Abstract
Nipple adenomas (NAs) are benign neoplasms composed of papillary hyperplasia of the epithelium of the major lactiferous ducts. Patients with NA may report bloody nipple discharge and clinically may resemble Paget disease, raising concern for malignancy. Mammographically, NAs are often occult. US can show a hypervascular circumscribed mass centered within the nipple with varying echogenicity. Diagnosis is usually made on punch biopsy or excision, but breast radiologists should be aware of this entity. Malignancy can be found elsewhere in the ipsilateral or contralateral breast, or very rarely may directly extend to involve an NA, but published experience with concurrent malignancies is small. We describe the radiologic-pathologic correlation of NAs.
Keywords: nipple adenoma, nipple discharge, histopathology
Key Messages.
Nipple adenomas are rare benign neoplasms that can clinically resemble Paget disease and cause nipple erosion.
Nipple adenomas are almost always mammographically occult but may be seen on US as hypervascular oval masses that are usually isoechoic to hypoechoic and confined to the nipple or intensely enhancing oval masses in the nipple with washout kinetics on MRI.
Further study is needed to clarify the relationship, if any, between nipple adenomas and concurrent or increased risk of breast malignancy.
Introduction
Described in 1955 by Jones as “florid papillomatosis of the nipple ducts,” nipple adenomas (NAs) are benign proliferative neoplasms of the epithelium of the major lactiferous ducts and involve the superficial duct orifices of the nipple (1,2). They present clinically as unilateral, palpable, or visible changes in the nipple with friable tissue (Figure 1) with or without erythema, or they may be clinically occult. The nipple can appear eroded or focally ulcerated with resultant bleeding that may be perceived as bloody nipple discharge, and an exudative crust can form that mimics eczematoid change, as in Paget disease (2–5). Nipple adenoma is a rare diagnosis, most often occurring in adult women with average age of 45 years (4,6–8). Nipple adenomas have been reported in baby girls as young as 5 months of age (9), ranging up to women aged 70 years and older (4,6). Rare reports have documented NA in men (10). Nipple adenomas typically require biopsy or surgical excision for diagnosis (11–14).
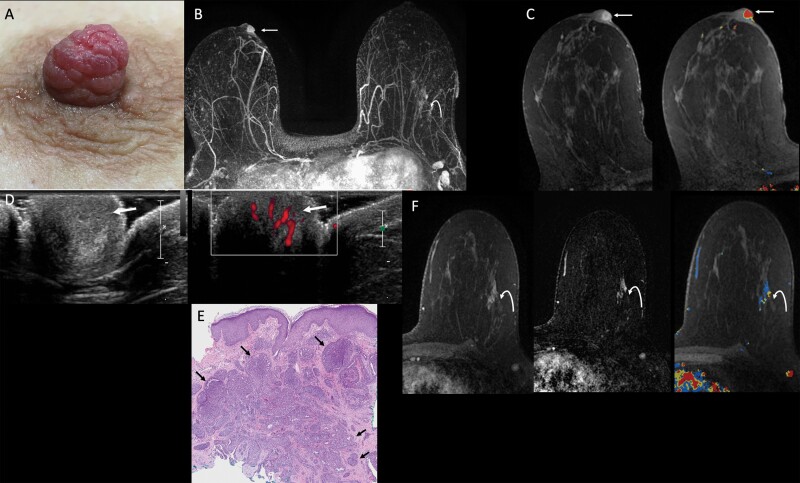
Figure 1.
Images of a 54-year-old female with a six-month history of spontaneous bloody right nipple discharge following poor healing of a nipple laceration. After initial mammographic and sonographic evaluation failed to show an etiology for the bloody discharge, she was referred for MRI. A: Clinical photograph demonstrates a prominent right nipple with hyperemic “raspberry-like” distortion of the nipple. B: Axial maximum intensity projection (MIP) image of first post-contrast MRI subtraction shows asymmetric strong enhancement of the right nipple (arrow) and non-mass enhancement in the outer left breast (curved arrow). C: Axial T1-weighted, fat-suppressed first post-contrast breast MRI shows a rim-enhancing 10-mm oval mass in the right nipple (left, arrow) with washout kinetics (right, arrow). D: MRI-directed transverse US of the right nipple (left) demonstrates a subtle, slightly hypoechoic, heterogeneous, 1-cm, ovoid mass within the right nipple (arrow), with internal vascularity on power Doppler (right, arrow). E: Histopathology (hematoxylin and eosin, 4x) from punch biopsy shows proliferating ductal structures with usual ductal hyperplasia consistent with nipple adenoma (arrows). Myoepithelial cell nuclei (which can be highlighted with immunohistochemical stain for p63) surround each individual ductal structure. F: Axial T1-weighted, fat-suppressed contrast-enhanced breast MRI with kinetic overlay better demonstrates a 3.3-cm linear non-mass enhancement in the outer left breast (curved arrow). The patient underwent MRI-guided biopsy, followed by breast-conserving surgery, with final pathology demonstrating two foci of invasive lobular carcinoma, measuring 1.6 cm and 0.8 cm, on a background of lobular carcinoma in situ. Two left axillary lymph nodes were negative for carcinoma. Surgical pathology from the right nipple confirmed nipple adenoma.
Imaging Findings
Nipple adenomas are often occult mammographically, secondary to small size and suboptimal evaluation of the nipple (8,13). The nipple may appear asymmetrically enlarged (15,16). Magnification views may be performed in the setting of nipple discharge, but calcifications are not typically seen in NA. Rather, suspicious calcifications or other suspicious findings may be noted elsewhere and may be due to concurrent malignancy (Figures 2 and 3).
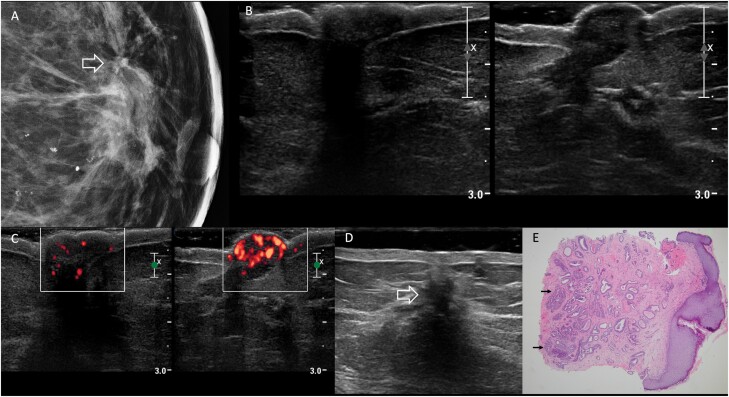
Figure 2.
Images of a 69-year-old woman with bloody left nipple discharge, retraction, and discoloration due to nipple adenoma. Physical examination reported firmness and bluish discoloration of the left nipple. A: Spot magnification mediolateral mammographic image of the left breast demonstrates an area of architectural distortion at the 12-o’clock position in the retroareolar left breast (arrow) with a few associated amorphous calcifications. The left nipple was mammographically unremarkable. B: On US, no discrete mass was seen in either nipple (right on left and left on right). C: Transverse power Doppler image shows hypervascularity of the left nipple (right-hand image) compared to the right. D: Additional transverse sonographic evaluation of the left breast (using a standoff pad) revealed a 15-mm hypoechoic, irregular mass at the 12-o’clock position, 1 cm from the nipple (open arrow), with posterior shadowing, corresponding to the mammographic distortion. US-guided core-needle biopsy showed radial scar with microcalcifications, ductal epithelial hyperplasia, and sclerosing adenosis; associated grade 1 ductal carcinoma in situ was found at excision. E: Histopathology (hematoxylin and eosin, 4x) from punch biopsy of the left nipple shows proliferation of irregular ductal structures (arrows), some of which demonstrate usual ductal hyperplasia, extending to multiple margins of the biopsy, consistent with nipple adenoma.
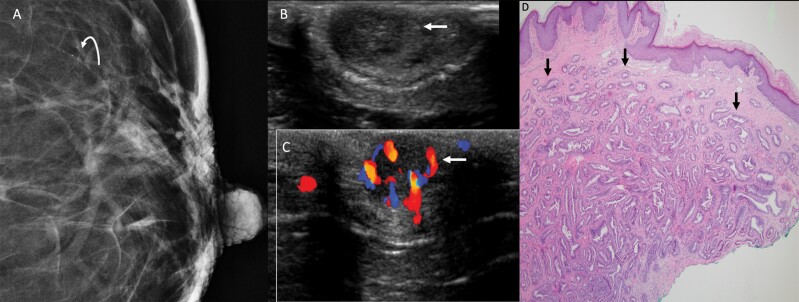
Figure 3.
This 66-year-old woman presented with non-spontaneous left bloody nipple discharge, with a nodular mass in the left nipple on exam. A: Spot magnification craniocaudal view of the left breast demonstrates fine linear calcifications spanning 5 mm approximately 3 cm from the nipple in the 1-o’clock position (curved arrow). No obvious nipple abnormality was evident mammographically. Transverse US of the left nipple (B) demonstrated a subtle, circumscribed, hypoechoic, 9-mm, oval mass (arrow) centered within the nipple, with minimal posterior enhancement, that was hypervascular on color Doppler (C) (arrow). D: Histopathology (hematoxylin and eosin, 4x) from nipple punch biopsy shows proliferation of irregular ductal structures (arrows) with usual ductal hyperplasia, consistent with nipple adenoma. This patient also underwent stereotactic left breast biopsy of the calcifications, which demonstrated ductal carcinoma in situ (DCIS), nuclear grade 2. Left segmental mastectomy including left nipple resection confirmed nipple adenoma. Atypical ductal hyperplasia, lobular carcinoma in situ, and atypical lobular hyperplasia were also seen in the 1-o’clock position, as well as multiple intraductal papillomas without atypia, ductal epithelial hyperplasia, and fibrocystic changes. No additional DCIS was seen in the surgical specimen.
On US, normal lactiferous ducts are hypo- to anechoic and extend from the nipple in a radial fashion (17). Sonographic evaluation of the nipple can be technically challenging, particularly because of acoustic shadowing deep to the nipple-areolar complex. Some techniques described to assist with optimal evaluation of the nipple on US include angling the probe from tissue adjacent to the nipple to evaluate tissues directly behind the nipple (using peripheral compression on the distal aspect of the transducer), using a glob of gel or a standoff pad (Figure 2), and using a rolled nipple technique (typically over the non-scanning index finger) to “flatten” the nipple itself and allow better visualization (13,18). Nipple adenomas can be seen as circumscribed, slightly hypoechoic masses centered within the nipple (Figures 1, 3 and 4). Internal vascularity is typically present (Figures 1–4) (8,12,13). Posterior features vary (8,15,19). Imaging features are indistinguishable from a papilloma within the nipple.
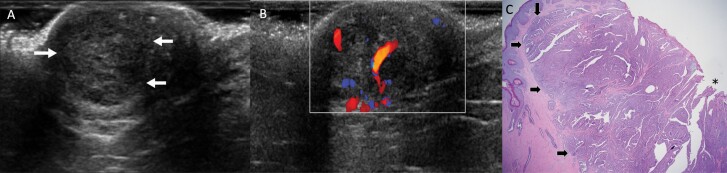
Figure 4.
Images of a 68-year-old postmenopausal female with itching and bleeding of the left nipple for several months due to nipple adenoma. On exam, the left nipple was swollen and erythematous with excoriation (not shown). Mammogram was unrevealing (not shown). Transverse US of the left breast (A) shows a subtle round circumscribed isoechoic mass (arrows) within the nipple, with posterior enhancement, and color Doppler (B) demonstrates increased internal vascularity within the mass. The patient was referred to surgery and underwent an excisional biopsy of the left nipple, which demonstrated nipple adenoma with focal skin ulceration, negative for malignancy. C: Histopathology (hematoxylin and eosin, 2x) demonstrates a predominantly papillary growth pattern (arrows) with epidermal ulceration (asterisk), consistent with nipple adenoma.
When seen on MRI, NAs can demonstrate hyperintense signal on pre-contrast T1- and T2-weighted imaging (19). Contrast-enhanced MRI will typically show avid homogeneous focal nipple enhancement with washout kinetics (Figure 1). Some NAs may be overlooked on MRI secondary to the normal nipple enhancement, but asymmetric increased enhancement can be observed (Figure 1) (13). Rim enhancement (Figure 1) and thickening of the nipple-areolar complex on MRI have uncommonly been observed (15,19).
Pathologic Findings
Early pathologic descriptions of NAs cited gray, firm, granular tumors on gross pathology, with microscopic cribriform patterns of papillary hyperplasia involving large lactiferous ducts (1,4). Di Bonito et al (3) described 13 cases of NA with clinical-pathologic correlates. Importantly, consistent with benign entities, a distinct double layer of epithelial and myoepithelial cells is maintained throughout the proliferation of ductules (3). The florid papillomatosis often seen in NA can mimic a large duct papilloma. Uncommonly, a superficially located solitary central intraductal papilloma can present with bloody nipple discharge but can be distinguished from NA as it is limited to a single ductal unit and lacks an adenomatous component that is present in NA (9,12).
Myoepithelial cells are arranged peripherally and can be highlighted with immunohistochemical stains, such as smooth muscle actin and p63 (3,15,20). There is no well-defined border to NA microscopically (Figures 1–4). The most common breast lesion in the differential diagnosis of NA is invasive ductal carcinoma, particularly tubular carcinoma, which can otherwise mimic sclerosing adenosis. Preservation of the myoepithelial cell layer rules out invasive ductal carcinoma.
The skin changes seen in NA can be attributed to ductal proliferation (Figure 4) in close proximity to the skin. The epithelium is easily abraded, causing the appearance of focal ulceration and possibly bleeding. In contrast, the skin changes in Paget disease result from infiltration of squamous epithelium by in situ neoplastic cells with disruption of squamous cell-cell junctions. The resulting scaling exudate and eczematoid changes are often rather extensive. There is often underlying ductal carcinoma in situ with or without invasive carcinoma in Paget disease. In contrast to ductal carcinoma in situ, necrosis is rarely observed in NA (2,4,21).
Discussion
Nipple adenoma is a benign entity that can be difficult to recognize clinically and on imaging, with presentation mimicking benign papilloma. The major diagnostic dilemma is distinguishing NA from Paget disease (3,17). Both NA and Paget disease can present with unilateral nipple enlargement or swelling, nipple nodules, skin changes including erosion, ulceration, or erythema, and/or sanguineous nipple discharge (4,5). Infection or inflammatory conditions have some overlapping features with NA but can be recognized as having distinct clinical presentation with erythema, edema, and skin thickening (12,19). Breast radiologists should include evaluation of the nipple itself and be aware of conditions that arise within the nipple.
Because of nonspecific clinical and imaging features, diagnosis largely relies on histologic and pathologic evaluation, with biopsy cited as the gold standard for diagnosis (14). Less aggressive histologic characteristics, including preserved myoepithelial layer and a lack of atypia, are consistent with benign etiology, although these are nonspecific and can be seen in other subareolar papillary-type tumors (1).
Traditionally, complete surgical excision is recommended, given the nonspecific presenting clinical findings, concern for malignant processes such as Paget disease associated with such findings (9,13), and to prevent recurrence (5,11,22). Delayed resection is suggested in pediatric cases because of the increased morbidity involved with partial nipple removal prior to breast maturity (9).
A search of our pathology database from 2007 to 2021 under an institutionally approved quality improvement protocol revealed 32 women with NA, 31 of which had adequate clinical data. One woman was diagnosed with NA at the age of 38 years upon surgical excision and had local recurrence approximately six years later. Of the 31 cases, 6 (19%) had concurrent breast malignancy at the time of NA diagnosis, including 3 of the presented cases (Figures 1–3). Four of these concurrent malignancies were elsewhere in the ipsilateral breast and 2 were in the contralateral breast. There is no known direct association of NA with malignancy, though the published experience with NA is small. A small case series of 5 patients reported concurrent diagnoses of either infiltrating ductal carcinoma or intraductal carcinoma (23). Another study demonstrated concurrent ipsilateral carcinoma in 14% (7/49) of women with NA (24). Importantly, there may be ascertainment bias in that women with current breast cancer will undergo careful clinical evaluation of their breasts by a breast surgeon that may lead to diagnosis of incidental NA. Nipple adenoma may be asymptomatic and occult on imaging, and thus never be reported in some patients (19). Additionally, patients presenting with concurrent NA and carcinomas may be more often reported in the literature. Further study is necessary to discern whether NA could comprise a type of high-risk lesion and potentially warrant, for example, MRI evaluation for concurrent malignancy prior to excision.
Conclusion
In conclusion, NA is a benign diagnosis involving the major lactiferous ducts. Although benign, the nonspecific clinical presentation and features resembling Paget disease account for the necessity of biopsy and/or resection. Imaging features are subtle and awareness of this entity may facilitate its recognition and radiologic-pathologic correlation. Imaging may show concurrent malignancy elsewhere. Nipple adenoma can locally recur if not completely excised. Breast radiologists should be aware of this uncommon cause of clinical change in the appearance of the nipple and perceived nipple discharge.
Contributor Information
Madeline E Leo, University of Pittsburgh Medical Center, Department of Radiology, Pittsburgh, PA, USA.
Gloria J Carter, Magee-Womens Hospital of UPMC, Department of Pathology, Pittsburgh, PA, USA.
Uzma Waheed, University of Pittsburgh School of Medicine, Magee-Womens Hospital of UPMC, Department of Radiology, Pittsburgh, PA, USA; Stanford University School of Medicine, Department of Radiology, Palo Alto, CA, USA.
Wendie A Berg, University of Pittsburgh School of Medicine, Magee-Womens Hospital of UPMC, Department of Radiology, Pittsburgh, PA, USA.
Funding
W.A.B. is supported by grants from the National Cancer Institute, R01CA187593, and the Breast Cancer Research Foundation, BCRF 020-015.
Conflict of Interest Statement
U.W. is a consultant to Becton Dickinson not related to this work. W.A.B. serves as Associate Editor for the Journal of Breast Imaging. As such, she was blinded to reviews and the management of this paper was performed by Dr Jennifer Harvey, Editor-in-Chief. W.A.B. is voluntary Chief Scientific Advisor to DenseBreast-info.org and discloses grant support to the Department of Radiology from Koios Medical, Inc., for which she is Principal Investigator, unrelated to this work. Other authors have nothing to disclose.
References
- 1. Jones DB. Florid papillomatosis of the nipple ducts. Cancer 1955;8(2):315–319. [DOI] [PubMed] [Google Scholar]
- 2. Lester SC, Lee AHS.. Nipple adenoma. In: World Health Organization Classification of Tumours, Breast Tumours. 5th ed. Vol 2. Lyons, France: International Agency for Research on Cancer, 2019:182–183. [Google Scholar]
- 3. Di Bonito M, Cantile M, Collina F, et al. Adenoma of the nipple: a clinicopathological report of 13 cases. Oncol Lett 2014;7(6):1839–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perzin KH, Lattes R.. Papillary adenoma of the nipple (florid papillomatosis, adenoma, adenomatosis). A clinicopathologic study. Cancer 1972;29(4):996–1009. [DOI] [PubMed] [Google Scholar]
- 5. Wang C, Wang X, Ma R.. Diagnosis and surgical treatment of nipple adenoma. ANZ J Surg 2015;85(6):444–447. [DOI] [PubMed] [Google Scholar]
- 6. Aftab K, Idrees R.. Nipple adenoma of breast: a masquerader of malignancy. J Coll Physicians Surg Pak JCPSP 2010;20(7):472–474. [PubMed] [Google Scholar]
- 7. Stone K, Wheeler A.. A review of anatomy, physiology, and benign pathology of the nipple. Ann Surg Oncol 2015;22(10):3236–3240. [DOI] [PubMed] [Google Scholar]
- 8. Parajuly SS, Peng YL, Zhu M, Gang YZ, Gyawali S.. Nipple adenoma of the breast: sonographic imaging findings. South Med J 2010;103(12):1280–1281. [DOI] [PubMed] [Google Scholar]
- 9. Clune JE, Kozakewich HP, VanBeek CA, Labow BI, Greene AK.. Nipple adenoma in infancy. J Pediatr Surg 2009;44(11):2219–2222. [DOI] [PubMed] [Google Scholar]
- 10. Fernandez-Flores A, Suarez-Peñaranda JM.. Immunophenotype of nipple adenoma in a male patient. Appl Immunohistochem Mol Morphol 2011;19(2):190–194. [DOI] [PubMed] [Google Scholar]
- 11. Ansari MS, Taghizadeh Fazli J, Ehsani A.. Dermoscopy of nipple adenoma. Clin Case Rep 2020;8(12):3254–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiorean A, Pintican RM, Szep M, et al. Nipple ultrasound: a pictorial essay. Korean J Radiol 2020;21(8):955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyons D, Wahab RA, Vijapura C, Mahoney MC.. The nipple-areolar complex: comprehensive imaging review. Clin Radiol 2021;76(3):172–184. [DOI] [PubMed] [Google Scholar]
- 14. Spohn GP, Trotter SC, Tozbikian G, Povoski SP.. Nipple adenoma in a female patient presenting with persistent erythema of the right nipple skin: case report, review of the literature, clinical implications, and relevancy to health care providers who evaluate and treat patients with dermatologic conditions of the breast skin. BMC Dermatol 2016;16(1):4. doi: 10.1186/s12895-016-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsubayashi RN, Adachi A, Yasumori K, et al. Adenoma of the nipple: correlation of magnetic resonance imaging findings with histologic features. J Comput Assist Tomogr 2006;30(1):148–150. [DOI] [PubMed] [Google Scholar]
- 16. Tsushimi T, Enoki T, Takemoto Y, et al. Adenoma of the nipple, focusing on the contrast-enhanced magnetic resonance imaging findings: report of a case. Surg Today 2011;41(8):1138–1141. [DOI] [PubMed] [Google Scholar]
- 17. Da Costa D, Taddese A, Cure ML, Gerson D, Poppiti R, Esserman LE.. Common and unusual diseases of the nipple-areolar complex. Radiographics 2007;27(Suppl 1):S65–S77. [DOI] [PubMed] [Google Scholar]
- 18. Yoon JH, Yoon H, Kim EK, Moon HJ, Park YV, Kim MJ.. Ultrasonographic evaluation of women with pathologic nipple discharge. Ultrason Seoul Korea 2017;36(4):310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Besim H, Deren O, Kaptanoğlu AF, Bas K, Çomunoğlu C, Alicioğlu B.. Florid papillomatosis of the nipple. Am Surg 2013;79(5):E214–E216. [PubMed] [Google Scholar]
- 20. Yang GZ, Li J, Ding HY.. Nipple adenoma: report of 18 cases with review of literatures. Zhonghua Bing Li Xue Za Zhi 2009;38(9):614–616. [PubMed] [Google Scholar]
- 21. Sanders MA, Brock JE, Harrison BT, et al. Nipple-invasive primary carcinomas: clinical, imaging, and pathologic features of breast carcinomas originating in the nipple. Arch Pathol Lab Med 2018;142(5):598–605. [DOI] [PubMed] [Google Scholar]
- 22. Tatterton MR, Fiddes R.. Nipple adenoma: a review of the literature. Ann Breast Surg 2019;3:29. [Google Scholar]
- 23. Jones MW, Tavassoli FA.. Coexistence of nipple duct adenoma and breast carcinoma: a clinicopathologic study of five cases and review of the literature. Mod Pathol 1995;8(6):633–636. [PubMed] [Google Scholar]
- 24. Rosen PP, Caicco JA.. Florid papillomatosis of the nipple. A study of 51 patients, including nine with mammary carcinoma. Am J Surg Pathol 1986;10(2):87–101. [PubMed] [Google Scholar]