41598-018-38214-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caffarel MM, Andradas C, Mira E, Pérez-Gómez E, Cerutti C, Moreno-Bueno G, et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer. (2010) 9:196. 10.1186/1476-4598-9-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dariš B, Tancer VM, Knez Ž, Ferk P. Cannabinoids in cancer treatment: therapeutic potential and legislation. Bosn J Basic Med Sci. (2019) 19:14–23. 10.17305/bjbms.2018.3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangal N, Erridge S, Habib N, Sadanandam A, Reebye V, Sodergren MH. Cannabinoids in the landscape of cancer. J Cancer Res Clin Oncol. (2021) 147:2507–4. 10.1007/s00432-021-03710-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu D, Hua LT, Ren YC, Cheng MA, Min CL, Chang C, et al. The Wedelolactone derivative inhibits estrogen receptor-mediated breast, endometrial, and ovarian cancer cells growth. BioMed Res Int. (2014) 2014:713263. 10.1155/2014/713263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. (2009) 14:277. [PubMed] [Google Scholar]
- 25.Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Agric Food Chem. (2011) 59:2056–61. 10.1021/jf104402t [DOI] [PubMed] [Google Scholar]
- 26.Patra S, Pradhan B, Nayak R, Behera C, Panda KC, Das S, et al. Apoptosis and autophagy modulating dietary phytochemicals in cancer therapeutics: current evidences and future perspectives. Phytother Res. (2021) 35:4194-214. 10.1002/ptr.7082 [DOI] [PubMed] [Google Scholar]
- 27.Jayakumar JK, Nirmala P, Kumar BAP, Kumar AP. Evaluation of protective effect of myricetin, a bioflavonoid in dimethyl benzanthracene-induced breast cancer in female Wistar rats. South Asian J Cancer. (2014) 3:107. 10.4103/2278-330X.130443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim ME, Ha TK, Yoon JH, Lee JS. Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Res. (2014) 34:701–6. [PubMed] [Google Scholar]
- 29.Sangwan V, Banerjee S, Jensen K, Chen Z, Chugh R, Dudeja V. Primary and liver metastasis-derived cell lines from KrasG12D; Trp53R172H; Pdx-1 Cre animals undergo apoptosis in response to triptolide. Pancreas. (2015) 44:583. 10.1097/MPA.0000000000000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y, Xie Q, Wu S, Yi D, Yu Y, Liu S. Myricetin induces apoptosis via endoplasmic reticulum stress and DNA double-strand breaks in human ovarian cancer cells. Mol Med Rep. (2016) 13:2094–100. 10.3892/mmr.2016.4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cijo GV, Vijayakumaran VV, Inoka MAD. ALC, Anbarasu K, Ragupathi NKD. Mechanism of action of flavonoids in prevention of inflammation-associated skin cancer. Curr Med Chem. (2016) 23:3697–716. 10.2174/0929867323666160627110342 [DOI] [PubMed] [Google Scholar]
- 32.Palanisamy UD, Ling LT, Manaharan T, Appleton D. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. (2011) 127:21–7. 10.1016/j.foodchem.2010.12.070 [DOI] [Google Scholar]
- 33.Ren Z, Zou W, Cui J, Liu L, Qing Y, Li Y. Geraniin suppresses tumor cell growth and triggers apoptosis in human glioma via inhibition of STAT3 signaling. Cytotechnology. (2017) 69:765–73. 10.1007/s10616-017-0085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Zhao M, Wu K, Chai X, Yu H, Tao Z. Immunomodulatory and anticancer activities of phenolics from emblica fruit (Phyllanthus emblica L). Food Chem. (2012) 131:685–90. 10.1016/j.foodchem.2011.09.063 [DOI] [Google Scholar]
- 35.Traber MG, Packer L. Vitamin E: beyond antioxidant function. Am J Clin Nutr. (1995) 62:1501S−9S. 10.1093/ajcn/62.6.1501S [DOI] [PubMed] [Google Scholar]
- 36.Korac RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn Rev. (2011) 5:164. 10.4103/0973-7847.91114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manu KA, Shanmugam MK, Ramachandran L, Li F, Fong CW, Kumar AP et al. First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway- Tocotrienol Enhances the Effect of Capecitabine in Gastric Cancer. Clin Cancer Res. (2012) 18:2220–9. 10.1158/1078-0432.CCR-11-2470 [DOI] [PubMed] [Google Scholar]
- 38.Chang C, Lee SO, Yeh S, Chang TM. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene. (2014) 33:3225–34. 10.1038/onc.2013.274 [DOI] [PubMed] [Google Scholar]
- 39.Tran AT, Ramalinga M, Kedir H, Clarke R, Kumar D. Autophagy inhibitor 3-methyladenine potentiates apoptosis induced by dietary tocotrienols in breast cancer cells. Eur J Nutr. (2015) 54:265–72. 10.1007/s00394-014-0707-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang JS, Zhang SJ Li Q, Liu YH, He N, Zhang J, et al. Tocotrienol-rich fraction (TRF) suppresses the growth of human colon cancer xenografts in Balb/C nude mice by the Wnt pathway. PLoS ONE. (2015) 10:e0122175. 10.1371/journal.pone.0122175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajasinghe L, Pindiprolu R, Razalli N, Wu Y, Gupta S. Delta tocotrienol inhibits MMP-9 dependent invasion and metastasis of non-small cell lung cancer (NSCLC) cell by suppressing Notch-1 mediated NF-_b and uPA pathways. FASEB J. (2015) 29:718–52. 10.1096/fasebj.29.1_supplement.752.18 [DOI] [Google Scholar]
- 42.Huang Y, Wu R, Su ZY, Guo Y, Zheng X, Yang CS, et al. A naturally occurring mixture of tocotrienols inhibits the growth of human prostate tumor, associated with epigenetic modifications of cyclin-dependent kinase inhibitors p21 and p27. J Nutr Biochem. (2017) 40:155–63. 10.1016/j.jnutbio.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 43.Guo JS, Wang SX, Li X, Zhu TR. Studies on the antibacterial constituents of Geranium sibiricum L. Acta Pharm Sin. (1987) 22:28–32. [PubMed] [Google Scholar]
- 44.Smith CD, Zhang X, Mooberry SL, Patterson GM, Moore RE. Cryptophycin: a new antimicrotubule agent active against drug-resistant cells. Cancer Res. (1994) 54:3779–84. [PubMed] [Google Scholar]
- 45.Dirsch VM, Gerbes AL, Vollmar AM. Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kappaB. Mol Pharmacol. (1998) 53:402–07. 10.1124/mol.53.3.402 [DOI] [PubMed] [Google Scholar]
- 46.Luesch H, Yoshida WY, Moore RE, Paul VJ, Corbett TH. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J Am Chem Soc. (2001) 123:54185423. 10.1021/ja010453j [DOI] [PubMed] [Google Scholar]
- 47.Chen HM, Wu YC, Chia YC, Chang FR, Hsu HK, Hsieh YC, et al. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. (2009) 286:161–71. 10.1016/j.canlet.2009.05.040 [DOI] [PubMed] [Google Scholar]
- 48.Wang HM, Pan JL, Chen CY, Chiu CC, Yang MH, Chang HW, et al. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. (2010) 45:1865–72. 10.1016/j.procbio.2010.05.023 [DOI] [Google Scholar]
- 49.Kounnis V, Chondrogiannis G, Mantzaris MD, Tzakos AG, Fokas D, Papanikolaou NA, et al. Microcystin LR shows cytotoxic activity against pancreatic cancer cells expressing the membrane OATP1B1 and OATP1B3 transporters. Anticancer Res. (2015) 35:5857–65. [PubMed] [Google Scholar]
- 50.Nazir T, Taha N, Islam A, Abraham S, Mahmood A, Mustafa M. Monocytopenia; Induction by vinorelbine, cisplatin and doxorubicin in breast, non-small cell lung and cervix cancer patients. Int J Health Sci. (2016) 10:542–7. 10.12816/0048904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanna V, Singh CK, Jashari R, Adhami VM, Chamcheu JC, Rady I, et al. Targeted nanoparticles encapsulating epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci Rep. (2017) 7:1–15. 10.1038/srep41573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou GL, Peng SF, Liao CL, Ho HC, Lu KW, Lien JC, et al. Casticin impairs cell growth and induces cell apoptosis via cell cycle arrest in human oral cancer SCC-4 cells. Environ Toxicol. (2018) 33:127–41. 10.1002/tox.22497 [DOI] [PubMed] [Google Scholar]
- 53.Sharma A, Ghani A, Sak K, Tuli HS, Sharma AK, Setzer WN, et al. Probing into therapeutic anti-cancer potential of apigenin: recent trends and future directions. Recent Pat Inflamm Allergy Drug Discov. (2019) 13:124–33. 10.2174/1872213X13666190816160240 [DOI] [PubMed] [Google Scholar]
- 54.Jang CH, Moon N, Oh J, Kim JS. Luteolin shifts Oxaliplatin-induced cell cycle arrest at G0/G1 to apoptosis in HCT116 human colorectal carcinoma cells. Nutrients. (2019) 11:770. 10.3390/nu11040770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox-Georgian D, Ramadoss N, Dona C, Basu C. Therapeutic and medicinal uses of terpenes. Med Plants. (2019) 12:333–59. 10.1007/978-3-030-31269-5_15 [DOI] [Google Scholar]
- 56.Farkhondeh T, Folgado SL, Pourbagher-Shahri AM, Ashrafizadeh M, Samarghandian S. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed Pharmacother. (2020) 127:110234. 10.1016/j.biopha.2020.110234 [DOI] [PubMed] [Google Scholar]
- 57.Sarkar S, Ponce NT, Banerjee A, Bandopadhyay R, Rajendran S, Lichtfouse E. Green polymeric nanomaterials for the photocatalytic degradation of dyes: a review. Env Chem Lett. (2020) 18:1569–80. 10.1007/s10311-020-01021-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanaoujiya R, Saroj SK, Srivastava S, Chaudhary MK. Renewable polysaccharide and biomedical application of nanomaterials. J Nanomat. (2022) 2022:1–16. 10.1155/2022/1050211 [DOI] [Google Scholar]
- 59.Morin-Crini N, Lichtfouse E, Torri G, Crini G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ Chem Lett. (2019) 17:1667–1692. 10.1007/s10311-019-00904-x [DOI] [Google Scholar]
- 60.Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. (2003) 125:13940–41. 267j. 10.1021/ja029267j [DOI] [PubMed] [Google Scholar]
- 61.Sharma M. Transdermal and intravenous nano drug delivery systems: present and future. In: Applications of Targeted Nano Drugs and Delivery Systems. Amsterdam: Elsevier; (2019). p. 499–550. [Google Scholar]
- 62.Bao Y, Ma J, Li N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr Polym. (2011) 84:76–82. 10.1016/j.carbpol.2010.10.061 [DOI] [Google Scholar]
- 63.Rather RA, Bhat MA, Shalla AH. An insight into synthetic and physiological aspects of superabsorbent hydrogels based on carbohydrate type polymers for various applications: a review. Carbohydr Polym Technol Appl. (2022) 3:100202. 10.1016/j.carpta.2022.100202 [DOI] [Google Scholar]
- 64.Li Z, Lin Z. Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate. (2021) 2:e21. 10.1002/agt2.21 [DOI] [Google Scholar]
- 65.Kansom T, Sajomsang W, Saeeng R, Charoensuksai P, Opanasopit P, Tonglairoum P. Apoptosis induction and antimigratory activity of andrographolide analog (3a1)-incorporated self-assembled nanoparticles in cancer cells. AAPS Pharm Sci Tech. (2018) 19:3123–33. 10.1208/s12249-018-1139-4 [DOI] [PubMed] [Google Scholar]
- 66.Zheng W, Li M, Lin Y, Zhan X. Encapsulation of verapamil and doxorubicin by MPEG-PLA to reverse drug resistance in ovarian cancer. Biomed Pharmacother. (2018) 108:565–73. 10.1016/j.biopha.2018.09.039 [DOI] [PubMed] [Google Scholar]
- 67.Cheng G, Zhang X, Chen Y, Lee RJ, Wang J, Yao J, et al. Anticancer activity of polymeric nanoparticles containing linoleic acid-SN38 (LA-SN38) conjugate in a murine model of colorectal cancer. Colloids Surf B. (2019) 181:822–9. 10.1016/j.colsurfb.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 68.Duse L, Agel MR, Pinnapireddy SR, Schäfer J, Selo MA, Ehrhardt C, et al. Photodynamic therapy of ovarian carcinoma cells with curcumin-loaded biodegradable polymeric nanoparticles. Pharmaceutics. (2019) 11:282. 10.3390/pharmaceutics11060282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nan Y. Lung carcinoma therapy using epidermal growth factor receptor-targeted lipid polymeric nanoparticles co-loaded with cisplatin and doxorubicin. Oncol Rep. (2019) 42:2087–96. 10.3892/or.2019.7323 [DOI] [PubMed] [Google Scholar]
- 70.Zu M, Ma L, Zhang X, Xie D, Kang Y, Xiao B. Chondroitin sulfate-functionalized polymeric nanoparticles for colon cancer-targeted chemotherapy. Colloids Surf B. (2019) 177:399–406. 10.1016/j.colsurfb.2019.02.031 [DOI] [PubMed] [Google Scholar]
- 71.Racoviceanu R, Trandafirescu C, Voicu M, Ghiulai R, Borcan F, Dehelean C, et al. Solid polymeric nanoparticles of albendazole: synthesis, physico-chemical characterization and biological activity. Molecules. (2020) 25:5130. 10.3390/molecules25215130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rani S, Sahoo RK, Nakhate KT, Ajazuddin Gupta U. Biotinylated HPMA centered polymeric nanoparticles for Bortezomib delivery. Int J Pharm. (2020) 579:119173. 10.1016/j.ijpharm.2020.119173 [DOI] [PubMed] [Google Scholar]
- 73.Liu C, Han Q, Liu H, Zhu C, Gui W, Yang X, et al. Precise engineering of Gemcitabine prodrug cocktails into single polymeric nanoparticles delivery for metastatic thyroid cancer cells. Drug Deliv. (2020) 27:1063–72. 10.1080/10717544.2020.1790693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, Chen C, Zhang X, He C, Zhao P, Li M, et al. Platinum complexes of curcumin delivered by dual-responsive polymeric nanoparticles improve chemotherapeutic efficacy based on the enhanced anti-metastasis activity and reduce side effects. Acta Pharm Sin B. (2020) 10:1106–21. 10.1016/j.apsb.2019.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ribeiro AF, Santos JF, Mattos RR, Barros EGO, Nasciutti LE, Cabral LM, et al. Characterization and in vitro antitumor activity of polymeric nanoparticles loaded with uncariatomentosa extract. An Acad Bras Cienc. (2020) 92:1–16. 10.1590/0001-3765202020190336 [DOI] [PubMed] [Google Scholar]
- 76.Lin H-H, Chen J-H, Wang C-J. Chemopreventive properties and molecular mechanisms of the bioactive compounds in Hibiscus sabdariffa Linne. Curr Med Chem. (2011) 18:1245–54. 10.2174/092986711795029663 [DOI] [PubMed] [Google Scholar]
- 77.Annaji M, Poudel I, Boddu SHS, Arnold RD, Tiwari AK, Babu RJ. Resveratrol-loaded nanomedicines for cancer applications. Cancer Rep. (2021) 4:e1353. 10.1002/cnr2.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mousa DS, El-Far AH, Saddiq AA, Sudha T, Mousa SA. Nanoformulated bioactive compounds derived from different natural products combat pancreatic cancer cell proliferation. Int J Nanomed. (2020) 15:2259–68. 10.2147/IJN.S238256 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Mouhid L, Corzo-Martínez M, Torres C, Vázquez L, Reglero G, Fornari T, et al. Improving in vivo efficacy of bioactive molecules: an overview of potentially antitumor phytochemicals and currently available lipid-based delivery systems. J Oncol. (2017) 2017: 7351976. 10.1155/2017/7351976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yun YH, Lee BK, Park K. Controlled drug delivery: historical perspective for the next generation. J Control Release. (2015) 219:2–7. 10.1016/j.jconrel.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vasile C, Pamfil D, Stoleru E, Baican M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules. (2020) 25:1539. 10.3390/molecules25071539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calzoni E, Cesaretti A, Polchi A, Michele AD, Tancini B, Emiliani C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J Funct Biomater. (2019) 10:1–15. 10.3390/jfb10010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Honey PJ, Rijo J, Anju A, Anoop KR. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm Sin B. (2014) 4:120–27. 10.1016/j.apsb.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. (2003) 2:347–60. 10.1038/nrd1088 [DOI] [PubMed] [Google Scholar]
- 85.Chivere VT, Kondiah PPD, Choonara YE, Pillay V. Nanotechnology-based biopolymeric oral delivery platforms for advanced cancer treatment. Cancers. (2020) 12:522. 10.3390/cancers12020522 [DOI] [PMC free article] [PubMed] [Google Scholar]