Abstract
Background
Gestational diabetes mellitus (GDM) is a common metabolic disorder. Hyperglycemia may cause gestational hypertension, increase the probability of infection, abnormal embryonic development, and increase the abortion rate. Oral hypoglycemic drugs may be another effective means of blood glucose control in addition to insulin injection. We included controlled clinical studies for meta-analysis to understand the effect of oral hypoglycemic drugs in gestational diabetes.
Methods
The databases were searched with the keywords “Glycemic control” & “gestational diabetes”: Embase (January, 2000–August, 2021), Pubmed (January, 2000–August, 2021), Web of Science (January, 2000–August, 2021), Ovid (January, 2000–August, 2021), and ClinicalTrials.org to obtain the randomized controlled trial (RCT) literatures related to the treatment of gestational diabetes with oral hypoglycemic drugs, after screening, the R language toolkit was used for the analysis.
Results
A total of 10 articles with a total of 1,938 patients were included, 7 studies used metformin as an hypoglycemic agent. Meta-analysis showed that oral metformin had no significant difference in fasting blood glucose levels after the intervention compared with insulin injection [MD = −0.35, 95%CI(−0.70,1.40), Z = 0.66, P = 0.51], with no significant difference in postprandial blood glucose levels after intervention [MD = −2.20, 95%CI(−5.94,1.55), Z = −1.15, P = 0.25], and no statistical difference in glycosylated hemoglobin [MD = 0.10, 95%CI(−0.17,−0.04), Z = −0.94, P = 0.31]. Metformin was more conducive to reducing maternal weight during pregnancy than insulin [MD = −1.55, 95%CI(−2.77,−0.34), Z = −2.5, P = 0.0123], metformin reduced the abortion rate compared with insulin [RR = 0.81, 95%CI(0.63,1.05), Z = −2.61, P = 0.015], and reduced cesarean section rate [RR = 0.66, 95%CI(0.49,0.90), Z = −3.95, P = 0.0001].
Discussion
The application of oral hypoglycemic drug metformin in blood glucose control of gestational diabetes can play a hypoglycemic effect equivalent to insulin and can control the weight of pregnant women, reduce the rate of abortion and cesarean section, and improve pregnancy outcomes.
Keywords: gestational diabetes mellitus, glycemic control, insulin, oral hypoglycemic agents, meta-analysis
Introduction
Gestational diabetes mellitus (GDM) is a common metabolic disorder that refers to varying degrees of abnormal glucose metabolism that occurs for the first time during pregnancy (1). It has been reported its incidence ranges from 1.32% to 3.75%. Gestational diabetes is specific, and the parturient has no history of diabetes before pregnancy (2). But due to a variety of physiological changes during pregnancy, the reabsorption of glucose by the renal tubules is weakened, so that the sugar content in the urine is high, which in turn can cause diabetes, which is gestational diabetes (3). The effect of gestational diabetes on maternal and fetal outcomes is related to the degree of glycemic control (4). Hyperglycemia may cause maternal gestational hypertension, increase the chance of infection, and may also cause abnormal embryonic development and increase the rate of miscarriage (5). Some patients can achieve the expected blood glucose range through lifestyle intervention, including changing lifestyle, reasonable diet, appropriate exercise, prevention of infection, and regular testing of blood glucose levels (6). However, some patients fail to reach the ideal blood glucose level and still need drugs for intervention (7). Injection of insulin is the most common method of blood glucose control. Besides, compared with insulin, that oral hypoglycemic agent is convenient to use and ideal for hypoglycemic effect. And the efficacy and safety of oral hypoglycemic drugs in GDM patients have been reported, but there is still a lack of systematic evaluation and comprehensive analysis in clinical practice (8). In order to understand the efficacy of oral hypoglycemic agents in the treatment of gestational diabetes, we included controlled clinical studies for meta-analysis to provide evidence for the clinical treatment of this disease.
Method
Inclusion of Studies
We followed the PICOS principle to develop inclusion criteria (P-participants, I-intervention, C-control, O-outcome, S-study type): (1) Study type: The literatures published after January, 2000 were limited to randomized controlled trials (RCTs), the language was English, and individual cases, guidelines, systematic analysis, and case-control studies of non-RCT studies were excluded. (2) Study subjects: The participants were pregnant women aged 18–45 years, 14–35 weeks of gestational age (GA), diagnosed with diabetes (we did not limit pregnant women to type 1 or type 2 diabetes), fasting blood glucose ≥7.0 mmol/L [126 mg/dl], and HbA1c ≥48 mmol/mol [≥6.5%] (9)). (3) Grouping and control: randomization must be taken in the study, we do not limit the randomization method (computer random number or manual random number), we do not limit the allocation concealment and blind method, but we will perform the quality assessment of the literature. (4) Intervention method: All patients were given routine prenatal care and iron, calcium, folic acid, and vitamin D supplementation after enrollment, all patients were given regulation from the diet and lifestyle, if the regulation failed (fasting blood glucose higher than 95 mg/dl and postprandial blood glucose higher than 40 mg/dl), the intervention was carried out. The control group was given conventional insulin injection, and the observation group was given hypoglycemic drugs (biguanides or Glinides). (5) Outcome indicators: The literature must provide observation indicators and statistical methods, provide outcome data, or indicate the accessible storage address of data.
Literature Search Strategy
Search database: Embase (January, 2000–August, 2021), Pubmed (January, 2000–August, 2021), Web of Science (January, 2000–August, 2021), Ovid (January, 2000–August, 2021), and ClinicalTrials.org. The search method was keyword rapid search, and the input keywords were: “Glycemic control” and “gestational diabetes.”
Selection of Literatures
SCREEN and inclusion of articles were done independently by two researchers, and in case of discrepancies during this process, a third person was consulted for agreement. After the initial search, we combined all retrieved articles with “. Enw” is reserved with suffix name and is managed uniformly after imported by Endnote X9 software. The software menu of “References” -> “find duplicates” allows the software to de duplication the retrieved literatures, and then browse the title and author of the literatures by manual method. For the literatures with a similar title and the same author, browse the abstract of the literatures. If the time, place, and number of participants of the study coincide, it is considered that the study is repeated. We only retain the literatures with the later publication time. By reading the title and abstract of the literature for preliminary screening, we remove the literature that obviously does not meet the inclusion requirements; for the remaining literature, we use the “Find full text” function of the software to obtain the full text of the literature. For some unobtainable literature, we search the database of the literature or the publication magazine to obtain the full text of the literature; if the literature cannot be obtained through the network, we try to contact the original author (Find via email) to obtain the original text; if it still fails, we exclude the literature. Literatures that were obtained were read and checked for completeness of literature data, and articles with missing data were excluded.
Data Extraction
After obtaining the full text of the literature, we use the self-made data table to extract the data information in the literature. Include the following contents: (1) Basic data of the literature: publication time, author, and region; (2) Characteristics of the study subjects: patient age, race, BMI, family history of diabetes, whether the first pregnancy, hypertension during pregnancy, fasting blood glucose, blood glucose (breakfast, lunch, and dinner), and glycosylated hemoglobin (HbA1c); (3) Literature intervention methods: grouping method, number of participants in each group, grouping intervention method, intervention time, and follow-up time; (4) Outcome data.
Outcome Indicators
Blood glucose control indicators: (a) fasting glycemia; (b) postprandial glycemia after lunch; (c) HbA1c postpartum;
Maternal situation and obstetric outcome indicators: (a) maternal weight gain; (b) abortion rate; (c) cesarean deliveries.
Statistical Methods
We used R language development environment (R version 4.1.2 released by “The R foundation for statistical computing”) to summarize and analyze the data of multiple studies. We entered the key data into CSV files, read the data under RGUI, and used Meta tool of RGUI environment (metabin/metacont/metainf/metabias/funnel) to obtain the summary data of continuous variables and binary variables. MD (mean, difference) effect size was used for continuous variables, and RR (Risk Ratio) effect size was used for dichotomous variables, with 95%CI as the confidence interval, and P < 0.05 was considered statistically significant. For the heterogeneity among different studies, I2 test was used for the analysis and Q check. The heterogeneity was not statistically significant when I2 < 50% or P ≥ 0.1, that means there was no (or acceptable) heterogeneity among the literatures, otherwise it indicated that there was heterogeneity among the literatures; if there was no statistical heterogeneity among the literatures, the fixed-effect model was used; if there was heterogeneity, the random effect model was used; the analysis results were presented in forest plot; publication bias was reported in the funnel plot.
Heterogeneity Investigation and Sensitivity Analysis
We try to analyze the heterogeneous literatures to determine the source of heterogeneity.
Results
Literature Screening Results
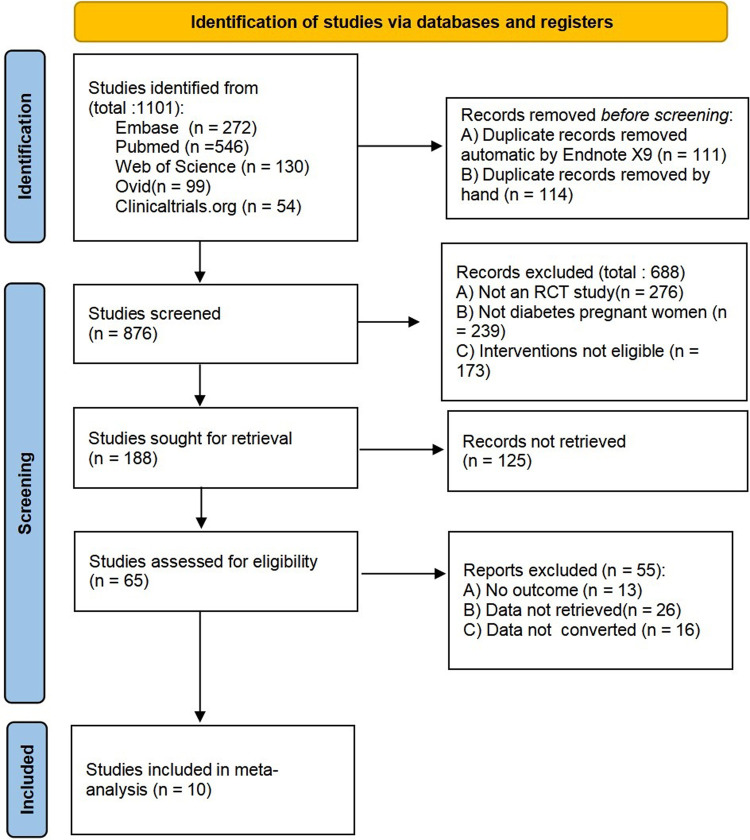
In this search, 1,101 literatures were initially searched, 10 literatures (10–19) were finally included, 1,938 patients were included, and we listed three typical cases for exclusion: (a) the literature (20) was a retrospective observational study, so it was excluded; (b) the literature (21) was a pilot study, the number of patients included was too small, 14 cases in total; (c) the literature (22) was an observational study, without comparative data. The selection flowchart is shown in Figure 1.
Figure 1.
The selection flow chart.
Basic Characteristics of Literatures
The published years of the studies included in this meta-analysis ranged from 2012 to 2021. The study subjects were all pregnant women with diabetes, aged 18–45 years. The minimum number of patients in the group was 32, and the maximum number was 253. Among them, three studies used glyburide as a hypoglycemic drug, while seven studies used metformin as a hypoglycemic drug, as shown in Table 1.
Table 1.
Basic characteristics, intervention measures, follow-up time, and outcome indicators of the included literatures.
| Author | Year of publication | Women age (years) | BMI (kg/m2) | Population (E/C) | Intervention group | Control group | Outcome indicators |
|---|---|---|---|---|---|---|---|
| Picón-César MJ et al. (10) | 2021 | 34.86 ± 4.83 | 30.42 ± 5.42 | 100/100 | Metformina Sandoz 850 mg/d, maximum 2,550 mg/d | Insulin 0.1 IU/kg/meal | (a) (b) (c) (d) (e) (f) |
| Kulshrestha V et al. (11) | 2021 | 29.7 ± 4.4 | 25.5 ± 4.0 | 49/50 | Metformin 1,000 mg twice daily | Insulin 0.1 IU/kg/meal | (d) (f) |
| Feig DS et al. (12) | 2016 | 34.7 ± 5.0 | 35.0 ± 7.1 | 253/249 | Metformin 1,000 mg twice daily | Insulin 0.1 IU/kg/meal | (c) (e) (f) |
| Casey BM et al. (13) | 2015 | 31.3 ± 6 | 29.0 ± 4.8 | 189/186 | Glyburide maximum of 20 mg per day | Insulin 0.1 IU/kg/meal | (d) (e) (f) |
| Beyuo T et al. (14) | 2015 | 33.51 ± 4.67 | 33.47 ± 6.95 | 113/117 | Metformin start dose 500 mg/d, max 2,500 mg/d | Insulin 0.1 IU/kg/meal | (a) (b) |
| Ainuddin J et al. (15) | 2015 | 30.6 ± 2.9 | N/A | 43/75 | Metformin start dose 500 mg/d, max 2,500 mg/d | Insulin 0.1 IU/kg/meal | (a) (b) (c) (d) (e) |
| Mirzamı M et al. (16) | 2015 | 29.50 ± 4.06 | N/A | 37/59 | 1.25 mg glyburide with morning meal | Insulin 0.4 unit/kg | (a) (b) |
| Spaulonci CP et al. (17) | 2013 | 31.93 ± 6.02 | 31.96 ± 4.75 | 47/47 | Initial metformin dose of 1,700 mg/d (850 mg three times a day) | Insulin 0.4 unit/kg | (a) (b) |
| Tempe A et al. (18) | 2013 | N/A | N/A | 32/32 | Glyburide 2.5 mg orally as the initial dose | Insulin 0.4 unit/kg | (e) (f) |
| Niromanesh S et al. (19) | 2012 | 30.7 ± 5.5 | 28.1 ± 4.0 | 80/80 | initial metformin dose of 500 mg | Insulin 0.7 U/kg/d | (a) (b) (c) (d) (f) |
Abbreviation: E indicates the intervention group and C indicates the control group.
Outcomes: (a) Fasting blood glucose; (b) Postprandial glycemia after lunch; (c) HbA1c; (d) Maternal weight gain; (e) Abortion rate; (f) Cesarean section rate.
Bias Risk Assessment and Quality Evaluation of the Included Literatures
The use of Cochrane handbook for systematic reviews of interventions for risk of bias assessment in the included literature is shown in Table 2, all literatures had a detailed description for randomization and drop-out cases, without selective reporting of risk of bias and other risks. The literatures (10, 16) reported blindness, while the literatures (18) did not specify allocation concealment, which may cause selective risk.
Table 2.
Risk of bias assessment and quality evaluation based on Cochrane Collaboration.
| Study | Random sequence generation | Classification hiding | Blind method | Data integrity | Optional reporting | Other bias | Quality evaluation |
|---|---|---|---|---|---|---|---|
| Picón-César MJ et al. (10) | Low | Low | Unclear | Low | Low | Low | B |
| Kulshrestha V et al. (11) | Low | Low | Low | Low | Low | Low | A |
| Feig DS et al. (12) | Low | Low | Low | Low | Low | Low | A |
| Casey BM et al. (13) | Low | Low | Low | Low | Low | Low | A |
| Beyuo T et al. (14) | Low | Low | Low | Low | Low | Low | A |
| Ainuddin J et al. (15) | Low | Low | Low | Low | Low | Low | A |
| Mirzamı M et al. (16) | Low | Low | Unclear | Low | Low | Low | B |
| Spaulonci CP et al. (17) | Low | Low | Low | Low | Low | Low | A |
| Tempe A et al. (18) | Low | Unclear | Low | Low | Low | Low | B |
| Niromanesh S et al. (19) | Low | Low | Low | Low | Low | Low | A |
Meta-Analysis Results
Fasting Blood Glucose (mg/dl)
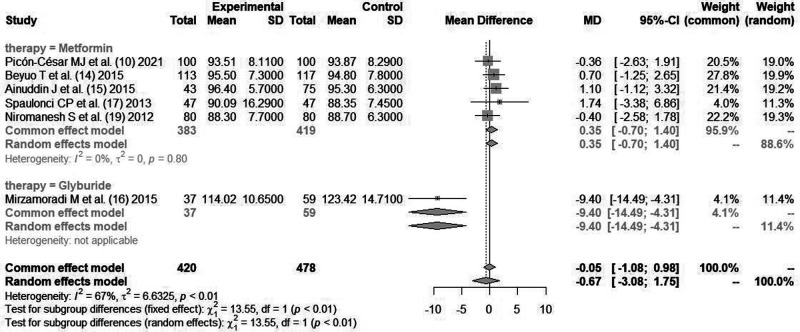
A total of six literatures (10, 14–17, 19) reported the fasting blood glucose of pregnant women after blood glucose control intervention, with heterogeneity between the literatures (I2 = 67%, P < 0.01). The random effect mode combined analysis was used. There was no statistically significant difference in fasting blood glucose level after intervention between hypoglycemic drugs and insulin [MD = −0.67, 95%CI(−3.08,1.75), Z = 0.87, P = 0.25].
The study was further divided into two subgroups according to hypoglycemic drugs (metformin group and glibenclamide group). Metformin included five literatures. There was no statistically significant heterogeneity between the literatures (I2 = 0%, P = 0.80). The pooled effect size for fasting blood glucose level after the intervention compared with insulin was [MD = −0.35, 95%CI(−0.70,1.40), Z = 0.66, P = 0.51]. The glibenclamide group contained only one article, and its effect size on fasting blood glucose compared with insulin was [MD = −9.40, 95%CI(−14.49,−4.31), Z = −3.62, P = 0.0003], as shown in Figure 2.
Figure 2.
Comparison of fasting glycemia after glycemic control during pregnancy.
Postprandial Glycemia After Lunch (mg/dl)
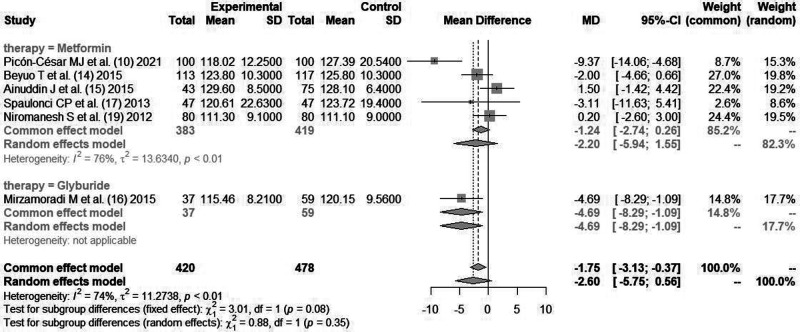
A total of six literatures (10, 14–17, 19) reported the blood glucose content of pregnant women after lunch after blood glucose control intervention, with heterogeneity between the literatures (I2 = 74%, P < 0.01). The random effects model combined analysis was used. There was no statistically significant difference in postprandial blood glucose level after intervention between hypoglycemic drugs and insulin [MD = −2.60, 95%CI(−5.75,0.56), Z = −1.61, P = 0.11].
The patients were further divided into two subgroups according to hypoglycemic drugs (metformin group and glibenclamide group). Metformin included five literatures. There was statistically significant heterogeneity between the literatures (I2 = 76%, P < 0.01). The pooled effect size for postprandial blood glucose level after the intervention compared with insulin was [MD = −2.20, 95%CI(−5.94,1.55), Z = −1.15, P = 0.25]. There was only one article in the glibenclamide group, and the effect size on blood glucose compared with insulin was [MD = −4.69, 95%CI(−8.29,−1.09)], as shown in Figure 3.
Figure 3.
Comparison of postprandial glycemia after lunch during pregnancy.
Glycosylated Hemoglobin (HbA1c) (%)
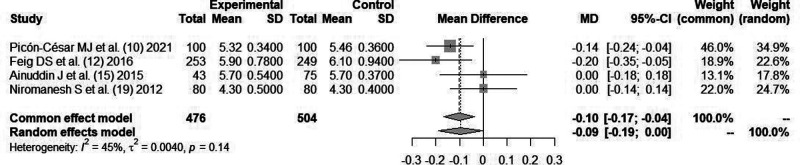
A total of four literatures (10, 12, 15, 19) reported the changes of glycated hemoglobin index after blood glucose control. All studies used metformin as the hypoglycemic agent. Since there was no statistical heterogeneity between the literatures (I2 = 45%, P = 0.14), the fixed-effect mode combined analysis was used. There was no statistical difference in glycosylated hemoglobin between metformin and insulin for blood glucose control [MD = 0.10, 95%CI(−0.17,−0.04), Z = −0.94, P = 0.31], as shown in Figure 4.
Figure 4.
Comparison of postpartum glycosylated hemoglobin (HbA1c postpartum) after glycemic control during pregnancy.
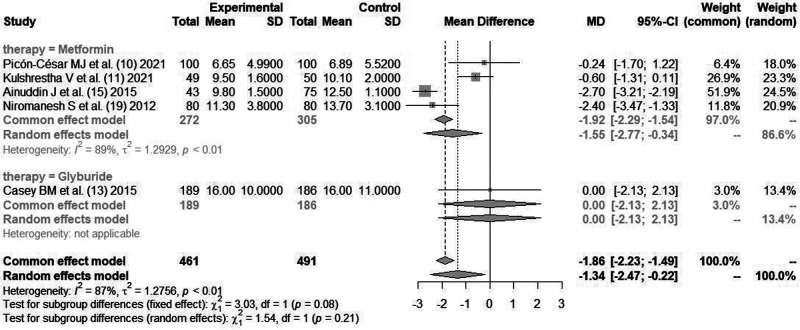
Maternal Weight Gain (kg)
Four literatures (10, 11, 15, 19) reported maternal weight gain indicators after glycemic control with metformin. Cause there was statistical heterogeneity between the literatures (I2 = 87%, P < 0.01), the random effects model combined analysis was used. There was a statistical difference in maternal weight gain between metformin and insulin for glycemic control [MD = −1.55, 95%CI(−2.77,−0.34), Z = −2.5, P = 0.0123], as shown in Figure 5.
Figure 5.
Comparison of maternal weight gain after blood glucose control during pregnancy.
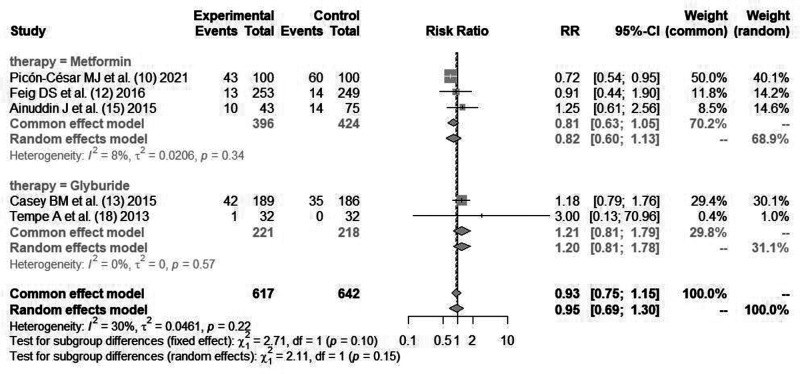
Abortion Rate
Three literatures (10, 12, 15) reported the indicators of maternal abortion rate after using metformin for blood glucose control. Cause there was no statistical heterogeneity between the literatures (I2 = 8%, P = 0.34), the fixed effect mode combined analysis was used. There was statistical difference in maternal abortion rate between metformin and insulin for blood glucose control [RR = 0.81, 95%CI(0.63,1.05), Z = −2.61, P = 0.015].
Two literatures (13, 18) reported the indicators of maternal abortion rate after using glibenclamide for blood glucose control. Cause there was no statistical heterogeneity between the literatures (I2 = 0%, P = 0.57), the fixed effect mode combined analysis was used. There was no statistical difference in maternal abortion rate between glibenclamide and insulin for blood glucose control [RR = 1.21, 95%CI(0.81,1.79), Z = 0.93, P = 0.35], as shown in Figure 6.
Figure 6.
Comparison of maternal abortion rate after blood glucose control during pregnancy.
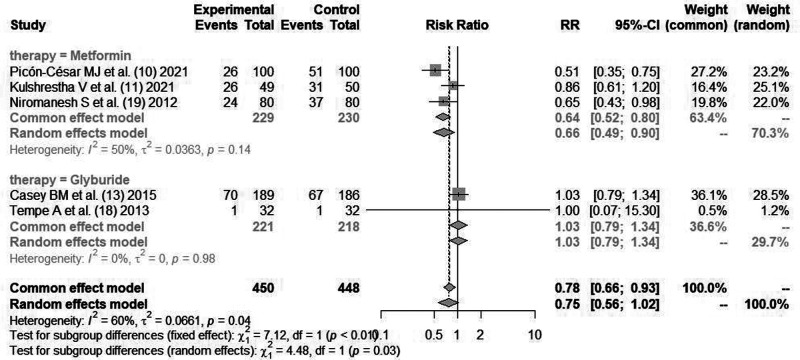
Cesarean Section Rate
Three literatures (10, 11, 19) reported the indicators of cesarean section rate of parturients after glycemic control with metformin. Cause there was statistical heterogeneity between the literatures (I2 = 50%, P = 0.14), the random effects model was used for combined analysis. There was statistical difference in cesarean section rate between metformin and insulin for glycemic control [RR = 0.66, 95%CI(0.49,0.90), Z = −3.95, P = 0.0001].
Two literatures (13, 18) reported the indicators of maternal cesarean section rate after using glibenclamide for blood glucose control. Cause there was no statistical heterogeneity between the literatures (I2 = 0%, P = 0.98), the fixed effect mode combined analysis was used. There was no statistical difference in maternal cesarean section rate between glibenclamide and insulin for blood glucose control [RR = 0.78, 95%CI(0.66,0.93), Z = 0.88, P = 0.44], as shown in Figure 7.
Figure 7.
Comparison of cesarean section rate after blood glucose control during pregnancy.
Heterogeneity Investigation and Sensitivity Analysis
In the analysis of fasting blood glucose, six articles (10, 14–17, 19) had heterogeneity (I2 = 67%, P < 0.01), but after being divided into two subgroups according to glucose-controlling drugs, five articles within the metformin group had no heterogeneity (I2 = 0%, P = 0.80), which suggested that glucose-controlling drugs were the greatest source of heterogeneity.
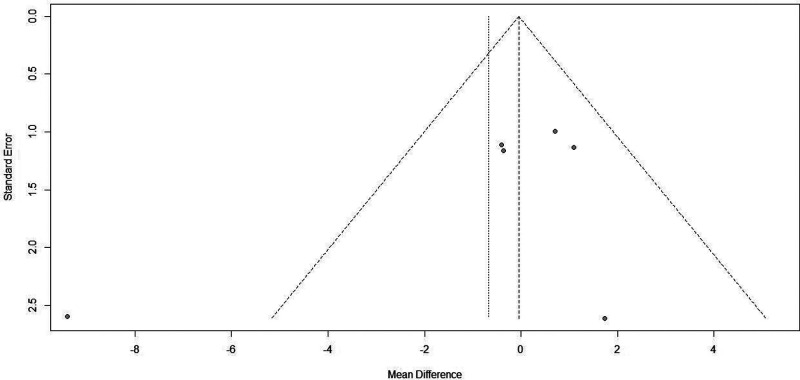
Analysis of Publication Bias
In the analysis of fasting blood glucose, the funnel plot showed that the two sides were not evenly distributed, suggesting the presence of publication bias, as shown in Figure 8.
Figure 8.
Funnel plot of fasting blood glucose indicators after blood glucose control during pregnancy.
Discussion
Ten RCTs with a total of 1,938 participants were included in this study, including seven studies using metformin as an oral hypoglycemic agent and three studies using glibenclamide as an oral hypoglycemic agent. The results of this study showed that the use of metformin as an oral hypoglycemic agent in gestational diabetes had no significant difference in glycemic control (fasting blood glucose, blood glucose, and glycosylated hemoglobin) compared with the utility of insulin injection, but the use of metformin could control maternal weight and improve pregnancy outcomes (reduce the rate of miscarriage and cesarean section). Both domestic and foreign guidelines recommend metformin as a first-line hypoglycemic drug. For patients with gestational diabetes, glucose control can be performed by intramuscular injection of insulin. Metformin, as a common hypoglycemic agent, promotes glucose uptake by target cells in the body, thereby regulating blood glucose levels (23). Studies (24) have revealed that metformin is mainly absorbed by the small intestine after oral administration, is not metabolized by the liver in the body, is mainly excreted unchanged by the kidney with the urine, and metformin itself has no hepatorenal toxicity and can be used normally in patients with normal liver and kidney function, so it has no negative impact on maternal and fetal outcomes. In addition, metformin belongs to the biguanide class of hypoglycemic agents, which control blood glucose by oral administration and can improve insulin therapy by improving insulin sensitivity, so it can be used in combination with insulin to better control blood glucose (25). But it is worth noting that during insulin therapy, the dose needs to be continuously adjusted, otherwise it will lead to hypoglycemic symptoms in patients, whether the blood glucose level is too high or too low, which will affect the safety of mothers and infants (26).
Glibenclamide is the second generation of sulfonylurea long-acting secretagogue, which produces the hypoglycemic effect by stimulating insulin cells to release insulin. It is suitable for mild and moderate non-insulin-dependent diabetes mellitus with unsatisfactory efficacy when diet is used alone. The results of the literature showed that the glibenclamide used as a glucose-controlling drug during pregnancy was superior to insulin therapy in lowering fasting blood glucose, but the evidence was insufficient cause too few articles were included. In a study by Moore LE et al. (27), metformin was compared with glibenclamide in gestational diabetes and found to have a 2.1-fold higher rate of glucose control failure with metformin than with glibenclamide. The efficacy and safety of glibenclamide remain to be deeply explored by more RCT studies.
The results of the study by Ashoush S et al. (28) showed that metformin in combination with insulin may be a better option for some patients whose glycemic control cannot be achieved with metformin. Literature (15) counted the cost of oral hypoglycemic agents using metformin throughout pregnancy, which was 4.02 ± 1.1 USD, much less than 24.83 ± 8.3 USD using insulin, which shows that metformin has the advantage of low price.
In this study, there was still heterogeneity in the metformin application group (blood glucose index), which may be related to the dynamic application adjustment of metformin in the study. Some patients failed to respond to oral metformin in regulating blood glucose and still needed insulin, which may bias the results. Although 10 included literatures were good, some literatures did not describe allocation concealment and blind method, which may cause implementation bias. Funnel plot showed possible publication bias, the number of included literatures was small, and the sample of participants was also small. The relevant studies still need to be supported by evidence from the study with higher quality RCT.
Summary
The results of this meta-analysis showed that the application of oral hypoglycemic drug metformin in the blood glucose control of gestational diabetes can play a hypoglycemic effect equivalent to insulin, control the weight of pregnant women, reduce the rate of abortion and cesarean section, and improve pregnancy outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
TW is mainly responsible for the writing, data analysis, and research design of the article. The corresponding author is WZ, and she is responsible for ensuring that the descriptions are accurate and agreed by all authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Wilkie G, Orr L, Leung K, Leftwich H. Comparison of intrapartum glycemic management strategies in pregnant women with type 1 diabetes. J Matern Fetal Neonatal Med (2021) 6:1–5. 10.1080/14767058.2021.2004114 [DOI] [PubMed] [Google Scholar]
- 2.Liu F, Sui W, Zhou ZF, Mi Y, He TQ, Li ZB, et al. Development of gestational diabetes mellitus in women with periodontitis in early pregnancy: a population-based clinical study. J Clin Periodontol (2021) 49:164–76. 10.1111/jcpe.13578 [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Wang H, Li M, Zhao R. Chemerin levels and its genetic variants are associated with gestational diabetes mellitus: a hospital-based study in a Chinese cohort. Gene (2022) 807:145888. 10.1016/j.gene.2021.145888 [DOI] [PubMed] [Google Scholar]
- 4.Murphy HR, Rayman G, Duffield K, Lewis KS, Kelly S, Johal B, et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care (2007) 30:2785–91. 10.2337/dc07-0500 [DOI] [PubMed] [Google Scholar]
- 5.Gomes-Filho IS, Pereira EC, Cruz SS, Adan LF, Vianna MI, Passos-Soares JS, et al. Relationship among mothers’ glycemic level, periodontitis, and birth weight. J Periodontol (2016) 87:238–47. 10.1902/jop.2015.150423 [DOI] [PubMed] [Google Scholar]
- 6.Walsh JM, Mahony RM, Culliton M, Foley ME, McAuliffe FM. Impact of a low glycemic index diet in pregnancy on markers of maternal and fetal metabolism and inflammation. Reprod Sci (2014) 21:1378–81. 10.1177/1933719114525275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care (2013) 36:1877–83. 10.2337/dc12-2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gojnic M, Perovic M, Pervulov M, Ljubic A. The effects of adjuvant insulin therapy among pregnant women with IGT who fail to achieve the moderate physical levels by diet and desired physical activity. J Matern Fetal Neonatal Med (2012) 25:2028–34. 10.3109/14767058.2012.672598 [DOI] [PubMed] [Google Scholar]
- 9.Cao Q, Hu Y, Fu J, Huang X, Wu L, Zhang J, et al. Gestational metformin administration in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized control studies. J Obstet Gynaecol Res (2021) 47:4148–57. 10.1111/jog.15044 [DOI] [PubMed] [Google Scholar]
- 10.Picón-César MJ, Molina-Vega M, Suárez-Arana M, González-Mesa E, Sola-Moyano AP, Roldan-López R, et al. Metformin for gestational diabetes study: metformin vs insulin in gestational diabetes: glycemic control and obstetrical and perinatal outcomes: prospective randomized trial. Am J Obstet Gynecol (2021) 225:517. E1–17. 10.1016/j.ajog.2021.04.229 [DOI] [PubMed] [Google Scholar]
- 11.Kulshrestha V, Balani S, Kachhawa G, Vanamail P, Kumari R, Sharma JB, et al. Efficacy of myoinositol in treatment of gestational diabetes mellitus in Asian Indian women: a pilot randomized clinical trial. Eur J Obstet Gynecol Reprod Biol (2021) 260:42–7. 10.1016/j.ejogrb.2021.02.017 [DOI] [PubMed] [Google Scholar]
- 12.Feig DS, Murphy K, Asztalos E, Tomlinson G, Sanchez J, Zinman B, et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multi-center randomized controlled trial. BMC Pregnancy Childbirth (2016) 16:173. 10.1186/s12884-016-0954-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey BM, Duryea EL, Abbassi-Ghanavati M, Tudela CM, Shivvers SA, McIntire DD, et al. Glyburide in women with mild gestational diabetes: a randomized controlled trial. Obstet Gynecol (2015) 126:303–9. 10.1097/AOG.0000000000000967 [DOI] [PubMed] [Google Scholar]
- 14.Beyuo T, Obed SA, Adjepong-Yamoah KK, Bugyei KA, Oppong SA, Marfoh K. Metformin versus insulin in the management of pre-gestational diabetes mellitus in pregnancy and gestational diabetes mellitus at the Korle Bu Teaching Hospital: a randomized clinical trial. PLoS One (2015) 10:125712. 10.1371/journal.pone.0125712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ainuddin J, Karim N, Hasan AA, Naqvi SA. Metformin versus insulin treatment in gestational diabetes in pregnancy in a developing country: a randomized control trial. Diabetes Res Clin Pract (2015) 107:290–9. 10.1016/j.diabres.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 16.Mirzamı M, Heidar Z, Faalpoor Z, Naeiji Z, Jamali R. Comparison of glyburide and insulin in women with gestational diabetes mellitus and associated perinatal outcome: a randomized clinical trial. Acta Med Iran (2015) 53:97–103. dx.doi.org/ [PubMed] [Google Scholar]
- 17.Spaulonci CP, Bernardes LS, Trindade TC, Zugaib M, Francisco RP. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol (2013) 209: 34.e1–347. 10.1016/j.ajog.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 18.Tempe A, Mayanglambam RD. Glyburide as treatment option for gestational diabetes mellitus. J Obstet Gynaecol Res (2013) 39:1147–52. 10.1111/jog.12042 [DOI] [PubMed] [Google Scholar]
- 19.Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract (2012) 98:422–9. 10.1016/j.diabres.2012.09.031 [DOI] [PubMed] [Google Scholar]
- 20.Pellonperä O, Rönnemaa T, Ekblad U, Vahlberg T, Tertti K. The effects of metformin treatment of gestational diabetes on maternal weight and glucose tolerance postpartum – a prospective follow-up study. Acta Obstet Gynecol Scand (2016) 95:79–87. 10.1111/aogs.12788 [DOI] [PubMed] [Google Scholar]
- 21.Refuerzo JS, Gowen R, Pedroza C, Hutchinson M, Blackwell SC, Ramin S. A pilot randomized, controlled trial of metformin versus insulin mellitus in women with type 2 diabetes mellitus during pregnancy. Am J Perinatol (2015) 30:163–70. 10.1055/s-0034-1378144 [DOI] [PubMed] [Google Scholar]
- 22.Herrera KM, Ou JP, Persad MD, Bernasko J, Garretto D, Garry D. Risk of metformin failure in the treatment of women with gestational diabetes. J Perinat Med (2021) 49:1084–8. 10.1515/jpm-2020-0435 [DOI] [PubMed] [Google Scholar]
- 23.Molina-Vega M, Picón-César MJ, Gutiérrez-Repiso C, Fearnández-Valero A, Lima-Rubio F, González-Romero S, et al. Metformin action over gut microbiota is related to weight and glycemic control in gestational diabetes mellitus: a randomized trial. Biomed Pharmacother (2021) 145:112465. 10.1016/j.biopha.2021.112465 [DOI] [PubMed] [Google Scholar]
- 24.Martine-Edith G, Johnson W, Hunsicker E, Hamer M, Petherick ES. Associations between maternal characteristics and pharmaceutical treatment of gestational diabetes: an analysis of the UK Born in Bradford (BiB) cohort study. BMJ Open (2021) 11:e053753. 10.1136/bmjopen-2021-053753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estrella J, Wiley V, Simmons D, Hng TM, McLean M. Effect of maternal metformin treatment in pregnancy on neonatal metabolism: evidence from newborn metabolic screening. Diabetes Care (2021) 44:2536–41. 10.2337/dc21-0327 [DOI] [PubMed] [Google Scholar]
- 26.Mathiesen ER, Kinsley B, Amiel SA, Heller S, McCance D, Duran S, et al. Maternal glycemic control and hypoglycemia in type 1 diabetic pregnancy: a randomized trial of insulin aspart versus human insulin in 322 pregnant women. Diabetes Care (2007) 30(4):771–6. 10.2337/dc06-1887 [DOI] [PubMed] [Google Scholar]
- 27.Moore LE, Clokey D, Rappaport VJ, Curet LB. Metformin compared with glyburide in gestational diabetes: a randomized controlled trial. Obstet Gynecol (2010) 115:55–9. 10.1097/AOG.0b013e3181c52132 [DOI] [PubMed] [Google Scholar]
- 28.Ashoush S, El-Said M, Fathi H, Abdelnaby M. Identification of poor metformin, requiring supplemental insulin, during randomization responders of metformin for the control of gestational diabetes mellitus. J Obstet Gynaecol Res (2016) 42:640–7. 10.1111/jog.12950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.